Potential Role of Intracranial Mast Cells in Neuroinflammation and Neuropathology Associated with Food Allergy
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and BLG Sensitization
2.2. Blood and Tissue Collection
2.3. Enzyme-Linked Immunosorbent Assays (ELISAs)
2.3.1. BLG-Specific IgE and IgG1
2.3.2. Histamine and Mast Cell Protease-1 (MCPT-1)
2.3.3. Serum BLG
2.4. Behavior Analysis
2.4.1. Open-Field Test (OFT)
2.4.2. Elevated Zero Maze (EZM)
2.4.3. Tail Suspension Test (TST)
2.5. Western Blotting
2.6. Immunohistochemical Staining
2.6.1. Brain
2.6.2. Dura Mater
2.7. Black Gold II Staining
2.8. MC Staining and Quantification
2.9. Densitometric Analysis of Histological Staining
2.10. Statistical Analysis
3. Results
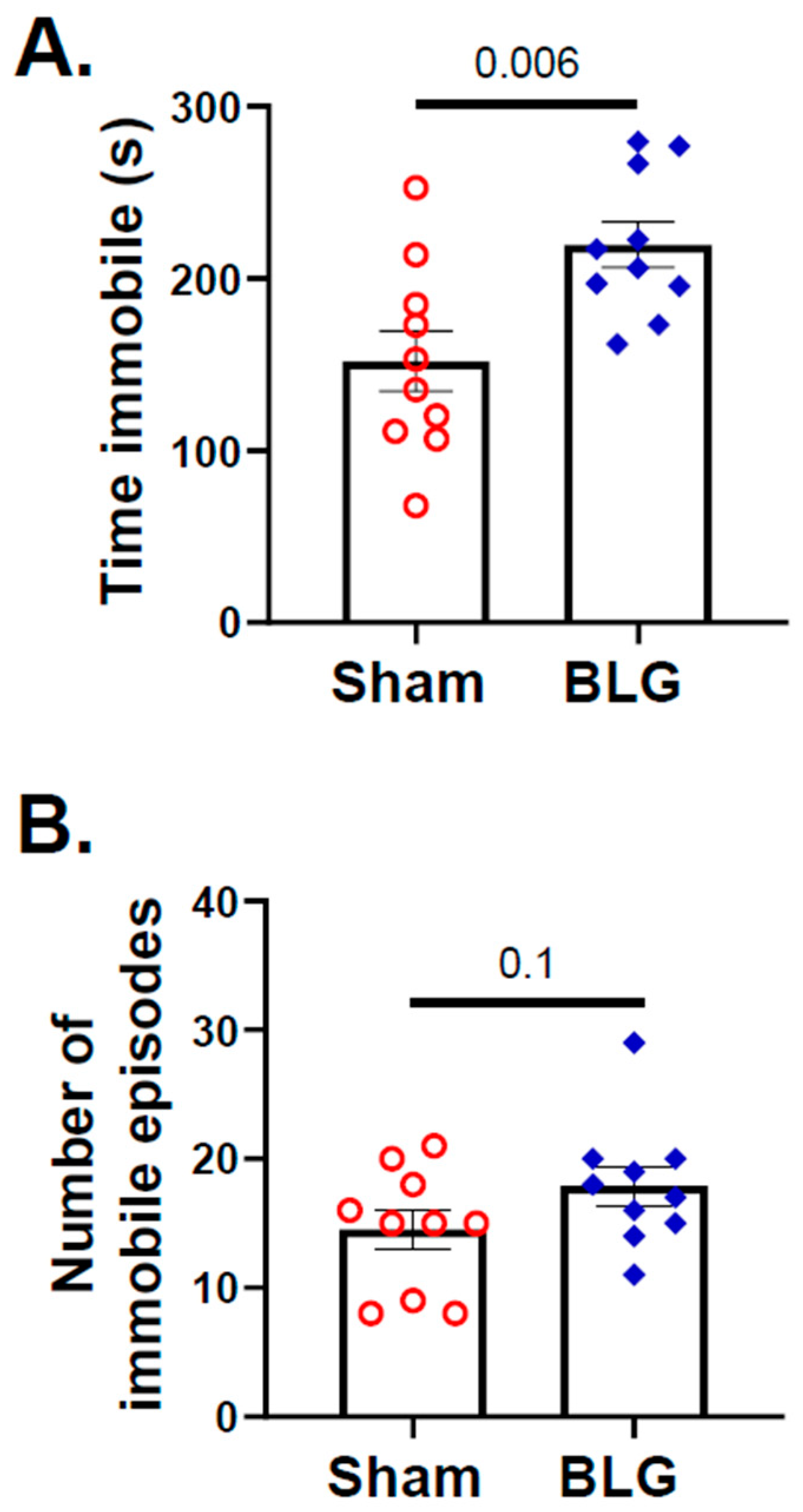
3.1. Repeated Allergen Consumption Resulted in Asymptomatic Hypersensitivity with Decreased Mobility and Depression-like Behavior in the BLG-Sensitized Mice
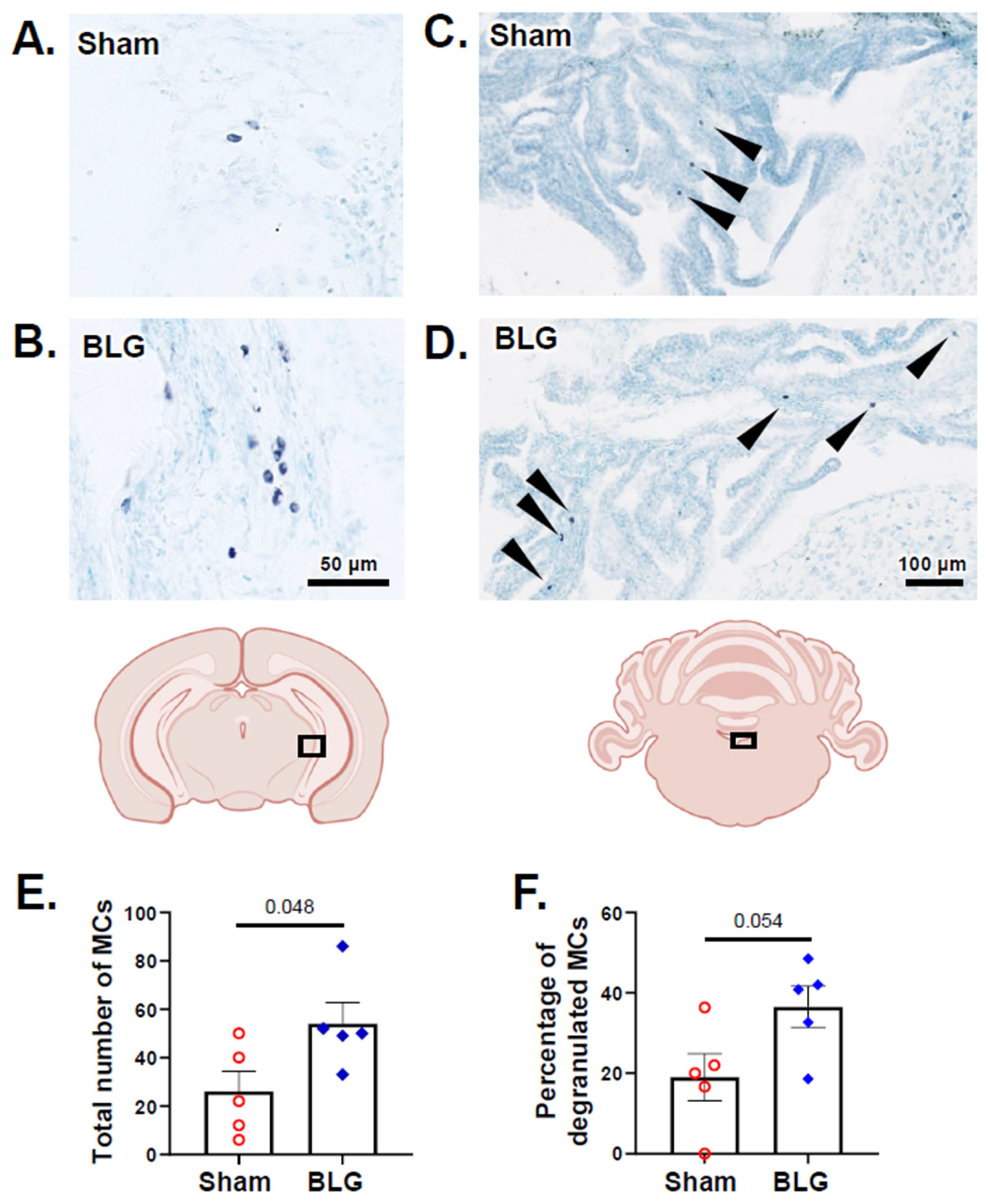
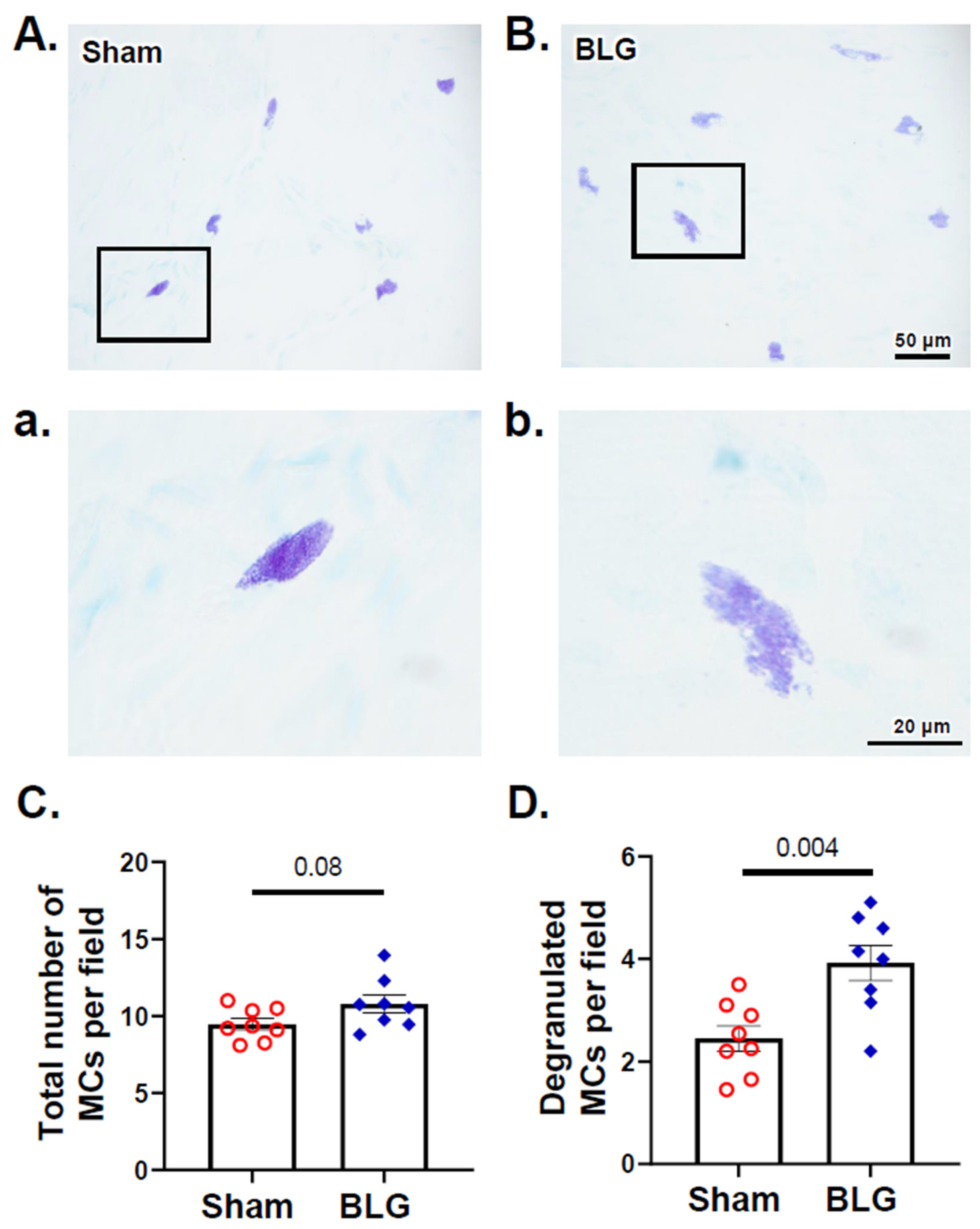
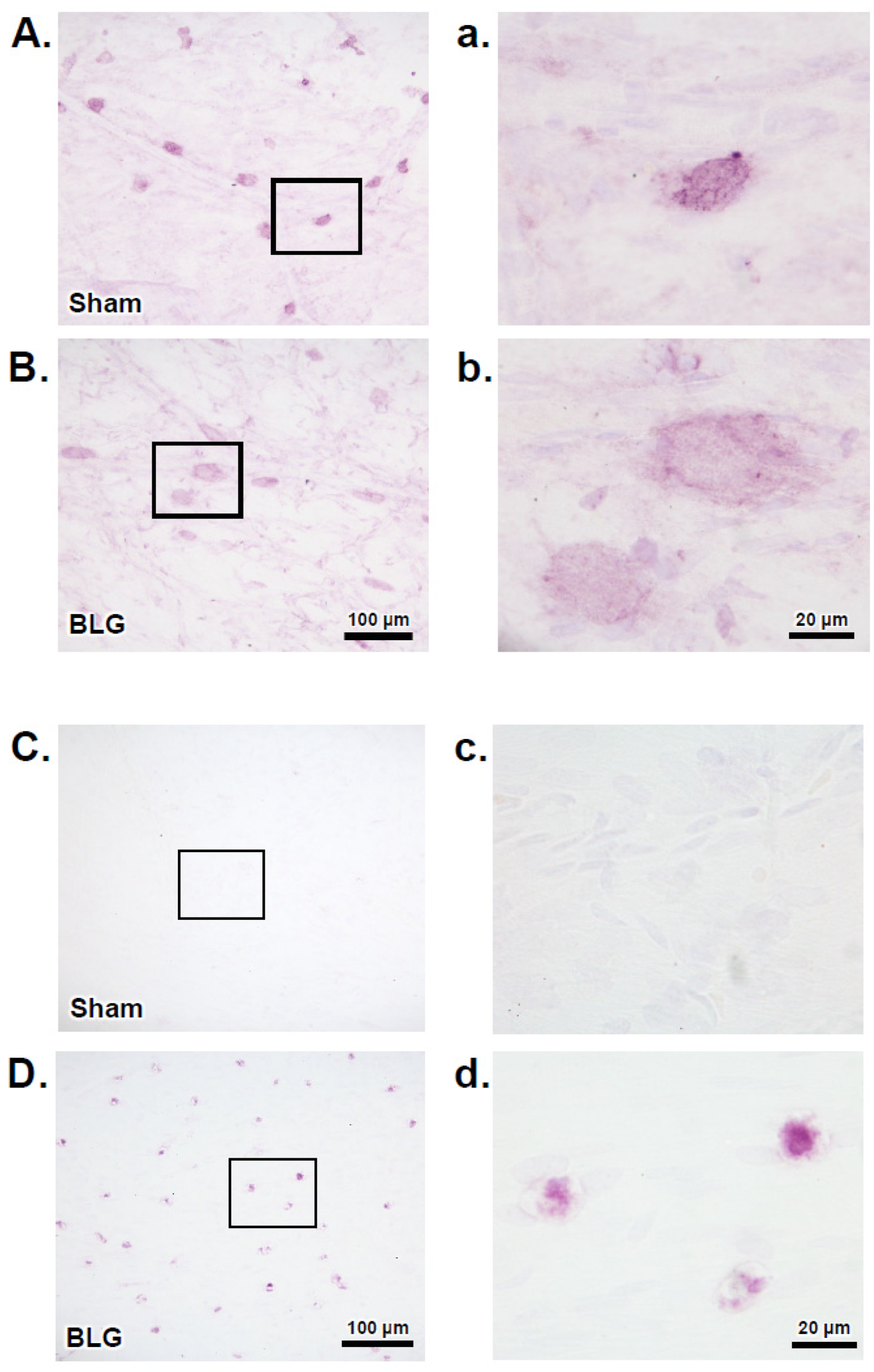
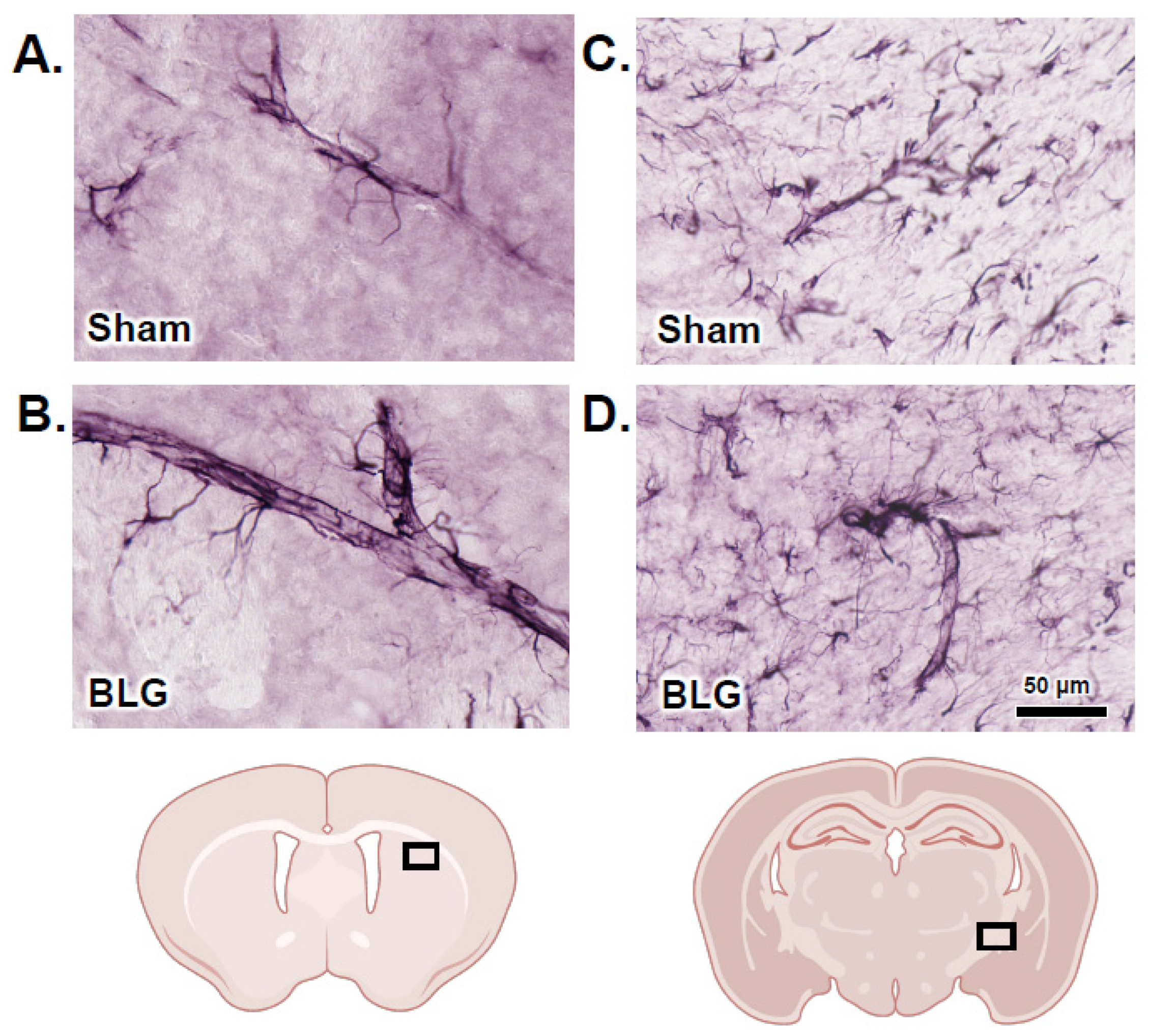
3.2. MCs Accumulated in the Brain and Meninges of the BLG-Sensitized Mice and Displayed Activated Morphology
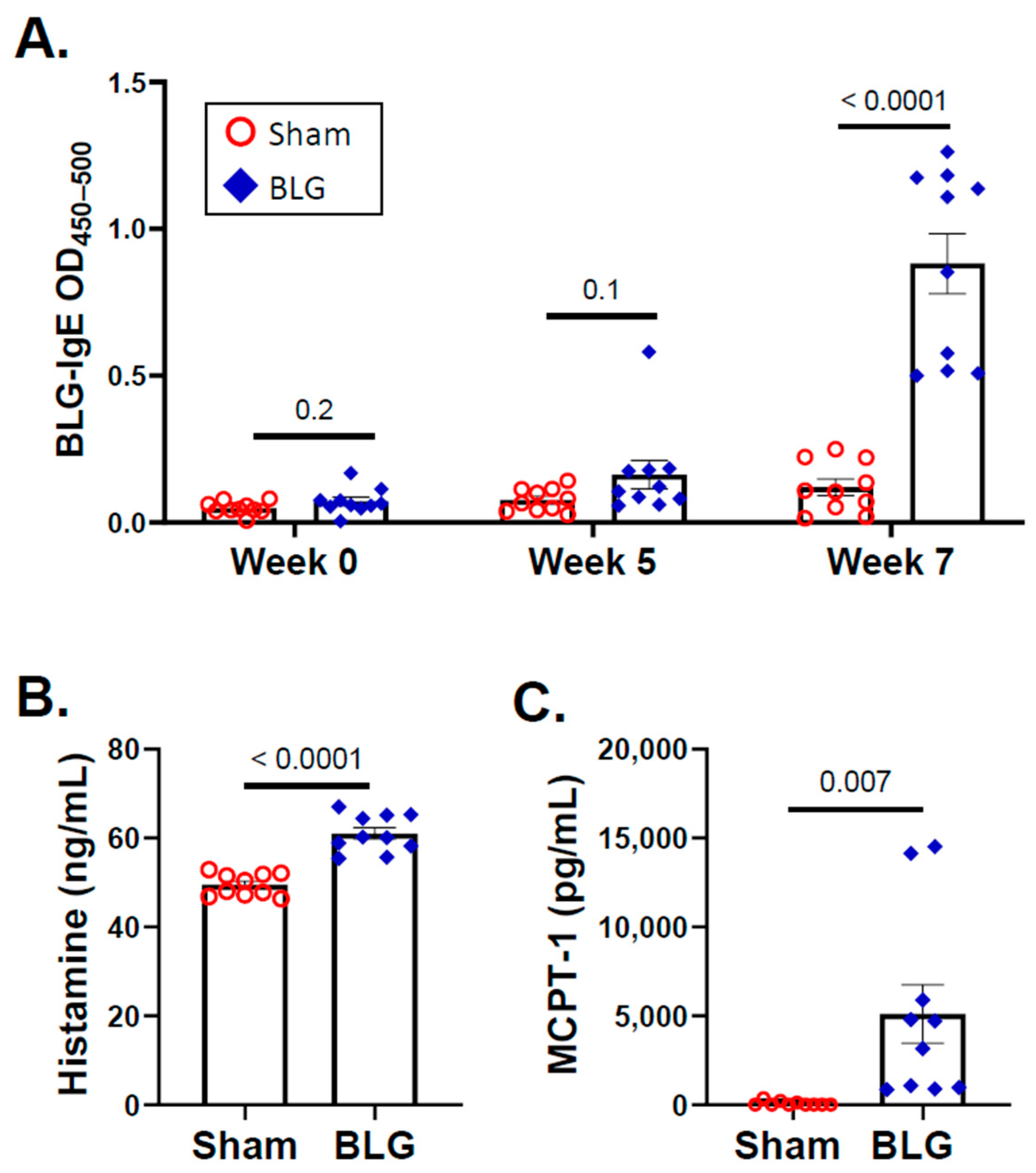
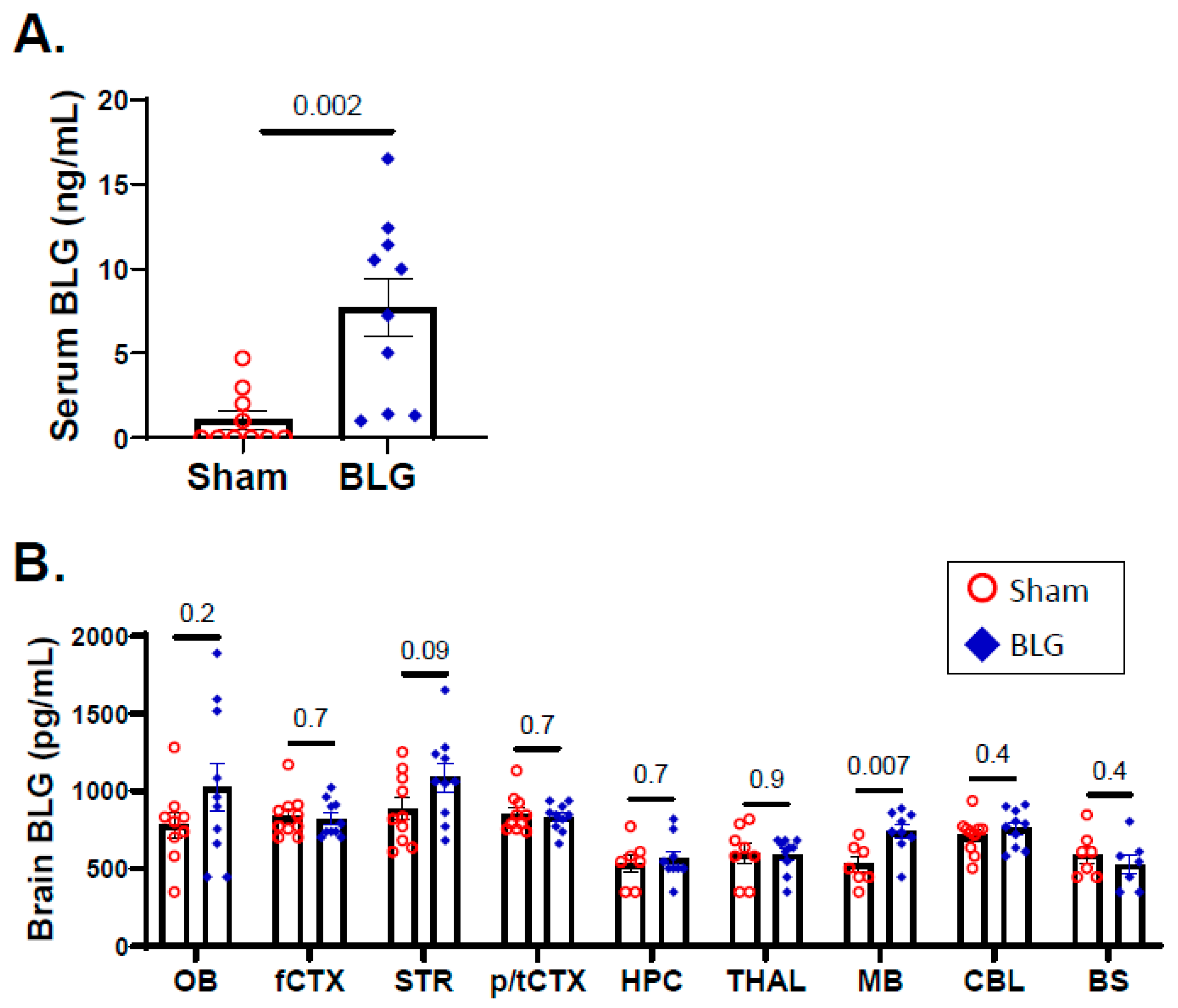
3.3. Intracranial MCs Are Likely Activated by Circulating Allergens in the CMA Mice during the Repeated Allergen Exposure
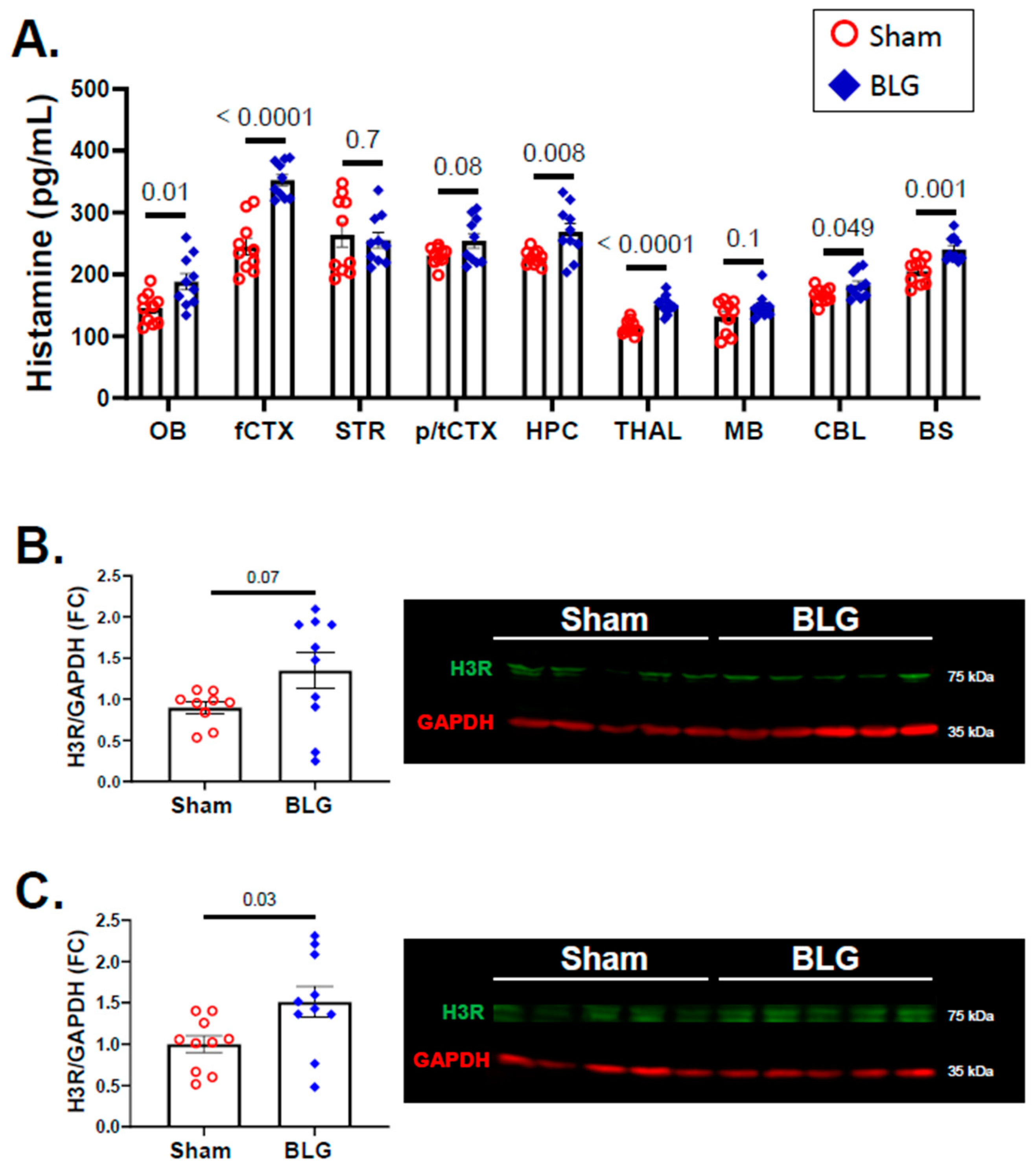
3.4. Histamine Levels and H3R Expression Were Regionally Elevated in Brains of the BLG-Sensitized Mice after the Repeated Allergen Consumption
3.5. Activation of Intracranial MCs Was Associated with BBB Impairment and Perivascular Astrogliosis
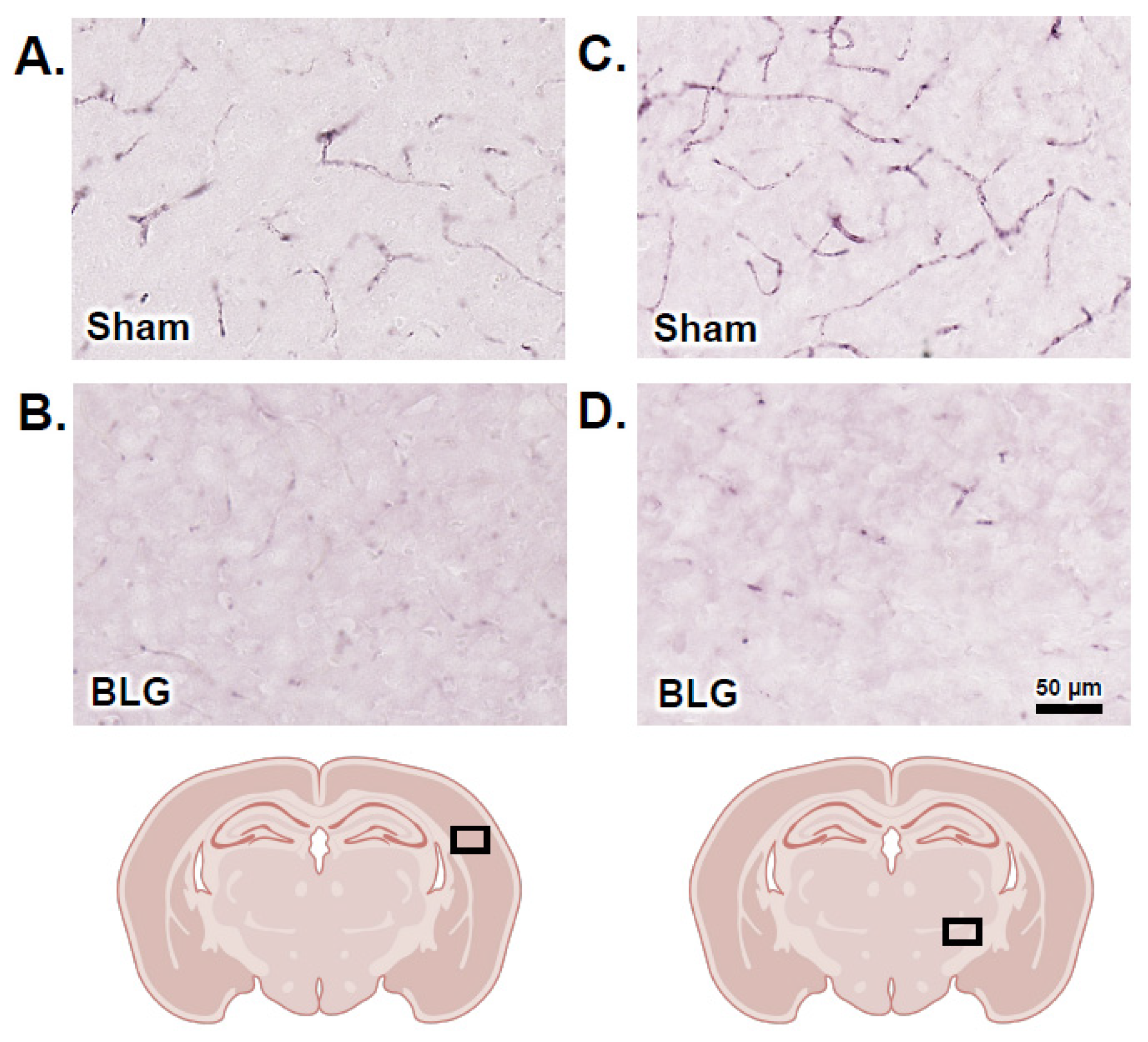
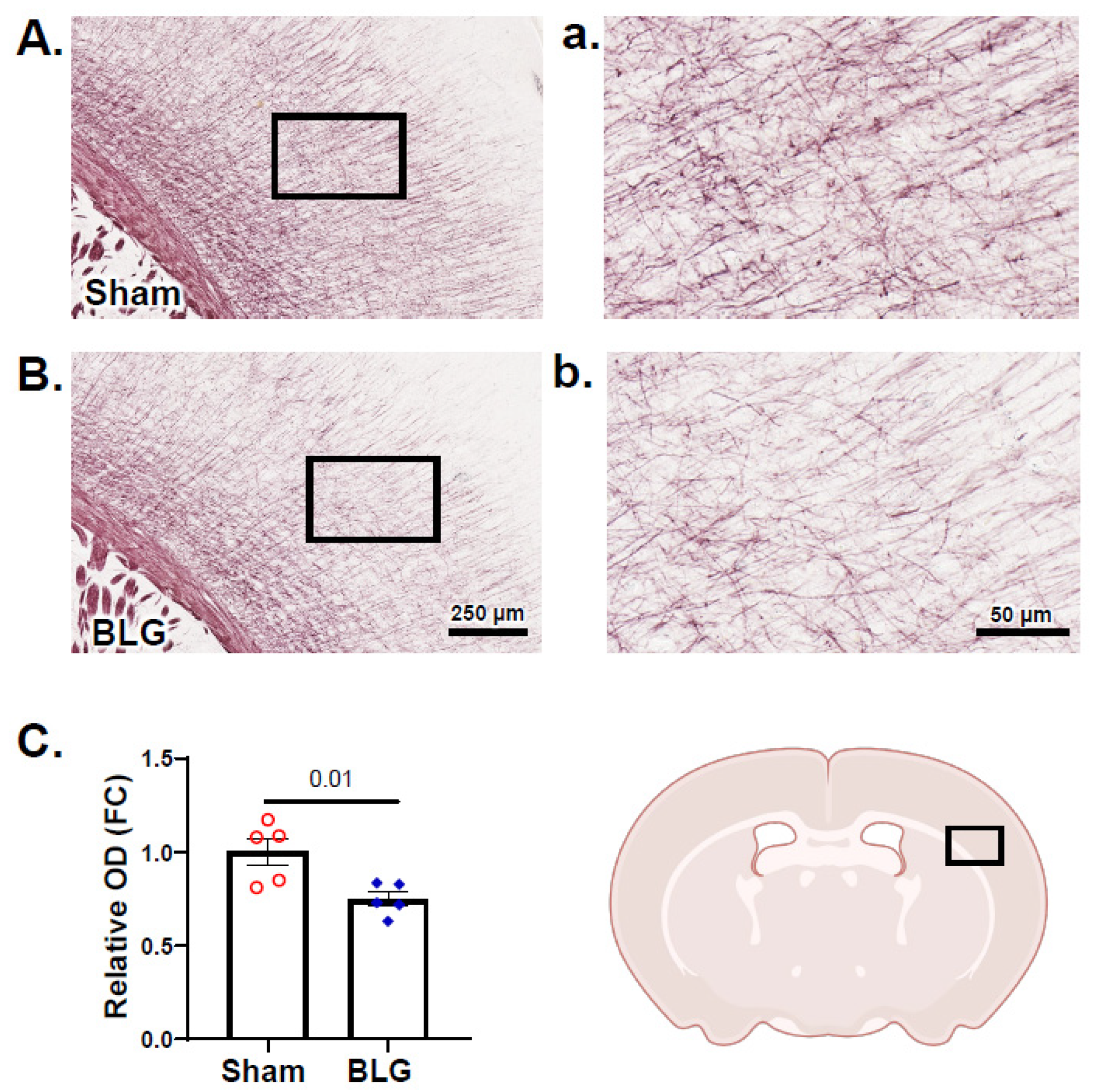
3.6. Cortical Demyelination Was Evident in the BLG-Sensitized Mice after the Repeated Allergen Exposure
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Schwartz, L.B.; Irani, A.M.; Roller, K.; Castells, M.C.; Schechter, N.M. Quantitation of histamine, tryptase, and chymase in dispersed human T and TC mast cells. J. Immunol. 1987, 138, 2611–2615. [Google Scholar] [PubMed]
- Zhang, S.; Anderson, D.F.; Bradding, P.; Coward, W.R.; Baddeley, S.M.; MacLeod, J.D.; McGill, J.I.; Church, M.K.; Holgate, S.T.; Roche, W.R. Human mast cells express stem cell factor. J. Pathol. 1998, 186, 59–66. [Google Scholar] [CrossRef]
- Grutzkau, A.; Kruger-Krasagakes, S.; Baumeister, H.; Schwarz, C.; Kogel, H.; Welker, P.; Lippert, U.; Henz, B.M.; Moller, A. Synthesis, storage, and release of vascular endothelial growth factor/vascular permeability factor (VEGF/VPF) by human mast cells: Implications for the biological significance of VEGF206. Mol. Biol. Cell 1998, 9, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, G.; Forsberg-Nilsson, K.; Xiang, Z.; Hallbook, F.; Nilsson, K.; Metcalfe, D.D. Human mast cells express functional TrkA and are a source of nerve growth factor. Eur. J. Immunol. 1997, 27, 2295–2301. [Google Scholar] [CrossRef] [PubMed]
- Olszewski, M.B.; Groot, A.J.; Dastych, J.; Knol, E.F. TNF trafficking to human mast cell granules: Mature chain-dependent endocytosis. J. Immunol. 2007, 178, 5701–5709. [Google Scholar] [CrossRef] [PubMed]
- Wilson, S.J.; Shute, J.K.; Holgate, S.T.; Howarth, P.H.; Bradding, P. Localization of interleukin (IL)-4 but not IL-5 to human mast cell secretory granules by immunoelectron microscopy. Clin. Exp. Allergy 2000, 30, 493–500. [Google Scholar] [CrossRef] [PubMed]
- Kimura, Y.; Pawankar, R.; Aoki, M.; Niimi, Y.; Kawana, S. Mast cells and T cells in Kimura’s disease express increased levels of interleukin-4, interleukin-5, eotaxin and RANTES. Clin. Exp. Allergy 2002, 32, 1787–1793. [Google Scholar] [CrossRef]
- Gombert, M.; Dieu-Nosjean, M.C.; Winterberg, F.; Bunemann, E.; Kubitza, R.C.; Da Cunha, L.; Haahtela, A.; Lehtimaki, S.; Muller, A.; Rieker, J.; et al. CCL1-CCR8 interactions: An axis mediating the recruitment of T cells and Langerhans-type dendritic cells to sites of atopic skin inflammation. J. Immunol. 2005, 174, 5082–5091. [Google Scholar] [CrossRef]
- Okayama, Y.; Kawakami, T. Development, Migration, and Survival of Mast Cells. Immunol. Res. 2006, 34, 97–115. [Google Scholar] [CrossRef]
- Rivera, J.; Gilfillan, A.M. Molecular regulation of mast cell activation. J. Allergy Clin. Immunol. 2006, 117, 1214–1225. [Google Scholar] [CrossRef]
- Zhao, W.; Kepley, C.L.; Morel, P.A.; Okumoto, L.M.; Fukuoka, Y.; Schwartz, L.B. Fc gamma RIIa, not Fc gamma RIIb, is constitutively and functionally expressed on skin-derived human mast cells. J. Immunol. 2006, 177, 694–701. [Google Scholar] [CrossRef] [PubMed]
- Malbec, O.; Daeron, M. The mast cell IgG receptors and their roles in tissue inflammation. Immunol. Rev. 2007, 217, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Arock, M.; Ross, E.; Lai-Kuen, R.; Averlant, G.; Gao, Z.; Abraham, S.N. Phagocytic and tumor necrosis factor alpha response of human mast cells following exposure to gram-negative and gram-positive bacteria. Infect. Immun. 1998, 66, 6030–6034. [Google Scholar] [CrossRef]
- Supajatura, V.; Ushio, H.; Nakao, A.; Okumura, K.; Ra, C.; Ogawa, H. Protective roles of mast cells against enterobacterial infection are mediated by Toll-like receptor 4. J. Immunol. 2001, 167, 2250–2256. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.A.; Leal-Berumen, I.; Croitoru, K.; Marshall, J.S. Rat peritoneal mast cells produce IFN-gamma following IL-12 treatment but not in response to IgE-mediated activation. J. Immunol. 1996, 157, 2123–2128. [Google Scholar] [PubMed]
- Mousli, M.; Hugli, T.E.; Landry, Y.; Bronner, C. Peptidergic pathway in human skin and rat peritoneal mast cell activation. Immunopharmacology 1994, 27, 1–11. [Google Scholar] [CrossRef]
- Li, W.W.; Guo, T.Z.; Liang, D.Y.; Sun, Y.; Kingery, W.S.; Clark, J.D. Substance P signaling controls mast cell activation, degranulation, and nociceptive sensitization in a rat fracture model of complex regional pain syndrome. Anesthesiology 2012, 116, 882–895. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Kempuraj, D.; Tagen, M.; Conti, P.; Kalogeromitros, D. Differential release of mast cell mediators and the pathogenesis of inflammation. Immunol. Rev. 2007, 217, 65–78. [Google Scholar] [CrossRef]
- Forsythe, P. Mast Cells in Neuroimmune Interactions. Trends Neurosci. 2019, 42, 43–55. [Google Scholar] [CrossRef]
- Lu, L.F.; Lind, E.F.; Gondek, D.C.; Bennett, K.A.; Gleeson, M.W.; Pino-Lagos, K.; Scott, Z.A.; Coyle, A.J.; Reed, J.L.; Van Snick, J.; et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature 2006, 442, 997–1002. [Google Scholar] [CrossRef]
- Samoszuk, M.; Kanakubo, E.; Chan, J.K. Degranulating mast cells in fibrotic regions of human tumors and evidence that mast cell heparin interferes with the growth of tumor cells through a mechanism involving fibroblasts. BMC Cancer 2005, 5, 121. [Google Scholar] [CrossRef] [PubMed]
- Garbuzenko, E.; Nagler, A.; Pickholtz, D.; Gillery, P.; Reich, R.; Maquart, F.X.; Levi-Schaffer, F. Human mast cells stimulate fibroblast proliferation, collagen synthesis and lattice contraction: A direct role for mast cells in skin fibrosis. Clin. Exp. Allergy 2002, 32, 237–246. [Google Scholar] [CrossRef]
- Toth-Jakatics, R.; Jimi, S.; Takebayashi, S.; Kawamoto, N. Cutaneous malignant melanoma: Correlation between neovascularization and peritumor accumulation of mast cells overexpressing vascular endothelial growth factor. Hum. Pathol. 2000, 31, 955–960. [Google Scholar] [CrossRef] [PubMed]
- McHale, C.; Mohammed, Z.; Gomez, G. Human Skin-Derived Mast Cells Spontaneously Secrete Several Angiogenesis-Related Factors. Front. Immunol. 2019, 10, 1445. [Google Scholar] [CrossRef] [PubMed]
- Florenzano, F.; Bentivoglio, M. Degranulation, density, and distribution of mast cells in the rat thalamus: A light and electron microscopic study in basal conditions and after intracerebroventricular administration of nerve growth factor. J. Comp. Neurol. 2000, 424, 651–669. [Google Scholar] [CrossRef]
- Silverman, A.J.; Sutherland, A.K.; Wilhelm, M.; Silver, R. Mast cells migrate from blood to brain. J. Neurosci. 2000, 20, 401–408. [Google Scholar] [CrossRef]
- Khalil, M.; Ronda, J.; Weintraub, M.; Jain, K.; Silver, R.; Silverman, A.J. Brain mast cell relationship to neurovasculature during development. Brain Res. 2007, 1171, 18–29. [Google Scholar] [CrossRef]
- Khalil, M.H.; Silverman, A.J.; Silver, R. Mast cells in the rat brain synthesize gonadotropin-releasing hormone. J. Neurobiol. 2003, 56, 113–124. [Google Scholar] [CrossRef]
- Goldschmidt, R.C.; Hough, L.B.; Glick, S.D. Rat brain mast cells: Contribution to brain histamine levels. J. Neurochem. 1985, 44, 1943–1947. [Google Scholar] [CrossRef]
- Zhuang, X.; Silverman, A.J.; Silver, R. Brain mast cell degranulation regulates blood-brain barrier. J. Neurobiol. 1996, 31, 393–403. [Google Scholar] [CrossRef]
- Nautiyal, K.M.; Ribeiro, A.C.; Pfaff, D.W.; Silver, R. Brain mast cells link the immune system to anxiety-like behavior. Proc. Natl. Acad. Sci. USA 2008, 105, 18053–18057. [Google Scholar] [CrossRef] [PubMed]
- Biran, V.; Cochois, V.; Karroubi, A.; Arrang, J.M.; Charriaut-Marlangue, C.; Heron, A. Stroke induces histamine accumulation and mast cell degranulation in the neonatal rat brain. Brain Pathol. 2008, 18, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Lozada, A.; Maegele, M.; Stark, H.; Neugebauer, E.M.; Panula, P. Traumatic brain injury results in mast cell increase and changes in regulation of central histamine receptors. Neuropathol. Appl. Neurobiol. 2005, 31, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Lock, C.; Hermans, G.; Pedotti, R.; Brendolan, A.; Schadt, E.; Garren, H.; Langer-Gould, A.; Strober, S.; Cannella, B.; Allard, J.; et al. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002, 8, 500–508. [Google Scholar] [CrossRef]
- Shanahan, L.; Zucker, N.; Copeland, W.E.; Costello, E.J.; Angold, A. Are children and adolescents with food allergies at increased risk for psychopathology? J. Psychosom. Res. 2014, 77, 468–473. [Google Scholar] [CrossRef]
- Costa-Pinto, F.A.; Basso, A.S. Neural and behavioral correlates of food allergy. Chem. Immunol. Allergy 2012, 98, 222–239. [Google Scholar] [CrossRef]
- Topal, E.; Catal, F.; Soylu, N.; Ozcan, O.O.; Celiksoy, M.H.; Babayigit, A.; Erge, D.; Karakoc, H.T.; Sancak, R. Psychiatric disorders and symptoms severity in pre-school children with cow’s milk allergy. Allergol. Immunopathol. 2016, 44, 445–449. [Google Scholar] [CrossRef]
- Moura, D.S.; Georgin-Lavialle, S.; Gaillard, R.; Hermine, O. Neuropsychological features of adult mastocytosis. Immunol. Allergy Clin. N. Am. 2014, 34, 407–422. [Google Scholar] [CrossRef]
- Georgin-Lavialle, S.; Gaillard, R.; Moura, D.; Hermine, O. Mastocytosis in adulthood and neuropsychiatric disorders. Transl. Res. 2016, 174, 77–85.e1. [Google Scholar] [CrossRef]
- Harcha, P.A.; Vargas, A.; Yi, C.; Koulakoff, A.A.; Giaume, C.; Saez, J.C. Hemichannels Are Required for Amyloid beta-Peptide-Induced Degranulation and Are Activated in Brain Mast Cells of APPswe/PS1dE9 Mice. J. Neurosci. 2015, 35, 9526–9538. [Google Scholar] [CrossRef][Green Version]
- Secor, V.H.; Secor, W.E.; Gutekunst, C.A.; Brown, M.A. Mast cells are essential for early onset and severe disease in a murine model of multiple sclerosis. J. Exp. Med. 2000, 191, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Ferro, M.A.; Van Lieshout, R.J.; Ohayon, J.; Scott, J.G. Emotional and behavioral problems in adolescents and young adults with food allergy. Allergy 2016, 71, 532–540. [Google Scholar] [CrossRef] [PubMed]
- de Theije, C.G.; Bavelaar, B.M.; Lopes da Silva, S.; Korte, S.M.; Olivier, B.; Garssen, J.; Kraneveld, A.D. Food allergy and food-based therapies in neurodevelopmental disorders. Pediatr. Allergy Immunol. 2014, 25, 218–226. [Google Scholar] [CrossRef] [PubMed]
- Lyall, K.; Van de Water, J.; Ashwood, P.; Hertz-Picciotto, I. Asthma and Allergies in Children With Autism Spectrum Disorders: Results From the CHARGE Study. Autism Res. 2015, 8, 567–574. [Google Scholar] [CrossRef]
- Germundson, D.L.; Smith, N.A.; Vendsel, L.P.; Kelsch, A.V.; Combs, C.K.; Nagamoto-Combs, K. Oral sensitization to whey proteins induces age- and sex-dependent behavioral abnormality and neuroinflammatory responses in a mouse model of food allergy: A potential role of mast cells. J. Neuroinflamm. 2018, 15, 120. [Google Scholar] [CrossRef]
- Germundson, D.L.; Vendsel, L.P.; Nagamoto-Combs, K. Region-specific regulation of central histaminergic H3 receptor expression in a mouse model of cow’s milk allergy. Brain Res. 2020, 1749, 147148. [Google Scholar] [CrossRef]
- Smith, N.A.; Germundson, D.L.; Combs, C.K.; Vendsel, L.P.; Nagamoto-Combs, K. Astrogliosis Associated With Behavioral Abnormality in a Non-anaphylactic Mouse Model of Cow’s Milk Allergy. Front. Cell. Neurosci. 2019, 13, 320. [Google Scholar] [CrossRef]
- Smith, N.A.; Germundson, D.L.; Gao, P.; Hur, J.; Floden, A.M.; Nagamoto-Combs, K. Anxiety-like behavior and intestinal microbiota changes as strain-and sex-dependent sequelae of mild food allergy in mouse models of cow’s milk allergy. Brain Behav. Immun. 2021, 95, 122–141. [Google Scholar] [CrossRef]
- Germundson, D.L.; Nagamoto-Combs, K. Isotype-Specific Detection of Serum Immunoglobulins Against Allergens. Methods Mol. Biol. 2021, 2223, 159–167. [Google Scholar] [CrossRef]
- Bailey, K.R.; Pavlova, M.N.; Rohde, A.D.; Hohmann, J.G.; Crawley, J.N. Galanin receptor subtype 2 (GalR2) null mutant mice display an anxiogenic-like phenotype specific to the elevated plus-maze. Pharmacol. Biochem. Behav. 2007, 86, 8–20. [Google Scholar] [CrossRef][Green Version]
- Tanda, K.; Nishi, A.; Matsuo, N.; Nakanishi, K.; Yamasaki, N.; Sugimoto, T.; Toyama, K.; Takao, K.; Miyakawa, T. Abnormal social behavior, hyperactivity, impaired remote spatial memory, and increased D1-mediated dopaminergic signaling in neuronal nitric oxide synthase knockout mice. Mol. Brain 2009, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Maeta, K.; Hattori, S.; Ikutomo, J.; Edamatsu, H.; Bilasy, S.E.; Miyakawa, T.; Kataoka, T. Comprehensive behavioral analysis of mice deficient in Rapgef2 and Rapgef6, a subfamily of guanine nucleotide exchange factors for Rap small GTPases possessing the Ras/Rap-associating domain. Mol. Brain 2018, 11, 27. [Google Scholar] [CrossRef] [PubMed]
- Mayorga, A.J.; Lucki, I. Limitations on the use of the C57BL/6 mouse in the tail suspension test. Psychopharmacology 2001, 155, 110–112. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Nagamoto-Combs, K.; Manocha, G.D.; Puig, K.; Combs, C.K. An improved approach to align and embed multiple brain samples in a gelatin-based matrix for simultaneous histological processing. J. Neurosci. Methods 2016, 261, 155–160. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernandez, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Nishida, K.; Yamasaki, S.; Ito, Y.; Kabu, K.; Hattori, K.; Tezuka, T.; Nishizumi, H.; Kitamura, D.; Goitsuka, R.; Geha, R.S.; et al. FcεRI-mediated mast cell degranulation requires calcium-independent microtubule-dependent translocation of granules to the plasma membrane. J. Cell Biol. 2005, 170, 115–126. [Google Scholar] [CrossRef]
- Schilling, L.; Wahl, M. Opening of the blood-brain barrier during cortical superfusion with histamine. Brain Res. 1994, 653, 289–296. [Google Scholar] [CrossRef]
- Shelestak, J.; Singhal, N.; Frankle, L.; Tomor, R.; Sternbach, S.; McDonough, J.; Freeman, E.; Clements, R. Increased blood-brain barrier hyperpermeability coincides with mast cell activation early under cuprizone administration. PLoS ONE 2020, 15, e0234001. [Google Scholar] [CrossRef]
- Berghoff, S.A.; Duking, T.; Spieth, L.; Winchenbach, J.; Stumpf, S.K.; Gerndt, N.; Kusch, K.; Ruhwedel, T.; Mobius, W.; Saher, G. Blood-brain barrier hyperpermeability precedes demyelination in the cuprizone model. Acta Neuropathol. Commun. 2017, 5, 94. [Google Scholar] [CrossRef]
- Johnson, D.; Seeldrayers, P.A.; Weiner, H.L. The role of mast cells in demyelination. 1. Myelin proteins are degraded by mast cell proteases and myelin basic protein and P2 can stimulate mast cell degranulation. Brain Res. 1988, 444, 195–198. [Google Scholar] [CrossRef]
- Nicolaou, N.; Poorafshar, M.; Murray, C.; Simpson, A.; Winell, H.; Kerry, G.; Harlin, A.; Woodcock, A.; Ahlstedt, S.; Custovic, A. Allergy or tolerance in children sensitized to peanut: Prevalence and differentiation using component-resolved diagnostics. J. Allergy Clin. Immunol. 2010, 125, 191–197.e13. [Google Scholar] [CrossRef] [PubMed]
- Neeland, M.R.; Andorf, S.; Manohar, M.; Dunham, D.; Lyu, S.C.; Dang, T.D.; Peters, R.L.; Perrett, K.P.; Tang, M.L.K.; Saffery, R.; et al. Mass cytometry reveals cellular fingerprint associated with IgE+ peanut tolerance and allergy in early life. Nat. Commun. 2020, 11, 1091. [Google Scholar] [CrossRef] [PubMed]
- Kraus, S.; Arber, N. Inflammation and colorectal cancer. Curr. Opin. Pharmacol. 2009, 9, 405–410. [Google Scholar] [CrossRef]
- Kolb, H.; Mandrup-Poulsen, T. The global diabetes epidemic as a consequence of lifestyle-induced low-grade inflammation. Diabetologia 2010, 53, 10–20. [Google Scholar] [CrossRef]
- Amherd-Hoekstra, A.; Naher, H.; Lorenz, H.M.; Enk, A.H. Psoriatic arthritis: A review. J. Dtsch. Dermatol. Ges. 2010, 8, 332–339. [Google Scholar] [CrossRef]
- Ghosh, S.; Mitchell, R. Impact of inflammatory bowel disease on quality of life: Results of the European Federation of Crohn’s and Ulcerative Colitis Associations (EFCCA) patient survey. J. Crohn’s Colitis 2007, 1, 10–20. [Google Scholar] [CrossRef]
- Miller, A.H.; Maletic, V.; Raison, C.L. Inflammation and its discontents: The role of cytokines in the pathophysiology of major depression. Biol. Psychiatry 2009, 65, 732–741. [Google Scholar] [CrossRef]
- Waldmann, T.A.; Iio, A.; Ogawa, M.; McIntyre, O.R.; Strober, W. The metabolism of IgE. Studies in normal individuals and in a patient with IgE myeloma. J. Immunol. 1976, 117, 1139–1144. [Google Scholar]
- Guichard, A.; Cruz-Moreno, B.; Aguilar, B.; van Sorge, N.M.; Kuang, J.; Kurkciyan, A.A.; Wang, Z.; Hang, S.; Pineton de Chambrun, G.P.; McCole, D.F.; et al. Cholera toxin disrupts barrier function by inhibiting exocyst-mediated trafficking of host proteins to intestinal cell junctions. Cell Host Microbe 2013, 14, 294–305. [Google Scholar] [CrossRef]
- Theoharides, T.C.; Konstantinidou, A.D. Corticotropin-releasing hormone and the blood-brain-barrier. Front. Biosci. 2007, 12, 1615–1628. [Google Scholar] [CrossRef] [PubMed]
- Dimitriadou, V.; Pang, X.; Theoharides, T.C. Hydroxyzine inhibits experimental allergic encephalomyelitis (EAE) and associated brain mast cell activation. Int. J. Immunopharmacol. 2000, 22, 673–684. [Google Scholar] [CrossRef]
- Toru, H.; Ra, C.; Nonoyama, S.; Suzuki, K.; Yata, J.; Nakahata, T. Induction of the high-affinity IgE receptor (FcεRI) on human mast cells by IL-4. Int. Immunol. 1996, 8, 1367–1373. [Google Scholar] [CrossRef] [PubMed]
- Thompson, H.L.; Thomas, L.; Metcalfe, D.D. Murine mast cells attach to and migrate on laminin-, fibronectin-, and matrigel-coated surfaces in response to FcεRI-mediated signals. Clin. Exp. Allergy 1993, 23, 270–275. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.; Rudy, R.F.; Cai, T.; Du, R. Cerebral Artery Diameter in Inbred Mice Varies as a Function of Strain. Front. Neuroanat. 2018, 12, 10. [Google Scholar] [CrossRef]
- Ghanavati, S.; Lerch, J.P.; Sled, J.G. Automatic anatomical labeling of the complete cerebral vasculature in mouse models. Neuroimage 2014, 95, 117–128. [Google Scholar] [CrossRef]
- Rozniecki, J.J.; Hauser, S.L.; Stein, M.; Lincoln, R.; Theoharides, T.C. Elevated mast cell tryptase in cerebrospinal fluid of multiple sclerosis patients. Ann. Neurol. 1995, 37, 63–66. [Google Scholar] [CrossRef]
- Hu, W.; Chen, Z. The roles of histamine and its receptor ligands in central nervous system disorders: An update. Pharmacol. Ther. 2017, 175, 116–132. [Google Scholar] [CrossRef]
- Arrang, J.M.; Garbarg, M.; Schwartz, J.C. Auto-inhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature 1983, 302, 832–837. [Google Scholar] [CrossRef]
- Abbott, N.J. Astrocyte-endothelial interactions and blood-brain barrier permeability. J. Anat. 2002, 200, 629–638. [Google Scholar] [CrossRef]
- Chen, Y.; Zhen, W.; Guo, T.; Zhao, Y.; Liu, A.; Rubio, J.P.; Krull, D.; Richardson, J.C.; Lu, H.; Wang, R. Histamine Receptor 3 negatively regulates oligodendrocyte differentiation and remyelination. PLoS ONE 2017, 12, e0189380. [Google Scholar] [CrossRef]
- Couturier, N.; Zappulla, J.P.; Lauwers-Cances, V.; Uro-Coste, E.; Delisle, M.B.; Clanet, M.; Montagne, L.; Van der Valk, P.; Bo, L.; Liblau, R.S. Mast cell transcripts are increased within and outside multiple sclerosis lesions. J. Neuroimmunol. 2008, 195, 176–185. [Google Scholar] [CrossRef]
- Olsson, Y. Mast cells in plaques of multiple sclerosis. Acta Neurol. Scand. 1974, 50, 611–618. [Google Scholar] [CrossRef]




| Antibody | Host | Clonality | Product No. | Dilution | Company |
|---|---|---|---|---|---|
| α-tubulin | Mouse | Monoclonal | sc-8035 | 1:1000 | Santa-Cruz Biotechnology, Inc. |
| Glyceraldeyde-3-phosphate (GAPDH) | Mouse | Monoclonal | sc-32233 | 1:1000 | Santa-Cruz Biotechnology, Inc. |
| Histamine H3 receptor (H3R) | Rabbit | Polyclonal | AHR-003 | 1:500 | Alomone Labs |
| Myelin basic protein (MBP) | Rabbit | Polyclonal | PA5-78397 | 1:5000 | Invitrogen, Thermo Fisher Scientific |
| Mast cell protease-1 (MCPT-1) | Rabbit | Monoclonal | MA5-38007 | 1:500 | Invitrogen, Thermo Fisher Scientific |
| Proteolipid protein 1 (PLP1) | Rabbit | Polyclonal | PA3-150 | 1:500 | Invitrogen, Thermo Fisher Scientific |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Germundson, D.L.; Nagamoto-Combs, K. Potential Role of Intracranial Mast Cells in Neuroinflammation and Neuropathology Associated with Food Allergy. Cells 2022, 11, 738. https://doi.org/10.3390/cells11040738
Germundson DL, Nagamoto-Combs K. Potential Role of Intracranial Mast Cells in Neuroinflammation and Neuropathology Associated with Food Allergy. Cells. 2022; 11(4):738. https://doi.org/10.3390/cells11040738
Chicago/Turabian StyleGermundson, Danielle L., and Kumi Nagamoto-Combs. 2022. "Potential Role of Intracranial Mast Cells in Neuroinflammation and Neuropathology Associated with Food Allergy" Cells 11, no. 4: 738. https://doi.org/10.3390/cells11040738
APA StyleGermundson, D. L., & Nagamoto-Combs, K. (2022). Potential Role of Intracranial Mast Cells in Neuroinflammation and Neuropathology Associated with Food Allergy. Cells, 11(4), 738. https://doi.org/10.3390/cells11040738






