Mice with Targeted Knockout of Tetraspanin 3 Exhibit Reduced Trabecular Bone Mass Caused by Decreased Osteoblast Functions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tspan3 KO Mice
2.2. Micro-CT Evaluation
2.3. Histology
2.4. Serum Alkaline Phosphatase (ALP) Assay
2.5. Statistical Analysis
3. Results
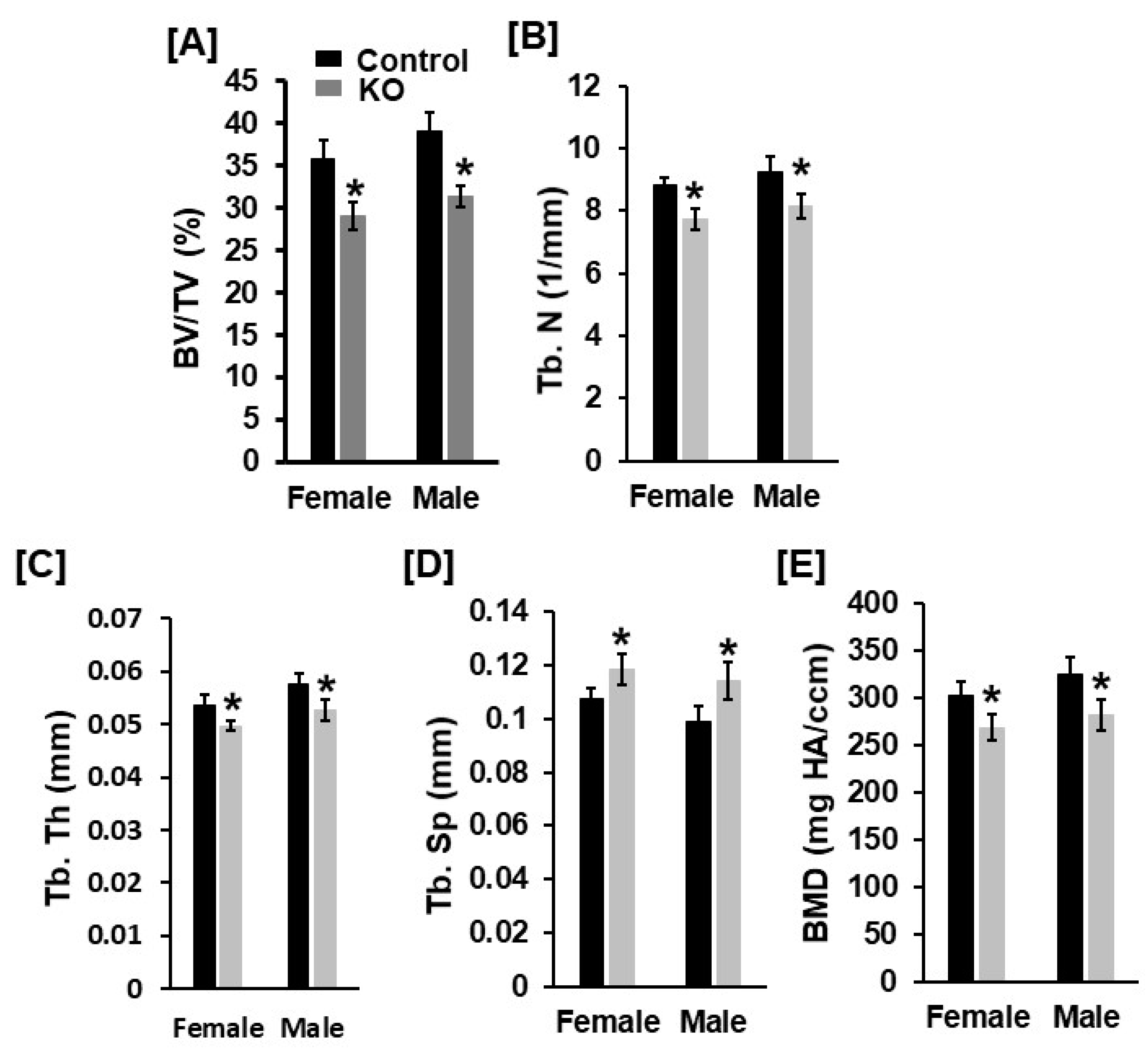
3.1. Mice with Disruption of Tspan3 Exhibit Low Bone Mineral Density
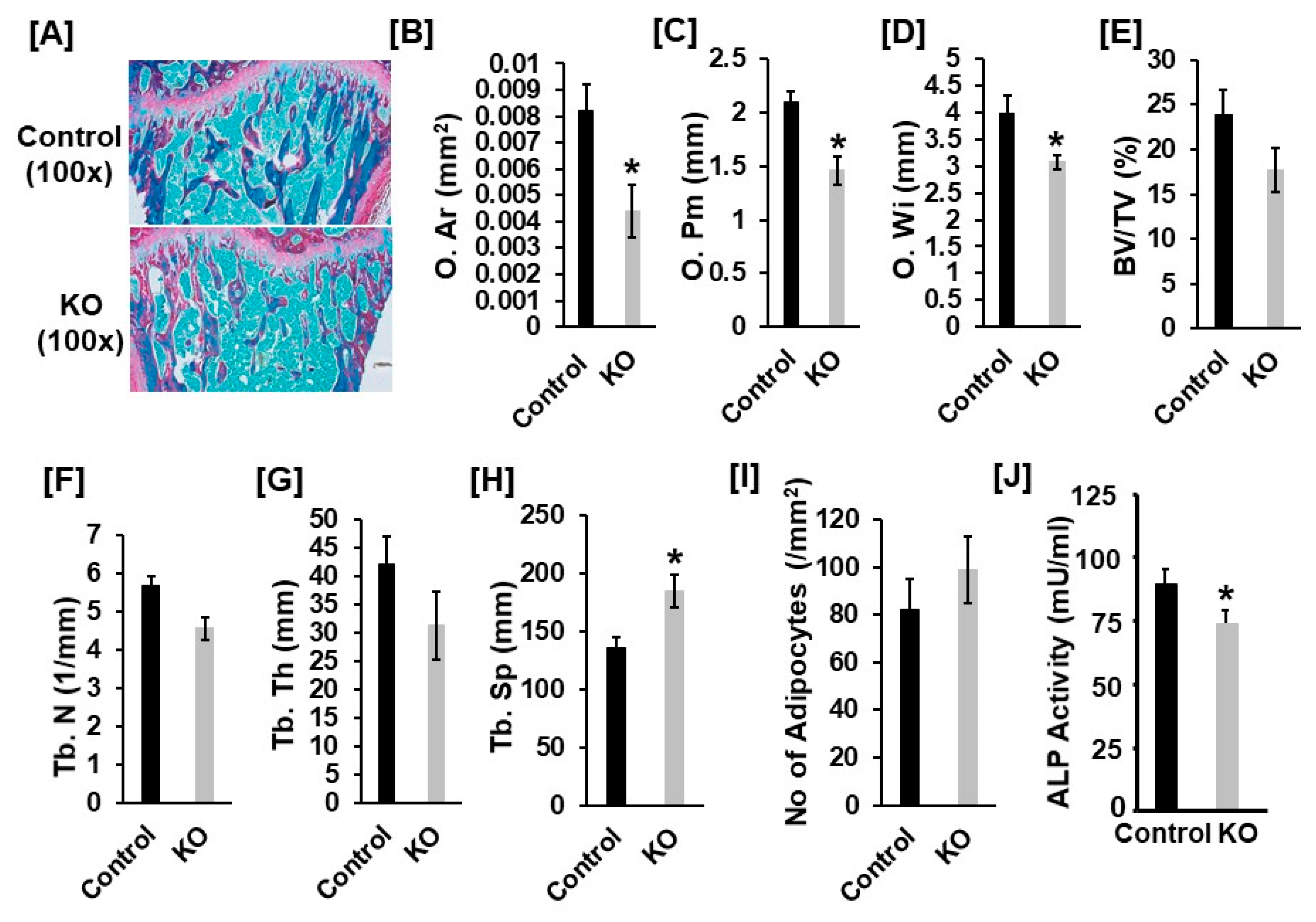
3.2. Disruption of Tspan3 in Mice Does Not Influence Cortical Bone Mass
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hemler, M.E. Tetraspanin functions and associated microdomains. Nat. Rev. Mol. Cell Biol. 2005, 6, 801–811. [Google Scholar] [CrossRef] [PubMed]
- Termini, C.M.; Gillette, J.M. Tetraspanins Function as Regulators of Cellular Signaling. Front. Cell Dev. Biol. 2017, 5, 34. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiwari-Woodruff, S.K.; Buznikov, A.G.; Vu, T.Q.; Micevych, P.E.; Chen, K.; Kornblum, H.I.; Bronstein, J.M. Osp/Claudin-11 Forms a Complex with a Novel Member of the Tetraspanin Super Family and β1 Integrin and Regulates Proliferation and Migration of Oligodendrocytes. J. Cell Biol. 2001, 153, 295–306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lindsey, R.C.; Xing, W.; Pourteymoor, S.; Godwin, C.; Gow, A.; Mohan, S. Novel Role for Claudin-11 in the Regulation of Osteoblasts via Modulation of ADAM10-Mediated Notch Signaling. J. Bone Miner. Res. 2019, 34, 1910–1922. [Google Scholar] [CrossRef] [PubMed]
- Kwon, H.Y.; Bajaj, J.; Ito, T.; Blevins, A.; Konuma, T.; Weeks, J.; Lytle, N.K.; Koechlein, C.S.; Rizzieri, D.; Chuah, C.; et al. Tetraspanin 3 Is Required for the Development and Propagation of Acute Myelogenous Leukemia. Cell Stem Cell 2015, 17, 152–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Müller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef]
- Xing, W.; Pourteymoor, S.; Mohan, S. Ascorbic acid regulates osterix expression in osteoblasts by activation of prolyl hydroxylase and ubiquitination-mediated proteosomal degradation pathway. Physiol. Genom. 2011, 43, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Xing, W.; Kim, J.; Wergedal, J.; Chen, S.-T.; Mohan, S. Ephrin B1 Regulates Bone Marrow Stromal Cell Differentiation and Bone Formation by Influencing TAZ Transactivation via Complex Formation with NHERF1. Mol. Cell. Biol. 2010, 30, 711–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: A 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013, 28, 2–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beamer, W.; Donahue, L.; Rosen, C.; Baylink, D. Genetic variability in adult bone density among inbred strains of mice. Bone 1996, 18, 397–403. [Google Scholar] [CrossRef]
- Qin, X.; Wergedal, J.E.; Rehage, M.; Tran, K.; Newton, J.; Lam, P.; Baylink, D.J.; Mohan, S. Pregnancy-Associated Plasma Protein-A Increases Osteoblast Proliferation in Vitro and Bone Formation in Vivo. Endocrinology 2006, 147, 5653–5661. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Farley, J.R.; Hall, S.L.; Tanner, M.A.; Wergedal, J.E. Specific activity of skeletal alkaline phosphatase in human osteoblast-line cells regulated by phosphate, phosphate esters, and phosphate analogs and release of alkaline phosphatase activity inversely regulated by calcium. J. Bone Miner. Res. 2009, 9, 497–508. [Google Scholar] [CrossRef] [PubMed]
- Seipold, L.; Damme, M.; Prox, J.; Rabe, B.; Kasparek, P.; Sedlacek, R.; Altmeppen, H.; Willem, M.; Boland, B.; Glatzel, M.; et al. Tetraspanin 3: A central endocytic membrane component regulating the expression of ADAM10, presenilin and the amyloid precursor protein. Biochim. Biophys. Acta 2017, 1864, 217–230. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.-O.; Lee, Y.D.; Kim, H.; Kim, M.K.; Song, M.-K.; Lee, Z.H.; Kim, H.-H. Tetraspanin 7 regulates sealing zone formation and the bone-resorbing activity of osteoclasts. Biochem. Biophys. Res. Commun. 2016, 477, 1078–1084. [Google Scholar] [CrossRef] [PubMed]
- Bergsma, A.; Ganguly, S.S.; Dick, D.; Williams, B.; Miranti, C.K. Global deletion of tetraspanin CD82 attenuates bone growth and enhances bone marrow adipogenesis. Bone 2018, 113, 105–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Shi, H.; Szymczak, L.; Aydin, T.; Yun, S.; Constas, K.; Schaeffer, A.; Ranjan, S.; Kubba, S.; Alam, E.; et al. Promotion of Bone Morphogenetic Protein Signaling by Tetraspanins and Glycosphingolipids. PLoS Genet. 2015, 11, e1005221. [Google Scholar] [CrossRef] [PubMed] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xing, W.; Pourteymoor, S.; Kesavan, C.; Gomez, G.A.; Mohan, S. Mice with Targeted Knockout of Tetraspanin 3 Exhibit Reduced Trabecular Bone Mass Caused by Decreased Osteoblast Functions. Cells 2022, 11, 977. https://doi.org/10.3390/cells11060977
Xing W, Pourteymoor S, Kesavan C, Gomez GA, Mohan S. Mice with Targeted Knockout of Tetraspanin 3 Exhibit Reduced Trabecular Bone Mass Caused by Decreased Osteoblast Functions. Cells. 2022; 11(6):977. https://doi.org/10.3390/cells11060977
Chicago/Turabian StyleXing, Weirong, Sheila Pourteymoor, Chandrasekhar Kesavan, Gustavo A. Gomez, and Subburaman Mohan. 2022. "Mice with Targeted Knockout of Tetraspanin 3 Exhibit Reduced Trabecular Bone Mass Caused by Decreased Osteoblast Functions" Cells 11, no. 6: 977. https://doi.org/10.3390/cells11060977
APA StyleXing, W., Pourteymoor, S., Kesavan, C., Gomez, G. A., & Mohan, S. (2022). Mice with Targeted Knockout of Tetraspanin 3 Exhibit Reduced Trabecular Bone Mass Caused by Decreased Osteoblast Functions. Cells, 11(6), 977. https://doi.org/10.3390/cells11060977




