New Aspects of Corpus Luteum Regulation in Physiological and Pathological Conditions: Involvement of Adipokines and Neuropeptides
Abstract
:1. Introduction
2. Corpus Luteum Structure and Physiology
3. Characteristic of Adipokines and Neuropeptides, Their Receptors, and Mechanism of Action
3.1. Leptin
3.2. Adiponectin
3.3. Apelin
3.4. Visfatin
3.5. Vaspin
3.6. Chemerin
3.7. Orexins
3.8. Ghrelin
3.9. Kisspeptin
3.10. Phoenixin
4. Expression and Function of Adipokines and Neuropeptides in the Corpus Luteum
4.1. Leptin
4.2. Adiponectin
4.3. Apelin
4.4. Visfatin
4.5. Vaspin
4.6. Chemerin
4.7. Orexins
4.8. Ghrelin
4.9. Kisspeptin
4.10. Phoenixin
5. Involvement of Adipokines and Neuropeptides in Corpus Luteum Pathology
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arosh, J.A.; Banu, S.K.; Chapdelaine, P.; Madore, E.; Sirois, J.; Fortier, M.A. Prostaglandin biosynthesis, transport, and signaling in corpus luteum: A basis for autoregulation of luteal function. Endocrinology 2004, 145, 2551–2560. [Google Scholar] [CrossRef] [PubMed]
- Ricke, W.A.; Redmer, D.A.; Reynolds, L.P. Growth and cellular proliferation of pig corpora lutea throughout the oestrous cycle. J. Reprod Fert 1999, 117, 369–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bopp, B.; Shoupe, D. Luteal phase defects. J. Reprod. Med. 1993, 38, 348–356. [Google Scholar] [PubMed]
- Coomarasamy, A.; Williams, H.; Truchanowicz, E.; Seed, P.T.; Small, R.; Quenby, S.; Gupta, P.; Dawood, F.; Koot, Y.E.; Bender Atik, R.; et al. A randomized trial of progesterone in women with recurrent miscarriages. N. Eng. J. Med. 2015, 373, 2141–2148. [Google Scholar] [CrossRef] [PubMed]
- Bowen-Shauver, J.M.; Gibori, G. The corpus luteum of pregnancy. Ovary 2014, 4, 201–230. [Google Scholar]
- Sorianello, E.; Fritz, S.; Beyer, C.; Hales, D.B.; Mayerhofer, A.; Libertun, C.; Lux-Lantos, V. Development of an experimental ovarian tumor: Immunocytochemical analysis. Europ. J. Endocr. 2002, 147, 387–395. [Google Scholar] [CrossRef] [Green Version]
- Numanoglu, C.; Guler, S.; Ozaydin, I.; Han, A.; Ulker, V.; Akbayir, O. Stromal luteoma of the ovary: A rare ovarian pathology. J. Obstet. Gynaecol. 2015, 35, 420–421. [Google Scholar] [CrossRef]
- Choi, J.R.; Levine, D.; Finberg, H. Luteoma of pregnancy: Sonographic findings in two cases. J. Ultrasound Med. 2000, 19, 877–881. [Google Scholar] [CrossRef]
- Evans, M.C.; Anderson, G.M. Neuroendocrine integration of nutritional signals on reproduction. J. Mol. Endocrinol. 2017, 58, 107–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchell, M.; Armstrong, D.T.; Robker, R.L.; Norman, R.J. Adipokines: Implications for female fertility and obesity. Reproduction 2005, 130, 583–597. [Google Scholar] [CrossRef] [Green Version]
- Billert, M.; Rak, A.; Nowak, K.W.; Skrzypski, M. Phoenixin: More than reproductive peptide. Int. J. Mol. Sci. 2020, 21, 8378. [Google Scholar] [CrossRef] [PubMed]
- Skorupskaite, K.; George, J.T.; Anderson, R.A. The kisspeptin-GnRH pathway in human reproductive health and disease. Hum. Reprod. Update 2014, 20, 485–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, B.D. Luteinization. In The Ovary; Leung, P.C.K., Adashi, E.Y., Eds.; Elsevier Inc.: San Diego, CA, USA, 2004; pp. 85–200. [Google Scholar]
- Reynolds, L.P.; Grazul-Bilska, A.T.; Redmer, D.A. Angiogenesis in the corpus luteum. Endocrine 2000, 12, 1–9. [Google Scholar] [CrossRef]
- Davis, J.S.; Rueda, B.R.; Spanel-Borowski, K. Microvascular endothelial cells of the corpus luteum. Reprod. Biol. Endocrinol. 2003, 10, 89. [Google Scholar] [CrossRef] [Green Version]
- Maroni, D.; Davis, J.S. Transforming growth factor Beta 1 stimulates profibrotic activities of luteal fibroblasts in cows. Biol. Reprod. 2012, 127, 1–11. [Google Scholar] [CrossRef]
- Walusimbi, S.S.; Pate, J.L. Physiology and endocrinology symposium: Role of immune cells in the corpus luteum. J. Anim. Sci. 2013, 91, 1650–1659. [Google Scholar] [CrossRef] [PubMed]
- Murphy, B.D. Models of luteinization. Biol. Reprod. 2000, 63, 2–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meidan, R.; Levy, N. Endothelin-1 receptors and biosynthesis in the corpus luteum: Molecular and physiological implications. Domest. Anim. Endocrinol. 2002, 23, 287–298. [Google Scholar] [CrossRef]
- Niswender, G.D. Molecular control of luteal secretion of progesterone. Reproduction 2002, 123, 333–339. [Google Scholar] [CrossRef] [PubMed]
- Stocco, D.M.; Clark, B.J. Role of the steroidogenic acute regulatory protein (StAR) in steroidogenesis. Biochem. Pharmacol. 1996, 51, 197–205. [Google Scholar] [CrossRef]
- Niswender, G.D.; Juengel, J.L.; Silva, P.J.; Rollyson, M.K.; McIntush, E.W. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000, 80, 1–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, B.D.; Gévry, N.; Ruiz-Cortés, T.; Coté, F.; Downey, B.R.; Sirois, J. Formation and early development of the corpus luteum in pigs. Reprod. Suppl. 2001, 58, 47–63. [Google Scholar] [CrossRef]
- Pratt, B.R.; Butcher, R.L.; Inskeep, E.K. Antiluteolytic effect of the conceptus and of PGE2 in ewes. J. Anim. Sci. 1977, 45, 784–791. [Google Scholar] [CrossRef] [PubMed]
- Magness, R.R.; Huie, J.M.; Hoyer, G.L.; Huecksteadt, T.P.; Reynolds, L.P.; Seperich, G.J.; Whysong, G.; Weems, C.W. Effect of chronic ipsilateral or contralateral intrauterine infusion of prostaglandin E2 (PGE2) on luteal function of unilaterally ovariectomized ewes. Prostaglandins Med. 1981, 6, 389–401. [Google Scholar] [CrossRef]
- Diaz, F.J.; Anderson, L.E.; Wu, Y.L.; Rabot, A.; Tsai, S.J.; Wiltbank, M.C. Regulation of progesterone and prostaglandin F2alpha production in the CL. Mol. Cell. Endocrinol. 2002, 191, 65–80. [Google Scholar] [CrossRef]
- Wiltbank, M.C.; Salih, S.M.; Atli, M.O.; Luo, W.; Bormann, C.L.; Ottobre, J.S.; Vezina, C.M.; Mehta, V.; Diaz, F.J.; Tsai, S.J.; et al. Comparison of endocrine and cellular mechanisms regulating the corpus luteum of primates and ruminants. Anim. Reprod. 2012, 9, 242–259. [Google Scholar]
- Stocco, C.; Telleria, C.; Gibori, G. The molecular control of corpus luteum formation, function, and regression. Endocr. Rev. 2007, 28, 117–149. [Google Scholar] [CrossRef] [PubMed]
- Ahima, R.S.; Prabakaran, D.; Mantzoros, C.; Qu, D.; Lowell, B.; Maratos-Flier, E.; Flier, J.S. Role of leptin in the neuroendocrine response to fasting. Nature 1996, 382, 250–252. [Google Scholar] [CrossRef] [PubMed]
- Saladin, R.; De Vos, P.; Guerre-Millo, M.; Leturque, A.; Girard, J.; Staels, B.; Auwerx, J. Transient increase in obese gene expression after food intake or insulin administration. Nature 1995, 12, 527–539. [Google Scholar] [CrossRef] [PubMed]
- Denver, R.J.; Bonett, R.M.; Boorse, G.C. Evolution of leptin structure and function. Neuroendocrinology 2011, 94, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Seroussi, E.; Cinnamon, Y.; Yosefi, S.; Genin, O.; Smith, J.G.; Rafati, N.; Bornelöv, S.; Andersson, L.; Friedman-Einat, M. Identification of the long-sought leptin in chicken and duck: Expression pattern of the highly GC-Rich avian leptin fits an autocrine/paracrine rather than endocrine function. Endocrinology 2016, 157, 737–751. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, H.K.; Ahima, R.S. Physiology of leptin: Energy homeostasis, neuroendocrine function and metabolism. Metabolism 2015, 64, 24–34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardi, O.; Estienne, A.; Reverchon, M.; Bigot, Y.; Froment, P.; Dupont, J. Adipokines in metabolic and reproductive functions in birds: An overview of current knowns and unknowns. Mol. Cell. Endocrinol. 2021, 534, 111370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, Y.; Heiman, M.; DiMarchi, R. Leptin: Structure, function and biology. Vitam. Horm. 2005, 71, 345–372. [Google Scholar] [CrossRef] [PubMed]
- Tartaglia, L.A.; Dembski, M.; Weng, X.; Deng, N.; Culpepper, J.; Devos, R.; Richards, G.J.; Campfield, L.A.; Clark, F.T.; Deeds, J.; et al. Identification and expression cloning of a leptin receptor, OB-R. Cell 1995, 83, 1263–1271. [Google Scholar] [CrossRef] [Green Version]
- Minokoshi, Y.; Kim, Y.-B.; Peroni, O.D.; Fryer, L.G.D.; Müller, C.; Carling, D.; Kahn, B.B. Leptin stimulates fatty-acid oxidation by activating AMP-Activated protein kinase. Nature 2002, 415, 339–343. [Google Scholar] [CrossRef]
- Estienne, A.; Brossaud, A.; Reverchon, M.; Ramé, C.; Froment, P.; Dupont, J. Adipokines expression and effects in oocyte maturation, fertilization and early embryo development: Lessons from mammals and birds. Int. J. Mol. Sci. 2020, 21, 3581. [Google Scholar] [CrossRef] [PubMed]
- Maeda, K.; Okubo, K.; Shimomura, I.; Funahashi, T.; Matsuzawa, Y.; Matsubara, K. CDNA cloning and expression of a novel adipose specific collagen-like factor, ApM1 (AdiposeMost Abundant Gene Transcript 1). Biochem. Biophys. Res. Commun. 1996, 221, 286–289. [Google Scholar] [CrossRef]
- Hu, E.; Liang, P.; Spiegelman, B.M. AdipoQ is a novel adipose-specific gene dysregulated in obesity. J. Biol. Chem. 1996, 271, 10697–10703. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arita, Y.; Kihara, S.; Ouchi, N.; Takahashi, M.; Maeda, K.; Miyagawa, J.; Hotta, K.; Shimomura, I.; Nakamura, T.; Miyaoka, K.; et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem. Biophys. Res. Commun. 1999, 257, 79–83. [Google Scholar] [CrossRef]
- Choi, H.M.; Doss, H.M.; Kim, K.S. Multifaceted physiological roles of adiponectin in inflammation and diseases. Int. J. Mol. Sci. 2020, 21, 1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fisher, F.F.M.; Trujillo, M.E.; Hanif, W.; Barnett, A.H.; McTernan, P.G.; Scherer, P.E.; Kumar, S. Serum high molecular weight complex of adiponectin correlates better with glucose tolerance than total serum adiponectin in Indo-Asian males. Diabetologia 2005, 48, 1084–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, M.; Liu, F. Regulation of adiponectin multimerization, signaling and function. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 25–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hendricks, G.L.; Hadley, J.A.; Krzysik-Walker, S.M.; Prabhu, K.S.; Vasilatos-Younken, R.; Ramachandran, R. Unique profile of chicken adiponectin, a predominantly heavy molecular weight multimer, and relationship to visceral adiposity. Endocrinology 2009, 150, 3092–3100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramachandran, R.; Maddineni, S.; Ocón-Grove, O.; Hendricks, G.; Vasilatos-Younken, R.; Hadley, J.A. Expression of adiponectin and its receptors in avian species. Gen. Comp. Endocrinol. 2013, 190, 88–95. [Google Scholar] [CrossRef]
- Yamauchi, T.; Kamon, J.; Ito, Y.; Tsuchida, A.; Yokomizo, T.; Kita, S.; Sugiyama, T.; Miyagishi, M.; Hara, K.; Tsunoda, M.; et al. Cloning of adiponectin receptors that mediate antidiabetic metabolic effects. Nature 2003, 423, 762–769. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, S.; Kita, S.; Obata, Y.; Fujishima, Y.; Nagao, H.; Masuda, S.; Tanaka, Y.; Nishizawa, H.; Funahashi, T.; Takagi, J.; et al. The unique Prodomain of T-Cadherin plays a key role in adiponectin binding with the essential extracellular cadherin repeats 1 and 2. J. Biol. Chem. 2017, 292, 7840–7849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diot, M.; Reverchon, M.; Rame, C.; Froment, P.; Brillard, J.-P.; Brière, S.; Levêque, G.; Guillaume, D.; Dupont, J. Expression of adiponectin, chemerin and Visfatin in plasma and different tissues during a laying season in turkeys. Reprod. Biol. Endocrinol. 2015, 13, 81. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Z.; Li, J.; Luo, L.; Li, X.; Liu, M.; Gao, M.; Yin, Y.; Luan, X. Molecular cloning and expression analysis of adiponectin and its receptors (AdipoR1 and AdipoR2) in the hypothalamus of the Huoyan goose during different stages of the egg-laying cycle. Reprod. Biol. Endocrinol. 2015, 13, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dadson, K.; Chasiotis, H.; Wannaiampikul, S.; Tungtrongchitr, R.; Xu, A.; Sweeney, G. Adiponectin mediated APPL1-AMPK signaling induces cell migration, MMP activation, and collagen remodeling in cardiac fibroblasts: Adiponectin action in cardiac fibroblasts. J. Cel. Biochem. 2014, 115, 785–793. [Google Scholar] [CrossRef] [PubMed]
- Xu, N.; Zhang, Y.; Doycheva, D.M.; Ding, Y.; Zhang, Y.; Tang, J.; Guo, H.; Zhang, J.H. Adiponectin attenuates neuronal apoptosis induced by hypoxia-ischemia via the activation of AdipoR1/APPL1/LKB1/AMPK pathway in neonatal rats. Neuropharmacology 2018, 133, 415–428. [Google Scholar] [CrossRef] [PubMed]
- Yan, J.; Gan, L.; Chen, D.; Sun, C. Adiponectin impairs chicken preadipocytes differentiation through P38 MAPK/ATF-2 and TOR/P70 S6 kinase pathways. PLoS ONE 2013, 8, e77716. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabrolle, C.; Tosca, L.; Dupont, J. Regulation of adiponectin and its receptors in rat ovary by human chorionic gonadotrophin treatment and potential involvement of adiponectin in granulosa cell steroidogenesis. Reproduction 2007, 133, 719–731. [Google Scholar] [CrossRef] [PubMed]
- Chabrolle, C.; Tosca, L.; Ramé, C.; Lecomte, P.; Royère, D.; Dupont, J. Adiponectin increases insulin-like growth factor i-induced progesterone and estradiol secretion in human granulosa cells. Fertil. Steril. 2009, 92, 1988–1996. [Google Scholar] [CrossRef]
- Richards, J.S.; Liu, Z.; Kawai, T.; Tabata, K.; Watanabe, H.; Suresh, D.; Kuo, F.-T.; Pisarska, M.D.; Shimada, M. Adiponectin and its receptors modulate granulosa cell and cumulus cell functions, fertility, and early embryo development in the mouse and human. Fertil. Steril. 2012, 98, 471–479.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chabrolle, C.; Tosca, L.; Crochet, S.; Tesseraud, S.; Dupont, J. Expression of adiponectin and its receptors (AdipoR1 and AdipoR2) in chicken ovary: Potential role in ovarian steroidogenesis. Domest. Anim. Endocrinol. 2007, 33, 480–487. [Google Scholar] [CrossRef] [PubMed]
- Mellouk, N.; Ramé, C.; Delaveau, J.; Rat, C.; Maurer, E.; Froment, P.; Dupont, J. Adipokines Expression profile in liver, adipose tissue and muscle during chicken embryo development. Gen. Comp. Endocrinol. 2018, 267, 146–156. [Google Scholar] [CrossRef]
- Tatemoto, K.; Hosoya, M.; Habata, Y.; Fujii, R.; Kakegawa, T.; Zou, M.-X.; Kawamata, Y.; Fukusumi, S.; Hinuma, S.; Kitada, C.; et al. Isolation and characterization of a novel endogenous peptide ligand for the human APJ receptor. Biochem. Biophys. Res. Commun. 1998, 251, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.K.; Saldivia, V.R.; Nguyen, T.; Cheng, R.; George, S.R.; O’Dowd, B.F. Modification of the terminal residue of apelin-13 antagonizes its hypotensive action. Endocrinology 2005, 146, 231–236. [Google Scholar] [CrossRef]
- O’Carroll, A.M.; Lolait, S.J.; Harris, L.E.; Pope, G.R. The apelin receptor APJ: Journey from an orphan to a multifaceted regulator of homeostasis. J. Endocrinol. 2013, 219, R13–R35. [Google Scholar] [CrossRef]
- O’Dowd, B.F.; Heiber, M.; Chan, A.; Heng, H.H.Q.; Tsui, L.-C.; Kennedy, J.L.; Shi, X.; Petronis, A.; George, S.R.; Nguyen, T. A human gene that shows identity with the gene encoding the angiotensin receptor is located on chromosome 11. Gene 1993, 136, 355–360. [Google Scholar] [CrossRef]
- Masri, B.; Morin, N.; Pedebernade, L.; Knibiehler, B.; Audigier, Y. The apelin receptor is coupled to Gi1 or Gi2 protein and is differentially desensitized by apelin fragments. J. Biol. Chem. 2006, 281, 18317–18326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estienne, A.; Bongrani, A.; Reverchon, M.; Ramé, C.; Ducluzeau, P.-H.; Froment, P.; Dupont, J. Involvement of novel adipokines, chemerin, Visfatin, Resistin and apelin in reproductive functions in normal and pathological conditions in humans and animal models. Int. J. Mol. Sci. 2019, 20, 4431. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pope, G.R.; Roberts, E.M.; Lolait, S.J.; O’Carroll, A.-M. Central and peripheral apelin receptor distribution in the mouse: Species differences with rat. Peptides 2012, 33, 139–148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rak, A.; Drwal, E.; Rame, C.; Knapczyk-Stwora, K.; Słomczyńska, M.; Dupont, J.; Gregoraszczuk, E.L. Expression of apelin and apelin receptor (APJ) in porcine ovarian follicles and in vitro effect of apelin on steroidogenesis and proliferation through APJ activation and different signaling pathways. Theriogenology 2017, 96, 126–135. [Google Scholar] [CrossRef] [PubMed]
- Różycka, M.; Kurowska, P.; Grzesiak, M.; Kotula-Balak, M.; Tworzydło, W.; Rame, C.; Gregoraszczuk, E.; Dupont, J.; Rak, A. Apelin and apelin receptor at different stages of corpus luteum development and effect of apelin on progesterone secretion and 3β-Hydroxysteroid dehydrogenase (3β-HSD) in pigs. Anim. Reprod. Sci. 2018, 192, 251–260. [Google Scholar] [CrossRef] [PubMed]
- Roche, J.; Ramé, C.; Reverchon, M.; Mellouk, N.; Rak, A.; Froment, P.; Dupont, J. Apelin (APLN) regulates progesterone secretion and oocyte maturation in bovine ovarian cells. Reproduction 2017, 153, 589–603. [Google Scholar] [CrossRef] [Green Version]
- Samal, B.; Sun, Y.; Stearns, G.; Xie, C.; Suggs, S.; Mcniece, I. Cloning and characterization of the CDNA encoding a novel human Pre-B-Cell Colony-Enhancing factor. Mol. Cell. Biol. 1994, 14, 7. [Google Scholar] [CrossRef]
- Rongvaux, A.; Shea, R.J.; Mulks, M.H.; Gigot, D.; Urbain, J.; Leo, O.; Andris, F. Pre-B-Cell colony-enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide Phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur. J. Immunol. 2002, 32, 3225–3234. [Google Scholar] [CrossRef]
- Revollo, J.R.; Grimm, A.A.; Imai, S. The Regulation of nicotinamide adenine dinucleotide biosynthesis by Nampt/PBEF/Visfatin in Mammals. Curr. Opin. Gastroenterol. 2007, 23, 164–170. [Google Scholar] [CrossRef]
- Yoon, M.J.; Yoshida, M.; Johnson, S.; Takikawa, A.; Usui, I.; Tobe, K.; Nakagawa, T.; Yoshino, J.; Imai, S. SIRT1-Mediated ENAMPT secretion from adipose tissue regulates hypothalamic NAD+ and function in mice. Cell Metab. 2015, 21, 706–717. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Audrito, V.; Managò, A.; Zamporlini, F.; Rulli, E.; Gaudino, F.; Madonna, G.; D’Atri, S.; Antonini Cappellini, G.C.; Ascierto, P.A.; Massi, D.; et al. Extracellular nicotinamide Phosphoribosyltransferase (ENAMPT) is a novel marker for patients with BRAF-Mutated metastatic melanoma. Oncotarget 2018, 9, 18997–19005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Meng, F.; Song, C.; Wang, Y.; Leung, F.C. Characterization of chicken Visfatin gene: CDNA cloning, tissue distribution, and promoter analysis. Poult. Sci. 2012, 91, 2885–2894. [Google Scholar] [CrossRef] [PubMed]
- Krzysik-Walker, S.M.; Hadley, J.A.; Pesall, J.E.; McFarland, D.C.; Vasilatos-Younken, R.; Ramachandran, R. Nampt/Visfatin/PBEF affects expression of myogenic regulatory factors and is regulated by Interleukin-6 in chicken skeletal muscle cells. Comp. Biochem. Physiol. Part A Mol. Integr. Physiol. 2011, 159, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Krzysik-Walker, S.M.; Ocón-Grove, O.M.; Maddineni, S.R.; Hendricks, G.L.; Ramachandran, R. Is Visfatin an adipokine or myokine? Evidence for greater Visfatin expression in skeletal muscle than visceral fat in chickens. Endocrinology 2008, 149, 1543–1550. [Google Scholar] [CrossRef]
- Xie, H.; Tang, S.-Y.; Luo, X.-H.; Huang, J.; Cui, R.-R.; Yuan, L.-Q.; Zhou, H.-D.; Wu, X.-P.; Liao, E.-Y. Insulin-Like Effects of Visfatin on human osteoblasts. Calcif. Tissue Int. 2007, 80, 201–210. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.E.P.; Onyango, D.J.; Ramanjaneya, M.; Conner, A.C.; Patel, S.T.; Dunmore, S.J.; Randeva, H.S. Visfatin regulates insulin secretion, insulin receptor Signalling and MRNA expression of diabetes-related genes in mouse pancreatic β-Cells. J. Mol. Endocrinol. 2010, 44, 171–178. [Google Scholar] [CrossRef]
- Jacques, C.; Holzenberger, M.; Mladenovic, Z.; Salvat, C.; Pecchi, E.; Berenbaum, F.; Gosset, M. Proinflammatory Actions of Visfatin/Nicotinamide Phosphoribosyltransferase (Nampt) involve regulation of insulin signaling pathway and Nampt enzymatic Activity. J. Biol. Chem. 2012, 287, 15100–15108. [Google Scholar] [CrossRef] [Green Version]
- Managò, A.; Audrito, V.; Mazzola, F.; Sorci, F.; Gaudino, F.; Gizzi, K.; Vitale, N.; Incarnato, D.; Minazzato, G.; Ianniello, A.; et al. Extracellular nicotinate phosphoribosyltransferase binds Toll like receptor 4 and mediates inflammation. Nat. Commun. 2019, 10, 4116. [Google Scholar] [CrossRef]
- Romacho, T.; Valencia, I.; Ramos-González, M.; Vallejo, S.; López-Esteban, M.; Lorenzo, O.; Cannata, P.; Romero, A.; Hipólito-Luengo, A.S.; Gómez-Cerezo, J.F.; et al. Visfatin/eNampt induces endothelial dysfunction in vivo: A role for Toll-Like Receptor 4 and NLRP3 inflammasome. Sci. Rep. 2020, 10, 5386. [Google Scholar] [CrossRef] [Green Version]
- Ons, E.; Gertler, A.; Buyse, J.; Lebihan-Duval, E.; Bordas, A.; Goddeeris, B.; Dridi, S. Visfatin gene expression in chickens is sex and tissue dependent. Dom. Anim. Endocrinol. 2010, 38, 63–74. [Google Scholar] [CrossRef]
- Reverchon, M.; Rame, C.; Bunel, A.; Chen, W.; Froment, P.; Dupont, J. VISFATIN (NAMPT) Improves in Vitro IGF1-Induced Steroidogenesis and IGF1 Receptor Signaling Through SIRT1 in bovine granulosa Cells1. Biol. Reprod. 2016, 54, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Choi, K.-H.; Joo, B.-S.; Sun, S.-T.; Park, M.-J.; Son, J.-B.; Joo, J.-K.; Lee, K.-S. Administration of Visfatin during superovulation improves developmental competency of oocytes and fertility potential in aged female mice. Fertil. Steril. 2012, 97, 1234–1241.e3. [Google Scholar] [CrossRef] [PubMed]
- Reverchon, M.; Cornuau, M.; Cloix, L.; Rame, C.; Guerif, F.; Royere, D.; Dupont, J. Visfatin is expressed in human granulosa cells: Regulation by metformin through AMPK/SIRT1 pathways and its role in steroidogenesis. Mol. Hum. Reprod. 2013, 19, 313–326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diot, M.; Reverchon, M.; Ramé, C.; Baumard, Y.; Dupont, J. Expression and effect of NAMPT (Visfatin) on progesterone secretion in hen granulosa cells. Reproduction 2015, 150, 53–63. [Google Scholar] [CrossRef] [Green Version]
- Riammer, S.; Garten, A.; Schaab, M.; Grunewald, S.; Kiess, W.; Kratzsch, J.; Paasch, U. Nicotinamide Phosphoribosyltransferase production in human spermatozoa is influenced by maturation stage. Andrology 2016, 4, 1045–1053. [Google Scholar] [CrossRef]
- Jeremy, M.; Gurusubramanian, G.; Roy, V.K. Localization pattern of Visfatin (NAMPT) in d-galactose induced aged rat testis. Ann. Anat. 2017, 211, 46–54. [Google Scholar] [CrossRef]
- Hida, K.; Wada, J.; Eguchi, J.; Zhang, H.; Baba, M.; Seida, A.; Hashimoto, I.; Okada, T.; Yasuhara, A.; Nakatsuka, A.; et al. Visceral adipose tissue-derived serine protease inhibitor: A unique insulin-sensitizing adipocytokine in obesity. Proc. Natl. Acad. Sci. USA 2005, 102, 10610–10615. [Google Scholar] [CrossRef] [Green Version]
- Heiker, J.T.; Klöting, N.; Kovacs, P.; Kuettner, E.B.; Sträter, N.; Schultz, S.; Kern, M.; Stumvoll, M.; Blüher, M.; Beck-Sickinger, A.G. Vaspin inhibits kallikrein 7 by serpin mechanism. Cell. Mol. Life Sci. 2013, 70, 2569–2583. [Google Scholar] [CrossRef] [Green Version]
- Ulbricht, D.; Tindall, C.A.; Oertwig, K.; Hanke, S.; Sträter, N.; Heiker, J.T. Kallikrein-related peptidase 14 is the second KLK protease targeted by the serpin vaspin. Biol. Chem. 2018, 399, 1079–1084. [Google Scholar] [CrossRef]
- Heiker, J.T. Vaspin (serpinA12) in obesity, insulin resistance, and inflammation. J. Pept. Sci. 2014, 20, 299–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oertwig, K.; Ulbricht, D.; Hanke, S.; Pippel, J.; Bellmann-Sickert, K.; Sträter, N.; Heiker, J.T. Glycosylation of human vaspin (SERPINA12) and its impact on serpin activity, heparin binding and thermal stability. Biochim. Biophys. Acta Proteins Proteom 2017, 1865, 1188–1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Körner, A.; Neef, M.; Friebe, D.; Erbs, S.; Kratzsch, J.; Dittrich, K.; Blüher, S.; Kapellen, T.M.; Kovacs, P.; Stumvoll, M.; et al. Vaspin is related to gender, puberty and deteriorating insulin sensitivity in children. Int. J. Obes. 2011, 35, 578–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saalbach, A.; Vester, K.; Rall, K.; Tremel, J.; Anderegg, U.; Beck-Sickinger, A.G.; Blüher, M.; Simon, J.C. Vaspin-a link of obesity and psoriasis? Exp. Dermat. 2012, 21, 309–312. [Google Scholar] [CrossRef] [PubMed]
- Caminos, J.E.; Bravo, S.B.; Garcés, M.F.; González, C.R.; Cepeda, L.A.; González, A.C.; Nogueiras, R.; Gallego, R.; García-Caballero, T.; Cordido, F.; et al. Vaspin and amylin are expressed in human and rat placenta and regulated by nutritional status. Histol. Histopathol. 2009, 24, 979–990. [Google Scholar] [CrossRef]
- Klöting, N.; Kovacs, P.; Kern, M.; Heiker, J.T.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Beck-Sickinger, A.G.; Blüher, M. Central vaspin administration acutely reduces food intake and has sustained blood glucose-lowering effects. Diabetologia 2011, 54, 1819–1823. [Google Scholar] [CrossRef] [Green Version]
- Kurowska, P.; Mlyczyńska, E.; Barbe, A.; Staub, C.; Gregoraszczuk, E.; Dupont, J.; Rak, A. Vaspin in the pig ovarian follicles: Expression and regulation by different hormones. Reproduction 2019, 158, 135–146. [Google Scholar] [CrossRef] [PubMed]
- Feng, R.; Li, Y.; Wang, C.; Luo, C.; Liu, L.; Chuo, F.; Li, Q.; Sun, C. Higher vaspin levels in subjects with obesity and type 2 diabetes mellitus: A meta-analysis. Diabetes Res. Clin. Pract. 2014, 106, 88–94. [Google Scholar] [CrossRef]
- Vehapoğlu, A.; Ustabas, F.; Ozgen, T.I.; Terzioglu, S.; Cermik, B.B.; Ozen, O.F. Role of circulating adipocytokines vaspin, apelin, and visfatin in the loss of appetite in underweight children: A pilot trial. J. Pediatr. Endocrinol. Metab. 2015, 28, 1065–1071. [Google Scholar] [CrossRef]
- Klöting, N.; Berndt, J.; Kralisch, S.; Kovacs, P.; Fasshauer, M.; Schön, M.R.; Stumvoll, M.; Blüher, M. Vaspin gene expression in human adipose tissue: Association with obesity and type 2 diabetes. Biochem. Biophys. Res. Commun. 2006, 339, 430–436. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Jurek, M.; Klimczyk, D.; Dupont, J.; Rak, A. Review: Vaspin (SERPINA12) expression and function in endocrine cells. Cells 2021, 10, 1710. [Google Scholar] [CrossRef] [PubMed]
- Nakatsuka, A.; Wada, J.; Iseda, I.; Teshigawara, S.; Higashio, K.; Murakami, K.; Kanzaki, M.; Inoue, K.; Terami, T.; Katayama, A.; et al. Vaspin is an adipokine ameliorating ER stress in obesity as a ligand for cell-surface GRP78/MTJ-1 complex. Diabetes 2012, 61, 2823–2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, I.M.; Abdelmalek, D.H.; Elfiky, A.A. GRP78: A cell’s response to stress. Life Sci. 2019, 226, 156–163. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lee, J.; Liem, D.; Ping, P. HSPA5 Gene encoding Hsp70 chaperone BiP in the endoplasmic reticulum. Gene 2017, 618, 14–23. [Google Scholar] [CrossRef] [PubMed]
- Chevalier, M.; Rhee, H.; Elguindi, E.C.; Blond, S.Y. Interaction of Murine BiP/GRP78 with the DnaJ Homologue MTJ. J. Biol. Chem. 2000, 275, 19620–19627. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cree, L.; Hammond, E.R.; Shelling, A.; Berg, M.C.; Peek, J.C.; Green, M.P. Maternal age and ovarian stimulation independently affect oocyte mtDNA copy number and cumulus cell gene expression in bovine clones. Hum. Reprod. 2015, 30, 1410–1420. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, Y.; Sun, M.; Shan, Y.; Zheng, X.; Ma, H.; Ma, W.; Wang, Z.; Pei, X.; Wang, Y. Endoplasmic reticulum stress-mediated apoptotic pathway is involved in corpus luteum regression in rats. Reprod. Sci. 2015, 22, 572–584. [Google Scholar] [CrossRef] [Green Version]
- Thon, M.; Hosoi, T.; Yoshii, M.; Ozawa, K. Leptin induced GRP78 expression through the PI3K-mTOR pathway in neuronal cells. Sci. Rep. 2014, 4, 7096. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbe, A.; Kurowska, P.; Mlyczyńska, E.; Ramé, C.; Staub, C.; Venturi, E.; Billon, Y.; Rak, A.; Dupont, J. Adipokines expression profiles in both plasma and peri renal adipose tissue in Large White and Meishan sows: A possible involvement in the fattening and the onset of puberty. Gen. Comp. Endocrinol. 2020, 299, 113584. [Google Scholar] [CrossRef]
- Mote, P.L.; Tillman, J.B.; Spindler, S.R. Glucose regulation of GRP78 gene expression. Mech. Ageing Dev. 1998, 104, 149–158. [Google Scholar] [CrossRef]
- Lee, A.S. The glucose-regulated proteins: Stress induction and clinical applications. Trends Biochem. Sci. 2001, 26, 504–510. [Google Scholar] [CrossRef]
- Zhang, C. Roles of Grp78 in female mammalian reproduction. Adv. Anat. Embryol. Cell Biol. 2017, 222, 129–155. [Google Scholar] [CrossRef] [PubMed]
- Dores-Silva, P.R.; Cauvi, D.M.; Coto, A.L.S.; Kiraly, V.T.R.; Borges, J.C.; De Maio, A. Interaction of HSPA5 (Grp78, BIP) with negatively charged phospholipid membranes via oligomerization involving the N-terminal end domain. Cell Stress Chaperones. 2020, 25, 979–991. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Li, X.; Wu, Y.; Duan, R.; Zhang, J.; Du, F.; Zhang, Q.; Li, Y.; Li, N. Effects of vaspin on pancreatic β cell secretion via PI3K/Akt and NF-κB signaling pathways. PLoS ONE 2017, 12, e0189722. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Xu, F.; Pei, H.X.; Zhu, X.; Lin, X.; Song, C.Y.; Liang, Q.H.; Liao, E.Y.; Yuan, L.Q. Vaspin regulates the osteogenic differentiation of MC3T3-E1 through the PI3K-Akt/miR-34c loop. Sci. Rep. 2016, 6, 25578. [Google Scholar] [CrossRef] [Green Version]
- Jung, C.H.; Lee, M.J.; Kang, Y.M.; Lee, Y.; Yoon, H.K.; Kang, S.-W.; Lee, W.J.; Park, J.Y. Vaspin inhibits cytokine-induced nuclear factor-kappa B activation and adhesion molecule expression via AMP-activated protein kinase activation in vascular endothelial cells. Cardiovas. Diabetol. 2014, 13, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Jiang, Y.; Shan, P.F.; Shen, J.; Liang, Q.H.; Cui, R.R.; Liu, Y.; Liu, G.Y.; Wu, S.S.; Lu, Q.; et al. Vaspin attenuates the apoptosis of human osteoblasts through ERK signaling pathway. Amino Acids 2013, 44, 961–968. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Dupont, J.; Rak, A. Role of vaspin in porcine ovary: Effect on signaling pathways and steroid synthesis via GRP78 receptor and protein kinase A. Biol. Reprod. 2020, 102, 1290–1305. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Opydo-Chanek, M.; Dupont, J.; Rak, A. In vitro effects of vaspin on porcine granulosa cell proliferation, cell cycle progression, and apoptosis by activation of GRP78 receptor and several kinase signaling pathways including MAP3/1, AKT, and STAT3. Int. J. Mol. Sci. 2019, 20, 5816. [Google Scholar] [CrossRef] [Green Version]
- Kurowska, P.; Mlyczyńska, E.; Estienne, A.; Barbe, A.; Rajska, I.; Soból, K.; Poniedziałek-Kempny, K.; Dupont, J.; Rak, A. Expression and impact of Vaspin on in vitro oocyte maturation through MAP3/1 and PRKAA1 Signalling pathways. Int. J. Mol. Sci. 2020, 21, 9342. [Google Scholar] [CrossRef]
- Meder, W.; Wendland, M.; Busmann, A.; Kutzleb, C.; Spodsberg, N.; John, H.; Richter, R.; Schleuder, D.; Meyer, M.; Forssmann, W.G. Characterization of human circulating TIG2 as a ligand for the orphan receptor ChemR23. FEBS Letters 2003, 555, 495–499. [Google Scholar] [CrossRef] [Green Version]
- Wittamer, V.; Franssen, J.D.; Vulcano, M.; Mirjolet, J.F.; le Poul, E.; Migeotte, I.; Brézillon, S.; Tyldesley, R.; Blanpain, C.; Detheux, M.; et al. Specific Recruitment of antigen-presenting cells by chemerin, a novel processed ligand from human inflammatory fluids. J. Exp. Med. 2003, 198, 977–985. [Google Scholar] [CrossRef] [PubMed]
- Nagpal, S.; Patel, S.; Jacobe, H.; DiSepio, D.; Ghosn, C.; Malhotra, M.; Teng, M.; Duvic, M.; Chandraratna, R.A.S. Tazarotene-Induced Gene 2 (TIG2), a novel retinoid-responsive gene in skin. J. Invest. Dermatol. 1997, 109, 91–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Zhang, J.; Lei, T.; Chen, X.; Zhang, Y.; Zhou, L.; Yu, A.; Chen, Z.; Yang, Z. Cloning of porcine chemerin, ChemR23 and GPR1 and their involvement in regulation of lipogenesis. BMB Reports 2010, 43, 491–498. [Google Scholar] [CrossRef] [PubMed]
- Du, X.-Y.; Leung, L.L.K. Proteolytic regulatory mechanism of chemerin bioactivity. Acta Biochim. Biophys. Sin. Shanghai 2009, 41, 973–979. [Google Scholar] [CrossRef] [Green Version]
- Zabel, B.A.; Allen, S.J.; Kulig, P.; Allen, J.A.; Cichy, J.; Handel, T.M.; Butcher, E.C. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J. Biol. Chem. 2005, 280, 34661–34666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mattern, A.; Zellmann, T.; Beck-Sickinger, A.G. Processing, signaling, and physiological function of chemerin. IUBMB Life 2014, 66, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Brunetti, L.; di Nisio, C.; Recinella, L.; Chiavaroli, A.; Leone, S.; Ferrante, C.; Orlando, G.; Vacca, M. Effects of Vaspin, chemerin and Omentin-1 on feeding behavior and hypothalamic peptide gene expression in the rat. Peptides 2011, 32, 1866–1871. [Google Scholar] [CrossRef]
- Garces, M.F.; Sanchez, E.; Acosta, B.J.; Angel, E.; Ruíz, A.I.; Rubio-Romero, J.A.; Diéguez, C.; Nogueiras, R.; Caminos, J.E. Expression and regulation of chemerin during rat pregnancy. Placenta 2012, 33, 373–378. [Google Scholar] [CrossRef]
- Roh, S.G.; Song, S.H.; Choi, K.C.; Katoh, K.; Wittamer, V.; Parmentier, M.; Sasaki, S.-I. Chemerin—A new adipokine that modulates adipogenesis via its own receptor. Biochem. Biophys. Res. Commun. 2007, 362, 1013–1018. [Google Scholar] [CrossRef] [Green Version]
- Song, S.H.; Fukui, K.; Nakajima, K.; Kozakai, T.; Sasaki, S.; Roh, S.G.; Katoh, K. Cloning, Expression analysis, and regulatory mechanisms of bovine chemerin and chemerin receptor. Domest. Anim. Endocrinol. 2010, 39, 97–105. [Google Scholar] [CrossRef] [PubMed]
- Goralski, K.B.; McCarthy, T.C.; Hanniman, E.A.; Zabel, B.A.; Butcher, E.C.; Parlee, S.D.; Muruganandan, S.; Sinal, C.J. Chemerin, a novel adipokine that regulates adipogenesis and adipocyte metabolism. J. Biol. Chem. 2007, 282, 28175–28188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bozaoglu, K.; Bolton, K.; McMillan, J.; Zimmet, P.; Jowett, J.; Collier, G.; Walder, K.; Segal, D. Chemerin is a novel adipokine associated with obesity and metabolic syndrome. Endocrinology 2007, 148, 4687–4694. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.E.; Muruganandan, S.; Ernst, M.C.; Parlee, S.D.; Zabel, B.A.; Butcher, E.C.; Sinal, C.J.; Goralski, K.B. Chemokine-like Receptor 1 regulates skeletal muscle cell myogenesis. Am. J. Physiol. Cell Physiol. 2012, 302, 1621–1631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garces, M.F.; Sanchez, E.; Ruíz-Parra, A.I.; Rubio-Romero, J.A.; Angel-Müller, E.; Suarez, M.A.; Bohórquez, L.F.; Bravo, S.B.; Nogueiras, R.; Diéguez, C.; et al. Serum chemerin levels during normal human pregnancy. Peptides 2013, 42, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, N.; Kiezun, M.; Dobrzyn, K.; Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Zaobidna, E.; Bogus-Nowakowska, K.; Wyrebek, J.; Bors, K.; et al. Expression of Chemerin and its receptors in the porcine hypothalamus and plasma chemerin levels during the Oestrous cycle and early pregnancy. Int. J. Mol. Sci. 2019, 20, 3887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Edinger, A.L.; Hoffman, T.L.; Sharron, M.; Lee, B.; O’Dowd, B.; Doms, R.W. Use of GPR1, GPR15, and STRL33 as coreceptors by diverse human immunodeficiency virus type 1 and simian immunodeficiency virus envelope proteins. Virology 1998, 249, 367–378. [Google Scholar] [CrossRef] [Green Version]
- Migeotte, I.; Franssen, J.D.; Goriely, S.; Willems, F.; Parmentier, M. Distribution and regulation of expression of the putative human chemokine receptor HCR in leukocyte populations. Eur. J. Immunol. 2002, 32, 494–501. [Google Scholar] [CrossRef]
- Reverchon, M.; Bertoldo, M.J.; Ramé, C.; Froment, P.; Dupont, J. CHEMERIN (RARRES2) Decreases in vitro granulosa cell steroidogenesis and blocks oocyte meiotic progression in bovine species1. Biol. Reprod. 2014, 90, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Zabel, B.A.; Nakae, S.; Zúñiga, L.; Kim, J.Y.; Ohyama, T.; Alt, C.; Pan, J.; Suto, H.; Soler, D.; Allen, S.J.; et al. Mast cell-expressed orphan receptor CCRL2 binds chemerin and is required for optimal induction of IgE-Mediated passive cutaneous anaphylaxis. J. Exp. Med. 2008, 205, 2207–2220. [Google Scholar] [CrossRef]
- Reverchon, M.; Cornuau, M.; Ramé, C.; Guerif, F.; Royère, D.; Dupont, J. Chemerin inhibits IGF-1-induced progesterone and estradiol secretion in human granulosa cells. Hum. Reprod. 2012, 27, 1790–1800. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Lecea, L.; Sutcliffe, G.J.; Fabre, V. Hypocretins/Orexins as integrators of physiological information: Lessons from mutant animals. Neuropeptides 2002, 36, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Sakurai, T.; Amemiya, A.; Ishii, M.; Matsuzaki, I.; Chemelli, R.M.; Tanaka, H.; Williams, S.C.; Richardson, J.A.; Kozlowski, G.P.; Wilson, S.; et al. Orexins and orexin receptors: A family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 1998, 92, 573–585. [Google Scholar] [CrossRef] [Green Version]
- De Lecea, L.; Kilduff, T.S.; Peyron, C.; Gao, X.-B.; Foye, P.E.; Danielson, P.E.; Fukuhara, C.; Battenberg, E.L.F.; Gautvik, V.T.; Bartlett, F.S.; et al. The hypocretins: Hypothalamus-Specific peptides with neuroexcitatory activity. Proc. Natl. Acad. Sci. USA 1998, 95, 322. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Malek, M.; Marklund, S.; Dyer, C.; Matteri, R.; Rothschild, M. Linkage and physical mapping of the porcine Prepro-Orexin gene. Mamm. Genome 2000, 11, 342–343. [Google Scholar] [CrossRef] [PubMed]
- Nambu, T.; Sakurai, T.; Mizukami, K.; Hosoya, Y.; Yanagisawa, M.; Goto, K. Distribution of orexin neurons in the adult rat brain. Brain Res. 1999, 827, 243–260. [Google Scholar] [CrossRef]
- Peyron, C.; Tighe, D.K.; van den Pol, A.N.; de Lecea, L.; Heller, H.C.; Sutcliffe, J.G.; Kilduff, T.S. Neurons containing hypocretin (Orexin) project to multiple neuronal systems. J. Neurosci. 1998, 18, 9996. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chemelli, R.M.; Willie, J.T.; Sinton, C.M.; Elmquist, J.K.; Scammell, T.; Lee, C.; Richardson, J.A.; Clay Williams, S.; Xiong, Y.; Kisanuki, Y.; et al. Narcolepsy in orexin knockout mice: Molecular genetics of sleep regulation. Cell 1999, 98, 437–451. [Google Scholar] [CrossRef] [Green Version]
- Aston-Jones, G.; Smith, R.J.; Moorman, D.E.; Richardson, K.A. Role of lateral hypothalamic orexin neurons in reward processing and addiction. Neuropharmacology 2009, 56, 112. [Google Scholar] [CrossRef] [Green Version]
- Eriksson, K.S.; Sergeeva, O.A.; Haas, H.L.; Selbach, O. Orexins/Hypocretins and aminergic systems. Acta Physiol. Oxf. 2010, 198, 263–275. [Google Scholar] [CrossRef]
- Watanabe, S.; Kuwaki, T.; Yanagisawa, M.; Fukuda, Y.; Shimoyama, M. Persistent pain and stress activate pain-inhibitory orexin pathways. Neuroreport 2005, 16, 5–8. [Google Scholar] [CrossRef] [PubMed]
- Furlong, T.M.; Vianna, D.M.L.; Liu, L.; Carrive, P. Hypocretin/Orexin contributes to the expression of some but not all forms of stress and arousal. Eur. J. Neurosci. 2009, 30, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Uramura, K.; Nambu, T.; Yada, T.; Goto, K.; Yanagisawa, M.; Sakurai, T. Orexin-Induced hyperlocomotion and stereotypy are mediated by the dopaminergic system. Brain Res. 2000, 873, 181–187. [Google Scholar] [CrossRef]
- Jöhren, O.; Neidert, S.J.; Kummer, M.; Dendorfer, A.; Dominiak, P. Prepro-Orexin and orexin receptor MRNAs are differentially expressed in peripheral tissues of male and female rats. Endocrinology 2011, 142, 3324–3331. [Google Scholar] [CrossRef] [PubMed]
- Sun, G.; Tian, Z.; Yao, Y.; Li, H.; Higuchi, T. Central and/or peripheral immunoreactivity of orexin-a in pregnant rats and women. J. Mol. Endocrinol. 2006, 36, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomasik, P.J.; Spodaryk, M.; Sztefko, K. Plasma concentrations of orexins in children. Ann. Nutr. Metab. 2004, 48, 215–220. [Google Scholar] [CrossRef]
- Kaminski, T.; Nitkiewicz, A.; Smolinska, N. Changes in plasma orexin a and orexin b concentrations during the estrous cycle of the pig. Peptides 2013, 39, 175–177. [Google Scholar] [CrossRef]
- Silveyra, P.; Lux-Lantos, V.; Libertun, C. Both orexin receptors are expressed in rat ovaries and fluctuate with the estrous cycle: Effects of orexin receptor antagonists on gonadotropins and ovulation. Am. J. Physiol. Endocrinol. Metab. 2007, 293, E977–E985. [Google Scholar] [CrossRef] [Green Version]
- Silveyra, P.; Catalano, P.N.; Lux-Lantos, V.; Libertun, C. Impact of Proestrous milieu on expression of orexin receptors and Prepro-Orexin in rat hypothalamus and hypophysis: Actions of Cetrorelix and nembutal. Am. J. Physiol. Endocrinol. Metab. 2007, 292, E820–E828. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, T.; Smolinska, N.; Nitkiewicz, A.; Przala, J. Expression of orexin receptors 1 (OX1R) and 2 (OX2R) in the porcine pituitary during the Oestrous cycle. Anim. Reprod. Sci. 2010, 117, 111–118. [Google Scholar] [CrossRef]
- Nitkiewicz, A.; Smolinska, N.; Przala, J.; Kaminski, T. Expression of orexin receptors 1 (OX1R) and 2 (OX2R) in the porcine ovary during the Oestrous cycle. Regul. Pept. 2010, 165, 186–190. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, T.; Smolinska, N.; Kiezun, M.; Dobrzyn, K.; Szeszko, K.; Maleszka, A. Effect of Orexin B on CYP17A1 and CYP19A3 Expression and Oestradiol, Oestrone and testosterone secretion in the porcine uterus during early pregnancy and the Oestrous cycle. Animal 2018, 12, 1921–1932. [Google Scholar] [CrossRef] [PubMed]
- Maleszka, A.; Smolinska, N.; Nitkiewicz, A.; Kiezun, M.; Chojnowska, K.; Dobrzyn, K.; Jazowska, J.; Kaminski, T. Expression of Orexin A and B in the Porcine Hypothalamus during the Oestrous Cycle. J. Physiol. Pharmacol. 2013, 64, 55–63. [Google Scholar] [PubMed]
- Nitkiewicz, A.; Smolinska, N.; Maleszka, A.; Chojnowska, K.; Kaminski, T. Expression of orexins and their precursor in the porcine ovary and the influence of orexins on ovarian steroidogenesis in pigs. Anim. Reprod. Sci. 2014, 148, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Kukkonen, J.P.; Leonard, C.S.; Kukkonen, J.P.; Leonard, C.S. Orexin/Hypocretin Receptor Signalling Cascades. Br. J. Pharmacol. 2014, 171, 314–331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ammoun, S.; Lindholm, D.; Wootz, H.; Åkerman, K.E.O.; Kukkonen, J.P. G-Protein-Coupled OX1 Orexin/Hcrtr-1 hypocretin receptors induce caspase-dependent and -independent cell death through P38 Mitogen-/Stress-Activated protein kinase. J. Biol. Chem. 2006, 281, 834–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanjaneya, M.; Conner, A.C.; Chen, J.; Kumar, P.; Brown, J.E.P.; Jöhren, O.; Lehnert, H.; Stanfield, P.R.; Randeva, H.S. Orexin-Stimulated MAP kinase cascades are activated through multiple G-Protein Signalling pathways in human H295R adrenocortical cells: Diverse roles for Orexins A and B. J. Endocrinol. 2009, 202, 249–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramanjaneya, M.; Conner, A.C.; Chen, J.; Stanfield, P.R.; Randeva, H.S. Orexins stimulate steroidogenic acute regulatory protein expression through multiple signaling pathways in human adrenal H295R cells. Endocrinology 2008, 149, 4106–4115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ekholm, M.E.; Johansson, L.; Kukkonen, J.P. IP3-Independent Signalling of OX1 Orexin/Hypocretin Receptors to Ca2+ Influx and ERK. Biochem. Biophys. Res. Commun. 2007, 353, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Wenzel, J.; Grabinski, N.; Knopp, C.A.; Dendorfer, A.; Ramanjaneya, M.; Randeva, H.S.; Ehrhart-Bornstein, M.; Dominiak, P.; Jöhren, O. Hypocretin/Orexin increases the expression of steroidogenic enzymes in human adrenocortical NCI H295R cells. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2009, 297, 1601–1609. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kang, C.; Zhang, Q.; Zhu, W.; Cai, C.; Sun, X.; Jin, M. Transcription analysis of the responses of porcine heart to Erysipelothrix Rhusiopathiae. PLoS ONE 2017, 12, e0185548. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Zhao, Y.; Zheng, D.; Ju, S.; Shen, Y.; Guo, L. Orexin A affects ins-1 rat insulinoma cell proliferation via orexin receptor 1 and the AKT signaling pathway. Int. J. Endocrinol. 2013, 2013, 854623. [Google Scholar] [CrossRef] [PubMed]
- Urbańska, A.; Sokołowska, P.; Woldan-Tambor, A.; Biegańska, K.; Brix, B.; Jöhren, O.; Namiecińska, M.; Zawilska, J.B. Orexins/Hypocretins Acting at Gi Protein-Coupled OX2 Receptors Inhibit Cyclic AMP Synthesis in the Primary Neuronal Cultures. J. Mol. Neurosci. 2011, 46, 10–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Liu, S.; Kakizaki, M.; Hirose, Y.; Ishikawa, Y.; Funato, H.; Yanagisawa, M.; Yu, Y.; Liu, Q. Orexin/Hypocretin activates MTOR complex 1 (MTORC1) via an Erk/Akt-Independent and calcium-stimulated lysosome v-ATPase pathway. J. Biol. Chem. 2014, 289, 31950–31959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Woldan-Tambor, A.; Biegańska, K.; Wiktorowska-Owczarek, A.; Zawilska, J.B. Activation of Orexin/Hypocretin Type 1 receptors stimulates CAMP synthesis in primary cultures of rat astrocytes. Pharmacol. Rep. 2011, 63, 717–723. [Google Scholar] [CrossRef]
- Marcus, J.N.; Aschkenasi, C.J.; Lee, C.E.; Chemelli, R.M.; Saper, C.B.; Yanagisawa, M.; Elmquist, J.K. Differential expression of orexin receptors 1 and 2 in the rat brain. J. Comp. Neurol. 2001, 435, 6–25. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, P.; Yu, H.; MacNeil, D.J.; van der Ploeg, L.H.T.; Guan, X.M. Distribution of orexin receptor MRNA in the rat brain. FEBS Lett. 1998, 438, 71–75. [Google Scholar] [CrossRef] [Green Version]
- Lu, X.Y.; Bagnol, D.; Burke, S.; Akil, H.; Watson, S.J. Differential distribution and regulation of OX1 and OX2 Orexin/Hypocretin receptor messenger RNA in the brain upon fasting. Horm. Behav. 2000, 37, 335–344. [Google Scholar] [CrossRef]
- Digby, J.E.; Chen, J.; Tang, J.Y.; Lehnert, H.; Matthews, R.N.; Randeva, H.S. Orexin receptor expression in human adipose tissue: Effects of Orexin-A and Orexin-B. J. Endocrinol. 2006, 191, 129–136. [Google Scholar] [CrossRef]
- Nakabayashi, M.; Suzuki, T.; Takahashi, K.; Totsune, K.; Muramatsu, Y.; Kaneko, C.; Date, F.; Takeyama, J.; Darnel, A.D.; Moriya, T.; et al. Orexin-A expression in human peripheral tissues. Mol. Cell. Endocrinol. 2003, 205, 43–50. [Google Scholar] [CrossRef]
- Randeva, H.S.; Karteris, E.; Grammatopoulos, D.; Hillhouse, E.W. Expression of Orexin-a and functional orexin Type 2 receptors in the human adult adrenals: Implications for adrenal function and energy homeostasis. J. Clin. Endocrinol. Metab. 2001, 86, 4808–4813. [Google Scholar] [CrossRef]
- Dehan, P.; Canon, C.; Trooskens, G.; Rehli, M.; Munaut, C.; van Criekinge, W.; Delvenne, P. Expression of Type 2 Orexin Receptor in human endometrium and its epigenetic silencing in endometrial cancer. J. Clin. Endocrinol. Metab. 2013, 98, 1549–1557. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sassek, M.; Pruszynska-Oszmalek, E.; Nowak, K.W. Orexin A modulates endocrine function and viability of porcine pancreatic islets. J. Physiol. Pharmacol. 2017, 68, 815–821. [Google Scholar]
- Pruszynska-Oszmalek, E.; Kolodziejski, P.A.; Kaczmarek, P.; Sassek, M.; Szczepankiewicz, D.; Mikula, R.; Nowak, K.W. Orexin a but not orexin b regulates lipid metabolism and leptin secretion in isolated porcine adipocytes. Dom. Anim. Endocrinol. 2018, 63, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Smolinska, N.; Kiezun, M.; Dobrzyn, K.; Szeszko, K.; Maleszka, A.; Kaminski, T. Expression of the orexin system in the porcine uterus, conceptus and trophoblast during early pregnancy. Animal 2015, 9, 1820–1831. [Google Scholar] [CrossRef] [Green Version]
- Jöhren, O.; Brüggemann, N.; Dendorfer, A.; Dominiak, P. Gonadal steroids differentially regulate the messenger ribonucleic acid expression of pituitary orexin Type 1 receptors and adrenal orexin Type 2 receptors. Endocrinology 2003, 144, 1219–1225. [Google Scholar] [CrossRef] [PubMed]
- Jöhren, O.; Neidert, S.J.; Kummer, M.; Dominiak, P. Sexually dimorphic expression of Prepro-Orexin MRNA in the rat hypothalamus. Peptides 2002, 23, 1177–1180. [Google Scholar] [CrossRef]
- Russell, S.H.; Small, C.J.; Dakin, C.L.; Abbott, C.R.; Morgan, D.G.A.; Ghatei, M.A.; Bloom, S.R. The central effects of Orexin-A in the hypothalamic-pituitary- adrenal axis in vivo and in vitro in male rats. J. Neuroendocrinol. 2001, 13, 561–566. [Google Scholar] [CrossRef] [PubMed]
- Kanenishi, K.; Ueno, M.; Momose, S.; Kuwabara, H.; Tanaka, H.; Sato, C.; Kobayashi, T.; Hino, O.; Sakamoto, H.; Hata, T. Prepro-Orexin MRNA expression in the rat brain is increased during pregnancy. Neurosci. Lett. 2004, 368, 73–77. [Google Scholar] [CrossRef] [PubMed]
- Porkka-Heiskanen, T.; Kalinchuk, A.; Alanko, L.; Huhtaniemi, I.; Stenberg, D. Orexin A and B levels in the hypothalamus of female rats: The effects of the estrous cycle and age. Eur. J. Endocrinol. 2004, 150, 737–742. [Google Scholar] [CrossRef] [Green Version]
- Kaminski, T.; Smolinska, N.; Nitkiewicz, A.; Przała, J. Expression of Orexin Receptors 1 (OX1R) and 2 (OX2R) in the Porcine Hypothalamus during the Oestrous Cycle. J. Physiol. Pharmacol. 2010, 61, 363–371. [Google Scholar] [PubMed]
- Russell, S.H.; Small, C.J.; Kennedy, A.R.; Stanley, S.A.; Seth, A.; Murphy, K.G.; Taheri, S.; Ghatei, M.A.; Bloom, S.R. Orexin A Interactions in the Hypothalamo-Pituitary Gonadal Axis. Endocrinology 2001, 142, 5294–5302. [Google Scholar] [CrossRef] [PubMed]
- Iqbal, J.; Pompolo, S.; Sakurai, T.; Clarke, I.J. Evidence that orexin-containing neurones provide direct input to gonadotropin-releasing hormone neurones in the ovine hypothalamus. J. Neuroendocrinol. 2001, 13, 1033–1041. [Google Scholar] [CrossRef] [PubMed]
- Tamura, T.; Irahara, M.; Tezuka, M.; Kiyokawa, M.; Aono, T. Orexins, orexigenic hypothalamic neuropeptides, suppress the pulsatile secretion of luteinizing hormone in ovariectomized female rats. Biochem. Biophys. Res. Commun. 1999, 264, 759–762. [Google Scholar] [CrossRef] [PubMed]
- Cataldi, N.I.; Lux Lantos, V.A.R.; Libertun, C. Orexin A and B in vitro modify orexins receptors expression and gonadotropins secretion of anterior pituitary cells of proestrous rats. Regul. Pept. 2014, 188, 25–30. [Google Scholar] [CrossRef]
- Ciccimarra, R.; Bussolati, S.; Grasselli, F.; Grolli, S.; Ragionieri, L.; Ravanetti, F.; Botti, M.; Gazza, F.; Cacchioli, A.; Di, R.; et al. Orexin system in swine ovarian follicles. Domest. Anim. Endocrinol. 2018, 62, 49–59. [Google Scholar] [CrossRef]
- Dobrzyn, K.; Smolinska, N.; Kiezun, M.; Szeszko, K.; Rytelewska, E.; Kisielewska, K.; Gudelska, M.; Kaminski, T. The in vitro effect of progesterone on the orexin system in porcine uterine tissues during early pregnancy. Acta Vet. Scand. 2018, 60, 76. [Google Scholar] [CrossRef]
- Kojima, M.; Hosoda, H.; Date, Y.; Nakazato, M.; Matsuo, H.; Kangawa, K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature 1999, 402, 656–660. [Google Scholar] [CrossRef]
- Kanamoto, N.; Akamizu, T.; Tagami, T.; Hataya, Y.; Moriyama, K.; Takaya, K.; Hosoda, H.; Kojima, M.; Kangawa, K.; Nakao, K. Genomic structure and characterization of the 5′-Flanking region of the human ghrelin gene. Endocrinology 2004, 145, 4144–4153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Au, C.M.C.; Furness, J.B.; Brown, K.A. Ghrelin and breast cancer: Emerging roles in obesity, estrogen regulation, and cancer. Front. Oncol. 2017, 6, 265. [Google Scholar] [CrossRef] [Green Version]
- Wajnrajch, M.P.; Ten, I.S.; Gertner, J.M.; Leibel, R.L. Genomic organization of the human GHRELIN gene. Int. J. Disabil. Hum. Develop. 2000, 1, 231–233. [Google Scholar] [CrossRef]
- Kaiya, H. Ghrelin. In Handbook of Hormones; Academic Press: Cambridge, MA, USA, 2016; p. 183-e21A-7. [Google Scholar] [CrossRef]
- Hosoda, H.; Kojima, M.; Matsuo, H.; Kangawa, K. Purification and characterization of rat Des-Gln14-Ghrelin, a second endogenous ligand for the growth hormone secretagogue receptor. J. Biol. Chem. 2000, 275, 21995–22000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueberberg, B.; Unger, N.; Saeger, W.; Mann, K.; Petersenn, S. Expression of ghrelin and its receptor in human tissues. Horm. Metab. Res. 2009, 41, 814–821. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Lei, Z.; Su, J.; Chen, S. Expression of ghrelin in the porcine Hypothalamo–Pituitary–Ovary axis during the estrous cycle. Anim. Reprod. Sci. 2008, 109, 356–367. [Google Scholar] [CrossRef] [PubMed]
- Toshinai, K.; Mondal, M.S.; Nakazato, M.; Date, Y.; Murakami, N.; Kojima, M.; Kangawa, K.; Matsukura, S. Upregulation of ghrelin expression in the stomach upon fasting, insulin-induced hypoglycemia, and leptin administration. Biochem. Biophys. Res. Commun. 2001, 281, 1220–1225. [Google Scholar] [CrossRef] [PubMed]
- Furness, J.B.; Hunne, B.; Matsuda, N.; Yin, L.; Russo, D.; Kato, I.; Fujimiya, M.; Patterson, M.; McLeod, J.; Andrews, Z.B.; et al. Investigation of the presence of ghrelin in the central nervous system of the rat and mouse. Neuroscience 2011, 193, 1–9. [Google Scholar] [CrossRef]
- Du, G.M.; Shi, Z.M.; Wei, X.H.; Liu, M.J.; Zhang, L.; Zhao, R.Q. Expression of gastric ghrelin and H+–K+-ATPase MRNA in weanling piglets and effect of ghrelin on H+–K+-ATPase expression and activity in gastric mucosal cells in vitro. Res. Vet. Sci. 2007, 82, 99–104. [Google Scholar] [CrossRef]
- Date, Y.; Nakazato, M.; Hashiguchi, S.; Dezaki, K.; Mondal, M.S.; Hosoda, H.; Kojima, M.; Kangawa, K.; Arima, T.; Matsuo, H.; et al. Ghrelin is present in pancreatic α-cells of humans and rats and stimulates insulin secretion. Diabetes 2002, 51, 124–129. [Google Scholar] [CrossRef] [Green Version]
- Arnes, L.; Hill, J.T.; Gross, S.; Magnuson, M.A.; Sussel, L. Ghrelin expression in the mouse pancreas defines a unique multipotent progenitor population. PLoS ONE 2012, 7, e52026. [Google Scholar] [CrossRef] [Green Version]
- Mostafa, A.F.; Samir, S.M. What is the effect of ghrelin on rat uterine contractility in vitro? J. Basic. Clin. Physiol. Pharmacol. 2013, 24, 137–142. [Google Scholar] [CrossRef]
- Vitari, F.; di Giancamillo, A.; Deponti, D.; Carollo, V.; Domeneghini, C. Distribution of ghrelin-producing cells in the gastrointestinal tract of pigs at different ages. Vet. Res. Commun. 2012, 36, 71–80. [Google Scholar] [CrossRef] [PubMed]
- Lin, T.; Meng, Q.; Sui, D.; Peng, D.; Li, Y.; Liu, X.; Xie, L.; Li, N. Molecular cloning and expression analysis of porcine ghrelin O-acyltransferase. Biochem. Gen. 2011, 49, 576–586. [Google Scholar] [CrossRef] [PubMed]
- Cummings, D.E.; Purnell, J.Q.; Frayo, R.S.; Schmidova, K.; Wisse, B.E.; Weigle, D.S. A Preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes 2001, 50, 1714–1719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tschöp, M.; Weyer, C.; Tataranni, P.A.; Devanarayan, V.; Ravussin, E.; Heiman, M.L. Circulating ghrelin levels are decreased in human obesity. Diabetes 2001, 50, 707–709. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hosoda, H.; Kojima, M.; Matsuo, H.; Kangawa, K. Ghrelin and Des-Acyl Ghrelin: Two major forms of rat ghrelin peptide in gastrointestinal tissue. Biochem. Biophys. Res. Commun. 2000, 279, 909–913. [Google Scholar] [CrossRef]
- Scrimgeour, K.; Gresham, M.J.; Giles, L.R.; Thomson, P.C.; Wynn, P.C.; Newman, R.E. Ghrelin secretion is more closely aligned to energy balance than with feeding behaviour in the grower pig. J. Endocrinol. 2008, 198, 135–145. [Google Scholar] [CrossRef] [Green Version]
- Tschöp, M.; Smiley, D.L.; Heiman, M.L. Ghrelin induces adiposity in rodents. Nature 2000, 407, 908–913. [Google Scholar] [CrossRef]
- Nagaya, N.; Uematsu, M.; Kojima, M.; Ikeda, Y.; Yoshihara, F.; Shimizu, W.; Hosoda, H.; Hirota, Y.; Ishida, H.; Mori, H.; et al. Chronic administration of ghrelin improves left ventricular dysfunction and attenuates development of cardiac cachexia in rats with heart failure. Circulation 2001, 104, 1430–1435. [Google Scholar] [CrossRef] [Green Version]
- Masuda, Y.; Tanaka, T.; Inomata, N.; Ohnuma, N.; Tanaka, S.; Itoh, Z.; Hosoda, H.; Kojima, M.; Kangawa, K. Ghrelin stimulates gastric acid secretion and motility in rats. Biochem. Biophys. Res. Commun. 2000, 276, 905–908. [Google Scholar] [CrossRef]
- Wren, A.M.; Small, C.J.; Ward, H.L.; Murphy, K.G.; Dakin, C.L.; Taheri, S.; Kennedy, A.R.; Roberts, G.H.; Morgan, D.G.A.; Ghatei, M.A.; et al. The novel hypothalamic peptide ghrelin stimulates food intake and growth hormone secretion. Endocrinology 2000, 141, 4325–4328. [Google Scholar] [CrossRef]
- Diano, S.; Farr, S.A.; Benoit, S.C.; McNay, E.C.; da Silva, I.; Horvath, B.; Gaskin, F.S.; Nonaka, N.; Jaeger, L.B.; Banks, W.A.; et al. Ghrelin controls hippocampal spine synapse density and memory performance. Nat. Neurosci. 2006, 9, 381–388. [Google Scholar] [CrossRef] [PubMed]
- Jerlhag, E.; Egecioglu, E.; Landgren, S.; Salomé, N.; Heilig, M.; Moechars, D.; Datta, R.; Perrissoud, D.; Dickson, S.L.; Engel, J.A. Requirement of central ghrelin signaling for alcohol reward. Proc. Natl. Acad. Sci. USA 2009, 106, 11318–11323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Perello, M.; Sakata, I.; Birnbaum, S.; Chuang, J.C.; Osborne-Lawrence, S.; Rovinsky, S.A.; Woloszyn, J.; Yanagisawa, M.; Lutter, M.; Zigman, J.M. Ghrelin increases the rewarding value of high fat diet in an orexin-dependent manner. Biol. Psychiatry 2010, 67, 880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, C.; Haage, D.; Taube, M.; Egecioglu, E.; Salomé, N.; Dickson, S.L. Central administration of ghrelin alters emotional responses in rats: Behavioural, electrophysiological and molecular evidence. Neuroscience 2011, 180, 201–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howard, A.D.; Feighner, S.D.; Cully, D.F.; Arena, J.P.; Liberator, P.A.; Rosenblum, C.I.; Hamelin, M.; Hreniuk, D.L.; Palyha, O.C.; Anderson, J.; et al. A receptor in pituitary and hypothalamus that functions in growth hormone release. Science 1996, 273, 974–977. [Google Scholar] [CrossRef]
- Feighner, S.D.; Howard, A.D.; Prendergast, K.; Palyha, O.C.; Hreniuk, D.L.; Nargund, R.; Underwood, D.; Tata, J.R.; Dean, D.C.; Tan, C.P.; et al. Structural requirements for the activation of the human growth hormone secretagogue receptor by peptide and nonpeptide secretagogues. Mol. Endocrinol. 1998, 12, 137–145. [Google Scholar] [CrossRef] [Green Version]
- McKee, K.K.; Palyha, O.C.; Feighner, S.D.; Hreniuk, D.L.; Tan, C.P.; Phillips, M.S.; Smith, R.G.; van der Ploeg, L.H.T.; Howard, A.D. Molecular analysis of rat pituitary and hypothalamic growth hormone secretagogue receptors. Mol. Endocrinol. 1997, 11, 415–423. [Google Scholar] [CrossRef]
- Suzuki, A.; Ishida, Y.; Aizawa, S.; Sakata, I.; Tsutsui, C.; Mondal, A.; Kanako, K.; Sakai, T. Molecular identification of GHS-R and GPR38 in Suncus Murinus. Peptides 2012, 36, 29–38. [Google Scholar] [CrossRef]
- Chan, C.B.; Leung, P.K.; Wise, H.; Cheng, C.H.K. Signal transduction mechanism of the seabream growth hormone secretagogue receptor. FEBS Lett. 2004, 577, 147–153. [Google Scholar] [CrossRef]
- Kohno, D.; Gao, H.-Z.; Muroya, S.; Kikuyama, S.; Yada, T. Ghrelin directly interacts with Neuropeptide-Y-Containing neurons in the rat arcuate nucleus. Diabetes 2003, 52, 948–956. [Google Scholar] [CrossRef] [Green Version]
- Kohno, D.; Sone, H.; Minokoshi, Y.; Yada, T. Ghrelin raises [Ca2+] via AMPK in hypothalamic arcuate nucleus NPY neurons. Biochem. Biophys. Res. Commun. 2008, 366, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Chen, Q.; Wang, L.; Li, G. Ghrelin Induces Cell Migration through GHSR1a-Mediated PI3K/Akt/ENOS/NO Signaling pathway in endothelial progenitor cells. Metabolism 2013, 62, 743–752. [Google Scholar] [CrossRef] [PubMed]
- Grey, C.L.; Chang, J.P. Ghrelin-Induced growth hormone release from goldfish pituitary cells involves voltage-sensitive calcium channels. Gen. Comp. Endocrinol. 2009, 160, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Rak-Mardyla, A.; Gregoraszczuk, E.L. ERK 1/2 and PI-3 kinase pathways as a potential mechanism of ghrelin action on cell proliferation and apoptosis in the porcine ovarian follicular cells. J. Physiol. Pharmacol. 2010, 61, 451–458. [Google Scholar] [PubMed]
- Yokote, R.; Sato, M.; Matsubara, S.; Ohye, H.; Niimi, M.; Murao, K.; Takahara, J. Molecular cloning and gene expression of growth hormone-releasing peptide receptor in rat tissues. Peptides 1998, 19, 15–20. [Google Scholar] [CrossRef]
- Korbonits, M.; Bustin, S.A.; Kojima, M.; Jordan, S.; Adams, E.F.; Lowe, D.G.; Kangawa, K.; Grossman, A.B. The expression of the growth hormone secretagogue receptor ligand ghrelin in normal and abnormal human pituitary and other neuroendocrine tumors. J. Clin. Endocrinol. Metab. 2001, 86, 881–887. [Google Scholar] [CrossRef]
- Gnanapavan, S.; Kola, B.; Bustin, S.A.; Morris, D.G.; McGee, P.; Fairclough, P.; Bhattacharya, S.; Carpenter, R.; Grossman, A.B.; Korbonits, M. The tissue distribution of the MRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J. Clin. Endocrinol. Metab. 2002, 87, 2988–2991. [Google Scholar] [CrossRef]
- Gaytan, F.; Barreiro, M.L.; Chopin, L.K.; Herington, A.C.; Morales, C.; Pinilla, L.; Casanueva, F.F.; Aguilar, E.; Diéguez, C.; Tena-Sempere, M. Immunolocalization of ghrelin and its functional receptor, the Type 1a growth hormone secretagogue receptor, in the cyclic human ovary. J. Clin. Endocrinol. Metab. 2003, 88, 879–887. [Google Scholar] [CrossRef]
- Tawadros, N.; Salamonsen, L.A.; Dimitriadis, E.; Chen, C. Facilitation of decidualization by locally produced ghrelin in the human endometrium. Mol. Hum. Reprod. 2007, 13, 483–489. [Google Scholar] [CrossRef] [Green Version]
- Sakata, I.; Nakamura, K.; Yamazaki, M.; Matsubara, M.; Hayashi, Y.; Kangawa, K.; Sakai, T. Ghrelin-Producing Cells Exist as Two Types of Cells, Closed- and Opened-Type Cells, in the rat gastrointestinal tract. Peptides 2002, 23, 531–536. [Google Scholar] [CrossRef]
- Mori, K.; Yoshimoto, A.; Takaya, K.; Hosoda, K.; Ariyasu, H.; Yahata, K.; Mukoyama, M.; Sugawara, A.; Hosoda, H.; Kojima, M.; et al. Kidney produces a novel acylated peptide, ghrelin. FEBS Lett. 2000, 486, 213–216. [Google Scholar] [CrossRef] [Green Version]
- Wierup, N.; Yang, S.; McEvilly, R.J.; Mulder, H.; Sundler, F. Ghrelin is expressed in a novel endocrine cell type in developing rat islets and inhibits insulin secretion from INS-1 (832/13) Cells. J. Histochem. Cytochem. 2004, 52, 301–310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreiro, M.L.; Suominen, J.S.; Gaytán, F.; Pinilla, L.; Chopin, L.K.; Casanueva, F.F.; Diéguez, C.; Aguilar, E.; Toppari, J.; Tena-Sempere, M. Developmental, stage-specific, and hormonally regulated expression of growth hormone secretagogue receptor messenger RNA in rat testis. Biol. Reprod. 2003, 68, 1631–1640. [Google Scholar] [CrossRef] [PubMed]
- Fang, F.; Liu, Y.; Zhao, X.; Li, Y.; Zhang, Y.; Zhang, X. The association between testicular ghrelin receptor MRNA and serum testosterone levels in Immunocastrated boars. Anim. Reprod. Sci. 2012, 135, 62–67. [Google Scholar] [CrossRef] [PubMed]
- Rak, A.; Szczepankiewicz, D.; Gregoraszczuk, E.Ł. Expression of ghrelin receptor, GHSR-1a, and its functional role in the porcine ovarian follicles. Growth Horm. IGF Res. 2009, 19, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Kluge, M.; Schüssler, P.; Schmidt, D.; Uhr, M.; Steiger, A. Ghrelin suppresses secretion of luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in women. J. Clin. Endocrinol. Metab. 2012, 97, E448–E451. [Google Scholar] [CrossRef] [Green Version]
- Rak-Mardyła, A.; Wróbel, A.; Gregoraszczuk, E.L. Ghrelin negatively affects the function of ovarian follicles in mature pigs by direct action on basal and gonadotropin-stimulated steroidogenesis. Reprod Sci. 2015, 22, 469–475. [Google Scholar] [CrossRef] [Green Version]
- Rak, A.; Gregoraszczuk, E.L. Modulatory effect of ghrelin in prepubertal porcine ovarian follicles. J. Physiol. Pharmacol. 2008, 59, 781–793. [Google Scholar] [PubMed]
- Sirotkin, A.V.; Grossmann, R.; María-Peon, M.T.; Roa, J.; Tena-Sempere, M.; Klein, S. Novel expression and functional role of ghrelin in chicken ovary. Mol. Cell Endocrinol. 2006, 257–258, 15–25. [Google Scholar] [CrossRef]
- Sirini, M.A.; Anchordoquy, J.P.; Quintana, S.; Furnus, C.; Relling, A.E.; Anchordoquy, J.M. Expression of ghrelin and its receptor mRNA in bovine oocyte and cumulus cells. Int J. Fertil Steril. 2019, 12, 335–338. [Google Scholar] [CrossRef]
- Waśko, R.; Komarowska, H.; Warenik-Szymankiewicz, A.; Sowiński, J. Elevated ghrelin plasma levels in patients with polycystic ovary syndrome. Horm Metab Res. 2004, 36, 170–173. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-H.; Miele, M.E.; Hicks, D.J.; Phillips, K.K.; Trent, J.M.; Weissman, B.E.; Welch, D.R. KiSS-1, a Novel human malignant melanoma metastasis-suppressor gene. J. Natl. Cancer Inst. 1996, 88, 1731–1737. [Google Scholar] [CrossRef]
- Kotani, M.; Detheux, M.; Vandenbogaerde, A.; Communi, D.; Vanderwinden, J.-M.; Le Poul, E.; Brézillon, S.; Tyldesley, R.; Suarez-Huerta, N.; Vandeput, F.; et al. The metastasis suppressor gene KiSS-1 Encodes Kisspeptins, the Natural ligands of the orphan G protein-coupled receptor GPR54. J. Biol. Chem. 2001, 276, 34631–34636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roch, G.J.; Busby, E.R.; Sherwood, N.M. Evolution of reproductive neurohormones. In Handbook of Neuroendocrinology; Academic Press: Cambridge, MA, USA, 2012; pp. 73–94. [Google Scholar] [CrossRef]
- Ohtaki, T.; Shintani, Y.; Honda, S.; Matsumoto, H.; Hori, A.; Kanehashi, K.; Terao, Y.; Kumano, S.; Takatsu, Y.; Masuda, Y.; et al. Metastasis suppressor gene KiSS-1 encodes peptide ligand of a G-Protein-Coupled receptor. Nature 2001, 411, 613–617. [Google Scholar] [CrossRef] [PubMed]
- Beltramo, M.; Robert, V.; Decourt, C. The Kisspeptin system in domestic animals: What we know and what we still need to understand of its role in reproduction. Dom. Anim. Endocrinol. 2020, 73, 106466. [Google Scholar] [CrossRef] [PubMed]
- Bianco, S.D.C.; Kaiser, U.B. Molecular biology of the kisspeptin receptor: Signaling, function, and mutations. Adv. Exp. Med. Biol. 2013, 784, 133–158. [Google Scholar] [CrossRef]
- Evans, B.J.; Wang, Z.; Mobley, L.; Khosravi, D.; Fujii, N.; Navenot, J.-M.; Peiper, S.C. Physical association of GPR54 C-Terminal with protein phosphatase 2A. Biochem. Biophys. Res. Commun. 2008, 377, 1067–1071. [Google Scholar] [CrossRef]
- Zhang, C.; Roepke, T.A.; Kelly, M.J.; Ronnekleiv, O.K. Kisspeptin Depolarizes gonadotropin-releasing hormone neurons through activation of TRPC-Like cationic channels. J. Neurosci. 2008, 28, 4423–4434. [Google Scholar] [CrossRef]
- Kanda, S.; Oka, Y. Structure, synthesis, and phylogeny of kisspeptin and its receptor. Adv. Exp. Med. Biol. 2013, 784, 9–26. [Google Scholar] [CrossRef]
- Seminara, S.B.; Messager, S.; Chatzidaki, E.E.; Thresher, R.R.; Acierno, J.S.; Jr Shagoury, J.K.; Bo-Abbas, Y.; Kuohung, W.; Schwinof, K.M.; Hendrick, A.G.; et al. The GPR54 gene as a regulator of puberty. N. Engl. J. Med. 2003, 349, 1614–1627. [Google Scholar] [CrossRef] [Green Version]
- de Roux, N.; Genin, E.; Carel, J.-C.; Matsuda, F.; Chaussain, J.-L.; Milgrom, E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-Derived peptide receptor GPR54. Proc. Natl. Acad. Sci. USA 2003, 100, 10972–10976. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, J.T.; Acohido, B.V.; Clifton, D.K.; Steiner, R.A. KiSS-1 Neurones are direct targets for leptin in the Ob/Ob mouse. J. Neuroendocrinol. 2006, 18, 298–303. [Google Scholar] [CrossRef]
- Pinto, F.M.; Cejudo-Román, A.; Ravina, C.G.; Fernández-Sánchez, M.; Martín-Lozano, D.; Illanes, M.; Tena-Sempere, M.; Candenas, M.L. Characterization of the Kisspeptin system in human spermatozoa: Kisspeptin system in human sperm. Int. J. Androl. 2012, 35, 63–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garcia-Ortega, J.; Pinto, F.M.; Fernandez-Sanchez, M.; Prados, N.; Cejudo-Roman, A.; Almeida, T.A.; Hernandez, M.; Romero, M.; Tena-Sempere, M.; Candenas, L. Expression of neurokinin B/NK3 Receptor and Kisspeptin/KISS1 receptor in human granulosa cells. Hum. Reprod. 2014, 29, 2736–2746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yosten, G.L.; Lyu, R.M.; Hsueh, A.J.; Avsian-Kretchmer, O.; Chang, J.K.; Tullock, C.W.; Dun, S.L.; Dun, N.; Samson, W.K. A novel reproductive peptide, phoenixin. J. Neuroendocrinol. 2013, 25, 206–215. [Google Scholar] [CrossRef] [PubMed]
- Jing, F.C.; Zhang, J.; Feng, C.; Nian, Y.Y.; Wang, J.H.; Hu, H.; Yang, B.D.; Sun, X.M.; Zheng, J.Y.; Yin, X.R. Potential rat model of anxiety-like gastric hypersensitivity induced by sequential stress. World J. Gastroenterol. 2017, 23, 7594–7608. [Google Scholar] [CrossRef] [PubMed]
- Pałasz, A.; Rojczyk, E.; Bogus, K.; Worthington, J.J.; Wiaderkiewicz, R. The novel neuropeptide phoenixin is highly co-expressed with nesfatin-1 in the rat hypothalamus, an immunohistochemical stud. Neurosci. Lett. 2015, 592, 17–21. [Google Scholar] [CrossRef] [PubMed]
- Rocca, C.; Scavello, F.; Granieri, M.C.; Pasqua, T.; Amodio, N.; Imbrogno, S.; Gattuso, A.; Mazza, R.; Cerra, M.C.; Angelone, T. Phoenixin-14: Detection and novel physiological implications in cardiac modulation and cardioprotection. Cell. Mol. Life Sci. 2018, 75, 743–756. [Google Scholar] [CrossRef] [PubMed]
- Billert, M.; Kolodziejski, P.A.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Phoenixin-14 stimulates proliferation and insulin secretion in insulin producing INS-1E cells. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 118533. [Google Scholar] [CrossRef] [PubMed]
- Prinz, P.; Scharner, S.; Friedrich, T.; Schalla, M.; Goebel-Stengel, M.; Rose, M.; Stengel, A. Central and peripheral expression sites of phoenixin-14 immunoreactivity in rats. Biochem. Biophys. Res. Commun. 2017, 493, 195–201. [Google Scholar] [CrossRef] [PubMed]
- Billert, M.; Wojciechowicz, T.; Jasaszwili, M.; Szczepankiewicz, D.; Wasko, J.; Kazmierczak, S.; Strowski, M.Z.; Nowak, K.W.; Skrzypski, M. Phoenixin-14 stimulates diferentiation of 3T3-L1 preadipocytes via cAMP/Epac-dependent mechanism. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 1449–1457. [Google Scholar] [CrossRef] [PubMed]
- Kalamon, N.; Blaszczyk, K.; Szlaga, A.; Billert, M.; Skrzypski, M.; Pawlicki, P.; Gorowska-Wojtowicz, E.; Kotula-Balak, M.; Blasiak, A.; Rak, A. Levels of the neuropeptide phoenixin-14 and its receptor GRP173 in the hypothalamus, ovary and periovarian adipose tissue in rat model of polycystic ovary syndrome. Biochem. Biophys. Res. Commun. 2020, 528, 628–635. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, X.P.; Nakamura, T.; Osuka, S.; Bayasula, B.; Nakanishi, N.; Kasahara, Y.; Muraoka, A.; Hayashi, S.; Nagai, T.; Murase, T.; et al. Effect of the neuropeptide phoenixin and its receptor GPR173 during folliculogenesis. Reproduction 2019, 158, 25–34. [Google Scholar] [CrossRef] [PubMed]
- Schalla, A.M.; Stengel, A. Phoenixin-A Pleiotropic Gut-Brain Peptide. Int. J. Mol. Sci. 2018, 19, 1726. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsumoto, M.; Saito, T.; Takasaki, J.; Kamohara, M.; Sugimoto, T.; Kabayashi, M.; Tadokoro, M.; Matsumoto, S.; Ohishi, T.; Furuichi, K. An evolutionarily conserved G-protein coupled receptor family, sreb, expressed in the central nervous system. Biochem. Biophys. Res. Commun. 2000, 272, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Larco, D.O.; Semsarzadeh, N.N.; Cho-Clark, M.; Mani, S.K.; Wu, T.J. The novel actions of the metabolite GnRH-(1–5) are mediated by a G protein-coupled receptor. Front. Endocrinol. 2013, 4, 83–89. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davidson, J.S.; Flanagan, C.A.; Zhou, W.; Becker, I.I.; Elario, R.; Emeran, W.; Sealfon, S.C.; Millar, R.P. Identification of N-glycosylation sites in the gonadotropin-releasing hormone receptor: Role in receptor expression but not ligand binding. Mol. Cell. Endocrinol. 1995, 107, 241–245. [Google Scholar] [CrossRef]
- Zhang, R.; Cai, H.; Fatima, N.; Buczko, E.; Dufau, M.L. Functional glycosylation sites of the rat luteinizing hormone receptor required for ligand binding. J. Biol. Chem. 1995, 270, 21722–21728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Larco, D.O.; Cho-Clark, M.; Mani, S.K.; Wu, T.J. The metabolite GnRH-(1–5) inhibits the migration of immortalized GnRH neurons. Endocrinology 2013, 154, 783–795. [Google Scholar] [CrossRef]
- Larco, D.O.; Cho-Clark, M.; Mani, S.K.; Wu, T.J. β-arrestin 2 is a mediator of GnRH-(1–5) signaling in immortalized GnRH neurons. Endocrinology 2013, 154, 4726–4736. [Google Scholar] [CrossRef] [Green Version]
- Treen, A.K.; Luo, V.; Belsham, D.D. Phoenixin activates immortalized GnRH and kisspeptin neurons through the novel receptor GPR173. Mol. Endocrinol. 2016, 30, 872–888. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mcilwraith, E.K.; Belsham, D.D. Phoenixin: Uncovering its receptor, signaling and functions. Acta Pharmacol. Sin. 2018, 39, 774–778. [Google Scholar] [CrossRef] [PubMed]
- Ullah, K.; Rahman, T.; Wu, D.D.; Lin, X.H.; Liu, Y.; Guo, X.Y.; Leung, P.; Zhang, R.J.; Huang, H.F.; Sheng, J.Z. Phoenixin-14 concentrations are increased in association with luteinizing hormone and nesfatin-1 concentrations in women with polycystic ovary syndrome. Clin. Chim. Acta 2017, 471, 243–247. [Google Scholar] [CrossRef] [PubMed]
- Jiang, J.H.; He, Z.; Peng, Y.L.; Jin, W.D.; Mu, J.; Xue, H.X.; Wang, Z.; Chang, M.; Wang, R. Effects of phoenixin-14 on anxiolytic-like behavior in mice. Behav. Brain Res. 2015, 286, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Rajeswari, J.J.; Unniappan, S. Phoenixin-20 Stimulates mRNAs Encoding Hypothalamo-Pituitary-Gonadal Hormones, is Pro-Vitellogenic, and promotes oocyte maturation in zebrafish. Sci. Rep. 2020, 10, 6264. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suszka-Świtek, A.; Pałasz, A.; Filipczak, Ł.; Menezes, I.C.; Mordecka-Chamera, K.; Angelono, T.; Bogus, K.; Bacopoulou, J.J.; Worthington, J.J.; Wiaderkiewicz, R. The GnRH analogues affect novel neuropeptide SMIM20/phoenixin and GPR173 receptor expressions in the female rat hypothalamic-pituitary-gonadal (HPG) axis. Clin. Exp. Pharmacol. Physiol. 2019, 46, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Chen, H.P.; Zhai, Y.; Jiang, D.N.; Liu, J.Y.; Tian, C.X.; Wu, T.L.; Zhu, C.H.; Deng, S.P.; Li, G.L. Phoenixin: Expression at different ovarian development stages and effects on genes ralated to reproduction in spotted scat, Scatophagus argus. Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2019, 228, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Löffler, S.; Aust, G.; Köhler, U.; Spanel-Borowski, K. Evidence of leptin expression in normal and polycystic human ovaries. Mol. Hum. Reprod. 2001, 7, 1143–1149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Archanco, M.; Muruzábal, F.J.; Llopiz, D.; Garayoa, M.; Gómez-Ambrosi, J.; Frühbeck, G.; Burrell, M.A. Leptin expression in the rat ovary depends on estrous cycle. J. Histochem. Cytochem. 2003, 51, 1269–1277. [Google Scholar] [CrossRef] [Green Version]
- Smolinska, N.; Kaminski, T.; Siawrys, G.; Przala, J. Leptin gene and protein expression in the ovary during the oestrous cycle and early pregnancy in pigs. Reprod. Domest. Anim. 2010, 45, e174–e183. [Google Scholar] [CrossRef]
- Gregoraszczuk, E.Ł.; Ptak, A.; Wojciechowicz, T.; Nowak, K. Action of IGF-I on expression of the long form of the leptin receptor (ObRb) in the prepubertal period and throughout the estrous cycle in the mature pig ovary. J. Reprod. Develop. 2007, 53, 289–295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phoophitphong, D.; Srisuwatanasagul, S.; Tummaruk, P. Leptin immunohistochemical staining in the porcine ovary. Anat. Histol. Embryol. 2017, 46, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, M.; Schilffarth, S.; Schams, D.; Meyer, H.H.D.; Berisha, B. The Expression of leptin and its receptor during different physiological stages in the bovine ovary. Mol. Reprod.Develop. 2010, 77, 174–181. [Google Scholar] [CrossRef] [PubMed]
- Galvão, A.; Tramontano, A.; Rebordão, M.R.; Amaral, A.; Bravo, P.P.; Szóstek, A.; Skarzynski, D.; Mollo, A.; Ferreira-Dias, G. Opposing roles of leptin and ghrelin in the equine corpus luteum regulation: An in vitro study. Mediators Inflamm. 2014, 2014, 682193. [Google Scholar] [CrossRef] [PubMed]
- Ramirez, M.A.; Arellano, A.A.; Xie, F.; Benavides, E.A.; Katchko, R.A.; Ayala, L.; Calderon, A.; Flores, R.A.; Escudero, J.M.; Keisler, D.H.; et al. The role of leptin in the development of the corpus luteum. In Leptin Production, Regulation and Functions; Nova Science Publishers Inc.: New York, NY, USA, 2017; pp. 107–130. [Google Scholar]
- Kumar, L.; Panda, R.P.; Hyder, I.; Yadav, V.P.; Sastry, K.V.H.; Sharma, G.T.; Mahapatra, R.K.; Bag, S.; Bhure, S.K.; Das, G.K.; et al. Expression of leptin and its receptor in corpus luteum during estrous cycle in buffalo (Bubalus Bubalis). Anim. Reprod. Sci. 2012, 135, 8–17. [Google Scholar] [CrossRef] [PubMed]
- Balogh, O.; Kowalewski, M.P.; Reichler, I.M. Leptin and Leptin receptor gene expression in the canine corpus luteum during diestrus, pregnancy and after Aglepristone-induced Luteolysis. Reprod. Domes. Anim. 2012, 47, 40–42. [Google Scholar] [CrossRef]
- Ryan, N.K.; van der Hoek, K.H.; Robertson, S.A.; Norman, R.J. Leptin and leptin receptor expression in the rat ovary. Endocrinology 2003, 144, 5006–5013. [Google Scholar] [CrossRef] [Green Version]
- Zamorano, P.L.; Mahesh, V.B.; de Sevilla, L.M.; Chorich, L.P.; Bhat, G.K.; Brann, D.W. Expression and localization of the leptin receptor in endocrine and neuroendocrine tissues of the rat. Neuroendocrinology 1997, 65, 223–228. [Google Scholar] [CrossRef] [PubMed]
- Bogacka, I.; Przala, J.; Siawrys, G.; Kaminski, T.; Smolinska, N. The Expression of short from of leptin receptor gene during early pregnancy in the pig examined by quantitative real time RT-PCR. J. Physiol. Pharmacol. 2006, 57, 479–489. [Google Scholar]
- Lin, J.; Barb, C.R.; Matteri, R.L.; Kraeling, R.R.; Chen, X.; Meinersmann, R.J.; Rampacek, G.B. Long form leptin receptor MRNA expression in the brain, pituitary, and other tissues in the pig. Domes. Anim. Endocrinol. 2000, 19, 53–61. [Google Scholar] [CrossRef]
- Ruiz-Cortés, T.Z.; Men, T.; Palin, M.-F.; Downey, B.R.; Lacroix, D.A.; Murphy, B.D. Porcine leptin receptor: Molecular structure and expression in the ovary. Mol. Reprod. Dev. 2000, 56, 465–474. [Google Scholar] [CrossRef]
- Smolinska, N.; Kaminski, T.; Siawrys, G.; Przala, J. Long form of leptin receptor gene and protein expression in the porcine ovary during the estrous cycle and early pregnancy. Reprod. Biol. 2007, 7, 17–39. [Google Scholar] [PubMed]
- Green, A.E.; O’Neil, J.S.; Swan, K.F.; Bohm, R.P.; Ratterree, M.S.; Henson, M.C. Leptin receptor transcripts are constitutively expressed in placenta and adipose tissue with advancing baboon pregnancy. Proc. Soc. Exp. Biol. Med. 2000, 223, 362–366. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, L.T.; Robinson, R.S.; Marsters, P.; Campbell, B.K.; Mann, G.E.; Hunter, M.G. Leptin in the bovine corpus luteum: Receptor expression and effects on progesterone production. Mol. Reprod. Develop. 2007, 74, 724–729. [Google Scholar] [CrossRef] [PubMed]
- Ryan, N.K.; Woodhouse, C.M.; van der Hoek, K.H.; Gilchrist, R.B.; Armstrong, D.T.; Norman, R.J. Expression of leptin and its receptor in the murine ovary: Possible role in the regulation of oocyte maturation. Biol. Reprod. 2002, 66, 1548–1554. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zerani, M.; Boiti, C.; Zampini, D.; Brecchia, G.; Dall’Aglio, C.; Ceccarelli, P.; Gobbetti, A. Ob Receptor in rabbit ovary and leptin in vitro regulation of corpora lutea. J. Endocrinol. 2004, 183, 279–288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallelli, M.F.; Bianchi, C.; Lombardo, D.; Rey, F.; Rodriguez, F.M.; Castillo, V.A.; Miragaya, M. Leptin and IGF1 Receptors in Alpaca (Vicugna Pacos) ovaries. Anim. Reprod. Sci. 2019, 200, 96–104. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, S.; Nishii, N.; Yamanaka, A.; Kitagawa, H.; Asano, M.; Tsubota, T.; Suzuki, M. Leptin Receptor (Ob-R) Expression in the Ovary and Uterus of the Wild Japanese Black Bear (Ursus Thibetanus Japonicus). J. Reprod. Develop. 2009, 55, 110–115. [Google Scholar] [CrossRef] [Green Version]
- Siawrys, G.; Smolinska, N. In vitro effects of luteinizing hormone, progesterone and oestradiol-17β on leptin gene expression and leptin secretion by porcine luteal cells obtained in early pregnancy. J. Physiol. Pharmacol. 2013, 64, 513–520. [Google Scholar]
- Siawrys, G.; Smolinska, N. Direct in vitro effect of LH and steroids on leptin gene expression and leptin secretion by porcine luteal cells during the mid-luteal phase of the estrous cycle. Reprod. Biol. 2012, 12, 317–323. [Google Scholar] [CrossRef] [PubMed]
- Gregoraszczuk, E.Ł.; Ptak, A. In vitro effect of leptin on growth hormone (GH)- and Insulin-Like Growth Factor-I (IGF-I)-Stimulated progesterone secretion and apoptosis in developing and mature corpora lutea of pig ovaries. J. Reprod. Develop. 2005, 51, 727–733. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reshma, R.; Mishra, S.R.; Thakur, N.; Parmar, M.S.; Somal, A.; Bharti, M.K.; Pandey, S.; Chandra, V.; Chouhan, V.S.; Verma, M.R.; et al. Modulatory role of leptin on ovarian functions in water buffalo (Bubalus Bubalis). Theriogenology 2016, 86, 1720–1739. [Google Scholar] [CrossRef] [PubMed]
- Nicklin, L.T.; Robinson, R.S.; Campbell, B.K.; Hunter, M.G.; Mann, G.E. Leptin infusion during the early luteal phase in ewes does not affect progesterone production. Domest. Anim. Endocrinol. 2007, 33, 240–244. [Google Scholar] [CrossRef] [PubMed]
- Sirotkin, A.V.; Tarko, A.; Kotwica, J.; Alrezaki, A.; Harrath, A.H. Interrelationships between metabolic hormones, leptin and ghrelin, and oil-related contaminants in control of oxytocin and prostaglandin f release by feline ovaries. Reprod. Biol. 2020, 20, 254–258. [Google Scholar] [CrossRef] [PubMed]
- Norambuena, M.C.; Hernández, F.; Maureira, J.; Rubilar, C.; Alfaro, J.; Silva, G.; Silva, M.; Ulloa-Leal, C. Effects of leptin administration on development, vascularization and function of corpus luteum in alpacas submitted to pre-ovulatory fasting. Anim. Reprod. Sci. 2017, 182, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Wiles, J.R.; Katchko, R.A.; Benavides, E.A.; O’Gorman, C.W.; Escudero, J.M.; Keisler, D.H.; Stanko, R.L.; Garcia, M.R. The effect of leptin on luteal angiogenic factors during the luteal phase of the estrous cycle in goats. Anim. Reprod. Sci. 2014, 148, 121–129. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katchko, R.A.; Wiles, J.R.; Aguirre, E.A.; O’Gorman, C.W.; Keisler, D.H.; Stanko, R.L.; Garcia, M.R. Leptin decreases angiogenic factors in the developing porcine corpus luteum. Biol. Reprod. 2009, 81, 578. [Google Scholar] [CrossRef]
- Maillard, V.; Uzbekova, S.; Guignot, F.; Perreau, C.; Ramé, C.; Coyral-Castel, S.; Dupont, J. Effect of adiponectin on bovine granulosa cell steroidogenesis, oocyte maturation and embryo development. Reprod. Biol. Endocrinol. 2010, 8, 23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tabandeh, M.R.; Hosseini, A.; Saeb, M.; Kafi, M.; Saeb, S. Changes in the gene expression of adiponectin and adiponectin receptors (AdipoR1 and AdipoR2) in ovarian follicular cells of dairy cow at different stages of development. Theriogenology 2010, 73, 659–669. [Google Scholar] [CrossRef]
- Campos, D.B.; Albornoz, M.; Papa, P.C.; Palin, M.-F.; Bordignon, V.; Murphy, B.D. Relationship between adiponectin and fertility in the female pig. Reprod. Fertil. Develop. 2015, 27, 458. [Google Scholar] [CrossRef]
- Maleszka, A.; Smolinska, N.; Nitkiewicz, A.; Kiezun, M.; Chojnowska, K.; Dobrzyn, K.; Szwaczek, H.; Kaminski, T. Adiponectin Expression in the porcine ovary during the Oestrous cycle and its effect on ovarian steroidogenesis. Int. J. Endocrinol. 2014, 2014, 957076. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.; Thakre, A.; Bahiram, K.B.; Sardar, V.M.; Dudhe, S.D.; Korde, J.P.; Bonde, S.W.; Kurkure, N.V. Abundance of Adiponectin MRNA Transcript in the buffalo corpus luteum during the estrous cycle and effects on progesterone secretion in vitro. Anim. Reprod. Sci. 2019, 208, 106110. [Google Scholar] [CrossRef]
- Ledoux, S.; Campos, D.B.; Lopes, F.L.; Dobias-Goff, M.; Palin, M.-F.; Murphy, B.D. Adiponectin Induces periovulatory changes in ovarian follicular cells. Endocrinology 2006, 147, 5178–5186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murakami, M.; Naraba, H.; Tanioka, T.; Semmyo, N.; Nakatani, Y.; Kojima, F.; Ikeda, T.; Fueki, M.; Ueno, A.; Oh-ishi, S.; et al. Regulation of prostaglandin E2 biosynthesis by inducible membrane-associated prostaglandin E2 synthase that acts in concert with Cyclooxygenase-2. J. Biol. Chem. 2000, 275, 32783–32792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakurai, T.; Tamura, K.; Okamoto, S.; Hara, T.; Kogo, H. Possible Role of cyclooxygenase ii in the acquisition of ovarian luteal function in Rodents1. Biol. Reprod. 2003, 69, 835–842. [Google Scholar] [CrossRef] [PubMed]
- Anuradha; Krishna, A. Modulation of Ovarian steroidogenesis by adiponectin during delayed embryonic development of Cynopterus Sphinx. J. Steroid. Biochem. Mol. Biol. 2014, 143, 291–305. [Google Scholar] [CrossRef] [PubMed]
- Shirasuna, K.; Shimizu, T.; Sayama, K.; Asahi, T.; Sasaki, M.; Berisha, B.; Schams, D.; Miyamoto, A. Expression and localization of apelin and its receptor APJ in the bovine corpus luteum during the estrous cycle and prostaglandin F2alpha-induced luteolysis. Reproduction 2008, 135, 519–525. [Google Scholar] [CrossRef] [Green Version]
- Schilffarth, S.; Antoni, B.; Schams, D.; Meyer, H.H.; Berisha, B. The expression of apelin and its receptor APJ during different physiological stages in the bovine ovary. Int. J. Biol. Sci. 2009, 5, 344–350. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mercati, F.; Scocco, P.; Maranesi, M.; Acuti, G.; Petrucci, L.; Cocci, P.; Renzi, A.; De Felice, E.; Dall’Aglio, C. Apelin system detection in the reproductive apparatus of ewes grazing on semi-natural pasture. Theriogenology 2019, 139, 156–166. [Google Scholar] [CrossRef]
- Xu, F.; Stouffer, R.L. Dynamic expression of apelin and its receptor in the primate preovulatory follicle and corpus luteum during the menstrual cycle. Biol. Reprod. 2012, 1, 169. [Google Scholar] [CrossRef]
- Pirino, C.; Maranesi, M.; Polisca, A.; Troisi, A.; Dall’Aglio, C. The immunohistochemical presence and distribution of ghrelin, apelin and their receptors in dog ovaries. Microbiol. Res. 2017, 8, 28–30. [Google Scholar] [CrossRef] [Green Version]
- Thakre, A.; Gupta, M.; Magar, S.P.; Bahiram, K.B.; Sardar, V.M.; Korde, J.P.; Bonde, S.W.; Hyder, I. Transcriptional and translational abundance of visfatin (NAMPT) in buffalo ovary during estrous cycle and its in vitro effect on steroidogenesis. Domes. Anim. Endocrinol. 2021, 75, 106583. [Google Scholar] [CrossRef]
- Annie, L.; Gurusubramanian, G.; Roy, V.K. Changes in the localization of ovarian visfatin protein and its possible role during estrous cycle of mice. Acta Histochem. 2020, 122, 151630. [Google Scholar] [CrossRef]
- Kurowska, P.; Mlyczyńska, E.; Dawid, M.; Grzesiak, M.; Dupont, J.; Rak, A. The role of vaspin in porcine corpus luteum. J. Endocrinol. 2020, 247, 283–294. [Google Scholar] [CrossRef] [PubMed]
- Kurowska, P.; Mlyczyńska, E.; Dupont, J.; Rak, A. Novel insights on the corpus luteum function: Role of vaspin on porcine luteal cell angiogenesis, proliferation and apoptosis by activation of GRP78 receptor and MAP3/1 kinase pathways. Int. J. Mol. Sci. 2020, 21, 6823. [Google Scholar] [CrossRef] [PubMed]
- Rytelewska, E.; Kisielewska, K.; Kiezun, M.; Kamil, D.; Gudelska, M.; Rak, A.; Dupont, J.; Kaminska, B.; Kaminski, T.; Smolinska, N. Expression of chemerin and its receptors in the ovaries of prepubertal and mature gilts. Mol. Reprod. Develop. 2020, 87, 739–762. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Kim, J.Y.; Xue, K.; Liu, J.Y.; Leader, A.; Tsang, B.K. Chemerin, a novel regulator of3follicular steroidogenesis and its potential involvement in polycystic ovarian syndrome. Endocrinology 2012, 153, 5600–5611. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yao, J.; Li, Z.; Fu, Y.; Wu, R.; Wang, Y.; Liu, C.; Yang, L.; Zhang, H. Involvement of obesity-associated upregulation of chemerin/chemokine-like receptor 1 in oxidative stress and apoptosis in ovaries and granulosa cells. Biochem. Biophys. Res. Commun. 2019, 510, 449–455. [Google Scholar] [CrossRef] [PubMed]
- Luangsay, S.; Wittamer, V.; Bondue, B.; de Henau, O.; Rouger, L.; Brait, M.; Franssen, J.-D.; de Nadai, P.; Huaux, F.; Parmentier, M. Mouse ChemR23 is expressed in dendritic cell subsets and macrophages, and mediates an anti-inflammatory activity of chemerin in a lung disease model. J. Immunol. 2009, 183, 6489–6499. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.L.; Ren, L.R.; Sun, L.F.; Huang, C.; Xiao, T.X.; Wang, B.B.; Chen, J.; Zabel, B.A.; Ren, P.; Zhang, J.V. The role of GPR1 signaling in mice corpus luteum. J. Endocrinol. 2016, 230, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Rytelewska, E.; Kiezun, M.; Kisielewska, K.; Gudelska, M.; Dobrzyn, K.; Kaminska, B.; Kaminski, T.; Smolinska, N. Chemerin as a modulator of ovarian steroidogenesis in pigs: An in vitro study. Theriogenology 2021, 160, 95–101. [Google Scholar] [CrossRef]
- Rytelewska, E.; Kiezun, M.; Zaobidna, E.; Gudelska, M.; Kisielewska, K.; Dobrzyn, K.; Kaminski, T.; Smolinska, N. Chemerin as a modulator of angiogenesis and apoptosis processes in the corpus luteum of pigs: An in vitro study. Biol. Reprod. 2021, 105, 1002–1015. [Google Scholar] [CrossRef]
- Makowczenko, K.G.; Jastrzebski, J.P.; Szeszko, K.; Smolinska, N.; Paukszto, L.; Dobrzyn, K.; Kiezun, M.; Rytelewska, E.; Kaminska, B.; Kaminski, T. Transcription analysis of the chemerin impact on gene expression profile in the luteal cells of gilts. Genes 2020, 11, 651. [Google Scholar] [CrossRef]
- Ragionieri, L.; Ravanetti, F.; di Lecce, R.; Botti, M.; Ciccimarra, R.; Bussolati, S.; Basini, G.; Gazza, F.; Cacchioli, A. Immunolocalization of Orexin A and its receptors in the different structures of the porcine ovary. Ann. Anat. 2018, 218, 214–226. [Google Scholar] [CrossRef]
- Basini, G.; Ciccimarra, R.; Bussolati, S.; Grolli, S.; Ragionieri, L.; Ravanetti, F.; Botti, M.; Gazza, F.; Cacchioli, A.; di Lecce, R.; et al. Orexin A in swine corpus luteum. Domes. Anim. Endocrinol. 2018, 64, 38–48. [Google Scholar] [CrossRef]
- Cataldi, N.I.; Lux-Lantos, V.A.R.; Libertun, C. Effects of Orexins A and B on expression of orexin receptors and progesterone release in luteal and granulosa ovarian cells. Regul. Pep. 2012, 178, 56–63. [Google Scholar] [CrossRef]
- Sardar, V.M.; Gupta, M.; Korde, J.P.; Bahiram, K.B.; Bambode, K.P.; Bonde, S.W. Expression profile of orexin system in corpus luteum of buffalo during estrous cycle. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1268–1276. [Google Scholar] [CrossRef]
- Grasselli, F.; Bussolati, S.; Grolli, S.; di Lecce, R.; Dall’aglio, C.; Basini, G. Effects of Orexin B on swine granulosa and endothelial cells. Animals 2021, 11, 1812. [Google Scholar] [CrossRef]
- Voisin, T.; Firar, A.E.; Rouyer-Fessard, C.; Gratio, V.; Laburthe, M. A Hallmark of Immunoreceptor, the Tyrosine-Based Inhibitory Motif ITIM, is present in the G Protein-Coupled receptor OX1R for Orexins and drives apoptosis: A novel mechanism. FASEB J. 2008, 22, 1993–2002. [Google Scholar] [CrossRef] [Green Version]
- Voisin, T.; Firar, A.E.; Avondo, V.; Laburthe, M. Orexin-Induced apoptosis: The key role of the seven-transmembrane domain orexin type 2 receptor. Endocrinology 2006, 147, 4977–4984. [Google Scholar] [CrossRef] [Green Version]
- Fujita, S.; Hasegawa, T.; Nishiyama, Y.; Fujisawa, S.; Nakano, Y.; Nada, T.; Iwata, N.; Kamada, Y.; Masuyama, H.; Otsuka, F. Interaction between Orexin A and bone morphogenetic protein system on progesterone biosynthesis by rat granulosa cells. J. Steroid Biochem. Mol. Biol. 2018, 181, 73–79. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Zu, N.; Zhang, C.S.; Xie, M.Y.; Liu, Y.Z.; Xu, X.J. Orexin A promotes granulosa cell secretion of progesterone in sheep. Iran. J. Vet. Res. 2019, 20, 136–142. [Google Scholar]
- Xie, M.; Han, D.; Xu, X. Orexin A Promotes progesterone secretion in luteinized granulose cells of mongolian ovis aries ovary by PRRT2 and ABCG1 genes. Zygote 2021, 29, 286–292. [Google Scholar] [CrossRef]
- Gupta, M.; Dangi, S.; Chouhan, V.S.; Hyder, I.; Babitha, V.; Yadav, V.P.; Khan, F.A.; Sonwane, A.; Singh, G.; Das, G.K.; et al. Expression and localization of ghrelin and its functional receptor in corpus luteum during different stages of estrous cycle and the modulatory role of ghrelin on progesterone production in cultured luteal cells in buffalo. Domest Anim Endocrinol. 2014, 48, 21–32. [Google Scholar] [CrossRef]
- Caminos, J.E.; Tena-Sempere, M.; Gaytan, F.; Sanchez-Criado, J.E.; Barreiro, M.L.; Nogueiras, R.; Casanueva, F.F.; Aguilar, E.; Dieguez, C. Expression of ghrelin in the cyclic and pregnant rat ovary. Endocrinology 2003, 144, 1594–1602. [Google Scholar] [CrossRef] [Green Version]
- Rak-Mardyła, A.; Gregoraszczuk, E.L.; Karpeta, A.; Duda, M. Expression of ghrelin and the ghrelin receptor in different stages of porcine corpus luteum development and the inhibitory effects of ghrelin on progesterone secretion, 3-hydroxysteroid dehydrogenase (3-honestly significant difference (HSD)) activity and protein expression. Theriogenology 2012, 77, 1505–1512. [Google Scholar] [CrossRef] [PubMed]
- Du, C.; Xilingaowa-Cao, G.; Wang, C.; Li, H.; Zhao, Y.; Siqingaowa-Cao, J. Expression of orexigenic peptide ghrelin in sheep ovary. Domes. Anim. Endocrinol. 2009, 36, 89–98. [Google Scholar] [CrossRef] [PubMed]
- Chandra, V.; Ram, H.; Sharma, A.K. Expression profile of ghrelin and ghrelin receptor in cyclic goat ovary. Ind. J. Anim. Sci. 2012, 82, 984–987. [Google Scholar]
- Tropea, A.; Tiberi, F.; Minici, F. Ghrelin affects the release of luteolytic and luteotropic factors in human luteal cells. J. Clin. Endocrinol. Metab. 2007, 92, 3239–3245. [Google Scholar] [CrossRef] [Green Version]
- Deaver, S.E.; Hoyer, P.B.; Dial, S.M.; Field, M.E.; Collier, R.J.; Rhoads, M.L. Localization of ghrelin and its receptor in the reproductive tract of Holstein heifers. J. Diary Sci. 2013, 96, 150–157. [Google Scholar] [CrossRef]
- Jain, A.; Tripti, J.; Abhijit, M. Expression and immunohistochemical localization of ghrelin gene in Biffalo (Babulus Bubalis) corpus luteum. Ind. J. Anim. Res. 2012, 46, 61–65. [Google Scholar]
- Romani, F.; Lanzone, A.; Tropea, A.; Familiari, A.; Scarinci, E.; Sali, M.; Delogu, G.; Catino, S.; Apa, R. In vitro effect of unacylated ghrelin and obestatin on human luteal cell function. Fertil. Steril. 2012, 97, 991–996. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, M.L.; Gaytan, F.; Caminos, J.E.; Pinilla, L.; Casanueva, F.F.; Aguilar, E.; Dieguez, C.; Tena-Sempere, M. Cellular location and hormonal regulation of ghrelin expression in rat testis. Biol. Reprod. 2002, 67, 1768–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kheradmand, A.; Dezfoulian, O.; Alirezaei, M. Ghrelin is a regulator of cellular apoptosis and proliferation in the rat ovary. Int. J. Pept. Res. Ther. 2014, 20, 289–298. [Google Scholar] [CrossRef]
- Komarowska, H.; Waśko, K.; Iwanik, P.; Majewski, L.; Rafińska, A.; Warenik-Szymankiewicz, J.; Sowiński, J. Ghrelin ovarian cell expression in patients with polycystic ovary syndrome: An immunohistochemical evaluation. Horm. Metab. Res. 2006, 38, 783–788. [Google Scholar] [CrossRef]
- Alirezaei, M.; Dezfoulian, O.; Abasi, M.; Sooktehzari, A. Ghrelin role in apoptosis and proliferation of ovine ovarian follicles and corpus luteum. Small Rum. Res. 2017, 157, 1–7. [Google Scholar] [CrossRef]
- Castellano, J.M.; Gaytan, M.; Roa, J.; Vigo, E.; Navarro, V.M.; Bellido, C.; Dieguez, C.; Aguilar, E.; Sánchez-Criado, J.E.; Pellicer, A.; et al. Expression of KiSS-1 in Rat Ovary: Putative local regulator of ovulation? Endocrinology 2006, 147, 4852–4862. [Google Scholar] [CrossRef]
- Peng, J.; Tang, M.; Zhang, B.-P.; Zhang, P.; Zhong, T.; Zong, T.; Yang, B.; Kuang, H.-B. Kisspeptin Stimulates Progesterone Secretion via the Erk1/2 Mitogen-Activated protein kinase signaling pathway in rat luteal cells. Fertil. Steril. 2013, 99, 1436–1443.e1. [Google Scholar] [CrossRef] [PubMed]
- Laoharatchatathanin, T.; Terashima, R.; Yonezawa, T.; Kurusu, S.; Kawaminami, M. Augmentation of Metastin/Kisspeptin MRNA Expression by the Proestrous luteinizing hormone surge in granulosa cells of rats: Implications for luteinization. Biol. Reprod. 2015, 93, 1–9. [Google Scholar] [CrossRef]
- Cielesh, M.; McGrath, B.; Scott, C.; Norman, S.; Stephen, C. The Localization of Kisspeptin and Kisspeptin receptor in the canine ovary during different stages of the reproductive cycle. Reprod. Domest. Anim. 2017, 52, 24–28. [Google Scholar] [CrossRef] [Green Version]
- Tanyapanyachon, P.; Amelkina, O.; Chatdarong, K. The Expression of Kisspeptin and its receptor in the domestic cat ovary and uterus in different stages of the ovarian cycle. Theriogenology 2018, 117, 40–48. [Google Scholar] [CrossRef] [PubMed]
- Mishra, G.K.; Patra, M.K.; Singh, L.K.; Upmanyu, V.; Chakravarti, S.; Singh, S.K.; Das, G.K.; Kumar, H.; Krisnaswami, N. Kiss1 and its receptor: Molecular Characterization and immunolocalization in the hypothalamus and corpus luteum of the buffalo. Anim. Biotechnol. 2019, 30, 342–351. [Google Scholar] [CrossRef]
- Mishra, G.K.; Patra, M.K.; Singh, L.K.; Upmanyu, V.; Chakravarti, S.; Karikalan, M.; Bag, S.; Singh, S.K.; Das, G.K.; Kumar, H.; et al. Expression and functional role of kisspeptin and its receptor in the cyclic corpus luteum of buffalo (Bubalus Bubalis). Theriogenology 2019, 130, 71–78. [Google Scholar] [CrossRef]
- Maranesi, M.; Petrucci, L.; Leonardi, L.; Bufalari, A.; Parillo, F.; Boiti, C.; Zerani, M. Kisspeptin/Kisspeptin Receptor system in pseudopregnant rabbit corpora lutea: Presence and function. Sci. Rep. 2019, 9, 5044. [Google Scholar] [CrossRef]
- Inoue, N.; Sasagawa, K.; Ikai, K.; Sasaki, Y.; Tomikawa, J.; Oishi, S.; Fujii, N.; Uenoyama, Y.; Ohmori, Y.; Yamamoto, N.; et al. Kisspeptin neurons mediate reflex ovulation in the musk shrew (Suncus Murinus). Proc. Natl. Acad. Sci. USA 2011, 108, 17527–17532. [Google Scholar] [CrossRef] [Green Version]
- Stephens, S.B.Z.; Tolson, K.P.; Rouse, M.L.; Poling, M.C.; Hashimoto-Partyka, M.K.; Mellon, P.L.; Kauffman, A.S. Absent Progesterone signaling in kisspeptin neurons disrupts the LH surge and impairs fertility in female mice. Endocrinology 2015, 156, 3091–3097. [Google Scholar] [CrossRef] [PubMed]
- Anuradha; Krishna, A. Kisspeptin regulates ovarian steroidogenesis during delayed embryonic development in the fruit bat, cynopterus sphinx. Mol. Reprod. Dev. 2017, 84, 1155–1167. [Google Scholar] [CrossRef]
- Clarke, S.A.; Dhillo, W.S. Phoenixin and its role in reproductive hormone release. Semin. Reprod. Med. 2019, 37, 191–196. [Google Scholar] [CrossRef]
- Mick, D.U.; Dennerlein, S.; Wiese, H.; Reinhold, R.; Pacheu-Grau, D.; Lorenzi, I.; Sasarman, F.; Weraarpachai, W.; Shoubridge, E.A.; Warscheid, B.; et al. Mitrac links mitochondrial protein translocation to respiratory-chain assembly and translational regulation. Cell 2012, 151, 1528–1541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennerlein, S.; Oeljeklaus, S.; Jans, D.; Hellwig, C.; Bareth, B.; Jakobs, S.; Deckers, M.; Warscheid, B.; Rehling, P. MITRAC7 acts as a COX1-specific chaperone and reveals a checkpoint during cytochrome coxidase assembly. Cell Reprod. 2015, 12, 1644–1655. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, G.L. Pathology of the corpus luteum of cows. Theriogenology 1996, 45, 1413–1428. [Google Scholar] [CrossRef]
- Hallatt, J.G.; Steele Jr, C.H.; Snyder, M. Ruptured corpus luteum with hemoperitoneum: A study of 173 surgical cases. Am. J. Obstet. Gynecol. 1984, 149, 5–9. [Google Scholar] [CrossRef]
- Spinelli, C.; Di Giacomo, M.; Mucci, N.; Massart, F. Hemorrhagic corpus luteum cysts: An unusual problem for pediatric surgeons. J. Pediatric Adolesc. Gynecol. 2009, 22, 163–167. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Kito, S.; Sumi, N.; Sato, K. A study of the central cavity in the bovine corpus luteum. Vet. Rec. 1998, 123, 180–183. [Google Scholar] [CrossRef] [PubMed]
- McEntee, K. Reproductive Pathology of Domestic Mammals; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Atri, M. Ectopic pregnancy versus corpus luteum cyst revisited: Best doppler predictors. J. Ultrasound Med. 2003, 22, 1181–1184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gröhn, Y.T.; Hertl, J.A.; Harman, J.L. Effect of early lactation milk yield on reproductive disorders in dairy cows. Am. J. Vet. Res. 1994, 55, 1521–1528. [Google Scholar] [PubMed]
- Farin, P.W.; Youngquist, R.S.; Parfet, J.R.; Garverick, H.A. Diagnosis of luteal and follicular ovarian cysts by palpation per rectum and linear-array ultrasonography in dairy cows. J. Am. Vet. Med. Assoc. 1992, 200, 1085–1089. [Google Scholar]
- Rizzo, A.; Piccinno, M.; Ceci, E.; Pantaleo, M.; Mutinati, M.; Roncetti, M.; Sciorsci, R.L. Kisspeptin and bovine follicular cysts. Vet. Ital. 2018, 54, 29–31. [Google Scholar] [CrossRef]
- Naldini, A.; Carraro, F. Role of inflammatory mediators in angiogenesis. Curr. Drug Targets Inflamm. Allergy 2005, 4, 3–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Voronov, E.; Carmi, Y.; Apte, R.N. The role IL-1 in tumor-mediated angiogenesis. Front. Physiol. 2014, 5, 114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brännström, M.; Giesecke, L.; Moore, I.C.; van den Heuvel, C.J.; Robertson, S.A. Leukocyte subpopulations in the rat corpus luteum during pregnancy and pseudopregnancy. Biol. Reprod. 1994, 50, 1161–1167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reibiger, I.; Spanel-Borowski, K. Difference in localization of eosinophils and mast cells in the bovine ovary. J. Reprod. Fertil. 2000, 118, 243–250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aust, G.; Simchen, C.; Heider, U.; Hmeidan, F.A.; Blumenauer, V.; Spanel-Borowski, K. Eosinophils in the human corpus luteum: The role of RANTES and eotaxin in eosinophil attraction into periovulatory structures. Mol. Hum. Reprod. 2000, 6, 1085–1091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, J.M.; Van Der Maaten, M.J.; Whetstone, C.A. Effects of a bovine herpesvirus-1 isolate on reproductive function in heifers: Classification as a type-2 (infectious pustular vulvovaginitis) virus by restriction endonuclease analysis of viral DNA. Am. J. Vet. Res. 1988, 49, 1653–1656. [Google Scholar] [PubMed]
- Miller, J.M.; Van Der Maaten, M.J. Experimentally induced infectious bovine rhinotracheitis virus infection during early pregnancy: Effect on the bovine corpus luteum and conceptus. Am. J. Vet. Res. 1986, 47, 223–228. [Google Scholar]
- Robinson, N.A.; Leslie, K.E.; Walton, J.S. Effect of treatment with progesterone on pregnancy rate and plasma concentrations of progesterone in Holstein cows. J. Dairy Sci. 1989, 72, 202–207. [Google Scholar] [CrossRef]
- Kaneko, K.; Nakamura, M.; Sato, R. Influence of Trueperella pyogenes in uterus on corpus luteum lifespan in cycling cows. Theriogenology 2013, 79, 803–808. [Google Scholar] [CrossRef]
- Peter, A.T.; Bosu, W.T.K. Effects of intrauterine infection on the function of the corpora lutea formed after first postpartum ovulations in dairy cows. Theriogenology 1987, 27, 593–609. [Google Scholar] [CrossRef]
- Williams, E.J.; Fischer, D.P.; Noakes, D.E.; England, G.C.; Rycroft, A.; Dobson, H.; Sheldon, I.M. The relationship between uterine pathogen growth density and ovarian function in the postpartum dairy cow. Theriogenology 2007, 68, 549–559. [Google Scholar] [CrossRef] [Green Version]
- Galvao, K.N.; Santos, J.E.P. Recent advances in the immunology and uterine microbiology of healthy cows and cows that develop uterine disease. Tur. J. Vet. Anim. Sci. 2014, 38, 577–588. [Google Scholar] [CrossRef]
- Lian, Y.; Zhao, F.; Wang, W. Central leptin resistance and hypothalamic inflammation are involved in letrozole-induced polycystic ovary syndrome rats. Biochem. Biophys. Res. Commun. 2016, 476, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Xita, N.; Papassotiriou, I.; Georgiou, I.; Vounatsou, M.; Margeli, A.; Tsatsoulis, A. The adiponectin-to-leptin ratio in women with polycystic ovary syndrome: Relation to insulin resistance and proinflammatory markers. Metabolism 2007, 56, 766–771. [Google Scholar] [CrossRef] [PubMed]
- Roberts, J.S. Veterinary Obstetrics and Genital Diseases: (Theriogenology); CBS Publishers: New Delhi, India, 1986. [Google Scholar]
- Chorlton, I.; Norris, H.J.; King, F.M. Malignant reticuloendothelial disease involving the ovary as a primary manifestation. A series of 19 lymphomas and 1 granulocytic sarcoma. Cancer 1974, 34, 397–407. [Google Scholar] [CrossRef]
- Dogra, S.; Neelakantan, D.; Patel, M.M.; Griesel, B.; Olson, A.; Woo, S. Adipokine Apelin/APJ pathway promotes peritoneal dissemination of ovarian cancer cells by regulating lipid metabolism. Mol. Cancer Res. 2021, 19, 1534–1545. [Google Scholar] [CrossRef] [PubMed]
- Kato, S.; Abarzua-Catalan, L.; Trigo, C.; Delpiano, A.; Sanhueza, C.; García, K.; Cuello, M. Leptin stimulates migration and invasion and maintains cancer stem-like properties in ovarian cancer cells: An explanation for poor outcomes in obese women. Oncotarget 2015, 6, 21100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffmann, M.; Rak, A.; Ptak, A. Bisphenol A and its derivatives decrease expression of chemerin, which reverses its stimulatory action in ovarian cancer cells. Toxicol. Lett. 2018, 291, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Falsetti, L.; Pasinetti, E.; Mazzani, M.D.; Gastaldi, A. Weight loss and menstrual cycle: Clinical and endocrinological evaluation. Gynecol. Endocrinol. 1992, 6, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Malik, P.; Rao, K.A. Luteal Phase Defect. In The Infertility Manual; Rao, K.A., Ed.; Jaypee Brothers Medical Publishers: New Delhi, India, 2018; pp. 106–112. [Google Scholar]
- Murtagh, E.; Risk Factor Collaboration. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: A pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. Lancet 2017, 390, 2627–2642. [Google Scholar]
- Nillius, S.J.; Wide, L. Gonadotrophin-releasing hormone treatment for induction of follicular maturation and ovulation in amenorrhoeic women with anorexia nervosa. BMJ 1973, 3, 405–408. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Practice Committee of the American Society for Reproductive Medicine. Obesity and reproduction: An educational bulletin. Fertil. Steril. 2008, 90, S21–S29. [Google Scholar] [CrossRef]
- Jain, A.; Polotsky, A.J.; Rochester, D.; Berga, S.L.; Loucks, T.; Zeitlian, G.; Santoro, N. Pulsatile luteinizing hormone amplitude and progesterone metabolite excretion are reduced in obese women. J. Clin. Endocrinol. Metab. 2007, 92, 2468–2473. [Google Scholar] [CrossRef] [PubMed]
- Kuokkanen, S.; Polotsky, A.J.; Chosich, J.; Bradford, A.P.; Jasinska, A.; Phang, T.; Appt, S.E. Corpus luteum as a novel target of weight changes that contribute to impaired female reproductive physiology and function. Syst. Biol. Reprod. Med. 2016, 62, 227–242. [Google Scholar] [CrossRef] [Green Version]
- Latif, R.; Rafique, N.; Salem, A.M.; AlSheikh, M.H.; Chathoth, S. Correlation between circulatory Kisspeptin and Adipokines in normal and over-weight Saudi females during menstrual cycle. Biol. Rhythm Res. 2018, 49, 169–174. [Google Scholar] [CrossRef]
- Loucks, A.B. Effects of exercise training on the menstrual cycle: Existence and mechanisms. Med. Sci. Sports Exerc. 1990, 22, 275–280. [Google Scholar] [CrossRef] [PubMed]
- De Souza, M.J.; Miller, B.E.; Loucks, A.B.; Luciano, A.A.; Pescatello, L.S.; Campbell, C.; Lasley, B. High frequency of luteal phase deficiency and anovulation in recreational women runners: Blunted elevation in follicle-stimulating hormone observed during luteal-follicular transition. J. Clin. Endocrinol. Metab. 1998, 83, 4220–4232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ginsburg, K.A. Luteal phase defect: Etiology, diagnosis, and management. Endocrinol. Metab. Clin. North. Am. 1992, 21, 85–104. [Google Scholar] [CrossRef]
- Plinta, R.; Olszanecka-Glinianowicz, M.; Drosdzol-Cop, A.; Chudek, J.; Skrzypulec-Plinta, V. The effect of three-month pre-season preparatory period and short-term exercise on plasma leptin, adiponectin, visfatin, and ghrelin levels in young female handball and basketball players. J. Endocrinol. Investig. 2012, 35, 595–601. [Google Scholar] [CrossRef]
- De Souza, M.J.; Van Heest, J.; Demers, L.M.; Lasley, B.L. Luteal phase deficiency in recreational runners: Evidence for a hypometabolic state. J. Clin. Endocrinol. Metab. 2003, 88, 337–346. [Google Scholar] [CrossRef] [Green Version]
- Kraemer, R.R.; Castracane, V.D. Exercise and humoral mediators of peripheral energy balance: Ghrelin and adiponectin. Exp. Biol. Med. Maywood 2007, 232, 184–194. [Google Scholar] [CrossRef]
- Verma, I.; Sood, R.; Juneja, S.; Kaur, S. Prevalence of hypothyroidism in infertile women and evaluation of response of treatment for hypothyroidism on infertility. Int. J. Appl. Basic. Med. Res. 2012, 2, 17–19. [Google Scholar] [CrossRef] [Green Version]
- Loucks, A.B.; Laughlin, G.A.; Mortola, J.F.; Girton, L.; Nelson, J.C.; Yen, S.S. Hypothalamic-pituitary-thyroidal function in eumenorrheic and amenorrheic athletes. J. Clin. Endocrinol. Metab. 1992, 75, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Naficy, H.; Behjatnia, Y. The effect of thyroid extract on luteal phase deficiency. Acta Med. Iran. 1975, 18, 55–60. [Google Scholar] [PubMed]
- Thong, F.S.; McLean, C.; Graham, T.E. Plasma leptin in female athletes: Relationship with body fat, reproductive, nutritional, and endocrine factors. J. Appl. Physiol. (1985) 2000, 88, 2037–2044. [Google Scholar] [CrossRef] [PubMed]
- Cunha-Filho, J.S.; Gross, J.L.; de Souza, C.A.B.; Lemos, N.A.; Giugliani, C.; Freitas, F.; Passos, E.P. Physiopathological aspects of corpus luteum defect in infertile patients with mild/minimal endometriosis. J. Assist. Reprod. Genet. 2013, 20, 117–121. [Google Scholar] [CrossRef] [PubMed]
- Moeloek, F.A.; Moegny, E. Endometriosis and luteal phase defect. Asia Oceania J. Obstet. Gynaecol. 1993, 19, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Jacobs, M.H.; Balasch, J.; Gonzalez-Merelo, J.M.; Vanrell, J.A.; Wheeler, C.; Strauss, J.F.; Lyttle, C.R. Endometrial cytosolic and nuclear progesterone receptors in the luteal phase defect. J. Clin. Endocrinol. Metab. 1987, 64, 472–475. [Google Scholar] [CrossRef]
- Daly, D.C.; Maslar, I.A.; Rosenberg, S.M.; Tohan, N.; Riddick, D.H. Prolactin production by luteal phase defect endometrium. Am. J. Obstet. Gynecol. 1981, 140, 587–591. [Google Scholar] [CrossRef]
- He, Y.E. Prolactin secretion in patients with endometriosis and its relationship to luteal phase defect and infertility. Zhonghua Fu Chan Ke Za Zhi 1993, 28, 14–17. [Google Scholar] [PubMed]
- Tennekoon, K.H.; Eswaramohan, T.; Karunanayake, E.H. Effect of leptin on prolactin and insulin-like growth factor-I secretion by cultured rat endometrial stromal cells. Fertil. Steril. 2007, 88, 193–199. [Google Scholar] [CrossRef]
- Farin, P.W.; Estill, C.T. Infertility due to abnormalities of the ovaries in cattle. Vet. Clin. North. Am. Food Anim. Pract. 1993, 9, 291–308. [Google Scholar] [CrossRef] [PubMed]
- Seguin, B. Altering estrous cycles in cows by intrauterine infusion. In Current Therapy in Theriogenology; Morrow, D.A., Ed.; W. B. Saunders. Co.: Philadelphia, PA, USA, 1980; pp. 177–188. [Google Scholar]
- Stabenfeldt, G.H.; Hughes, J.P.; Neely, D.P.; Kindahl, H.; Edqvist, L.E.; Gustafsson, B. Physiologic and pathophysiologic aspects of prostaglandin F2 alpha during the reproductive cycle. J. Am. Vet. Med. Assoc. 1980, 176, 1187–1194. [Google Scholar] [PubMed]
- Garrett, J.E.; Geisert, R.D.; Zavy, M.T.; Gries, L.K.; Wettemann, R.P.; Buchanan, D.S. Effect of exogenous progesterone on prostaglandin F2 alpha release and the interestrous interval in the bovine. Prostaglandins 1988, 36, 85–96. [Google Scholar] [CrossRef]
- Ginther, O.J. Effect of progesterone on length of estrous cycle in cattle. Am. J. Vet. Res. 1970, 31, 493–496. [Google Scholar] [PubMed]
- Tesarik, J.; Conde-López, C.; Galán-Lázaro, M.; Mendoza-Tesarik, R. Luteal Phase in Assisted Reproductive Technology. Front. Reprod. Health 2020, 2, 595183. [Google Scholar] [CrossRef]
- Patel, B.G.; Rudnicki, M.; Yu, J.; Shu, Y.; Taylor, R.N. Progesterone resistance in endometriosis: Origins, consequences and interventions. Acta Obstet. Gynecol. Scand. 2017, 96, 623–632. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Savaris, R.F.; Groll, J.M.; Young, S.L.; DeMayo, F.J.; Jeong, J.W.; Hamilton, A.E.; Giudice, L.C.; Lessey, B.A. Progesterone resistance in PCOS endometrium: A microarray analysis in clomiphene citrate-treated and artificial menstrual cycles. J. Clin. Endocrinol. Metab. 2011, 96, 1737–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhillo, W.S.; Chaudhri, O.B.; Thompson, E.L.; Murphy, K.G.; Patterson, M.; Ramachandran, R.; Nijher, G.K.; Amber, V.; Kokkinos, A.; Donaldson, M.; et al. Kisspeptin-54 stimulates gonadotropin release most potently during the preovulatory phase of the menstrual cycle in women. J. Clin. Endocrinol. Metab. 2007, 92, 3958–3966. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jayasena, C.N.; Nijher, G.M.; Chaudhri, O.B.; Murphy, K.G.; Ranger, A.; Lim, A.; Patel, D.; Mehta, A.; Todd, C.; Ramachandran, R.; et al. Subcutaneous injection of kisspeptin-54 acutely stimulates gonadotropin secretion in women with hypothalamic amenorrhea, but chronic administration causes tachyphylaxis. J. Clin. Endocrinol. Metab. 2009, 94, 4315–4323. [Google Scholar] [CrossRef]
- Tan, B.K.; Heutling, D.; Chen, J.; Farhatullah, S.; Adya, R.; Keay, S.D.; Kennedy, C.R.; Lehnert, H.; Randeva, H.S. Metformin decreases the adipokine vaspin in overweight women with polycystic ovary syndrome concomitant with improvement in insulin sensitivity and a decrease in insulin resistance. Diabetes 2008, 57, 1501–1507. [Google Scholar] [CrossRef] [Green Version]
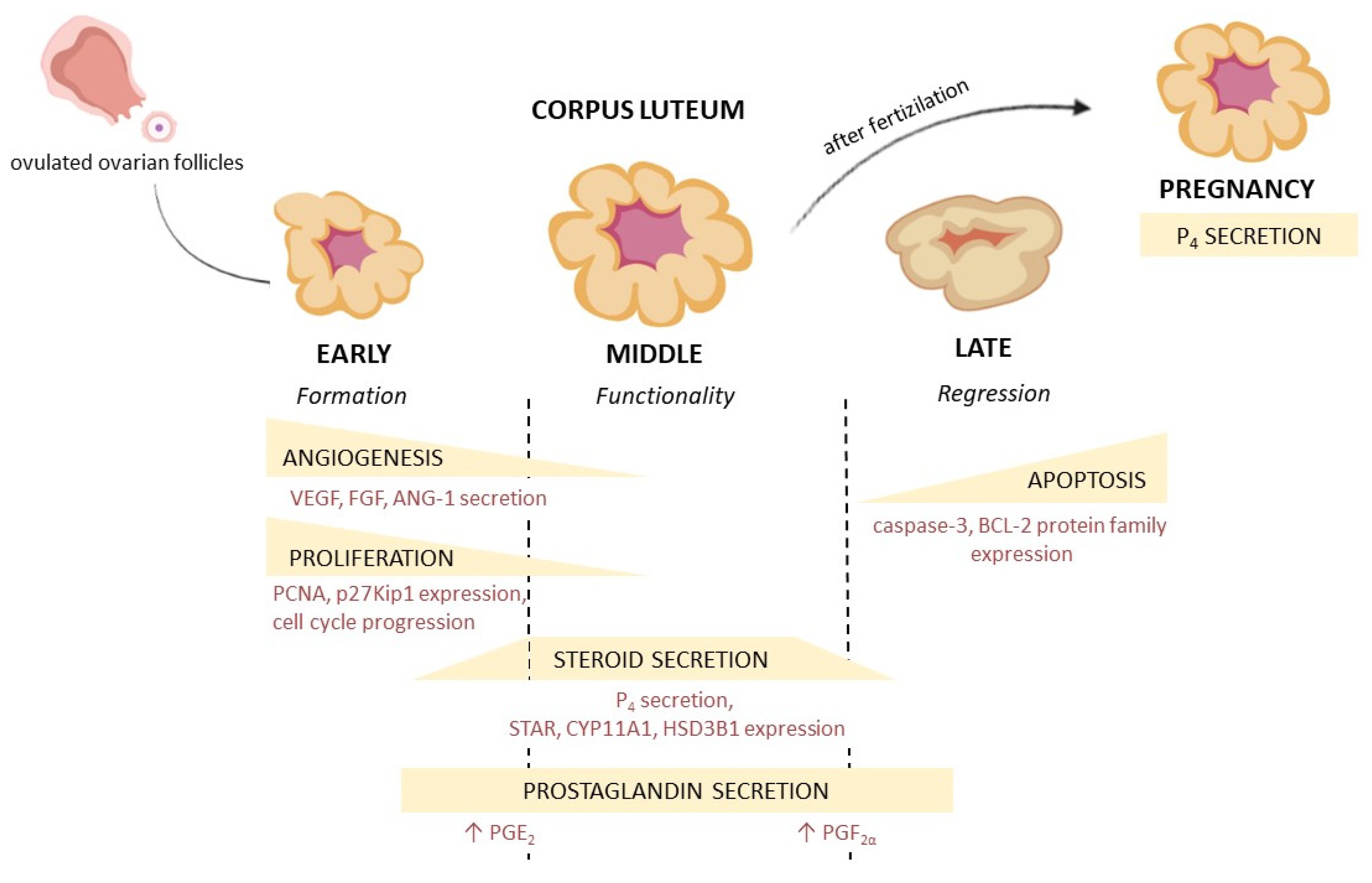


| Adipokine | Species Expression | |
|---|---|---|
| Gene | Protein | |
| leptin | human, rat, pig (EC, PR), cattle, horse, goat, water buffalo, dog (EC, PR) | human, rat, pig (EC, PR), cattle, horse, goat, water buffalo |
| LEPR | human, baboon (PR), cattle (EC, PR), dog, rat, pig (EC, PR), horse, water buffalo | rat, pig (EC, PR), horse, water buffalo, mouse, rabbit, alpaca, Japanese black bear |
| adiponectin | rat, cattle, pig, water buffalo | rat, cattle, pig, water buffalo |
| AdipoR1, AdipoR2 | rat, cattle, pig, water buffalo | rat, cattle, pig, water buffalo |
| apelin | cattle, rhesus monkey, pig, mouse | cattle, sheep, pig, dog |
| APJ | cattle, rhesus monkey, pig, mouse | cattle, sheep, pig, dog |
| visfatin | water buffalo, cattle, pig (EC, PR) | water buffalo, cattle, mouse, pig (EC, PR) |
| vaspin | pig | pig |
| chemerin | pig (EC, PR), cattle, rat, mouse | pig (EC, PR), cattle, rat |
| CMKLR1 | pig (EC, PR), cattle, mouse, rat | pig (EC, PR), cattle, rat |
| CCRL2 | pig (EC, PR), cattle | pig (EC, PR), cattle |
| GPR1 | mouse, pig (EC, PR), cattle | mouse, pig (EC, PR), cattle |
| orexin | pig, rat, water buffalo | pig, rat |
| OXR1, OXR2 | pig, rat, water buffalo | pig, rat |
| ghrelin | pig, rat (EC, PR), horse, cattle, water buffalo, goat, sheep | pig, rat (EC, PR), human, horse, cattle, water buffalo, sheep |
| GHSR | pig, human, horse, cattle, water buffalo, goat | pig, human, horse, cattle, water buffalo, |
| kisspeptin | rat, water buffalo | rat, water buffalo, dog, cat, rabbit (PR) |
| Kiss1R | rat, water buffalo | rat, dog, cat, water buffalo, rabbit (PR) |
| phoenixin | pig | pig, human |
| GPR173 | pig | pig |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mlyczyńska, E.; Kieżun, M.; Kurowska, P.; Dawid, M.; Pich, K.; Respekta, N.; Daudon, M.; Rytelewska, E.; Dobrzyń, K.; Kamińska, B.; et al. New Aspects of Corpus Luteum Regulation in Physiological and Pathological Conditions: Involvement of Adipokines and Neuropeptides. Cells 2022, 11, 957. https://doi.org/10.3390/cells11060957
Mlyczyńska E, Kieżun M, Kurowska P, Dawid M, Pich K, Respekta N, Daudon M, Rytelewska E, Dobrzyń K, Kamińska B, et al. New Aspects of Corpus Luteum Regulation in Physiological and Pathological Conditions: Involvement of Adipokines and Neuropeptides. Cells. 2022; 11(6):957. https://doi.org/10.3390/cells11060957
Chicago/Turabian StyleMlyczyńska, Ewa, Marta Kieżun, Patrycja Kurowska, Monika Dawid, Karolina Pich, Natalia Respekta, Mathilde Daudon, Edyta Rytelewska, Kamil Dobrzyń, Barbara Kamińska, and et al. 2022. "New Aspects of Corpus Luteum Regulation in Physiological and Pathological Conditions: Involvement of Adipokines and Neuropeptides" Cells 11, no. 6: 957. https://doi.org/10.3390/cells11060957
APA StyleMlyczyńska, E., Kieżun, M., Kurowska, P., Dawid, M., Pich, K., Respekta, N., Daudon, M., Rytelewska, E., Dobrzyń, K., Kamińska, B., Kamiński, T., Smolińska, N., Dupont, J., & Rak, A. (2022). New Aspects of Corpus Luteum Regulation in Physiological and Pathological Conditions: Involvement of Adipokines and Neuropeptides. Cells, 11(6), 957. https://doi.org/10.3390/cells11060957










