The Sonic Hedgehog Pathway Modulates Survival, Proliferation, and Differentiation of Neural Progenitor Cells under Inflammatory Stress In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Isolation and Culture of Neuronal Precursors Cells (NPCs)
2.2. Treatment Characteristics and Study Design
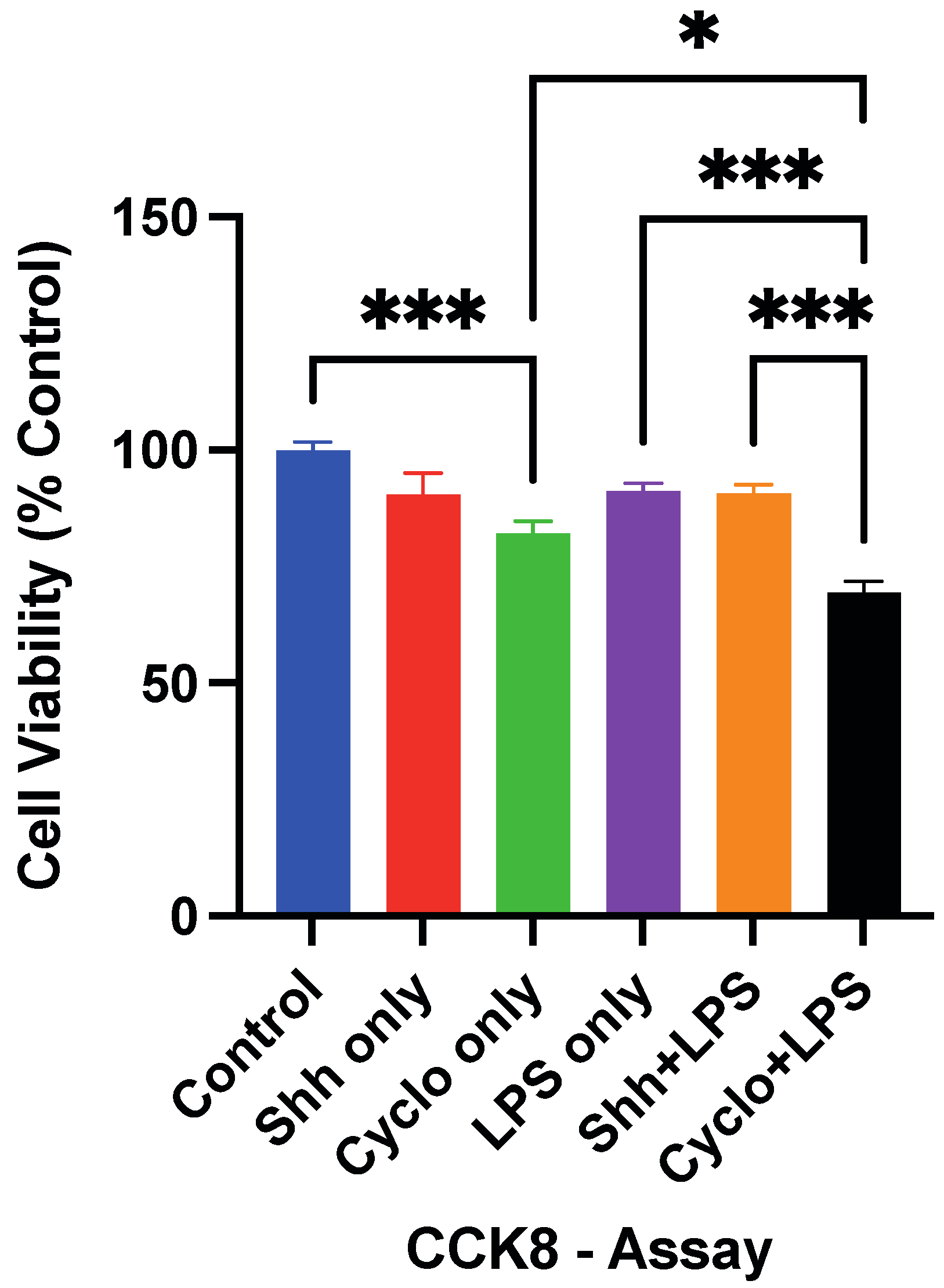
2.3. Cell-Count-Kit 8 (CCK-8) Viable Cell Quantification
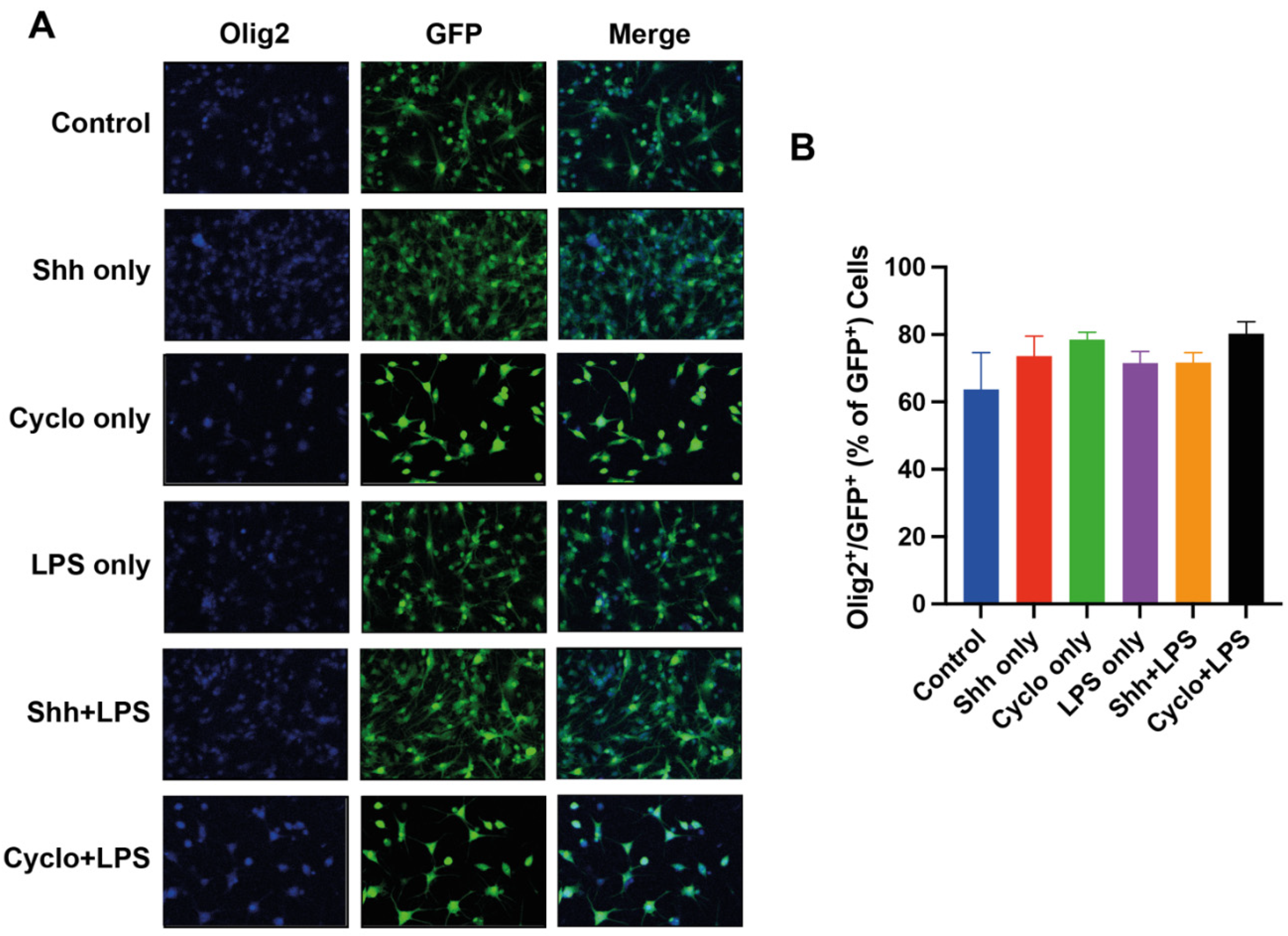
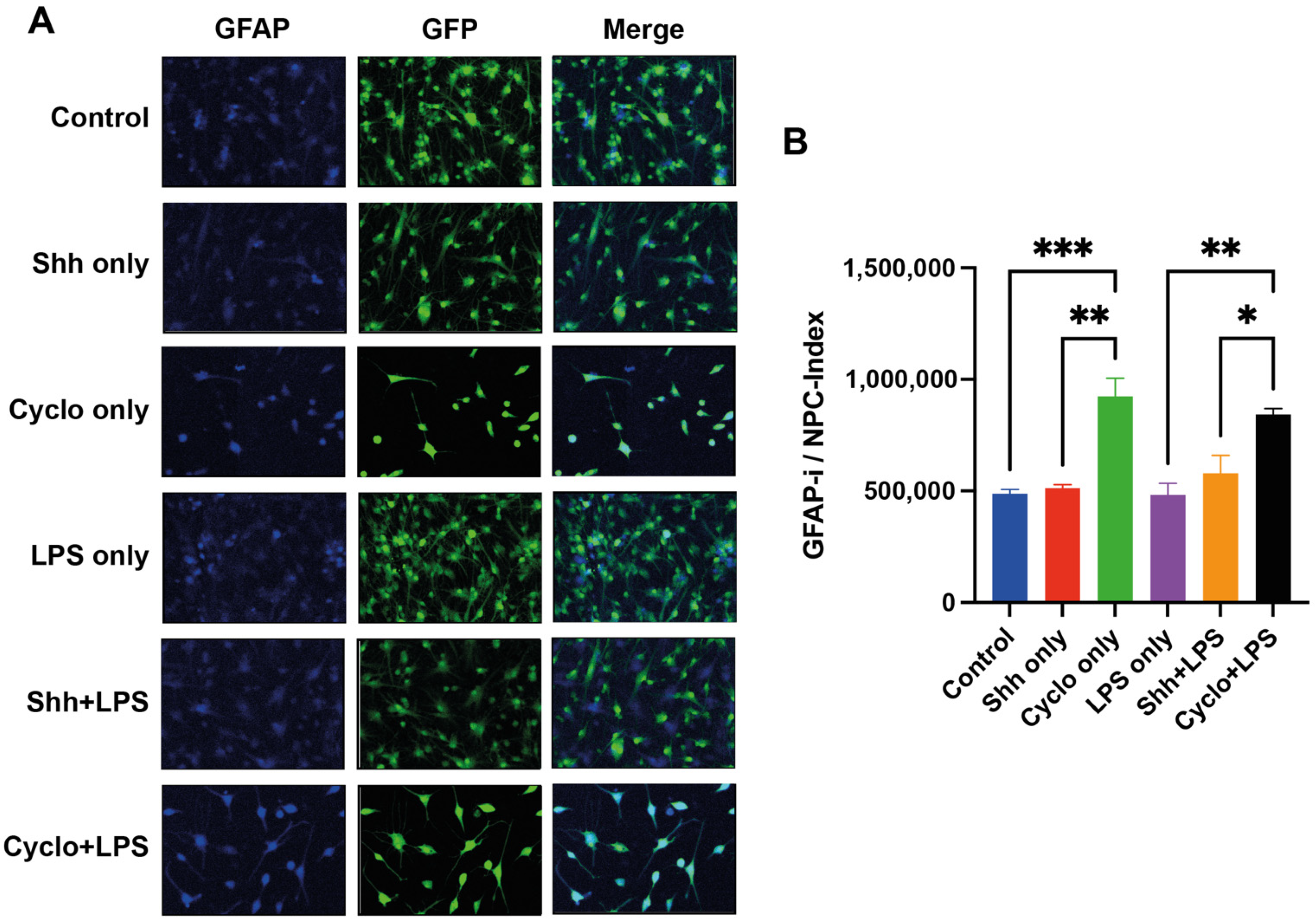
2.4. Immunofluorescence Staining and Imaging Analysis
2.5. Western Blot
2.6. Statistical Analysis
3. Results
3.1. The Survival of NPCs Is Influenced by Shh-Signaling
3.2. Stimulation of the Shh-Pathway Enhances the Proliferation of NPCs
3.3. The Oligodendroglial Differentiation of NPCs Is Not Affected by Shh-Pathway Modulation
3.4. The Shh-Pathway Is Associated with Pro-Neuronal Differentiation of NPCs
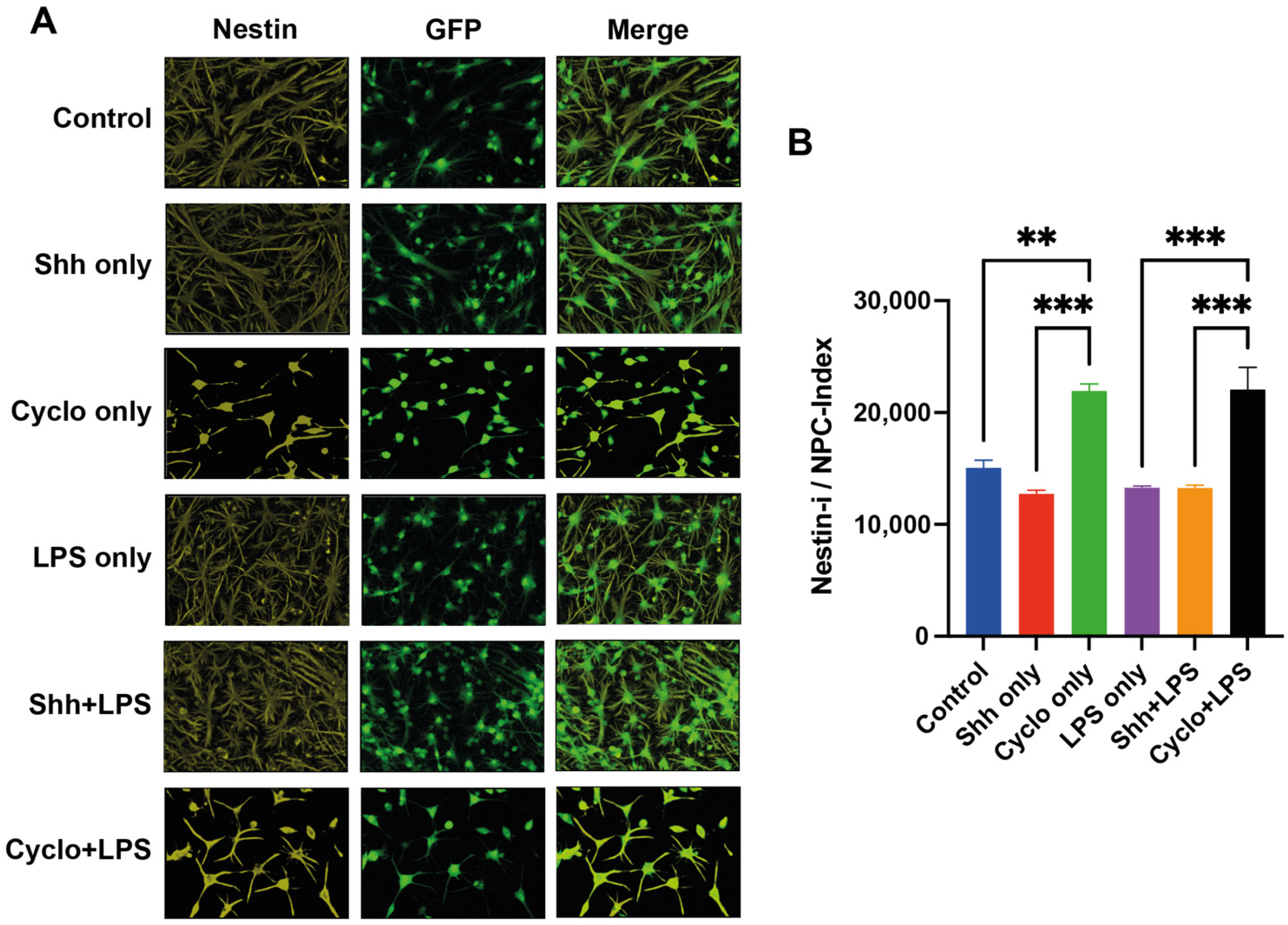
3.5. Blockage of the Shh-Pathway Is Related to Astroglial Differentiation of NPCs
3.6. Deprivation of Shh-Signaling in NPCs Is Associated with Less Differentiation
4. Discussions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Carballo, G.B.; Honorato, J.R.; de Lopes, G.P.F.; Spohr, T. A highlight on Sonic hedgehog pathway. Cell Commun. Signal 2018, 16, 11. [Google Scholar] [CrossRef]
- Ahn, S.; Joyner, A.L. In vivo analysis of quiescent adult neural stem cells responding to Sonic hedgehog. Nature 2005, 437, 894–897. [Google Scholar] [CrossRef] [PubMed]
- Machold, R.; Hayashi, S.; Rutlin, M.; Muzumdar, M.D.; Nery, S.; Corbin, J.G.; Gritli-Linde, A.; Dellovade, T.; Porter, J.A.; Rubin, L.L.; et al. Sonic hedgehog is required for progenitor cell maintenance in telencephalic stem cell niches. Neuron 2003, 39, 937–950. [Google Scholar] [CrossRef] [Green Version]
- Palma, V.; Lim, D.A.; Dahmane, N.; Sanchez, P.; Brionne, T.C.; Herzberg, C.D.; Gitton, Y.; Carleton, A.; Alvarez-Buylla, A.; Ruiz i Altaba, A. Sonic hedgehog controls stem cell behavior in the postnatal and adult brain. Development 2005, 132, 335–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tickle, C.; Towers, M. Sonic Hedgehog Signaling in Limb Development. Front. Cell Dev. Biol. 2017, 5, 14. [Google Scholar] [CrossRef] [Green Version]
- Dassule, H.R.; Lewis, P.; Bei, M.; Maas, R.; McMahon, A.P. Sonic hedgehog regulates growth and morphogenesis of the tooth. Development 2000, 127, 4775–4785. [Google Scholar] [CrossRef]
- Hosoya, A.; Shalehin, N.; Takebe, H.; Shimo, T.; Irie, K. Sonic Hedgehog Signaling and Tooth Development. Int. J. Mol. Sci. 2020, 21, 1587. [Google Scholar] [CrossRef] [Green Version]
- Memi, F.; Zecevic, N.; Radonjic, N. Multiple roles of Sonic Hedgehog in the developing human cortex are suggested by its widespread distribution. Brain Struct. Funct. 2018, 223, 2361–2375. [Google Scholar] [CrossRef] [Green Version]
- Patten, I.; Placzek, M. The role of Sonic hedgehog in neural tube patterning. Cell Mol. Life Sci 2000, 57, 1695–1708. [Google Scholar] [CrossRef]
- Arvidsson, A.; Collin, T.; Kirik, D.; Kokaia, Z.; Lindvall, O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat. Med. 2002, 8, 963–970. [Google Scholar] [CrossRef] [Green Version]
- Yousefifard, M.; Rahimi-Movaghar, V.; Nasirinezhad, F.; Baikpour, M.; Safari, S.; Saadat, S.; Moghadas Jafari, A.; Asady, H.; Razavi Tousi, S.M.; Hosseini, M. Neural stem/progenitor cell transplantation for spinal cord injury treatment; A systematic review and meta-analysis. Neuroscience 2016, 322, 377–397. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, S.D.; Yang, J.L.; Hwang, W.C.; Yang, D.I. Emerging roles of sonic hedgehog in adult neurological diseases: Neurogenesis and beyond. Int. J. Mol. Sci. 2018, 19, 2423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Younsi, A.; Zheng, G.; Tail, M.; Harms, A.K.; Roth, J.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K. Sonic Hedgehog modulates the inflammatory response and improves functional recovery after spinal cord injury in a thoracic contusion-compression model. Eur. Spine J. 2021, 30, 1509–1520. [Google Scholar] [CrossRef] [PubMed]
- Sari, I.N.; Phi, L.T.H.; Jun, N.; Wijaya, Y.T.; Lee, S.; Kwon, H.Y. Hedgehog signaling in cancer: A prospective therapeutic target for eradicating cancer stem cells. Cells 2018, 7, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Skoda, A.M.; Simovic, D.; Karin, V.; Kardum, V.; Vranic, S.; Serman, L. The role of the Hedgehog signaling pathway in cancer: A comprehensive review. Bosn. J. Basic Med. Sci. 2018, 18, 8–20. [Google Scholar] [CrossRef]
- Riobo-Del Galdo, N.A.; Lara Montero, A.; Wertheimer, E.V. Role of hedgehog signaling in breast cancer: Pathogenesis and therapeutics. Cells 2019, 8, 375. [Google Scholar] [CrossRef] [Green Version]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic spinal cord injury-repair and regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Siddiqui, A.M.; Khazaei, M.; Fehlings, M.G. Translating mechanisms of neuroprotection, regeneration, and repair to treatment of spinal cord injury. Prog. Brain Res. 2015, 218, 15–54. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic spinal cord injury: An overview of pathophysiology, models and acute injury mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef] [Green Version]
- Tominaga, H.; Ishiyama, M.; Ohseto, F.; Sasamoto, K.; Hamamoto, T.; Suzuki, K.; Watanabe, M. A water-soluble tetrazolium salt useful for colorimetric cell viability assay. Anal. Commun. 1999, 36, 47–50. [Google Scholar] [CrossRef]
- Younsi, A.; Zheng, G.; Scherer, M.; Riemann, L.; Zhang, H.; Tail, M.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K. Three growth factors induce proliferation and differentiation of neural precursor cells in vitro and support cell-transplantation after spinal cord injury in vivo. Stem Cells Int. 2020, 2020, 5674921. [Google Scholar] [CrossRef] [PubMed]
- Furlan, J.C.; Gulasingam, S.; Craven, B.C. Epidemiology of war-related spinal cord injury among combatants: A systematic review. Global Spine J. 2019, 9, 545–558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hachem, L.D.; Ahuja, C.S.; Fehlings, M.G. Assessment and management of acute spinal cord injury: From point of injury to rehabilitation. J. Spinal Cord Med. 2017, 40, 665–675. [Google Scholar] [CrossRef] [PubMed]
- Sandrow-Feinberg, H.R.; Houlé, J.D. Exercise after spinal cord injury as an agent for neuroprotection, regeneration and rehabilitation. Brain Res. 2015, 1619, 12–21. [Google Scholar] [CrossRef] [Green Version]
- Fakhoury, M. Spinal cord injury: Overview of experimental approaches used to restore locomotor activity. Rev. Neurosci. 2015, 26, 397–405. [Google Scholar] [CrossRef]
- Weston, N.M.; Sun, D. The potential of stem cells in treatment of traumatic brain injury. Curr. Neurol. Neurosci. Rep. 2018, 18, 1. [Google Scholar] [CrossRef]
- Marklund, N.; Bellander, B.M.; Godbolt, A.K.; Levin, H.; McCrory, P.; Thelin, E.P. Treatments and rehabilitation in the acute and chronic state of traumatic brain injury. J. Intern. Med. 2019, 285, 608–623. [Google Scholar] [CrossRef] [Green Version]
- Hutson, T.H.; Di Giovanni, S. The translational landscape in spinal cord injury: Focus on neuroplasticity and regeneration. Nat. Rev. Neurol. 2019, 15, 732–745. [Google Scholar] [CrossRef]
- Assinck, P.; Duncan, G.J.; Hilton, B.J.; Plemel, J.R.; Tetzlaff, W. Cell transplantation therapy for spinal cord injury. Nat. Neurosci. 2017, 20, 637–647. [Google Scholar] [CrossRef]
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal cord injury: Pathophysiology, treatment strategies, associated challenges, and future implications. Cell Tissue Res. 2019, 377, 125–151. [Google Scholar] [CrossRef]
- Nusslein-Volhard, C.; Wieschaus, E. Mutations affecting segment number and polarity in Drosophila. Nature 1980, 287, 795–801. [Google Scholar] [CrossRef] [PubMed]
- Xia, Y.P.; Dai, R.L.; Li, Y.N.; Mao, L.; Xue, Y.M.; He, Q.W.; Huang, M.; Huang, Y.; Mei, Y.W.; Hu, B. The protective effect of sonic hedgehog is mediated by the phosphoinositide [corrected] 3-kinase/AKT/Bcl-2 pathway in cultured rat astrocytes under oxidative stress. Neuroscience 2012, 209, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pitter, K.L.; Tamagno, I.; Feng, X.; Ghosal, K.; Amankulor, N.; Holland, E.C.; Hambardzumyan, D. The SHH/Gli pathway is reactivated in reactive glia and drives proliferation in response to neurodegeneration-induced lesions. Glia 2014, 62, 1595–1607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amankulor, N.M.; Hambardzumyan, D.; Pyonteck, S.M.; Becher, O.J.; Joyce, J.A.; Holland, E.C. Sonic hedgehog pathway activation is induced by acute brain injury and regulated by injury-related inflammation. J. Neurosci. 2009, 29, 10299–10308. [Google Scholar] [CrossRef] [Green Version]
- Ji, H.; Miao, J.; Zhang, X.; Du, Y.; Liu, H.; Li, S.; Li, L. Inhibition of sonic hedgehog signaling aggravates brain damage associated with the down-regulation of Gli1, Ptch1 and SOD1 expression in acute ischemic stroke. Neurosci. Lett 2012, 506, 1–6. [Google Scholar] [CrossRef]
- Jia, Y.; Yang, J.; Lu, T.; Pu, X.; Chen, Q.; Ji, L.; Luo, C. Repair of spinal cord injury in rats via exosomes from bone mesenchymal stem cells requires sonic hedgehog. Regen. Ther. 2021, 18, 309–315. [Google Scholar] [CrossRef]
- Balordi, F.; Fishell, G. Mosaic removal of hedgehog signaling in the adult SVZ reveals that the residual wild-type stem cells have a limited capacity for self-renewal. J. Neurosci. 2007, 27, 14248–14259. [Google Scholar] [CrossRef]
- Bambakidis, N.C.; Theodore, N.; Nakaji, P.; Harvey, A.; Sonntag, V.K.; Preul, M.C.; Miller, R.H. Endogenous stem cell proliferation after central nervous system injury: Alternative therapeutic options. Neurosurg. Focus 2005, 19, E1. [Google Scholar] [CrossRef]
- Jin, K.; Wang, X.; Xie, L.; Mao, X.O.; Zhu, W.; Wang, Y.; Shen, J.; Mao, Y.; Banwait, S.; Greenberg, D.A. Evidence for stroke-induced neurogenesis in the human brain. Proc. Natl. Acad. Sci. USA 2006, 103, 13198–13202. [Google Scholar] [CrossRef] [Green Version]
- Yamazaki, K.; Kawabori, M.; Seki, T.; Houkin, K. Clinical trials of stem cell treatment for spinal cord injury. Int. J. Mol. Sci. 2020, 21, 3994. [Google Scholar] [CrossRef]
- Muniswami, D.M.; Kanthakumar, P.; Kanakasabapathy, I.; Tharion, G. Motor recovery after transplantation of bone marrow mesenchymal stem cells in rat models of spinal cord injury. Ann. Neurosci. 2019, 25, 126–140. [Google Scholar] [CrossRef] [PubMed]
- Younsi, A.; Zheng, G.; Riemann, L.; Scherer, M.; Zhang, H.; Tail, M.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K. Long-term effects of neural precursor cell transplantation on secondary injury processes and functional recovery after severe cervical contusion-compression spinal cord injury. Int. J. Mol. Sci. 2021, 22, 13106. [Google Scholar] [CrossRef] [PubMed]
- Fischer, I.; Dulin, J.N.; Lane, M.A. Transplanting neural progenitor cells to restore connectivity after spinal cord injury. Nat. Rev. Neurosci. 2020, 21, 366–383. [Google Scholar] [CrossRef] [PubMed]
- Younsi, A.; Zheng, G.; Scherer, M.; Riemann, L.; Zhang, H.; Tail, M.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K. Treadmill training improves survival and differentiation of transplanted neural precursor cells after cervical spinal cord injury. Stem Cell Res. 2020, 45, 101812. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Fu, C.; Xiong, F.; He, C.; Wei, Q. Stem cell therapy for spinal cord injury. Cell Transpl. 2021, 30, 963689721989266. [Google Scholar] [CrossRef]
- Zholudeva, L.V.; Lane, M.A. Transplanting Cells for spinal cord repair: Who, what, when, where and why? Cell Transpl. 2019, 28, 388–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- LaPlaca, M.C.; Simon, C.M.; Prado, G.R.; Cullen, D.K. CNS injury biomechanics and experimental models. Prog. Brain Res. 2007, 161, 13–26. [Google Scholar] [CrossRef]
- Salvador, E.; Burek, M.; Förster, C.Y. An in vitro model of traumatic brain injury. Methods Mol. Biol. 2018, 1717, 219–227. [Google Scholar] [CrossRef]
- Huan, X.; Oumei, C.; Hongmei, Q.; Junxia, Y.; Xiaojiao, M.; Qingsong, J. PDE9 inhibition promotes proliferation of neural stem cells via cGMP-PKG pathway following oxygen-glucose deprivation/reoxygenation injury in vitro. Neurochem. Int. 2020, 133, 104630. [Google Scholar] [CrossRef]
- Yang, J.; Huang, J.; Shen, C.; Cheng, W.; Yu, P.; Wang, L.; Tang, F.; Guo, S.; Yang, Q.; Zhang, J. Resveratrol treatment in different time-attenuated neuronal apoptosis after oxygen and glucose deprivation/reoxygenation via enhancing the activation of nrf-2 signaling pathway in vitro. Cell Transpl. 2018, 27, 1789–1797. [Google Scholar] [CrossRef]
- Cheng, W.; Yu, P.; Wang, L.; Shen, C.; Song, X.; Chen, J.; Tang, F.; Yang, Q. Sonic hedgehog signaling mediates resveratrol to increase proliferation of neural stem cells after oxygen-glucose deprivation/reoxygenation injury in vitro. Cell Physiol. Biochem. 2015, 35, 2019–2032. [Google Scholar] [CrossRef] [PubMed]
- Fehlings, M.G.; Nguyen, D.H. Immunoglobulin G: A potential treatment to attenuate neuroinflammation following spinal cord injury. J. Clin. Immunol. 2010, 30 (Suppl. 1), S109–S112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oyinbo, C.A. Secondary injury mechanisms in traumatic spinal cord injury: A nugget of this multiply cascade. Acta Neurobiol. Exp. 2011, 71, 281–299. [Google Scholar]
- Kipp, M.; Norkute, A.; Johann, S.; Lorenz, L.; Braun, A.; Hieble, A.; Gingele, S.; Pott, F.; Richter, J.; Beyer, C. Brain-region-specific astroglial responses in vitro after lps exposure. J. Mol. Neurosci. 2008, 35, 235–243. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, C.; Ferrari, D.; Zalfa, C.; Soncini, M.; Mazzoccoli, G.; Facchini, F.A.; Marongiu, L.; Granucci, F.; Copetti, M.; Vescovi, A.L.; et al. Toll-like receptor 4 modulation influences human neural stem cell proliferation and differentiation. Cell Death Dis. 2018, 9, 280. [Google Scholar] [CrossRef] [Green Version]
- Okun, E.; Griffioen, K.J.; Son, T.G.; Lee, J.H.; Roberts, N.J.; Mughal, M.R.; Hutchison, E.; Cheng, A.; Arumugam, T.V.; Lathia, J.D.; et al. TLR2 activation inhibits embryonic neural progenitor cell proliferation. J. Neurochem. 2010, 114, 462–474. [Google Scholar] [CrossRef] [Green Version]
- Rietdijk, C.D.; Wezel, R.J.A.v.; Garssen, J.; Kraneveld, A.D. Neuronal toll-like receptors and neuro-immunity in Parkinson’s disease, Alzheimer’s disease and stroke. Neuroimmunol. Neuroinflammation 2016, 3, 27–37. [Google Scholar] [CrossRef] [Green Version]
- De Filippis, L.; Peri, F. The Role of TLR4 in Neural Stem Cells–Mediated Neurogenesis and Neuroinflammation; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; pp. 129–141. [Google Scholar]
- Olson, J.K.; Miller, S.D. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004, 173, 3916–3924. [Google Scholar] [CrossRef] [Green Version]
- Petralia, R.S.; Wang, Y.X.; Mattson, M.P.; Yao, P.J. Sonic hedgehog distribution within mature hippocampal neurons. Commun. Integr. Biol. 2011, 4, 775–777. [Google Scholar] [CrossRef] [Green Version]
- Haldipur, P.; Bharti, U.; Govindan, S.; Sarkar, C.; Iyengar, S.; Gressens, P.; Mani, S. Expression of Sonic hedgehog during cell proliferation in the human cerebellum. Stem Cells Dev. 2012, 21, 1059–1068. [Google Scholar] [CrossRef]
- Garcia, A.D.; Petrova, R.; Eng, L.; Joyner, A.L. Sonic hedgehog regulates discrete populations of astrocytes in the adult mouse forebrain. J. Neurosci. 2010, 30, 13597–13608. [Google Scholar] [CrossRef] [PubMed]
- Nye, M.D.; Almada, L.L.; Fernandez-Barrena, M.G.; Marks, D.L.; Elsawa, S.F.; Vrabel, A.; Tolosa, E.J.; Ellenrieder, V.; Fernandez-Zapico, M.E. The transcription factor GLI1 interacts with SMAD proteins to modulate transforming growth factor β-induced gene expression in a p300/CREB-binding protein-associated factor (PCAF)-dependent manner. J. Biol. Chem. 2014, 289, 15495–15506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mastrangelo, E.; Milani, M. Role and inhibition of GLI1 protein in cancer. Lung Cancer 2018, 9, 35–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, G.; Mehler, M.F.; Zhao, J.; Yu Yung, S.; Kessler, J.A. Sonic hedgehog and BMP2 exert opposing actions on proliferation and differentiation of embryonic neural progenitor cells. Dev. Biol. 1999, 215, 118–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Y.; Li, M.; Xu, X.; Song, M.; Tao, H.; Bai, Y. Green tea epigallocatechin-3-gallate (EGCG) promotes neural progenitor cell proliferation and sonic hedgehog pathway activation during adult hippocampal neurogenesis. Mol. Nutr. Food Res. 2012, 56, 1292–1303. [Google Scholar] [CrossRef] [PubMed]
- Sims, J.R.; Lee, S.W.; Topalkara, K.; Qiu, J.; Xu, J.; Zhou, Z.; Moskowitz, M.A. Sonic hedgehog regulates ischemia/hypoxia-induced neural progenitor proliferation. Stroke 2009, 40, 3618–3626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Zhang, Z.G.; Gregg, S.R.; Zhang, R.L.; Jiao, Z.; LeTourneau, Y.; Liu, X.; Feng, Y.; Gerwien, J.; Torup, L.; et al. The Sonic hedgehog pathway mediates carbamylated erythropoietin-enhanced proliferation and differentiation of adult neural progenitor cells. J. Biol. Chem. 2007, 282, 32462–32470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, X.; Li, Y.; Li, S.; Li, H.; Yang, C.; Lin, J. The role of Shh signalling pathway in central nervous system development and related diseases. Cell Biochem. Funct. 2021, 39, 180–189. [Google Scholar] [CrossRef]
- Prajerova, I.; Honsa, P.; Chvatal, A.; Anderova, M. Distinct effects of sonic hedgehog and Wnt-7a on differentiation of neonatal neural stem/progenitor cells in vitro. Neuroscience 2010, 171, 693–711. [Google Scholar] [CrossRef]
- Chen, E.; Xu, D.; Lan, X.; Jia, B.; Sun, L.; Zheng, J.C.; Peng, H. A novel role of the STAT3 pathway in brain inflammation-induced human neural progenitor cell differentiation. Curr. Mol. Med. 2013, 13, 1474–1484. [Google Scholar] [CrossRef] [Green Version]
- Peng, H.; Whitney, N.; Wu, Y.; Tian, C.; Dou, H.; Zhou, Y.; Zheng, J. HIV-1-infected and/or immune-activated macrophage-secreted TNF-alpha affects human fetal cortical neural progenitor cell proliferation and differentiation. Glia 2008, 56, 903–916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mao, S.; Li, X.; Wang, J.; Ding, X.; Zhang, C.; Li, L. miR-17-92 facilitates neuronal differentiation of transplanted neural stem/precursor cells under neuroinflammatory conditions. J. Neuroinflammation 2016, 13, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.S.; Yu, K.; Rho, J.Y.; Lee, E.; Han, J.S.; Koo, D.B.; Cho, Y.S.; Kim, J.; Lee, K.K.; Han, Y.M. Cyclopamine treatment of human embryonic stem cells followed by culture in human astrocyte medium promotes differentiation into nestin- and GFAP-expressing astrocytic lineage. Life Sci. 2006, 80, 154–159. [Google Scholar] [CrossRef] [PubMed]
- Ortega, M.C.; Cases, O.; Merchán, P.; Kozyraki, R.; Clemente, D.; de Castro, F. Megalin mediates the influence of sonic hedgehog on oligodendrocyte precursor cell migration and proliferation during development. Glia 2012, 60, 851–866. [Google Scholar] [CrossRef]
- Nery, S.; Wichterle, H.; Fishell, G. Sonic hedgehog contributes to oligodendrocyte specification in the mammalian forebrain. Development 2001, 128, 527–540. [Google Scholar] [CrossRef]
- Namchaiw, P.; Wen, H.; Mayrhofer, F.; Chechneva, O.; Biswas, S.; Deng, W. Temporal and partial inhibition of GLI1 in neural stem cells (NSCs) results in the early maturation of NSC derived oligodendrocytes in vitro. Stem Cell Res. Ther. 2019, 10, 272. [Google Scholar] [CrossRef]
- Araujo, G.L.; Araujo, J.A.; Schroeder, T.; Tort, A.B.; Costa, M.R. Sonic hedgehog signaling regulates mode of cell division of early cerebral cortex progenitors and increases astrogliogenesis. Front. Cell Neurosci. 2014, 8, 77. [Google Scholar] [CrossRef] [Green Version]
- Hill, S.A.; Fu, M.; Garcia, A.D.R. Sonic hedgehog signaling in astrocytes. Cell Mol. Life Sci. 2021, 78, 1393–1403. [Google Scholar] [CrossRef]
- Wechsler-Reya, R.J.; Scott, M.P. Control of neuronal precursor proliferation in the cerebellum by Sonic Hedgehog. Neuron 1999, 22, 103–114. [Google Scholar] [CrossRef] [Green Version]



| Treatment 1 | Treatment 2 | Group Name |
|---|---|---|
| Untreated | Shh | Shh only |
| Cyclopamine | Cyclopamine only | |
| none | Control | |
| LPS | Shh | Shh+LPS |
| Cyclopamine | Cyclopamine+LPS | |
| none | LPS only |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tail, M.; Zhang, H.; Zheng, G.; Hatami, M.; Skutella, T.; Unterberg, A.; Zweckberger, K.; Younsi, A. The Sonic Hedgehog Pathway Modulates Survival, Proliferation, and Differentiation of Neural Progenitor Cells under Inflammatory Stress In Vitro. Cells 2022, 11, 736. https://doi.org/10.3390/cells11040736
Tail M, Zhang H, Zheng G, Hatami M, Skutella T, Unterberg A, Zweckberger K, Younsi A. The Sonic Hedgehog Pathway Modulates Survival, Proliferation, and Differentiation of Neural Progenitor Cells under Inflammatory Stress In Vitro. Cells. 2022; 11(4):736. https://doi.org/10.3390/cells11040736
Chicago/Turabian StyleTail, Mohamed, Hao Zhang, Guoli Zheng, Maryam Hatami, Thomas Skutella, Andreas Unterberg, Klaus Zweckberger, and Alexander Younsi. 2022. "The Sonic Hedgehog Pathway Modulates Survival, Proliferation, and Differentiation of Neural Progenitor Cells under Inflammatory Stress In Vitro" Cells 11, no. 4: 736. https://doi.org/10.3390/cells11040736
APA StyleTail, M., Zhang, H., Zheng, G., Hatami, M., Skutella, T., Unterberg, A., Zweckberger, K., & Younsi, A. (2022). The Sonic Hedgehog Pathway Modulates Survival, Proliferation, and Differentiation of Neural Progenitor Cells under Inflammatory Stress In Vitro. Cells, 11(4), 736. https://doi.org/10.3390/cells11040736






