Asymptomatic Hyperuricemia Promotes Recovery from Ischemic Organ Injury by Modulating the Phenotype of Macrophages
Abstract
1. Introduction
2. Materials and Methods
2.1. Animal Model of Ischemia-Reperfusion-Induced Acute Kidney Injury
2.2. Assessment of Kidney Excretory Function
2.3. Assessment of Serum Uric Acid
2.4. Histological Analysis
2.4.1. Light Microscopy
2.4.2. Electron Microscopy
2.5. RNA Preparation and Real-Time Quantitative RT-qPCR
2.6. Flow Cytometry Analysis
2.7. In Vitro Cell Culture Experiments
2.8. Oxidative Stress Analysis
2.9. Uric Acid Uptake Assay
2.10. Analysis of Open Access Gene Expression Profiles
2.11. Single Cell RNA-Sequencing
2.12. Bioinformatic Analysis of Single Cell RNA-Sequencing
2.13. Statistical Analysis
3. Results
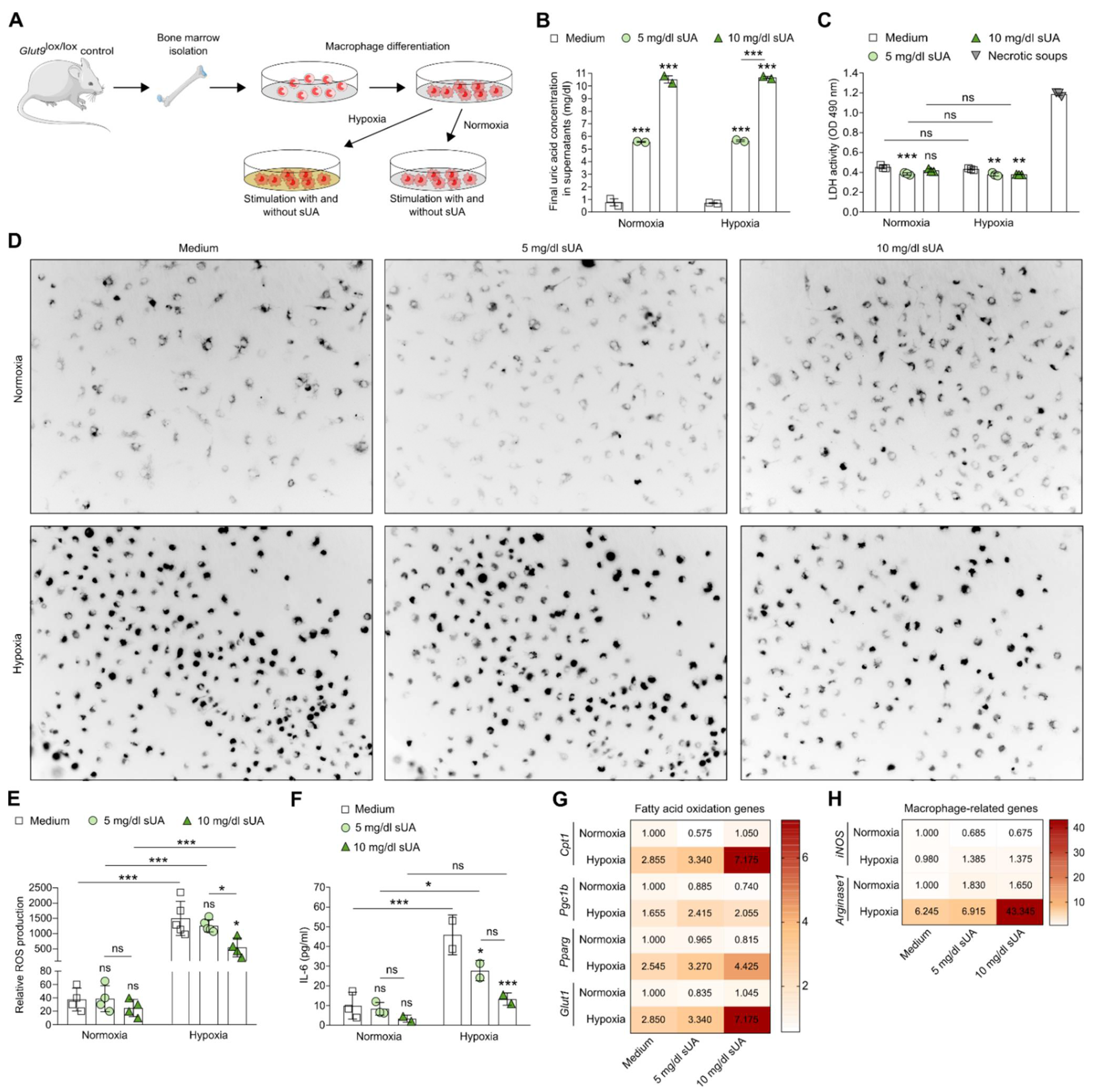
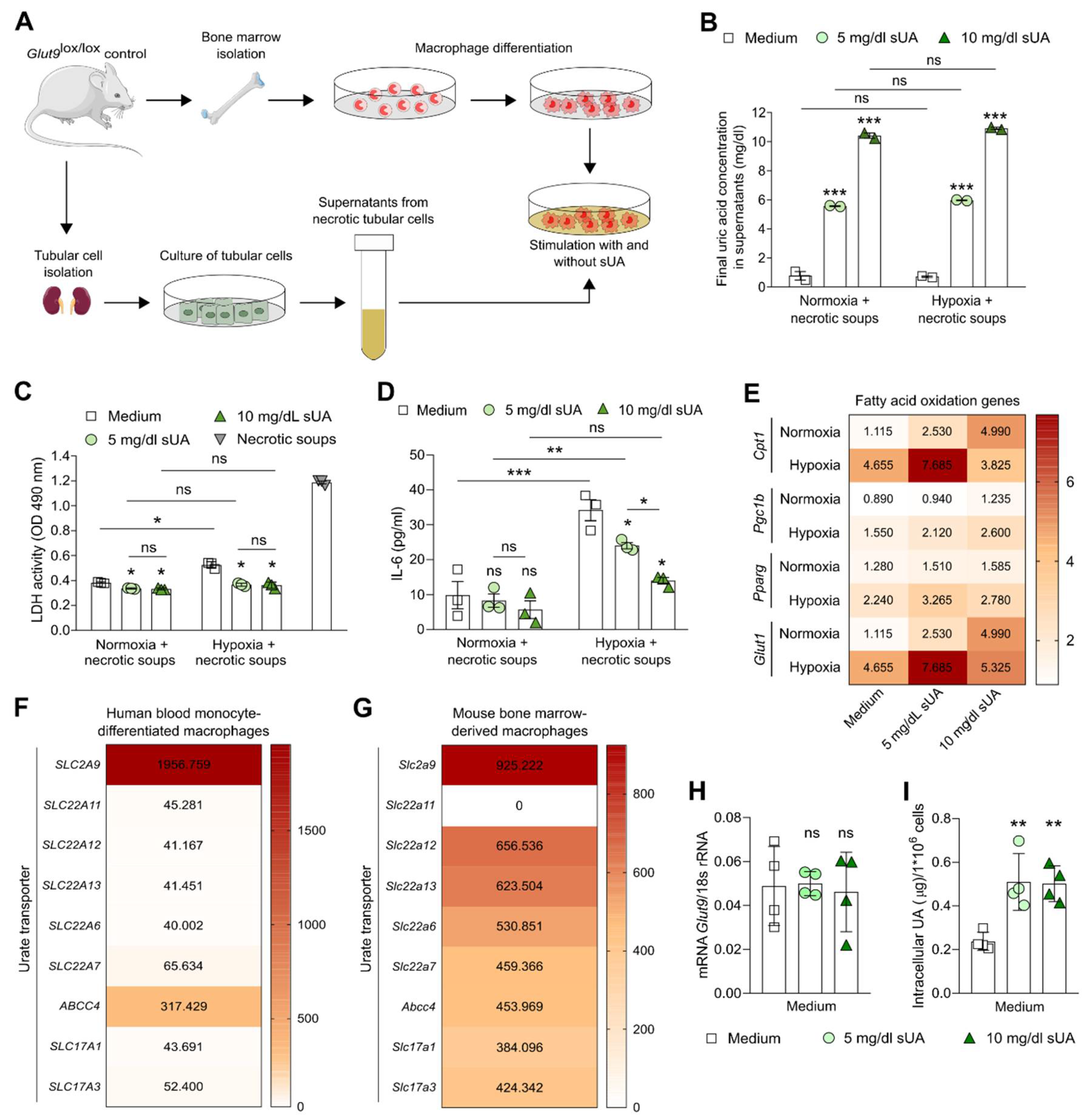
3.1. Soluble Uric Acid Inhibits the Pro-Inflammatory Function of Activated Macrophages and Increases Fatty Acid Oxidation
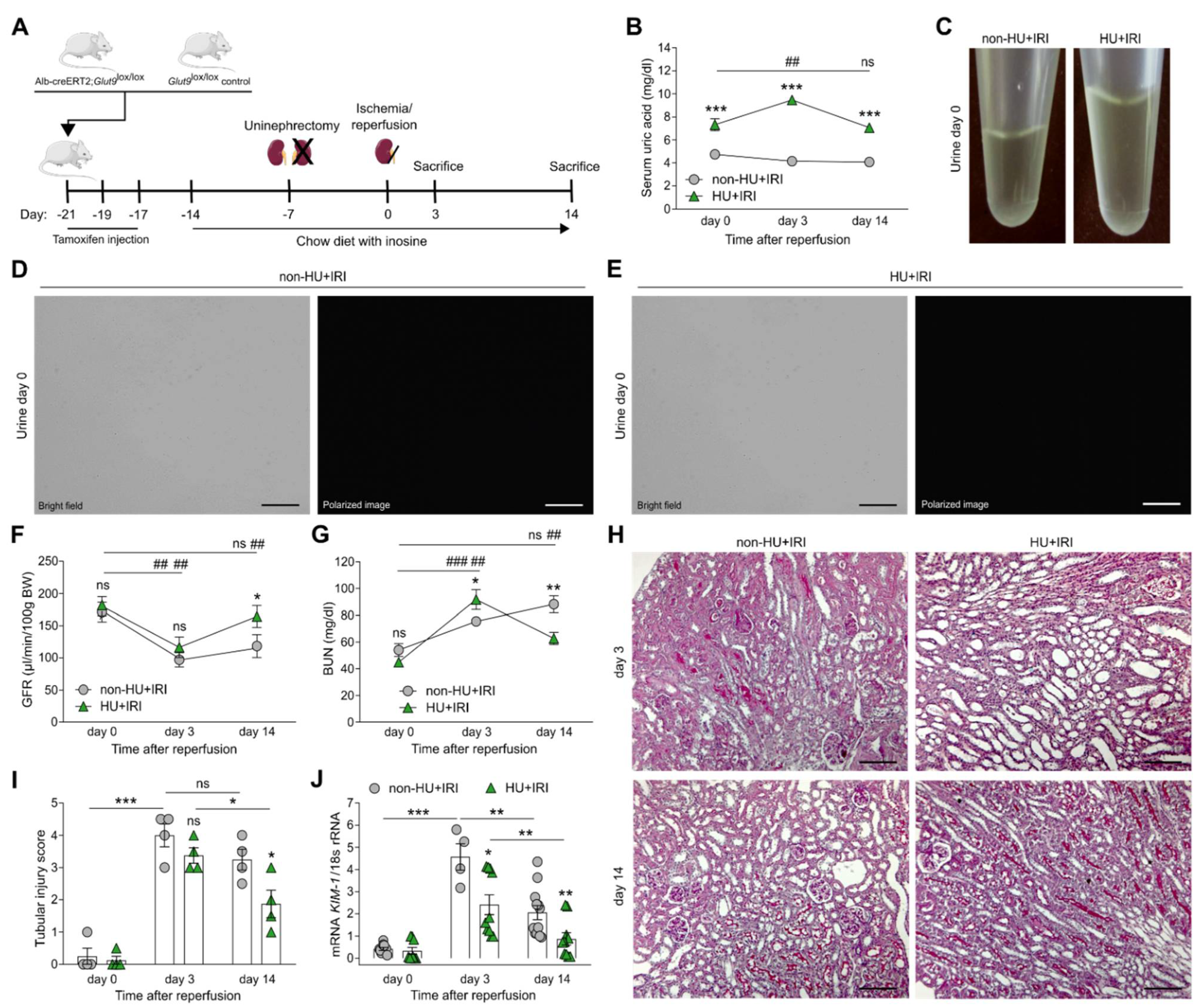
3.2. Asymptomatic Hyperuricemia Improves Kidney Function upon Acute Organ Injury
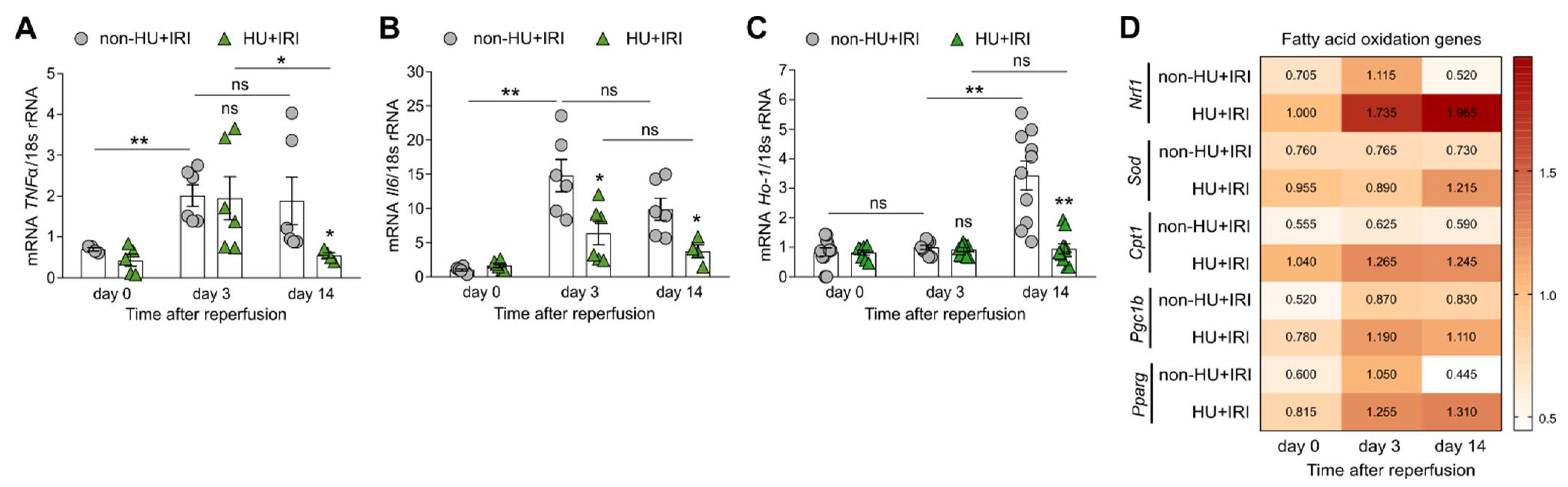
3.3. Asymptomatic Hyperuricemia Restores Tubular Integrity and Enhances Fatty Acid Oxidation and Mitochondria Dynamics after Acute Organ Injury
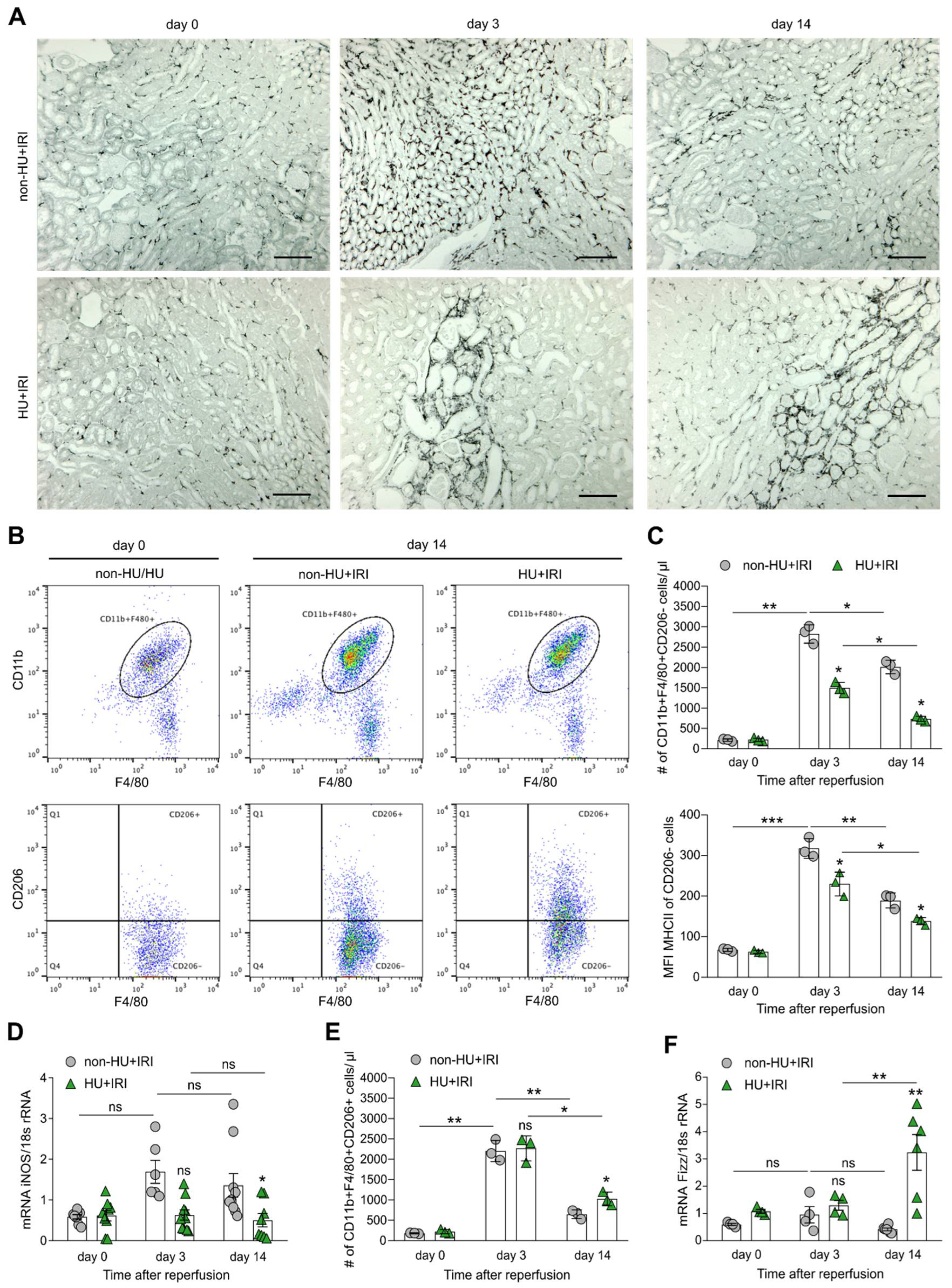
3.4. Asymptomatic Hyperuricemia Drives an Anti-Inflammatory M2-like Macrophage Phenotype in Acute Organ Injury
3.5. Asymptomatic Hyperuricemia Reduces Interstitial Fibrosis in Acute Organ Injury to Chronic Kidney Disease Transition
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Hoste, E.A.J.; Kellum, J.A.; Selby, N.M.; Zarbock, A.; Palevsky, P.M.; Bagshaw, S.M.; Goldstein, S.L.; Cerda, J.; Chawla, L.S. Global epidemiology and outcomes of acute kidney injury. Nat. Rev. Nephrol. 2018, 14, 607–625. [Google Scholar] [CrossRef]
- Kellum, J.A.; Romagnani, P.; Ashuntantang, G.; Ronco, C.; Zarbock, A.; Anders, H.J. Acute kidney injury. Nat. Rev. Dis. Primers. 2021, 7, 52. [Google Scholar] [CrossRef]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef]
- Suarez-Alvarez, B.; Liapis, H.; Anders, H.J. Links between coagulation, inflammation, regeneration, and fibrosis in kidney pathology. Lab. Invest. 2016, 96, 378–390. [Google Scholar] [CrossRef]
- Anders, H.J. Immune system modulation of kidney regeneration-mechanisms and implications. Nat. Reviews. Nephrol. 2014, 10, 347–358. [Google Scholar] [CrossRef]
- He, L.; Wei, Q.; Liu, J.; Yi, M.; Liu, Y.; Liu, H.; Sun, L.; Peng, Y.; Liu, F.; Venkatachalam, M.A.; et al. AKI on CKD: Heightened injury, suppressed repair, and the underlying mechanisms. Kidney Int. 2017, 92, 1071–1083. [Google Scholar] [CrossRef]
- Van den Bossche, J.; O’Neill, L.A.; Menon, D. Macrophage Immunometabolism: Where Are We (Going)? Trends Immunol. 2017, 38, 395–406. [Google Scholar] [CrossRef]
- Huang, S.C.; Smith, A.M.; Everts, B.; Colonna, M.; Pearce, E.L.; Schilling, J.D.; Pearce, E.J. Metabolic Reprogramming Mediated by the mTORC2-IRF4 Signaling Axis Is Essential for Macrophage Alternative Activation. Immunity 2016, 45, 817–830. [Google Scholar] [CrossRef]
- Pearce, E.L.; Pearce, E.J. Metabolic pathways in immune cell activation and quiescence. Immunity 2013, 38, 633–643. [Google Scholar] [CrossRef]
- Szeto, H.H. Pharmacologic Approaches to Improve Mitochondrial Function in AKI and CKD. J. Am. Soc. Nephrol. 2017, 28, 2856–2865. [Google Scholar] [CrossRef]
- Shimada, M.; Johnson, R.J.; May, W.S., Jr.; Lingegowda, V.; Sood, P.; Nakagawa, T.; Van, Q.C.; Dass, B.; Ejaz, A.A. A novel role for uric acid in acute kidney injury associated with tumour lysis syndrome. Nephrol. Dial. Transplant. 2009, 24, 2960–2964. [Google Scholar] [CrossRef]
- Galardy, P.J.; Hochberg, J.; Perkins, S.L.; Harrison, L.; Goldman, S.; Cairo, M.S. Rasburicase in the prevention of laboratory/clinical tumour lysis syndrome in children with advanced mature B-NHL: A Children’s Oncology Group Report. Br. J. Haematol. 2013, 163, 365–372. [Google Scholar] [CrossRef]
- Preitner, F.; Laverriere-Loss, A.; Metref, S.; Da Costa, A.; Moret, C.; Rotman, S.; Bazin, D.; Daudon, M.; Sandt, C.; Dessombz, A.; et al. Urate-induced acute renal failure and chronic inflammation in liver-specific Glut9 knockout mice. Am. J. Physiol. Renal. Physiol. 2013, 305, F786–F795. [Google Scholar] [CrossRef][Green Version]
- Sanchez-Lozada, L.G.; Tapia, E.; Santamaria, J.; Avila-Casado, C.; Soto, V.; Nepomuceno, T.; Rodriguez-Iturbe, B.; Johnson, R.J.; Herrera-Acosta, J. Mild hyperuricemia induces vasoconstriction and maintains glomerular hypertension in normal and remnant kidney rats. Kidney Int. 2005, 67, 237–247. [Google Scholar] [CrossRef]
- Sautin, Y.Y.; Nakagawa, T.; Zharikov, S.; Johnson, R.J. Adverse effects of the classic antioxidant uric acid in adipocytes: NADPH oxidase-mediated oxidative/nitrosative stress. Am. J. Physiol. Cell Physiol. 2007, 293, C584–C596. [Google Scholar] [CrossRef]
- Roncal, C.A.; Mu, W.; Croker, B.; Reungjui, S.; Ouyang, X.; Tabah-Fisch, I.; Johnson, R.J.; Ejaz, A.A. Effect of elevated serum uric acid on cisplatin-induced acute renal failure. Am. J. Physiol. Renal. Physiol. 2007, 292, F116–F122. [Google Scholar] [CrossRef]
- Braga, T.T.; Davanso, M.R.; Mendes, D.; de Souza, T.A.; de Brito, A.F.; Cruz, M.C.; Hiyane, M.I.; de Lima, D.S.; Nunes, V.; de Fatima Giarola, J.; et al. Sensing soluble uric acid by Naip1-Nlrp3 platform. Cell Death Dis. 2021, 12, 158. [Google Scholar] [CrossRef]
- Kim, S.M.; Lee, S.H.; Kim, Y.G.; Kim, S.Y.; Seo, J.W.; Choi, Y.W.; Kim, D.J.; Jeong, K.H.; Lee, T.W.; Ihm, C.G.; et al. Hyperuricemia-induced NLRP3 activation of macrophages contributes to the progression of diabetic nephropathy. Am. J. Physiol. Renal. Physiol. 2015, 308, F993–F1003. [Google Scholar] [CrossRef]
- Martinez-Reyes, C.P.; Manjarrez-Reyna, A.N.; Mendez-Garcia, L.A.; Aguayo-Guerrero, J.A.; Aguirre-Sierra, B.; Villalobos-Molina, R.; Lopez-Vidal, Y.; Bobadilla, K.; Escobedo, G. Uric Acid Has Direct Proinflammatory Effects on Human Macrophages by Increasing Proinflammatory Mediators and Bacterial Phagocytosis Probably via URAT1. Biomolecules 2020, 10, 576. [Google Scholar] [CrossRef]
- Braga, T.T.; Forni, M.F.; Correa-Costa, M.; Ramos, R.N.; Barbuto, J.A.; Branco, P.; Castoldi, A.; Hiyane, M.I.; Davanso, M.R.; Latz, E.; et al. Soluble Uric Acid Activates the NLRP3 Inflammasome. Sci. Rep. 2017, 7, 39884. [Google Scholar] [CrossRef]
- Crisan, T.O.; Cleophas, M.C.P.; Novakovic, B.; Erler, K.; van de Veerdonk, F.L.; Stunnenberg, H.G.; Netea, M.G.; Dinarello, C.A.; Joosten, L.A.B. Uric acid priming in human monocytes is driven by the AKT-PRAS40 autophagy pathway. Proc. Natl. Acad. Sci. USA 2017, 114, 5485–5490. [Google Scholar] [CrossRef]
- Mulay, S.R.; Steiger, S.; Shi, C.; Anders, H.J. A guide to crystal-related and nano- or microparticle-related tissue responses. FEBS J. 2020, 287, 818–832. [Google Scholar] [CrossRef]
- Honarpisheh, M.; Foresto-Neto, O.; Desai, J.; Steiger, S.; Gomez, L.A.; Popper, B.; Boor, P.; Anders, H.J.; Mulay, S.R. Phagocytosis of environmental or metabolic crystalline particles induces cytotoxicity by triggering necroptosis across a broad range of particle size and shape. Sci. Rep. 2017, 7, 15523. [Google Scholar] [CrossRef]
- Mulay, S.R.; Anders, H.J. Crystallopathies. N. Engl. J. Med. 2016, 374, 2465–2476. [Google Scholar] [CrossRef]
- Ma, Q.; Honarpisheh, M.; Li, C.; Sellmayr, M.; Lindenmeyer, M.; Bohland, C.; Romagnani, P.; Anders, H.J.; Steiger, S. Soluble Uric Acid Is an Intrinsic Negative Regulator of Monocyte Activation in Monosodium Urate Crystal-Induced Tissue Inflammation. J. Immunol. 2020, 205, 789–800. [Google Scholar] [CrossRef]
- Preitner, F.; Pimentel, A.; Metref, S.; Berthonneche, C.; Sarre, A.; Moret, C.; Rotman, S.; Centeno, G.; Firsov, D.; Thorens, B. No development of hypertension in the hyperuricemic liver-Glut9 knockout mouse. Kidney Int. 2015, 87, 940–947. [Google Scholar] [CrossRef]
- Sellmayr, M.; Hernandez Petzsche, M.R.; Ma, Q.; Kruger, N.; Liapis, H.; Brink, A.; Lenz, B.; Angelotti, M.L.; Gnemmi, V.; Kuppe, C.; et al. Only Hyperuricemia with Crystalluria, but not Asymptomatic Hyperuricemia, Drives Progression of Chronic Kidney Disease. J. Am. Soc. Nephrol. 2020, 31, 2773–2792. [Google Scholar] [CrossRef]
- Marschner, J.A.; Schafer, H.; Holderied, A.; Anders, H.J. Optimizing Mouse Surgery with Online Rectal Temperature Monitoring and Preoperative Heat Supply. Effects on Post-Ischemic Acute Kidney Injury. PLoS ONE 2016, 11, e0149489. [Google Scholar] [CrossRef]
- Nakazawa, D.; Kumar, S.V.; Marschner, J.; Desai, J.; Holderied, A.; Rath, L.; Kraft, F.; Lei, Y.; Fukasawa, Y.; Moeckel, G.W.; et al. Histones and Neutrophil Extracellular Traps Enhance Tubular Necrosis and Remote Organ Injury in Ischemic AKI. J. Am. Soc. Nephrol. 2017, 28, 1753–1768. [Google Scholar] [CrossRef]
- Schreiber, A.; Shulhevich, Y.; Geraci, S.; Hesser, J.; Stsepankou, D.; Neudecker, S.; Koenig, S.; Heinrich, R.; Hoecklin, F.; Pill, J.; et al. Transcutaneous measurement of renal function in conscious mice. Am. J. Physiol. Renal. Physiol. 2012, 303, F783–F788. [Google Scholar] [CrossRef]
- Steiger, S.; Grill, J.F.; Ma, Q.; Bauerle, T.; Jordan, J.; Smolle, M.; Bohland, C.; Lech, M.; Anders, H.J. Anti-Transforming Growth Factor beta IgG Elicits a Dual Effect on Calcium Oxalate Crystallization and Progressive Nephrocalcinosis-Related Chronic Kidney Disease. Front. Immunol. 2018, 9, 619. [Google Scholar] [CrossRef] [PubMed]
- Lech, M.; Avila-Ferrufino, A.; Allam, R.; Segerer, S.; Khandoga, A.; Krombach, F.; Garlanda, C.; Mantovani, A.; Anders, H.J. Resident dendritic cells prevent postischemic acute renal failure by help of single Ig IL-1 receptor-related protein. J. Immunol. 2009, 183, 4109–4118. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Thomasova, D.; Ryu, M.; Anders, H.J. MDM2 (murine double minute-2) links inflammation and tubular cell healing during acute kidney injury in mice. Kidney Int. 2012, 81, 1199–1211. [Google Scholar] [CrossRef] [PubMed]
- Han, S.J.; Jang, H.S.; Noh, M.R.; Kim, J.; Kong, M.J.; Kim, J.I.; Park, J.W.; Park, K.M. Mitochondrial NADP(+)-Dependent Isocitrate Dehydrogenase Deficiency Exacerbates Mitochondrial and Cell Damage after Kidney Ischemia-Reperfusion Injury. J. Am. Soc. Nephrol. 2017, 28, 1200–1215. [Google Scholar] [CrossRef]
- Lech, M.; Anders, H.J. Expression profiling by real-time quantitative polymerase chain reaction (RT-qPCR). Methods Mol. Biol. 2014, 1169, 133–142. [Google Scholar] [CrossRef]
- Li, N.; Steiger, S.; Fei, L.; Li, C.; Shi, C.; Salei, N.; Schraml, B.U.; Zheng, Z.; Anders, H.J.; Lichtnekert, J. IRF8-Dependent Type I Conventional Dendritic Cells (cDC1s) Control Post-Ischemic Inflammation and Mildly Protect Against Post-Ischemic Acute Kidney Injury and Disease. Front. Immunol. 2021, 12, 685559. [Google Scholar] [CrossRef]
- Mills, E.L.; Kelly, B.; Logan, A.; Costa, A.S.H.; Varma, M.; Bryant, C.E.; Tourlomousis, P.; Dabritz, J.H.M.; Gottlieb, E.; Latorre, I.; et al. Succinate Dehydrogenase Supports Metabolic Repurposing of Mitochondria to Drive Inflammatory Macrophages. Cell 2016, 167, 457–470.e13. [Google Scholar] [CrossRef]
- Leal-Calvo, T.; Martins, B.L.; Bertoluci, D.F.; Rosa, P.S.; de Camargo, R.M.; Germano, G.V.; Brito de Souza, V.N.; Pereira Latini, A.C.; Moraes, M.O. Large-Scale Gene Expression Signatures Reveal a Microbicidal Pattern of Activation in Mycobacterium leprae-Infected Monocyte-Derived Macrophages With Low Multiplicity of Infection. Front. Immunol. 2021, 12, 647832. [Google Scholar] [CrossRef]
- Askovich, P.S.; Ramsey, S.A.; Diercks, A.H.; Kennedy, K.A.; Knijnenburg, T.A.; Aderem, A. Identifying novel transcription factors involved in the inflammatory response by using binding site motif scanning in genomic regions defined by histone acetylation. PLoS ONE 2017, 12, e0184850. [Google Scholar] [CrossRef]
- Lazzeri, E.; Angelotti, M.L.; Peired, A.; Conte, C.; Marschner, J.A.; Maggi, L.; Mazzinghi, B.; Lombardi, D.; Melica, M.E.; Nardi, S.; et al. Endocycle-related tubular cell hypertrophy and progenitor proliferation recover renal function after acute kidney injury. Nat. Commun. 2018, 9, 1344. [Google Scholar] [CrossRef]
- Dobin, A.; Davis, C.A.; Schlesinger, F.; Drenkow, J.; Zaleski, C.; Jha, S.; Batut, P.; Chaisson, M.; Gingeras, T.R. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics 2013, 29, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Lun, A.T.; McCarthy, D.J.; Marioni, J.C. A step-by-step workflow for low-level analysis of single-cell RNA-seq data with Bioconductor. F1000Res 2016, 5, 2122. [Google Scholar] [CrossRef] [PubMed]
- Haghverdi, L.; Lun, A.T.L.; Morgan, M.D.; Marioni, J.C. Batch effects in single-cell RNA-sequencing data are corrected by matching mutual nearest neighbors. Nat. Biotechnol. 2018, 36, 421–427. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Chen, C.; Song, Y.; Cai, Q.; Li, J.; Tang, Y.; Han, X.; Qu, W.; Chen, A.; Wang, H.; et al. Hypoxia modifies the polarization of macrophages and their inflammatory microenvironment, and inhibits malignant behavior in cancer cells. Oncol. Lett. 2019, 18, 5871–5878. [Google Scholar] [CrossRef]
- Delprat, V.; Tellier, C.; Demazy, C.; Raes, M.; Feron, O.; Michiels, C. Cycling hypoxia promotes a pro-inflammatory phenotype in macrophages via JNK/p65 signaling pathway. Sci. Rep. 2020, 10, 882. [Google Scholar] [CrossRef]
- Diaz-Bulnes, P.; Saiz, M.L.; Lopez-Larrea, C.; Rodriguez, R.M. Crosstalk Between Hypoxia and ER Stress Response: A Key Regulator of Macrophage Polarization. Front. Immunol. 2019, 10, 2951. [Google Scholar] [CrossRef]
- Tran, M.; Tam, D.; Bardia, A.; Bhasin, M.; Rowe, G.C.; Kher, A.; Zsengeller, Z.K.; Akhavan-Sharif, M.R.; Khankin, E.V.; Saintgeniez, M.; et al. PGC-1alpha promotes recovery after acute kidney injury during systemic inflammation in mice. J. Clin. Investig. 2011, 121, 4003–4014. [Google Scholar] [CrossRef]
- Svensson, K.; Schnyder, S.; Cardel, B.; Handschin, C. Loss of Renal Tubular PGC-1alpha Exacerbates Diet-Induced Renal Steatosis and Age-Related Urinary Sodium Excretion in Mice. PLoS ONE 2016, 11, e0158716. [Google Scholar] [CrossRef]
- Mulay, S.R.; Honarpisheh, M.M.; Foresto-Neto, O.; Shi, C.; Desai, J.; Zhao, Z.B.; Marschner, J.A.; Popper, B.; Buhl, E.M.; Boor, P.; et al. Mitochondria Permeability Transition versus Necroptosis in Oxalate-Induced AKI. J. Am. Soc. Nephrol. 2019, 30, 1857–1869. [Google Scholar] [CrossRef]
- Tran, M.T.; Zsengeller, Z.K.; Berg, A.H.; Khankin, E.V.; Bhasin, M.K.; Kim, W.; Clish, C.B.; Stillman, I.E.; Karumanchi, S.A.; Rhee, E.P.; et al. PGC1alpha drives NAD biosynthesis linking oxidative metabolism to renal protection. Nature 2016, 531, 528–532. [Google Scholar] [CrossRef]
- Jesinkey, S.R.; Funk, J.A.; Stallons, L.J.; Wills, L.P.; Megyesi, J.K.; Beeson, C.C.; Schnellmann, R.G. Formoterol restores mitochondrial and renal function after ischemia-reperfusion injury. J. Am. Soc. Nephrol. 2014, 25, 1157–1162. [Google Scholar] [CrossRef] [PubMed]
- Mulay, S.R.; Linkermann, A.; Anders, H.J. Necroinflammation in Kidney Disease. J. Am. Soc. Nephrol. 2016, 27, 27–39. [Google Scholar] [CrossRef] [PubMed]
- Ames, B.N.; Cathcart, R.; Schwiers, E.; Hochstein, P. Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc. Natl. Acad. Sci. USA 1981, 78, 6858–6862. [Google Scholar] [CrossRef]
- Lech, M.; Grobmayr, R.; Weidenbusch, M.; Anders, H.J. Tissues use resident dendritic cells and macrophages to maintain homeostasis and to regain homeostasis upon tissue injury: The immunoregulatory role of changing tissue environments. Mediat. Inflamm. 2012, 2012, 951390. [Google Scholar] [CrossRef] [PubMed]
- Cervantes-Silva, M.P.; Cox, S.L.; Curtis, A.M. Alterations in mitochondrial morphology as a key driver of immunity and host defence. EMBO Rep. 2021, 22, e53086. [Google Scholar] [CrossRef]
- Wang, Y.; Li, N.; Zhang, X.; Horng, T. Mitochondrial metabolism regulates macrophage biology. J. Biol. Chem. 2021, 297, 100904. [Google Scholar] [CrossRef]
- Li, Y.; He, Y.; Miao, K.; Zheng, Y.; Deng, C.; Liu, T.M. Imaging of macrophage mitochondria dynamics in vivo reveals cellular activation phenotype for diagnosis. Theranostics 2020, 10, 2897–2917. [Google Scholar] [CrossRef]
- Martinon, F.; Petrilli, V.; Mayor, A.; Tardivel, A.; Tschopp, J. Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 2006, 440, 237–241. [Google Scholar] [CrossRef]
- Desai, J.; Steiger, S.; Anders, H.J. Molecular Pathophysiology of Gout. Trends Mol. Med. 2017, 23, 756–768. [Google Scholar] [CrossRef]
- Dai, Y.; Cao, Y.; Zhang, Z.; Vallurupalli, S.; Mehta, J.L. Xanthine Oxidase Induces Foam Cell Formation through LOX-1 and NLRP3 Activation. Cardiovasc. Drugs Ther. 2017, 31, 19–27. [Google Scholar] [CrossRef]
- Huang, T.T.; Hao, D.L.; Wu, B.N.; Mao, L.L.; Zhang, J. Uric acid demonstrates neuroprotective effect on Parkinson’s disease mice through Nrf2-ARE signaling pathway. Biochem. Biophys. Res. Commun. 2017, 493, 1443–1449. [Google Scholar] [CrossRef] [PubMed]
- Chamorro, A.; Amaro, S.; Castellanos, M.; Segura, T.; Arenillas, J.; Marti-Fabregas, J.; Gallego, J.; Krupinski, J.; Gomis, M.; Canovas, D.; et al. Safety and efficacy of uric acid in patients with acute stroke (URICO-ICTUS): A randomised, double-blind phase 2b/3 trial. Lancet Neurol. 2014, 13, 453–460. [Google Scholar] [CrossRef]
- Chamorro, A.; Amaro, S.; Castellanos, M.; Gomis, M.; Urra, X.; Blasco, J.; Arenillas, J.F.; Roman, L.S.; Munoz, R.; Macho, J.; et al. Uric acid therapy improves the outcomes of stroke patients treated with intravenous tissue plasminogen activator and mechanical thrombectomy. Int. J. Stroke 2017, 12, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Cheungpasitporn, W.; Thongprayoon, C.; Harrison, A.M.; Erickson, S.B. Admission hyperuricemia increases the risk of acute kidney injury in hospitalized patients. Clin. Kidney J. 2016, 9, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Erol, T.; Tekin, A.; Katircibasi, M.T.; Sezgin, N.; Bilgi, M.; Tekin, G.; Zumrutdal, A.; Sezgin, A.T.; Muderrisoglu, H. Efficacy of allopurinol pretreatment for prevention of contrast-induced nephropathy: A randomized controlled trial. Int. J. Cardiol. 2013, 167, 1396–1399. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Bhawani, G.; Kumari, N.; Murthy, K.S.; Lalwani, V.; Raju Ch, N. Comparative study of renal protective effects of allopurinol and N-acetyl-cysteine on contrast induced nephropathy in patients undergoing cardiac catheterization. J. Clin. Diagn. Res. 2014, 8, HC03-07. [Google Scholar] [CrossRef]
- Haryono, A.; Nugrahaningsih, D.A.A.; Sari, D.C.R.; Romi, M.M.; Arfian, N. Reduction of Serum Uric Acid Associated with Attenuation of Renal Injury, Inflammation and Macrophages M1/M2 Ratio in Hyperuricemic Mice Model. Kobe J. Med. Sci. 2018, 64, E107–E114. [Google Scholar]
- Romi, M.M.; Arfian, N.; Tranggono, U.; Setyaningsih, W.A.W.; Sari, D.C.R. Uric acid causes kidney injury through inducing fibroblast expansion, Endothelin-1 expression, and inflammation. BMC Nephrol. 2017, 18, 326. [Google Scholar] [CrossRef]
- Yang, Z.; Xiaohua, W.; Lei, J.; Ruoyun, T.; Mingxia, X.; Weichun, H.; Li, F.; Ping, W.; Junwei, Y. Uric acid increases fibronectin synthesis through upregulation of lysyl oxidase expression in rat renal tubular epithelial cells. Am. J. Physiol. Renal. Physiol. 2010, 299, F336–F346. [Google Scholar] [CrossRef]
- Khosla, U.M.; Zharikov, S.; Finch, J.L.; Nakagawa, T.; Roncal, C.; Mu, W.; Krotova, K.; Block, E.R.; Prabhakar, S.; Johnson, R.J. Hyperuricemia induces endothelial dysfunction. Kidney Int. 2005, 67, 1739–1742. [Google Scholar] [CrossRef]
- Tung, Y.T.; Lin, L.C.; Liu, Y.L.; Ho, S.T.; Lin, C.Y.; Chuang, H.L.; Chiu, C.C.; Huang, C.C.; Wu, J.H. Antioxidative phytochemicals from Rhododendron oldhamii Maxim. leaf extracts reduce serum uric acid levels in potassium oxonate-induced hyperuricemic mice. BMC Complement Altern. Med. 2015, 15, 423. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.W.; Wang, C.P.; Wang, X.; Zhang, Y.L.; Liu, L.; Wang, R.W.; Ye, J.F.; Hu, L.S.; Kong, L.D. Uricosuric and nephroprotective properties of Ramulus Mori ethanol extract in hyperuricemic mice. J. Ethnopharmacol. 2012, 143, 896–904. [Google Scholar] [CrossRef] [PubMed]
- Patschan, D.; Patschan, S.; Gobe, G.G.; Chintala, S.; Goligorsky, M.S. Uric acid heralds ischemic tissue injury to mobilize endothelial progenitor cells. J. Am. Soc. Nephrol. 2007, 18, 1516–1524. [Google Scholar] [CrossRef] [PubMed]
- Steiger, S.; Ma, Q.; Anders, H.J. The case for evidence-based medicine for the association between hyperuricaemia and CKD. Nat. Rev. Nephrol. 2020, 16, 422. [Google Scholar] [CrossRef]
- Lan, R.; Geng, H.; Singha, P.K.; Saikumar, P.; Bottinger, E.P.; Weinberg, J.M.; Venkatachalam, M.A. Mitochondrial Pathology and Glycolytic Shift during Proximal Tubule Atrophy after Ischemic AKI. J. Am. Soc. Nephrol. 2016, 27, 3356–3367. [Google Scholar] [CrossRef]
- Kang, H.M.; Ahn, S.H.; Choi, P.; Ko, Y.A.; Han, S.H.; Chinga, F.; Park, A.S.; Tao, J.; Sharma, K.; Pullman, J.; et al. Defective fatty acid oxidation in renal tubular epithelial cells has a key role in kidney fibrosis development. Nat. Med. 2015, 21, 37–46. [Google Scholar] [CrossRef]
- Ash, S.R.; Cuppage, F.E. Shift toward anaerobic glycolysis in the regenerating rat kidney. Am. J. Pathol. 1970, 60, 385–402. [Google Scholar]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Funk, J.A.; Schnellmann, R.G. Persistent disruption of mitochondrial homeostasis after acute kidney injury. Am. J. Physiol. Renal. Physiol. 2012, 302, F853–F864. [Google Scholar] [CrossRef]
- Perry, H.M.; Huang, L.; Wilson, R.J.; Bajwa, A.; Sesaki, H.; Yan, Z.; Rosin, D.L.; Kashatus, D.F.; Okusa, M.D. Dynamin-Related Protein 1 Deficiency Promotes Recovery from AKI. J. Am. Soc. Nephrol. 2018, 29, 194–206. [Google Scholar] [CrossRef]
- Gall, J.M.; Wang, Z.; Bonegio, R.G.; Havasi, A.; Liesa, M.; Vemula, P.; Borkan, S.C. Conditional knockout of proximal tubule mitofusin 2 accelerates recovery and improves survival after renal ischemia. J. Am. Soc. Nephrol. 2015, 26, 1092–1102. [Google Scholar] [CrossRef] [PubMed]
- Fedorova, L.V.; Sodhi, K.; Gatto-Weis, C.; Puri, N.; Hinds, T.D., Jr.; Shapiro, J.I.; Malhotra, D. Peroxisome proliferator-activated receptor delta agonist, HPP593, prevents renal necrosis under chronic ischemia. PLoS ONE 2013, 8, e64436. [Google Scholar] [CrossRef] [PubMed]
- Tang, C.; Han, H.; Yan, M.; Zhu, S.; Liu, J.; Liu, Z.; He, L.; Tan, J.; Liu, Y.; Liu, H.; et al. PINK1-PRKN/PARK2 pathway of mitophagy is activated to protect against renal ischemia-reperfusion injury. Autophagy 2018, 14, 880–897. [Google Scholar] [CrossRef] [PubMed]
- Chales, G. How should we manage asymptomatic hyperuricemia? Joint Bone Spine 2019, 86, 437–443. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving bioscience research reporting: The ARRIVE guidelines for reporting animal research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gnemmi, V.; Li, Q.; Ma, Q.; De Chiara, L.; Carangelo, G.; Li, C.; Molina-Van den Bosch, M.; Romagnani, P.; Anders, H.-J.; Steiger, S. Asymptomatic Hyperuricemia Promotes Recovery from Ischemic Organ Injury by Modulating the Phenotype of Macrophages. Cells 2022, 11, 626. https://doi.org/10.3390/cells11040626
Gnemmi V, Li Q, Ma Q, De Chiara L, Carangelo G, Li C, Molina-Van den Bosch M, Romagnani P, Anders H-J, Steiger S. Asymptomatic Hyperuricemia Promotes Recovery from Ischemic Organ Injury by Modulating the Phenotype of Macrophages. Cells. 2022; 11(4):626. https://doi.org/10.3390/cells11040626
Chicago/Turabian StyleGnemmi, Viviane, Qiubo Li, Qiuyue Ma, Letizia De Chiara, Giulia Carangelo, Chenyu Li, Mireia Molina-Van den Bosch, Paola Romagnani, Hans-Joachim Anders, and Stefanie Steiger. 2022. "Asymptomatic Hyperuricemia Promotes Recovery from Ischemic Organ Injury by Modulating the Phenotype of Macrophages" Cells 11, no. 4: 626. https://doi.org/10.3390/cells11040626
APA StyleGnemmi, V., Li, Q., Ma, Q., De Chiara, L., Carangelo, G., Li, C., Molina-Van den Bosch, M., Romagnani, P., Anders, H.-J., & Steiger, S. (2022). Asymptomatic Hyperuricemia Promotes Recovery from Ischemic Organ Injury by Modulating the Phenotype of Macrophages. Cells, 11(4), 626. https://doi.org/10.3390/cells11040626







