Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation
Abstract
1. Introduction
2. Current Challenges in Lung Transplantation
2.1. Donor Shortage and Expansion of Donor Pool
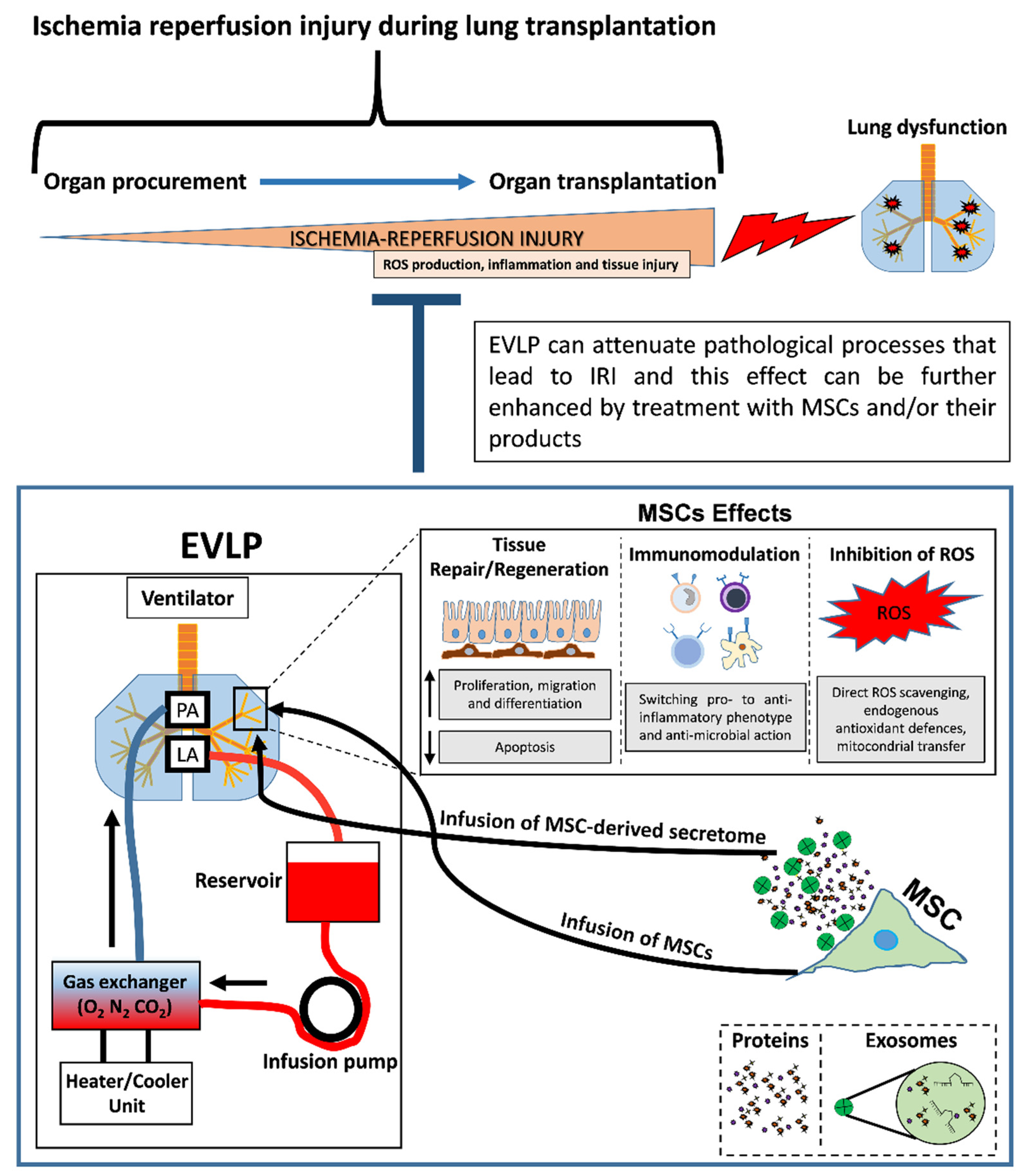
2.2. Lung Preservation by Ex Vivo Lung Perfusion (EVLP)
3. Mesenchymal Stromal/Stem Cells (MSCs) and Their Therapeutic Effects
3.1. Biological Role of MSCs
3.2. Therapeutic Properties of MSCs
4. Mesenchymal Stromal/Stem Cell (MSC)-based Therapeutic Approaches to Improving Lung Transplantation
4.1. Therapeutic Effects of MSCs on Ischemia-Reperfusion Injury (IRI)
4.2. MSCs as Therapeutic Tool to Improve EVLP
5. Conclusions and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- van der Mark, S.C.; Hoek, R.A.S.; Hellemons, M.E. Developments in lung transplantation over the past decade. Eur. Respir Rev. 2020, 29, 190132. [Google Scholar] [CrossRef] [PubMed]
- Forgie, K.A.; Fialka, N.; Freed, D.H.; Nagendran, J. Lung Transplantation, Pulmonary Endothelial Inflammation, and Ex-Situ Lung Perfusion: A Review. Cells 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Keshavamurthy, S.; Rodgers-Fischl, P. Donation after circulatory death (DCD)-lung procurement. Indian. J. Thorac. Cardiovasc. Surg. 2021, 37, 425–432. [Google Scholar] [CrossRef] [PubMed]
- Opelz, G.; Dohler, B.; Ruhenstroth, A.; Cinca, S.; Unterrainer, C.; Stricker, L.; Scherer, S.; Gombos, P.; Susal, C.; Daniel, V.; et al. The collaborative transplant study registry. Transpl. Rev. (Orlando). 2013, 27, 43–45. [Google Scholar] [CrossRef]
- Shepherd, H.M.; Gauthier, J.M.; Puri, V.; Kreisel, D.; Nava, R.G. Advanced considerations in organ donors. J. Thorac Dis. 2021, 13, 6528–6535. [Google Scholar] [CrossRef]
- Thabut, G.; Mal, H. Outcomes after lung transplantation. J. Thorac Dis. 2017, 9, 2684–2691. [Google Scholar] [CrossRef]
- Gagliotti, C.; Morsillo, F.; Moro, M.L.; Masiero, L.; Procaccio, F.; Vespasiano, F.; Pantosti, A.; Monaco, M.; Errico, G.; Ricci, A.; et al. Infections in liver and lung transplant recipients: A national prospective cohort. Eur. J. Clin. Microbiol. Infect. Dis. 2018, 37, 399–407. [Google Scholar] [CrossRef]
- Sun, H.; Deng, M.; Chen, W.; Liu, M.; Dai, H.; Wang, C. Graft dysfunction and rejection of lung transplant, a review on diagnosis and management. Clin. Respir. J. 2022, 16, 5–12. [Google Scholar] [CrossRef]
- Bozso, S.; Vasanthan, V.; Luc, J.G.; Kinaschuk, K.; Freed, D.; Nagendran, J. Lung transplantation from donors after circulatory death using portable ex vivo lung perfusion. Can. Respir. J. 2015, 22, 47–51. [Google Scholar] [CrossRef][Green Version]
- Laubach, V.E.; Sharma, A.K. Mechanisms of lung ischemia-reperfusion injury. Curr. Opin. Organ. Transplant. 2016, 21, 246–252. [Google Scholar] [CrossRef]
- de Perrot, M.; Liu, M.; Waddell, T.K.; Keshavjee, S. Ischemia-reperfusion-induced lung injury. Am. J. Respir. Crit. Care Med. 2003, 167, 490–511. [Google Scholar] [CrossRef]
- Kawashima, M.; Juvet, S.C. The role of innate immunity in the long-term outcome of lung transplantation. Ann. Transl. Med. 2020, 8, 412. [Google Scholar] [CrossRef] [PubMed]
- Charles, E.J.; Huerter, M.E.; Wagner, C.E.; Sharma, A.K.; Zhao, Y.; Stoler, M.H.; Mehaffey, J.H.; Isbell, J.M.; Lau, C.L.; Tribble, C.G.; et al. Donation After Circulatory Death Lungs Transplantable Up to Six Hours After Ex Vivo Lung Perfusion. Ann. Thorac. Surg. 2016, 102, 1845–1853. [Google Scholar] [CrossRef] [PubMed]
- Cypel, M.; Yeung, J.C.; Machuca, T.; Chen, M.; Singer, L.G.; Yasufuku, K.; de Perrot, M.; Pierre, A.; Waddell, T.K.; Keshavjee, S. Experience with the first 50 ex vivo lung perfusions in clinical transplantation. J. Thorac. Cardiovasc. Surg. 2012, 144, 1200–1206. [Google Scholar] [CrossRef]
- Huerter, M.E.; Sharma, A.K.; Zhao, Y.; Charles, E.J.; Kron, I.L.; Laubach, V.E. Attenuation of Pulmonary Ischemia-Reperfusion Injury by Adenosine A2B Receptor Antagonism. Ann. Thorac. Surg. 2016, 102, 385–393. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Martens, A.; Boada, M.; Vanaudenaerde, B.M.; Verleden, S.E.; Vos, R.; Verleden, G.M.; Verbeken, E.K.; Van Raemdonck, D.; Schols, D.; Claes, S.; et al. Steroids can reduce warm ischemic reperfusion injury in a porcine donation after circulatory death model with ex vivo lung perfusion evaluation. Transpl. Int. 2016, 29, 1237–1246. [Google Scholar] [CrossRef]
- Nakajima, D.; Date, H. Ex vivo lung perfusion in lung transplantation. Gen. Thorac. Cardiovasc. Surg. 2021, 69, 625–630. [Google Scholar] [CrossRef] [PubMed]
- Gallo, A.; Cuscino, N.; Contino, F.; Bulati, M.; Pampalone, M.; Amico, G.; Zito, G.; Carcione, C.; Centi, C.; Bertani, A.; et al. Changes in the Transcriptome Profiles of Human Amnion-Derived Mesenchymal Stromal/Stem Cells Induced by Three-Dimensional Culture: A Potential Priming Strategy to Improve Their Properties. Int. J. Mol. Sci. 2022, 23, 863. [Google Scholar] [CrossRef]
- Konala, V.B.; Mamidi, M.K.; Bhonde, R.; Das, A.K.; Pochampally, R.; Pal, R. The current landscape of the mesenchymal stromal cell secretome: A new paradigm for cell-free regeneration. Cytotherapy 2016, 18, 13–24. [Google Scholar] [CrossRef]
- Lo Nigro, A.; Gallo, A.; Bulati, M.; Vitale, G.; Paini, D.S.; Pampalone, M.; Galvagno, D.; Conaldi, P.G.; Miceli, V. Amnion-Derived Mesenchymal Stromal/Stem Cell Paracrine Signals Potentiate Human Liver Organoid Differentiation: Translational Implications for Liver Regeneration. Front. Med. (Lausanne) 2021, 8, 746298. [Google Scholar] [CrossRef]
- Miceli, V.; Bulati, M.; Iannolo, G.; Zito, G.; Gallo, A.; Conaldi, P.G. Therapeutic Properties of Mesenchymal Stromal/Stem Cells: The Need of Cell Priming for Cell-Free Therapies in Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 763. [Google Scholar] [CrossRef]
- Jimenez-Puerta, G.J.; Marchal, J.A.; Lopez-Ruiz, E.; Galvez-Martin, P. Role of Mesenchymal Stromal Cells as Therapeutic Agents: Potential Mechanisms of Action and Implications in Their Clinical Use. J. Clin. Med. 2020, 9, 445. [Google Scholar] [CrossRef] [PubMed]
- Madrigal, M.; Rao, K.S.; Riordan, N.H. A review of therapeutic effects of mesenchymal stem cell secretions and induction of secretory modification by different culture methods. J. Transl. Med. 2014, 12, 260. [Google Scholar] [CrossRef]
- Mohammadipoor, A.; Antebi, B.; Batchinsky, A.I.; Cancio, L.C. Therapeutic potential of products derived from mesenchymal stem/stromal cells in pulmonary disease. Respir. Res. 2018, 19, 218. [Google Scholar] [CrossRef]
- Wick, K.D.; Leligdowicz, A.; Zhuo, H.; Ware, L.B.; Matthay, M.A. Mesenchymal stromal cells reduce evidence of lung injury in patients with ARDS. JCI Insight 2021, 6, e148983. [Google Scholar] [CrossRef] [PubMed]
- Abreu, S.C.; Weiss, D.J.; Rocco, P.R. Extracellular vesicles derived from mesenchymal stromal cells: A therapeutic option in respiratory diseases? Stem Cell Res. Ther. 2016, 7, 53. [Google Scholar] [CrossRef] [PubMed]
- Morrison, T.J.; Jackson, M.V.; Cunningham, E.K.; Kissenpfennig, A.; McAuley, D.F.; O’Kane, C.M.; Krasnodembskaya, A.D. Mesenchymal Stromal Cells Modulate Macrophages in Clinically Relevant Lung Injury Models by Extracellular Vesicle Mitochondrial Transfer. Am. J. Respir. Crit. Care Med. 2017, 196, 1275–1286. [Google Scholar] [CrossRef] [PubMed]
- Schmelzer, E.; Miceli, V.; Chinnici, C.M.; Bertani, A.; Gerlach, J.C. Effects of Mesenchymal Stem Cell Coculture on Human Lung Small Airway Epithelial Cells. BioMed Res. Int. 2020, 2020, 9847579. [Google Scholar] [CrossRef]
- Tian, W.; Liu, Y.; Zhang, B.; Dai, X.; Li, G.; Li, X.; Zhang, Z.; Du, C.; Wang, H. Infusion of mesenchymal stem cells protects lung transplants from cold ischemia-reperfusion injury in mice. Lung 2015, 193, 85–95. [Google Scholar] [CrossRef]
- La Francesca, S.; Ting, A.E.; Sakamoto, J.; Rhudy, J.; Bonenfant, N.R.; Borg, Z.D.; Cruz, F.F.; Goodwin, M.; Lehman, N.A.; Taggart, J.M.; et al. Multipotent adult progenitor cells decrease cold ischemic injury in ex vivo perfused human lungs: An initial pilot and feasibility study. Transplant. Res. 2014, 3, 19. [Google Scholar] [CrossRef]
- Lee, J.W.; Fang, X.; Gupta, N.; Serikov, V.; Matthay, M.A. Allogeneic human mesenchymal stem cells for treatment of E. coli endotoxin-induced acute lung injury in the ex vivo perfused human lung. Proc. Natl. Acad. Sci. USA 2009, 106, 16357–16362. [Google Scholar] [CrossRef] [PubMed]
- McAuley, D.F.; Curley, G.F.; Hamid, U.I.; Laffey, J.G.; Abbott, J.; McKenna, D.H.; Fang, X.; Matthay, M.A.; Lee, J.W. Clinical grade allogeneic human mesenchymal stem cells restore alveolar fluid clearance in human lungs rejected for transplantation. Am. J. Physiol. Lung Cell Mol. Physiol. 2014, 306, L809–L815. [Google Scholar] [CrossRef]
- Ullah, M.; Kodam, S.P.; Mu, Q.; Akbar, A. Microbubbles versus Extracellular Vesicles as Therapeutic Cargo for Targeting Drug Delivery. ACS Nano 2021, 15, 3612–3620. [Google Scholar] [CrossRef] [PubMed]
- Lonati, C.; Bassani, G.A.; Brambilla, D.; Leonardi, P.; Carlin, A.; Maggioni, M.; Zanella, A.; Dondossola, D.; Fonsato, V.; Grange, C.; et al. Mesenchymal stem cell-derived extracellular vesicles improve the molecular phenotype of isolated rat lungs during ischemia/reperfusion injury. J. Heart Lung Transplant. 2019, 38, 1306–1316. [Google Scholar] [CrossRef]
- Stone, M.L.; Zhao, Y.; Robert Smith, J.; Weiss, M.L.; Kron, I.L.; Laubach, V.E.; Sharma, A.K. Mesenchymal stromal cell-derived extracellular vesicles attenuate lung ischemia-reperfusion injury and enhance reconditioning of donor lungs after circulatory death. Respir. Res. 2017, 18, 212. [Google Scholar] [CrossRef] [PubMed]
- Varkouhi, A.K.; Jerkic, M.; Ormesher, L.; Gagnon, S.; Goyal, S.; Rabani, R.; Masterson, C.; Spring, C.; Chen, P.Z.; Gu, F.X.; et al. Extracellular Vesicles from Interferon-gamma-primed Human Umbilical Cord Mesenchymal Stromal Cells Reduce Escherichia coli-induced Acute Lung Injury in Rats. Anesthesiology 2019, 130, 778–790. [Google Scholar] [CrossRef]
- Chailakhyan, R.K.; Aver’yanov, A.V.; Zabozlaev, F.G.; Sobolev, P.A.; Sorokina, A.V.; Akul’shin, D.A.; Gerasimov, Y.V. Comparison of the efficiency of transplantation of bone marrow multipotent mesenchymal stromal cells cultured under normoxic and hypoxic conditions and their conditioned media on the model of acute lung injury. Bull. Exp. Biol. Med. 2014, 157, 138–142. [Google Scholar] [CrossRef]
- Hayes, M.; Curley, G.F.; Masterson, C.; Devaney, J.; O’Toole, D.; Laffey, J.G. Mesenchymal stromal cells are more effective than the MSC secretome in diminishing injury and enhancing recovery following ventilator-induced lung injury. Intensive Care Med. Exp. 2015, 3, 29. [Google Scholar] [CrossRef]
- Hwang, B.; Liles, W.C.; Waworuntu, R.; Mulligan, M.S. Pretreatment with bone marrow-derived mesenchymal stromal cell-conditioned media confers pulmonary ischemic tolerance. J. Thorac. Cardiovasc. Surg. 2016, 151, 841–849. [Google Scholar] [CrossRef]
- Lu, H.; Poirier, C.; Cook, T.; Traktuev, D.O.; Merfeld-Clauss, S.; Lease, B.; Petrache, I.; March, K.L.; Bogatcheva, N.V. Conditioned media from adipose stromal cells limit lipopolysaccharide-induced lung injury, endothelial hyperpermeability and apoptosis. J. Transl. Med. 2015, 13, 67. [Google Scholar] [CrossRef]
- Miceli, V.; Bertani, A.; Chinnici, C.M.; Bulati, M.; Pampalone, M.; Amico, G.; Carcione, C.; Schmelzer, E.; Gerlach, J.C.; Conaldi, P.G. Conditioned Medium from Human Amnion-Derived Mesenchymal Stromal/Stem Cells Attenuating the Effects of Cold Ischemia-Reperfusion Injury in an In Vitro Model Using Human Alveolar Epithelial Cells. Int. J. Mol. Sci. 2021, 22, 510. [Google Scholar] [CrossRef]
- Moreira, A.; Naqvi, R.; Hall, K.; Emukah, C.; Martinez, J.; Moreira, A.; Dittmar, E.; Zoretic, S.; Evans, M.; Moses, D.; et al. Effects of mesenchymal stromal cell-conditioned media on measures of lung structure and function: A systematic review and meta-analysis of preclinical studies. Stem Cell Res. Ther. 2020, 11, 399. [Google Scholar] [CrossRef] [PubMed]
- Shologu, N.; Scully, M.; Laffey, J.G.; O’Toole, D. Human Mesenchymal Stem Cell Secretome from Bone Marrow or Adipose-Derived Tissue Sources for Treatment of Hypoxia-Induced Pulmonary Epithelial Injury. Int. J. Mol. Sci. 2018, 19, 2996. [Google Scholar] [CrossRef]
- Khush, K.K.; Cherikh, W.S.; Chambers, D.C.; Goldfarb, S.; Hayes, D., Jr.; Kucheryavaya, A.Y.; Levvey, B.J.; Meiser, B.; Rossano, J.W.; Stehlik, J.; et al. The International Thoracic Organ Transplant Registry of the International Society for Heart and Lung Transplantation: Thirty-fifth Adult Heart Transplantation Report-2018; Focus Theme: Multiorgan Transplantation. J. Heart Lung Transplant. 2018, 37, 1155–1168. [Google Scholar] [CrossRef] [PubMed]
- Lyu, D.M.; Goff, R.R.; Chan, K.M. The Lung Allocation Score and Its Relevance. Semin. Respir. Crit. Care Med. 2021, 42, 346–356. [Google Scholar] [CrossRef] [PubMed]
- Valapour, M.; Skeans, M.A.; Heubner, B.M.; Smith, J.M.; Hertz, M.I.; Edwards, L.B.; Cherikh, W.S.; Callahan, E.R.; Snyder, J.J.; Israni, A.K.; et al. OPTN/SRTR 2013 Annual Data Report: Lung. Am. J. Transplant. 2015, 15 (Suppl. 2), 1–28. [Google Scholar] [CrossRef]
- Weill, D. Access to Lung Transplantation. The Long and Short of It. Am. J. Respir. Crit. Care Med. 2016, 193, 605–606. [Google Scholar] [CrossRef]
- Krutsinger, D.; Reed, R.M.; Blevins, A.; Puri, V.; De Oliveira, N.C.; Zych, B.; Bolukbas, S.; Van Raemdonck, D.; Snell, G.I.; Eberlein, M. Lung transplantation from donation after cardiocirculatory death: A systematic review and meta-analysis. J. Heart Lung Transplant. 2015, 34, 675–684. [Google Scholar] [CrossRef]
- Reeb, J.; Keshavjee, S.; Cypel, M. Expanding the lung donor pool: Advancements and emerging pathways. Curr. Opin. Organ. Transplant. 2015, 20, 498–505. [Google Scholar] [CrossRef]
- Halpern, S.D.; Hasz, R.D.; Abt, P.L. Incidence and distribution of transplantable organs from donors after circulatory determination of death in U.S. intensive care units. Ann. Am. Thorac. Soc. 2013, 10, 73–80. [Google Scholar] [CrossRef]
- Levvey, B.J.; Harkess, M.; Hopkins, P.; Chambers, D.; Merry, C.; Glanville, A.R.; Snell, G.I. Excellent clinical outcomes from a national donation-after-determination-of-cardiac-death lung transplant collaborative. Am. J. Transplant. 2012, 12, 2406–2413. [Google Scholar] [CrossRef] [PubMed]
- Meers, C.; Van Raemdonck, D.; Verleden, G.M.; Coosemans, W.; Decaluwe, H.; De Leyn, P.; Nafteux, P.; Lerut, T. The number of lung transplants can be safely doubled using extended criteria donors; a single-center review. Transpl. Int. 2010, 23, 628–635. [Google Scholar] [CrossRef]
- Wigfield, C. Donation after cardiac death for lung transplantation: A review of current clinical practice. Curr. Opin. Organ. Transplant. 2014, 19, 455–459. [Google Scholar] [CrossRef] [PubMed]
- Gomez-de-Antonio, D.; Campo-Canaveral, J.L.; Crowley, S.; Valdivia, D.; Cordoba, M.; Moradiellos, J.; Naranjo, J.M.; Ussetti, P.; Varela, A. Clinical lung transplantation from uncontrolled non-heart-beating donors revisited. J. Heart Lung Transplant. 2012, 31, 349–353. [Google Scholar] [CrossRef] [PubMed]
- Munshi, L.; Keshavjee, S.; Cypel, M. Donor management and lung preservation for lung transplantation. Lancet Respir. Med. 2013, 1, 318–328. [Google Scholar] [CrossRef]
- Steen, S.; Ingemansson, R.; Eriksson, L.; Pierre, L.; Algotsson, L.; Wierup, P.; Liao, Q.; Eyjolfsson, A.; Gustafsson, R.; Sjoberg, T. First human transplantation of a nonacceptable donor lung after reconditioning ex vivo. Ann. Thorac. Surg. 2007, 83, 2191–2194. [Google Scholar] [CrossRef] [PubMed]
- Steen, S.; Sjoberg, T.; Pierre, L.; Liao, Q.; Eriksson, L.; Algotsson, L. Transplantation of lungs from a non-heart-beating donor. Lancet 2001, 357, 825–829. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Liu, M.; Anraku, M.; Chen, F.; Karolak, W.; Sato, M.; Laratta, J.; Azad, S.; Madonik, M.; et al. Normothermic ex vivo lung perfusion in clinical lung transplantation. Gen. Thorac. Cardiovasc Surg. 2011, 364, 1431–1440. [Google Scholar] [CrossRef]
- Cypel, M.; Yeung, J.C.; Hirayama, S.; Rubacha, M.; Fischer, S.; Anraku, M.; Sato, M.; Harwood, S.; Pierre, A.; Waddell, T.K.; et al. Technique for prolonged normothermic ex vivo lung perfusion. J. Heart Lung Transplant. 2008, 27, 1319–1325. [Google Scholar] [CrossRef]
- Machuca, T.N.; Cypel, M. Ex vivo lung perfusion. J. Thorac Dis. 2014, 6, 1054–1062. [Google Scholar]
- Tikkanen, J.M.; Cypel, M.; Machuca, T.N.; Azad, S.; Binnie, M.; Chow, C.W.; Chaparro, C.; Hutcheon, M.; Yasufuku, K.; de Perrot, M.; et al. Functional outcomes and quality of life after normothermic ex vivo lung perfusion lung transplantation. J. Heart Lung Transplant. 2015, 34, 547–556. [Google Scholar] [CrossRef]
- Iske, J.; Hinze, C.A.; Salman, J.; Haverich, A.; Tullius, S.G.; Ius, F. The potential of ex vivo lung perfusion on improving organ quality and ameliorating ischemia reperfusion injury. Am. J. Transplant. 2021, 21, 3831–3839. [Google Scholar] [CrossRef] [PubMed]
- Wagner, C.E.; Pope, N.H.; Charles, E.J.; Huerter, M.E.; Sharma, A.K.; Salmon, M.D.; Carter, B.T.; Stoler, M.H.; Lau, C.L.; Laubach, V.E.; et al. Ex vivo lung perfusion with adenosine A2A receptor agonist allows prolonged cold preservation of lungs donated after cardiac death. J. Thorac. Cardiovasc. Surg. 2016, 151, 538–545. [Google Scholar] [CrossRef]
- Cypel, M.; Liu, M.; Rubacha, M.; Yeung, J.C.; Hirayama, S.; Anraku, M.; Sato, M.; Medin, J.; Davidson, B.L.; de Perrot, M.; et al. Functional repair of human donor lungs by IL-10 gene therapy. Sci. Transl. Med. 2009, 1, 4ra9. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, D.; Watanabe, Y.; Ohsumi, A.; Pipkin, M.; Chen, M.; Mordant, P.; Kanou, T.; Saito, T.; Lam, R.; Coutinho, R.; et al. Mesenchymal stromal cell therapy during ex vivo lung perfusion ameliorates ischemia-reperfusion injury in lung transplantation. J. Heart Lung Transplant. 2019, 38, 1214–1223. [Google Scholar] [CrossRef]
- Zuk, P.A.; Zhu, M.; Ashjian, P.; De Ugarte, D.A.; Huang, J.I.; Mizuno, H.; Alfonso, Z.C.; Fraser, J.K.; Benhaim, P.; Hedrick, M.H. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell. 2002, 13, 4279–4295. [Google Scholar] [CrossRef] [PubMed]
- Colter, D.C.; Class, R.; DiGirolamo, C.M.; Prockop, D.J. Rapid expansion of recycling stem cells in cultures of plastic-adherent cells from human bone marrow. Proc. Natl. Acad. Sci. USA 2000, 97, 3213–3218. [Google Scholar] [CrossRef] [PubMed]
- Gronthos, S.; Mankani, M.; Brahim, J.; Robey, P.G.; Shi, S. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc. Natl. Acad. Sci. USA 2000, 97, 13625–13630. [Google Scholar] [CrossRef]
- Miranda, J.P.; Filipe, E.; Fernandes, A.S.; Almeida, J.M.; Martins, J.P.; De la Fuente, A.; Abal, M.; Barcia, R.N.; Cruz, P.; Cruz, H.; et al. The Human Umbilical Cord Tissue-Derived MSC Population UCX((R)) Promotes Early Motogenic Effects on Keratinocytes and Fibroblasts and G-CSF-Mediated Mobilization of BM-MSCs When Transplanted In Vivo. Cell Transplant. 2015, 24, 865–877. [Google Scholar] [CrossRef]
- Parolini, O.; Alviano, F.; Bagnara, G.P.; Bilic, G.; Buhring, H.J.; Evangelista, M.; Hennerbichler, S.; Liu, B.; Magatti, M.; Mao, N.; et al. Concise review: Isolation and characterization of cells from human term placenta: Outcome of the first international Workshop on Placenta Derived Stem Cells. Stem Cells 2008, 26, 300–311. [Google Scholar] [CrossRef]
- Keating, A. Mesenchymal stromal cells: New directions. Cell Stem Cell 2012, 10, 709–716. [Google Scholar] [CrossRef] [PubMed]
- Hass, R.; Kasper, C.; Bohm, S.; Jacobs, R. Different populations and sources of human mesenchymal stem cells (MSC): A comparison of adult and neonatal tissue-derived MSC. Cell Commun. Signal. CCS 2011, 9, 12. [Google Scholar] [CrossRef]
- Miceli, V.; Pampalone, M.; Vella, S.; Carreca, A.P.; Amico, G.; Conaldi, P.G. Comparison of Immunosuppressive and Angiogenic Properties of Human Amnion-Derived Mesenchymal Stem Cells between 2D and 3D Culture Systems. Stem Cells Int. 2019, 2019, 7486279. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological functions of mesenchymal stem cells and clinical implications. Cell Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Parolini, O.; Soncini, M.; Evangelista, M.; Schmidt, D. Amniotic membrane and amniotic fluid-derived cells: Potential tools for regenerative medicine? Regen. Med. 2009, 4, 275–291. [Google Scholar] [CrossRef] [PubMed]
- Pittenger, M.F.; Mackay, A.M.; Beck, S.C.; Jaiswal, R.K.; Douglas, R.; Mosca, J.D.; Moorman, M.A.; Simonetti, D.W.; Craig, S.; Marshak, D.R. Multilineage potential of adult human mesenchymal stem cells. Science 1999, 284, 143–147. [Google Scholar] [CrossRef]
- Bani-Yaghoub, M.; Wilson, P.; Hengstschlager, M.; Nikaido, T.; Pei, D. Amniotic stem cells: Potential in regenerative medicine. Stem Cells Int. 2012, 2012, 530674. [Google Scholar] [CrossRef] [PubMed]
- Kern, S.; Eichler, H.; Stoeve, J.; Kluter, H.; Bieback, K. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells 2006, 24, 1294–1301. [Google Scholar] [CrossRef]
- Degirmenci, B.; Valenta, T.; Dimitrieva, S.; Hausmann, G.; Basler, K. GLI1-expressing mesenchymal cells form the essential Wnt-secreting niche for colon stem cells. Nature 2018, 558, 449–453. [Google Scholar] [CrossRef]
- Kolf, C.M.; Cho, E.; Tuan, R.S. Mesenchymal stromal cells. Biology of adult mesenchymal stem cells: Regulation of niche, self-renewal and differentiation. Arthritis Res. Ther. 2007, 9, 204. [Google Scholar] [CrossRef][Green Version]
- Wosczyna, M.N.; Konishi, C.T.; Perez Carbajal, E.E.; Wang, T.T.; Walsh, R.A.; Gan, Q.; Wagner, M.W.; Rando, T.A. Mesenchymal Stromal Cells Are Required for Regeneration and Homeostatic Maintenance of Skeletal Muscle. Cell Rep. 2019, 27, 2029–2035 e5. [Google Scholar] [CrossRef]
- Morrison, S.J.; Scadden, D.T. The bone marrow niche for haematopoietic stem cells. Nature 2014, 505, 327–334. [Google Scholar] [CrossRef]
- Aggarwal, S.; Pittenger, M.F. Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 2005, 105, (4), 1815–1822. [Google Scholar] [CrossRef]
- Bulati, M.; Miceli, V.; Gallo, A.; Amico, G.; Carcione, C.; Pampalone, M.; Conaldi, P.G. The Immunomodulatory Properties of the Human Amnion-Derived Mesenchymal Stromal/Stem Cells Are Induced by INF-gamma Produced by Activated Lymphomonocytes and Are Mediated by Cell-To-Cell Contact and Soluble Factors. Front. Immunol. 2020, 11, 54. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, G.; Fox, J.; Ashton, B.; Middleton, J. Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells 2007, 25, 2739–2749. [Google Scholar] [CrossRef]
- Cheng, Z.; Ou, L.; Zhou, X.; Li, F.; Jia, X.; Zhang, Y.; Liu, X.; Li, Y.; Ward, C.A.; Melo, L.G.; et al. Targeted migration of mesenchymal stem cells modified with CXCR4 gene to infarcted myocardium improves cardiac performance. Mol. Ther. J. Am. Soc. Gene Ther. 2008, 16, 571–579. [Google Scholar] [CrossRef]
- Henschler, R.; Deak, E.; Seifried, E. Homing of Mesenchymal Stem Cells. Transfus. Med. Hemotherapy: Off. Organ. Der Dtsch. Ges. Fur Transfus. Und Immunhamatol. 2008, 35, 306–312. [Google Scholar] [CrossRef]
- Galipeau, J.; Sensebe, L. Mesenchymal Stromal Cells: Clinical Challenges and Therapeutic Opportunities. Cell Stem Cell 2018, 22, 824–833. [Google Scholar] [CrossRef] [PubMed]
- Phinney, D.G.; Galipeau, J.; Krampera, M.; Martin, I.; Shi, Y.; Sensebe, L. MSCs: Science and trials. Nat. Med. 2013, 19, 812. [Google Scholar] [CrossRef] [PubMed]
- Squillaro, T.; Peluso, G.; Galderisi, U. Clinical Trials With Mesenchymal Stem Cells: An Update. Cell Transplant. 2016, 25, 829–848. [Google Scholar] [CrossRef] [PubMed]
- Trounson, A.; McDonald, C. Stem Cell Therapies in Clinical Trials: Progress and Challenges. Cell Stem Cell 2015, 17, 11–22. [Google Scholar] [CrossRef]
- Wei, X.; Yang, X.; Han, Z.P.; Qu, F.F.; Shao, L.; Shi, Y.F. Mesenchymal stem cells: A new trend for cell therapy. Acta Pharmacol. Sin. 2013, 34, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, S.A.; Roobrouck, V.D.; Verfaillie, C.M.; Van Gool, S.W. Immunological characteristics of human mesenchymal stem cells and multipotent adult progenitor cells. Immunol. Cell Biol. 2013, 91, 32–39. [Google Scholar] [CrossRef] [PubMed]
- Le Blanc, K.; Tammik, C.; Rosendahl, K.; Zetterberg, E.; Ringden, O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp. Hematol. 2003, 31, 890–896. [Google Scholar] [CrossRef]
- Ullah, I.; Subbarao, R.B.; Rho, G.J. Human mesenchymal stem cells - current trends and future prospective. Biosci. Rep. 2015, 35, e00191. [Google Scholar] [CrossRef]
- Poggi, A.; Zocchi, M.R. Immunomodulatory Properties of Mesenchymal Stromal Cells: Still Unresolved “Yin and Yang”. Curr. Stem Cell Res. Ther. 2019, 14, 344–350. [Google Scholar] [CrossRef]
- Fu, Y.; Karbaat, L.; Wu, L.; Leijten, J.; Both, S.K.; Karperien, M. Trophic Effects of Mesenchymal Stem Cells in Tissue Regeneration. Tissue Eng.. Part. B Rev. 2017, 23, 515–528. [Google Scholar] [CrossRef]
- Tao, H.; Han, Z.; Han, Z.C.; Li, Z. Proangiogenic Features of Mesenchymal Stem Cells and Their Therapeutic Applications. Stem Cells Int. 2016, 2016, 1314709. [Google Scholar] [CrossRef]
- Stavely, R.; Nurgali, K. The emerging antioxidant paradigm of mesenchymal stem cell therapy. Stem Cells Transl. Med. 2020, 9, 985–1006. [Google Scholar] [CrossRef]
- Harrell, C.R.; Fellabaum, C.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Molecular Mechanisms Responsible for Therapeutic Potential of Mesenchymal Stem Cell-Derived Secretome. Cells 2019, 8, 467. [Google Scholar] [CrossRef] [PubMed]
- Wu, X.; Jiang, J.; Gu, Z.; Zhang, J.; Chen, Y.; Liu, X. Mesenchymal stromal cell therapies: Immunomodulatory properties and clinical progress. Stem Cell Res. Ther. 2020, 11, 345. [Google Scholar] [CrossRef] [PubMed]
- Horton, J.A.; Hudak, K.E.; Chung, E.J.; White, A.O.; Scroggins, B.T.; Burkeen, J.F.; Citrin, D.E. Mesenchymal stem cells inhibit cutaneous radiation-induced fibrosis by suppressing chronic inflammation. Stem Cells 2013, 31, 2231–2241. [Google Scholar] [CrossRef]
- Jankowski, M.; Dompe, C.; Sibiak, R.; Wasiatycz, G.; Mozdziak, P.; Jaskowski, J.M.; Antosik, P.; Kempisty, B.; Dyszkiewicz-Konwinska, M. In Vitro Cultures of Adipose-Derived Stem Cells: An Overview of Methods, Molecular Analyses, and Clinical Applications. Cells 2020, 9, 1783. [Google Scholar] [CrossRef] [PubMed]
- Leuning, D.G.; Beijer, N.R.M.; du Fosse, N.A.; Vermeulen, S.; Lievers, E.; van Kooten, C.; Rabelink, T.J.; Boer, J. The cytokine secretion profile of mesenchymal stromal cells is determined by surface structure of the microenvironment. Sci. Rep. 2018, 8, 7716. [Google Scholar] [CrossRef] [PubMed]
- Miceli, V.; Chinnici, C.M.; Bulati, M.; Pampalone, M.; Amico, G.; Schmelzer, E.; Gerlach, J.C.; Conaldi, P.G. Comparative study of the production of soluble factors in human placenta-derived mesenchymal stromal/stem cells grown in adherent conditions or as aggregates in a catheter-like device. Biochem. Biophys. Res. Commun. 2020, 522, 171–176. [Google Scholar] [CrossRef]
- Nikolits, I.; Nebel, S.; Egger, D.; Kress, S.; Kasper, C. Towards Physiologic Culture Approaches to Improve Standard Cultivation of Mesenchymal Stem Cells. Cells 2021, 10, 886. [Google Scholar] [CrossRef]
- Newman, R.E.; Yoo, D.; LeRoux, M.A.; Danilkovitch-Miagkova, A. Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm. Allergy Drug Targets 2009, 8, 110–123. [Google Scholar] [CrossRef]
- Foronjy, R.F.; Majka, S.M. The potential for resident lung mesenchymal stem cells to promote functional tissue regeneration: Understanding microenvironmental cues. Cells 2012, 1, 874. [Google Scholar] [CrossRef]
- Gnecchi, M.; Zhang, Z.; Ni, A.; Dzau, V.J. Paracrine mechanisms in adult stem cell signaling and therapy. Circ. Res. 2008, 103, 1204–1219. [Google Scholar] [CrossRef]
- Eefting, F.; Rensing, B.; Wigman, J.; Pannekoek, W.J.; Liu, W.M.; Cramer, M.J.; Lips, D.J.; Doevendans, P.A. Role of apoptosis in reperfusion injury. Cardiovasc. Res. 2004, 61, 414–426. [Google Scholar] [CrossRef]
- Li, J.H.; Zhang, N.; Wang, J.A. Improved anti-apoptotic and anti-remodeling potency of bone marrow mesenchymal stem cells by anoxic pre-conditioning in diabetic cardiomyopathy. J. Endocrinol. Investig. 2008, 31, 103–110. [Google Scholar] [CrossRef]
- Lee, S.C.; Kim, J.O.; Kim, S.J. Secretome from human adipose-derived stem cells protects mouse liver from hepatic ischemia-reperfusion injury. Surgery 2015, 157, 934–943. [Google Scholar] [CrossRef]
- Gyorgy, B.; Szabo, T.G.; Pasztoi, M.; Pal, Z.; Misjak, P.; Aradi, B.; Laszlo, V.; Pallinger, E.; Pap, E.; Kittel, A.; et al. Membrane vesicles, current state-of-the-art: Emerging role of extracellular vesicles. Cell Mol. Life Sci. 2011, 68, 2667–2688. [Google Scholar] [CrossRef] [PubMed]
- Harrell, C.R.; Jovicic, N.; Djonov, V.; Arsenijevic, N.; Volarevic, V. Mesenchymal Stem Cell-Derived Exosomes and Other Extracellular Vesicles as New Remedies in the Therapy of Inflammatory Diseases. Cells 2019, 8, 1605. [Google Scholar] [CrossRef] [PubMed]
- Tomasoni, S.; Longaretti, L.; Rota, C.; Morigi, M.; Conti, S.; Gotti, E.; Capelli, C.; Introna, M.; Remuzzi, G.; Benigni, A. Transfer of growth factor receptor mRNA via exosomes unravels the regenerative effect of mesenchymal stem cells. Stem Cells Dev. 2013, 22, 772–780. [Google Scholar] [CrossRef]
- van den Akker, F.; Vrijsen, K.R.; Deddens, J.C.; Buikema, J.W.; Mokry, M.; van Laake, L.W.; Doevendans, P.A.; Sluijter, J.P.G. Suppression of T cells by mesenchymal and cardiac progenitor cells is partly mediated via extracellular vesicles. Heliyon 2018, 4, e00642. [Google Scholar] [CrossRef] [PubMed]
- Gatti, S.; Bruno, S.; Deregibus, M.C.; Sordi, A.; Cantaluppi, V.; Tetta, C.; Camussi, G. Microvesicles derived from human adult mesenchymal stem cells protect against ischaemia-reperfusion-induced acute and chronic kidney injury. Nephrol. Dial. Transplant. Off. Publ. Eur. Dial. Transpl. Assoc. - Eur. Ren. Assoc. 2011, 26, 1474–1483. [Google Scholar] [CrossRef]
- Wilkey, B.J.; Abrams, B.A. Mitigation of Primary Graft Dysfunction in Lung Transplantation: Current Understanding and Hopes for the Future. Semin. Cardiothorac. Vasc. Anesth. 2020, 24, 54–66. [Google Scholar] [CrossRef]
- Christie, J.D.; Kotloff, R.M.; Ahya, V.N.; Tino, G.; Pochettino, A.; Gaughan, C.; DeMissie, E.; Kimmel, S.E. The effect of primary graft dysfunction on survival after lung transplantation. Am. J. Respir. Crit. Care Med. 2005, 171, 1312–1316. [Google Scholar] [CrossRef]
- Jarvinen, L.; Badri, L.; Wettlaufer, S.; Ohtsuka, T.; Standiford, T.J.; Toews, G.B.; Pinsky, D.J.; Peters-Golden, M.; Lama, V.N. Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J. Immunol. 2008, 181, 4389–4396. [Google Scholar] [CrossRef]
- Chambers, D.C.; Enever, D.; Lawrence, S.; Sturm, M.J.; Herrmann, R.; Yerkovich, S.; Musk, M.; Hopkins, P.M. Mesenchymal Stromal Cell Therapy for Chronic Lung Allograft Dysfunction: Results of a First-in-Man Study. Stem Cells Transl. Med. 2017, 6, 1152–1157. [Google Scholar] [CrossRef]
- Guillamat-Prats, R.; Camprubi-Rimblas, M.; Puig, F.; Herrero, R.; Tantinya, N.; Serrano-Mollar, A.; Artigas, A. Alveolar Type II Cells or Mesenchymal Stem Cells: Comparison of Two Different Cell Therapies for the Treatment of Acute Lung Injury in Rats. Cells 2020, 9, 1816. [Google Scholar] [CrossRef]
- Ahn, S.Y.; Park, W.S.; Kim, Y.E.; Sung, D.K.; Sung, S.I.; Ahn, J.Y.; Chang, Y.S. Vascular endothelial growth factor mediates the therapeutic efficacy of mesenchymal stem cell-derived extracellular vesicles against neonatal hyperoxic lung injury. Exp. Mol. Med. 2018, 50, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Aliotta, J.M.; Pereira, M.; Wen, S.; Dooner, M.S.; Del Tatto, M.; Papa, E.; Goldberg, L.R.; Baird, G.L.; Ventetuolo, C.E.; Quesenberry, P.J.; et al. Exosomes induce and reverse monocrotaline-induced pulmonary hypertension in mice. Cardiovasc. Res. 2016, 110, 319–330. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Chen, L.; Wu, X.; Lin, J.; Fang, J.; Chen, X.; Wei, S.; Xu, J.; Gao, Q.; Kang, M. Ischemia postconditioning and mesenchymal stem cells engraftment synergistically attenuate ischemia reperfusion-induced lung injury in rats. J. Surg. Res. 2012, 178, 81–91. [Google Scholar] [CrossRef] [PubMed]
- Devaney, J.; Horie, S.; Masterson, C.; Elliman, S.; Barry, F.; O’Brien, T.; Curley, G.F.; O’Toole, D.; Laffey, J.G. Human mesenchymal stromal cells decrease the severity of acute lung injury induced by E. coli in the rat. Thorax 2015, 70, 625–635. [Google Scholar] [CrossRef] [PubMed]
- Gulasi, S.; Atici, A.; Yilmaz, S.N.; Polat, A.; Yilmaz, M.; Lacin, M.T.; Orekici, G.; Celik, Y. Mesenchymal stem cell treatment in hyperoxia-induced lung injury in newborn rats. Pediatrics Int. Off. J. Jpn. Pediatric Soc. 2016, 58, 206–213. [Google Scholar] [CrossRef]
- Huh, J.W.; Kim, S.Y.; Lee, J.H.; Lee, J.S.; Van Ta, Q.; Kim, M.; Oh, Y.M.; Lee, Y.S.; Lee, S.D. Bone marrow cells repair cigarette smoke-induced emphysema in rats. Am. J. Physiol. Lung Cell Mol. Physiol. 2011, 301, L255–L266. [Google Scholar] [CrossRef]
- Ionescu, L.; Byrne, R.N.; van Haaften, T.; Vadivel, A.; Alphonse, R.S.; Rey-Parra, G.J.; Weissmann, G.; Hall, A.; Eaton, F.; Thebaud, B. Stem cell conditioned medium improves acute lung injury in mice: In vivo evidence for stem cell paracrine action. Am. J. Physiol. Lung Cell Mol. Physiol. 2012, 303, L967–L977. [Google Scholar] [CrossRef]
- Kennelly, H.; Mahon, B.P.; English, K. Human mesenchymal stromal cells exert HGF dependent cytoprotective effects in a human relevant pre-clinical model of COPD. Sci. Rep. 2016, 6, 38207. [Google Scholar] [CrossRef]
- Kim, Y.S.; Kim, J.Y.; Cho, R.; Shin, D.M.; Lee, S.W.; Oh, Y.M. Adipose stem cell-derived nanovesicles inhibit emphysema primarily via an FGF2-dependent pathway. Exp. Mol. Med. 2017, 49, e284. [Google Scholar] [CrossRef] [PubMed]
- Ni, S.; Wang, D.; Qiu, X.; Pang, L.; Song, Z.; Guo, K. Bone marrow mesenchymal stem cells protect against bleomycin-induced pulmonary fibrosis in rat by activating Nrf2 signaling. Int. J. Clin. Exp. Pathol. 2015, 8, 7752–7761. [Google Scholar] [PubMed]
- Pacienza, N.; Santa-Cruz, D.; Malvicini, R.; Robledo, O.; Lemus-Larralde, G.; Bertolotti, A.; Marcos, M.; Yannarelli, G. Mesenchymal Stem Cell Therapy Facilitates Donor Lung Preservation by Reducing Oxidative Damage during Ischemia. Stem Cells Int. 2019, 2019, 8089215. [Google Scholar] [CrossRef]
- Sadeghi, S.; Mosaffa, N.; Hashemi, S.M.; Mehdi Naghizadeh, M.; Ghazanfari, T. The immunomodulatory effects of mesenchymal stem cells on long term pulmonary complications in an animal model exposed to a sulfur mustard analog. Int. Immunopharmacol. 2020, 80, 105879. [Google Scholar] [CrossRef] [PubMed]
- Shen, Q.; Chen, B.; Xiao, Z.; Zhao, L.; Xu, X.; Wan, X.; Jin, M.; Dai, J.; Dai, H. Paracrine factors from mesenchymal stem cells attenuate epithelial injury and lung fibrosis. Mol. Med. Rep. 2015, 11, 2831–2837. [Google Scholar] [CrossRef]
- Wakayama, H.; Hashimoto, N.; Matsushita, Y.; Matsubara, K.; Yamamoto, N.; Hasegawa, Y.; Ueda, M.; Yamamoto, A. Factors secreted from dental pulp stem cells show multifaceted benefits for treating acute lung injury in mice. Cytotherapy 2015, 17, 1119–1129. [Google Scholar] [CrossRef] [PubMed]
- Wittwer, T.; Rahmanian, P.; Choi, Y.H.; Zeriouh, M.; Karavidic, S.; Neef, K.; Christmann, A.; Piatkowski, T.; Schnapper, A.; Ochs, M.; et al. Mesenchymal stem cell pretreatment of non-heart-beating-donors in experimental lung transplantation. J. Cardiothorac. Surg. 2014, 9, 151. [Google Scholar] [CrossRef][Green Version]
- Zhu, Y.G.; Feng, X.M.; Abbott, J.; Fang, X.H.; Hao, Q.; Monsel, A.; Qu, J.M.; Matthay, M.A.; Lee, J.W. Human mesenchymal stem cell microvesicles for treatment of Escherichia coli endotoxin-induced acute lung injury in mice. Stem Cells 2014, 32, 116–125. [Google Scholar] [CrossRef]
- Sun, C.K.; Yen, C.H.; Lin, Y.C.; Tsai, T.H.; Chang, L.T.; Kao, Y.H.; Chua, S.; Fu, M.; Ko, S.F.; Leu, S.; et al. Autologous transplantation of adipose-derived mesenchymal stem cells markedly reduced acute ischemia-reperfusion lung injury in a rodent model. J. Transl. Med. 2011, 9, 118. [Google Scholar] [CrossRef]
- Mordant, P.; Nakajima, D.; Kalaf, R.; Iskender, I.; Maahs, L.; Behrens, P.; Coutinho, R.; Iyer, R.K.; Davies, J.E.; Cypel, M.; et al. Mesenchymal stem cell treatment is associated with decreased perfusate concentration of interleukin-8 during ex vivo perfusion of donor lungs after 18-hour preservation. J. Heart Lung Transplant. 2016, 35, 1245–1254. [Google Scholar] [CrossRef]
- Gennai, S.; Monsel, A.; Hao, Q.; Park, J.; Matthay, M.A.; Lee, J.W. Microvesicles Derived From Human Mesenchymal Stem Cells Restore Alveolar Fluid Clearance in Human Lungs Rejected for Transplantation. Am. J. Transplant. 2015, 15, 2404–2412. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.Y.; Yiang, G.T.; Liao, W.T.; Tsai, A.P.; Cheng, Y.L.; Cheng, P.W.; Li, C.Y.; Li, C.J. Current Mechanistic Concepts in Ischemia and Reperfusion Injury. Cell. Physiol. Biochem. 2018, 46, 1650–1667. [Google Scholar] [CrossRef] [PubMed]
- Slegtenhorst, B.R.; Dor, F.J.; Rodriguez, H.; Voskuil, F.J.; Tullius, S.G. Ischemia/reperfusion Injury and its Consequences on Immunity and Inflammation. Curr. Transplant. Rep. 2014, 1, 147–154. [Google Scholar] [CrossRef] [PubMed]
- Ponticelli, C.E. The impact of cold ischemia time on renal transplant outcome. Kidney Int. 2015, 87, 272–275. [Google Scholar] [CrossRef]
- Oliva, J. Therapeutic Properties of Mesenchymal Stem Cell on Organ Ischemia-Reperfusion Injury. Int. J. Mol. Sci. 2019, 20, 5511. [Google Scholar] [CrossRef]
- Takamura, M.; Usui, S.; Inoue, O.; Ootsuji, H.; Takashima, S.I.; Nomura, A.; Kato, T.; Murai, H.; Furusho, H.; Sakai, Y.; et al. Adipose-derived regenerative cells exert beneficial effects on systemic responses following myocardial ischemia/reperfusion. Cardiol. J. 2016, 23, 685–693. [Google Scholar] [CrossRef] [PubMed]
- Cui, X.; He, Z.; Liang, Z.; Chen, Z.; Wang, H.; Zhang, J. Exosomes From Adipose-derived Mesenchymal Stem Cells Protect the Myocardium Against Ischemia/Reperfusion Injury Through Wnt/beta-Catenin Signaling Pathway. J. Cardiovasc. Pharmacol. 2017, 70, 225–231. [Google Scholar] [CrossRef]
- Mias, C.; Trouche, E.; Seguelas, M.H.; Calcagno, F.; Dignat-George, F.; Sabatier, F.; Piercecchi-Marti, M.D.; Daniel, L.; Bianchi, P.; Calise, D.; et al. Ex vivo pretreatment with melatonin improves survival, proangiogenic/mitogenic activity, and efficiency of mesenchymal stem cells injected into ischemic kidney. Stem Cells 2008, 26, 1749–1757. [Google Scholar] [CrossRef]
- Haga, H.; Yan, I.K.; Borrelli, D.A.; Matsuda, A.; Parasramka, M.; Shukla, N.; Lee, D.D.; Patel, T. Extracellular vesicles from bone marrow-derived mesenchymal stem cells protect against murine hepatic ischemia/reperfusion injury. Liver Transpl. 2017, 23, 791–803. [Google Scholar] [CrossRef]
- Li, S.; Zheng, X.; Li, H.; Zheng, J.; Chen, X.; Liu, W.; Tai, Y.; Zhang, Y.; Wang, G.; Yang, Y. Mesenchymal Stem Cells Ameliorate Hepatic Ischemia/Reperfusion Injury via Inhibition of Neutrophil Recruitment. J. Immunol. Res. 2018, 2018, 7283703. [Google Scholar] [CrossRef]
- Zare, M.A.; Zare, A.; Azarpira, N.; Pakbaz, S. The protective effect of bone marrow-derived mesenchymal stem cells in liver ischemia/reperfusion injury via down-regulation of miR-370. Iran. J. Basic Med. Sci. 2019, 22, 683–689. [Google Scholar]
- Lu, W.; Si, Y.I.; Ding, J.; Chen, X.; Zhang, X.; Dong, Z.; Fu, W. Mesenchymal stem cells attenuate acute ischemia-reperfusion injury in a rat model. Exp. Ther. Med. 2015, 10, 2131–2137. [Google Scholar] [CrossRef] [PubMed]
- Neizer, H.; Singh, G.B.; Gupta, S.; Singh, S.K. Addressing donor-organ shortages using extended criteria in lung transplantation. Ann. Cardiothorac. Surg. 2020, 9, 49–50. [Google Scholar] [CrossRef]
- Rosso, L.; Zanella, A.; Righi, I.; Barilani, M.; Lazzari, L.; Scotti, E.; Gori, F.; Mendogni, P. Lung transplantation, ex-vivo reconditioning and regeneration: State of the art and perspectives. J. Thorac Dis. 2018, 10, S2423–S2430. [Google Scholar] [CrossRef] [PubMed]
- Lonati, C.; Bassani, G.A.; Brambilla, D.; Leonardi, P.; Carlin, A.; Faversani, A.; Gatti, S.; Valenza, F. Influence of ex vivo perfusion on the biomolecular profile of rat lungs. FASEB J. 2018, 32, 5532–5549. [Google Scholar] [CrossRef]
- Roffia, V.; De Palma, A.; Lonati, C.; Di Silvestre, D.; Rossi, R.; Mantero, M.; Gatti, S.; Dondossola, D.; Valenza, F.; Mauri, P.; et al. Proteome Investigation of Rat Lungs subjected to Ex Vivo Perfusion (EVLP). Molecules 2018, 23, 3061. [Google Scholar] [CrossRef] [PubMed]
- Shin, J.; Hsin, M.K.; Baciu, C.; Chen, Y.; Zamel, R.; Machuca, T.; Yeung, J.; Cypel, M.; Keshavjee, S.; Liu, M. Use of metabolomics to identify strategies to improve and prolong ex vivo lung perfusion for lung transplants. J. Heart Lung Transplant. 2021, 40, 525–535. [Google Scholar] [CrossRef]
- Wilson, J.G.; Liu, K.D.; Zhuo, H.; Caballero, L.; McMillan, M.; Fang, X.; Cosgrove, K.; Vojnik, R.; Calfee, C.S.; Lee, J.W.; et al. Mesenchymal stem (stromal) cells for treatment of ARDS: A phase 1 clinical trial. Lancet Respir. Med. 2015, 3, 24–32. [Google Scholar] [CrossRef]
- Walter, J.; Ware, L.B.; Matthay, M.A. Mesenchymal stem cells: Mechanisms of potential therapeutic benefit in ARDS and sepsis. Lancet Respir. Med. 2014, 2, 1016–1026. [Google Scholar] [CrossRef]
| Use of Cells or Their Products | Study Model | Effects Due to MSC Treatment | Mechanisms | References |
|---|---|---|---|---|
| BM-MSCs | Mouse lung IRI | Protection against cold IRI in lung transplants | Improved arterial blood oxygenation capacity, reduced levels of pro-inflammatory cytokine and cell apoptosis | [29] |
| MSC-derived EVs | Rat lung IRI and EVLP | Improved tissue integrity and metabolism | Decrease in vascular resistance and rise in perfusate NO metabolites; Up-regulation of genes involved in the resolution of both inflammation and oxidative stress | [34] |
| UC-MSCs and UC-MSC-derived EVs | Mouse lung IRI | Attenuation of lung dysfunction and injury by improving the efficacy of EVLP | Decreased levels of edema, neutrophil infiltration and myeloperoxidase; decrease in pro-inflammatory cytokines and increase in KGF, PGE2 and IL-10; | [35] |
| UC-MSC-derived EVs | E. coli-induced rat lung injury | Increased survival | Enhanced phagocytosis of E. coli | [36] |
| BM-MSCs and BM-MSC-derived CM | Rat lung Injury | Attenuation of lung injury | Reduced levels of pro-inflammatory cytokine | [37] |
| BM-MSCs and BM-MSC-derived CM | Ventilator-induced rat lung injury | Reduction in injury and improvement in recovery | Reduced levels of edema, neutrophil, and alveolar IL-6 concentrations | [38] |
| BM-MSC-derived CM | Rat lung IRI | Protection against lung IRI | Decrease in both pro-inflammatory cytokines and infiltrating inflammatory cells, and increase in both M2-like macrophages and regulatory T cells | [39] |
| AdMSC-derived CM | LPS-induced mouse lung injury | Reduction in ARDS indices | Reduced endothelial barrier hyperpermeability and activation of pro-inflammatory and pro-apoptotic pathways in endothelium. | [40] |
| AMSC-derived CM | In vitro model of human lung IRI | Attenuation of IRI effects by improving the efficacy of in vitro EVLP | Increase in anti-inflammatory factors and up-regulation of anti-apoptotic factors | [41] |
| BM-MSCs and AdMSC-derived CM | Rat and human alveolar epithelial cell injury | Decreased cell injury | Decrease in pro-inflammatory factors and increase in anti-inflammatory factors; inhibition of p38 MAPK and translocation of Bcl-2 to the nucleus; Increased expression of cytoprotective glucose-regulated proteins | [43] |
| MSCs | Swine lung IRI | Attenuation of ischemic injury in donor lungs during EVLP and attenuation of IRI after transplantation | Increased levels of HGF and IL-4 and decreased levels of TNFα and cell death markers | [65] |
| BM-MSCs | HCL- and LPS-induced rat lung injury | Decreased inflammation | Decrease in proinflammatory cytokines, neutrophil infiltration, hemorrhage and interstitial edema | [122] |
| UC-MSCs and UC-MSC-derived EVs | Rat neonatal hyperoxic lung injuries | Attenuation of hyperoxic lung injuries | Increased alveolarization and angiogenesis; decrease in alveolar epithelial cell death, macrophages and cytokines in lung | [123] |
| BM-MSC-derived EVs | Mouse pulmonary arterial hypertension | Reduction in pulmonary vascular remodeling and right ventricle hypertrophy | Increased levels of anti-inflammatory and anti-proliferative miRs including miRs-34a,-122,-124, and -127. | [124] |
| BM-MSCs | Rat lung IRI | Attenuation of lung pathologic injury | Reduced myeloperoxidase production, decreased levels of of pro-inflammatory cytokine and cell apoptosis in lung tissue | [125] |
| BM-MSCs | E. coli-induced rat pneumonia | Reduction in lung injury; improvement in survival; reduction in lung bacterial load and suppression of inflammation | Enhanced macrophage phagocytic capacity and increase in lung and systemic concentrations of the antimicrobial peptide LL37 | [126] |
| BM-MSCs | Hyperoxia-induced rat lung injury | Mitigation of emphysema | Increased number of alveoli and decrease in α-SMA expression by myofibroblasts | [127] |
| BM-MSCs and BM-MSC-derived CM | Cigarette-smoke-induced rat emphysema | Alleviation of emphysema and increase in the number of small pulmonary vessels | Decrease in pulmonary artery medial wall thickness and reduction in apoptosis in lungs with emphysema | [128] |
| BM-MSCs and BM-MSC-derived CM | LPS-induced mouse lung injury | Resolution of lung injury by attenuating lung inflammation | Decrease in neutrophils and increase in M2 in BAL | [129] |
| BM-MSCs and BM-MSC-derived CM | Mouse chronic obstructive pulmonary disease | Reduction in injury | Reduced levels of inflammation, fibrosis and apoptotic and increased production of HGF | [130] |
| AdMSC-derived EVs | Elastase-induced mouse emphysema | Reduction in lung emphysema | Increased levels of FGF2 | [131] |
| BM-MSCs | Bleomycin-induced rat pulmonary fibrosis | Decreased fibrosis | Attenuation of NRF2, NQO1, HO-1, γ-GCS, lipid peroxidation, and increase in SOD activity | [132] |
| UC-MSCs | Rat lung IRI | Reduction in Oxidative stress damage and inflammation | Reduced levels of MPO activity and neutrophil markers; reduction in reactive oxygen species production | [133] |
| AdMSCs and AdMSC-derived CM | Sulfur mustard-induced mouse lung injury | Reduction in progressive histopathologic changes in the lung | Reducd levels of both M1 and M2 cells, TNF-α and IL-1β | [134] |
| BM-MSC-derived CM | Bleomycin-induced rat pulmonary fibrosis | Protection against lung fibrosis | Decrease in lung inflammation, fibrotic scores, collagen deposition, and cell apoptosis | [135] |
| SHEDs and SHED-derived CM | Bleomycin-induced mouse pulmonary fibrosis | Attenuation of lung injury and improvement in survival rate | Reduced levels of pro-inflammatory factors and increased levels of anti-inflammatory factors and M2 cells | [136] |
| BM-MSCs | Swine lung transplantation | Improvement in dynamic lung compliance | Reduced intrapulmonary edema | [137] |
| BM-MSC-derived EVs | E. Coli-induced mouse lung Injury | Reduction in lung edema and inflammation | Decrease in lung protein permeability, neutrophils and macrophage inflammatory protein-2 levels in the BAL fluid; increase in KGF in BAL | [138] |
| AdMSCs | Rat lung IRI | Attenuation of lung damage after IRI | Suppression of oxidative stress and inflammatory reaction | [139] |
| UC-MSCs | Swine lung IRI | Attenuation of IRI by improving the efficacy of EVLP | Increased levels of VEGF and decreased concentration of circulating IL-8 | [140] |
| BM-MSCs | Human lung IRI and EVLP | Decreased cold ischemic injury | Decrease in pro-inflammatory cytokines and increase in anti-inflammatory cytokines | [30] |
| BM-MSCs and BM-MSC-derived CM | E. coli-induced human lung injury | Increase in alveolar fluid clearance in lungs during EVLP | KGF secretion | [31] |
| BM-MSCs | Human lungs rejected for transplantation and subjected to prolonged ischemic time | Restoration of alveolar fluid clearance | KGF secretion | [32] |
| BM-MSC-derived EVs | Human lungs rejected for transplantation | Increase in alveolar fluid clearance in donor lungs during EVLP | Improved airway and hemodynamic parameters | [141] |
| AdMSCs | Clinical trial | Attenuation of IRI and host immunological reaction towards the graft | Not determined | NCT04714801 |
| BM-MSCs | Clinical trial | Attenuation of graft rejection and bronchiolitis obliteran syndrome (BOS) | Not determined | NCT02181712 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miceli, V.; Bertani, A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells 2022, 11, 826. https://doi.org/10.3390/cells11050826
Miceli V, Bertani A. Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells. 2022; 11(5):826. https://doi.org/10.3390/cells11050826
Chicago/Turabian StyleMiceli, Vitale, and Alessandro Bertani. 2022. "Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation" Cells 11, no. 5: 826. https://doi.org/10.3390/cells11050826
APA StyleMiceli, V., & Bertani, A. (2022). Mesenchymal Stromal/Stem Cells and Their Products as a Therapeutic Tool to Advance Lung Transplantation. Cells, 11(5), 826. https://doi.org/10.3390/cells11050826






