Transit Amplifying Progenitors in the Cerebellum: Similarities to and Differences from Transit Amplifying Cells in Other Brain Regions and between Species
Abstract
:1. Introduction
2. Traditional Models of Transit Amplification in Neural Progenitors
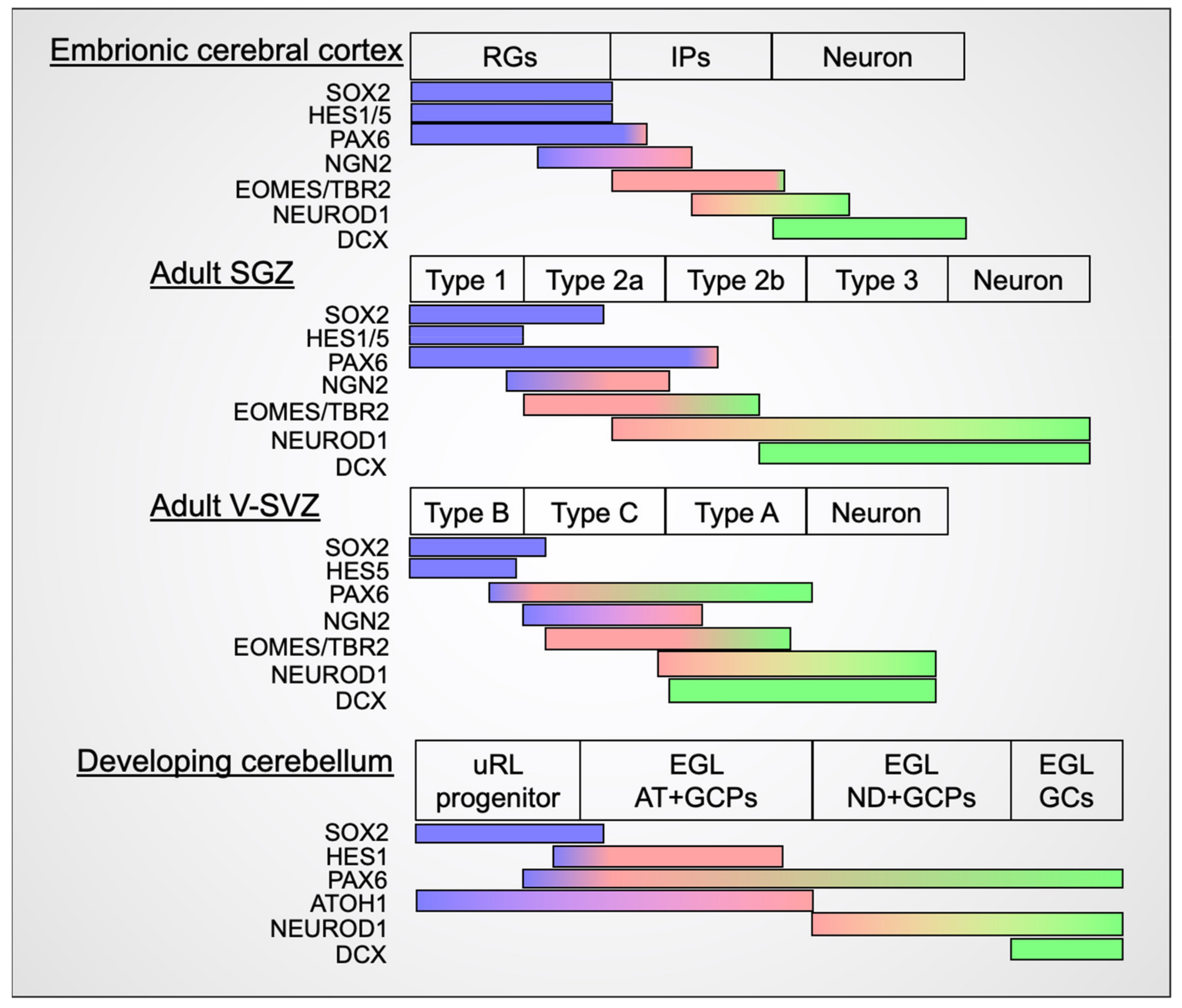
2.1. Embryonic Neurogenesis in the Mammalian Cerebral Cortex
2.2. Adult Neurogenesis in Mammals
2.2.1. SGZ of the Hippocampal Dentate Gyrus
2.2.2. V-SVZ of the LV
2.2.3. Other Regions
2.3. Neurogenesis in the Drosophila CNS
3. Transit Amplification of Mammalian Cerebellar Granule Cell Progenitors
3.1. Identification of Novel Transit Amplifying Progenitors in the Mouse EGL
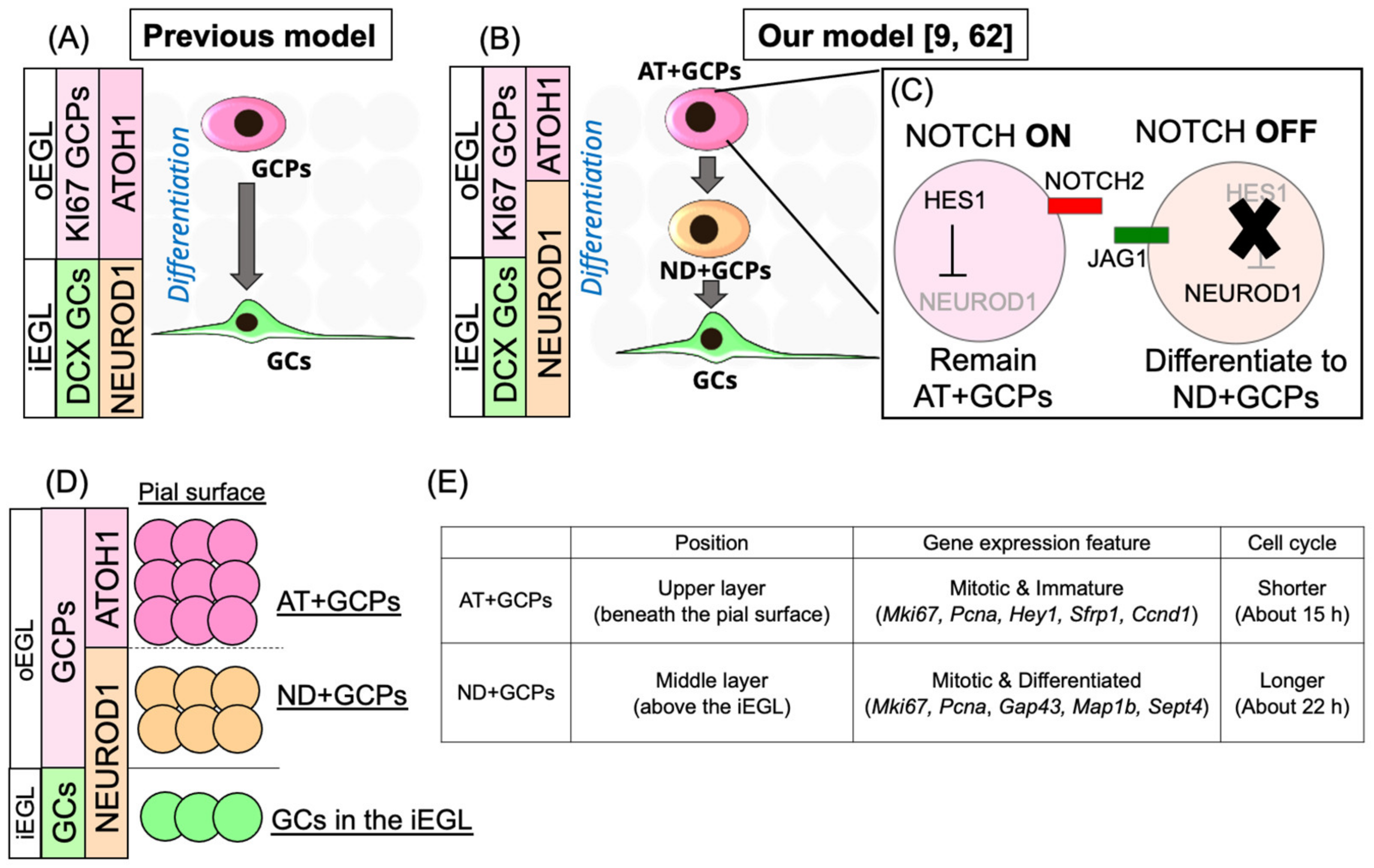
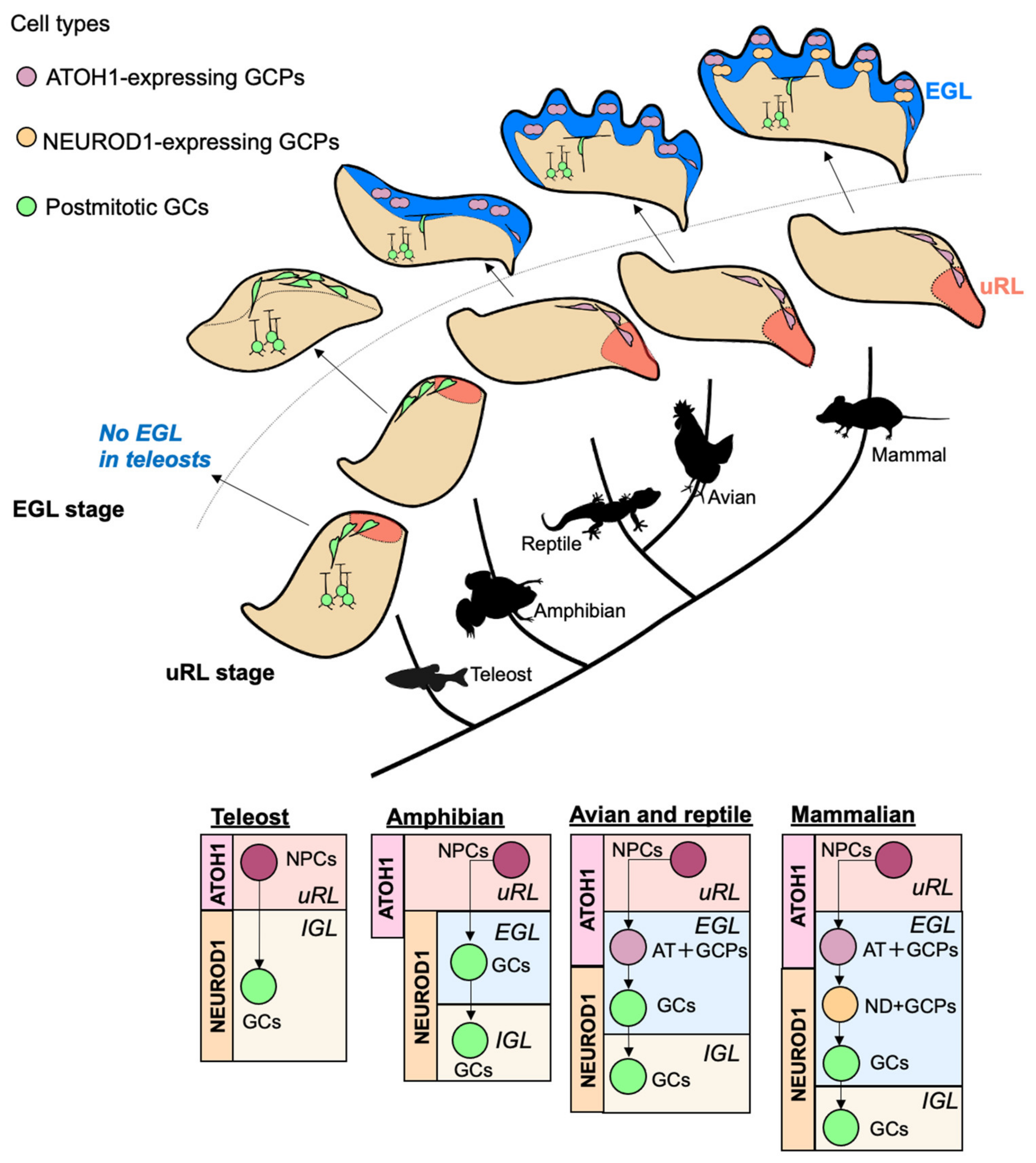
3.2. Comparisons of Transit Amplification of GC-Lineage Cells in Vertebrates
3.2.1. Fish, Amphibians, Reptiles, and Birds
3.2.2. Rodents
3.2.3. Humans
4. Conclusions and Perspectives
| uRL | EGL | ATOH1 Expression in the EGL | AT + GCPs | ND + GCPs | |
|---|---|---|---|---|---|
| Fish [67,68] | Yes | No | No | No | No |
| Amphibian [69] | Yes | Yes (non-proliferative) | Yes | No | No |
| Reptile [70] | Yes | Yes | Yes | Yes | Unknown |
| Chick [71,84] | Yes | Yes | Yes | Yes | No |
| Mouse [9,61] | Yes | Yes | Yes | Yes | Yes |
| Human [61,80,81] | Yes | Yes | Yes | Yes | Yes |
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rangel-Huerta, E.; Maldonado, E. Transit-Amplifying Cells in the Fast Lane from Stem Cells towards Differentiation. Stem Cells Int. 2017, 2017, 7602951. [Google Scholar] [CrossRef]
- Jurkowski, M.P.; Bettio, L.; Woo, E.K.; Patten, A.; Yau, S.Y.; Gil-Mohapel, J. Beyond the Hippocampus and the SVZ: Adult Neurogenesis Throughout the Brain. Front. Cell. Neurosci. 2020, 14, 576444. [Google Scholar] [CrossRef] [PubMed]
- De Sousa, E.M.F.; de Sauvage, F.J. Cellular Plasticity in Intestinal Homeostasis and Disease. Cell Stem Cell 2019, 24, 54–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alonso, L.; Fuchs, E. Stem cells in the skin: Waste not, Wnt not. Genes Dev. 2003, 17, 1189–1200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, N.; Guillemot, F. Neurogenesis in the embryonic and adult brain: Same regulators, different roles. Front. Cell. Neurosci. 2014, 8, 396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dwyer, N.D.; Chen, B.; Chou, S.J.; Hippenmeyer, S.; Nguyen, L.; Ghashghaei, H.T. Neural Stem Cells to Cerebral Cortex: Emerging Mechanisms Regulating Progenitor Behavior and Productivity. J. Neurosci. 2016, 36, 11394–11401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Vry, J.; Martinez-Martinez, P.; Losen, M.; Temel, Y.; Steckler, T.; Steinbusch, H.W.; De Baets, M.H.; Prickaerts, J. In vivo electroporation of the central nervous system: A non-viral approach for targeted gene delivery. Prog. Neurobiol. 2010, 92, 227–244. [Google Scholar] [CrossRef]
- Butts, T.; Green, M.J.; Wingate, R.J. Development of the cerebellum: Simple steps to make a ‘little brain’. Development 2014, 141, 4031–4041. [Google Scholar] [CrossRef] [Green Version]
- Miyashita, S.; Owa, T.; Seto, Y.; Yamashita, M.; Aida, S.; Sone, M.; Ichijo, K.; Nishioka, T.; Kaibuchi, K.; Kawaguchi, Y.; et al. Cyclin D1 controls development of cerebellar granule cell progenitors through phosphorylation and stabilization of ATOH1. EMBO J. 2021, 40, e105712. [Google Scholar] [CrossRef]
- Hofman, M.A. Evolution of the human brain: When bigger is better. Front. Neuroanat. 2014, 8, 15. [Google Scholar] [CrossRef] [Green Version]
- Herculano-Houzel, S. The human brain in numbers: A linearly scaled-up primate brain. Front. Hum. Neurosci. 2009, 3, 31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goncalves, J.T.; Schafer, S.T.; Gage, F.H. Adult Neurogenesis in the Hippocampus: From Stem Cells to Behavior. Cell 2016, 167, 897–914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alvarez-Buylla, A.; Lim, D.A. For the long run: Maintaining germinal niches in the adult brain. Neuron 2004, 41, 683–686. [Google Scholar] [CrossRef] [Green Version]
- Jiang, Y.; Reichert, H. Drosophila neural stem cells in brain development and tumor formation. J. Neurogenet. 2014, 28, 181–189. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardenas, A.; Villalba, A.; de Juan Romero, C.; Pico, E.; Kyrousi, C.; Tzika, A.C.; Tessier-Lavigne, M.; Ma, L.; Drukker, M.; Cappello, S.; et al. Evolution of Cortical Neurogenesis in Amniotes Controlled by Robo Signaling Levels. Cell 2018, 174, 590–606.e21. [Google Scholar] [CrossRef] [Green Version]
- Barnea, A.; Pravosudov, V. Birds as a model to study adult neurogenesis: Bridging evolutionary, comparative and neuroethological approaches. Eur. J. Neurosci. 2011, 34, 884–907. [Google Scholar] [CrossRef]
- Zupanc, G.K.H. Adult neurogenesis in the central nervous system of teleost fish: From stem cells to function and evolution. J. Exp. Biol. 2021, 224, jeb226357. [Google Scholar] [CrossRef]
- Vaid, S.; Huttner, W.B. Transcriptional Regulators and Human-Specific/Primate-Specific Genes in Neocortical Neurogenesis. Int. J. Mol. Sci. 2020, 21, 4614. [Google Scholar] [CrossRef]
- Loo, L.; Simon, J.M.; Xing, L.; McCoy, E.S.; Niehaus, J.K.; Guo, J.; Anton, E.S.; Zylka, M.J. Single-cell transcriptomic analysis of mouse neocortical development. Nat. Commun. 2019, 10, 134. [Google Scholar] [CrossRef] [Green Version]
- Ruan, X.; Kang, B.; Qi, C.; Lin, W.; Wang, J.; Zhang, X. Progenitor cell diversity in the developing mouse neocortex. Proc. Natl. Acad. Sci. USA 2021, 118, e2018866118. [Google Scholar] [CrossRef]
- Bedogni, F.; Hevner, R.F. Cell-Type-Specific Gene Expression in Developing Mouse Neocortex: Intermediate Progenitors Implicated in Axon Development. Front. Mol. Neurosci. 2021, 14, 686034. [Google Scholar] [CrossRef] [PubMed]
- Smart, I.H.; Dehay, C.; Giroud, P.; Berland, M.; Kennedy, H. Unique morphological features of the proliferative zones and postmitotic compartments of the neural epithelium giving rise to striate and extrastriate cortex in the monkey. Cereb. Cortex 2002, 12, 37–53. [Google Scholar] [CrossRef] [PubMed]
- Hansen, D.V.; Lui, J.H.; Parker, P.R.; Kriegstein, A.R. Neurogenic radial glia in the outer subventricular zone of human neocortex. Nature 2010, 464, 554–561. [Google Scholar] [CrossRef] [PubMed]
- Fietz, S.A.; Kelava, I.; Vogt, J.; Wilsch-Brauninger, M.; Stenzel, D.; Fish, J.L.; Corbeil, D.; Riehn, A.; Distler, W.; Nitsch, R.; et al. OSVZ progenitors of human and ferret neocortex are epithelial-like and expand by integrin signaling. Nat. Neurosci. 2010, 13, 690–699. [Google Scholar] [CrossRef]
- Gertz, C.C.; Lui, J.H.; LaMonica, B.E.; Wang, X.; Kriegstein, A.R. Diverse behaviors of outer radial glia in developing ferret and human cortex. J. Neurosci. 2014, 34, 2559–2570. [Google Scholar] [CrossRef]
- Paul, V.; Tonchev, A.B.; Henningfeld, K.A.; Pavlakis, E.; Rust, B.; Pieler, T.; Stoykova, A. Scratch2 modulates neurogenesis and cell migration through antagonism of bHLH proteins in the developing neocortex. Cereb. Cortex 2014, 24, 754–772. [Google Scholar] [CrossRef] [Green Version]
- Lv, X.; Ren, S.Q.; Zhang, X.J.; Shen, Z.; Ghosh, T.; Xianyu, A.; Gao, P.; Li, Z.; Lin, S.; Yu, Y.; et al. TBR2 coordinates neurogenesis expansion and precise microcircuit organization via Protocadherin 19 in the mammalian cortex. Nat. Commun. 2019, 10, 3946. [Google Scholar] [CrossRef] [Green Version]
- Hasenpusch-Theil, K.; Laclef, C.; Colligan, M.; Fitzgerald, E.; Howe, K.; Carroll, E.; Abrams, S.R.; Reiter, J.F.; Schneider-Maunoury, S.; Theil, T. A transient role of the ciliary gene Inpp5e in controlling direct versus indirect neurogenesis in cortical development. eLife 2020, 9, e58162. [Google Scholar] [CrossRef]
- Das, R.M.; Storey, K.G. Apical abscission alters cell polarity and dismantles the primary cilium during neurogenesis. Science 2014, 343, 200–204. [Google Scholar] [CrossRef] [Green Version]
- Altman, J.; Das, G.D. Autoradiographic and histological evidence of postnatal hippocampal neurogenesis in rats. J. Comp. Neurol. 1965, 124, 319–335. [Google Scholar] [CrossRef]
- Gould, E.; McEwen, B.S.; Tanapat, P.; Galea, L.A.; Fuchs, E. Neurogenesis in the dentate gyrus of the adult tree shrew is regulated by psychosocial stress and NMDA receptor activation. J. Neurosci. 1997, 17, 2492–2498. [Google Scholar] [CrossRef]
- Gould, E.; Tanapat, P.; McEwen, B.S.; Flugge, G.; Fuchs, E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc. Natl. Acad. Sci. USA 1998, 95, 3168–3171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eriksson, P.S.; Perfilieva, E.; Bjork-Eriksson, T.; Alborn, A.M.; Nordborg, C.; Peterson, D.A.; Gage, F.H. Neurogenesis in the adult human hippocampus. Nat. Med. 1998, 4, 1313–1317. [Google Scholar] [CrossRef] [PubMed]
- Doetsch, F.; Caille, I.; Lim, D.A.; Garcia-Verdugo, J.M.; Alvarez-Buylla, A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell 1999, 97, 703–716. [Google Scholar] [CrossRef] [Green Version]
- Hodge, R.D.; Kahoud, R.J.; Hevner, R.F. Transcriptional control of glutamatergic differentiation during adult neurogenesis. Cell. Mol. Life Sci. 2012, 69, 2125–2134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponti, G.; Obernier, K.; Guinto, C.; Jose, L.; Bonfanti, L.; Alvarez-Buylla, A. Cell cycle and lineage progression of neural progenitors in the ventricular-subventricular zones of adult mice. Proc. Natl. Acad. Sci. USA 2013, 110, E1045–E1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.; Liu, F.; Liu, Y.Y.; Zhao, C.H.; You, Y.; Wang, L.; Zhang, J.; Wei, B.; Ma, T.; Zhang, Q.; et al. Identification and characterization of neuroblasts in the subventricular zone and rostral migratory stream of the adult human brain. Cell Res. 2011, 21, 1534–1550. [Google Scholar] [CrossRef] [Green Version]
- Gonzalez-Perez, O.; Alvarez-Buylla, A. Oligodendrogenesis in the subventricular zone and the role of epidermal growth factor. Brain Res. Rev. 2011, 67, 147–156. [Google Scholar] [CrossRef] [Green Version]
- Menn, B.; Garcia-Verdugo, J.M.; Yaschine, C.; Gonzalez-Perez, O.; Rowitch, D.; Alvarez-Buylla, A. Origin of oligodendrocytes in the subventricular zone of the adult brain. J. Neurosci. 2006, 26, 7907–7918. [Google Scholar] [CrossRef]
- Kokoeva, M.V.; Yin, H.; Flier, J.S. Neurogenesis in the hypothalamus of adult mice: Potential role in energy balance. Science 2005, 310, 679–683. [Google Scholar] [CrossRef]
- Lee, D.A.; Bedont, J.L.; Pak, T.; Wang, H.; Song, J.; Miranda-Angulo, A.; Takiar, V.; Charubhumi, V.; Balordi, F.; Takebayashi, H.; et al. Tanycytes of the hypothalamic median eminence form a diet-responsive neurogenic niche. Nat. Neurosci. 2012, 15, 700–702. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernier, P.J.; Bedard, A.; Vinet, J.; Levesque, M.; Parent, A. Newly generated neurons in the amygdala and adjoining cortex of adult primates. Proc. Natl. Acad. Sci. USA 2002, 99, 11464–11469. [Google Scholar] [CrossRef] [Green Version]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ernst, A.; Alkass, K.; Bernard, S.; Salehpour, M.; Perl, S.; Tisdale, J.; Possnert, G.; Druid, H.; Frisen, J. Neurogenesis in the striatum of the adult human brain. Cell 2014, 156, 1072–1083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luzzati, F.; De Marchis, S.; Fasolo, A.; Peretto, P. Neurogenesis in the caudate nucleus of the adult rabbit. J. Neurosci. 2006, 26, 609–621. [Google Scholar] [CrossRef]
- Bedard, A.; Cossette, M.; Levesque, M.; Parent, A. Proliferating cells can differentiate into neurons in the striatum of normal adult monkey. Neurosci. Lett. 2002, 328, 213–216. [Google Scholar] [CrossRef]
- Bello, B.C.; Izergina, N.; Caussinus, E.; Reichert, H. Amplification of neural stem cell proliferation by intermediate progenitor cells in Drosophila brain development. Neural Dev. 2008, 3, 5. [Google Scholar] [CrossRef] [Green Version]
- Bowman, S.K.; Rolland, V.; Betschinger, J.; Kinsey, K.A.; Emery, G.; Knoblich, J.A. The tumor suppressors Brat and Numb regulate transit-amplifying neuroblast lineages in Drosophila. Dev. Cell 2008, 14, 535–546. [Google Scholar] [CrossRef] [Green Version]
- Boone, J.Q.; Doe, C.Q. Identification of Drosophila type II neuroblast lineages containing transit amplifying ganglion mother cells. Dev. Neurobiol. 2008, 68, 1185–1195. [Google Scholar] [CrossRef] [Green Version]
- Azevedo, F.A.; Carvalho, L.R.; Grinberg, L.T.; Farfel, J.M.; Ferretti, R.E.; Leite, R.E.; Jacob Filho, W.; Lent, R.; Herculano-Houzel, S. Equal numbers of neuronal and nonneuronal cells make the human brain an isometrically scaled-up primate brain. J. Comp. Neurol. 2009, 513, 532–541. [Google Scholar] [CrossRef]
- Herculano-Houzel, S.; Mota, B.; Lent, R. Cellular scaling rules for rodent brains. Proc. Natl. Acad. Sci. USA 2006, 103, 12138–12143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chedotal, A. Should I stay or should I go? Becoming a granule cell. Trends Neurosci. 2010, 33, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Wang, V.Y.; Zoghbi, H.Y. Genetic regulation of cerebellar development. Nat. Rev. Neurosci. 2001, 2, 484–491. [Google Scholar] [CrossRef] [PubMed]
- Goldowitz, D.; Hamre, K. The cells and molecules that make a cerebellum. Trends Neurosci. 1998, 21, 375–382. [Google Scholar] [CrossRef]
- Ryan, K.E.; Kim, P.S.; Fleming, J.T.; Brignola, E.; Cheng, F.Y.; Litingtung, Y.; Chiang, C. Lkb1 regulates granule cell migration and cortical folding of the cerebellar cortex. Dev. Biol. 2017, 432, 165–177. [Google Scholar] [CrossRef] [PubMed]
- Owa, T.; Taya, S.; Miyashita, S.; Yamashita, M.; Adachi, T.; Yamada, K.; Yokoyama, M.; Aida, S.; Nishioka, T.; Inoue, Y.U.; et al. Meis1 Coordinates Cerebellar Granule Cell Development by Regulating Pax6 Transcription, BMP Signaling and Atoh1 Degradation. J. Neurosci. 2018, 38, 1277–1294. [Google Scholar] [CrossRef] [Green Version]
- El Nagar, S.; Chakroun, A.; Le Greneur, C.; Figarella-Branger, D.; Di Meglio, T.; Lamonerie, T.; Billon, N. Otx2 promotes granule cell precursor proliferation and Shh-dependent medulloblastoma maintenance in vivo. Oncogenesis 2018, 7, 60. [Google Scholar] [CrossRef] [Green Version]
- Chang, C.H.; Zanini, M.; Shirvani, H.; Cheng, J.S.; Yu, H.; Feng, C.H.; Mercier, A.L.; Hung, S.Y.; Forget, A.; Wang, C.H.; et al. Atoh1 Controls Primary Cilia Formation to Allow for SHH-Triggered Granule Neuron Progenitor Proliferation. Dev. Cell 2019, 48, 184–199.e5. [Google Scholar] [CrossRef] [Green Version]
- Xenaki, D.; Martin, I.B.; Yoshida, L.; Ohyama, K.; Gennarini, G.; Grumet, M.; Sakurai, T.; Furley, A.J. F3/contactin and TAG1 play antagonistic roles in the regulation of sonic hedgehog-induced cerebellar granule neuron progenitor proliferation. Development 2011, 138, 519–529. [Google Scholar] [CrossRef] [Green Version]
- Shiraishi, R.D.; Miyashita, S.; Yamashita, M.; Adachi, T.; Shimoda, M.M.; Owa, T.; Hoshino, M. Expression of transcription factors and signaling molecules in the cerebellar granule cell development. Gene Expr. Patterns 2019, 34, 119068. [Google Scholar] [CrossRef]
- Behesti, H.; Kocabas, A.; Buchholz, D.E.; Carroll, T.S.; Hatten, M.E. Altered temporal sequence of transcriptional regulators in the generation of human cerebellar granule cells. eLife 2021, 10, e67074. [Google Scholar] [CrossRef] [PubMed]
- Adachi, T.; Miyashita, S.; Yamashita, M.; Shimoda, M.; Okonechnikov, K.; Chavez, L.; Kool, M.; Pfister, S.M.; Inoue, T.; Kawauchi, D.; et al. Notch Signaling between Cerebellar Granule Cell Progenitors. eNeuro 2021, 8, ENEURO.0468-20.2021. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Maeda, T.; Lee, J.E. NeuroD is required for differentiation of the granule cells in the cerebellum and hippocampus. Genes Dev. 1999, 13, 1647–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimojo, H.; Ohtsuka, T.; Kageyama, R. Oscillations in notch signaling regulate maintenance of neural progenitors. Neuron 2008, 58, 52–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sugahara, F.; Murakami, Y.; Pascual-Anaya, J.; Kuratani, S. Reconstructing the ancestral vertebrate brain. Dev. Growth Differ. 2017, 59, 163–174. [Google Scholar] [CrossRef] [Green Version]
- Sugahara, F.; Pascual-Anaya, J.; Oisi, Y.; Kuraku, S.; Aota, S.; Adachi, N.; Takagi, W.; Hirai, T.; Sato, N.; Murakami, Y.; et al. Evidence from cyclostomes for complex regionalization of the ancestral vertebrate brain. Nature 2016, 531, 97–100. [Google Scholar] [CrossRef]
- Butts, T.; Modrell, M.S.; Baker, C.V.; Wingate, R.J. The evolution of the vertebrate cerebellum: Absence of a proliferative external granule layer in a non-teleost ray-finned fish. Evol. Dev. 2014, 16, 92–100. [Google Scholar] [CrossRef] [Green Version]
- Chaplin, N.; Tendeng, C.; Wingate, R.J. Absence of an external germinal layer in zebrafish and shark reveals a distinct, anamniote ground plan of cerebellum development. J. Neurosci. 2010, 30, 3048–3057. [Google Scholar] [CrossRef] [Green Version]
- Butts, T.; Hanzel, M.; Wingate, R.J. Transit amplification in the amniote cerebellum evolved via a heterochronic shift in NeuroD1 expression. Development 2014, 141, 2791–2795. [Google Scholar] [CrossRef] [Green Version]
- Macri, S.; Di-Poi, N. Heterochronic Developmental Shifts Underlying Squamate Cerebellar Diversity Unveil the Key Features of Amniote Cerebellogenesis. Front. Cell Dev. Biol. 2020, 8, 593377. [Google Scholar] [CrossRef]
- Hanzel, M.; Rook, V.; Wingate, R.J.T. Mitotic granule cell precursors undergo highly dynamic morphological transitions throughout the external germinal layer of the chick cerebellum. Sci. Rep. 2019, 9, 15218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sultan, F.; Braitenberg, V. Shapes and sizes of different mammalian cerebella. A study in quantitative comparative neuroanatomy. J. Hirnforsch. 1993, 34, 79–92. [Google Scholar] [PubMed]
- Lee, J.K.; Cho, J.H.; Hwang, W.S.; Lee, Y.D.; Reu, D.S.; Suh-Kim, H. Expression of neuroD/BETA2 in mitotic and postmitotic neuronal cells during the development of nervous system. Dev. Dyn. 2000, 217, 361–367. [Google Scholar] [CrossRef]
- Barton, R.A.; Venditti, C. Rapid evolution of the cerebellum in humans and other great apes. Curr. Biol. 2014, 24, 2440–2444. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sereno, M.I.; Diedrichsen, J.; Tachrount, M.; Testa-Silva, G.; d’Arceuil, H.; De Zeeuw, C. The human cerebellum has almost 80% of the surface area of the neocortex. Proc. Natl. Acad. Sci. USA 2020, 117, 19538–19543. [Google Scholar] [CrossRef]
- Marzban, H.; Del Bigio, M.R.; Alizadeh, J.; Ghavami, S.; Zachariah, R.M.; Rastegar, M. Cellular commitment in the developing cerebellum. Front. Cell. Neurosci. 2014, 8, 450. [Google Scholar] [CrossRef] [Green Version]
- Volpe, J.J. Cerebellum of the premature infant: Rapidly developing, vulnerable, clinically important. J. Child Neurol. 2009, 24, 1085–1104. [Google Scholar] [CrossRef] [Green Version]
- Abraham, H.; Tornoczky, T.; Kosztolanyi, G.; Seress, L. Cell formation in the cortical layers of the developing human cerebellum. Int. J. Dev. Neurosci. 2001, 19, 53–62. [Google Scholar] [CrossRef]
- Haldipur, P.; Bharti, U.; Alberti, C.; Sarkar, C.; Gulati, G.; Iyengar, S.; Gressens, P.; Mani, S. Preterm delivery disrupts the developmental program of the cerebellum. PLoS ONE 2011, 6, e23449. [Google Scholar] [CrossRef] [Green Version]
- Haldipur, P.; Aldinger, K.A.; Bernardo, S.; Deng, M.; Timms, A.E.; Overman, L.M.; Winter, C.; Lisgo, S.N.; Razavi, F.; Silvestri, E.; et al. Spatiotemporal expansion of primary progenitor zones in the developing human cerebellum. Science 2019, 366, 454–460. [Google Scholar] [CrossRef]
- Aldinger, K.A.; Thomson, Z.; Phelps, I.G.; Haldipur, P.; Deng, M.; Timms, A.E.; Hirano, M.; Santpere, G.; Roco, C.; Rosenberg, A.B.; et al. Spatial and cell type transcriptional landscape of human cerebellar development. Nat. Neurosci. 2021, 24, 1163–1175. [Google Scholar] [CrossRef]
- Stoodley, C.J.; Limperopoulos, C. Structure-function relationships in the developing cerebellum: Evidence from early-life cerebellar injury and neurodevelopmental disorders. Semin. Fetal Neonatal Med. 2016, 21, 356–364. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boutin, C.; Hardt, O.; de Chevigny, A.; Core, N.; Goebbels, S.; Seidenfaden, R.; Bosio, A.; Cremer, H. NeuroD1 induces terminal neuronal differentiation in olfactory neurogenesis. Proc. Natl. Acad. Sci. USA 2010, 107, 1201–1206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Green, M.J.; Myat, A.M.; Emmenegger, B.A.; Wechsler-Reya, R.J.; Wilson, L.J.; Wingate, R.J. Independently specified Atoh1 domains define novel developmental compartments in rhombomere 1. Development 2014, 141, 389–398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bani-Yaghoub, M.; Tremblay, R.G.; Lei, J.X.; Zhang, D.; Zurakowski, B.; Sandhu, J.K.; Smith, B.; Ribecco-Lutkiewicz, M.; Kennedy, J.; Walker, P.R.; et al. Role of Sox2 in the development of the mouse neocortex. Dev. Biol. 2006, 295, 52–66. [Google Scholar] [CrossRef] [Green Version]
- Ferri, A.L.; Cavallaro, M.; Braida, D.; Di Cristofano, A.; Canta, A.; Vezzani, A.; Ottolenghi, S.; Pandolfi, P.P.; Sala, M.; DeBiasi, S.; et al. Sox2 deficiency causes neurodegeneration and impaired neurogenesis in the adult mouse brain. Development 2004, 131, 3805–3819. [Google Scholar] [CrossRef] [Green Version]
- Ahlfeld, J.; Favaro, R.; Pagella, P.; Kretzschmar, H.A.; Nicolis, S.; Schuller, U. Sox2 requirement in sonic hedgehog-associated medulloblastoma. Cancer Res. 2013, 73, 3796–3807. [Google Scholar] [CrossRef] [Green Version]
- Gleeson, J.G.; Lin, P.T.; Flanagan, L.A.; Walsh, C.A. Doublecortin is a microtubule-associated protein and is expressed widely by migrating neurons. Neuron 1999, 23, 257–271. [Google Scholar] [CrossRef] [Green Version]
- Ohtsuka, T.; Kageyama, R. Hes1 overexpression leads to expansion of embryonic neural stem cell pool and stem cell reservoir in the postnatal brain. Development 2021, 148, dev189191. [Google Scholar] [CrossRef]
- Bansod, S.; Kageyama, R.; Ohtsuka, T. Hes5 regulates the transition timing of neurogenesis and gliogenesis in mammalian neocortical development. Development 2017, 144, 3156–3167. [Google Scholar] [CrossRef] [Green Version]
- Sueda, R.; Imayoshi, I.; Harima, Y.; Kageyama, R. High Hes1 expression and resultant Ascl1 suppression regulate quiescent vs. active neural stem cells in the adult mouse brain. Genes Dev. 2019, 33, 511–523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lugert, S.; Basak, O.; Knuckles, P.; Haussler, U.; Fabel, K.; Gotz, M.; Haas, C.A.; Kempermann, G.; Taylor, V.; Giachino, C. Quiescent and active hippocampal neural stem cells with distinct morphologies respond selectively to physiological and pathological stimuli and aging. Cell Stem Cell 2010, 6, 445–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imayoshi, I.; Sakamoto, M.; Yamaguchi, M.; Mori, K.; Kageyama, R. Essential roles of Notch signaling in maintenance of neural stem cells in developing and adult brains. J. Neurosci. 2010, 30, 3489–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Masserdotti, G.; Badaloni, A.; Green, Y.S.; Croci, L.; Barili, V.; Bergamini, G.; Vetter, M.L.; Consalez, G.G. ZFP423 coordinates Notch and bone morphogenetic protein signaling, selectively up-regulating Hes5 gene expression. J. Biol. Chem. 2010, 285, 30814–30824. [Google Scholar] [CrossRef] [Green Version]
- Englund, C.; Fink, A.; Lau, C.; Pham, D.; Daza, R.A.; Bulfone, A.; Kowalczyk, T.; Hevner, R.F. Pax6, Tbr2, and Tbr1 are expressed sequentially by radial glia, intermediate progenitor cells, and postmitotic neurons in developing neocortex. J. Neurosci. 2005, 25, 247–251. [Google Scholar] [CrossRef] [Green Version]
- Maekawa, M.; Takashima, N.; Arai, Y.; Nomura, T.; Inokuchi, K.; Yuasa, S.; Osumi, N. Pax6 is required for production and maintenance of progenitor cells in postnatal hippocampal neurogenesis. Genes Cells 2005, 10, 1001–1014. [Google Scholar] [CrossRef]
- Nacher, J.; Varea, E.; Blasco-Ibanez, J.M.; Castillo-Gomez, E.; Crespo, C.; Martinez-Guijarro, F.J.; McEwen, B.S. Expression of the transcription factor Pax 6 in the adult rat dentate gyrus. J. Neurosci. Res. 2005, 81, 753–761. [Google Scholar] [CrossRef]
- Kohwi, M.; Osumi, N.; Rubenstein, J.L.; Alvarez-Buylla, A. Pax6 is required for making specific subpopulations of granule and periglomerular neurons in the olfactory bulb. J. Neurosci. 2005, 25, 6997–7003. [Google Scholar] [CrossRef] [Green Version]
- Roybon, L.; Deierborg, T.; Brundin, P.; Li, J.Y. Involvement of Ngn2, Tbr and NeuroD proteins during postnatal olfactory bulb neurogenesis. Eur. J. Neurosci. 2009, 29, 232–243. [Google Scholar] [CrossRef]
- Yeung, J.; Ha, T.J.; Swanson, D.J.; Choi, K.; Tong, Y.; Goldowitz, D. Wls provides a new compartmental view of the rhombic lip in mouse cerebellar development. J. Neurosci. 2014, 34, 12527–12537. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, T.; Pontious, A.; Englund, C.; Daza, R.A.; Bedogni, F.; Hodge, R.; Attardo, A.; Bell, C.; Huttner, W.B.; Hevner, R.F. Intermediate neuronal progenitors (basal progenitors) produce pyramidal-projection neurons for all layers of cerebral cortex. Cereb. Cortex 2009, 19, 2439–2450. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodge, R.D.; Kowalczyk, T.D.; Wolf, S.A.; Encinas, J.M.; Rippey, C.; Enikolopov, G.; Kempermann, G.; Hevner, R.F. Intermediate progenitors in adult hippocampal neurogenesis: Tbr2 expression and coordinate regulation of neuronal output. J. Neurosci. 2008, 28, 3707–3717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brill, M.S.; Ninkovic, J.; Winpenny, E.; Hodge, R.D.; Ozen, I.; Yang, R.; Lepier, A.; Gascon, S.; Erdelyi, F.; Szabo, G.; et al. Adult generation of glutamatergic olfactory bulb interneurons. Nat. Neurosci. 2009, 12, 1524–1533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hevner, R.F.; Hodge, R.D.; Daza, R.A.; Englund, C. Transcription factors in glutamatergic neurogenesis: Conserved programs in neocortex, cerebellum, and adult hippocampus. Neurosci. Res. 2006, 55, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Kuwabara, T.; Hsieh, J.; Muotri, A.; Yeo, G.; Warashina, M.; Lie, D.C.; Moore, L.; Nakashima, K.; Asashima, M.; Gage, F.H. Wnt-mediated activation of NeuroD1 and retro-elements during adult neurogenesis. Nat. Neurosci. 2009, 12, 1097–1105. [Google Scholar] [CrossRef] [Green Version]
- Gao, Z.; Ure, K.; Ables, J.L.; Lagace, D.C.; Nave, K.A.; Goebbels, S.; Eisch, A.J.; Hsieh, J. Neurod1 is essential for the survival and maturation of adult-born neurons. Nat. Neurosci. 2009, 12, 1090–1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miyashita, S.; Hoshino, M. Transit Amplifying Progenitors in the Cerebellum: Similarities to and Differences from Transit Amplifying Cells in Other Brain Regions and between Species. Cells 2022, 11, 726. https://doi.org/10.3390/cells11040726
Miyashita S, Hoshino M. Transit Amplifying Progenitors in the Cerebellum: Similarities to and Differences from Transit Amplifying Cells in Other Brain Regions and between Species. Cells. 2022; 11(4):726. https://doi.org/10.3390/cells11040726
Chicago/Turabian StyleMiyashita, Satoshi, and Mikio Hoshino. 2022. "Transit Amplifying Progenitors in the Cerebellum: Similarities to and Differences from Transit Amplifying Cells in Other Brain Regions and between Species" Cells 11, no. 4: 726. https://doi.org/10.3390/cells11040726
APA StyleMiyashita, S., & Hoshino, M. (2022). Transit Amplifying Progenitors in the Cerebellum: Similarities to and Differences from Transit Amplifying Cells in Other Brain Regions and between Species. Cells, 11(4), 726. https://doi.org/10.3390/cells11040726






