Bryostatin-1 Attenuates Ischemia-Elicited Neutrophil Transmigration and Ameliorates Graft Injury after Kidney Transplantation
Abstract
:1. Introduction
2. Materials and Methods
2.1. Human Samples
2.2. Experimental Design for In Vitro Studies
2.3. Maintenance of HUVECs
2.4. Isolation of Human Neutrophils
2.5. In Vitro Model of Hypoxia and Normoxia
2.6. Neutrophil Transmigration
2.7. Myeloperoxidase (MPO) Assay
2.8. FITC-Dextran Permeability Assay
2.9. Animals
2.10. Experimental Design and Treatment Algorithm for In Vivo Studies
2.11. Anesthesia and Monitoring during Graft Procurement
2.12. Graft Procurement
2.13. Anesthesia and Monitoring during Autotransplantation
2.14. Orthotopic Kidney Autotransplantation
2.15. MPO Assay for Tissue Samples
2.16. Blood and Urine Analysis
2.17. Histological Evaluation
2.18. Transmission Electron Microscopy
2.19. Quantitative Polymerase Chain Reaction (qPCR)
2.20. Statistics
3. Results
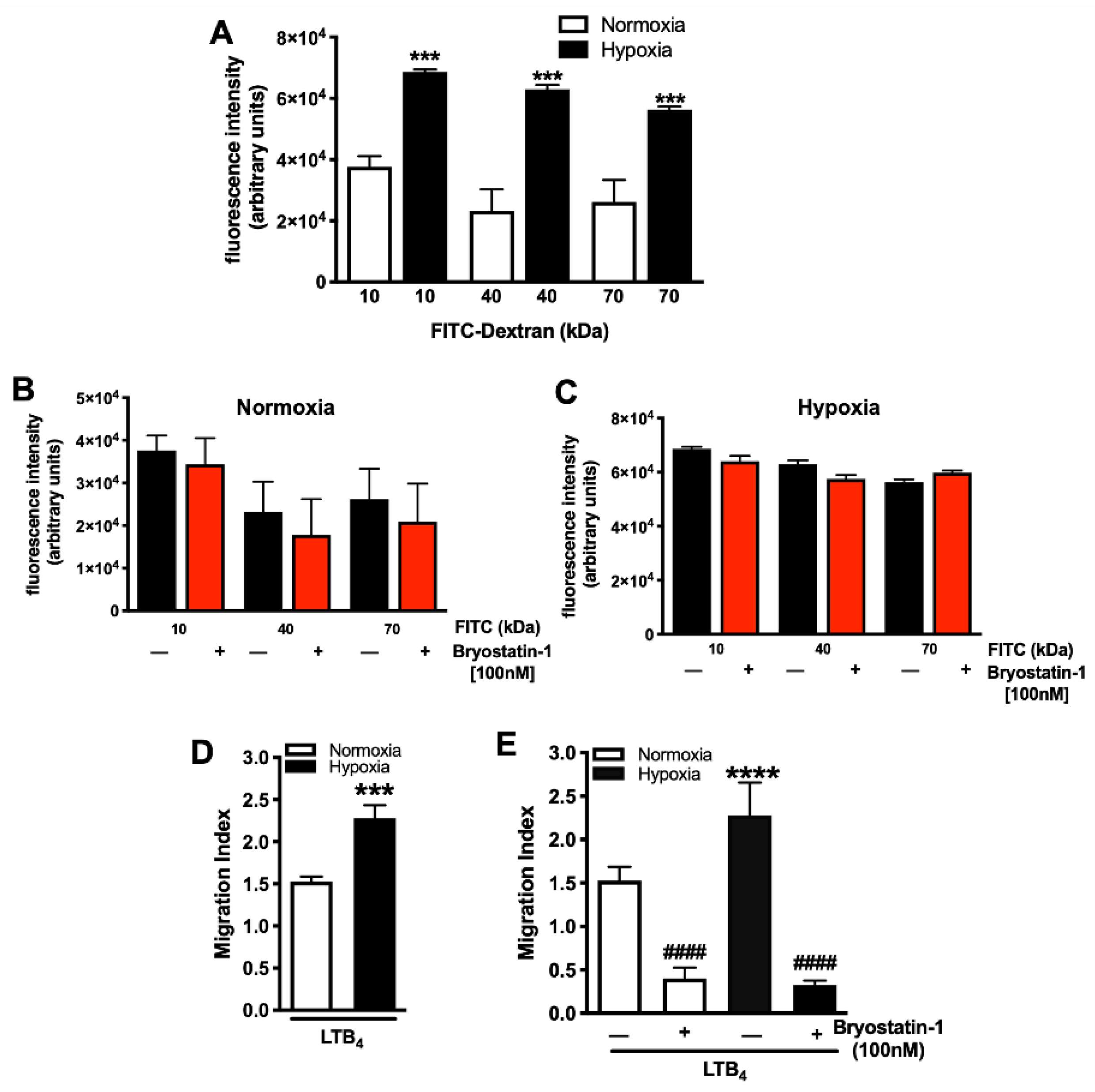
3.1. Bryostatin-1 Reduces Hypoxia-Elicited Neutrophil TEM
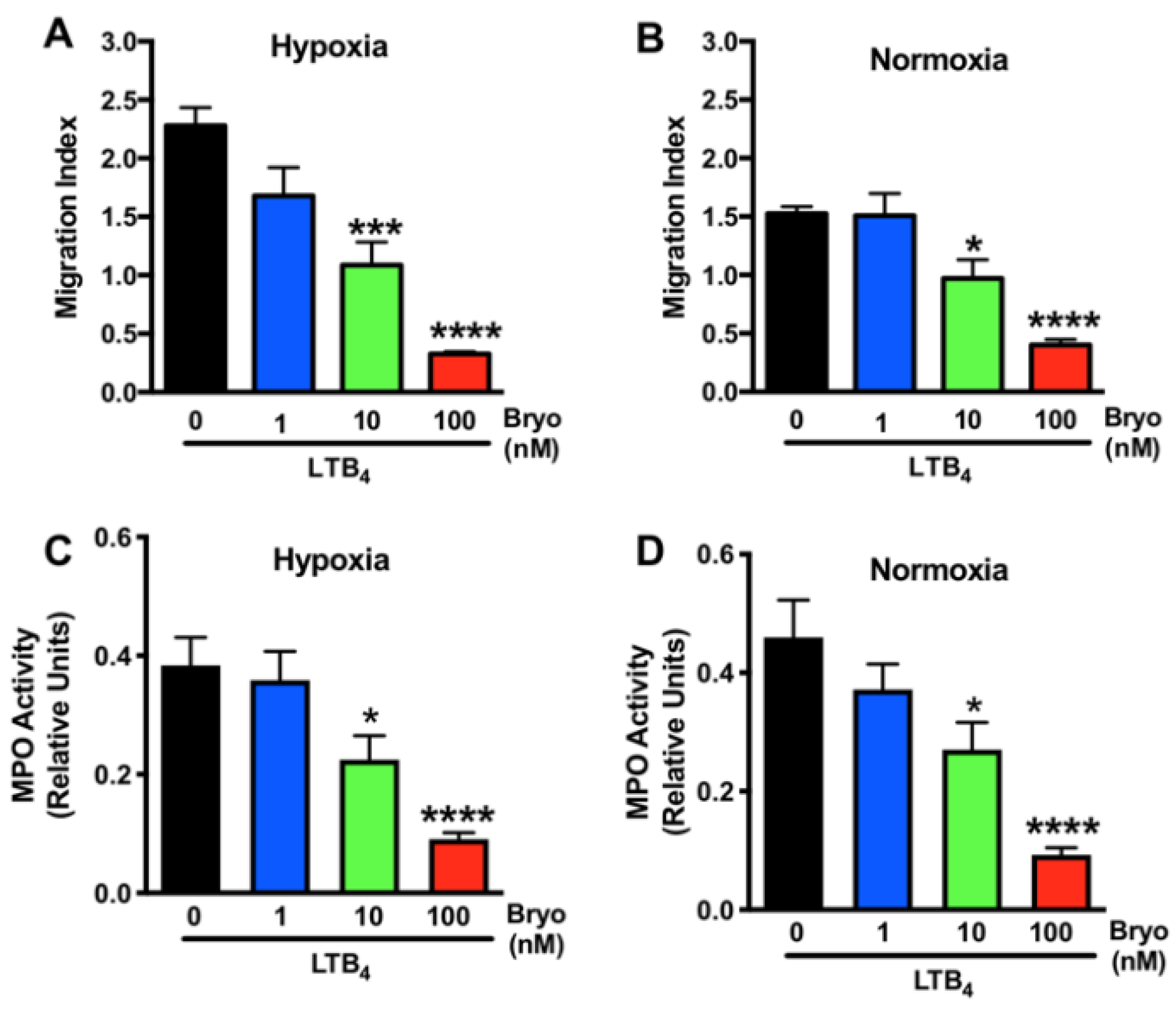
3.2. Dose-Dependent Effects of Bryostatin-1 in Altering Neutrophil Activation and Transmigration
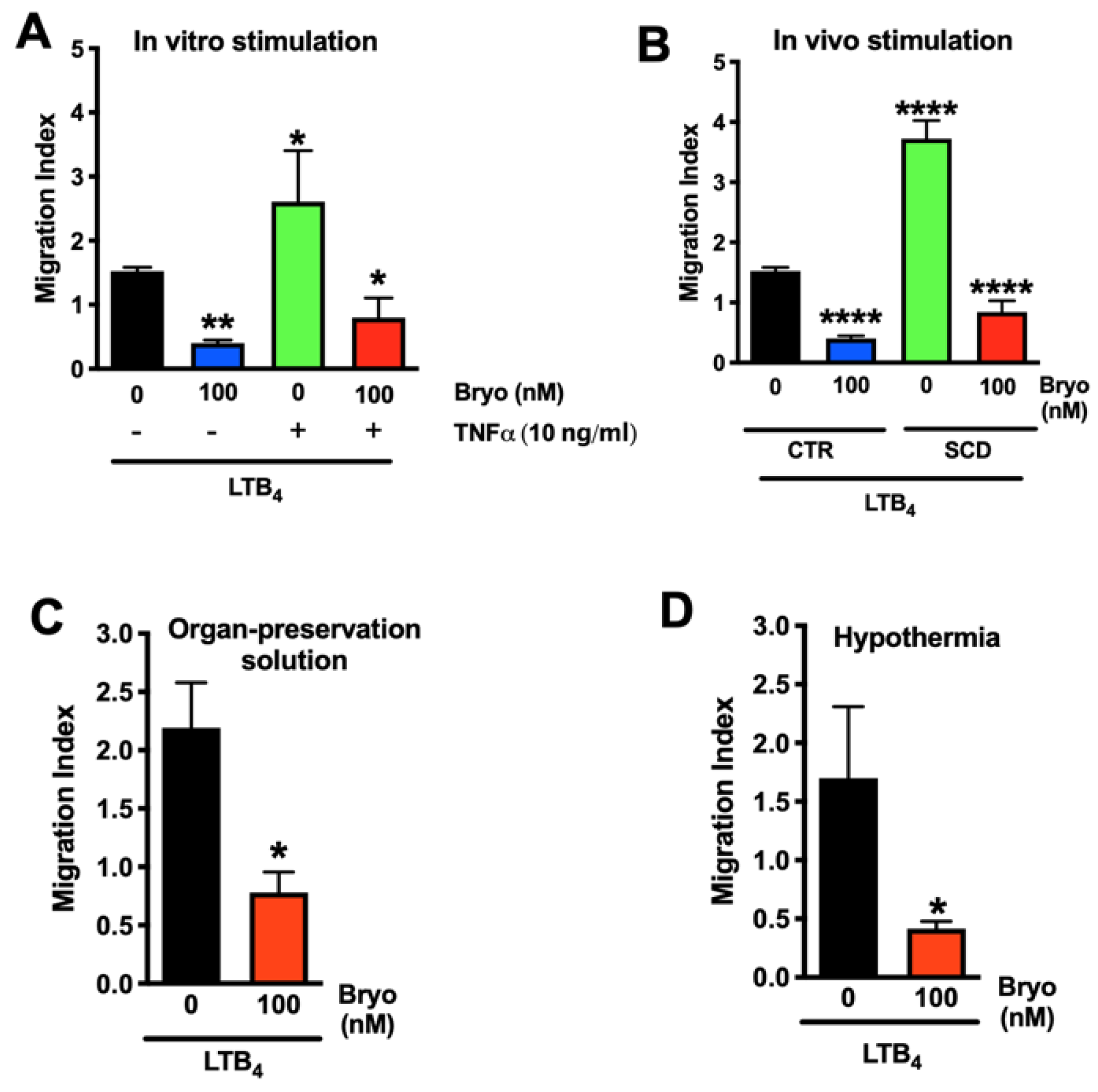
3.3. Bryostatin-1 Reduces Neutrophil TEM under Clinically Relevant Conditions
3.4. Bryostatin-1 Acts by Altering Neutrophil-Endothelial Cell Crosstalk Pathways
3.5. Bryostatin-1 Attenuates Neutrophil Transmigration in Renal Autografts
3.6. Bryostatin-1 Protects Renal Autografts from Ischemia-Reperfusion Injury
3.7. Bryostatin-1 Improves Kidney Function following Renal Autotransplantation
4. Discussion
5. Conclusions
6. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hart, A.; Lentine, K.L.; Smith, J.M.; Miller, J.M.; Skeans, M.A.; Prentice, M.; Robinson, A.; Foutz, J.; Booker, S.E.; Israni, A.K.; et al. OPTN/SRTR 2019 Annual Data Report: Kidney. Am. J. Transplant. 2021, 21 (Suppl. S2), 21–137. [Google Scholar] [CrossRef] [PubMed]
- Debout, A.; Foucher, Y.; Trebern-Launay, K.; Legendre, C.; Kreis, H.; Mourad, G.; Garrigue, V.; Morelon, E.; Buron, F.; Rostaing, L.; et al. Each additional hour of cold ischemia time significantly increases the risk of graft failure and mortality following renal transplantation. Kidney Int. 2015, 87, 343–349. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eltzschig, H.K.; Eckle, T. Ischemia and reperfusion--from mechanism to translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kasimsetty, S.G.; McKay, D.B. Ischemia as a factor affecting innate immune responses in kidney transplantation. Curr. Opin. Nephrol. Hypertens. 2016, 25, 3–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zens, T.J.; Danobeitia, J.S.; Leverson, G.; Chlebeck, P.J.; Zitur, L.J.; Redfield, R.R.; D’Alessandro, A.M.; Odorico, S.; Kaufman, D.B.; Fernandez, L.A. The impact of kidney donor profile index on delayed graft function and transplant outcomes: A single-center analysis. Clin. Transplant. 2018, 32, e13190. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Song, S.H.; Lee, J.Y.; Kim, D.G.; Lee, J.G.; Kim, B.S.; Kim, M.S.; Huh, K.H. The recovery status from delayed graft function can predict long-term outcome after deceased donor kidney transplantation. Sci. Rep. 2017, 7, 13725. [Google Scholar] [CrossRef] [PubMed]
- Basile, D.P.; Yoder, M.C. Renal endothelial dysfunction in acute kidney ischemia reperfusion injury. Cardiovasc. Hematol. Disord. Drug Targets 2014, 14, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ponticelli, C. Ischaemia-reperfusion injury: A major protagonist in kidney transplantation. Nephrol. Dial. Transplant. 2014, 29, 1134–1140. [Google Scholar] [CrossRef] [PubMed]
- Bonventre, J.V.; Yang, L. Cellular pathophysiology of ischemic acute kidney injury. J. Clin. Investig. 2011, 121, 4210–4221. [Google Scholar] [CrossRef] [PubMed]
- Miura, M.; Fu, X.; Zhang, Q.W.; Remick, D.G.; Fairchild, R.L. Neutralization of Gro alpha and macrophage inflammatory protein-2 attenuates renal ischemia/reperfusion injury. Am. J. Pathol. 2001, 159, 2137–2145. [Google Scholar] [CrossRef]
- Awad, A.S.; Rouse, M.; Huang, L.; Vergis, A.L.; Reutershan, J.; Cathro, H.P.; Linden, J.; Okusa, M.D. Compartmentalization of neutrophils in the kidney and lung following acute ischemic kidney injury. Kidney Int. 2009, 75, 689–698. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kelly, K.J.; Williams, W.W., Jr.; Colvin, R.B.; Meehan, S.M.; Springer, T.A.; Gutierrez-Ramos, J.C.; Bonventre, J.V. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J. Clin. Investig. 1996, 97, 1056–1063. [Google Scholar] [CrossRef] [PubMed]
- Riera, M.; Torras, J.; Herrero, I.; Valles, J.; Paubert-Braquet, M.; Cruzado, J.M.; Alsina, J.; Grinyo, J.M. Neutrophils accentuate renal cold ischemia-reperfusion injury. Dose-dependent protective effect of a platelet-activating factor receptor antagonist. J. Pharmacol. Exp. Ther. 1997, 280, 786–794. [Google Scholar] [PubMed]
- Rouschop, K.M.; Roelofs, J.J.; Claessen, N.; da Costa Martins, P.; Zwaginga, J.J.; Pals, S.T.; Weening, J.J.; Florquin, S. Protection against renal ischemia reperfusion injury by CD44 disruption. J. Am. Soc. Nephrol. 2005, 16, 2034–2043. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiao, H.; Kohda, Y.; McLeroy, P.; Craig, L.; Housini, I.; Star, R.A. Alpha-melanocyte-stimulating hormone protects against renal injury after ischemia in mice and rats. J. Clin. Investig. 1997, 99, 1165–1172. [Google Scholar] [CrossRef] [PubMed]
- Kolaczkowska, E.; Kubes, P. Neutrophil recruitment and function in health and inflammation. Nat. Rev. Immunol. 2013, 13, 159–175. [Google Scholar] [CrossRef] [PubMed]
- Lorenowicz, M.J.; Fernandez-Borja, M.; Hordijk, P.L. cAMP signaling in leukocyte transendothelial migration. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 1014–1022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carpenter, A.C.; Alexander, J.S. Endothelial PKC delta activation attenuates neutrophil transendothelial migration. Inflamm. Res. 2008, 57, 216–229. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.; Nichols, A.; Song, J.C.; Mammen, J.; Calvo, I.; Worrell, R.T.; Cuppoletti, J.; Matlin, K.; Matthews, J.B. Bryostatin-1 attenuates TNF-induced epithelial barrier dysfunction: Role of novel PKC isozymes. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G703–G712. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Adair, C.D.; Coe, L.; Weeks, J.W.; Lewis, D.F.; Alexander, J.S. Activation of endothelial cells in preeclampsia: Increased neutrophil-endothelial adhesion correlates with up-regulation of adhesion molecule P-selectin in human umbilical vein endothelial cells isolated from preeclampsia. J. Soc. Gynecol. Investig. 1998, 5, 237–243. [Google Scholar] [CrossRef] [PubMed]
- Ansari, J.; Senchenkova, E.Y.; Vital, S.A.; Al-Yafeai, Z.; Kaur, G.; Sparkenbaugh, E.M.; Orr, A.W.; Pawlinski, R.; Hebbel, R.P.; Granger, D.N.; et al. Targeting the AnxA1/Fpr2/ALX pathway regulates neutrophil function, promoting thromboinflammation resolution in sickle cell disease. Blood 2021, 137, 1538–1549. [Google Scholar] [CrossRef]
- Liu, W.J.; Ernst, L.; Doorschodt, B.; Bednarsch, J.; Becker, F.; Nakatake, R.; Masano, Y.; Neumann, U.P.; Lang, S.A.; Boor, P.; et al. Orthotopic Kidney Auto-Transplantation in a Porcine Model Using 24 Hours Organ Preservation And Continuous Telemetry. J. Vis. Exp. 2020, 21, e61591. [Google Scholar] [CrossRef]
- Schreinemachers, M.C.; Doorschodt, B.M.; Florquin, S.; van den Bergh Weerman, M.A.; Reitsma, J.B.; Lai, W.; Sitzia, M.; Minor, T.M.; Tolba, R.H.; van Gulik, T.M. Improved preservation and microcirculation with POLYSOL after transplantation in a porcine kidney autotransplantation model. Nephrol. Dial. Transplant. 2009, 24, 816–824. [Google Scholar] [CrossRef] [Green Version]
- Duvigneau, J.C.; Hartl, R.T.; Groiss, S.; Gemeiner, M. Quantitative simultaneous multiplex real-time PCR for the detection of porcine cytokines. J. Immunol. Methods 2005, 306, 16–27. [Google Scholar] [CrossRef]
- Mohr, A.; Brockmann, J.G.; Becker, F. HTK-N: Modified Histidine-Tryptophan-Ketoglutarate Solution-A Promising New Tool in Solid Organ Preservation. Int. J. Mol. Sci. 2020, 21, 6468. [Google Scholar] [CrossRef]
- Kageyama, S.; Hirao, H.; Nakamura, K.; Ke, B.; Zhang, M.; Ito, T.; Aziz, A.; Oncel, D.; Kaldas, F.M.; Busuttil, R.W.; et al. Recipient HO-1 inducibility is essential for posttransplant hepatic HO-1 expression and graft protection: From bench-to-bedside. Am. J. Transplant. 2019, 19, 356–367. [Google Scholar] [CrossRef] [Green Version]
- Uchida, Y.; Freitas, M.C.; Zhao, D.; Busuttil, R.W.; Kupiec-Weglinski, J.W. The inhibition of neutrophil elastase ameliorates mouse liver damage due to ischemia and reperfusion. Liver Transplant. 2009, 15, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Nakazawa, D.; Kumar, S.V.; Marschner, J.; Desai, J.; Holderied, A.; Rath, L.; Kraft, F.; Lei, Y.; Fukasawa, Y.; Moeckel, G.W.; et al. Histones and Neutrophil Extracellular Traps Enhance Tubular Necrosis and Remote Organ Injury in Ischemic AKI. J. Am. Soc. Nephrol. 2017, 28, 1753–1768. [Google Scholar] [CrossRef] [Green Version]
- Okusa, M.D.; Linden, J.; Huang, L.; Rieger, J.M.; Macdonald, T.L.; Huynh, L.P. A(2A) adenosine receptor-mediated inhibition of renal injury and neutrophil adhesion. Am. J. Physiol. Renal Physiol. 2000, 279, F809–F818. [Google Scholar] [CrossRef] [Green Version]
- Kish, D.D.; Gorbachev, A.V.; Parameswaran, N.; Gupta, N.; Fairchild, R.L. Neutrophil expression of Fas ligand and perforin directs effector CD8 T cell infiltration into antigen-challenged skin. J. Immunol. 2012, 189, 2191–2202. [Google Scholar] [CrossRef] [Green Version]
- Kreisel, D.; Sugimoto, S.; Zhu, J.; Nava, R.; Li, W.; Okazaki, M.; Yamamoto, S.; Ibrahim, M.; Huang, H.J.; Toth, K.A.; et al. Emergency granulopoiesis promotes neutrophil-dendritic cell encounters that prevent mouse lung allograft acceptance. Blood 2011, 118, 6172–6182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scozzi, D.; Ibrahim, M.; Menna, C.; Krupnick, A.S.; Kreisel, D.; Gelman, A.E. The Role of Neutrophils in Transplanted Organs. Am. J. Transplant. 2017, 17, 328–335. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Safaeinejad, F.; Bahrami, S.; Redl, H.; Niknejad, H. Inhibition of Inflammation, Suppression of Matrix Metalloproteinases, Induction of Neurogenesis, and Antioxidant Property Make Bryostatin-1 a Therapeutic Choice for Multiple Sclerosis. Front. Pharmacol. 2018, 9, 625. [Google Scholar] [CrossRef]
- Tan, Z.; Lucke-Wold, B.P.; Logsdon, A.F.; Turner, R.C.; Tan, C.; Li, X.; Hongpaison, J.; Alkon, D.L.; Simpkins, J.W.; Rosen, C.L.; et al. Bryostatin extends tPA time window to 6 h following middle cerebral artery occlusion in aged female rats. Eur. J. Pharmacol. 2015, 764, 404–412. [Google Scholar] [CrossRef] [Green Version]
- Kornberg, M.D.; Smith, M.D.; Shirazi, H.A.; Calabresi, P.A.; Snyder, S.H.; Kim, P.M. Bryostatin-1 alleviates experimental multiple sclerosis. Proc. Natl. Acad. Sci. USA 2018, 115, 2186–2191. [Google Scholar] [CrossRef] [Green Version]
- Ariza, M.E.; Ramakrishnan, R.; Singh, N.P.; Chauhan, A.; Nagarkatti, P.S.; Nagarkatti, M. Bryostatin-1, a naturally occurring antineoplastic agent, acts as a Toll-like receptor 4 (TLR-4) ligand and induces unique cytokines and chemokines in dendritic cells. J. Biol. Chem. 2011, 286, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedewald, J.J.; Rabb, H. Inflammatory cells in ischemic acute renal failure. Kidney Int. 2004, 66, 486–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klausner, J.M.; Paterson, I.S.; Goldman, G.; Kobzik, L.; Rodzen, C.; Lawrence, R.; Valeri, C.R.; Shepro, D.; Hechtman, H.B. Postischemic renal injury is mediated by neutrophils and leukotrienes. Am. J. Physiol. 1989, 256, F794–F802. [Google Scholar] [CrossRef]
- Chen, L.; Shi, D.; Guo, M. The roles of PKC-delta and PKC-epsilon in myocardial ischemia/reperfusion injury. Pharmacol. Res. 2021, 170, 105716. [Google Scholar] [CrossRef]
- Inagaki, K.; Chen, L.; Ikeno, F.; Lee, F.H.; Imahashi, K.; Bouley, D.M.; Rezaee, M.; Yock, P.G.; Murphy, E.; Mochly-Rosen, D. Inhibition of delta-protein kinase C protects against reperfusion injury of the ischemic heart In Vivo. Circulation 2003, 108, 2304–2307. [Google Scholar] [CrossRef] [Green Version]
- Chen, L.; Hahn, H.; Wu, G.; Chen, C.H.; Liron, T.; Schechtman, D.; Cavallaro, G.; Banci, L.; Guo, Y.; Bolli, R.; et al. Opposing cardioprotective actions and parallel hypertrophic effects of delta PKC and epsilon PKC. Proc. Natl. Acad. Sci. USA 2001, 98, 11114–11119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Inagaki, K.; Hahn, H.S.; Dorn, G.W., 2nd; Mochly-Rosen, D. Additive protection of the ischemic heart ex vivo by combined treatment with delta-protein kinase C inhibitor and epsilon-protein kinase C activator. Circulation 2003, 108, 869–875. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chou, W.H.; Choi, D.S.; Zhang, H.; Mu, D.; McMahon, T.; Kharazia, V.N.; Lowell, C.A.; Ferriero, D.M.; Messing, R.O. Neutrophil protein kinase Cdelta as a mediator of stroke-reperfusion injury. J. Clin. Investig. 2004, 114, 49–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, N.; Kim, S.H.; Lee, S.M. Differential consequences of protein kinase C activation during early and late hepatic ischemic preconditioning. J. Physiol. Sci. 2012, 62, 199–209. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.K.; Hongpaisan, J.; Alkon, D.L. Postischemic PKC activation rescues retrograde and anterograde long-term memory. Proc. Natl. Acad. Sci. USA 2009, 106, 14676–14680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, M.K.; Hongpaisan, J.; Nelson, T.J.; Alkon, D.L. Poststroke neuronal rescue and synaptogenesis mediated In Vivo by protein kinase C in adult brains. Proc. Natl. Acad. Sci. USA 2008, 105, 13620–13625. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Zhang, G.; Song, Z.; Xiang, X.; Shu, S.; Liu, Z.; Yang, D.; Wei, Q.; Dong, Z. Protein Kinase C-delta Mediates Kidney Tubular Injury in Cold Storage-Associated Kidney Transplantation. J. Am. Soc. Nephrol. 2020, 31, 1050–1065. [Google Scholar] [CrossRef] [PubMed]
- Castor, T.P. Bryoid Composition, Methods of Making and Use Thereof. U.S. Patent 10,954,248, 23 March 2021. [Google Scholar]
- Castor, T.P. Supercritical Fluid Isolation of Bryostatin 1, SBIR Phase II Final Report. SBIR Grant No. 5 R44 CA 64017-03, 21 April 2001. [Google Scholar]
- Castor, T.P. Method and Apparatus for Isolating Therapeutic Compositions from Source Materials. U.S. Patent 5,750,709, 12 May 1998. [Google Scholar]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Becker, F.; Kebschull, L.; Rieger, C.; Mohr, A.; Heitplatz, B.; Van Marck, V.; Hansen, U.; Ansari, J.; Reuter, S.; Strücker, B.; et al. Bryostatin-1 Attenuates Ischemia-Elicited Neutrophil Transmigration and Ameliorates Graft Injury after Kidney Transplantation. Cells 2022, 11, 948. https://doi.org/10.3390/cells11060948
Becker F, Kebschull L, Rieger C, Mohr A, Heitplatz B, Van Marck V, Hansen U, Ansari J, Reuter S, Strücker B, et al. Bryostatin-1 Attenuates Ischemia-Elicited Neutrophil Transmigration and Ameliorates Graft Injury after Kidney Transplantation. Cells. 2022; 11(6):948. https://doi.org/10.3390/cells11060948
Chicago/Turabian StyleBecker, Felix, Linus Kebschull, Constantin Rieger, Annika Mohr, Barbara Heitplatz, Veerle Van Marck, Uwe Hansen, Junaid Ansari, Stefan Reuter, Benjamin Strücker, and et al. 2022. "Bryostatin-1 Attenuates Ischemia-Elicited Neutrophil Transmigration and Ameliorates Graft Injury after Kidney Transplantation" Cells 11, no. 6: 948. https://doi.org/10.3390/cells11060948






