An Optimized Workflow for the Analysis of Metabolic Fluxes in Cancer Spheroids Using Seahorse Technology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Cell Cultures
2.2. Spheroid Formation Protocols
2.3. Imaging Analysis of 3D Models
2.4. Seahorse XFe96 Assay Preparation and Running on 3D Cultures
2.4.1. XFe96 Spheroid Microplate Coating
2.4.2. Spheroid Transfer onto the Assay Microplate
2.4.3. Sensor Cartridge Hydration and Loading
2.4.4. Normalization on Area
2.4.5. Protein Content Assay for Normalization
2.4.6. DNA Content Assay Normalization
2.5. Spheroid Digestion and Cell Count
2.6. Seahorse XFe96 Assay on 2D Cultures
2.7. Statistical Analysis
3. Results
3.1. The Single Spheroid Protocol Produces Spheroids Homogeneous in Size and Shape

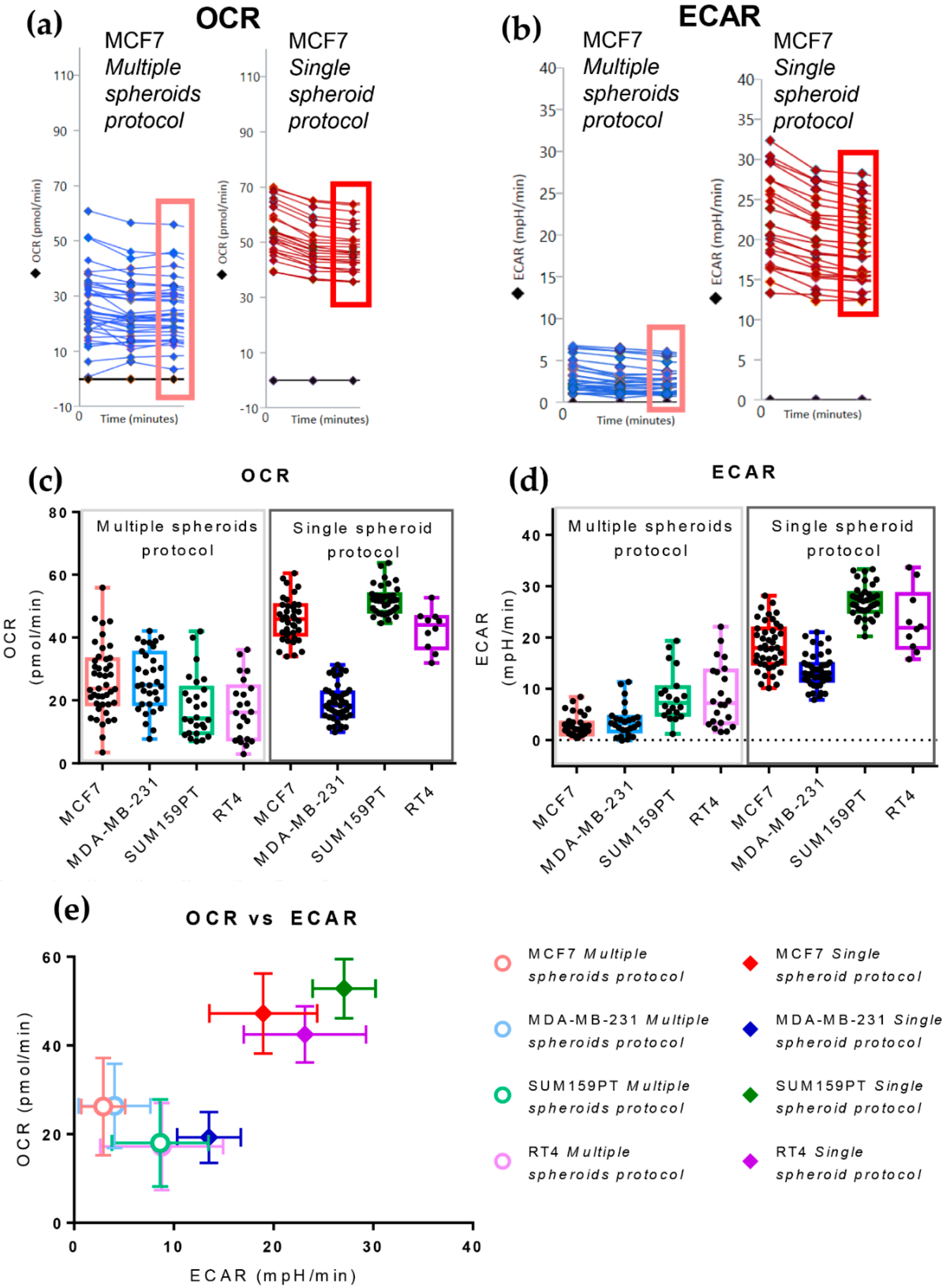
3.2. The Single Spheroid Protocol Allows More Accurate Determination of Oxygen Consumption Rate and Extracellular Acidification Rate by Seahorse XFe96 under Basal and Drug-Perturbed Conditions
3.3. The Single Spheroid Protocol Allows More Accurate Determination of Oxygen Consumption Rate and Extracellular Acidification Rate by Seahorse XFe96 Drug-Perturbed Conditions
- Oligomycin: causes an OCR decrease due to ATP synthase inhibition. The difference between basal respiration and the lowest OCR value measured after oligomycin injection represents the ATP produced by the mitochondria, contributing to meeting the cell’s energy needs under basal conditions (ATP linked respiration). The difference between the lowest OCR value measured after oligomycin injection and non-mitochondrial respiration (defined below) is called the proton leak and represents the remaining basal respiration not coupled to ATP production. Therefore, it can be a sign of mitochondrial damage or can be used as a mechanism to regulate mitochondrial ATP production.
- Carbonyl cyanide-4 (trifluoromethoxy) phenylhydrazone (FCCP): disrupts the proton gradient required for ATP synthesis, uncoupling oxygen consumption from oxidative phosphorylation. It increases OCR due to the attempt of the cells to rescue the disrupted mitochondrial membrane potential through the enhancement of electron transport chain activity. This treatment allows the calculation of the Maximal respiration and Spare respiratory capacity, which reflect the capability of the cell to respond to an energetic demand, such as in a stressful condition.
- A mixture of Rotenone and Antimycin A: these two drugs inhibit complex I and III of the electron transport chain, respectively, enabling the assessment of non-mitochondrial respiration.
3.4. The Cell Line of Origin Distinguishes the Metabolic Phenotype of Spheroids More Than Their Dimension
3.5. Growth in 3D Differentially Affects Metabolic Plasticity in MCF7 and MDA-MB-231 Cancer Cell Lines

4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pupo, E.; Avanzato, D.; Middonti, E.; Bussolino, F.; Lanzetti, L. KRAS-driven metabolic rewiring reveals novel actionable targets in cancer. Front. Oncol. 2019, 9, 848. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dong, Y.; Tu, R.; Liu, H.; Qing, G. Regulation of cancer cell metabolism: Oncogenic MYC in the driver’s seat. Signal Transduct. Target. Ther. 2020, 5, 124. [Google Scholar] [CrossRef]
- Maya-Mendoza, A.; Ostrakova, J.; Kosar, M.; Hall, A.; Duskova, P.; Mistrik, M.; Merchut-Maya, J.M.; Hodny, Z.; Bartkova, J.; Christensen, C.; et al. Myc and Ras oncogenes engage different energy metabolism programs and evoke distinct patterns of oxidative and DNA replication stress. Mol. Oncol. 2015, 9, 601–616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyssiotis, C.A.; Kimmelman, A.C. Metabolic Interactions in the Tumor Microenvironment. Trends Cell Biol. 2017, 27, 863–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sutherland, R.M. Cell and environment interactions in tumor microregions: The multicell spheroid model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Kelm, J.M.; Timmins, N.E.; Brown, C.J.; Fussenegger, M.; Nielsen, L.K. Method for generation of homogeneous multicellular tumor spheroids applicable to a wide variety of cell types. Biotechnol. Bioeng. 2003, 83, 173–180. [Google Scholar] [CrossRef]
- Langhans, S.A. Three-dimensional in vitro cell culture models in drug discovery and drug repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef]
- Unger, C.; Kramer, N.; Walzl, A.; Scherzer, M.; Hengstschläger, M.; Dolznig, H. Modeling human carcinomas: Physiologically relevant 3D models to improve anti-cancer drug development. Adv. Drug Deliv. Rev. 2014, 79, 50–67. [Google Scholar] [CrossRef]
- Jensen, C.; Teng, Y. Is It Time to Start Transitioning From 2D to 3D Cell Culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [Green Version]
- Zanoni, M.; Cortesi, M.; Zamagni, A.; Arienti, C.; Pignatta, S.; Tesei, A. Modeling neoplastic disease with spheroids and organoids. J. Hematol. Oncol. 2020, 13, 97. [Google Scholar] [CrossRef] [PubMed]
- Chaicharoenaudomrung, N.; Kunhorm, P.; Noisa, P. Three-dimensional cell culture systems as an in vitro platform for cancer and stem cell modeling. World J. Stem Cells 2019, 11, 1065–1083. [Google Scholar] [CrossRef]
- Costa, E.C.; Moreira, A.F.; de Melo-Diogo, D.; Gaspar, V.M.; Carvalho, M.P.; Correia, I.J. 3D tumor spheroids: An overview on the tools and techniques used for their analysis. Biotechnol. Adv. 2016, 34, 1427–1441. [Google Scholar] [CrossRef] [PubMed]
- Rogers, G.W.; Trockmorton, H.; Burroughs, S.E. Targeting Energy Metabolism for Cancer Therapeutic Discovery Using Agilent Seahorse XF Technology: Agilent 2019. Available online: https://www.agilent.com/cs/library/applications/application-energy-metabolism-cancer-theraputics-discovery-cell-analysis-5994-1017en-agilent.pdf (accessed on 29 January 2022).
- Pasquale, V.; Ducci, G.; Campioni, G.; Ventrici, A.; Assalini, C.; Busti, S.; Vanoni, M.; Vago, R.; Sacco, E. Profiling and Targeting of Energy and Redox Metabolism in Grade 2 Bladder Cancer Cells with Different Invasiveness Properties. Cells 2020, 9, 2669. [Google Scholar] [CrossRef] [PubMed]
- Porporato, P.E.; Payen, V.L.; Pérez-Escuredo, J.; De Saedeleer, C.J.; Danhier, P.; Copetti, T.; Dhup, S.; Tardy, M.; Vazeille, T.; Bouzin, C.; et al. A mitochondrial switch promotes tumor metastasis. Cell Rep. 2014, 8, 754–766. [Google Scholar] [CrossRef] [Green Version]
- Viale, A.; Pettazzoni, P.; Lyssiotis, C.A.; Ying, H.; Sánchez, N.; Marchesini, M.; Carugo, A.; Green, T.; Seth, S.; Giuliani, V.; et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature 2014, 514, 628–632. [Google Scholar] [CrossRef] [Green Version]
- Raggi, C.; Taddei, M.L.; Sacco, E.; Navari, N.; Correnti, M.; Piombanti, B.; Pastore, M.; Campani, C.; Pranzini, E.; Iorio, J.; et al. Mitochondrial oxidative metabolism contributes to a cancer stem cell phenotype in cholangiocarcinoma. J. Hepatol. 2021, 74, 1373–1385. [Google Scholar] [CrossRef]
- Parri, M.; Ippolito, L.; Cirri, P.; Ramazzotti, M.; Chiarugi, P. Metabolic cell communication within tumour microenvironment: Models, methods and perspectives. Curr. Opin. Biotechnol. 2020, 63, 210–219. [Google Scholar] [CrossRef]
- Damiani, C.; Gaglio, D.; Sacco, E.; Alberghina, L.; Vanoni, M. Systems metabolomics: From metabolomic snapshots to design principles. Curr. Opin. Biotechnol. 2020, 63, 190–199. [Google Scholar] [CrossRef]
- Kam, Y.; Rogers, G.W.; Jastromb, N.; Dranka, B.P. Methods and Strategies for Normalizing XF Metabolic Data to Cellular Parameters: Agilent 2018, p. 8. Available online: https://www.agilent.com/cs/library/technicaloverviews/public/Methods_and_Strategies_for_Normalizing_Tech_Overview_022118.pdf (accessed on 29 January 2022).
- Agilent Cell Analysis Publications Alert October 2020. Available online: https://seahorseinfo.agilent.com/acton/media/10967/agilent-cell-analysis-publications-alert-october-2020#cancer (accessed on 28 January 2022).
- Jiang, L.; Shestov, A.A.; Swain, P.; Yang, C.; Parker, S.J.; Wang, Q.A.; Terada, L.S.; Adams, N.D.; McCabe, M.T.; Pietrak, B.; et al. Reductive carboxylation supports redox homeostasis during anchorage-independent growth. Nature 2016, 532, 255–258. [Google Scholar] [CrossRef]
- Russell, S.; Wojtkowiak, J.; Neilson, A.; Gillies, R.J. Metabolic Profiling of healthy and cancerous tissues in 2D and 3D. Sci. Rep. 2017, 7, 15285. [Google Scholar] [CrossRef]
- Taddeo, E.P.; Stiles, L.; Sereda, S.; Ritou, E.; Wolf, D.M.; Abdullah, M.; Swanson, Z.; Wilhelm, J.; Bellin, M.; McDonald, P.; et al. Individual islet respirometry reveals functional diversity within the islet population of mice and human donors. Mol. Metab. 2018, 16, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Pelicano, H.; Zhang, W.; Liu, J.; Hammoudi, N.; Dai, J.; Xu, R.H.; Pusztai, L.; Huang, P. Mitochondrial dysfunction in some triple-negative breast cancer cell lines: Role of mTOR pathway and therapeutic potential. Breast Cancer Res. 2014, 16, 434. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lanning, N.J.; Castle, J.P.; Singh, S.J.; Leon, A.N.; Tovar, E.A.; Sanghera, A.; MacKeigan, J.P.; Filipp, F.V.; Graveel, C.R. Metabolic profiling of triple-negative breast cancer cells reveals metabolic vulnerabilities. Cancer Metab. 2017, 5, 6. [Google Scholar] [CrossRef]
- Guha, M.; Srinivasan, S.; Raman, P.; Jiang, Y.; Kaufman, B.A.; Taylor, D.; Dong, D.; Chakrabarti, R.; Picard, M.; Carstens, R.P.; et al. Aggressive triple negative breast cancers have unique molecular signature on the basis of mitochondrial genetic and functional defects. Biochim. Biophys. Acta-Mol. Basis Dis. 2018, 1864, 1060–1071. [Google Scholar] [CrossRef]
- Durand, R.E. Multicell spheroids as a model for cell kinetic studies. Cell Tissue Kinet. 1990, 23, 141–159. [Google Scholar] [PubMed]
- Zanoni, M.; Piccinini, F.; Arienti, C.; Zamagni, A.; Santi, S.; Polico, R.; Bevilacqua, A.; Tesei, A. 3D tumor spheroid models for in vitro therapeutic screening: A systematic approach to enhance the biological relevance of data obtained. Sci. Rep. 2016, 6, 19103. [Google Scholar] [CrossRef]
- Nunes, A.S.; Barros, A.S.; Costa, E.C.; Moreira, A.F.; Correia, I.J. 3D tumor spheroids as in vitro models to mimic in vivo human solid tumors resistance to therapeutic drugs. Biotechnol. Bioeng. 2019, 116, 206–226. [Google Scholar] [CrossRef] [Green Version]
- Mittler, F.; Obeïd, P.; Rulina, A.V.; Haguet, V.; Gidrol, X.; Balakirev, M.Y. High-content monitoring of drug effects in a 3D spheroid model. Front. Oncol. 2017, 7, 293. [Google Scholar] [CrossRef] [Green Version]
- Weeber, F.; Ooft, S.N.; Dijkstra, K.K.; Voest, E.E. Tumor Organoids as a Pre-clinical Cancer Model for Drug Discovery. Cell Chem. Biol. 2017, 24, 1092–1100. [Google Scholar] [CrossRef]
- Korving, J.; Moll, J.; Voest, E.E.; Weeber, F.; de Ligt, J.; Rottenberg, S.; Bounova, G.; Boj, S.F.; Kopper, O.; Vries, R.G.J.; et al. A Living Biobank of Breast Cancer Organoids Captures Disease Heterogeneity. Cell 2017, 172, 373–386.e10. [Google Scholar]
- Vlachogiannis, G.; Hedayat, S.; Vatsiou, A.; Jamin, Y.; Fernández-Mateos, J.; Khan, K.; Lampis, A.; Eason, K.; Huntingford, I.; Burke, R.; et al. Patient-derived organoids model treatment response of metastatic gastrointestinal cancers. Science 2018, 359, 920–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeRose, Y.S.; Gligorich, K.M.; Wang, G.; Georgelas, A.; Bowman, P.; Courdy, S.J.; Welm, A.L.; Welm, B.E. Patient-Derived Models of Human Breast Cancer: Protocols for In Vitro and In Vivo Applications in Tumor Biology and Translational Medicine. Curr. Protoc. Pharmacol. 2013, 60, 1–43. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, J. Systems Biology of Metabolism: A Driver for Developing Personalized and Precision Medicine. Cell Metab. 2017, 25, 572–579. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jones, A.E.; Sheng, L.; Acevedo, A.; Veliova, M.; Shirihai, O.S.; Stiles, L.; Divakaruni, A.S. Forces, fluxes, and fuels: Tracking mitochondrial metabolism by integrating measurements of membrane potential, respiration, and metabolites. Am. J. Physiol.-Cell Physiol. 2021, 320, C80–C91. [Google Scholar] [CrossRef] [PubMed]
- Nolfi-Donegan, D.; Braganza, A.; Shiva, S. Mitochondrial electron transport chain: Oxidative phosphorylation, oxidant production, and methods of measurement. Redox Biol. 2020, 37, 101674. [Google Scholar] [CrossRef]
- Wu, D.; Ma, Y.; Cao, Y.; Zhang, T. Mitochondrial toxicity of nanomaterials. Sci. Total Environ. 2020, 702, 134994. [Google Scholar] [CrossRef]
- Freyer, J.P.; Sutherland, R.M. Selective Dissociation and Characterization of Cells from Different Regions of Muiticell Tumor Spheroids. Cancer Res. 1980, 40, 3956–3965. [Google Scholar]
- Sutherland, R.M.; Sordat, B.; Bamat, J.; Gabbert, H.; Bourrat, B.; Mueller-Klieser, W. Oxygenation and Differentiation in Multicellular Spheroids of Human Colon Carcinoma. Cancer Res. 1986, 46, 5320–5329. [Google Scholar]
- Walenta, S.; Dotsch, J.; Bourrat-Flock, B.; Mueller-Klieser, W. Size-dependent oxygenation and energy status in multicellular tumor spheroids. Adv. Exp. Med. Biol. 1990, 277, 889–893. [Google Scholar]
- Murphy, K.C.; Hung, B.P.; Browne-Bourne, S.; Zhou, D.; Yeung, J.; Genetos, D.C.; Leach, J.K. Measurement of oxygen tension within mesenchymal stem cell spheroids. J. R. Soc. Interface 2017, 14, 20160851. [Google Scholar] [CrossRef] [PubMed]
- Bonanomi, M.; Salmistraro, N.; Fiscon, G.; Conte, F.; Paci, P.; Bravatà, V.; Forte, G.I.; Volpari, T.; Scorza, M.; Mastroianni, F.; et al. Transcriptomics and metabolomics integration reveals redox-dependent metabolic rewiring in breast cancer cells. Cancers 2021, 13, 5058. [Google Scholar] [CrossRef] [PubMed]
- Leung, B.M.; Lesher-Perez, S.C.; Matsuoka, T.; Moraes, C.; Takayama, S. Media additives to promote spheroid circularity and compactness in hanging drop platform. Biomater. Sci. 2015, 3, 336–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Damiani, C.; Maspero, D.; Di Filippo, M.; Colombo, R.; Pescini, D.; Graudenzi, A.; Westerhoff, H.V.; Alberghina, L.; Vanoni, M.; Mauri, G. Integration of single-cell RNA-seq data into population models to characterize cancer metabolism. PLoS Comput. Biol. 2019, 15, e1006733. [Google Scholar] [CrossRef]
- Wei, D.; Xu, M.; Wang, Z.; Tong, J. The Development of Single-Cell Metabolism and Its Role in Studying Cancer Emergent Properties. Front. Oncol. 2022, 11, 814085. [Google Scholar] [CrossRef]
- Xiao, Z.; Dai, Z.; Locasale, J.W. Metabolic landscape of the tumor microenvironment at single cell resolution. Nat. Commun. 2019, 10, 3763. [Google Scholar] [CrossRef]
- Argüello, R.J.; Combes, A.J.; Char, R.; Gigan, J.P.; Baaziz, A.I.; Bousiquot, E.; Camosseto, V.; Samad, B.; Tsui, J.; Yan, P.; et al. SCENITH: A Flow Cytometry-Based Method to Functionally Profile Energy Metabolism with Single-Cell Resolution. Cell Metab. 2020, 32, 1063–1075.e7. [Google Scholar] [CrossRef]
- Gómez-Cebrián, N.; Domingo-Ortí, I.; Poveda, J.L.; Vicent, M.J.; Puchades-Carrasco, L.; Pineda-Lucena, A. Multi-omic approaches to breast cancer metabolic phenotyping: Applications in diagnosis, prognosis, and the development of novel treatments. Cancers 2021, 13, 4544. [Google Scholar] [CrossRef]
- Wu, D.; Harrison, D.L.; Szasz, T.; Yeh, C.F.; Shentu, T.P.; Meliton, A.; Huang, R.T.; Zhou, Z.; Mutlu, G.M.; Huang, J.; et al. Single-cell metabolic imaging reveals a SLC2A3-dependent glycolytic burst in motile endothelial cells. Nat. Metab. 2021, 3, 714–727. [Google Scholar] [CrossRef]
- Harrison, D.; Wu, D.; Huang, J.; Fang, Y. Single-cell lactate production rate as a measure of glycolysis in endothelial cells. STAR Protoc. 2021, 2, 100807. [Google Scholar] [CrossRef]
- Noel, P.; Muñoz, R.; Rogers, G.W.; Neilson, A.; Von Hoff, D.D.; Han, H. Preparation and metabolic assay of 3-dimensional spheroid co-cultures of pancreatic cancer cells and fibroblasts. J. Vis. Exp. 2017, 2017, 56081. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franchi-Mendes, T.; Eduardo, R.; Domenici, G.; Brito, C. 3D cancer models: Depicting cellular crosstalk within the tumour microenvironment. Cancers 2021, 13, 4610. [Google Scholar] [CrossRef] [PubMed]



| Cell Line | Protocol | Area (μm2) | Roundness | ||
|---|---|---|---|---|---|
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | ||
| MCF7 | Multiple spheroids | 95,997.4 (n = 93) ± 48,223.0 | 50.2 | 0.54 (n = 93) ± 0.19 | 35.2 |
| Single spheroid | 421,135.9 (n = 75) ± 36,417.5 | 8.6 | 0.82 (n = 75) ± 0.07 | 8.7 | |
| MDA-MB-231 | Multiple spheroids | 156,971.5 (n = 88) ± 94,030.3 | 59.9 | 0.49 (n = 88) ± 0.12 | 24.6 |
| Single spheroid | 415,612.7 (n = 56) ± 73,802.6 | 17.8 | 0.61 (n = 56) ± 0.07 | 11.2 | |
| SUM159PT | Multiple spheroids | 137,404.0 (n = 25) ± 54,377.4 | 39.6 | 0.69 (n = 25) ± 0.12 | 18.1 |
| Single spheroid | 267,606.1 (n = 45) ± 34,643.6 | 12.9 | 0.67 (n = 45) ± 0.08 | 12.2 | |
| RT4 | Multiple spheroids | 333,372.0 (n = 27) ± 226,341.7 | 67.9 | 0.44 (n = 27) ± 0.15 | 33.1 |
| Single spheroid | 483,380.8 (n = 11) ± 18,691.7 | 3.9 | 0.66 (n = 11) ± 0.079 | 12.0 | |
| Cell line | Protocol | Basal OCR | Basal ECAR | ||
|---|---|---|---|---|---|
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | ||
| MCF7 | Multiple spheroids | 26.20 ± 10.99 | 41.9 | 2.68 ± 2.05 | 76.3 |
| Single spheroid | 45.76 ± 6.68 | 14.6 | 18.33 ± 4.58 | 25.0 | |
| MDA-MB-231 | Multiple spheroids | 26.36 ± 9.49 | 36.0 | 3.72 ± 3.06 | 82.2 |
| Single spheroid | 19.26 ± 5.74 | 29.8 | 13.53 ± 3.19 | 23.6 | |
| SUM159PT | Multiple spheroids | 18.00 ± 9.83 | 54.6 | 8.61 ± 4.95 | 57.5 |
| Single spheroid | 52.00 ± 4.69 | 8.8 | 27.08 ± 3.16 | 11.7 | |
| RT4 | Multiple spheroids | 17.21 ± 9.82 | 57.1 | 8.79 ± 6.16 | 70.1 |
| Single spheroid | 42.48 ± 6.32 | 14.9 | 23.14 ± 6.13 | 26.5 | |
| Drug Treatment | Measurement | Multiple Spheroids Protocol | Single Spheroid Protocol | ||
|---|---|---|---|---|---|
| Mean ± SD | CV (%) | Mean ± SD | CV (%) | ||
| Basal | 1 | 26.99 ± 12.56 | 46.5 | 54.24 ± 9.25 | 17.1 |
| 2 | 24.64 ± 11.13 | 45.2 | 48.90 ± 8.53 | 17.4 | |
| 3 | 24.85 ± 11.13 | 44.8 | 47.54 ± 8.34 | 17.5 | |
| Oligomycin | 4 | 21.15 ± 9.60 | 45.4 | 47.97 ± 8.31 | 17.3 |
| 5 | 16.79 ± 8.48 | 50.5 | 46.21 ± 8.13 | 17.6 | |
| 6 | 14.75 ± 7.76 | 52.6 | 44.16 ± 7.91 | 17.9 | |
| 7 | 13.91 ± 7.87 | 56.6 | 42.54 ± 7.72 | 18.2 | |
| 8 | 13.82 ± 8.06 | 58.3 | 41.01 ± 7.60 | 18.5 | |
| FCCP | 9 | 39.51 ± 16.53 | 41.8 | 73.77 ± 10.58 | 14.3 |
| 10 | 35.34 ± 15.04 | 42.6 | 75.22 ± 10.41 | 13.8 | |
| 11 | 32.58 ± 14.29 | 43.9 | 76.33 ± 10.35 | 13.6 | |
| 12 | 30.55 ± 14.48 | 47.4 | 77.31 ± 10.28 | 13.3 | |
| Rotenone/Antimycin A | 13 | 17.33 ± 9.01 | 52.0 | 60.53 ± 8.86 | 14.6 |
| 14 | 12.78 ± 6.92 | 54.1 | 38.72 ± 9.19 | 23.7 | |
| 15 | 11.97 ± 6.89 | 57.5 | 28.34 ± 8.50 | 30.0 | |
| 16 | 11.47 ± 6.35 | 55.4 | 22.11 ± 7.55 | 34.1 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campioni, G.; Pasquale, V.; Busti, S.; Ducci, G.; Sacco, E.; Vanoni, M. An Optimized Workflow for the Analysis of Metabolic Fluxes in Cancer Spheroids Using Seahorse Technology. Cells 2022, 11, 866. https://doi.org/10.3390/cells11050866
Campioni G, Pasquale V, Busti S, Ducci G, Sacco E, Vanoni M. An Optimized Workflow for the Analysis of Metabolic Fluxes in Cancer Spheroids Using Seahorse Technology. Cells. 2022; 11(5):866. https://doi.org/10.3390/cells11050866
Chicago/Turabian StyleCampioni, Gloria, Valentina Pasquale, Stefano Busti, Giacomo Ducci, Elena Sacco, and Marco Vanoni. 2022. "An Optimized Workflow for the Analysis of Metabolic Fluxes in Cancer Spheroids Using Seahorse Technology" Cells 11, no. 5: 866. https://doi.org/10.3390/cells11050866
APA StyleCampioni, G., Pasquale, V., Busti, S., Ducci, G., Sacco, E., & Vanoni, M. (2022). An Optimized Workflow for the Analysis of Metabolic Fluxes in Cancer Spheroids Using Seahorse Technology. Cells, 11(5), 866. https://doi.org/10.3390/cells11050866






