Dissecting miRNA–Gene Networks to Map Clinical Utility Roads of Pharmacogenomics-Guided Therapeutic Decisions in Cardiovascular Precision Medicine
Abstract
:1. Introduction
2. Role of miRNAs in Cardiovascular System Physiology and Pathophysiology
3. Clinical Relevance of miRNA Networks in the Clinical Practice of CVDs
4. Experimental Data Collection of miRNA CVD Biomarkers and Bioinformatic Analysis
5. Atherosclerosis
6. Cardiomyopathy
7. Lipid Metabolism Disorder
8. The Clinical Implementation of the Biomarker miRNA Networks in the CVD Cluster of Atherosclerosis and Coronary Artery Disease
9. The Clinical Implementation of the Biomarker miRNA Networks in the CVD Cluster of Cardiomyopathy and Heart Failure
10. The Clinical Implementation of the Biomarker miRNA Networks in the CVD Cluster of Lipid Metabolism Disorders
11. Discussion
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Benjamin, E.J.; Muntner, P.; Alonso, A.; Bittencourt, M.S.; Callaway, C.W.; Carson, A.P.; Chamberlain, A.M.; Chang, A.R.; Cheng, S.; Das, S.R.; et al. Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation 2019, 139, e56–e528. [Google Scholar] [CrossRef]
- Vizirianakis, I.S. Nanomedicine and personalized medicine toward the application of pharmacotyping in clinical practice to improve drug-delivery outcomes. Nanomed.-Nanotechnol. Biol. Med. 2011, 7, 11–17. [Google Scholar] [CrossRef] [PubMed]
- Vizirianakis, I.S.; Miliotou, A.N.; Mystridis, G.A.; Andriotis, E.G.; Andreadis, I.I.; Papadopoulou, L.C.; Fatouros, D.G. Tackling pharmacological response heterogeneity by PBPK modeling to advance precision medicine productivity of nanotechnology and genomics therapeutics. Expert Rev. Precis. Med. Drug Dev. 2019, 4, 139–151. [Google Scholar] [CrossRef]
- Vizirianakis, I.S.; Fatouros, D.G. Personalized nanomedicine: Paving the way to the practical clinical utility of genomics and nanotechnology advancements. Adv. Drug Deliv. Rev. 2012, 64, 1359–1362. [Google Scholar] [CrossRef] [PubMed]
- Leopold, J.A.; Loscalzo, J. Emerging Role of Precision Medicine in Cardiovascular Disease. Circ. Res. 2018, 122, 1302–1315. [Google Scholar] [CrossRef] [PubMed]
- Zaiou, M.; El Amri, H. Cardiovascular pharmacogenetics: A promise for genomically-guided therapy and personalized medicine. Clin. Genet. 2017, 91, 355–370. [Google Scholar] [CrossRef]
- Holmes, M.V.; Richardson, T.G.; Ference, B.A.; Davies, N.M.; Smith, G.D. Integrating genomics with biomarkers and therapeutic targets to invigorate cardiovascular drug development. Nat. Rev. Cardiol. 2021, 18, 435–453. [Google Scholar] [CrossRef]
- Morton, S.U.; Quiat, D.; Seidman, J.G.; Seidman, C.E. Genomic frontiers in congenital heart disease. Nat. Rev. Cardiol. 2022, 19, 26–42. [Google Scholar] [CrossRef]
- Padmanabhan, S.; Dominiczak, A.F. Genomics of hypertension: The road to precision medicine. Nat. Rev. Cardiol. 2021, 18, 235–250. [Google Scholar] [CrossRef]
- Zhou, S.S.; Jin, J.P.; Wang, J.Q.; Zhang, Z.G.; Freedman, J.H.; Zheng, Y.; Cai, L. MiRNAs in cardiovascular diseases: Potential biomarkers, therapeutic targets and challenges review-article. Acta Pharmacol. Sin. 2018, 39, 1073–1084. [Google Scholar] [CrossRef] [Green Version]
- Siasos, G.; Bletsa, E.; Stampouloglou, P.K.; Oikonomou, E.; Tsigkou, V.; Paschou, S.A.; Vlasis, K.; Marinos, G.; Vavuranakis, M.; Stefanadis, C.; et al. MicroRNAs in cardiovascular disease. Hell. J. Cardiol. HJC Hell. Kardiol. Ep. 2020, 61, 165–173. [Google Scholar] [CrossRef] [PubMed]
- Akodad, M.; Mericskay, M.; Roubille, F. Micro-RNAs as promising biomarkers in cardiac diseases. Ann. Transl. Med. 2016, 4, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghafouri-Fard, S.; Gholipour, M.; Taheri, M. Role of microRNAs in the pathogenesis of coronary artery disease. Front. Cardiovasc. Med. 2021, 8, 632392. [Google Scholar] [CrossRef] [PubMed]
- Krammer, T.L.; Mayr, M.; Hackl, M. microRNAs as promising biomarkers of platelet activity in antiplatelet therapy monitoring. Int. J. Mol. Sci. 2020, 21, 3477. [Google Scholar] [CrossRef] [PubMed]
- Schulte, C.; Karakas, M.; Zeller, T. microRNAs in cardiovascular disease—Clinical application. Clin. Chem. Lab. Med. 2017, 55, 687–704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felekkis, K.; Papaneophytou, C. Challenges in Using Circulating Micro-RNAs as Biomarkers for Cardiovascular Diseases. Int. J. Mol. Sci. 2020, 21, 561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giassafaki, L.P.N.; Siqueira, S.; Panteris, E.; Psatha, K.; Chatzopoulou, F.; Aivaliotis, M.; Tzimagiorgis, G.; Mullertz, A.; Fatouros, D.G.; Vizirianakis, I.S. Towards analyzing the potential of exosomes to deliver microRNA therapeutics. J. Cell. Physiol. 2021, 236, 1529–1544. [Google Scholar] [CrossRef]
- Kaneto, C.M.; Nascimento, J.S.; Prado, M.; Mendonca, L.S.O. Circulating miRNAs as biomarkers in cardiovascular diseases. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 2234–2243. [Google Scholar] [CrossRef]
- Zamani, P.; Fereydouni, N.; Butler, A.E.; Navashenaq, J.G.; Sahebkar, A. The therapeutic and diagnostic role of exosomes in cardiovascular diseases. Trends Cardiovasc. Med. 2019, 29, 313–323. [Google Scholar] [CrossRef]
- Verduci, L.; Tarcitano, E.; Strano, S.; Yarden, Y.; Blandino, G. CircRNAs: Role in human diseases and potential use as biomarkers. Cell Death Dis. 2021, 12, 468. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Y.; Wang, Y.; Zhao, Y.; Ding, H.; Li, P. Circular RNAs: Functions and Clinical Significance in Cardiovascular Disease. Front. Cell Dev. Biol. 2020, 8, 584051. [Google Scholar] [CrossRef] [PubMed]
- Correia, C.C.M.; Rodrigues, L.F.; de Avila Pelozin, B.R.; Oliveira, E.M.; Fernandes, T. Long Non-Coding RNAs in Cardiovascular Diseases: Potential Function as Biomarkers and Therapeutic Targets of Exercise Training. Non-Coding RNA 2021, 7, 65. [Google Scholar] [CrossRef] [PubMed]
- Yeh, C.F.; Chang, Y.E.; Lu, C.Y.; Hsuan, C.F.; Chang, W.T.; Yang, K.C. Expedition to the missing link: Long noncoding RNAs in cardiovascular diseases. J. Biomed. Sci. 2020, 27, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beermann, J.; Piccoli, M.T.; Viereck, J.; Thum, T. Non-coding rnas in development and disease: Background, mechanisms, and therapeutic approaches. Physiol. Rev. 2016, 96, 1297–1325. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendell, J.T.; Olson, E.N. MicroRNAs in stress signaling and human disease. Cell 2012, 148, 1172–1187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carthew, R.W.; Sontheimer, E.J. Origins and Mechanisms of miRNAs and siRNAs. Cell 2009, 136, 642–655. [Google Scholar] [CrossRef] [Green Version]
- Ishizu, H.; Siomi, H.; Siomi, M.C. Biology of Piwi-interacting RNAs: New insights into biogenesis and function inside and outside of germlines. Genes Dev. 2012, 26, 2361–2373. [Google Scholar] [CrossRef] [Green Version]
- Schimmel, P. RNA Processing and Modifications: The emerging complexity of the tRNA world: Mammalian tRNAs beyond protein synthesis. Nat. Rev. Mol. Cell Biol. 2018, 19, 45–58. [Google Scholar] [CrossRef]
- Cordes, K.R.; Srivastava, D. MicroRNA regulation of cardiovascular development. Circ. Res. 2009, 104, 724–732. [Google Scholar] [CrossRef]
- Quiat, D.; Olson, E.N. MicroRNAs in cardiovascular disease: From pathogenesis to prevention and treatment. J. Clin. Investig. 2013, 123, 11–18. [Google Scholar] [CrossRef] [Green Version]
- De Rosa, S.; Curcio, A.; Indolfi, C. Emerging role of micrornas in cardiovascular diseases. Circ. J. 2014, 78, 567–575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Abdel-Mageed, A.B.; Adamidi, C.; Adelson, P.D.; Akat, K.M.; Alsop, E.; Ansel, K.M.; Arango, J.; Aronin, N.; Avsaroglu, S.K.; et al. The Extracellular RNA Communication Consortium: Establishing Foundational Knowledge and Technologies for Extracellular RNA Research. Cell 2019, 177, 231–242. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Rooij, E.; Sutherland, L.B.; Liu, N.; Williams, A.H.; McAnally, J.; Gerard, R.D.; Richardson, J.A.; Olson, E.N. A signature pattern of stress-responsive microRNAs that can evoke cardiac hypertrophy and heart failure. Proc. Natl. Acad. Sci. USA 2006, 103, 18255–18260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wronska, A.; Kurkowska-Jastrzebska, I.; Santulli, G. Application of microRNAs in diagnosis and treatment of cardiovascular disease. Acta Physiol. 2015, 213, 60–83. [Google Scholar] [CrossRef] [Green Version]
- Condorelli, G.; Latronico, M.V.G.; Cavarretta, E. MicroRNAs in cardiovascular diseases: Current knowledge and the road ahead. J. Am. Coll. Cardiol. 2014, 63, 2177–2187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, T.; Boeckel, J.N.; Groß, S.; Klotsche, J.; Palapies, L.; Leistner, D.; Pieper, L.; Stalla, G.K.; Lehnert, H.; Silber, S.; et al. Improved risk stratification in prevention by use of a panel of selected circulating microRNAs. Sci. Rep. 2017, 7, 4511. [Google Scholar] [CrossRef]
- Papageorgiou, N.; Tousoulis, D.; Androulakis, E.; Siasos, G.; Briasoulis, A.; Vogiatzi, G.; Kampoli, A.M.; Tsiamis, E.; Tentolouris, C.; Stefanadis, C. The Role of microRNAs in Cardiovascular Disease. Curr. Med. Chem. 2012, 19, 2605–2610. [Google Scholar] [CrossRef] [PubMed]
- Feinberg, M.W.; Moore, K.J. MicroRNA Regulation of Atherosclerosis. Circ. Res. 2016, 118, 703–720. [Google Scholar] [CrossRef] [Green Version]
- Hosen, M.R.; Goody, P.R.; Zietzer, A.; Nickenig, G.; Jansen, F. MicroRNAs as Master Regulators of Atherosclerosis: From Pathogenesis to Novel Therapeutic Options. Antioxid. Redox Signal. 2020, 33, 621–644. [Google Scholar] [CrossRef]
- MacRae, C.A.; Vasan, R.S. The future of genetics and genomics. Circulation 2016, 133, 2634–2639. [Google Scholar] [CrossRef]
- Lin, Y.; Qian, F.; Shen, L.; Chen, F.; Chen, J.; Shen, B. Computer-aided biomarker discovery for precision medicine: Data resources, models and applications. Brief. Bioinform. 2019, 20, 952–975. [Google Scholar] [CrossRef] [PubMed]
- Shi, R.; Ge, L.; Zhou, X.; Ji, W.J.; Lu, R.Y.; Zhang, Y.Y.; Zeng, S.; Liu, X.; Zhao, J.H.; Zhang, W.C.; et al. Decreased platelet miR-223 expression is associated with high on-clopidogrel platelet reactivity. Thromb. Res. 2013, 131, 508–513. [Google Scholar] [CrossRef] [PubMed]
- Willeit, P.; Zampetaki, A.; Dudek, K.; Kaudewitz, D.; King, A.; Kirkby, N.S.; Crosby-Nwaobi, R.; Prokopi, M.; Drozdov, I.; Langley, S.R.; et al. Circulating MicroRNAs as novel biomarkers for platelet activation. Circ. Res. 2013, 112, 595–600. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.Y.; Zhou, X.; Ji, W.J.; Shi, R.; Lu, R.Y.; Li, J.L.; Yang, G.H.; Luo, T.; Zhang, J.Q.; Zhao, J.H.; et al. Decreased circulating microRNA-223 level predicts high on-treatment platelet reactivity in patients with troponin-negative non-ST elevation acute coronary syndrome. J. Thromb. Thrombolysis 2014, 38, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Peng, L.; Liu, J.; Qin, L.; Liu, J.; Xi, S.; Lu, C.; Yin, T. Interaction between platelet-derived microRNAs and CYP2C19*2 genotype on clopidogrel antiplatelet responsiveness in patients with ACS. Thromb. Res. 2017, 157, 97–102. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Q.H.; Wang, Y.D.; Hao, Y.Y.; Juan, L.R.; Teng, M.X.; Zhang, X.J.; Li, M.M.; Wang, G.H.; Liu, Y.L. miR2Disease: A manually curated database for microRNA deregulation in human disease. Nucleic Acids Res. 2009, 37, D98–D104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruepp, A.; Kowarsch, A.; Schmidl, D.; Buggenthin, F.; Brauner, B.; Dunger, I.; Fobo, G.; Frishman, G.; Montrone, C.; Theis, F.J. PhenomiR: A knowledgebase for microRNA expression in diseases and biological processes. Genome Biol. 2010, 11, R6. [Google Scholar] [CrossRef] [Green Version]
- Ru, Y.B.; Kechris, K.J.; Tabakoff, B.; Hoffman, P.; Radcliffe, R.A.; Bowler, R.; Mahaffey, S.; Rossi, S.; Calin, G.A.; Bemis, L.; et al. The multiMiR R package and database: Integration of microRNA-target interactions along with their disease and drug associations. Nucleic Acids Res. 2014, 42, e133. [Google Scholar] [CrossRef]
- Hsu, S.D.; Lin, F.M.; Wu, W.Y.; Liang, C.; Huang, W.C.; Chan, W.L.; Tsai, W.T.; Chen, G.Z.; Lee, C.J.; Chiu, C.M.; et al. miRTarBase: A database curates experimentally validated microRNA-target interactions. Nucleic Acids Res. 2011, 39, D163–D169. [Google Scholar] [CrossRef] [Green Version]
- Xiao, F.F.; Zuo, Z.X.; Cai, G.S.; Kang, S.L.; Gao, X.L.; Li, T.B. miRecords: An integrated resource for microRNA-target interactions. Nucleic Acids Res. 2009, 37, D105–D110. [Google Scholar] [CrossRef]
- Li, P.P.D.; Fu, Y.X.; Ru, J.L.; Huang, C.; Du, J.F.; Zheng, C.L.; Chen, X.T.; Li, P.P.D.; Lu, A.P.; Yang, L.; et al. Insights from systems pharmacology into cardiovascular drug discovery and therapy. BMC Syst. Biol. 2014, 8, s12918–s13014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vizirianakis, I.S.; Chatzopoulou, F.; Papazoglou, A.S.; Karagiannidis, E.; Sofdis, G.; Stalikas, N.; Stefopoulos, C.; Kyritsis, K.A.; Mittas, N.; Theodoroula, N.F.; et al. The GEnetic Syntax Score: A genetic risk assessment implementation tool grading the complexity of coronary artery disease-rationale and design of the GESS study. BMC Cardiovasc. Disord. 2021, 21, 284. [Google Scholar] [CrossRef]
- Yu, G.C.; Wang, L.G.; Yan, G.R.; He, Q.Y. DOSE: An R/Bioconductor package for disease ontology semantic and enrichment analysis. Bioinformatics 2015, 31, 608–609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.C.; He, Q.Y. ReactomePA: An R/Bioconductor package for reactome pathway analysis and visualization. Mol. Biosyst. 2016, 12, 477–479. [Google Scholar] [CrossRef] [PubMed]
- Wu, T.; Hu, E.; Xu, S.; Chen, M.; Guo, P.; Dai, Z.; Feng, T.; Zhou, L.; Tang, W.; Zhan, L.; et al. ClusterProfiler 4.0: A universal enrichment tool for interpreting omics data. Innovation 2021, 2, 100141. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.C.; Wang, L.G.; Han, Y.Y.; He, Q.Y. ClusterProfiler: An R Package for Comparing Biological Themes among Gene Clusters. Omics-A J. Integr. Biol. 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Z.; Du, Z.D.; Payattakool, R.; Yu, P.S.; Chen, C.F. A new method to measure the semantic similarity of GO terms. Bioinformatics 2007, 23, 1274–1281. [Google Scholar] [CrossRef] [Green Version]
- Gu, Z.G.; Eils, R.; Schlesner, M. Complex heatmaps reveal patterns and correlations in multidimensional genomic data. Bioinformatics 2016, 32, 2847–2849. [Google Scholar] [CrossRef] [Green Version]
- Shannon, P.; Markiel, A.; Ozier, O.; Baliga, N.S.; Wang, J.T.; Ramage, D.; Amin, N.; Schwikowski, B.; Ideker, T. Cytoscape: A software environment for integrated models of biomolecular interaction networks. Genome Res. 2003, 13, 2498–2504. [Google Scholar] [CrossRef]
- Moreau, P.R.; Bosch, V.T.; Bouvy-Liivrand, M.; Ounap, K.; Ord, T.; Pulkkinen, H.H.; Polonen, P.; Heinaniemi, M.; Yla-Herttuala, S.; Laakkonen, J.P.; et al. Profiling of Primary and Mature miRNA Expression in Atherosclerosis-Associated Cell Types. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 2149–2167. [Google Scholar] [CrossRef]
- Conrad, T.; Marsico, A.; Gehre, M.; Orom, U.A. Microprocessor Activity Controls Differential miRNA Biogenesis In Vivo. Cell Rep. 2014, 9, 542–554. [Google Scholar] [CrossRef] [Green Version]
- Xiong, X.D.; Cho, M.; Cai, X.P.; Cheng, J.; Jing, X.; Cen, J.M.; Liu, X.; Yang, X.L.; Suh, Y. A common variant in pre-miR-146 is associated with coronary artery disease risk and its mature miRNA expression. Mutat. Res.-Fundam. Mol. Mech. Mutagenesis 2014, 761, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Quan, X.; Ji, Y.; Zhang, C.; Guo, X.; Zhang, Y.; Jia, S.; Ma, W.; Fan, Y.; Wang, C. Circulating MiR-146a May be a Potential Biomarker of Coronary Heart Disease in Patients with Subclinical Hypothyroidism. Cell. Physiol. Biochem. 2018, 45, 226–236. [Google Scholar] [CrossRef] [PubMed]
- Dang, L.T.H.; Lawson, N.D.; Fish, J.E. MicroRNA Control of Vascular Endothelial Growth Factor Signaling Output during Vascular Development. Arterioscler. Thromb. Vasc. Biol. 2013, 33, 193–200. [Google Scholar] [CrossRef] [Green Version]
- Carmeliet, P. Manipulating angiogenesis in medicine. J. Intern. Med. 2004, 255, 538–561. [Google Scholar] [CrossRef] [PubMed]
- Olsson, A.K.; Dimberg, A.; Kreuger, J.; Claesson-Welsh, L. VEGF receptor signalling—In control of vascular function. Nat. Rev. Mol. Cell Biol. 2006, 7, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Sundquist, K.; Svensson, P.J.; Rastkhani, H.; Palmer, K.; Memon, A.A.; Sundquist, J.; Zoller, B. Association of recurrent venous thromboembolism and circulating microRNAs. Clin. Epigenet. 2019, 11, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, Y.J.; Yuan, Y.Q.; Qiu, C.G. Underexpression of CACNA1C Caused by Overexpression of microRNA-29a Underlies the Pathogenesis of Atrial Fibrillation. Med. Sci. Monit. 2016, 22, 2175–2181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nickenig, G.; Harrison, D.G. The AT1-type angiotensin receptor in oxidative stress and atherogenesis. Part II: AT1 receptor regulation. Circulation 2002, 105, 530–536. [Google Scholar] [CrossRef] [Green Version]
- Zhu, N.; Zhang, D.; Chen, S.; Liu, X.; Lin, L.; Huang, X.; Guo, Z.; Liu, J.; Wang, Y.; Yuan, W.; et al. Endothelial enriched microRNAs regulate angiotensin II-induced endothelial inflammation and migration. Atherosclerosis 2011, 215, 286–293. [Google Scholar] [CrossRef]
- Zheng, L.; Xu, C.C.; Chen, W.D.; Shen, W.L.; Ruan, C.C.; Zhu, L.M.; Zhu, D.L.; Gao, P.J. MicroRNA-155 regulates angiotensin II type 1 receptor expression and phenotypic differentiation in vascular adventitial fibroblasts. Biochem. Biophys. Res. Commun. 2010, 400, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Haas, U.; Sczakiel, G.; Laufer, S.D. MicroRNA-mediated regulation of gene expression is affected by disease-associated SNPs within the 3′-UTR via altered RNA structure. RNA Biol. 2012, 9, 924–937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ceolotto, G.; Papparella, I.; Bortoluzzi, A.; Strapazzon, G.; Ragazzo, F.; Bratti, P.; Fabricio, A.S.C.; Squarcina, E.; Gion, M.; Palatini, P.; et al. Interplay between miR-155, AT1R A1166C polymorphism, and AT1R expression in young untreated hypertensives. Am. J. Hypertens. 2011, 24, 241–246. [Google Scholar] [CrossRef] [PubMed]
- Stanković, A.; Kolaković, A.; Živković, M.; Djurić, T.; Bundalo, M.; Končar, I.; Davidović, L.; Alavantić, D. Angiotensin receptor type 1 polymorphism A1166C is associated with altered AT1R and miR-155 expression in carotid plaque tissue and development of hypoechoic carotid plaques. Atherosclerosis 2016, 248, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, J.L.; Brown, M.S. A century of cholesterol and coronaries: From plaques to genes to statins. Cell 2015, 161, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurtz, C.L.; Fannin, E.E.; Toth, C.L.; Pearson, D.S.; Vickers, K.C.; Sethupathy, P. Inhibition of miR-29 has a significant lipid-lowering benefit through suppression of lipogenic programs in liver. Sci. Rep. 2015, 5, 12911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.X.; Gao, M.; Li, C.Z.; Yu, C.Z.; Yan, H.; Peng, C.; Li, Y.; Li, C.G.; Ma, Z.L.; Zhao, Y.; et al. Dicer1/miR-29/HMGCR axis contributes to hepatic free cholesterol accumulation in mouse non-alcoholic steatohepatitis. Acta Pharmacol. Sin. 2017, 38, 660–671. [Google Scholar] [CrossRef]
- Selitsky, S.R.; Dinh, T.A.; Toth, C.L.; Kurtz, C.L.; Honda, M.; Struck, B.R.; Kaneko, S.; Vickers, K.C.; Lemon, S.M.; Sethupathy, P. Transcriptomic analysis of chronic hepatitis b and c and liver cancer reveals microrna-mediated control of cholesterol synthesis programs. mBio 2015, 6, e01500-15. [Google Scholar] [CrossRef] [Green Version]
- Kirac, D.; Bayam, E.; Dagdelen, M.; Gezmis, H.; Sarikaya, S.; Pala, S.; Altunok, E.C.; Genc, E. HMGCR and ApoE mutations may cause different responses to lipid lowering statin therapy. Cell. Mol. Biol. 2017, 6, 43–48. [Google Scholar] [CrossRef]
- Bentzon, J.F.; Otsuka, F.; Virmani, R.; Falk, E. Mechanisms of plaque formation and rupture. Circ. Res. 2014, 114, 1852–1866. [Google Scholar] [CrossRef]
- Kolodgie, F.D.; Burke, A.P.; Farb, A.; Gold, H.K.; Yuan, J.; Narula, J.; Finn, A.V.; Virmani, R. The thin-cap fibroatheroma: A type of vulnerable plaque the major precursor lesion to acute coronary syndromes. Curr. Opin. Cardiol. 2001, 16, 285–292. [Google Scholar] [CrossRef] [PubMed]
- Misra, A.; Feng, Z.; Chandran, R.R.; Kabir, I.; Rotllan, N.; Aryal, B.; Sheikh, A.Q.; Ding, L.; Qin, L.; Fernández-Hernando, C.; et al. Integrin beta3 regulates clonality and fate of smooth muscle-derived atherosclerotic plaque cells. Nat. Commun. 2018, 9, 2073. [Google Scholar] [CrossRef] [PubMed]
- Motovska, Z.; Kvasnicka, J.; Widimsky, P.; Petr, R.; Hajkova, J.; Bobcikova, P.; Osmancik, P.; Odvodyova, D.; Katina, S. Platelet glycoprotein GP VI 13254C allele is an independent risk factor of premature myocardial infarction. Thromb. Res. 2010, 125, e61–e64. [Google Scholar] [CrossRef] [PubMed]
- Weiss, E.J.; Bray, P.F.; Tayback, M.; Schulman, S.P.; Kickler, T.S.; Becker, L.C.; Weiss, J.L.; Gerstenblith, G.; Goldschmidt-Clermont, P.J. A Polymorphism of a Platelet Glycoprotein Receptor as an Inherited Risk Factor for Coronary Thrombosis. N. Engl. J. Med. 1996, 334, 1090–1094. [Google Scholar] [CrossRef] [Green Version]
- Kucharska-Newton, A.M.; Monda, K.L.; Campbell, S.; Bradshaw, P.T.; Wagenknecht, L.E.; Boerwinkle, E.; Wasserman, B.A.; Heiss, G. Association of the platelet GPIIb/IIIa polymorphism with atherosclerotic plaque morphology: The Atherosclerosis Risk in Communities (ARIC) Study. Atherosclerosis 2011, 216, 151–156. [Google Scholar] [CrossRef] [Green Version]
- Evans, R.M.; Barish, G.D.; Wang, Y.X. PPARs and the complex journey to obesity. Nat. Med. 2004, 10, 355–361. [Google Scholar] [CrossRef]
- Torra, I.P.; Chinetti, G.; Duval, C.; Fruchart, J.C.; Staels, B. Peroxisome proliferator-activated receptors: From transcriptional control to clinical practice. Curr. Opin. Lipidol. 2001, 12, 245–254. [Google Scholar] [CrossRef]
- Tyagi, S.; Gupta, P.; Saini, A.; Kaushal, C.; Sharma, S. The peroxisome proliferator-activated receptor: A family of nuclear receptors role in various diseases. J. Adv. Pharm. Technol. Res. 2011, 2, 236–240. [Google Scholar] [CrossRef]
- Chandra, M.; Miriyala, S.; Panchatcharam, M. PPAR γ and Its Role in Cardiovascular Diseases. PPAR Res. 2017, 2017, 6404638. [Google Scholar] [CrossRef] [Green Version]
- Lee, E.K.; Lee, M.J.; Abdelmohsen, K.; Kim, W.; Kim, M.M.; Srikantan, S.; Martindale, J.L.; Hutchison, E.R.; Kim, H.H.; Marasa, B.S.; et al. miR-130 Suppresses Adipogenesis by Inhibiting Peroxisome Proliferator-Activated Receptor Expression. Mol. Cell. Biol. 2011, 31, 626–638. [Google Scholar] [CrossRef] [Green Version]
- Motawi, T.K.; Shaker, O.G.; Ismail, M.F.; Sayed, N.H. Peroxisome Proliferator-Activated Receptor Gamma in Obesity and Colorectal Cancer: The Role of Epigenetics. Sci. Rep. 2017, 7, 10714. [Google Scholar] [CrossRef] [PubMed]
- Lin, Q.; Gao, Z.; Alarcon, R.M.; Ye, J.; Yun, Z. A role of miR-27 in the regulation of adipogenesis. FEBS J. 2009, 276, 2348–2358. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.F.; Fu, W.M.; He, M.L.; Xie, W.D.; Lv, Q.; Wan, G.; Li, G.; Wang, H.; Lu, G.; Hu, X.; et al. MiRNA-20a promotes osteogenic differentiation of human mesenchymal stem cells by co-regulating BMP signaling. RNA Biol. 2011, 8, 829–838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oladi, M.; Nohtani, M.; Avan, A.; Mirhafez, S.R.; Tajbakhsh, A.; Ghasemi, F.; Asadi, A.; Elahdadi Salmani, M.; Mohammadi, A.; Hoseinzadeh, L.; et al. Impact of the C1431T polymorphism of the peroxisome proliferator activated receptor-gamma (PPAR-γ) gene on fasted serum lipid levels in patients with coronary artery disease. Ann. Nutr. Metab. 2015, 66, 149–154. [Google Scholar] [CrossRef] [PubMed]
- Lagou, V.; Scott, R.A.; Manios, Y.; Chen, T.L.J.; Wang, G.; Grammatikaki, E.; Kortsalioudaki, C.; Liarigkovinos, T.; Moschonis, G.; Roma-Giannikou, E.; et al. Impact of peroxisome proliferator-activated receptors γ and δ on adiposity in toddlers and preschoolers in the GENESIS study. Obesity 2008, 16, 913–918. [Google Scholar] [CrossRef]
- Ding, S.; Liu, L.; Zhuge, Q.C.; Yu, Z.; Zhang, X.; Xie, J.; Hong, W.; Wang, S.; Yang, Y.; Chen, B. The meta-analysis of the association of PPARG P12A, C161T polymorphism and coronary heart disease. Wien. Klin. Wochenschr. 2012, 124, 671–677. [Google Scholar] [CrossRef]
- Wang, P.; Wang, Q.; Yin, Y.; Yang, Z.; Li, W.; Liang, D.; Zhou, P. Association between peroxisome proliferator-activated receptor gamma gene polymorphisms and atherosclerotic diseases: A meta-analysis of case-control studies. J. Atheroscler. Thromb. 2015, 22, 912–925. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Chen, X.; Chen, L.; Chen, K.; Zhou, J.; Song, J. MiR-1-3p that correlates with left ventricular function of HCM can serve as a potential target and differentiate HCM from DCM. J. Transl. Med. 2018, 16, 161. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Xing, Q.; Zhou, X.; Li, J.; Li, Y.; Zhang, L.; Zhou, Q.; Tang, B. Circulating miRNA-21 is a promising biomarker for heart failure. Mol. Med. Rep. 2017, 16, 7766–7774. [Google Scholar] [CrossRef] [Green Version]
- Ranade, K.; Jorgenson, E.; Sheu, W.H.-H.; Pei, D.; Hsiung, C.A.; Chiang, F.T.; Chen, Y.D.I.; Pratt, R.; Olshen, R.A.; Curb, D.; et al. A polymorphism in the α1 adrenergic receptor is associated with resting heart rate. Am. J. Hum. Genet. 2002, 70, 935–942. [Google Scholar] [CrossRef] [Green Version]
- Börjesson, M.; Magnusson, Y.; Hjalmarson, Å.; Andersson, B. A novel polymorphism in the gene coding for the beta1-adrenergic receptor associated with survival in patients with heart failure. Eur. Heart J. 2000, 21, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Mak, T.W.; Hauck, L.; Grothe, D.; Billia, F. p53 regulates the cardiac transcriptome. Proc. Natl. Acad. Sci. USA 2017, 114, 2331–2336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nomura, S.; Satoh, M.; Fujita, T.; Higo, T.; Sumida, T.; Ko, T.; Yamaguchi, T.; Tobita, T.; Naito, A.T.; Ito, M.; et al. Cardiomyocyte gene programs encoding morphological and functional signatures in cardiac hypertrophy and failure. Nat. Commun. 2018, 9, 4435. [Google Scholar] [CrossRef] [PubMed]
- Rokavec, M.; Li, H.; Jiang, L.; Hermeking, H. The p53/miR-34 axis in development and disease. J. Mol. Cell Biol. 2014, 6, 214–230. [Google Scholar] [CrossRef] [Green Version]
- Lee, R.C.; Feinbaum, R.L.; Ambros, V. The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 1993, 75, 843–854. [Google Scholar] [CrossRef]
- Chen, C.Y.; Lee, D.S.; Choong, O.K.; Chang, S.K.; Hsu, T.; Nicholson, M.W.; Liu, L.W.; Lin, P.J.; Ruan, S.C.; Lin, S.W.; et al. Cardiac-specific microRNA-125b deficiency induces perinatal death and cardiac hypertrophy. Sci. Rep. 2021, 11, 2377. [Google Scholar] [CrossRef]
- Zhou, H.; Sen, L.I.N.; Youdong, H.U.; Dianxuan, G.U.O.; Wang, Y.; Xia, L.I. miR-125a-5p and miR-7 inhibits the proliferation, migration and invasion of vascular smooth muscle cell by targeting EGFR. Mol. Med. Rep. 2021, 24, 708. [Google Scholar] [CrossRef]
- Prasad, S.V.N.; Gupta, M.K.; Duan, Z.H.; Surampudi, V.S.K.; Liu, C.G.; Kotwal, A.; Moravec, C.S.; Starling, R.C.; Perez, D.M.; Sen, S.; et al. A unique microRNA profile in end-stage heart failure indicates alterations in specific cardiovascular signaling networks. PLoS ONE 2017, 12, e0170456. [Google Scholar] [CrossRef] [Green Version]
- Ritter, C.A.; Arteaga, C.L. The epidermal growth factor receptor-tyrosine kinase: A promising therapeutic target in solid tumors. Semin. Oncol. 2003, 30, 3–11. [Google Scholar] [CrossRef]
- Dyck, J.R.B.; Lopaschuk, G.D. Malonyl CoA Control of Fatty Acid Oxidation in the Ischemic Heart. J. Mol. Cell. Cardiol. 2002, 34, 1099–1109. [Google Scholar] [CrossRef]
- Gimbrone, M.A.; García-Cardeña, G. Endothelial Cell Dysfunction and the Pathobiology of Atherosclerosis. Circ. Res. 2016, 118, 620–636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Libby, P.; Ridker, P.M.; Hansson, G.K. Progress and challenges in translating the biology of atherosclerosis. Nature 2011, 473, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Thavarajah, T.; Gu, W.D.; Cai, J.J.; Xu, Q.B. Impact of miRNA in Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2018, 38, E159–E170. [Google Scholar] [CrossRef] [Green Version]
- Pereira-da-Silva, T.; Cruz, M.C.; Carrusca, C.; Ferreira, R.C.; Napoleao, P.; Carmo, M.M. Circulating microRNA profiles in different arterial territories of stable atherosclerotic disease: A systematic review. Am. J. Cardiovasc. Dis. 2018, 8, 1–13. [Google Scholar] [PubMed]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Koppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of MicroRNA-126 by Apoptotic Bodies Induces CXCL12-Dependent Vascular Protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef]
- Economou, E.K.; Oikonomou, E.; Siasos, G.; Papageorgiou, N.; Tsalamandris, S.; Mourouzis, K.; Papaioanou, S.; Tousoulis, D. The role of microRNAs in coronary artery disease: From pathophysiology to diagnosis and treatment. Atherosclerosis 2015, 241, 624–633. [Google Scholar] [CrossRef]
- Jansen, F.; Yang, X.; Proebsting, S.; Hoelscher, M.; Przybilla, D.; Baumann, K.; Schmitz, T.; Dolf, A.; Endl, E.; Franklin, B.S.; et al. MicroRNA expression in circulating microvesicles predicts cardiovascular events in patients with coronary artery disease. J. Am. Heart Assoc. 2014, 3, e001249. [Google Scholar] [CrossRef] [Green Version]
- Fichtlscherer, S.; De Rosa, S.; Fox, H.; Schwietz, T.; Fischer, A.; Liebetrau, C.; Weber, M.; Hamm, C.W.; Roxe, T.; Muller-Ardogan, M.; et al. Circulating MicroRNAs in Patients With Coronary Artery Disease. Circ. Res. 2010, 107, 677–684. [Google Scholar] [CrossRef] [Green Version]
- Santulli, G. microRNAs Distinctively Regulate Vascular Smooth Muscle and Endothelial Cells: Functional Implications in Angiogenesis, Atherosclerosis, and In-Stent Restenosis. Microrna Basic Sci. Mol. Biol. Clin. Pract. 2015, 887, 53–77. [Google Scholar] [CrossRef] [Green Version]
- Wei, Y.Y.; Nazari-Jahantigh, M.; Neth, P.; Weber, C.; Schober, A. MicroRNA-126,-145, and -155 A Therapeutic Triad in Atherosclerosis? Arterioscler. Thromb. Vasc. Biol. 2013, 33, 449–454. [Google Scholar] [CrossRef] [Green Version]
- Fang, Q.; Tian, M.; Wang, F.; Zhang, Z.H.; Du, T.Y.; Wang, W.; Yang, Y.; Li, X.Q.; Chen, G.Z.; Xiao, L.; et al. Amlodipine induces vasodilation via Akt2/Sp1-activated miR-21 in smooth muscle cells. Br. J. Pharmacol. 2019, 176, 2306–2320. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, Y.; Satoh, M.; Minami, Y.; Tabuchi, T.; Itoh, T.; Nakamura, M. Expression of miR-I 46a/b is associated with the Toll-like receptor 4 signal in coronary artery disease: Effect of renin-angiotensin system blockade and statins on miRNA-I 46a/b and Toll-like receptor 4 levels. Clin. Sci. 2010, 119, 395–405. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.J.; Liu, D.Q.; Chen, X.; Li, J.; Li, L.M.; Bian, Z.; Sun, F.; Lu, J.W.; Yin, Y.A.; Cai, X.; et al. Secreted Monocytic miR-150 Enhances Targeted Endothelial Cell Migration. Mol. Cell 2010, 39, 133–144. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.Q.; Shao, G.F.; Chen, X.L.; Yang, X.; Huang, X.Y.; Peng, P.; Ba, Y.N.; Zhang, L.; Jehangir, T.; Bu, S.Z.; et al. miRNA 206 and miRNA 574-5p are highly expression in coronary artery disease. Biosci. Rep. 2016, 36, e00295. [Google Scholar] [CrossRef] [PubMed]
- Li, H.Y.; Zhao, X.; Liu, Y.Z.; Meng, Z.; Wang, D.; Yang, F.; Shi, Q.W. Plasma MicroRNA-126-5p is Associated with the Complexity and Severity of Coronary Artery Disease in Patients with Stable Angina Pectoris. Cell. Physiol. Biochem. 2016, 39, 837–846. [Google Scholar] [CrossRef]
- Tang, Y.; Zhang, Y.C.; Chen, Y.; Xiang, Y.; Shen, C.X.; Li, Y.G. The role of miR-19b in the inhibition of endothelial cell apoptosis and its relationship with coronary artery disease. Sci. Rep. 2015, 5, 15132. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.W.; Huang, T.S.; Lo, H.H.; Huang, P.H.; Lin, C.C.; Chang, S.J.; Liao, K.H.; Tsai, C.H.; Chan, C.H.; Tsai, C.F.; et al. Deficiency of the MicroRNA-31-MicroRNA-720 Pathway in the Plasma and Endothelial Progenitor Cells from Patients With Coronary Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2014, 34, 857–869. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sayed, A.S.M.; Xia, K.; Li, F.; Deng, X.; Salma, U.; Li, T.B.; Deng, H.; Yang, D.F.; Zhou, H.Y.; Yang, T.L.; et al. The diagnostic value of circulating microRNAs for middle-aged (40-60-year-old) coronary artery disease patients. Clinics 2015, 70, 257–263. [Google Scholar] [CrossRef]
- Dong, J.; Liang, Y.Z.; Zhang, J.; Wu, L.J.; Wang, S.; Hua, Q.; Yan, Y.X. Potential Role of Lipometabolism-Related MicroRNAs in Peripheral Blood Mononuclear Cells as Biomarkers for Coronary Artery Disease. J. Atheroscler. Thromb. 2017, 24, 430–441. [Google Scholar] [CrossRef] [Green Version]
- Vindis, C.; Faccini, J.; Ruidavets, J.B.; Cordelier, P.; Martins, F.; Maoret, J.J.; Ferrieres, J.; Elbaz, M.; Bongard, V.; Ferrieres, J.; et al. Circulating miR-155, miR-145 and let-7c as diagnostic biomarkers of the coronary artery disease. Atherosclerosis 2017, 263, E277. [Google Scholar] [CrossRef] [Green Version]
- Zeller, T.; Keller, T.; Ojeda, F.; Reichlin, T.; Twerenbold, R.; Tzikas, S.; Wild, P.S.; Reiter, M.; Czyz, E.; Lackner, K.J.; et al. Assessment of microRNAs in patients with unstable angina pectoris. Eur. Heart J. 2014, 35, 2106–2114. [Google Scholar] [CrossRef]
- D’Alessandra, Y.; Devanna, P.; Limana, F.; Straino, S.; Di Carlo, A.; Brambilla, P.G.; Rubino, M.; Carena, M.C.; Spazzafumo, L.; De Simone, M.; et al. Circulating microRNAs are new and sensitive biomarkers of myocardial infarction. Eur. Heart J. 2010, 31, 2765–2773. [Google Scholar] [CrossRef] [Green Version]
- Thygesen, K.; Alpert, J.S.; Jaffe, A.S.; Simoons, M.L.; Chaitman, B.R.; White, H.D.; Katus, H.A.; Apple, F.S.; Lindahl, B.; Morrow, D.A.; et al. Third Universal Definition of Myocardial Infarction. Circulation 2012, 126, 2020–2035. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.R.; Li, N.; Zhang, Y.H.; Ran, Y.Q.; Pu, J.L. Circulating MicroRNAs are Promising Novel Biomarkers of Acute Myocardial Infarction. Intern. Med. 2011, 50, 1789–1795. [Google Scholar] [CrossRef] [Green Version]
- Ward, J.A.; Esa, N.; Pidikiti, R.; Freedman, J.E.; Keaney, J.F.; Tanriverdi, K.; Vitseva, O.; Ambros, V.; Lee, R.; McManus, D.D. Circulating Cell and Plasma microRNA Profiles Differ between Non-ST-Segment and ST-Segment-Elevation Myocardial Infarction. Fam. Med. Med. Sci. Res. 2013, 2, 108. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.D.; Guo, X.M.; Zhong, W.; Weng, R.Q.; Liu, J.; Gu, X.D.; Zhong, Z.X. Circulating MicroRNA Expression Profiles in Patients with Stable and Unstable Angina. Clinics 2020, 75, e1546. [Google Scholar] [CrossRef] [PubMed]
- Gao, H.; Guddeti, R.R.; Matsuzawa, Y.; Liu, L.P.; Su, L.X.; Guo, D.; Nie, S.P.; Du, J.; Zhang, M. Plasma Levels of microRNA-145 Are Associated with Severity of Coronary Artery Disease. PLoS ONE 2015, 10, e0123477. [Google Scholar] [CrossRef] [Green Version]
- Guo, J.F.; Zhang, Y.; Zheng, Q.X.; Zhang, Y.; Zhou, H.H.; Cui, L.M. Association between elevated plasma microRNA-223 content and severity of coronary heart disease. Scand. J. Clin. Lab. Investig. 2018, 78, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.Q.; Liang, C.; He, Z.Q.; Fan, M.; Wu, Z.G. Circulating miR-214 is associated with the severity of coronary artery disease. J. Geriatr. Cardiol. 2013, 10, 34–38. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.K.; Ma, J. Alteration in microRNA-155 level correspond to severity of coronary heart disease. Scand. J. Clin. Lab. Investig. 2018, 78, 219–223. [Google Scholar] [CrossRef]
- Zhong, J.F.; He, Y.; Chen, W.J.; Shui, X.R.; Chen, C.; Lei, W. Circulating microRNA-19a as a Potential Novel Biomarker for Diagnosis of Acute Myocardial Infarction. Int. J. Mol. Sci. 2014, 15, 20355–20364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gadde, S.; Rayner, K.J. Nanomedicine Meets microRNA: Current Advances in RNA-Based Nanotherapies for Atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2016, 36, e73–e79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.X.; Alkhoury, K.; Wang, Y.I.; Foster, G.A.; Radecke, C.E.; Tam, K.; Edwards, C.M.; Facciotti, M.T.; Armstrong, E.J.; Knowlton, A.A.; et al. IRF-1 and miRNA126 Modulate VCAM-1 Expression in Response to a High-Fat Meal. Circ. Res. 2012, 111, 1054–1064. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hergenreider, E.; Heydt, S.; Tréguer, K.; Boettger, T.; Horrevoets, A.J.G.; Zeiher, A.M.; Scheffer, M.P.; Frangakis, A.S.; Yin, X.; Mayr, M.; et al. Atheroprotective communication between endothelial cells and smooth muscle cells through miRNAs. Nat. Cell Biol. 2012, 14, 249–256. [Google Scholar] [CrossRef]
- Hu, S.; Huang, M.; Li, Z.; Jia, F.; Ghosh, Z.; Lijkwan, M.A.; Fasanaro, P.; Sun, N.; Wang, X.; Martelli, F.; et al. MicroRNA-210 as a novel therapy for treatment of ischemic heart disease. Circulation 2010, 122, S124–S131. [Google Scholar] [CrossRef] [Green Version]
- Briasoulis, A.; Tousoulis, D.; Vogiatzi, G.; Siasos, G.; Papageorgiou, N.; Oikonomou, E.; Genimata, V.; Konsola, T.; Stefanadis, C. MicroRNAs: Biomarkers for Cardiovascular Disease in Patients with Diabetes Mellitus. Curr. Top. Med. Chem. 2013, 13, 1533–1539. [Google Scholar] [CrossRef]
- Carè, A.; Catalucci, D.; Felicetti, F.; Bonci, D.; Addario, A.; Gallo, P.; Bang, M.L.; Segnalini, P.; Gu, Y.; Dalton, N.D.; et al. MicroRNA-133 controls cardiac hypertrophy. Nat. Med. 2007, 13, 613–618. [Google Scholar] [CrossRef]
- Chen, J.-F.; Murchison, E.P.; Tang, R.; Callis, T.E.; Tatsuguchi, M.; Deng, Z.; Rojas, M.; Hammond, S.M.; Schneider, M.D.; Selzman, C.H.; et al. Targeted deletion of Dicer in the heart leads to dilated cardiomyopathy and heart failure. Proc. Natl. Acad. Sci. USA 2008, 105, 2111–2116. [Google Scholar] [CrossRef] [Green Version]
- Cheng, Y.; Ji, R.; Yue, J.; Yang, J.; Liu, X.; Chen, H.; Dean, D.B.; Zhang, C. MicroRNAs Are Aberrantly Expressed in Hypertrophic Heart. Am. J. Pathol. 2007, 170, 1831–1840. [Google Scholar] [CrossRef] [Green Version]
- Halkein, J.; Tabruyn, S.P.; Ricke-Hoch, M.; Haghikia, A.; Nguyen, N.-Q.-N.; Scherr, M.; Castermans, K.; Malvaux, L.; Lambert, V.; Thiry, M.; et al. MicroRNA-146a is a therapeutic target and biomarker for peripartum cardiomyopathy. J. Clin. Investig. 2013, 123, 2143–2154. [Google Scholar] [CrossRef]
- Ikeda, S.; He, A.; Kong, S.W.; Lu, J.; Bejar, R.; Bodyak, N.; Lee, K.-H.; Ma, Q.; Kang, P.M.; Golub, T.R.; et al. MicroRNA-1 Negatively Regulates Expression of the Hypertrophy-Associated Calmodulin and Mef2a Genes. Mol. Cell. Biol. 2009, 29, 2193–2204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaguszewski, M.; Osipova, J.; Ghadri, J.-R.; Napp, L.C.; Widera, C.; Franke, J.; Fijalkowski, M.; Nowak, R.; Fijalkowska, M.; Volkmann, I.; et al. A signature of circulating microRNAs differentiates takotsubo cardiomyopathy from acute myocardial infarction. Eur. Heart J. 2014, 35, 999–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jakubik, D.; Fitas, A.; Eyileten, C.; Jarosz-Popek, J.; Nowak, A.; Czajka, P.; Wicik, Z.; Sourij, H.; Siller-Matula, J.M.; De Rosa, S.; et al. MicroRNAs and long non-coding RNAs in the pathophysiological processes of diabetic cardiomyopathy: Emerging biomarkers and potential therapeutics. Cardiovasc. Diabetol. 2021, 20, 55. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, Y.; Ono, K.; Horie, T.; Nishi, H.; Nagao, K.; Kinoshita, M.; Watanabe, S.; Baba, O.; Kojima, Y.; Shizuta, S.; et al. Increased MicroRNA-1 and MicroRNA-133a Levels in Serum of Patients With Cardiovascular Disease Indicate Myocardial Damage. Circ. Cardiovasc. Genet. 2011, 4, 446–454. [Google Scholar] [CrossRef]
- Zhao, Y.; Ransom, J.F.; Li, A.; Vedantham, V.; von Drehle, M.; Muth, A.N.; Tsuchihashi, T.; McManus, M.T.; Schwartz, R.J.; Srivastava, D. Dysregulation of Cardiogenesis, Cardiac Conduction, and Cell Cycle in Mice Lacking miRNA-1-2. Cell 2007, 129, 303–317. [Google Scholar] [CrossRef] [Green Version]
- Sucharov, C.; Bristow, M.R.; Port, J.D. miRNA expression in the failing human heart: Functional correlates. J. Mol. Cell. Cardiol. 2008, 45, 185–192. [Google Scholar] [CrossRef] [Green Version]
- Song, X.-W.; Li, Q.; Lin, L.; Wang, X.-C.; Li, D.-F.; Wang, G.-K.; Ren, A.-J.; Wang, Y.-R.; Qin, Y.-W.; Yuan, W.-J.; et al. MicroRNAs are dynamically regulated in hypertrophic hearts, and miR-199a is essential for the maintenance of cell size in cardiomyocytes. J. Cell. Physiol. 2010, 225, 437–443. [Google Scholar] [CrossRef]
- Oikonomou, E.; Zografos, T.; Papamikroulis, G.-A.; Siasos, G.; Vogiatzi, G.; Theofilis, P.; Briasoulis, A.; Papaioannou, S.; Vavuranakis, M.; Gennimata, V.; et al. Biomarkers in Atrial Fibrillation and Heart Failure. Curr. Med. Chem. 2019, 26, 873–887. [Google Scholar] [CrossRef]
- Pordzik, J.; Jakubik, D.; Jarosz-Popek, J.; Wicik, Z.; Eyileten, C.; De Rosa, S.; Indolfi, C.; Siller-Matula, J.M.; Czajka, P.; Postula, M. Significance of circulating microRNAs in diabetes mellitus type 2 and platelet reactivity: Bioinformatic analysis and review. Cardiovasc. Diabetol. 2019, 18, 113. [Google Scholar] [CrossRef] [Green Version]
- Klenke, S.; Eul, S.; Peters, J.; Neumann, T.; Adamzik, M.; Frey, U.H. Circulating miR-192 is a prognostic marker in patients with ischemic cardiomyopathy. Future Cardiol. 2018, 14, 283–289. [Google Scholar] [CrossRef]
- Schipper, M.E.I.; van Kuik, J.; de Jonge, N.; Dullens, H.F.J.; de Weger, R.A. Changes in Regulatory MicroRNA Expression in Myocardium of Heart Failure Patients on Left Ventricular Assist Device Support. J. Heart Lung Transplant. 2008, 27, 1282–1285. [Google Scholar] [CrossRef] [PubMed]
- Calderon-Dominguez, M.; Belmonte, T.; Quezada-Feijoo, M.; Ramos-Sánchez, M.; Fernández-Armenta, J.; Pérez-Navarro, A.; Cesar, S.; Peña-Peña, L.; Vea, À.; Llorente-Cortés, V.; et al. Emerging role of microRNAs in dilated cardiomyopathy: Evidence regarding etiology. Transl. Res. 2020, 215, 86–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, M.; Xu, L.; Liu, J.; Wang, D.; Li, Q.; Wang, L.; Li, P.; Chen, S.; Liu, T. Expression of Bcl-2 and microRNAs in cardiac tissues of patients with dilated cardiomyopathy. Mol. Med. Rep. 2017, 15, 359–365. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, K.-L.; Zhang, H.-F.; Shen, J.; Zhang, Q.; Li, X.-L. Circulating microRNAs levels in Chinese heart failure patients caused by dilated cardiomyopathy. Indian Heart J. 2013, 65, 12–16. [Google Scholar] [CrossRef] [Green Version]
- Sucharov, C.C.; Kao, D.P.; Port, J.D.; Karimpour-Fard, A.; Quaife, R.A.; Minobe, W.; Nunley, K.; Lowes, B.D.; Gilbert, E.M.; Bristow, M.R. Myocardial microRNAs associated with reverse remodeling in human heart failure. JCI Insight 2017, 2, e89169. [Google Scholar] [CrossRef] [Green Version]
- Bernardo, B.C.; Ooi, J.Y.; Matsumoto, A.; Tham, Y.K.; Singla, S.; Kiriazis, H.; Patterson, N.L.; Sadoshima, J.; Obad, S.; Lin, R.C. Sex differences in response to miRNA-34a therapy in mouse models of cardiac disease: Identification of sex-, disease- and treatment-regulated miRNAs. J. Physiol. 2016, 594, 5959–5974. [Google Scholar] [CrossRef] [Green Version]
- Schulte, C. Diagnostic and prognostic value of circulating microRNAs in heart failure with preserved and reduced ejection fraction. World J. Cardiol. 2015, 7, 843. [Google Scholar] [CrossRef]
- Vogel, B.; Keller, A.; Frese, K.S.; Leidinger, P.; Sedaghat-Hamedani, F.; Kayvanpour, E.; Kloos, W.; Backe, C.; Thanaraj, A.; Brefort, T.; et al. Multivariate miRNA signatures as biomarkers for non-ischaemic systolic heart failure. Eur. Heart J. 2013, 34, 2812–2823. [Google Scholar] [CrossRef] [Green Version]
- Nair, N.; Kumar, S.; Gongora, E.; Gupta, S. Circulating miRNA as novel markers for diastolic dysfunction. Mol. Cell. Biochem. 2013, 376, 33–40. [Google Scholar] [CrossRef]
- Watson, C.J.; Gupta, S.K.; O’Connell, E.; Thum, S.; Glezeva, N.; Fendrich, J.; Gallagher, J.; Ledwidge, M.; Grote-Levi, L.; McDonald, K.; et al. MicroRNA signatures differentiate preserved from reduced ejection fraction heart failure. Eur. J. Heart Fail. 2015, 17, 405–415. [Google Scholar] [CrossRef] [Green Version]
- Wong, L.L.; Armugam, A.; Sepramaniam, S.; Karolina, D.S.; Lim, K.Y.; Lim, J.Y.; Chong, J.P.C.; Ng, J.Y.X.; Chen, Y.-T.; Chan, M.M.Y.; et al. Circulating microRNAs in heart failure with reduced and preserved left ventricular ejection fraction. Eur. J. Heart Fail. 2015, 17, 393–404. [Google Scholar] [CrossRef] [PubMed]
- Peterlin, A.; Počivavšek, K.; Petrovič, D.; Peterlin, B. The Role of microRNAs in Heart Failure: A Systematic Review. Front. Cardiovasc. Med. 2020, 7, 161. [Google Scholar] [CrossRef] [PubMed]
- Vickers, K.C.; Palmisano, B.T.; Shoucri, B.M.; Shamburek, R.D.; Remaley, A.T. MicroRNAs are transported in plasma and delivered to recipient cells by high-density lipoproteins. Nat. Cell Biol. 2011, 17, 104. [Google Scholar] [CrossRef] [Green Version]
- Aryal, B.; Singh, A.K.; Rotllan, N.; Price, N.; Fernandez-Hernando, C. MicroRNAs and lipid metabolism. Curr. Opin. Lipidol. 2017, 28, 273–280. [Google Scholar] [CrossRef] [PubMed]
- Rayner, K.J.; Esau, C.C.; Hussain, F.N.; McDaniel, A.L.; Marshall, S.M.; van Gils, J.M.; Ray, T.D.; Sheedy, F.J.; Goedeke, L.; Liu, X.Q.; et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature 2011, 478, 404–407. [Google Scholar] [CrossRef] [Green Version]
- Wagschal, A.; Najafi-Shoushtari, S.H.; Wang, L.; Goedeke, L.; Sinha, S.; deLemos, A.S.; Black, J.C.; Ramírez, C.M.; Li, Y.; Tewhey, R.; et al. Genome-wide identification of microRNAs regulating cholesterol and triglyceride homeostasis. Nat. Med. 2015, 21, 1290–1297. [Google Scholar] [CrossRef] [Green Version]
- Horie, T.; Nishino, T.; Baba, O.; Kuwabara, Y.; Nakao, T.; Nishiga, M.; Usami, S.; Izuhara, M.; Sowa, N.; Yahagi, N.; et al. MicroRNA-33 regulates sterol regulatory element-binding protein 1 expression in mice. Nat. Commun. 2013, 4, 2883. [Google Scholar] [CrossRef] [Green Version]
- Esau, C.; Davis, S.; Murray, S.F.; Yu, X.X.; Pandey, S.K.; Pear, M.; Watts, L.; Booten, S.L.; Graham, M.; McKay, R.; et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell Metab. 2006, 3, 87–98. [Google Scholar] [CrossRef] [Green Version]
- Soh, J.; Iqbal, J.; Queiroz, J.; Fernandez-Hernando, C.; Hussain, M.M. MicroRNA-30c reduces hyperlipidemia and atherosclerosis in mice by decreasing lipid synthesis and lipoprotein secretion. Nat. Med. 2013, 19, 892–900. [Google Scholar] [CrossRef] [Green Version]
- Yang, K.; He, Y.S.; Wang, X.Q.; Lu, L.; Chen, Q.J.; Liu, J.; Sun, Z.; Shen, W.F. MiR-146a inhibits oxidized low-density lipoprotein-induced lipid accumulation and inflammatory response via targeting toll-like receptor 4. FEBS Lett. 2011, 585, 854–860. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.A.; Ceccarelli, R.; Lu, C.Y. Pharmacogenomic Biomarkers in US FDA-Approved Drug Labels (2000–2020). J. Pers. Med. 2021, 11, 179. [Google Scholar] [CrossRef]
- Adeniyi, O.; Ramamoorthy, A.; Schuck, R.; Sun, J.; Wilson, J.; Zineh, I.; Pacanowski, M. An Overview of Genomic Biomarker Use in Cardiovascular Disease Clinical Trials. Clin. Pharmacol. Ther. 2019, 106, 841–846. [Google Scholar] [CrossRef]
- Kalman, J.M.; Lavandero, S.; Mahfoud, F.; Nahrendorf, M.; Yacoub, M.H.; Zhao, D. Looking back and thinking forwards—15 years of cardiology and cardiovascular research. Nat. Reviews. Cardiol. 2019, 16, 651–660. [Google Scholar] [CrossRef] [PubMed]
- Colpaert, R.M.W.; Calore, M. Epigenetics and microRNAs in cardiovascular diseases. Genomics 2021, 113, 540–551. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Mannon, E.C.; Ray, S.C.; Ryan, M.J. Does sex matter? An update on the implementation of sex as a biological variable in research. Am. J. Physiol.-Ren. Physiol. 2020, 318, F329–F331. [Google Scholar] [CrossRef]
- Kang, W.; Eldfjell, Y.; Fromm, B.; Estivill, X.; Biryukova, I.; Friedländer, M.R. miRTrace reveals the organismal origins of microRNA sequencing data. Genome Biol. 2018, 19, 213. [Google Scholar] [CrossRef]
- Weber, M.; Baker, M.B.; Patel, R.S.; Quyyumi, A.A.; Bao, G.; Searles, C.D. MicroRNA Expression Profile in CAD Patients and the Impact of ACEI/ARB. Cardiol. Res. Pract. 2011, 2011, 532915. [Google Scholar] [CrossRef] [Green Version]
- Kaudewitz, D.; Zampetaki, A.; Mayr, M. MicroRNA Biomarkers for Coronary Artery Disease? Curr. Atheroscler. Rep. 2015, 17, 70. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gidlöf, O.; Andersson, P.; van der Pals, J.; Götberg, M.; Erlinge, D. Cardiospecific microRNA Plasma Levels Correlate with Troponin and Cardiac Function in Patients with ST Elevation Myocardial Infarction, Are Selectively Dependent on Renal Elimination, and Can Be Detected in Urine Samples. Cardiology 2011, 118, 217–226. [Google Scholar] [CrossRef] [Green Version]
- Singh, N.; Heggermont, W.; Fieuws, S.; Vanhaecke, J.; Van Cleemput, J.; De Geest, B. Endothelium-enriched microRNAs as diagnostic biomarkers for cardiac allograft vasculopathy. J. Heart Lung Transplant. 2015, 34, 1376–1384. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sato, F.; Tsuchiya, S.; Terasawa, K.; Tsujimoto, G. Intra-Platform Repeatability and Inter-Platform Comparability of MicroRNA Microarray Technology. PLoS ONE 2009, 4, e5540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Januzzi, J.L., Jr.; Canty, J.M.; Das, S.; DeFilippi, C.R.; Gintant, G.A.; Gutstein, D.E.; Jaffe, A.; Kaushik, E.P.; Leptak, C.; Mehta, C.; et al. Gaining Efficiency in Clinical Trials With Cardiac Biomarkers: JACC Review Topic of the Week. J. Am. Coll. Cardiol. 2021, 77, 1922–1933. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Swanson, K.M.; Rojas, R.L.; Wang, Z.; St Sauver, J.L.; Visscher, S.L.; Prokop, L.J.; Bielinski, S.J.; Wang, L.; Weinshilboum, R.; et al. Systematic review of the evidence on the cost-effectiveness of pharmacogenomics-guided treatment for cardiovascular diseases. Genet. Med. 2020, 22, 475–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Silverio, A.; Cavallo, P.; De Rosa, R.; Galasso, G. Big Health Data and Cardiovascular Diseases: A Challenge for Research, an Opportunity for Clinical Care. Front. Med. 2019, 6, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seetharam, K.; Brito, D.; Farjo, P.D.; Sengupta, P.P. The Role of Artificial Intelligence in Cardiovascular Imaging: State of the Art Review. Front. Cardiovasc. Med. 2020, 7, 618849. [Google Scholar] [CrossRef]
- Judd, R.M. Machine Learning in Medical Imaging: All Journeys Begin With a Single Step. JACC Cardiovasc. Imaging 2020, 13, 696–698. [Google Scholar] [CrossRef]
- Mittas, N.; Chatzopoulou, F.; Kyritsis, K.A.; Papagiannopoulos, C.I.; Theodoroula, N.F.; Papazoglou, A.S.; Karagiannidis, E.; Sofidis, G.; Moysidis, D.V.; Stalikas, N.; et al. A Risk-Stratification Machine Learning Framework for the Prediction of Coronary Artery Disease Severity: Insights from the GESS Trial. Front. Cardiovasc. Med. 2022, 8, 812182. [Google Scholar] [CrossRef]
- Hanna, J.; Hossain, G.S.; Kocerha, J. The Potential for microRNA Therapeutics and Clinical Research. Front. Genet. 2019, 10, 478. [Google Scholar] [CrossRef] [Green Version]
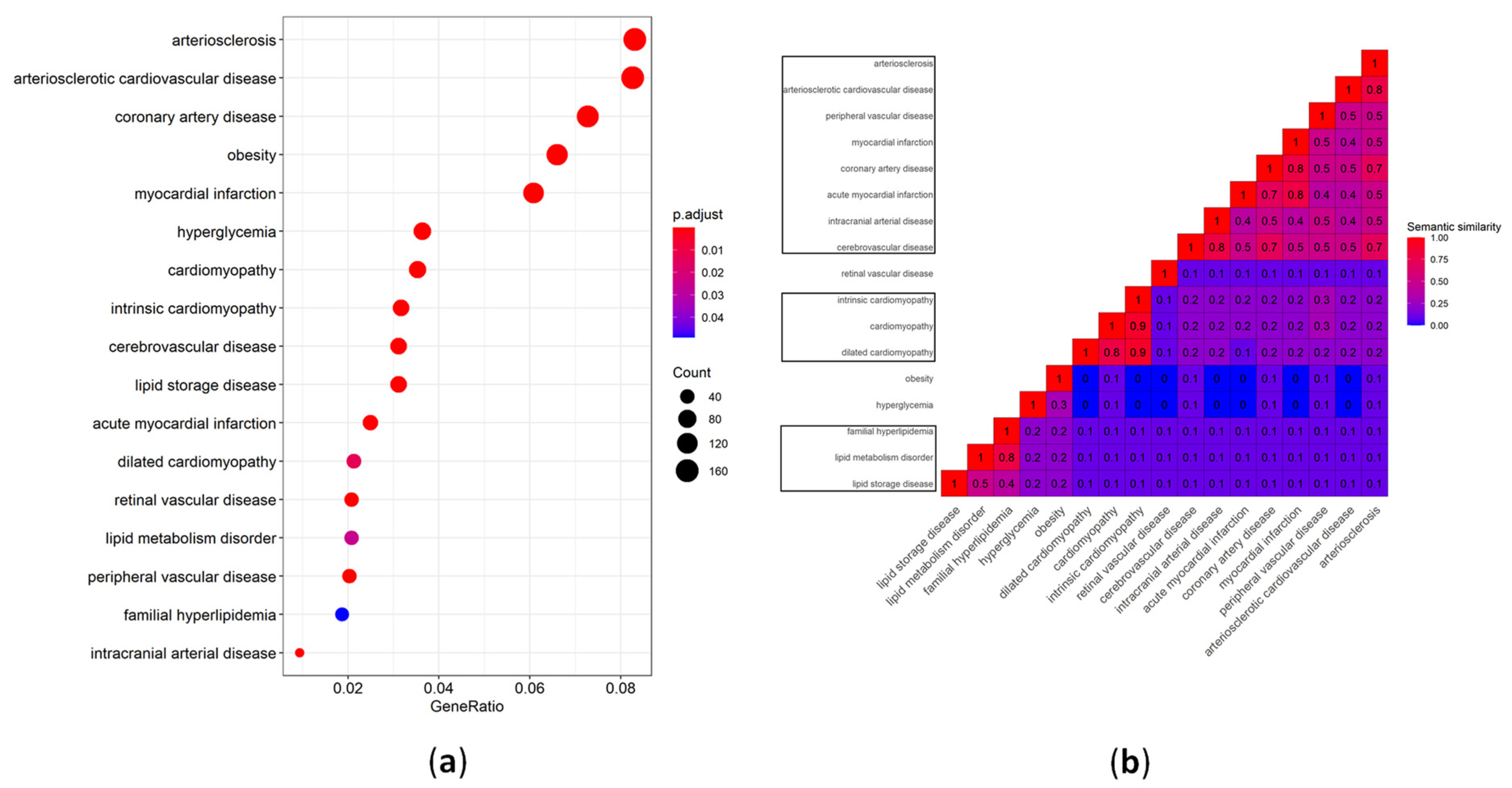

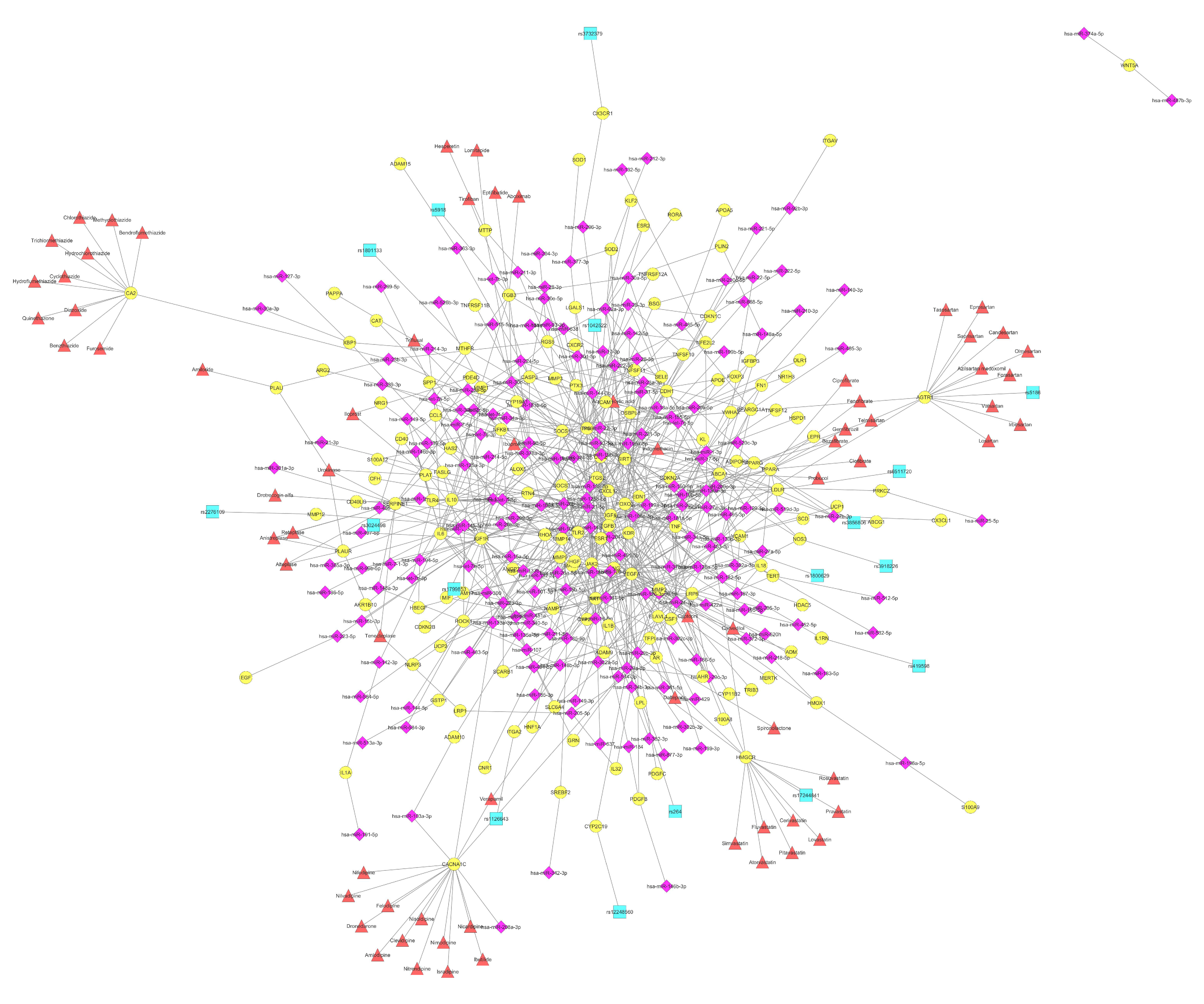
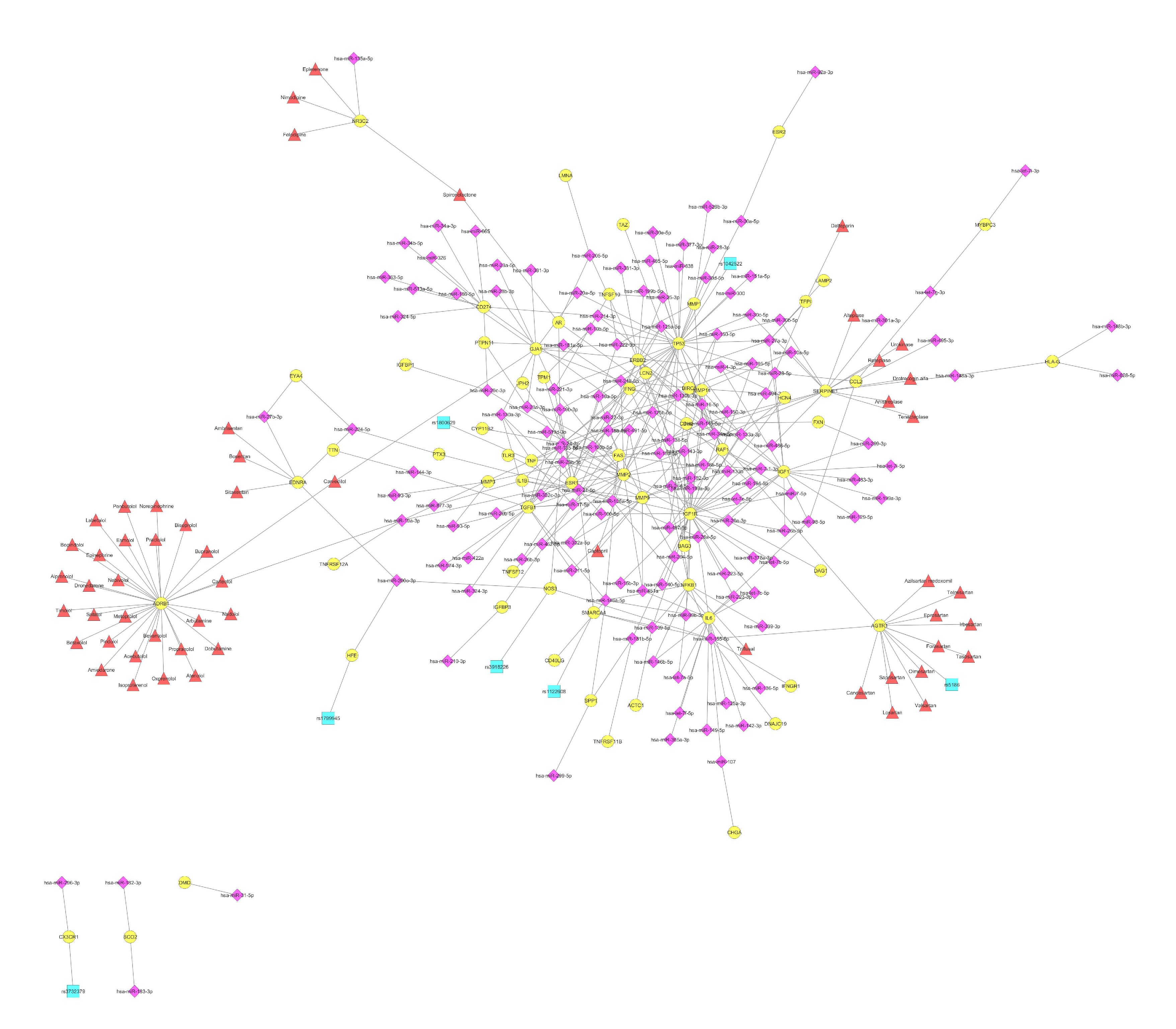
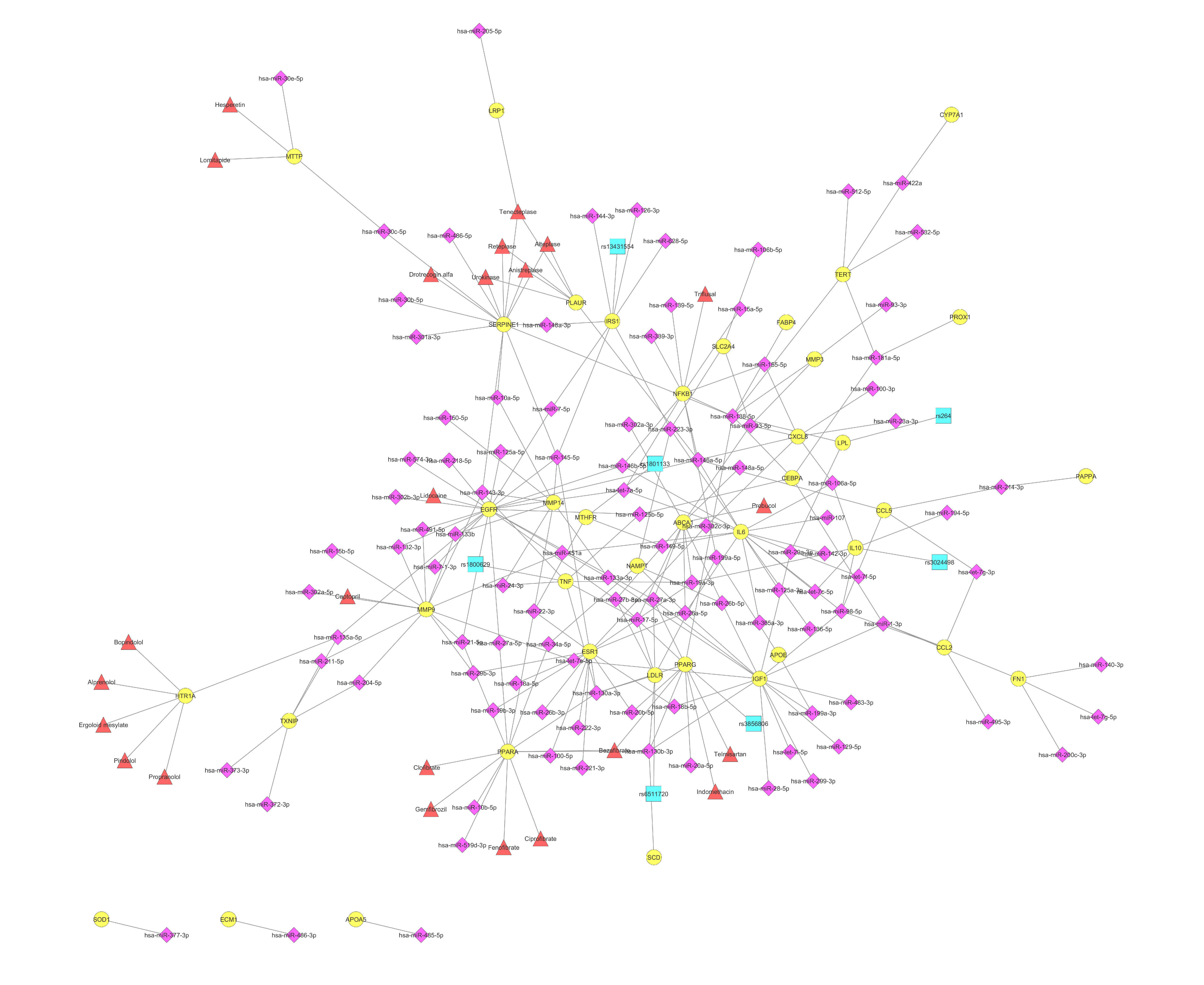
| Mature_Mirna | Targeted Genes | SNPs | Drugs | Disease_Drug | |
|---|---|---|---|---|---|
| 1 | miR-155-5p | 241 | 5 | 15 | Cardiac hypertrophy; hypertension |
| 2 | miR-21-5p | 153 | 4 | 11 | Myocardial infarction; heart failure; vascular disease; cardiac hypertrophy; cardiomyopathy, dilated; stroke |
| 3 | miR-145-5p | 151 | 3 | 9 | Vascular disease; supravalvar aortic stenosis |
| 4 | miR-34a-5p | 148 | 4 | 31 | Stroke |
| 5 | miR-125b-5p | 126 | 5 | 4 | Cardiac hypertrophy; heart failure; vascular disease; cardiomyopathy, dilated; supravalvar aortic stenosis; cardiovascular; cardiomyopathy, idiopathic dilated; stroke |
| 6 | miR-29a-3p | 123 | 7 | 28 | Cardiac hypertrophy; cardiomyopathy, dilated; stroke |
| 7 | miR-24-3p | 110 | 4 | 1 | Cardiac hypertrophy; heart failure; cardiovascular; cardiomyopathy, dilated; supravalvar aortic stenosis; stroke |
| 8 | miR-29b-3p | 109 | 6 | 15 | Cardiac hypertrophy; myocardial infarction; cardiomyopathy, dilated; stroke |
| 9 | miR-200c-3p | 98 | 3 | 5 | Cardiomyopathy, dilated |
| 10 | miR-17-5p | 95 | 3 | 8 | Cardiomyopathy, dilated; stroke |
| Atherosclerosis | Cardiomyopathy | Lipid Metabolism Disorder | ||||
|---|---|---|---|---|---|---|
| Mature_Mirna | Targeted Genes | Mature_Mirna | Targeted Genes | Mature_Mirna | Targeted Genes | |
| 1 | miR-146a-5p * | 18 | miR-21-5p | 10 | miR-138-5p * | 8 |
| 2 | miR-155-5p | 15 | miR-24-3p | 9 | miR-146a-5p * | 6 |
| 3 | miR-21-5p | 15 | miR-145-5p | 7 | miR-26a-5p * | 5 |
| 4 | miR-24-3p | 15 | miR-138-5p * | 7 | miR-27a-3p * | 5 |
| 5 | miR-29b-3p | 13 | miR-143-3p * | 7 | miR-145-5p | 5 |
| 6 | miR-143-3p * | 12 | miR-17-5p | 7 | miR-130a-3p * | 5 |
| 7 | miR-145-5p | 12 | miR-155-5p | 6 | miR-98-5p * | 5 |
| 8 | miR-221-3p * | 12 | miR-125b-5p | 6 | miR-130b-3p * | 5 |
| 9 | miR-126-3p * | 11 | miR-146a-5p * | 6 | miR-223-3p * | 4 |
| 10 | miR-138-5p * | 11 | miR-133b * | 5 | miR-1-3p * | 4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatzopoulou, F.; Kyritsis, K.A.; Papagiannopoulos, C.I.; Galatou, E.; Mittas, N.; Theodoroula, N.F.; Papazoglou, A.S.; Karagiannidis, E.; Chatzidimitriou, M.; Papa, A.; et al. Dissecting miRNA–Gene Networks to Map Clinical Utility Roads of Pharmacogenomics-Guided Therapeutic Decisions in Cardiovascular Precision Medicine. Cells 2022, 11, 607. https://doi.org/10.3390/cells11040607
Chatzopoulou F, Kyritsis KA, Papagiannopoulos CI, Galatou E, Mittas N, Theodoroula NF, Papazoglou AS, Karagiannidis E, Chatzidimitriou M, Papa A, et al. Dissecting miRNA–Gene Networks to Map Clinical Utility Roads of Pharmacogenomics-Guided Therapeutic Decisions in Cardiovascular Precision Medicine. Cells. 2022; 11(4):607. https://doi.org/10.3390/cells11040607
Chicago/Turabian StyleChatzopoulou, Fani, Konstantinos A. Kyritsis, Christos I. Papagiannopoulos, Eleftheria Galatou, Nikolaos Mittas, Nikoleta F. Theodoroula, Andreas S. Papazoglou, Efstratios Karagiannidis, Maria Chatzidimitriou, Anna Papa, and et al. 2022. "Dissecting miRNA–Gene Networks to Map Clinical Utility Roads of Pharmacogenomics-Guided Therapeutic Decisions in Cardiovascular Precision Medicine" Cells 11, no. 4: 607. https://doi.org/10.3390/cells11040607
APA StyleChatzopoulou, F., Kyritsis, K. A., Papagiannopoulos, C. I., Galatou, E., Mittas, N., Theodoroula, N. F., Papazoglou, A. S., Karagiannidis, E., Chatzidimitriou, M., Papa, A., Sianos, G., Angelis, L., Chatzidimitriou, D., & Vizirianakis, I. S. (2022). Dissecting miRNA–Gene Networks to Map Clinical Utility Roads of Pharmacogenomics-Guided Therapeutic Decisions in Cardiovascular Precision Medicine. Cells, 11(4), 607. https://doi.org/10.3390/cells11040607









