Human Sex Matters: Y-Linked Lysine Demethylase 5D Drives Accelerated Male Craniofacial Osteogenic Differentiation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Animal Surgery and Postoperative Care
2.3. Euthanasia and Sample Extraction
2.4. Micro-CT
2.5. Histology
2.6. Osteogenic Differentiation of Human Neural Crest Derived Inferior Turbinate Stem Cells
2.7. RNA Isolation and Sequencing
2.8. Generation of Lentiviral Vectors
2.9. siRNA-Mediated Knockdown and Pharmacological Inhibition of KDM5D
2.10. Western Blot
2.11. qPCR
2.12. Statistical Analysis
3. Results
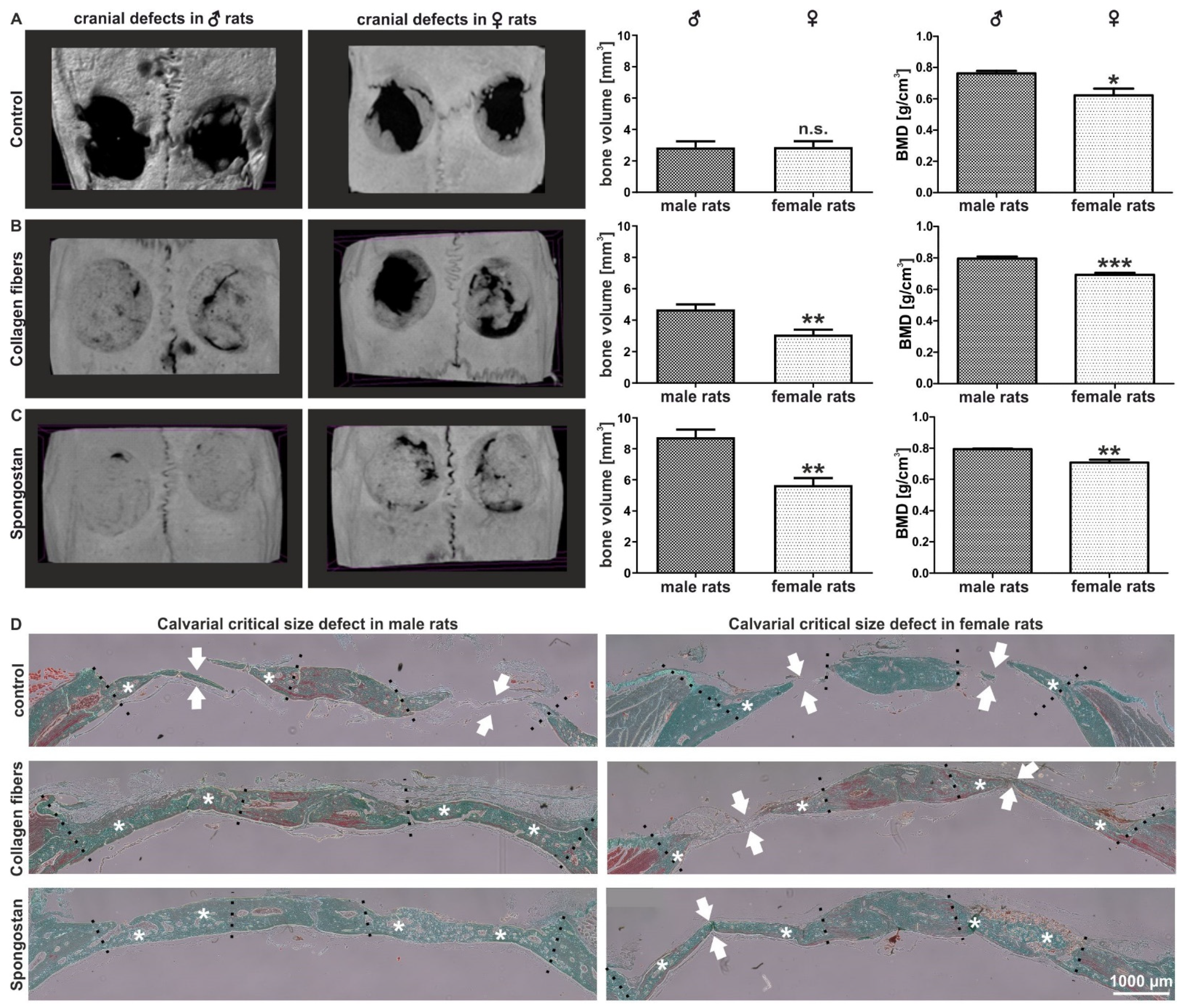
3.1. Calvarial Critical-Size Defects in Female Rats Showed Impaired Regeneration Compared to Male Animals
3.2. Female Adult Human Craniofacial Stem Cells Showed Strongly Delayed Osteogenic Differentiation
3.3. Transcriptome Analysis Revealed an Extreme Sexually Dimorphic Gene Expression
3.4. Identification of Y-Linked Lysin Demethylase 5D as a Novel Regulator of Osteogenic Differentiation in Male NCSCs
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wojcik, S.M.; Tantra, M.; Stepniak, B.; Man, K.-N.M.; Müller-Ribbe, K.; Begemann, M.; Ju, A.; Papiol, S.; Ronnenberg, A.; Gurvich, A.; et al. Genetic Markers of a Munc13 Protein Family Member, BAIAP3, Are Gender Specifically Associated with Anxiety and Benzodiazepine Abuse in Mice and Humans. Mol. Med. 2013, 19, 135–148. [Google Scholar] [CrossRef]
- Mitjans, M.; Begemann, M.; Ju, A.; Dere, E.; Wüstefeld, L.; Hofer, S.; Hassouna, I.; Balkenhol, J.; Oliveira, B.; van der Auwera, S.; et al. Sexual dimorphism of AMBRA1-related autistic features in human and mouse. Transl. Psychiatry 2017, 7, e1247. [Google Scholar] [CrossRef] [PubMed]
- Strube, P.; Mehta, M.; Baerenwaldt, A.; Trippens, J.; Wilson, C.J.; Ode, A.; Perka, C.; Duda, G.N.; Kasper, G. Sex-specific compromised bone healing in female rats might be associated with a decrease in mesenchymal stem cell quantity. Bone 2009, 45, 1065–1072. [Google Scholar] [CrossRef]
- Greiner, J.F.; Merten, M.; Kaltschmidt, C.; Kaltschmidt, B. Sexual dimorphisms in adult human neural, mesoderm-derived, and neural crest-derived stem cells. FEBS Lett. 2019, 593, 3338–3352. [Google Scholar] [CrossRef] [Green Version]
- Riggs, B.L.; Khosla, S.; Melton, L.J. Sex Steroids and the Construction and Conservation of the Adult Skeleton. Endocr. Rev. 2002, 23, 279–302. [Google Scholar] [CrossRef] [PubMed]
- Møller, A.M.J.; Delaissé, J.-M.; Olesen, J.B.; Madsen, J.S.; Canto, L.M.; Bechmann, T.; Rogatto, S.R.; Søe, K. Aging and menopause reprogram osteoclast precursors for aggressive bone resorption. Bone Res. 2020, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Pien, D.M.; Olmedo, D.G.; Guglielmotti, M.B. Influence of age and gender on peri-implant osteogenesis. Age and gender on peri-implant osteogenesis. Acta Odontol Latinoam 2001, 14, 9–13. [Google Scholar]
- Parker, M.J.; Raghavan, R.; Gurusamy, K. Incidence of Fracture-healing Complications after Femoral Neck Fractures. Clin. Orthop. Relat. Res. 2007, 458, 175–179. [Google Scholar] [CrossRef]
- Cawthon, P.M. Gender Differences in Osteoporosis and Fractures. Clin. Orthop. Relat. Res. 2011, 469, 1900–1905. [Google Scholar] [CrossRef] [Green Version]
- Ejiri, S.; Tanaka, M.; Watanabe, N.; Anwar, R.B.; Yamashita, E.; Yamada, K.; Ikegame, M. Estrogen deficiency and its effect on the jaw bones. J. Bone Miner. Metab. 2008, 26, 409–415. [Google Scholar] [CrossRef]
- E Compston, J.; McClung, M.R.; Leslie, W.D. Osteoporosis. Lancet 2019, 393, 364–376. [Google Scholar] [CrossRef]
- Khosla, S.; Oursler, M.J.; Monroe, D.G. Estrogen and the skeleton. Trends Endocrinol. Metab. 2012, 23, 576–581. [Google Scholar] [CrossRef] [Green Version]
- Zhou, S.; Zilberman, Y.; Wassermann, K.; Bain, S.D.; Sadovsky, Y.; Gazit, D. Estrogen modulates estrogen receptor α and β expression, osteogenic activity, and apoptosis in mesenchymal stem cells (MSCs) of osteoporotic mice. J. Cell. Biochem. 2001, 81, 144–155. [Google Scholar] [CrossRef] [PubMed]
- Meszaros, L.B.; Usas, A.; Cooper, G.M.; Huard, J. Effect of Host Sex and Sex Hormones on Muscle-Derived Stem Cell-Mediated Bone Formation and Defect Healing. Tissue Eng. Part A 2012, 18, 1751–1759. [Google Scholar] [CrossRef] [Green Version]
- A Corsi, K.; Pollett, J.B.; A Phillippi, J.; Usas, A.; Li, G.; Huard, J. Osteogenic Potential of Postnatal Skeletal Muscle-Derived Stem Cells Is Influenced by Donor Sex. J. Bone Miner. Res. 2007, 22, 1592–1602. [Google Scholar] [CrossRef]
- Aksu, A.E.; Rubin, J.P.; Dudas, J.R.; Marra, K.G. Role of Gender and Anatomical Region on Induction of Osteogenic Differentiation of Human Adipose-derived Stem Cells. Ann. Plast. Surg. 2008, 60, 306–322. [Google Scholar] [CrossRef]
- Hauser, S.; Widera, D.; Qunneis, F.; Müller, J.; Zander, C.; Greiner, J.; Strauss, C.; Lüningschrör, P.; Heimann, P.; Schwarze, H.; et al. Isolation of Novel Multipotent Neural Crest-Derived Stem Cells from Adult Human Inferior Turbinate. Stem Cells Dev. 2012, 21, 742–756. [Google Scholar] [CrossRef] [PubMed]
- Greiner, J.F.; Gottschalk, M.; Fokin, N.; Büker, B.; Kaltschmidt, B.P.; Dreyer, A.; Vordemvenne, T.; Kaltschmidt, C.; Hütten, A.; Kaltschmidt, B. Natural and synthetic nanopores directing osteogenic differentiation of human stem cells. Nanomed. Nanotechnol. Biol. Med. 2019, 17, 319–328. [Google Scholar] [CrossRef]
- Hofemeier, A.D.; Hachmeister, H.; Pilger, C.; Schürmann, M.; Greiner, J.F.; Nolte, L.; Sudhoff, H.; Kaltschmidt, C.; Huser, T.; Kaltschmidt, B. Label-free nonlinear optical microscopy detects early markers for osteogenic differentiation of human stem cells. Sci. Rep. 2016, 6, 26716. [Google Scholar] [CrossRef] [PubMed]
- Noden, D.M. The control of avian cephalic neural crest cytodifferentiation: I. Skeletal and connective tissues. Dev. Biol. 1978, 67, 296–312. [Google Scholar] [CrossRef]
- Graham, A.; Begbie, J.; McGonnell, I. Significance of the cranial neural crest. Dev. Dyn. 2003, 229, 5–13. [Google Scholar] [CrossRef] [PubMed]
- Rocha, M.; Beiriger, A.; Kushkowski, E.E.; Miyashita, T.; Singh, N.; Venkataraman, V.; Prince, V.E. From head to tail: Regionalization of the neural crest. Development 2020, 147. [Google Scholar] [CrossRef] [PubMed]
- Kaltschmidt, B.; Kaltschmidt, C.; Widera, D. Adult Craniofacial Stem Cells: Sources and Relation to the Neural Crest. Stem Cell Rev. Rep. 2011, 8, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Höving, A.L.; Windmöller, B.A.; Knabbe, C.; Kaltschmidt, B.; Kaltschmidt, C.; Greiner, J.F.W. Between Fate Choice and Self-Renewal—Heterogeneity of Adult Neural Crest-Derived Stem Cells. Front. Cell Dev. Biol. 2021, 9, 662754. [Google Scholar] [CrossRef]
- La Noce, M.; Mele, L.; Tirino, V.; Paino, F.; De Rosa, A.; Naddeo, P.; Papagerakis, P.; Papaccio, G.; Desiderio, V. Neural crest stem cell population in craniomaxillofacial development and tissue repair. Eur. Cells Mater. 2014, 28, 348–357. [Google Scholar] [CrossRef]
- Soto, J.; Ding, X.; Wang, A.; Li, S. Neural crest-like stem cells for tissue regeneration. STEM CELLS Transl. Med. 2021, 10, 681–693. [Google Scholar] [CrossRef]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef] [Green Version]
- Singhatanadgit, W.; Donos, N.; Olsen, I. Isolation and Characterization of Stem Cell Clones from Adult Human Ligament. Tissue Eng. Part A 2009, 15, 2625–2636. [Google Scholar] [CrossRef]
- Vordemvenne, T.; Wähnert, D.; Koettnitz, J.; Merten, M.; Fokin, N.; Becker, A.; Büker, B.; Vogel, A.; Kronenberg, D.; Stange, R.; et al. Bone Regeneration: A Novel Osteoinductive Function of Spongostan by the Interplay between Its Nano- and Microtopography. Cells 2020, 9, 654. [Google Scholar] [CrossRef] [Green Version]
- Wähnert, D.; Koettnitz, J.; Merten, M.; Kronenberg, D.; Stange, R.; Greiner, J.F.W.; Kaltschmidt, C.; Vordemvenne, T.; Kaltschmidt, B. Spongostan™ Leads to Increased Regeneration of a Rat Calvarial Critical Size Defect Compared to NanoBone® and Actifuse. Materials 2021, 14, 1961. [Google Scholar] [CrossRef]
- Spicer, P.P.; Kretlow, J.D.; Young, S.; Jansen, J.A.; Kasper, F.K.; Mikos, A.G. Evaluation of bone regeneration using the rat critical size calvarial defect. Nat. Protoc. 2012, 7, 1918–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greiner, J.F.; Hauser, S.; Widera, D.; Müller, J.; Qunneis, F.; Zander, C.; Martin, I.; Mallah, J.; Schuetzmann, D.; Prante, C.; et al. Efficient animal-serum free 3D cultivation method for adult human neural crest-derived stem cell therapeutics. Eur. Cells Mater. 2011, 22, 403–419. [Google Scholar] [CrossRef] [PubMed]
- Gregory, C.A.; Gunn, W.G.; Peister, A.; Prockop, D.J. An Alizarin red-based assay of mineralization by adherent cells in culture: Comparison with cetylpyridinium chloride extraction. Anal. Biochem. 2004, 329, 77–84. [Google Scholar] [CrossRef]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [Green Version]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [Green Version]
- Tang, Y.; Garson, K.; Li, L.; Vanderhyden, B.C. Optimization of lentiviral vector production using polyethylenimine-mediated transfection. Oncol. Lett. 2014, 9, 55–62. [Google Scholar] [CrossRef]
- Li, K.; Han, J.; Wang, Z. Histone modifications centric-regulation in osteogenic differentiation. Cell Death Discov. 2021, 7, 1–8. [Google Scholar] [CrossRef]
- Qin, G.; Li, Y.; Wang, H.; Yang, J.; Chen, Q.; Tang, H.; Wang, Y.; Zhang, M.; Jiang, T.; Lin, S.; et al. Lysine-Specific Demethylase 4A Regulates Osteogenic Differentiation via Regulating the Binding Ability of H3K9me3 with the Promoters of Runx2, Osterix and Osteocalcin. J. Biomed. Nanotechnol. 2020, 16, 899–909. [Google Scholar] [CrossRef] [PubMed]
- Shang, J.; Wang, H.; Fan, X.; Shangguan, L.; Liu, H. A genome wide analysis of alternative splicing events during the osteogenic differentiation of human cartilage endplate-derived stem cells. Mol. Med. Rep. 2016, 14, 1389–1396. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.N.; Green, J.; Wang, Z.; Deng, Y.; Qiao, M.; Peabody, M.; Zhang, Q.; Ye, J.; Yan, Z.; Denduluri, S.; et al. Bone Morphogenetic Protein (BMP) signaling in development and human diseases. Genes Dis. 2014, 1, 87–105. [Google Scholar] [CrossRef] [Green Version]
- Choi, J.; Bae, T.; Byambasuren, N.; Park, S.-H.; Jo, C.H.; Kim, D.; Hur, J.K.; Hwang, N.S. CRISPR-Cpf1 Activation of Endogenous BMP4 Gene for Osteogenic Differentiation of Umbilical-Cord-Derived Mesenchymal Stem Cells. Mol. Ther. - Methods Clin. Dev. 2020, 17, 309–316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheng, S.-L.; Shao, J.-S.; Charlton-Kachigian, N.; Loewy, A.P.; Towler, D.A. Msx2 Promotes Osteogenesis and Suppresses Adipogenic Differentiation of Multipotent Mesenchymal Progenitors. J. Biol. Chem. 2003, 278, 45969–45977. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dacic, S.; Kalajzic, I.; Visnjic, D.; Lichtler, A.C.; Rowe, D.W. Col1a1-Driven Transgenic Markers of Osteoblast Lineage Progression. J. Bone Miner. Res. 2001, 16, 1228–1236. [Google Scholar] [CrossRef] [PubMed]
- Grigoriadis, A.E.; Wang, Z.-Q.; Cecchini, M.G.; Hofstetter, W.; Felix, R.; Fleisch, H.A.; Wagner, E.F. c-Fos: A Key Regulator of Osteoclast-Macrophage Lineage Determination and Bone Remodeling. Science 1994, 266, 443–448. [Google Scholar] [CrossRef]
- Closs, E.I.; Murray, A.B.; Schmidt, J.; Schön, A.; Erfle, V.; Strauss, P.G. c-fos expression precedes osteogenic differentiation of cartilage cells in vitro. J. Cell Biol. 1990, 111, 1313–1323. [Google Scholar] [CrossRef] [Green Version]
- Ohta, S.; Yamamuro, T.; Lee, K.; Okumura, H.; Kasai, R.; Hiraki, Y.; Ikeda, T.; Iwasaki, R.; Kikuchi, H.; Konishi, J.; et al. Fracture healing induces expression of the proto-oncogenec-fosin vivo Possible involvement of the Fos protein in osteoblastic differentiation. FEBS Lett. 1991, 284, 42–45. [Google Scholar] [CrossRef] [Green Version]
- Lee, W.-R.; Ishikawa, T.; Umetani, M. The interaction between metabolism, cancer and cardiovascular disease, connected by 27-hydroxycholesterol. Clin. Lipidol. 2014, 9, 617–624. [Google Scholar] [CrossRef] [Green Version]
- Schüle, R.; Siddique, T.; Deng, H.-X.; Yang, Y.; Donkervoort, S.; Hansson, M.; Madrid, R.E.; Siddique, N.; Schöls, L.; Björkhem, I. Marked accumulation of 27-hydroxycholesterol in SPG5 patients with hereditary spastic paresis. J. Lipid Res. 2010, 51, 819–823. [Google Scholar] [CrossRef] [Green Version]
- Umetani, M.; Domoto, H.; Gormley, A.K.; Yuhanna, I.S.; Cummins, C.L.; Javitt, N.B.; Korach, K.S.; Shaul, P.W.; Mangelsdorf, D.J. 27-Hydroxycholesterol is an endogenous SERM that inhibits the cardiovascular effects of estrogen. Nat. Med. 2007, 13, 1185–1192. [Google Scholar] [CrossRef]
- DuSell, C.D.; Nelson, E.R.; Wang, X.; Abdo, J.; Mödder, U.I.; Umetani, M.; Gesty-Palmer, D.; Javitt, N.B.; Khosla, S.; McDonnell, D.P. The Endogenous Selective Estrogen Receptor Modulator 27-Hydroxycholesterol Is a Negative Regulator of Bone Homeostasis. Endocrinology 2010, 151, 3675–3685. [Google Scholar] [CrossRef] [Green Version]
- Fagerberg, L.; Hallström, B.M.; Oksvold, P.; Kampf, C.; Djureinovic, D.; Odeberg, J.; Habuka, M.; Tahmasebpoor, S.; Danielsson, A.; Edlund, K.; et al. Analysis of the Human Tissue-specific Expression by Genome-wide Integration of Transcriptomics and Antibody-based Proteomics. Mol. Cell. Proteom. 2014, 13, 397–406. [Google Scholar] [CrossRef] [Green Version]
- Li, L.; Li, Q.; Wang, Q.; Liu, L.; Li, R.; Liu, H.; He, Y.; Lash, G.E. Rare copy number variants in the genome of Chinese female children and adolescents with Turner syndrome. Biosci. Rep. 2019, 39, BSR20181305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Faienza, M.F.; Ventura, A.; Colucci, S.; Cavallo, L.; Grano, M.; Brunetti, G. Bone Fragility in Turner Syndrome: Mechanisms and Prevention Strategies. Front. Endocrinol. 2016, 7, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, B.; Yu, F.; Wang, C.; Li, B.; Liu, M.; Ye, L. Epigenetic Control of Mesenchymal Stem Cell Fate Decision via Histone Methyltransferase Ash1l. Stem Cells 2018, 37, 115–127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Wang, G.; Wang, Y.; Zhou, J.; Yuan, H.; Li, X.; Liu, Y.; Wang, B. Histone demethylase KDM7A reciprocally regulates adipogenic and osteogenic differentiation via regulation of C/EBPα and canonical Wnt signalling. J. Cell. Mol. Med. 2019, 23, 2149–2162. [Google Scholar] [CrossRef] [PubMed]
- Ge, W.; Shi, L.; Zhou, Y.; Liu, Y.; Ma, G.; Jiang, Y.; Xu, Y.; Zhang, X.; Feng, H. Inhibition of Osteogenic Differentiation of Human Adipose-Derived Stromal Cells by Retinoblastoma Binding Protein 2 Repression of RUNX2-Activated Transcription. Stem Cells 2011, 29, 1112–1125. [Google Scholar] [CrossRef]
- Hemming, S.; Cakouros, D.; Isenmann, S.; Cooper, L.; Menicanin, D.; Zannettino, A.; Gronthos, S. EZH2 and KDM6A Act as an Epigenetic Switch to Regulate Mesenchymal Stem Cell Lineage Specification. Stem Cells 2013, 32, 802–815. [Google Scholar] [CrossRef]
- Feng, Y.; Wan, P.; Yin, L. Long Noncoding RNA X-Inactive Specific Transcript (XIST) Promotes Osteogenic Differentiation of Periodontal Ligament Stem Cells by Sponging MicroRNA-214-3p. Med Sci. Monit. 2020, 26, e918932-1–e918932-8. [Google Scholar] [CrossRef]
- Ma, X.; Fan, C.; Wang, Y.; Du, Y.; Zhu, Y.; Liu, H.; Lv, L.; Liu, Y.; Zhou, Y. MiR-137 knockdown promotes the osteogenic differentiation of human adipose-derived stem cells via the LSD1/BMP2/SMAD4 signaling network. J. Cell. Physiol. 2019, 235, 909–919. [Google Scholar] [CrossRef]
- Yu, V.W.; Ambartsoumian, G.; Verlinden, L.; Moir, J.M.; Prud’Homme, J.; Gauthier, C.; Roughley, P.J.; St-Arnaud, R. FIAT represses ATF4-mediated transcription to regulate bone mass in transgenic mice. J. Cell Biol. 2005, 169, 591–601. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.; Domrachev, M.; Lash, A.E. Gene Expression Omnibus: NCBI gene expression and hybridization array data repository. Nucleic Acids Res. 2002, 30, 207–210. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Female Donors | Donor Age (y) | Passage of NCSCs |
|---|---|---|
| I | 52 | 4 |
| II | 26 | 4 |
| III | 20 | 4 |
| Male Donors | ||
| I | 26 | 3 |
| II | 44 | 4 |
| III | 22 | 4 |
| Gene Name | ENS ID | Down-/Upregulation in Female NCSCs Compared to Male NCSCs (log2 Fold Change) | p-Value |
|---|---|---|---|
| BMP4 | ENSG00000125378 | −1.2 | 0.014 |
| MSX2 | ENSG00000120149 | −0.8 | 0.013 |
| COL11A1 | ENSG00000060718 | −2.8 | 0.015 |
| COL4A4 | ENSG00000081052 | −1.2 | 0.043 |
| ITGA1 | ENSG00000213949 | −0.8 | 0.021 |
| FOS | ENSG00000170345 | −1.5 | 0.000 |
| BMP7 | ENSG00000101144 | 2.6 | 0.008 |
| MIR137HG | ENSG00000225206 | 1.0 | 0.037 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Merten, M.; Greiner, J.F.W.; Niemann, T.; Grosse Venhaus, M.; Kronenberg, D.; Stange, R.; Wähnert, D.; Kaltschmidt, C.; Vordemvenne, T.; Kaltschmidt, B. Human Sex Matters: Y-Linked Lysine Demethylase 5D Drives Accelerated Male Craniofacial Osteogenic Differentiation. Cells 2022, 11, 823. https://doi.org/10.3390/cells11050823
Merten M, Greiner JFW, Niemann T, Grosse Venhaus M, Kronenberg D, Stange R, Wähnert D, Kaltschmidt C, Vordemvenne T, Kaltschmidt B. Human Sex Matters: Y-Linked Lysine Demethylase 5D Drives Accelerated Male Craniofacial Osteogenic Differentiation. Cells. 2022; 11(5):823. https://doi.org/10.3390/cells11050823
Chicago/Turabian StyleMerten, Madlen, Johannes F. W. Greiner, Tarek Niemann, Meike Grosse Venhaus, Daniel Kronenberg, Richard Stange, Dirk Wähnert, Christian Kaltschmidt, Thomas Vordemvenne, and Barbara Kaltschmidt. 2022. "Human Sex Matters: Y-Linked Lysine Demethylase 5D Drives Accelerated Male Craniofacial Osteogenic Differentiation" Cells 11, no. 5: 823. https://doi.org/10.3390/cells11050823






