The Effect of Magnetic Field Gradient and Gadolinium-Based MRI Contrast Agent Dotarem on Mouse Macrophages
Abstract
:1. Introduction
2. Material and Methods
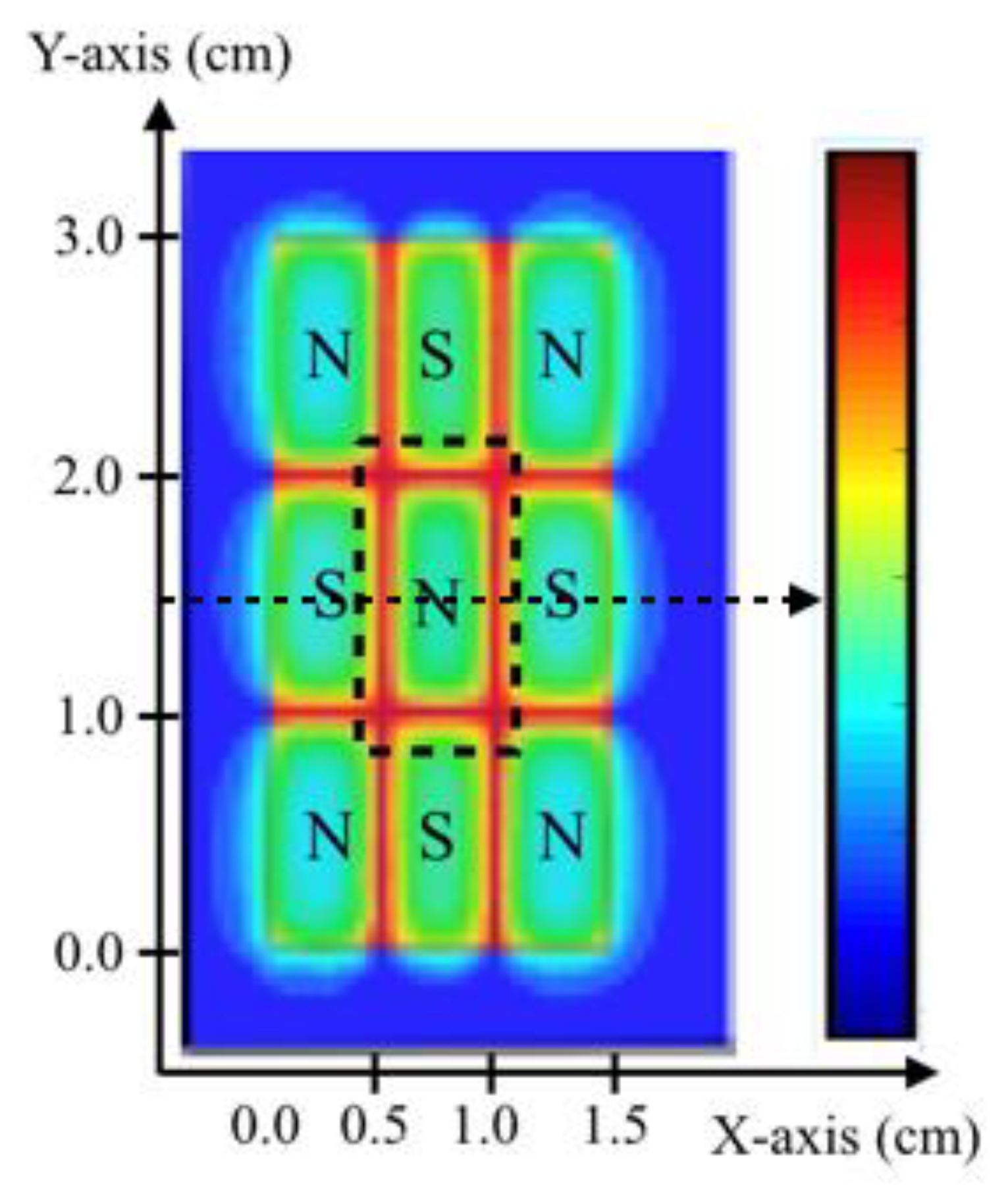
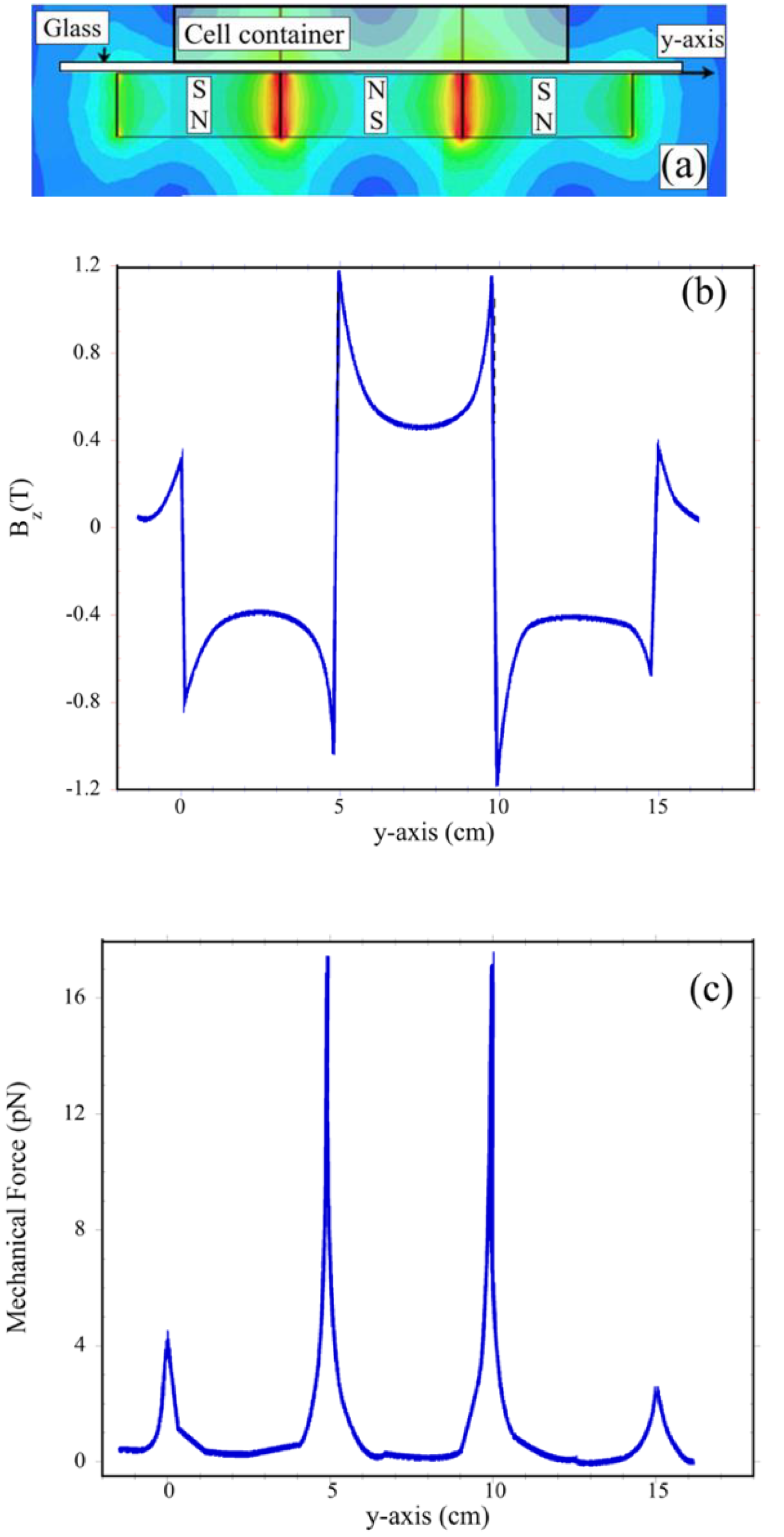
2.1. Magnetic Field Generation and Permanent Magnets’ Configuration
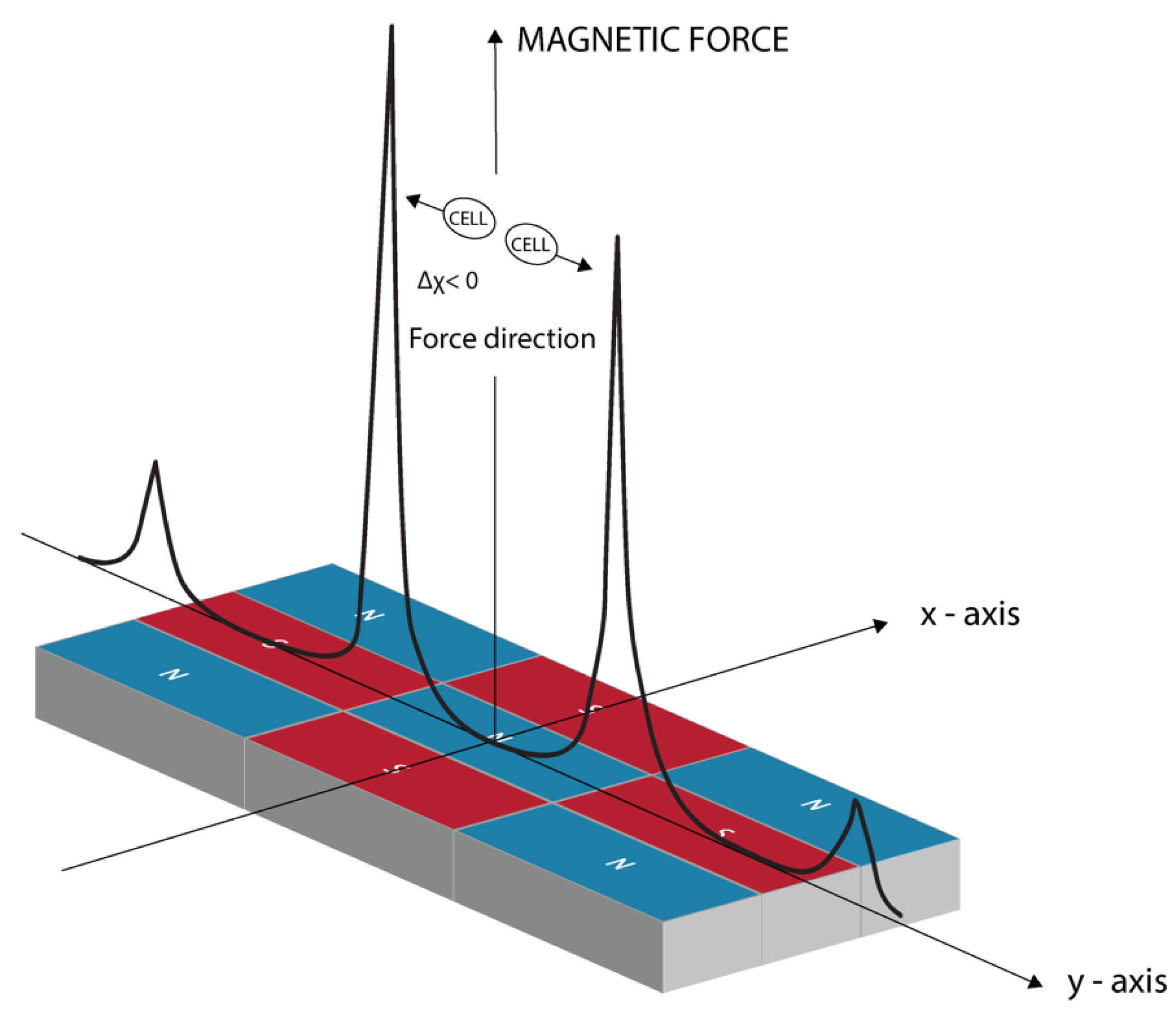
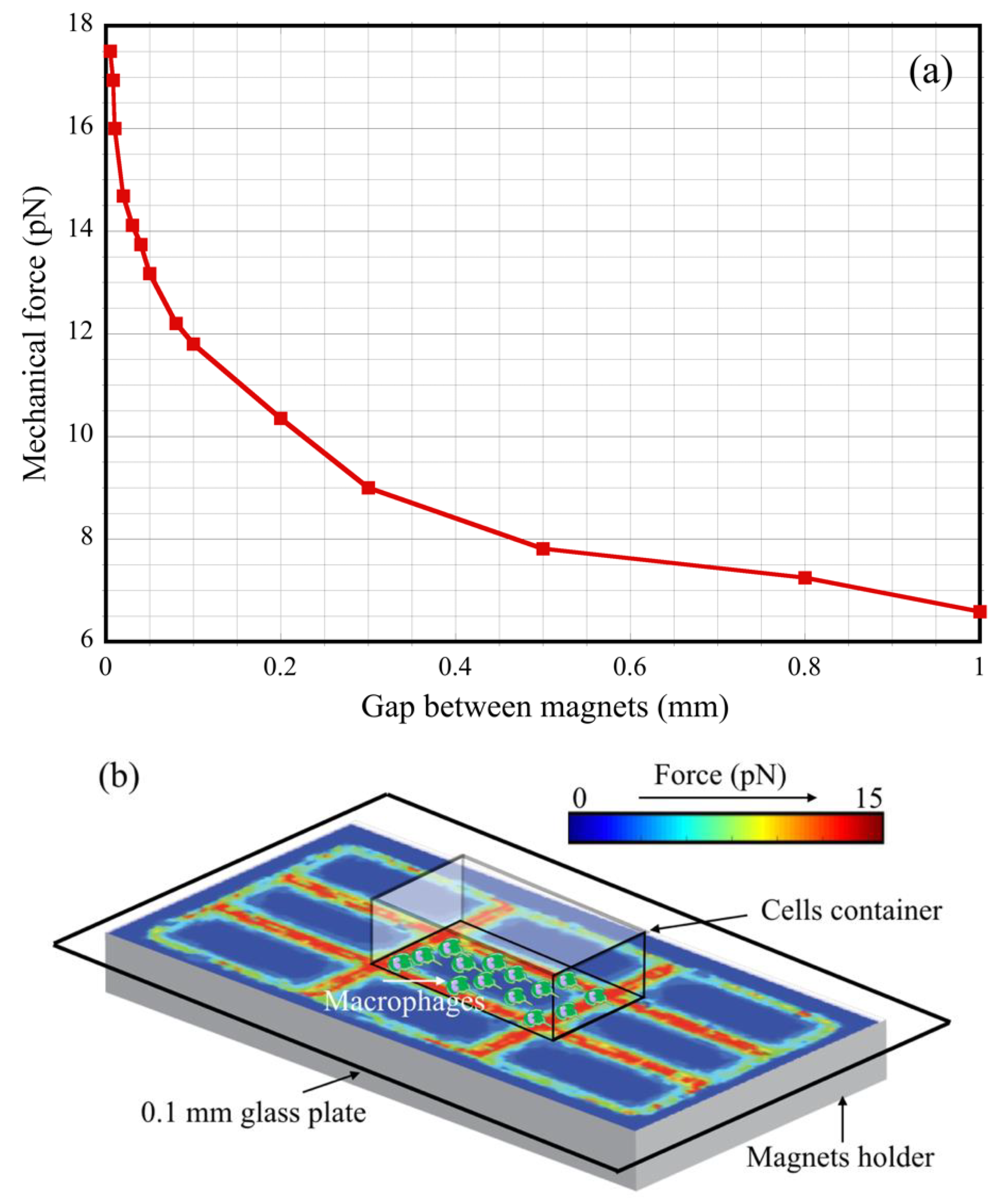
2.2. The Magnetic Field Gradient Created a Mechanical Force Acting on Macrophages
2.3. Macrophage Culture on Magnets
2.4. Mice
2.5. Dotarem Treatment of RAW 264.4 Cells
2.6. Western Blot Analysis
2.7. Immunostaining and Organelle Distribution Analysis
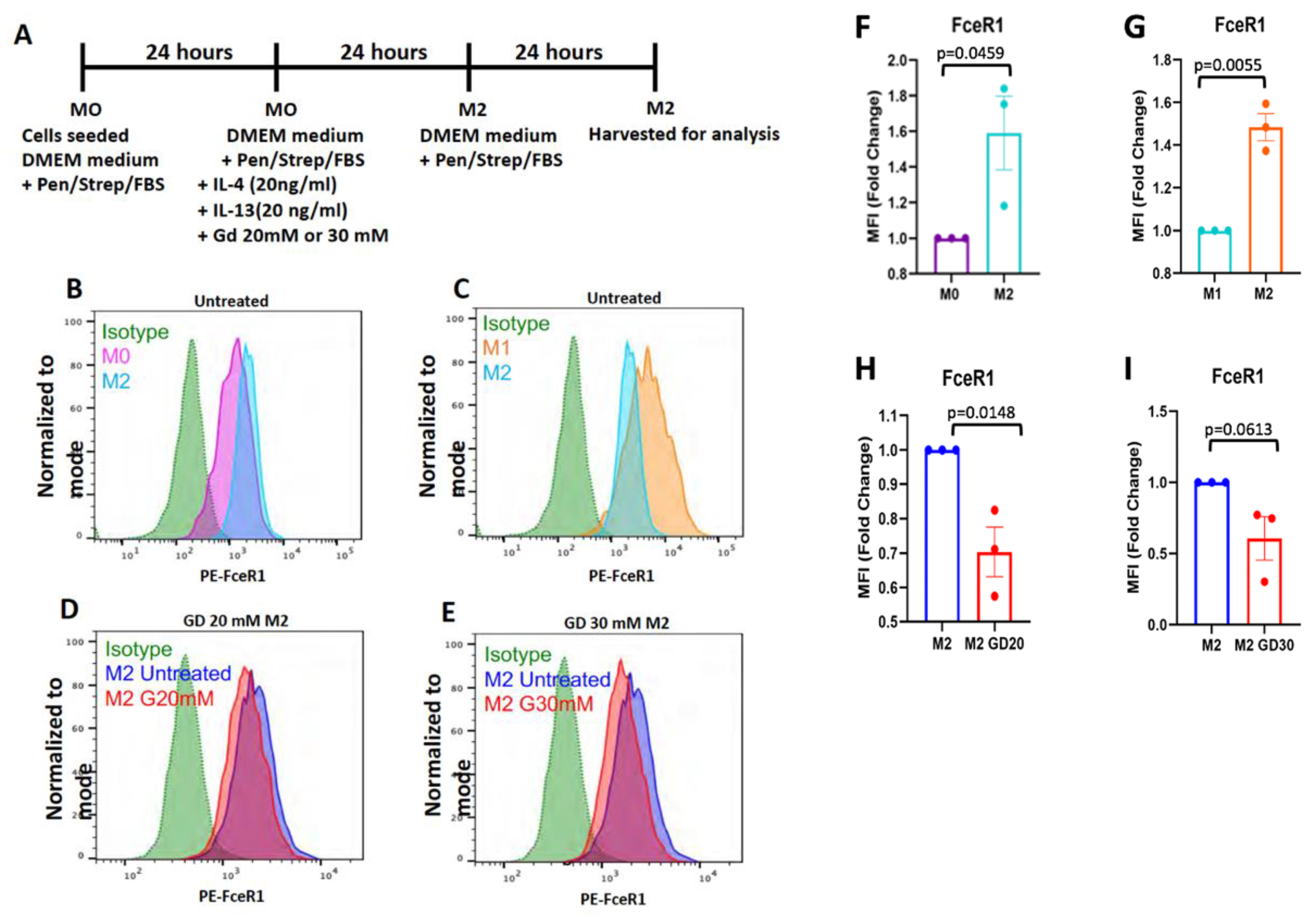
2.8. Flow Cytometry
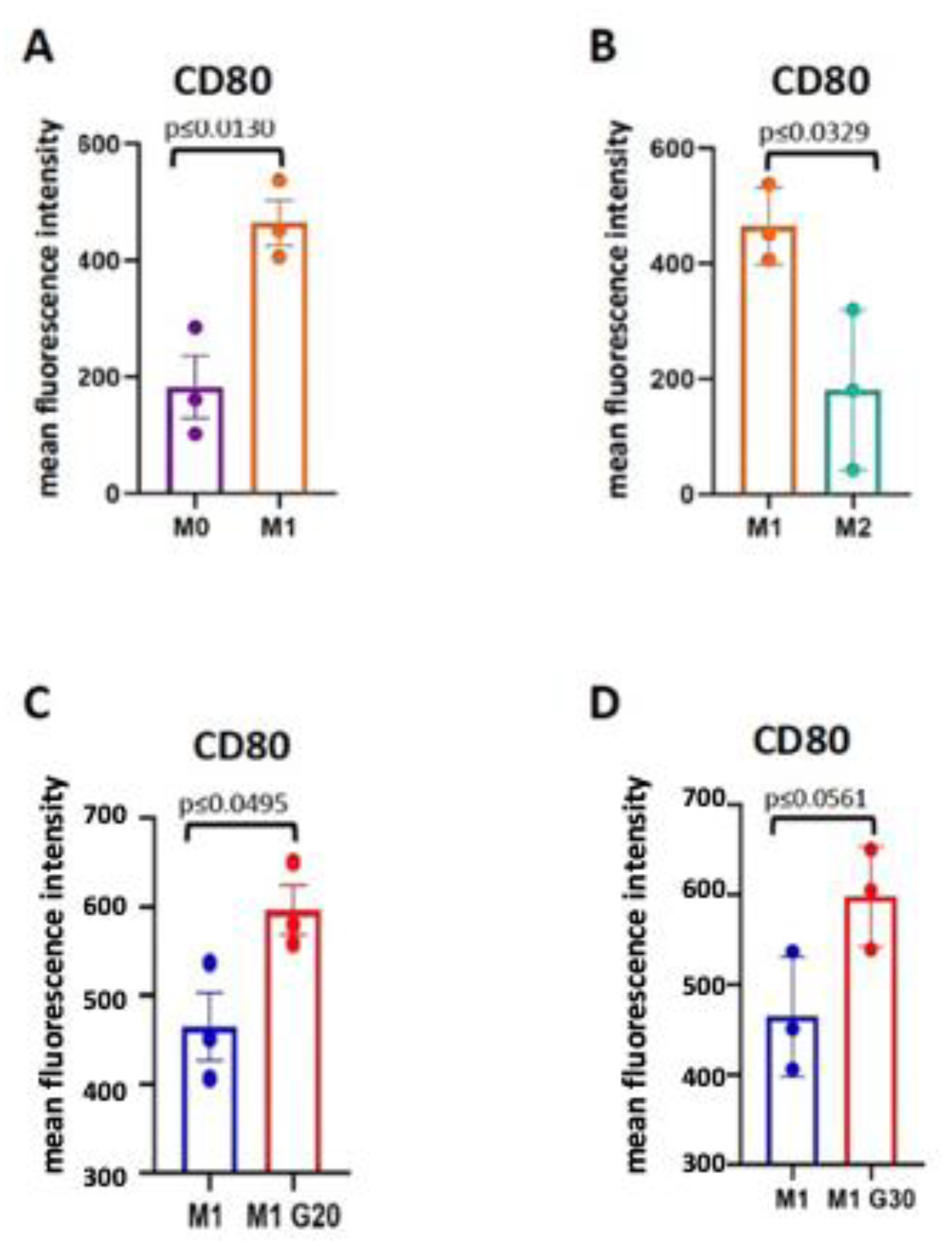
2.9. Macrophage Polarization
3. Results
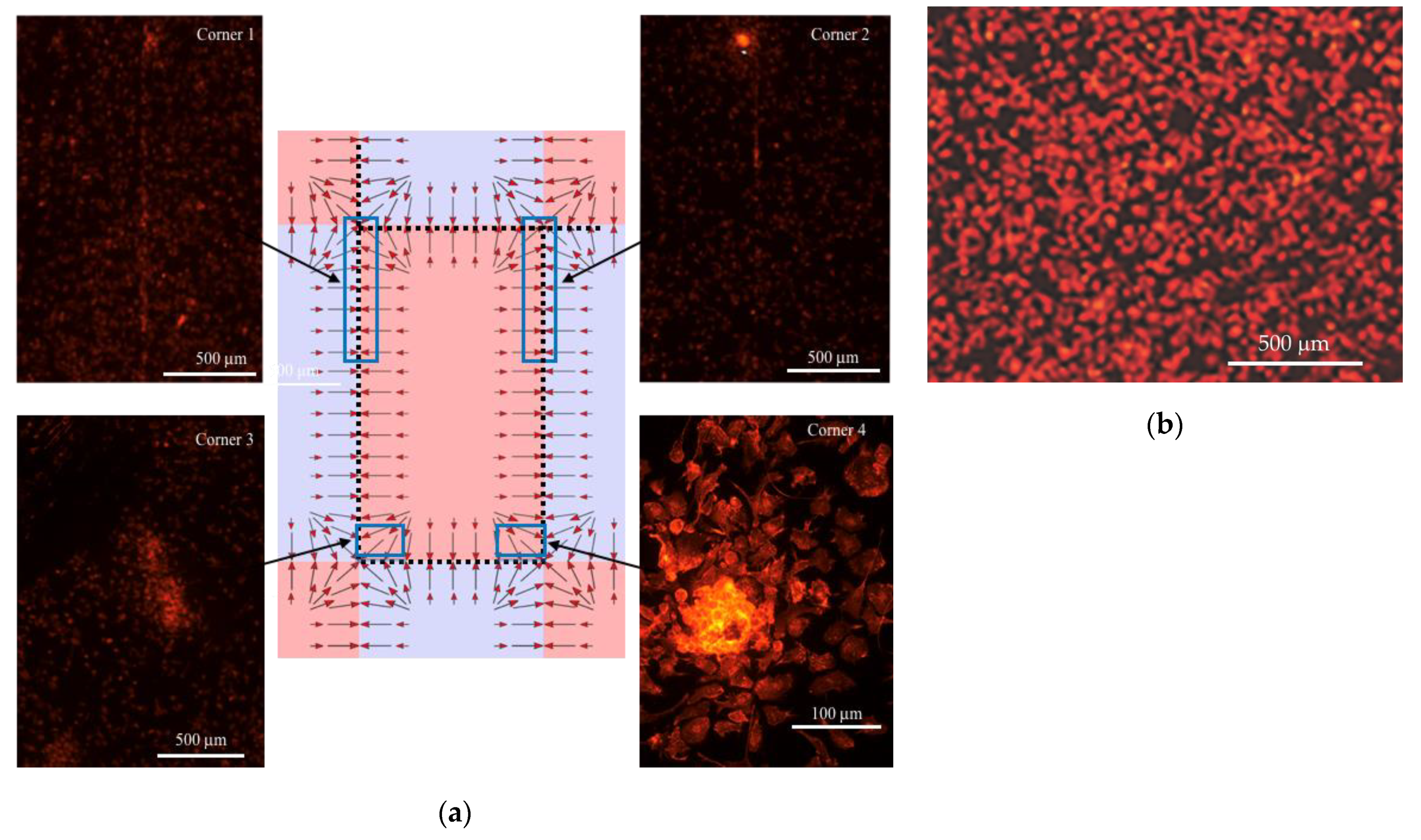
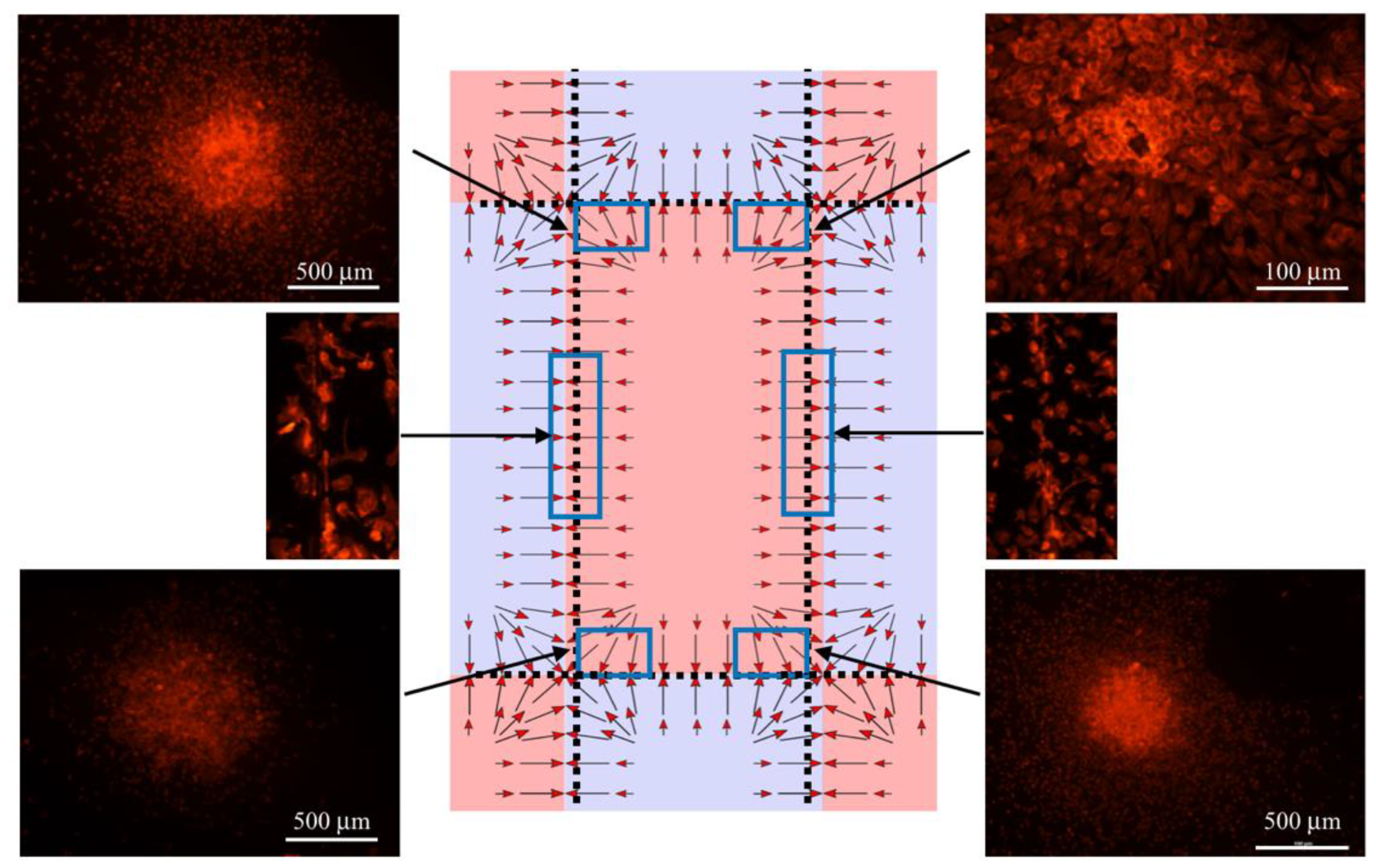
3.1. The Effect of the Magnetic Field on Macrophages
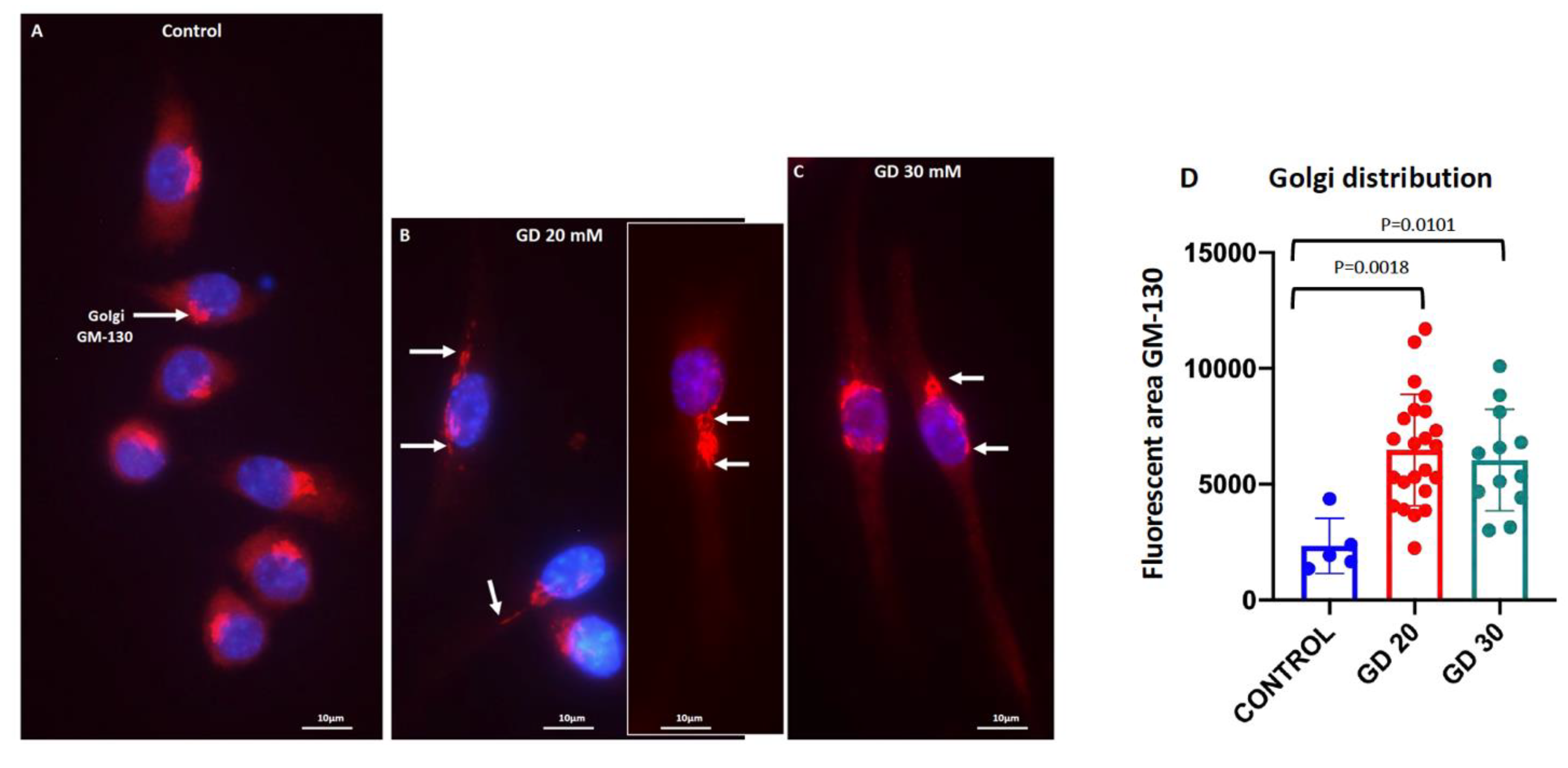
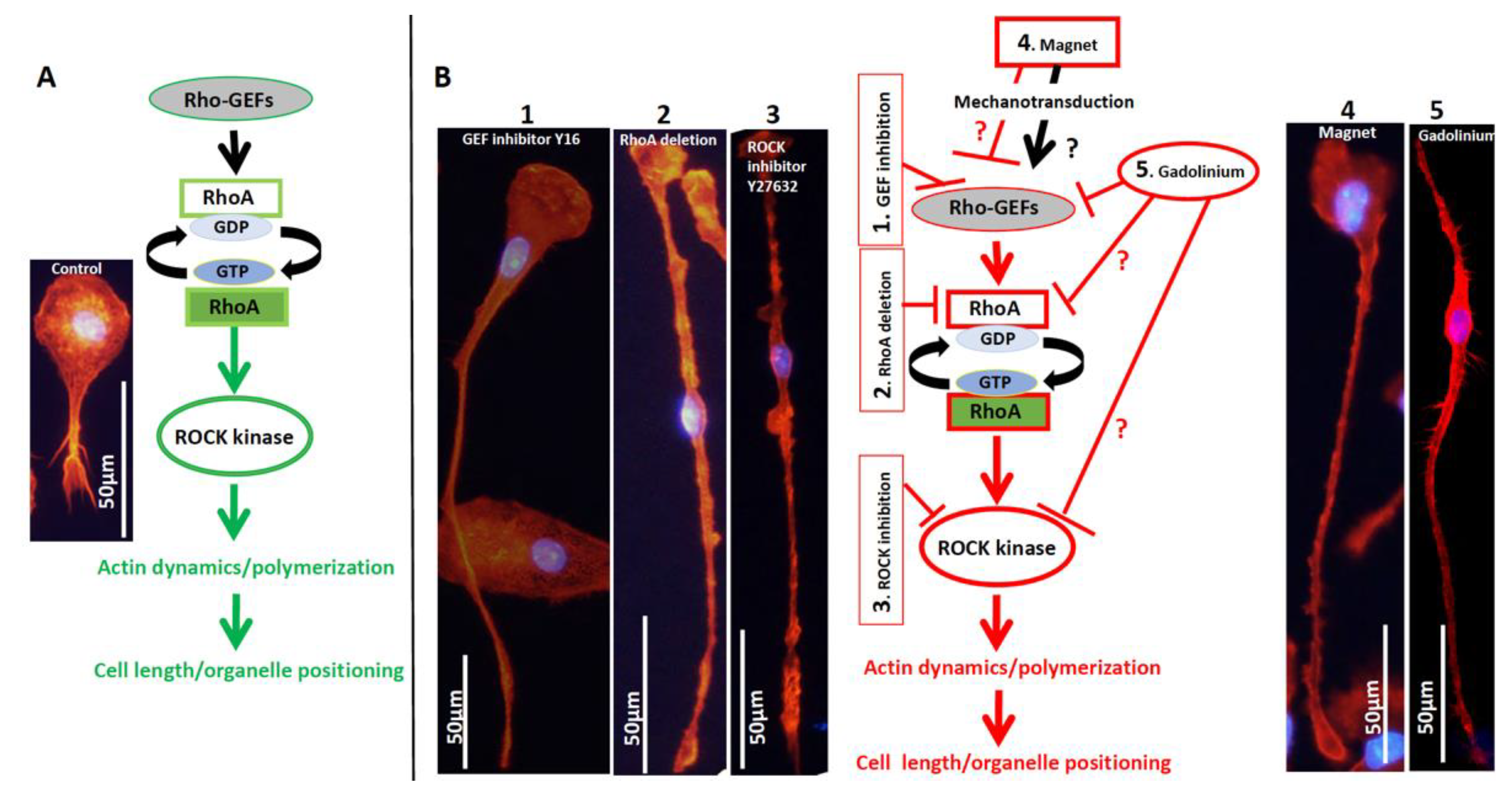
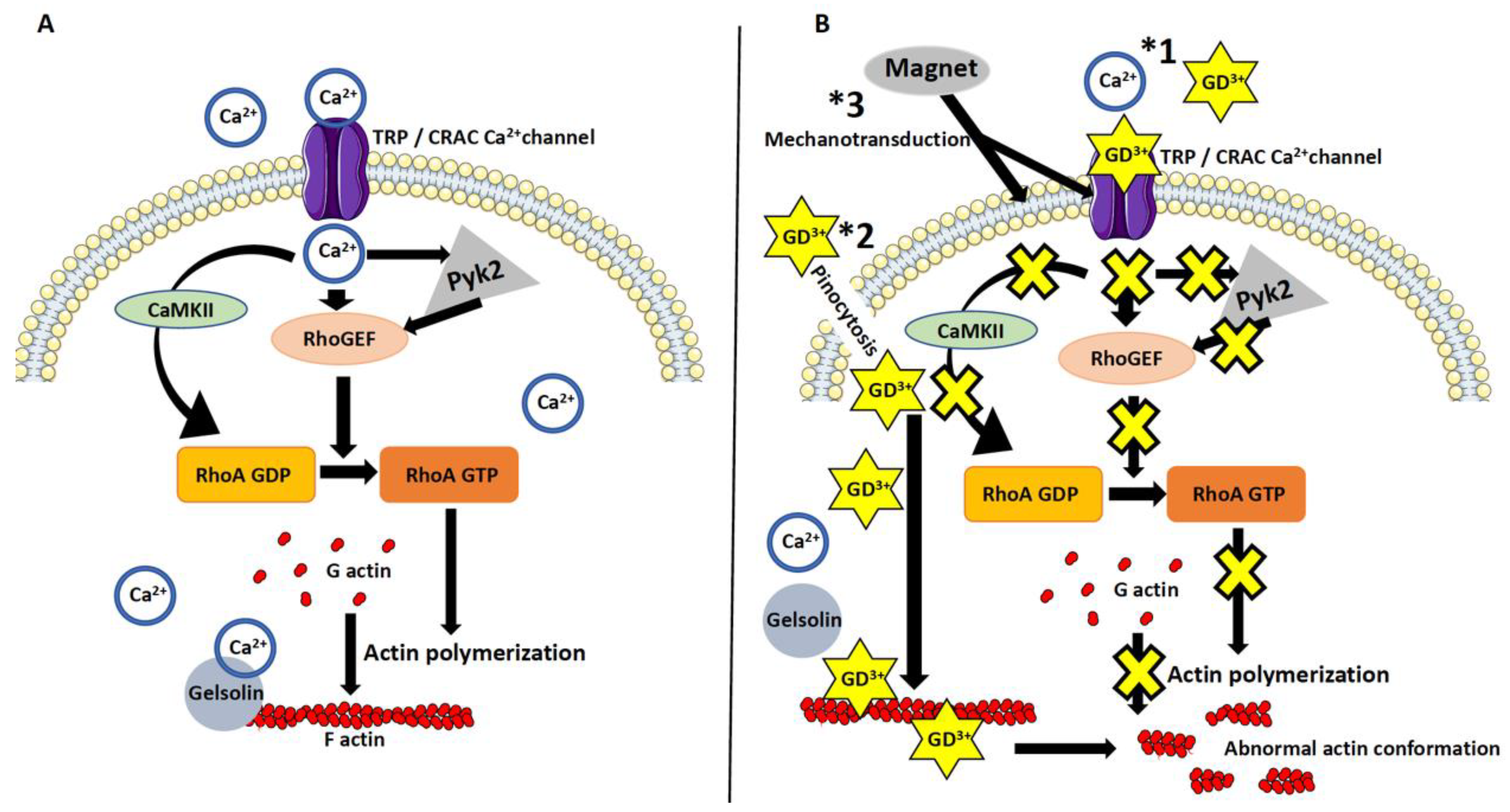
3.2. Dotarem Caused Cell Elongation and Redistribution of Cell Organelles
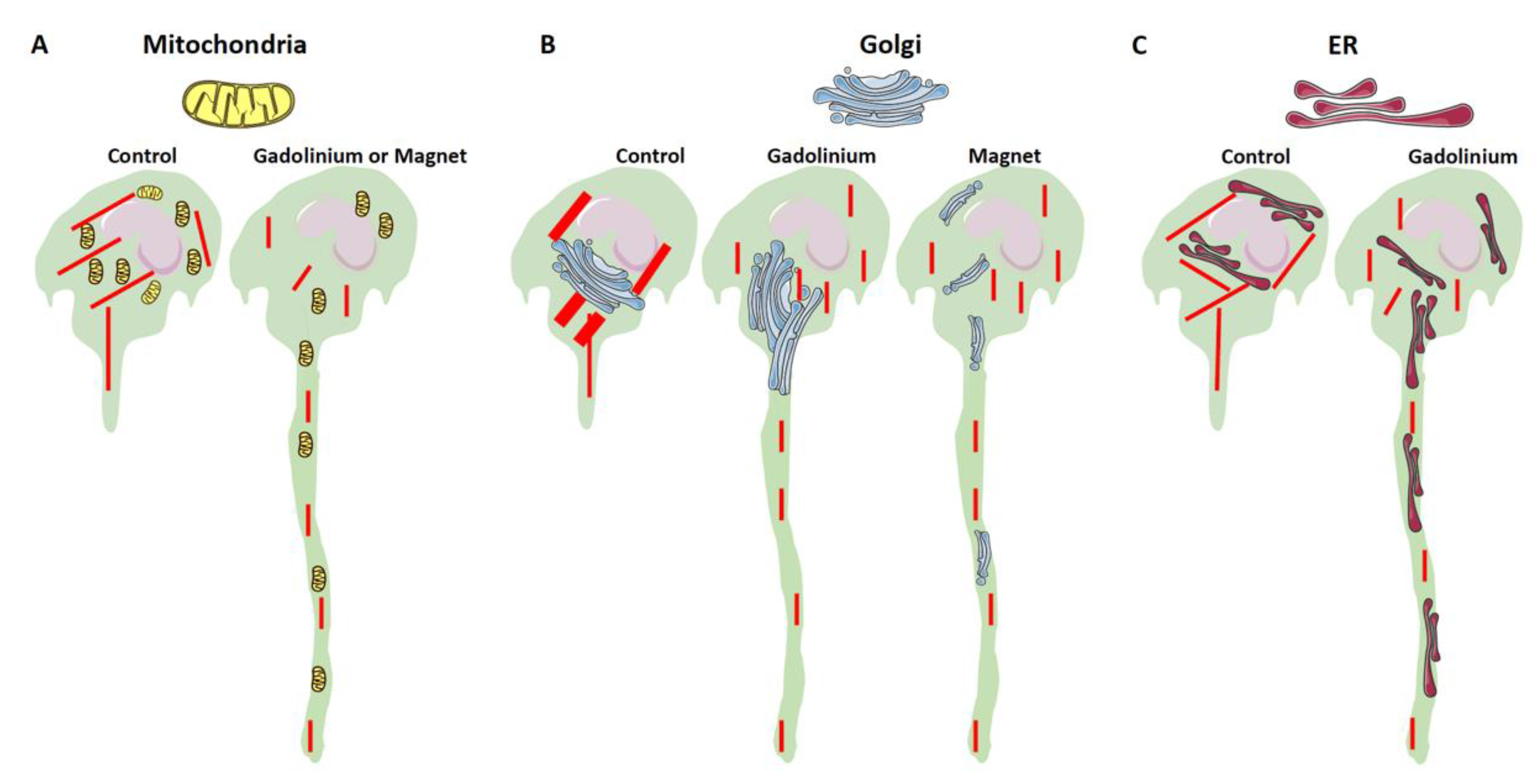
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cheng, Y.-C.N.; Haacke, E.M.; Thompson, M.R.; Venkatesan, R. Magnetic Resonance Imaging; Wiley Online Library: Hoboken, NJ, USA, 2014. [Google Scholar] [CrossRef] [Green Version]
- McRobbie, D.W.; Moore, E.A.; Graves, M.J.; Prince, M.R. MRI From Picture to Proton; Cambridge University Press: Cambridge, UK, 2006. [Google Scholar] [CrossRef]
- Vorst, A.; Rosen, A.; Kotsuka, Y. RF/Microwave Interaction with Biological Tissues; John Wiley & Sons: Hoboken, NJ, USA, 2006; p. 330. [Google Scholar]
- Theysohn, J.M.; Maderwald, S.; Kraff, O.; Moenninghoff, C.; Ladd, M.E.; Ladd, S.C. Subjective acceptance of 7 Tesla MRI for human imaging. MAGMA 2008, 21, 63–72. [Google Scholar] [CrossRef]
- Schenck, J.F. Safety of strong, static magnetic fields. J. Magn. Reson. Imaging 2000, 12, 2–19. [Google Scholar] [CrossRef]
- Miyakoshi, J. Effects of static magnetic fields at the cellular level. Prog. Biophys. Mol. Biol. 2005, 87, 213–223. [Google Scholar] [CrossRef]
- Buemi, M.; Marino, D.; Di Pasquale, G.; Floccari, F.; Senatore, M.; Aloisi, C.; Grasso, F.; Mondio, G.; Perillo, P.; Frisina, N.; et al. Cell proliferation/cell death balance in renal cell cultures after exposure to a static magnetic field. Nephron 2001, 87, 269–273. [Google Scholar] [CrossRef]
- Sormaala, M.J.; Ruohola, J.P.; Mattila, V.M.; Koskinen, S.K.; Pihlajamaki, H.K. Comparison of 1.5T and 3T MRI scanners in evaluation of acute bone stress in the foot. BMC Musculoskelet Disord. 2011, 12, 128. [Google Scholar] [CrossRef] [Green Version]
- Nagel, A.M.; Umathum, R.; Rosler, M.B.; Ladd, M.E.; Litvak, I.; Gor’kov, P.L.; Brey, W.W.; Schepkin, V.D. (39) K and (23) Na relaxation times and MRI of rat head at 21.1 T. NMR Biomed. 2016, 29, 759–766. [Google Scholar] [CrossRef]
- Neuner, I.; Arrubla, J.; Felder, J. Simultaneous EEG-fMRI acquisition at low, high and ultra-high magnetic fields up to 9.4 483 T: Perspectives and challenges. Neuroimage 2014, 102, 71–79. [Google Scholar] [CrossRef]
- Fraum, T.J.; Ludwig, D.R.; Bashir, M.R.; Fowler, K.J. Gadolinium-based contrast agents: A comprehensive risk assessment. J. Magn. Reson. Imaging 2017, 46, 338–353. [Google Scholar] [CrossRef]
- Cowling, T.; Frey, N. Macrocyclic and Linear Gadolinium Based Contrast Agents for Adults Undergoing Magnetic Resonance Imaging: A Review of Safety; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019; NBK546000. [Google Scholar]
- Xiao, Y.D.; Paudel, R.; Liu, J.; Ma, C.; Zhang, Z.S.; Zhou, S.K. MRI contrast agents: Classification and application (Review). Int. J. Mol. Med. 2016, 38, 1319–1326. [Google Scholar] [CrossRef] [Green Version]
- Idée, J.M.; Port, M.; Dencausse, A.; Lancelot, E.; Corot, C. Involvement of gadolinium chelates in the mechanism of nephrogenic systemic fibrosis: An update. Radiol. Clin. N. Am. 2009, 47, 855–869. [Google Scholar] [CrossRef]
- Idée, J.M.; Port, M.; Corot, C. Re: Chemical structure and stability of gadolinium chelates. Radiographics 2009, 29, 2099. [Google Scholar] [CrossRef] [Green Version]
- Idée, J.M.; Port, M.; Robic, C.; Medina, C.; Sabatou, M.; Corot, C. Role of thermodynamic and kinetic parameters in gadolinium chelate stability. J. Magn. Reson. Imaging 2009, 30, 1249–1258. [Google Scholar] [CrossRef]
- Hao, N.B.; Lü, M.H.; Fan, Y.H.; Cao, Y.L.; Zhang, Z.R.; Yang, S.M. Macrophages in tumor microenvironments and the progression of tumors. Clin. Dev. Immunol. 2012, 2012, 948098. [Google Scholar] [CrossRef] [Green Version]
- Malikova, H.; Holesta, M. Gadolinium contrast agents-are they really safe? J. Vasc. Access 2017, 18, 1–7. [Google Scholar] [CrossRef]
- Schlaudecker, J.D.; Bernheisel, C.R. Gadolinium-associated nephrogenic systemic fibrosis. Am. Fam. Physician 2009, 80, 711–714. [Google Scholar]
- Uosef, A.; Villagran, M.; Kubiak, J.Z.; Wosik, J.; Ghobrial, R.M.; Kloc, M. Side effects of gadolinium MRI contrast agents. Pediatr. I Med. Rodz. -Paediatr. Fam. Med. 2020, 16, 49–52. [Google Scholar] [CrossRef]
- Mallio, C.A.; Parillo, M.; Zobel, B.B.; Parizel, P.M.; Quattrocchi, C.C. Effect of Exposure to Gadodiamide and Brain Irradiation on T. J. Magn. Reson. Imaging 2020, 52, 1525–1530. [Google Scholar] [CrossRef]
- Ramalho, J.; Ramalho, M.; Jay, M.; Burke, L.M.; Semelka, R.C. Gadolinium toxicity and treatment. Magn. Reson. Imaging 2016, 34, 1394–1398. [Google Scholar] [CrossRef] [Green Version]
- Ramalho, J.; Semelka, R.C.; Ramalho, M.; Nunes, R.H.; AlObaidy, M.; Castillo, M. Gadolinium-Based Contrast Agent Accumulation and Toxicity: An Update. AJNR Am. J. Neuroradiol. 2016, 37, 1192–1198. [Google Scholar] [CrossRef] [Green Version]
- Kanda, T.; Nakai, Y.; Oba, H.; Toyoda, K.; Kitajima, K.; Furui, S. Gadolinium deposition in the brain. Magn. Reson. Imaging 2016, 34, 1346–1350. [Google Scholar] [CrossRef]
- Schenck, J.F. Physical interactions of static magnetic fields with living tissues. Prog. Biophys. Mol. Biol. 2005, 87, 185–204. [Google Scholar] [CrossRef]
- Landau, L.D.; Lifshitz, E.M.; Pitaevskii, L.P. Electrodynamics of Continuous Media; Pergamon: Oxford, UK; New York, NY, USA, 1984. [Google Scholar]
- Wosik, J.; Chen, W.; Qin, K.; Ghobrial, R.M.; Kubiak, J.Z.; Kloc, M. Magnetic Field Changes Macrophage Phenotype. Biophys J. 2018, 114, 2001–2013. [Google Scholar] [CrossRef] [Green Version]
- Giardini, P.A.; Fletcher, D.A.; Theriot, J.A. Compression forces generated by actin comet tails on lipid vesicles. Proc. Natl. Acad. Sci. USA 2003, 100, 6493–6498. [Google Scholar] [CrossRef] [Green Version]
- Noireaux, V.; Golsteyn, R.M.; Friederich, E.; Prost, J.; Antony, C.; Louvard, D.; Sykes, C. Growing an actin gel on spherical surfaces. Biophys J. 2000, 78, 1643–1654. [Google Scholar] [CrossRef] [Green Version]
- Balaban, N.Q.; Schwarz, U.S.; Riveline, D.; Goichberg, P.; Tzur, G.; Sabanay, I.; Mahalu, D.; Safran, S.; Bershadsky, A.; Addadi, L.; et al. Force and focal adhesion assembly: A close relationship studied using elastic micropatterned substrates. Nat. Cell Biol. 2001, 3, 466–472. [Google Scholar] [CrossRef]
- Stricker, J.; Aratyn-Schaus, Y.; Oakes, P.W.; Gardel, M.L. Spatiotemporal constraints on the force-dependent growth of focal adhesions. Biophys J. 2011, 100, 2883–2893. [Google Scholar] [CrossRef] [Green Version]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R.; Parsons, M.; Yang, M.T.; McLean, M.A.; Sligar, S.G.; Chen, C.S.; Ha, T.; et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2010, 466, 263–266. [Google Scholar] [CrossRef] [Green Version]
- Deguchi, S.; Ohashi, T.; Sato, M. Tensile properties of single stress fibers isolated from cultured vascular smooth muscle cells. J. Biomech 2006, 39, 2603–2610. [Google Scholar] [CrossRef]
- Zablotskii, V.; Polyakova, T.; Lunov, O.; Dejneka, A. How a High-Gradient Magnetic Field Could Affect Cell Life. Sci. Rep. 2016, 6, 37407. [Google Scholar] [CrossRef]
- Wosik, J.; Suarez-Villagran, M.; Miller, J.H.; Ghobrial, R.M.; Kloc, M. Macrophage phenotype bioengineered by magnetic, genetic, or pharmacologic interference. Immunol. Res. 2019, 67, 1–11. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Minze, L.J.; Kubiak, J.Z.; Li, X.C.; Ghobrial, R.M.; Kloc, M. Dissonant response of M0/M2 and M1 bone-marrow-derived macrophages to RhoA pathway interference. Cell Tissue Res. 2016, 366, 707–720. [Google Scholar] [CrossRef]
- Russell, E.; McMahon, S.J.; Russell, B.; Mohamud, H.; McGarry, C.K.; Schettino, G.; Prise, K.M. Effects of Gadolinium MRI Contrast Agents on DNA Damage and Cell Survival when Used in Combination with Radiation. Radiat Res. 2020, 194, 298–309. [Google Scholar] [CrossRef]
- Taciak, B.; Białasek, M.; Braniewska, A.; Sas, Z.; Sawicka, P.; Kiraga, Ł.; Rygiel, T.; Król, M. Evaluation of phenotypic and functional stability of RAW 264.7 cell line through serial passages. PLoS ONE 2018, 13, e0198943. [Google Scholar] [CrossRef]
- Uosef, A.; Vaughn, N.; Chu, X.; Elshawwaf, M.; Abdelshafy, A.A.A.; Elsaid, K.M.K.; Ghobrial, R.M.; Kloc, M. Siponimod (Mayzent) Downregulates RhoA and Cell Surface Expression of the S1P1 and CX3CR1 Receptors in Mouse RAW 264.7 Macrophages. Arch. Immunol. Ther. Exp. (Warsz) 2020, 68, 19. [Google Scholar] [CrossRef]
- Van Bergeijk, P.; Hoogenraad, C.C.; Kapitein, L.C. Right Time, Right Place: Probing the Functions of Organelle Positioning. Trends Cell Biol. 2016, 26, 121–134. [Google Scholar] [CrossRef]
- Kloc, M.; Uosef, A.; Villagran, M.; Zdanowski, R.; Kubiak, J.Z.; Wosik, J.; Ghobrial, R.M. RhoA- and Actin-Dependent Functions of Macrophages from the Rodent Cardiac Transplantation Model Perspective-Timing Is the Essence. Biology 2021, 10, 70. [Google Scholar] [CrossRef]
- Park, J.; Xi, H. The complete mitochondrial genome of. Mitochondrial DNA B 2019, 5, 355–357. [Google Scholar] [CrossRef]
- Nakamura, N. Emerging new roles of GM130, a cis-Golgi matrix protein, in higher order cell functions. J. Pharmacol. Sci. 2010, 112, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Kloc, M.; Chanana, P.; Vaughn, N.; Uosef, A.; Kubiak, J.Z.; Ghobrial, R.M. New Insights into Cellular Functions of Nuclear Actin. Biology 2021, 10, 304. [Google Scholar] [CrossRef]
- Esfahani, P.H.; Knöll, R. An Approach to Study Shape-Dependent Transcriptomics at a Single Cell Level. J. Vis. Exp. 2020, 165, e61577. [Google Scholar] [CrossRef]
- Chen, W.; Zhao, Y.; Li, X.C.; Kubiak, J.Z.; Ghobrial, R.M.; Kloc, M. Rho-specific Guanine nucleotide exchange factors (Rho-GEFs) inhibition affects macrophage phenotype and disrupts Golgi complex. Int. J. Biochem. Cell Biol. 2017, 93, 12–24. [Google Scholar] [CrossRef]
- Liu, Y.; Chen, W.; Wu, C.; Minze, L.J.; Kubiak, J.Z.; Li, X.C.; Kloc, M.; Ghobrial, R.M. Macrophage/monocyte-specific deletion of Ras homolog gene family member A (RhoA) downregulates fractalkine receptor and inhibits chronic rejection of mouse cardiac allografts. J. Heart Lung Transpl. 2017, 36, 340–354. [Google Scholar] [CrossRef] [Green Version]
- Bourne, G.W.; Trifaró, J.M. The gadolinium ion: A potent blocker of calcium channels and catecholamine release from cultured chromaffin cells. Neuroscience 1982, 7, 1615–1622. [Google Scholar] [CrossRef]
- Barden, J.A.; Dos Remedios, C.G. Binding stoichiometry of gadolinium to actin: Its effect on the actin-bound divalent cation. Biochem. Biophys Res Commun. 1979, 86, 529–535. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Zhu, L.; Yang, X.; He, F.; Wang, T.; Bao, T.; Lu, H.; Wang, H.; Yang, S. Inulin alleviates inflammation of alcoholic liver disease via SCFAs-inducing suppression of M1 and facilitation of M2 macrophages in mice. Int. Immunopharmacol. 2020, 78, 106062. [Google Scholar] [CrossRef]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Jaguin, M.; Houlbert, N.; Fardel, O.; Lecureur, V. Polarization profiles of human M-CSF-generated macrophages and comparison of M1-markers in classically activated macrophages from GM-CSF and M-CSF origin. Cell Immunol. 2013, 281, 51–61. [Google Scholar] [CrossRef]
- Shin, J.S.; Greer, A.M. The role of FcεRI expressed in dendritic cells and monocytes. Cell Mol. Life Sci. 2015, 72, 2349–2360. [Google Scholar] [CrossRef] [Green Version]
- Turner, H.; Kinet, J.P. Signalling through the high-affinity IgE receptor Fc epsilonRI. Nature 1999, 402, B24–B30. [Google Scholar] [CrossRef]
- Liu, Y.; Kubiak, J.Z.; Li, X.C.; Ghobrial, R.M.; Kloc, M. Macrophages and RhoA Pathway in Transplanted Organs. Results Probl. Cell Differ. 2017, 62, 365–376. [Google Scholar] [CrossRef]
- Nguyen, L.K.; Kholodenko, B.N.; von Kriegsheim, A. Rac1 and RhoA: Networks, loops and bistability. Small GTPases 2018, 9, 316–321. [Google Scholar] [CrossRef] [Green Version]
- Van Buul, J.D.; Geerts, D.; Huveneers, S. Rho GAPs and GEFs: Controling switches in endothelial cell adhesion. Cell Adhes. Migr. 2014, 8, 108–124. [Google Scholar] [CrossRef] [Green Version]
- Aspenström, P. Integration of signalling pathways regulated by small GTPases and calcium. Biochim. Biophys. Acta 2004, 1742, 51–58. [Google Scholar] [CrossRef] [Green Version]
- Ying, Z.; Giachini, F.R.; Tostes, R.C.; Webb, R.C. PYK2/PDZ-RhoGEF links Ca2+ signaling to RhoA. Arter. Thromb. Vasc. Biol. 2009, 29, 1657–1663. [Google Scholar] [CrossRef] [Green Version]
- Haws, H.J.; McNeil, M.A.; Hansen, M.D. Control of cell mechanics by RhoA and calcium fluxes during epithelial scattering. Tissue Barriers 2016, 4, e1187326. [Google Scholar] [CrossRef] [Green Version]
- Amano, M.; Nakayama, M.; Kaibuchi, K. Rho-kinase/ROCK: A key regulator of the cytoskeleton and cell polarity. Cytoskeleton (Hoboken) 2010, 67, 545–554. [Google Scholar] [CrossRef] [Green Version]
- Beceiro, S.; Radin, J.N.; Chatuvedi, R.; Piazuelo, M.B.; Horvarth, D.J.; Cortado, H.; Gu, Y.; Dixon, B.; Gu, C.; Lange, I.; et al. TRPM2 ion channels regulate macrophage polarization and gastric inflammation during Helicobacter pylori infection. Mucosal Immunol. 2017, 10, 493–507. [Google Scholar] [CrossRef]
- Janmey, P.A.; Kas, J.; Shah, J.V.; Allen, P.G.; Tang, J.X. Cytoskeletal networks and filament bundles: Regulation by proteins and polycations. Biol. Bull. 1998, 194, 334–335; discussion 335–336. [Google Scholar] [CrossRef]
- Janmey, P.A. The cytoskeleton and cell signaling: Component localization and mechanical coupling. Physiol. Rev. 1998, 78, 763–781. [Google Scholar] [CrossRef] [Green Version]
- Janmey, P.A.; Shah, J.V.; Janssen, K.P.; Schliwa, M. Viscoelasticity of intermediate filament networks. Subcell Biochem. 1998, 31, 381–397. [Google Scholar]
- Lin, C.Y.; Hilgenberg, L.G.; Smith, M.A.; Lynch, G.; Gall, C.M. Integrin regulation of cytoplasmic calcium in excitatory neurons depends upon glutamate receptors and release from intracellular stores. Mol. Cell Neurosci. 2008, 37, 770–780. [Google Scholar] [CrossRef] [Green Version]
- Olivares, S.; Green, R.M.; Henkel, A.S. Endoplasmic reticulum stress activates the hepatic activator protein 1 complex via mitogen activated protein kinase-dependent signaling pathways. PLoS ONE 2014, 9, e103828. [Google Scholar] [CrossRef] [Green Version]
- Gregory, R.B.; Barritt, G.J. Evidence that Ca2+-release-activated Ca2+ channels in rat hepatocytes are required for the maintenance of hormone-induced Ca2+ oscillations. Biochem. J. 2003, 370, 695–702. [Google Scholar] [CrossRef] [Green Version]
- Chauvet, S.; Jarvis, L.; Chevallet, M.; Shrestha, N.; Groschner, K.; Bouron, A. Pharmacological Characterization of the Native Store-Operated Calcium Channels of Cortical Neurons from Embryonic Mouse Brain. Front. Pharmacol. 2016, 7, 486. [Google Scholar] [CrossRef] [Green Version]
- Nagasawa, M.; Kojima, I. Translocation of TRPV2 channel induced by focal administration of mechanical stress. Physiol. Rep. 2015, 3, e12296. [Google Scholar] [CrossRef] [Green Version]
- Di Gregorio, E.; Ferrauto, G.; Gianolio, E.; Aime, S. Gd loading by hypotonic swelling: An efficient and safe route for cellular labeling. Contrast Media. Mol. Imaging 2013, 8, 475–486. [Google Scholar] [CrossRef]
- Yin, H.L. Gelsolin: Calcium- and polyphosphoinositide-regulated actin-modulating protein. Bioessays 1987, 7, 176–179. [Google Scholar] [CrossRef]
- Choi, J.W.; Moon, W.J. Gadolinium Deposition in the Brain: Current Updates. Korean J. Radiol. 2019, 20, 134–147. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chanana, P.; Uosef, A.; Vaughn, N.; Suarez-Villagran, M.; Ghobrial, R.M.; Kloc, M.; Wosik, J. The Effect of Magnetic Field Gradient and Gadolinium-Based MRI Contrast Agent Dotarem on Mouse Macrophages. Cells 2022, 11, 757. https://doi.org/10.3390/cells11050757
Chanana P, Uosef A, Vaughn N, Suarez-Villagran M, Ghobrial RM, Kloc M, Wosik J. The Effect of Magnetic Field Gradient and Gadolinium-Based MRI Contrast Agent Dotarem on Mouse Macrophages. Cells. 2022; 11(5):757. https://doi.org/10.3390/cells11050757
Chicago/Turabian StyleChanana, Priyanka, Ahmed Uosef, Nicole Vaughn, Martha Suarez-Villagran, Rafik M. Ghobrial, Malgorzata Kloc, and Jarek Wosik. 2022. "The Effect of Magnetic Field Gradient and Gadolinium-Based MRI Contrast Agent Dotarem on Mouse Macrophages" Cells 11, no. 5: 757. https://doi.org/10.3390/cells11050757
APA StyleChanana, P., Uosef, A., Vaughn, N., Suarez-Villagran, M., Ghobrial, R. M., Kloc, M., & Wosik, J. (2022). The Effect of Magnetic Field Gradient and Gadolinium-Based MRI Contrast Agent Dotarem on Mouse Macrophages. Cells, 11(5), 757. https://doi.org/10.3390/cells11050757






