Endocrine Regulation of Maturation and Sex Change in Groupers
Abstract
1. Introduction
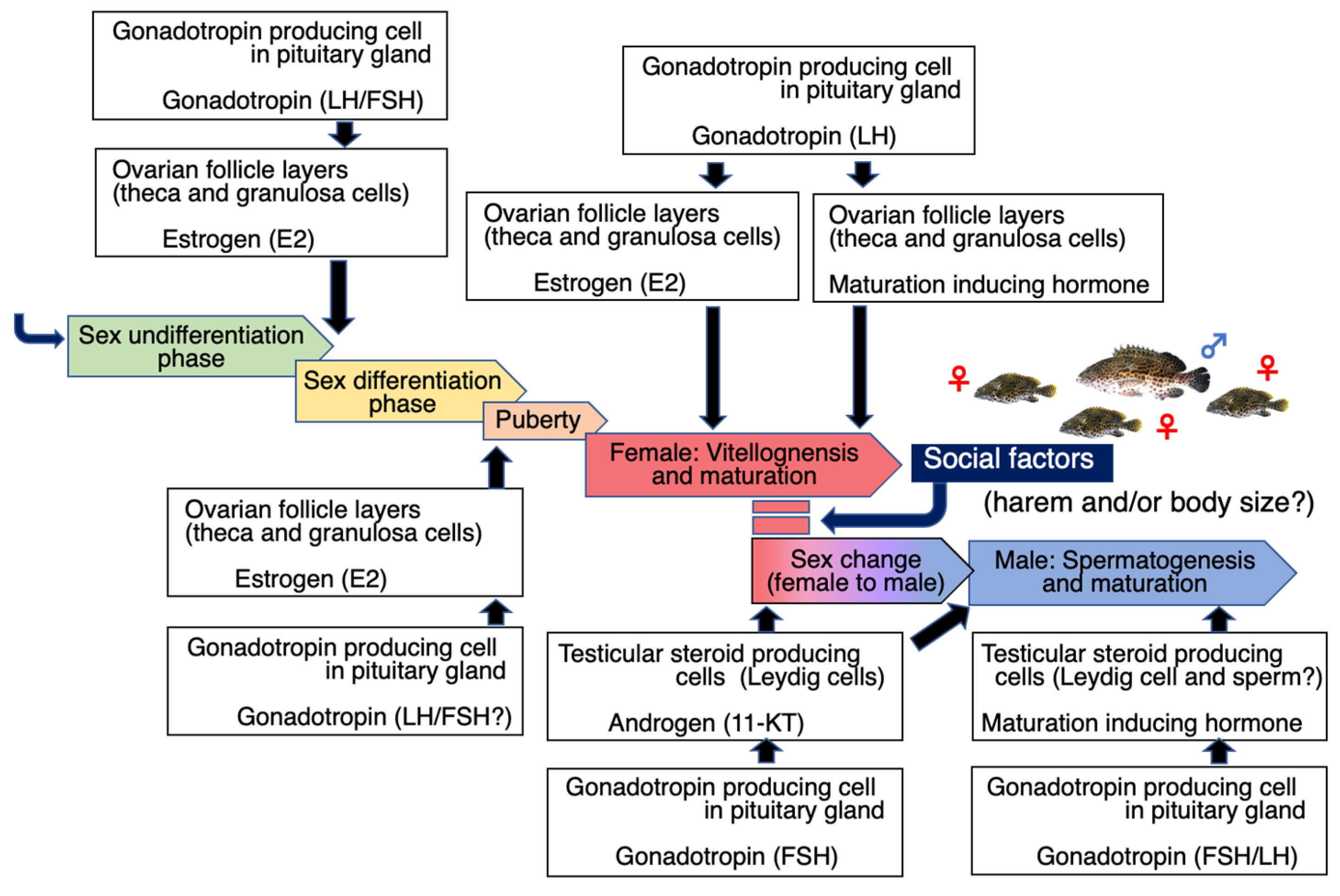
2. First Maturity (Puberty)
2.1. Mechanism of Maturation in Grouper
2.2. Vitellogenesis
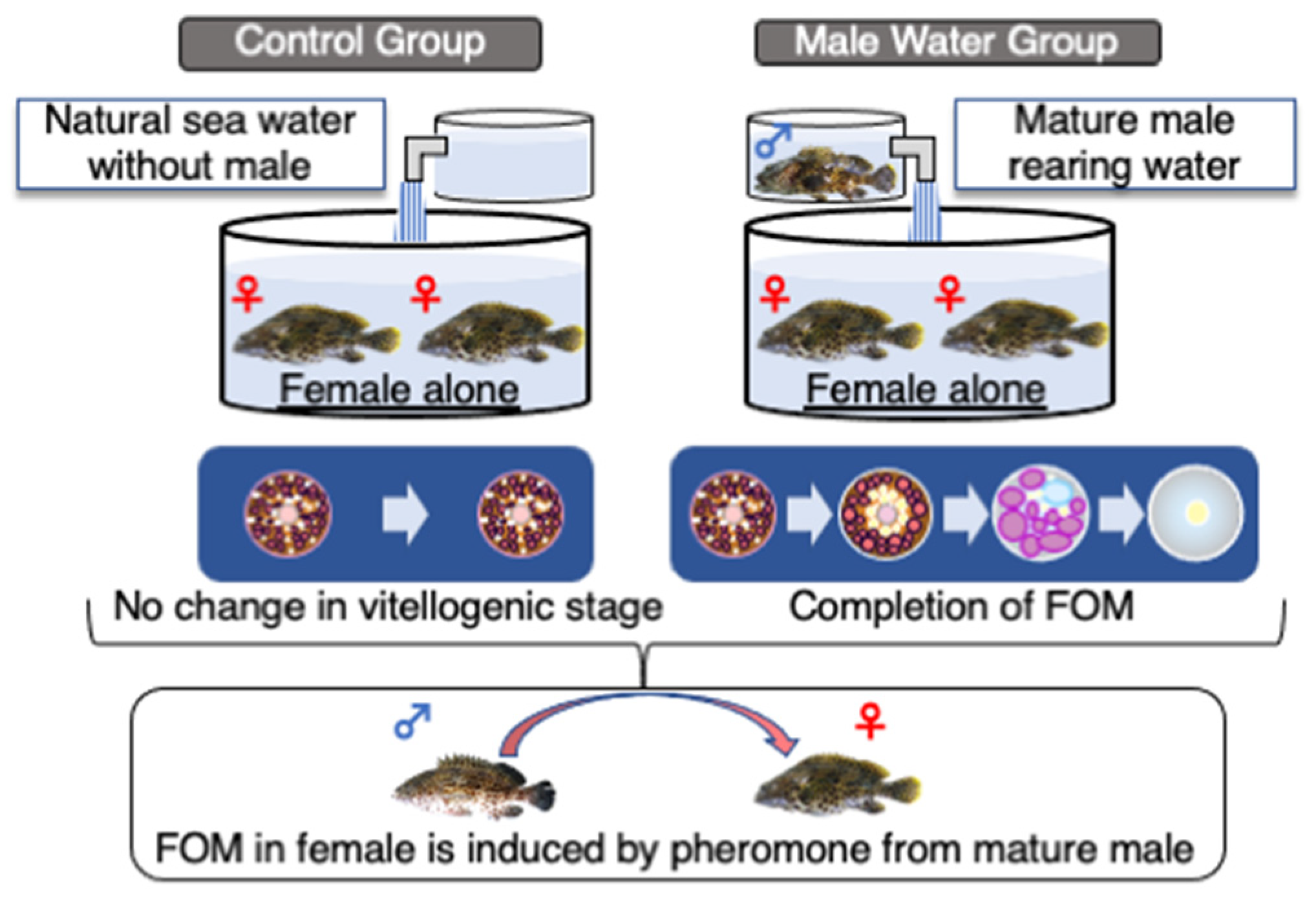
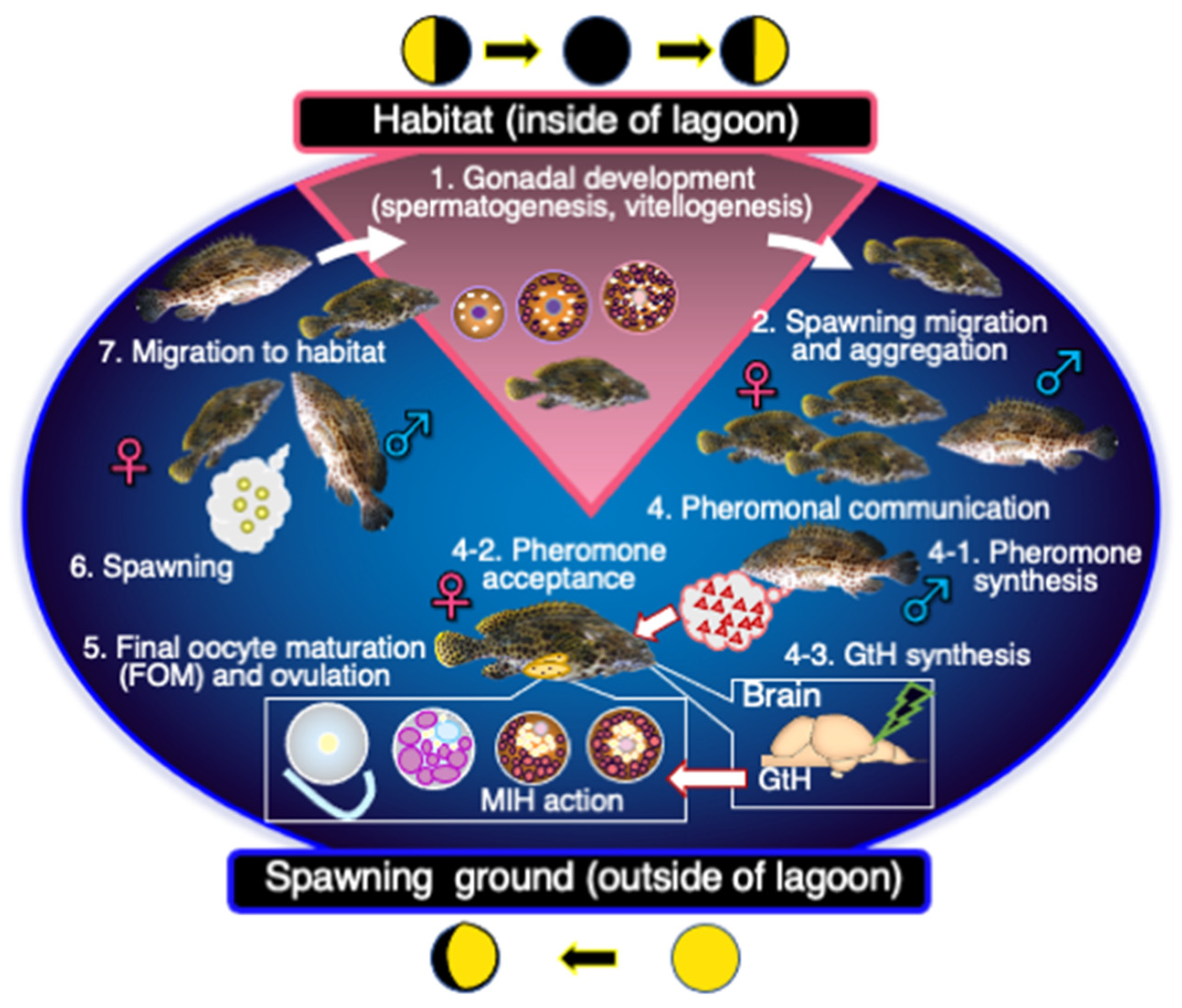
3. Final Maturation and Spawning
4. Sex Change and Males
4.1. Process of Sex Change
4.2. Male Maturation
5. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Smith, C.L. The Patterns of Sexually and the Classification of Serranid Fishes. Amer. Mus. Novit. 1965, 2207, 1–20. [Google Scholar]
- Liao, I.C.; Leaño, E.M. The Aquaculture of Groupers; Asian Fisheries Society, National Taiwan Ocean University: Taiwan, China, 2008. [Google Scholar]
- Jawad, L.A. Groupers of the World: A Field and Market Guide. Mar. Biol. Res. 2012, 8, 912–913. [Google Scholar] [CrossRef]
- Ni Lar, S.; Chuda, H.; Arakawa, T.; Mizuno, K.; Soyano, K. Ovarian Development and Final Oocyte Maturation in Cultured Sevenband Grouper Epinephelus Septemfasciatus. Fish. Sci. 2004, 70, 360–365. [Google Scholar] [CrossRef]
- Oh, S.-R.; Kang, H.-C.; Lee, C.-H.; Hur, S.-W.; Lee, Y.-D. Sex Reversal and Masculinization According to Growth in Longtooth Grouper Epinephelus Bruneus. Dev. Reprod. 2013, 17, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Okumura, S. Variation in Egg Size Spawned under Different Water Temperatures in the Red Spotted Grouper, Epinephelus Akaara. Aquacult. Sci. 2003, 51, 231–232. [Google Scholar] [CrossRef]
- Lee, Y.D.; Park, S.H.; Takemura, A.; Takano, K. Histological Observations of Seasonal Reproductive and Lunarrelated Spawning Cycles in the Female Honeycomb Grouper Epinephelus Merra in Okinawan Waters. Fish. Sci. 2002, 68, 872–877. [Google Scholar] [CrossRef]
- Soyano, K.; Masumoto, T.; Tanaka, H.; Takushima, M.; Nakamura, M. Lunar-Related Spawning in Honeycomb Grouper, Epinephelus Merra. Fish Physiol. Biochem. 2003, 28, 218. [Google Scholar] [CrossRef]
- Kawabe, K.; Kohno, H. Morphological Development of Larval and Juvenile Blacktip Grouper, Epinephelus Fasciatus. Fish. Sci. 2009, 75, 1239–1251. [Google Scholar] [CrossRef]
- Murata, R.; Karimata, H.; Alam, M.A.; Nakamura, M. Gonadal Sex Differentiation in the Malabar Grouper, Epinephelus Malabaricus. Aquaculture 2009, 293, 286–289. [Google Scholar] [CrossRef]
- Kobayashi, Y.; Nagahama, Y.; Nakamura, M. Diversity and Plasticity of Sex Determination and Differentiation in Fishes. Sex. Dev. 2013, 7, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Nanami, A.; Kawabata, Y.; Sato, T.; Yamaguchi, T.; Kawabe, R.; Soyano, K. Spawning Migration and Returning Behavior of White-Streaked Grouper Epinephelus Ongus Determined by Acoustic Telemetry. Mar. Biol. 2014, 161, 669–680. [Google Scholar] [CrossRef]
- Okuzawa, K. Puberty in Teleosts. Fish Physiol.Biochem. 2002, 26, 31–41. [Google Scholar] [CrossRef]
- Jalabert, B. Particularities of Reproduction and Oogenesis in Teleost Fish Compared to Mammals. Reprod. Nutr. Dev. 2005, 45, 261–279. [Google Scholar] [CrossRef]
- Carrillo, M.; Zanuy, S.; Felip, A.; Bayarri, M.J.; Molés, G.; Gómez, A. Hormonal and Environmental Control of Puberty in Perciform Fish: The Case of Sea Bass. Ann. N. Y. Acad. Sci. 2009, 1163, 49–59. [Google Scholar] [CrossRef] [PubMed]
- Taranger, G.L.; Carrillo, M.; Schulz, R.W.; Fontaine, P.; Zanuy, S.; Felip, A.; Weltzien, F.A.; Dufour, S.; Karlsen, Ø.; Norberg, B.; et al. Control of Puberty in Farmed Fish. Gen. Comp. Endocrinol. 2010, 165, 483–515. [Google Scholar] [CrossRef]
- Oh, S.B.; Lee, C.H.; Lee, Y.D. Induction of Puberty in Red Spotted Grouper, Epinephelus Akaara By Water Temperature. J. Aquacult. Res. Dev. 2018, 9, 1–7. [Google Scholar] [CrossRef]
- Ryu, Y.-W.; Hur, S.-W.; Hur, S.-P.; Lee, C.-H.; Lim, B.-S.; Lee, Y.-D. Characterization of Pubertal Development Phases in Female Longtooth Grouper, Epinephelus Bruneus via Classification of Bodyweight. Dev. Reprod. 2013, 17, 55–62. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Xu, W.-G.; Manabe, S.; Mushirobira, Y.; Nagae, M.; Soyano, K. Changes in Expression of Reproduction-Related Hormones in the Brain and Pituitary during Early Ovarian Differentiation and Development in the Red Spotted Grouper Epinephelus Akaara, with Emphasis on FSHβ and LHβ. Aquaculture 2020, 514, 734497. [Google Scholar] [CrossRef]
- Lubzens, E.; Young, G.; Bobe, J.; Cerdà, J. Oogenesis in Teleosts: How Fish Eggs Are Formed. Gen. Comp. Endocrinol. 2010, 165, 367–389. [Google Scholar] [CrossRef]
- Pierce, J.G.; Parsons, T.F. Glycoprotein Hormones: Stracture and Function. Ann. Rev. Biochem. 1981, 50, 465–495. [Google Scholar] [CrossRef] [PubMed]
- Hearn, M.T.W.; Gomme, P.T. Molecular Architecture and Biorecognition Processes of the Cystine Knot Protein Superfamily: Part I. The Glycoprotein Hormones. J. Mol. Recognit. 2000, 23, 223–278. [Google Scholar] [CrossRef]
- Hyeon, K.M.; Jeong, H.B.; Lim, B.S.; Hur, S.P.; Lee, Y.D.; Park, J.G.; Kim, S.J. Molecular Cloning of GnRH1 Gene and GTH CDNAs of the Protogynous Longtooth Grouper, Epinephelus Bruneus. Genes Genom. 2010, 32, 583–591. [Google Scholar] [CrossRef]
- Dennis, L.P.; Nocillado, J.; Palma, P.; Amagai, T.; Soyano, K.; Elizur, A. Development of a Giant Grouper Luteinizing Hormone (LH) Enzyme-Linked Immunosorbent Assay (ELISA) and Its Use towards Understanding Sexual Development in Grouper. Gen. Comp. Endocrinol. 2020, 296, 113542. [Google Scholar] [CrossRef] [PubMed]
- Naito, N.; Hyodo, S.; Okumoto, N.; Urano, A.; Nakai, Y. Cell and Tissue Research Oncorhynchus Mykiss, during Ovarian Development. Cell Tissue Res. 1991, 266, 457–467. [Google Scholar] [CrossRef]
- Amano, M.; Aida, K.; Okumoto, N.; Hasegawa, Y. Changes in Salmon GnRH and Chicken GnRH-2 Contents in the Brain and Pituitary and GTH Contents in the Pituitary in Female Masu Salmon, Oncorhynchus Masou, from Hatching through Ovulation. Zool. Sci. 1992, 9, 375–386. [Google Scholar]
- Gomez, J.M.; Weil, C.; Ollitrault, M.; le Bail, P.-Y.; Breton, B.; le Gac, F. Growth Hormone (GH) and Gonadotropin Subunit Gene Expression and Pituitary and Plasma Changes during Spermatogenesis and Oogenesis in Rainbow Trout (Oncorhynchus Mykiss). Gen. Comp. Endocrinol. 1999, 113, 413–428. [Google Scholar] [CrossRef]
- Ando, H.; Urano, A. Molecular Regulation of Gonadotropin Secretion by Gonadotropin-Releasing Hormone in Salmonid Fishes. Zool. Sci. 2005, 22, 379–389. [Google Scholar] [CrossRef][Green Version]
- Nagahama, Y. Endocrine Regulation of Gametogenesis in Fish. Int. J. Dev. BioI. 1994, 38, 217–229. [Google Scholar]
- Kobayashi, Y.; Alam, M.A.; Horiguchi, R.; Shimizu, A.; Nakamura, M. Sexually Dimorphic Expression of Gonadotropin Subunits in the Pituitary of Protogynous Honeycomb Grouper (Epinephelus Merra): Evidence That Follicle-Stimulating Hormone (FSH) Induces Gonadal Sex Change. Biol. Reprod. 2010, 82, 1030–1036. [Google Scholar] [CrossRef]
- Hara, A.; Hiramatsu, N.; Fujita, T. Vitellogenesis and Choriogenesis in Fishes. Fish. Sci. 2016, 82, 187–202. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Hara, A.; Hiramatsu, K.; Fukada, H.; Weber, G.M.; Denslow, N.D.; Sullivan, C.V. Vitellogenin-Derived Yolk Proteins of White Perch, Morone Americana: Purification, Characterization, and Vitellogenin-Receptor Binding 1. Biol. Reprod. 2002, 67, 655–667. [Google Scholar] [CrossRef] [PubMed]
- Hwang, I.J.; Baek, H.J. In Vitro Sex Steroid Metabolism in Red Spotted Grouper, Epinephelus Akaara during Oocyte Maturation. Dev. Reprod. 2021, 25, 75–82. [Google Scholar] [CrossRef] [PubMed]
- Johnson, K.; Thomas, P.; Wilson, R.R., Jr. Seasonal Cycles of Gonadal Development and Plasma Sex Steroid Levels in Epinephelus Morio, a Protogynous Grouper in the Eastern Gulf of Mexico. J. Fish Biol. 1998, 52, 502–518. [Google Scholar]
- Maldonado-García, M.; Gracia-López, V.; Kiewek-Martínez, M.; Carrillo, M.; Zanuy, S. Reproductive Cycle of Leopard Grouper Mycteroperca Rosacea (Streets, 1877) Held in Captivity: Relationship between Gonad Development and Sex Steroid Concentration. Lat. Am. J. Aquat. Res. 2018, 46, 83–90. [Google Scholar] [CrossRef]
- Palma, P.; Takemura, A.; Libunao, G.X.; Superio, J.; de Jesus-Ayson, E.G.; Ayson, F.; Nocillado, J.; Dennis, L.; Chan, J.; Thai, T.Q.; et al. Reproductive Development of the Threatened Giant Grouper Epinephelus Lanceolatus. Aquaculture 2019, 509, 1–7. [Google Scholar] [CrossRef]
- Mandich, A.; Massari, A.; Bottero, S.; Marino, G. Histological and Histochemical Study of Female Germ Cell Development in the Dusky Grouper Epinephelus Marginatus (Lowe, 1834). Eur. J. Histochem. 2002, 46, 87–100. [Google Scholar] [CrossRef]
- Matsubara, T.; Ohkubo, N.; Andoh, T.; Sullivan, C.V.; Hara, A. Two Forms of Vitellogenin, Yielding Two Distinct Lipovitellins, Play Different Roles during Oocyte Maturation and Early Development of Barfin Flounder, Verasper Moseri, a Marine Teleost That Spawns Pelagic Eggs. Dev. Biol. 1999, 213, 18–32. [Google Scholar] [CrossRef] [PubMed]
- Matsubara, T.; Nagae, M.; Ohkubo, N.; Andoh, T.; Sawaguchi, S.; Hiramatsu, N.; Sullivan, C.V.; Hara, A. Multiple Vitellogenins and Their Unique Roles in Marine Teleosts. Fish Physiol. Biochem. 2003, 28, 295–299. [Google Scholar] [CrossRef]
- Hiramatsu, N.; Todo, T.; Sullivan, C.V.; Schilling, J.; Reading, B.J.; Matsubara, T.; Ryu, Y.W.; Mizuta, H.; Luo, W.; Nishimiya, O.; et al. Ovarian Yolk Formation in Fishes: Molecular Mechanisms Underlying Formation of Lipid Droplets and Vitellogenin-Derived Yolk Proteins. Gen. Comp. Endocrinol. 2015, 221, 9–15. [Google Scholar] [CrossRef]
- Om, A.D.; Sharif, S.; Jasmani, S.; Sung, Y.Y.; Bolong, A.A. Molecular Characteristic of Giant Grouper (Epinephelus Lanceolatus)Vitellogenin. J. Aquac. Res. Dev. 2015, 6, 6. [Google Scholar] [CrossRef]
- Tsuchihashi, Y.; Takatori, Y.; Kuriyama, I.; Hanyuu, K.; Tsuji, M.; Tsumoto, K. Induced Spawning of Cultured Sevenband Grouper, Epinephelus Septemfasciatus, in September by Manipulation of Water Temperature and Photoperiod (in Japanese with English Abstract). Aquacult. Sci. 2007, 55, 395–402. [Google Scholar] [CrossRef]
- Lee, C.-H.; Hur, S.-W.; Kim, B.-H.; Soyano, K.; Lee, Y.-D. Induced Maturation and Fertilized Egg Production of the Red-Spotted Grouper, Epinephelus Akaara, Using Adaptive Physiology of Photoperiod and Water Temperature. Aquacult. Res. 2020, 51, 2084–2090. [Google Scholar] [CrossRef]
- Kawabe, K.; Kato, K.; Kimura, J. Year Round Spawning of Reared Blacktip Grouper, Epinephelus Fasciatus, in Chichi-Jima, Ogasawara Islands, Southern Japan (in Japanese with English Abstract). Suisanzoshoku Aquacult. Sci. 2000, 48, 467–473. [Google Scholar] [CrossRef]
- Kanemaru, T.; Nakamura, M.; Murata, R.; Kuroki, K.; Horie, H.; Uchida, K.; Senthilkumaran, B.; Kagawa, H. Induction of Sexual Maturation of the Female Honeycomb Grouper, Epinephelus Merra, in the Non-Breeding Season by Modulating Environmental Factors with GnRH Analogue Implantation. Aquaculture 2012, 358–359, 85–91. [Google Scholar] [CrossRef]
- Nagahama, Y.; Yamashita, M. Regulation of Oocyte Maturation in Fish. Dev. Growth Differ. 2008, 50, 1019. [Google Scholar] [CrossRef]
- Kagawa, H.; Tanaka, H.; Okuzawa, K.; Hirose, K. Development of Maturational Competence of Oocytes of Red Seabream, Pagrus Major, after Human Chorionic Gonadotropin Treatment in Vitro Requires RNA and Protein Synthesis. Gen. Comp. Endocrinol. 1994, 94, 199–206. [Google Scholar] [CrossRef]
- Yoshizaki, G.; Shusa, M.; Takeuchi, T.; Patiño, R. Gonadotropin-Dependent Oocyte Maturational Competence Requires Activation of the Protein Kinase A Pathway and Synthesis of RNA and Protein in Ovarian Follicles of Nibe, Nibea Mitsukurii (Teleostei, Sciaenidae). Fish Physiol. Biochem. 2001, 25, 201–208. [Google Scholar] [CrossRef]
- Teruya, K.; Masuma, S.; Hondo, Y.; Hamasaki, K. Spawning Season, Lunar-Related Spawning and Mating Systems in the Camouflage Grouper Epinephelus Polyphekadion at Ishigaki Island, Japan. Aquacult. Sci. 2008, 56, 359–368. [Google Scholar]
- Okumura, S.; Okamoto, K.; Oomori, R.; Nakazono, A. Spawning Behavior and Artificial Fertilization in Captive Reared Red Spotted Grouper, Epinephelus Akaara. Aquaculture 2002, 206, 165–173. [Google Scholar] [CrossRef]
- Amagai, T.; Izumida, D.; Murata, R.; Soyano, K. Male Pheromones Induce Ovulation in Female Honeycomb Groupers (Epinephelus Merra): A Comprehensive Study of Spawning Aggregation Behavior and Ovarian Development. Cells 2022, 11, 484. [Google Scholar] [CrossRef]
- Peter, R.E.; Yu, K.L. Neuroendocrine Regulation of Ovulation in Fishes: Basic and Applied Aspects. Rev. Fish Biol. Fish. 1997, 7, 173–197. [Google Scholar] [CrossRef]
- Chen, L.-C.; Martinich’, R.L. Pheromonal Stimulation and Metabolite Inhibition of Ovulation in the Zebrafish, Brachydanio Rerio. Fish. Bull. 1975, 73, 889–894. [Google Scholar]
- van den Hurk, R.; Schoonen, W.G.E.J.; van Zoelen, G.A.; Lambert, J.G.D. The Biosynthesis of Steroid Glucuronides in the Testis of the Zebrafish, Bmchydanio Rerio, and Their Pheromonal Function as Ovulation Inducers. Gen. Comp. Endocrinol. 1987, 68, 179–188. [Google Scholar] [CrossRef]
- Resink, J.W.; van den Berg, T.W.M.; van den Hurk, R.; Huisman, E.A.; van Oordt, P.G.W.J. Induction of Gonadotropin Release and Ovulation by Pheromones in the African Catfish, Clarias Gariepinus. Aquaculture 1989, 83, 167–177. [Google Scholar] [CrossRef]
- Huertas, M.; Almeida, O.G.; Canário, A.V.M.; Hubbard, P.C. Tilapia Male Urinary Pheromone Stimulates Female Reproductive Axis. Gen. Comp. Endocrinol. 2014, 196, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Scott, A.P.; Liley, N.R.; Vermeirssen, E.L.M. Urine of Reproductively Mature Female Rainbow Trout Oncorhynchus Mykiss. J. Fish Biol. 1994, 44, 131–147. [Google Scholar] [CrossRef]
- Chung-Davidson, Y.W.; Wang, H.; Siefkes, M.J.; Bryan, M.B.; Wu, H.; Johnson, N.S.; Li, W. Pheromonal Bile Acid 3-Ketopetromyzonol Sulfate Primes the Neuroendocrine System in Sea Lamprey. BMC Neurosci. 2013, 14, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Supii, A.I.; Arifati, D.; Widodo, M.S.; Kilawati, Y. Analysis of Male Urine as Pheromone to Increase Reproduction in Female Tiger Grouper (Epinephelus Fuscoquttatus). IOP Conf. Ser.: Mater. Sci. Eng. 2019, 515, 012044. [Google Scholar] [CrossRef]
- Meiri, I.; Gothilf, Y.; Zohar, Y.; Elizur, A. Physiological Changes in the Spawning Gilthead Seabream, Sparus Aurata, Succeeding the Removal of Males. J. Exp. Zool. 2002, 292, 555–564. [Google Scholar] [CrossRef]
- Soyano, K.; Sakakura, Y.; Hagiwara, A. Reproduction and Larviculture of Seven-Band Grouper, Epinephelus Septemfasciatus, in Japan. In The Aquaculture of Groupers; Liao, I.C., Leaño, E.M., Eds.; Asian Fisheries Society, National Taiwan Ocean University: Taiwan, China, 2008; pp. 1–27. [Google Scholar]
- Nakamura, M.; Kobayashi, Y.; Miura, S.; Alam, M.A.; Bhandari, R.K. Sex Change in Coral Reef Fish. Fish Physiol. Biochem. 2005, 31, 117–122. [Google Scholar] [CrossRef]
- Alam, M.A.; Nakamura, M. Determination of Sex and Gonadal Maturity in the Honeycomb Grouper, Epinephelus Merra, through Biopsy. Aquacult. Int. 2008, 16, 27–32. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Komuro, H.; Nakamura, S.; Higa, M.; Nakamura, M. Gonadal Restructuring and Correlative Steroid Hormone Profiles during Natural Sex Change in Protogynous Honeycomb Grouper (Epinephelus Merra). Zool. Sci. 2003, 20, 1399–1404. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, R.K.; Alam, M.A.; Higa, M.; Soyano, K.; Nakamura, M. Evidence That Estrogen Regulates the Sex Change of Honeycomb Grouper (Epinephelus Merra), a Protogynous Hermaphrodite Fish. J. Exp. Zool. Part A Comp. Exp. Biol. 2005, 303, 497–503. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Chen, H.; Peng, C.; Ye, Z.; Zhao, M.; Xiao, L.; Zhang, H.; Li, S.; Lin, H.; Zhang, Y. A Highly Efficient Method of Inducing Sex Change Using Social Control in the Protogynous Orange-Spotted Grouper (Epinephelus Coioides). Aquaculture 2020, 517, 734787. [Google Scholar] [CrossRef]
- Nakamura, M.; Hourigan, T.F.; Yamauchi, K.; Nagahama, Y.; Grau, E.G. Histological and Ultrastructural Evidence for the Role of Gonadal Steroid Hormones in Sex Change in the Protogynous Wrasse Thalassoma Duperrey. Environ. Biol. Fish. 1989, 24, 117–136. [Google Scholar] [CrossRef]
- Nozu, R.; Kojima, Y.; Nakamura, M. Short Term Treatment with Aromatase Inhibitor Induces Sex Change in the Protogynous Wrasse, Halichoeres Trimaculatus. Gen. Comp. Endocrinol. 2009, 161, 360–364. [Google Scholar] [CrossRef] [PubMed]
- Kroon, F.J.; Liley, N.R. The Role of Steroid Hormones in Protogynous Sex Change in the Blackeye Goby Coryphopterus Nicholsii (Teleostei: Gobiidae). Gen. Comp. Endocrinol. 2000, 118, 273–283. [Google Scholar] [CrossRef]
- Ochi, H. Mating Behavior and Sex Change of the Anemonefish, Amphiprion Clarkii, in the Temperate Waters of Southern Japan. Environ. Biol. Fish. 1989, 26, 257–275. [Google Scholar] [CrossRef]
- Nakamura, M.; Miura, S.; Nozu, R.; Kobayashi, Y. Opposite-Directional Sex Change in Functional Female Protandrous Anemonefish, Amphiprion Clarkii: Effect of Aromatase Inhibitor on the Ovarian Tissue. Zool. Lett. 2015, 1, 27. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kobayashi, Y.; Nakamura, M.; Sunobe, T.; Usami, T.; Kobayashi, T.; Manabe, H.; Paul-Prasanth, B.; Suzuki, N.; Nagahama, Y. Sex Change in the Gobiid Fish Is Mediated through Rapid Switching of Gonadotropin Receptors from Ovarian to Testicular Portion or Vice Versa. Endocrinology 2009, 150, 1503–1511. [Google Scholar] [CrossRef]
- Sprenger, D.; Dingemanse, N.J.; Dochtermann, N.A.; Theobald, J.; Walker, S.P.W. Aggressive Females Become Aggressive Males in a Sex-Changing Reef Fish. Ecol. Lett. 2012, 15, 986–992. [Google Scholar] [CrossRef]
- Solomon-Lane, T.K.; Crespi, E.J.; Grober, M.S. Stress and Serial Adult Metamorphosis: Multiple Roles for the Stress Axis in Socially Regulated Sex Change. Front. Neurosci. 2013, 7, 210. [Google Scholar] [CrossRef] [PubMed]
- Mackie, M.C. Socially Controlled Sex-Change in the Half-Moon Grouper, Epinephelus Rivulatus, at Ningaloo Reef, Western Australia. Coral Reefs 2003, 22, 133–142. [Google Scholar] [CrossRef]
- Chen, J.; Xiao, L.; Peng, C.; Ye, Z.; Wang, D.; Yang, Y.; Zhang, H.; Zhao, M.; Li, S.; Lin, H.; et al. Socially Controlled Male-to-Female Sex Reversal in the Protogynous Orange-Spotted Grouper, Epinephelus Coioides. J. Fish Biol. 2019, 94, 414–421. [Google Scholar] [CrossRef]
- Abu-Hakima, R. Aspects of the Reproductive Biology of the Grouper, Epinephelus Tauvina (Forskil), in Kuwaiti Waters. J. Fish Biol. 1987, 30, 213–222. [Google Scholar] [CrossRef]
- Alam, M.A.; Bhandari, R.K.; Kobayashi, Y.; Nakamura, S.; Soyano, K.; Nakamura, M. Changes in Androgen-Producing Cell Size and Circulating 11-Ketotestosterone Level during Female-Male Sex Change of Honeycomb Grouper Epinephelus Merra. Mol. Reprod. Dev. 2006, 73, 206–214. [Google Scholar] [CrossRef]
- Alam, M.A.; Komuro, H.; Bhandari, R.K.; Nakamura, S.; Soyano, K.; Nakamura, M. Immunohistochemical Evidence Identifying the Site of Androgen Production in the Ovary of the Protogynous Grouper Epinephelus Merra. Cell Tissue Res. 2005, 320, 323–329. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.; Nozu, R.; Mushirobira, Y.; Amagai, T.; Fushimi, J.; Kobayashi, Y.; Soyano, K.; Nagahama, Y.; Nakamura, M. Testicular Inducing Steroidogenic Cells Trigger Sex Change in Groupers. Sci. Rep. 2021, 11, 11117. [Google Scholar] [CrossRef]
- Alam, M.A.; Kobayashi, Y.; Hirai, T.; Nakamura, M. Isolation, Characterization and Expression Analyses of FSH Receptor in Protogynous Grouper. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 2010, 156, 364–371. [Google Scholar] [CrossRef]
- Glamuzina, B.; Glavic, N.; Skaramuca, B.; Kozul, V. Induced Sex Reversal of Dusky Grouper Epinephelus Marginatus (Lowe). Aquacult. Res. 1998, 29, 563–567. [Google Scholar] [CrossRef]
- Zhou, L.; Gui, J.F. Molecular Mechanisms Underlying Sex Change in Hermaphroditic Groupers. Fish Physiol. Biochem. 2010, 36, 181–193. [Google Scholar] [CrossRef] [PubMed]
- Todd, E.V.; Liu, H.; Muncaster, S.; Gemmell, N.J. Bending Genders: The Biology of Natural Sex Change in Fish. Sex. Dev. 2016, 10, 223–241. [Google Scholar] [CrossRef]
- Biran, J.; Levavi-Sivan, B. Endocrine Control of Reproduction, Fish. In Encyclopedia of Reproduction, 2nd ed.; Skinner, M.K., Ed.; Academic Press: Cambridge, MA, USA, 2018; Volume 6, pp. 362–368. [Google Scholar]
- Murata, R.; Karimata, H.; Kobayashi, Y.; Horiguchi, R.; Kishimoto, K.; Kimura, M.; Kobayashi, T.; Soyano, K.; Nakamura, M. Differentiation of Steroid-Producing Cells during Ovarian Differentiation in the Protogynous Malabar Grouper, Epinephelus Malabaricus. Int. J. Dev. Biol. 2011, 55, 619–625. [Google Scholar] [CrossRef]
- Pandian, T.J.; Sheela, S.G. Aquaculture Hormonal Induction of Sex Reversal in Fish. Aquaculture 1995, 138, 1–22. [Google Scholar] [CrossRef]
- Murata, R.; Karimata, H.; Alam, M.A.; Nakamura, M. Precocious Sex Change and Spermatogenesis in the Underyearling Malabar Grouper Epinephelus Malabaricus by Androgen Treatment. Aquacult. Res. 2010, 41, 303–308. [Google Scholar] [CrossRef]
- Bhandari, R.K.; Alam, M.A.; Soyano, K.; Nakamura, M. Induction of Female-to-Male Sex Change in the Honeycomb Grouper (Epinephelus Merra) by 11-Ketotestosterone Treatments. Zool. Sci. 2006, 23, 65–69. [Google Scholar] [CrossRef] [PubMed]
- Murata, R.; Kobayashi, Y.; Karimata, H.; Kishimoto, K.; Kimura, M.; Nakamura, M. Transient Sex Change in the Immature Malabar Grouper, Epinephelus Malabaricus, Androgen Treatment. Biol. Reprod. 2014, 91, 1–7. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, M.; Peng, C.; Wang, X.; Xiao, L.; Wang, D.; Chen, J.; Zhao, H.; Zhang, H.; Li, S.; et al. MT-Feeding-Induced Impermanent Sex Reversal in the Orange-Spotted Grouper during Sex Differentiation. Int. J. Mol. Sci. 2018, 19, 2828. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soyano, K.; Amagai, T.; Yamaguchi, T.; Mushirobira, Y.; Xu, W.-G.; Phạm, N.T.; Murata, R. Endocrine Regulation of Maturation and Sex Change in Groupers. Cells 2022, 11, 825. https://doi.org/10.3390/cells11050825
Soyano K, Amagai T, Yamaguchi T, Mushirobira Y, Xu W-G, Phạm NT, Murata R. Endocrine Regulation of Maturation and Sex Change in Groupers. Cells. 2022; 11(5):825. https://doi.org/10.3390/cells11050825
Chicago/Turabian StyleSoyano, Kiyoshi, Takafumi Amagai, Tomofumi Yamaguchi, Yuji Mushirobira, Wen-Gang Xu, Nhan Thành Phạm, and Ryosuke Murata. 2022. "Endocrine Regulation of Maturation and Sex Change in Groupers" Cells 11, no. 5: 825. https://doi.org/10.3390/cells11050825
APA StyleSoyano, K., Amagai, T., Yamaguchi, T., Mushirobira, Y., Xu, W.-G., Phạm, N. T., & Murata, R. (2022). Endocrine Regulation of Maturation and Sex Change in Groupers. Cells, 11(5), 825. https://doi.org/10.3390/cells11050825





