The Role of Amino Acids in Endothelial Biology and Function
Abstract
1. Introduction
2. Amino Acid in Biology and Function of Endothelial Cells
2.1. Non-Essential Amino Acids
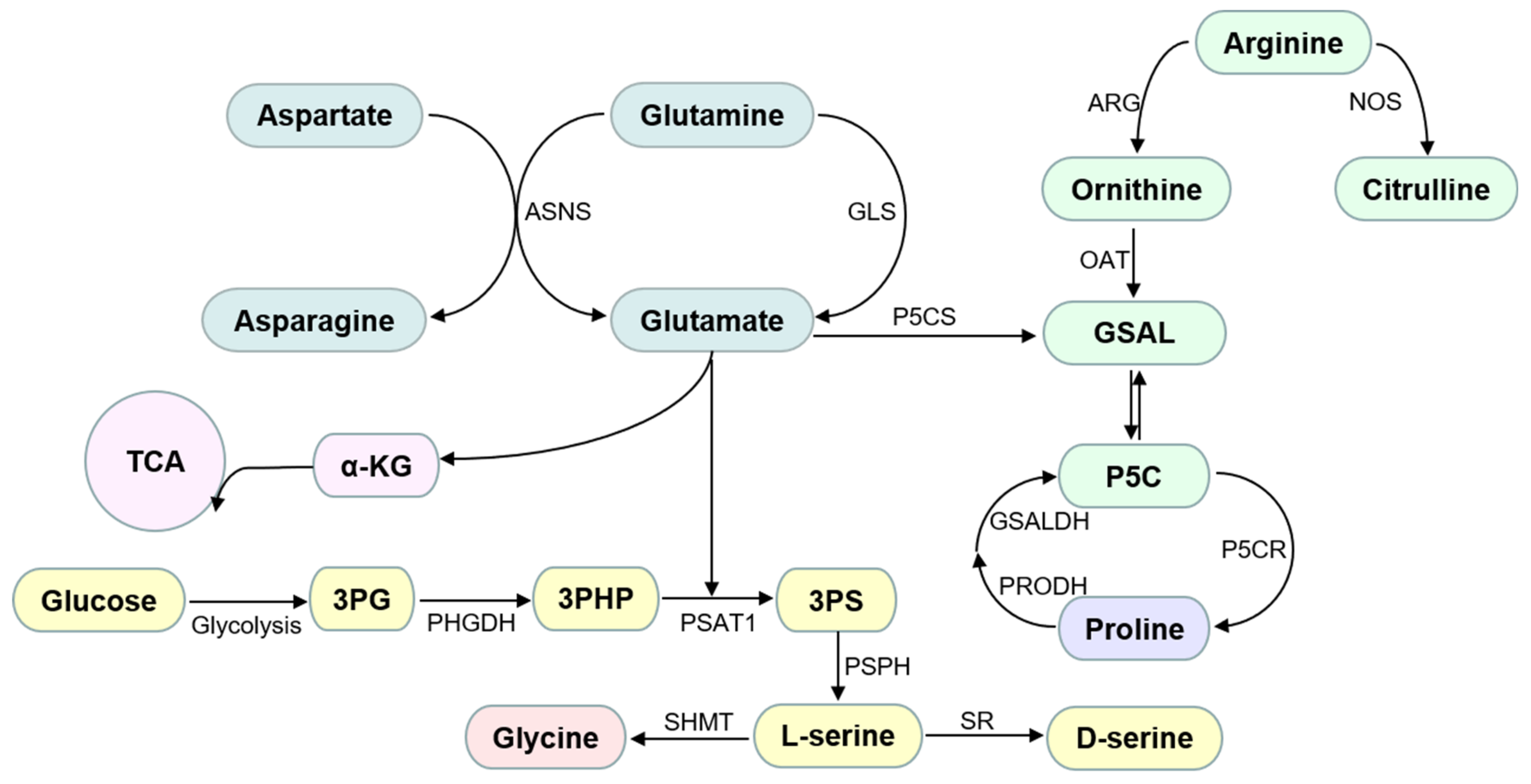
2.1.1. Glycine
2.1.2. Proline
2.1.3. Serine
2.1.4. Cysteine
2.1.5. Glutamine and Asparagine
2.1.6. Arginine
2.2. Essential Amino Acids
2.2.1. Tryptophan, Methionine, and Phenylalanine
2.2.2. Branched-Chain Amino Acids
3. Amino Acid Homeostasis Disruption as a Risk Factor of Vascular Complications in Ischemic Heart Disease
3.1. Macrovascular and Microvascular Complications in Diabetes
3.2. Hypertension
3.3. Hypercholesterolemia
4. Drugs That Restore Amino Acid Homeostasis for Improvement of Endothelial Dysfunction
5. Amino Acid Supplement for Improvement of Endothelial Dysfunction in Clinic
6. Current Challenge and Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Triggle, C.R.; Samuel, S.M.; Ravishankar, S.; Marei, I.; Arunachalam, G.; Ding, H. The endothelium: Influencing vascular smooth muscle in many ways. Can. J. Physiol. Pharm. 2012, 90, 713–738. [Google Scholar] [CrossRef]
- Ciccone, V.; Genah, S.; Morbidelli, L. Endothelium as a Source and Target of H2S to Improve Its Trophism and Function. Antioxid. 2021, 10, 486. [Google Scholar] [CrossRef]
- Boulanger, C.M. Endothelium. Arter. Thromb. Vasc. Biol. 2016, 36, e26–e31. [Google Scholar] [CrossRef]
- Michiels, C. Endothelial cell functions. J. Cell Physiol. 2003, 196, 430–443. [Google Scholar] [CrossRef]
- Nitz, K.; Lacy, M.; Atzler, D. Amino Acids and Their Metabolism in Atherosclerosis. Arter. Thromb. Vasc. Biol. 2019, 39, 319–330. [Google Scholar] [CrossRef]
- Jackson, A.A.; Shaw, J.C.; Barber, A.; Golden, M.H. Nitrogen metabolism in preterm infants fed human donor breast milk: The possible essentiality of glycine. Pediatr. Res. 1981, 15, 1454–1461. [Google Scholar] [CrossRef]
- Gersovitz, M.; Bier, D.; Matthews, D.; Udall, J.; Munro, H.N.; Young, V.R. Dynamic aspects of whole body glycine metabolism: Influence of protein intake in young adult and elderly males. Metabolism 1980, 29, 1087–1094. [Google Scholar] [CrossRef]
- Mahmood, K.; Emadi, A. 1-C Metabolism-Serine, Glycine, Folates-In Acute Myeloid Leukemia. Pharmaceuticals 2021, 14, 190. [Google Scholar] [CrossRef] [PubMed]
- Ducker, G.S.; Rabinowitz, J.D. One-Carbon Metabolism in Health and Disease. Cell Metab. 2017, 25, 27–42. [Google Scholar] [CrossRef] [PubMed]
- Cioffi, C.L. Modulation of Glycine-Mediated Spinal Neurotransmission for the Treatment of Chronic Pain. J. Med. Chem. 2018, 61, 2652–2679. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.; Han, G.; Ning, H.; Song, J.; Ran, N.; Yi, X.; Seow, Y.; Yin, H. Glycine Enhances Satellite Cell Proliferation, Cell Transplantation, and Oligonucleotide Efficacy in Dystrophic Muscle. Mol. Ther. 2020, 28, 1339–1358. [Google Scholar] [CrossRef]
- Bekri, A.; Liao, M.; Drapeau, P. Glycine Regulates Neural Stem Cell Proliferation During Development via Lnx1-Dependent Notch Signaling. Front. Mol. Neurosci. 2019, 12, 44. [Google Scholar] [CrossRef]
- Zhang, Y.; Jia, H.; Jin, Y.; Liu, N.; Chen, J.; Yang, Y.; Dai, Z.; Wang, C.; Wu, G.; Wu, Z. Glycine Attenuates LPS-Induced Apoptosis and Inflammatory Cell Infiltration in Mouse Liver. J. Nutr. 2020, 150, 1116–1125. [Google Scholar] [CrossRef]
- Maneikyte, J.; Bausys, A.; Leber, B.; Feldbacher, N.; Hoefler, G.; Kolb-Lenz, D.; Strupas, K.; Stiegler, P.; Schemmer, P. Dietary Glycine Prevents FOLFOX Chemotherapy-Induced Heart Injury: A Colorectal Cancer Liver Metastasis Treatment Model in Rats. Nutrients 2020, 12, 2634. [Google Scholar] [CrossRef]
- Van den Eynden, J.; Ali, S.S.; Horwood, N.; Carmans, S.; Brone, B.; Hellings, N.; Steels, P.; Harvey, R.J.; Rigo, J.M. Glycine and glycine receptor signalling in non-neuronal cells. Front. Mol. Neurosci. 2009, 2, 9. [Google Scholar] [CrossRef]
- Su, L.; Kong, X.; Loo, S.J.; Gao, Y.; Kovalik, J.P.; Su, X.; Ma, J.; Ye, L. Diabetic Endothelial Cells Differentiated From Patient iPSCs Show Dysregulated Glycine Homeostasis and Senescence Associated Phenotypes. Front. Cell Dev. Biol. 2021, 9, 667252. [Google Scholar] [CrossRef]
- Zhang, Y.; Ikejima, K.; Honda, H.; Kitamura, T.; Takei, Y.; Sato, N. Glycine prevents apoptosis of rat sinusoidal endothelial cells caused by deprivation of vascular endothelial growth factor. Hepatology 2000, 32, 542–546. [Google Scholar] [CrossRef]
- Hasegawa, S.; Ichiyama, T.; Sonaka, I.; Ohsaki, A.; Okada, S.; Wakiguchi, H.; Kudo, K.; Kittaka, S.; Hara, M.; Furukawa, S. Cysteine, histidine and glycine exhibit anti-inflammatory effects in human coronary arterial endothelial cells. Clin. Exp. Immunol. 2012, 167, 269–274. [Google Scholar] [CrossRef]
- Zakaria, E.R.; Joseph, B.; Hamidi, M.; Zeeshan, M.; Algamal, A.; Sartaj, F.; Althani, M.; Fadl, T.; Madan, D. Glycine improves peritoneal vasoreactivity to dialysis solutions in the elderly. Qatar Med. J. 2019, 2019, 19. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, J.; Chen, L.; Li, J.; Zhang, H.; Guo, X. Glycine Suppresses AGE/RAGE Signaling Pathway and Subsequent Oxidative Stress by Restoring Glo1 Function in the Aorta of Diabetic Rats and in HUVECs. Oxid. Med. Cell Longev. 2019, 2019, 4628962. [Google Scholar] [CrossRef]
- Ruiz-Ramirez, A.; Ortiz-Balderas, E.; Cardozo-Saldana, G.; Diaz-Diaz, E.; El-Hafidi, M. Glycine restores glutathione and protects against oxidative stress in vascular tissue from sucrose-fed rats. Clin. Sci. 2014, 126, 19–29. [Google Scholar] [CrossRef]
- Yu, Q.; Tai, Y.Y.; Tang, Y.; Zhao, J.; Negi, V.; Culley, M.K.; Pilli, J.; Sun, W.; Brugger, K.; Mayr, J.; et al. BOLA (BolA Family Member 3) Deficiency Controls Endothelial Metabolism and Glycine Homeostasis in Pulmonary Hypertension. Circulation 2019, 139, 2238–2255. [Google Scholar] [CrossRef]
- Bruns, H.; Petrulionis, M.; Schultze, D.; Al Saeedi, M.; Lin, S.; Yamanaka, K.; Ambrazevicius, M.; Strupas, K.; Schemmer, P. Glycine inhibits angiogenic signaling in human hepatocellular carcinoma cells. Amino Acids 2014, 46, 969–976. [Google Scholar] [CrossRef]
- Bruns, H.; Kazanavicius, D.; Schultze, D.; Saeedi, M.A.; Yamanaka, K.; Strupas, K.; Schemmer, P. Glycine inhibits angiogenesis in colorectal cancer: Role of endothelial cells. Amino Acids 2016, 48, 2549–2558. [Google Scholar] [CrossRef]
- Guo, D.; Murdoch, C.E.; Xu, H.; Shi, H.; Duan, D.D.; Ahmed, A.; Gu, Y. Vascular endothelial growth factor signaling requires glycine to promote angiogenesis. Sci. Rep. 2017, 7, 14749. [Google Scholar] [CrossRef]
- Tsuji-Tamura, K.; Sato, M.; Fujita, M.; Tamura, M. Glycine exerts dose-dependent biphasic effects on vascular development of zebrafish embryos. Biochem. Biophys. Res. Commun. 2020, 527, 539–544. [Google Scholar] [CrossRef]
- Tsuji-Tamura, K.; Sato, M.; Fujita, M.; Tamura, M. The role of PI3K/Akt/mTOR signaling in dose-dependent biphasic effects of glycine on vascular development. Biochem. Biophys. Res. Commun. 2020, 529, 596–602. [Google Scholar] [CrossRef]
- Karna, E.; Szoka, L.; Huynh, T.Y.L.; Palka, J.A. Proline-dependent regulation of collagen metabolism. Cell Mol. Life Sci. 2020, 77, 1911–1918. [Google Scholar] [CrossRef]
- Szabados, L.; Savoure, A. Proline: A multifunctional amino acid. Trends Plant. Sci. 2010, 15, 89–97. [Google Scholar] [CrossRef]
- Patriarca, E.J.; Cermola, F.; D’Aniello, C.; Fico, A.; Guardiola, O.; De Cesare, D.; Minchiotti, G. The Multifaceted Roles of Proline in Cell Behavior. Front. Cell Dev. Biol. 2021, 9, 728576. [Google Scholar] [CrossRef]
- Phang, J.M. Proline Metabolism in Cell Regulation and Cancer Biology: Recent Advances and Hypotheses. Antioxid Redox Signal. 2019, 30, 635–649. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Meininger, C.J.; Hawker, J.R., Jr.; Haynes, T.E.; Kepka-Lenhart, D.; Mistry, S.K.; Morris, S.M., Jr.; Wu, G. Regulatory role of arginase I and II in nitric oxide, polyamine, and proline syntheses in endothelial cells. Am. J. Physiol. Endocrinol. Metab. 2001, 280, E75–E82. [Google Scholar] [CrossRef] [PubMed]
- McAuslan, B.R.; Reilly, W.; Hannan, G.N.; Schindhelm, K.; Milthorpe, B.; Saur, B.A. Induction of endothelial cell migration by proline analogs and its relevance to angiogenesis. Exp. Cell Res. 1988, 176, 248–257. [Google Scholar] [CrossRef]
- Liu, N.; Dai, Z.; Zhang, Y.; Chen, J.; Yang, Y.; Wu, G.; Tso, P.; Wu, Z. Maternal L-proline supplementation enhances fetal survival, placental development, and nutrient transport in micedagger. Biol. Reprod. 2019, 100, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Reeds, P.J. Dispensable and indispensable amino acids for humans. J. Nutr. 2000, 130, 1835S–1840S. [Google Scholar] [CrossRef]
- Murtas, G.; Marcone, G.L.; Sacchi, S.; Pollegioni, L. L-serine synthesis via the phosphorylated pathway in humans. Cell Mol. Life Sci. 2020, 77, 5131–5148. [Google Scholar] [CrossRef]
- Maugard, M.; Vigneron, P.A.; Bolanos, J.P.; Bonvento, G. l-Serine links metabolism with neurotransmission. Prog. Neurobiol. 2021, 197, 101896. [Google Scholar] [CrossRef]
- El-Hattab, A.W.; Shaheen, R.; Hertecant, J.; Galadari, H.I.; Albaqawi, B.S.; Nabil, A.; Alkuraya, F.S. On the phenotypic spectrum of serine biosynthesis defects. J. Inherit. Metab. Dis. 2016, 39, 373–381. [Google Scholar] [CrossRef]
- Vandekeere, S.; Dubois, C.; Kalucka, J.; Sullivan, M.R.; Garcia-Caballero, M.; Goveia, J.; Chen, R.; Diehl, F.F.; Bar-Lev, L.; Souffreau, J.; et al. Serine Synthesis via PHGDH Is Essential for Heme Production in Endothelial Cells. Cell Metab. 2018, 28, 573–587.e513. [Google Scholar] [CrossRef]
- Maralani, M.N.; Movahedian, A.; Javanmard, S.H. Antioxidant and cytoprotective effects of L-Serine on human endothelial cells. Res. Pharm. Sci. 2012, 7, 209–215. [Google Scholar]
- Sim, W.C.; Han, I.; Lee, W.; Choi, Y.J.; Lee, K.Y.; Kim, D.G.; Jung, S.H.; Oh, S.H.; Lee, B.H. Inhibition of homocysteine-induced endoplasmic reticulum stress and endothelial cell damage by l-serine and glycine. Toxicol. Vitr. 2016, 34, 138–145. [Google Scholar] [CrossRef]
- Mishra, R.C.; Tripathy, S.; Desai, K.M.; Quest, D.; Lu, Y.; Akhtar, J.; Gopalakrishnan, V. Nitric oxide synthase inhibition promotes endothelium-dependent vasodilatation and the antihypertensive effect of L-serine. Hypertension 2008, 51, 791–796. [Google Scholar] [CrossRef]
- Mishra, R.C.; Tripathy, S.; Quest, D.; Desai, K.M.; Akhtar, J.; Dattani, I.D.; Gopalakrishnan, V. L-Serine lowers while glycine increases blood pressure in chronic L-NAME-treated and spontaneously hypertensive rats. J. Hypertens. 2008, 26, 2339–2348. [Google Scholar] [CrossRef]
- Mishra, R.C.; Tripathy, S.; Gandhi, J.D.; Balsevich, J.; Akhtar, J.; Desai, K.M.; Gopalakrishnan, V. Decreases in splanchnic vascular resistance contribute to hypotensive effects of L-serine in hypertensive rats. Am. J. Physiol. Heart Circ. Physiol. 2010, 298, H1789–H1796. [Google Scholar] [CrossRef]
- Yin, J.; Ren, W.; Yang, G.; Duan, J.; Huang, X.; Fang, R.; Li, C.; Li, T.; Yin, Y.; Hou, Y.; et al. L-Cysteine metabolism and its nutritional implications. Mol. Nutr. Food Res. 2016, 60, 134–146. [Google Scholar] [CrossRef]
- Lum, H.; Roebuck, K.A. Oxidant stress and endothelial cell dysfunction. Am. J. Physiol. Cell Physiol. 2001, 280, C719–C741. [Google Scholar] [CrossRef]
- Jain, S.K.; Velusamy, T.; Croad, J.L.; Rains, J.L.; Bull, R. L-cysteine supplementation lowers blood glucose, glycated hemoglobin, CRP, MCP-1, and oxidative stress and inhibits NF-kappaB activation in the livers of Zucker diabetic rats. Free Radic. Biol. Med. 2009, 46, 1633–1638. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. Glucose-6-phosphate dehydrogenase deficiency increases cell adhesion molecules and activates human monocyte-endothelial cell adhesion: Protective role of l-cysteine. Arch. Biochem. Biophys. 2019, 663, 11–21. [Google Scholar] [CrossRef]
- Parsanathan, R.; Jain, S.K. L-Cysteine in vitro can restore cellular glutathione and inhibits the expression of cell adhesion molecules in G6PD-deficient monocytes. Amino Acids 2018, 50, 909–921. [Google Scholar] [CrossRef]
- Kanikarla-Marie, P.; Jain, S.K. L-Cysteine supplementation reduces high-glucose and ketone-induced adhesion of monocytes to endothelial cells by inhibiting ROS. Mol. Cell Biochem. 2014, 391, 251–256. [Google Scholar] [CrossRef]
- Okumura, N.; Inoue, R.; Kakutani, K.; Nakahara, M.; Kinoshita, S.; Hamuro, J.; Koizumi, N. Corneal Endothelial Cells Have an Absolute Requirement for Cysteine for Survival. Cornea 2017, 36, 988–994. [Google Scholar] [CrossRef]
- Longchamp, A.; Mirabella, T.; Arduini, A.; MacArthur, M.R.; Das, A.; Trevino-Villarreal, J.H.; Hine, C.; Ben-Sahra, I.; Knudsen, N.H.; Brace, L.E.; et al. Amino Acid Restriction Triggers Angiogenesis via GCN2/ATF4 Regulation of VEGF and H2S Production. Cell 2018, 173, 117–129.e114. [Google Scholar] [CrossRef]
- Hine, C.; Harputlugil, E.; Zhang, Y.; Ruckenstuhl, C.; Lee, B.C.; Brace, L.; Longchamp, A.; Trevino-Villarreal, J.H.; Mejia, P.; Ozaki, C.K.; et al. Endogenous hydrogen sulfide production is essential for dietary restriction benefits. Cell 2015, 160, 132–144. [Google Scholar] [CrossRef]
- Saha, S.; Chakraborty, P.K.; Xiong, X.; Dwivedi, S.K.; Mustafi, S.B.; Leigh, N.R.; Ramchandran, R.; Mukherjee, P.; Bhattacharya, R. Cystathionine beta-synthase regulates endothelial function via protein S-sulfhydration. FASEB J. 2016, 30, 441–456. [Google Scholar] [CrossRef]
- Papapetropoulos, A.; Pyriochou, A.; Altaany, Z.; Yang, G.; Marazioti, A.; Zhou, Z.; Jeschke, M.G.; Branski, L.K.; Herndon, D.N.; Wang, R.; et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc. Natl. Acad. Sci. USA 2009, 106, 21972–21977. [Google Scholar] [CrossRef]
- Shibuya, N.; Mikami, Y.; Kimura, Y.; Nagahara, N.; Kimura, H. Vascular endothelium expresses 3-mercaptopyruvate sulfurtransferase and produces hydrogen sulfide. J. Biochem. 2009, 146, 623–626. [Google Scholar] [CrossRef]
- Zhao, W.; Zhang, J.; Lu, Y.; Wang, R. The vasorelaxant effect of H(2)S as a novel endogenous gaseous K(ATP) channel opener. EMBO J. 2001, 20, 6008–6016. [Google Scholar] [CrossRef]
- Bir, S.C.; Kolluru, G.K.; McCarthy, P.; Shen, X.; Pardue, S.; Pattillo, C.B.; Kevil, C.G. Hydrogen sulfide stimulates ischemic vascular remodeling through nitric oxide synthase and nitrite reduction activity regulating hypoxia-inducible factor-1alpha and vascular endothelial growth factor-dependent angiogenesis. J. Am. Heart Assoc. 2012, 1, e004093. [Google Scholar] [CrossRef]
- Kutz, J.L.; Greaney, J.L.; Santhanam, L.; Alexander, L.M. Evidence for a functional vasodilatatory role for hydrogen sulphide in the human cutaneous microvasculature. J. Physiol. 2015, 593, 2121–2129. [Google Scholar] [CrossRef]
- Kanagy, N.L.; Szabo, C.; Papapetropoulos, A. Vascular biology of hydrogen sulfide. Am. J. Physiol. Cell Physiol. 2017, 312, C537–C549. [Google Scholar] [CrossRef]
- Gorini, F.; Del Turco, S.; Sabatino, L.; Gaggini, M.; Vassalle, C. H2S as a Bridge Linking Inflammation, Oxidative Stress and Endothelial Biology: A Possible Defense in the Fight against SARS-CoV-2 Infection? Biomedicines 2021, 9, 1107. [Google Scholar] [CrossRef] [PubMed]
- Szabo, C. Hydrogen sulfide, an enhancer of vascular nitric oxide signaling: Mechanisms and implications. Am. J. Physiol. Cell Physiol. 2017, 312, C3–C15. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, S.; Wu, J.; Wu, S.; Xu, G.; Wei, D. Essential Role of Nonessential Amino Acid Glutamine in Atherosclerotic Cardiovascular Disease. DNA Cell Biol. 2020, 39, 8–15. [Google Scholar] [CrossRef] [PubMed]
- Unterluggauer, H.; Mazurek, S.; Lener, B.; Hutter, E.; Eigenbrodt, E.; Zwerschke, W.; Jansen-Durr, P. Premature senescence of human endothelial cells induced by inhibition of glutaminase. Biogerontology 2008, 9, 247–259. [Google Scholar] [CrossRef] [PubMed]
- Krutzfeldt, A.; Spahr, R.; Mertens, S.; Siegmund, B.; Piper, H.M. Metabolism of exogenous substrates by coronary endothelial cells in culture. J. Mol. Cell Cardiol. 1990, 22, 1393–1404. [Google Scholar] [CrossRef]
- Huang, H.; Vandekeere, S.; Kalucka, J.; Bierhansl, L.; Zecchin, A.; Bruning, U.; Visnagri, A.; Yuldasheva, N.; Goveia, J.; Cruys, B.; et al. Role of glutamine and interlinked asparagine metabolism in vessel formation. EMBO J. 2017, 36, 2334–2352. [Google Scholar] [CrossRef]
- Kim, B.; Li, J.; Jang, C.; Arany, Z. Glutamine fuels proliferation but not migration of endothelial cells. EMBO J 2017, 36, 2321–2333. [Google Scholar] [CrossRef]
- Yi, S.; Lin, K.; Jiang, T.; Shao, W.; Huang, C.; Jiang, B.; Li, Q.; Lin, D. NMR-based metabonomic analysis of HUVEC cells during replicative senescence. Aging 2020, 12, 3626–3646. [Google Scholar] [CrossRef]
- Pires, R.S.; Braga, P.G.S.; Santos, J.M.B.; Amaral, J.B.; Amirato, G.R.; Trettel, C.S.; Dos Santos, C.A.F.; Vaisberg, M.; Nali, L.H.S.; Vieira, R.P.; et al. l-Glutamine supplementation enhances glutathione peroxidase and paraoxonase-1 activities in HDL of exercising older individuals. Exp. Gerontol. 2021, 156, 111584. [Google Scholar] [CrossRef]
- Jing, L.; Wu, Q.; Wang, F. Glutamine induces heat-shock protein and protects against Escherichia coli lipopolysaccharide-induced vascular hyporeactivity in rats. Crit. Care 2007, 11, R34. [Google Scholar] [CrossRef]
- Pai, M.H.; Shih, Y.M.; Shih, J.M.; Yeh, C.L. Glutamine Administration Modulates Endothelial Progenitor Cell and Lung Injury in Septic Mice. Shock 2016, 46, 587–592. [Google Scholar] [CrossRef]
- Su, S.T.; Yeh, C.L.; Hou, Y.C.; Pai, M.H.; Yeh, S.L. Dietary glutamine supplementation enhances endothelial progenitor cell mobilization in streptozotocin-induced diabetic mice subjected to limb ischemia. J. Nutr. Biochem. 2017, 40, 86–94. [Google Scholar] [CrossRef]
- Pavlova, N.N.; Hui, S.; Ghergurovich, J.M.; Fan, J.; Intlekofer, A.M.; White, R.M.; Rabinowitz, J.D.; Thompson, C.B.; Zhang, J. As Extracellular Glutamine Levels Decline, Asparagine Becomes an Essential Amino Acid. Cell Metab. 2018, 27, 428–438.e425. [Google Scholar] [CrossRef]
- Liu, X.M.; Peyton, K.J.; Durante, W. Ammonia promotes endothelial cell survival via the heme oxygenase-1-mediated release of carbon monoxide. Free Radic. Biol. Med. 2017, 102, 37–46. [Google Scholar] [CrossRef]
- Lopez, M.J.; Mohiuddin, S.S. Biochemistry, Essential Amino Acids; StatPearls: Treasure Island, FL, USA, 2022. [Google Scholar]
- Oberkersch, R.E.; Santoro, M.M. Role of amino acid metabolism in angiogenesis. Vasc. Pharm. 2019, 112, 17–23. [Google Scholar] [CrossRef]
- Pollock, J.S.; Forstermann, U.; Mitchell, J.A.; Warner, T.D.; Schmidt, H.H.; Nakane, M.; Murad, F. Purification and characterization of particulate endothelium-derived relaxing factor synthase from cultured and native bovine aortic endothelial cells. Proc. Natl. Acad. Sci. USA 1991, 88, 10480–10484. [Google Scholar] [CrossRef]
- Costa, G.; Shushanof, M.; Bouskela, E.; Bottino, D. Oral L-Arginine (5 g/day) for 14 Days Improves Microcirculatory Function in Healthy Young Women and Healthy and Type 2 Diabetes Mellitus Elderly Women. J. Vasc. Res. 2022, 59, 24–33. [Google Scholar] [CrossRef]
- Adejare, A.; Oloyo, A.; Anigbogu, C.; Jaja, S. l-arginine Supplementation Increased Only Endothelium-Dependent Relaxation in Sprague-Dawley Rats Fed a High-Salt Diet by Enhancing Abdominal Aorta Endothelial Nitric Oxide Synthase Gene Expression. Clin. Med. Insights Cardiol. 2020, 14, 1179546820902843. [Google Scholar] [CrossRef]
- Khalaf, D.; Kruger, M.; Wehland, M.; Infanger, M.; Grimm, D. The Effects of Oral l-Arginine and l-Citrulline Supplementation on Blood Pressure. Nutrients 2019, 11, 1679. [Google Scholar] [CrossRef]
- Meirelles, C.M.; Matsuura, C.; Silva, R.S., Jr.; Guimaraes, F.F.; Gomes, P.S.C. Acute Effects of L-Arginine Supplementation on Oxygen Consumption Kinetics and Muscle Oxyhemoglobin and Deoxyhemoglobin during Treadmill Running in Male Adults. Int. J. Exerc. Sci. 2019, 12, 444–455. [Google Scholar]
- Alvares, T.S.; Conte, C.A.; Paschoalin, V.M.; Silva, J.T.; Meirelles Cde, M.; Bhambhani, Y.N.; Gomes, P.S. Acute l-arginine supplementation increases muscle blood volume but not strength performance. Appl. Physiol. Nutr. Metab. 2012, 37, 115–126. [Google Scholar] [CrossRef]
- Sudar-Milovanovic, E.; Obradovic, M.; Jovanovic, A.; Zaric, B.; Zafirovic, S.; Panic, A.; Radak, D.; Isenovic, E.R. Benefits of L-Arginine on Cardiovascular System. Mini Rev. Med. Chem. 2016, 16, 94–103. [Google Scholar] [CrossRef]
- Melhem, N.J.; Taleb, S. Tryptophan: From Diet to Cardiovascular Diseases. Int. J. Mol. Sci. 2021, 22, 9904. [Google Scholar] [CrossRef]
- Ramprasath, T.; Han, Y.M.; Zhang, D.; Yu, C.J.; Zou, M.H. Tryptophan Catabolism and Inflammation: A Novel Therapeutic Target For Aortic Diseases. Front. Immunol. 2021, 12, 731701. [Google Scholar] [CrossRef]
- Wang, Q.; Liu, D.; Song, P.; Zou, M.H. Tryptophan-kynurenine pathway is dysregulated in inflammation, and immune activation. Front. Biosci. (Landmark Ed) 2015, 20, 1116–1143. [Google Scholar] [CrossRef]
- Wirleitner, B.; Rudzite, V.; Neurauter, G.; Murr, C.; Kalnins, U.; Erglis, A.; Trusinskis, K.; Fuchs, D. Immune activation and degradation of tryptophan in coronary heart disease. Eur. J. Clin. Investig. 2003, 33, 550–554. [Google Scholar] [CrossRef]
- Metghalchi, S.; Ponnuswamy, P.; Simon, T.; Haddad, Y.; Laurans, L.; Clement, M.; Dalloz, M.; Romain, M.; Esposito, B.; Koropoulis, V.; et al. Indoleamine 2,3-Dioxygenase Fine-Tunes Immune Homeostasis in Atherosclerosis and Colitis through Repression of Interleukin-10 Production. Cell Metab. 2015, 22, 460–471. [Google Scholar] [CrossRef]
- Hoel, H.; Hove-Skovsgaard, M.; Hov, J.R.; Gaardbo, J.C.; Holm, K.; Kummen, M.; Rudi, K.; Nwosu, F.; Valeur, J.; Gelpi, M.; et al. Impact of HIV and Type 2 diabetes on Gut Microbiota Diversity, Tryptophan Catabolism and Endothelial Dysfunction. Sci. Rep. 2018, 8, 6725. [Google Scholar] [CrossRef]
- Broekhuizen, M.; Klein, T.; Hitzerd, E.; de Rijke, Y.B.; Schoenmakers, S.; Sedlmayr, P.; Danser, A.H.J.; Merkus, D.; Reiss, I.K.M. l-Tryptophan-Induced Vasodilation Is Enhanced in Preeclampsia: Studies on Its Uptake and Metabolism in the Human Placenta. Hypertension 2020, 76, 184–194. [Google Scholar] [CrossRef]
- Eleftheriadis, T.; Pissas, G.; Sounidaki, M.; Antoniadi, G.; Rountas, C.; Liakopoulos, V.; Stefanidis, L. Tryptophan depletion under conditions that imitate insulin resistance enhances fatty acid oxidation and induces endothelial dysfunction through reactive oxygen species-dependent and independent pathways. Mol. Cell Biochem. 2017, 428, 41–56. [Google Scholar] [CrossRef]
- Lee, L.Y.; Oldham, W.M.; He, H.; Wang, R.; Mulhern, R.; Handy, D.E.; Loscalzo, J. Interferon-gamma Impairs Human Coronary Artery Endothelial Glucose Metabolism by Tryptophan Catabolism and Activates Fatty Acid Oxidation. Circulation 2021, 144, 1612–1628. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.J.; Zhou, C.; Wei, Y.Y.; Li, H.H.; Lei, W.; Boeldt, D.S.; Wang, K.; Zheng, J. Differential Distribution of Tryptophan-Metabolites in Fetal and Maternal Circulations During Normotensive and Preeclamptic Pregnancies. Reprod. Sci. 2021, 29, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, M.; Ding, Y.; Wang, Q.; Zhang, W.; Song, P.; Zou, M.H. Activation of NAD(P)H oxidase by tryptophan-derived 3-hydroxykynurenine accelerates endothelial apoptosis and dysfunction in vivo. Circ. Res. 2014, 114, 480–492. [Google Scholar] [CrossRef] [PubMed]
- Pae, H.O.; Oh, G.S.; Lee, B.S.; Rim, J.S.; Kim, Y.M.; Chung, H.T. 3-Hydroxyanthranilic acid, one of L-tryptophan metabolites, inhibits monocyte chemoattractant protein-1 secretion and vascular cell adhesion molecule-1 expression via heme oxygenase-1 induction in human umbilical vein endothelial cells. Atherosclerosis 2006, 187, 274–284. [Google Scholar] [CrossRef]
- Chambers, J.C.; McGregor, A.; Jean-Marie, J.; Obeid, O.A.; Kooner, J.S. Demonstration of rapid onset vascular endothelial dysfunction after hyperhomocysteinemia: An effect reversible with vitamin C therapy. Circulation 1999, 99, 1156–1160. [Google Scholar] [CrossRef]
- Steed, M.M.; Tyagi, S.C. Mechanisms of cardiovascular remodeling in hyperhomocysteinemia. Antioxid Redox Signal. 2011, 15, 1927–1943. [Google Scholar] [CrossRef]
- Chambers, J.C.; Obeid, O.A.; Kooner, J.S. Physiological increments in plasma homocysteine induce vascular endothelial dysfunction in normal human subjects. Arter. Thromb. Vasc. Biol. 1999, 19, 2922–2927. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, C.; Yang, X.; Zhang, C. L-phenylalanine attenuates high salt-induced hypertension in Dahl SS rats through activation of GCH1-BH4. PLoS ONE 2021, 16, e0250126. [Google Scholar] [CrossRef]
- Heikal, L.; Starr, A.; Hussein, D.; Prieto-Lloret, J.; Aaronson, P.; Dailey, L.A.; Nandi, M. l-Phenylalanine Restores Vascular Function in Spontaneously Hypertensive Rats Through Activation of the GCH1-GFRP Complex. JACC Basic Transl. Sci. 2018, 3, 366–377. [Google Scholar] [CrossRef]
- Tan, R.; Li, J.; Liu, F.; Liao, P.; Ruiz, M.; Dupuis, J.; Zhu, L.; Hu, Q. Phenylalanine induces pulmonary hypertension through calcium-sensing receptor activation. Am. J. Physiol. Lung Cell Mol. Physiol. 2020, 319, L1010–L1020. [Google Scholar] [CrossRef]
- Mitchell, B.M.; Dorrance, A.M.; Webb, R.C. Phenylalanine improves dilation and blood pressure in GTP cyclohydrolase inhibition-induced hypertensive rats. J. Cardiovasc. Pharm. 2004, 43, 758–763. [Google Scholar] [CrossRef]
- Holecek, M. The BCAA-BCKA cycle: Its relation to alanine and glutamine synthesis and protein balance. Nutrition 2001, 17, 70. [Google Scholar] [CrossRef]
- Ruiz-Canela, M.; Toledo, E.; Clish, C.B.; Hruby, A.; Liang, L.; Salas-Salvado, J.; Razquin, C.; Corella, D.; Estruch, R.; Ros, E.; et al. Plasma Branched-Chain Amino Acids and Incident Cardiovascular Disease in the PREDIMED Trial. Clin. Chem. 2016, 62, 582–592. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Wang, Y.; You, H.; Hui, P.; Zheng, Y.; Du, J. Increased branched-chain amino acid levels are associated with long-term adverse cardiovascular events in patients with STEMI and acute heart failure. Life Sci. 2018, 209, 167–172. [Google Scholar] [CrossRef]
- Zhenyukh, O.; Gonzalez-Amor, M.; Rodrigues-Diez, R.R.; Esteban, V.; Ruiz-Ortega, M.; Salaices, M.; Mas, S.; Briones, A.M.; Egido, J. Branched-chain amino acids promote endothelial dysfunction through increased reactive oxygen species generation and inflammation. J. Cell Mol. Med. 2018, 22, 4948–4962. [Google Scholar] [CrossRef]
- Yang, Y.; Wu, Z.; Meininger, C.J.; Wu, G. L-Leucine and NO-mediated cardiovascular function. Amino Acids 2015, 47, 435–447. [Google Scholar] [CrossRef]
- Cojocaru, E.; Magdalena Leon-Constantin, M.; Ungureanu, C.; Trandafirescu, M.F.; Mastaleru, A.; Mihaela Trandafir, L.; Dumitru Petrariu, F.; Viola Badulescu, O.; Filip, N. Hypolipemiant Actions and Possible Cardioprotective Effects of Valine and Leucine: An Experimental Study. Medicina 2021, 57, 239. [Google Scholar] [CrossRef]
- Zhao, Y.; Dai, X.Y.; Zhou, Z.; Zhao, G.X.; Wang, X.; Xu, M.J. Leucine supplementation via drinking water reduces atherosclerotic lesions in apoE null mice. Acta Pharm. Sin. 2016, 37, 196–203. [Google Scholar] [CrossRef]
- Argyrakopoulou, G.; Kontrafouri, P.; Eleftheriadou, I.; Kokkinos, A.; Arapostathi, C.; Kyriaki, D.; Perrea, D.; Revenas, C.; Katsilambros, N.; Tentolouris, N. The Effect of the Oral Administration of Leucine on Endothelial Function, Glucose and Insulin Concentrations in Healthy Subjects. Exp. Clin. Endocrinol. Diabetes 2019, 127, 505–510. [Google Scholar] [CrossRef]
- Murata, K.; Moriyama, M. Isoleucine, an essential amino acid, prevents liver metastases of colon cancer by antiangiogenesis. Cancer Res. 2007, 67, 3263–3268. [Google Scholar] [CrossRef]
- Gauthier-Coles, G.; Vennitti, J.; Zhang, Z.; Comb, W.C.; Xing, S.; Javed, K.; Broer, A.; Broer, S. Quantitative modelling of amino acid transport and homeostasis in mammalian cells. Nat. Commun. 2021, 12, 5282. [Google Scholar] [CrossRef]
- Chaveroux, C.; Lambert-Langlais, S.; Cherasse, Y.; Averous, J.; Parry, L.; Carraro, V.; Jousse, C.; Maurin, A.C.; Bruhat, A.; Fafournoux, P. Molecular mechanisms involved in the adaptation to amino acid limitation in mammals. Biochimie 2010, 92, 736–745. [Google Scholar] [CrossRef]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef]
- Hu, X.; Guo, F. Amino Acid Sensing in Metabolic Homeostasis and Health. Endocr. Rev. 2021, 42, 56–76. [Google Scholar] [CrossRef]
- Husi, H.; Van Agtmael, T.; Mullen, W.; Bahlmann, F.H.; Schanstra, J.P.; Vlahou, A.; Delles, C.; Perco, P.; Mischak, H. Proteome-based systems biology analysis of the diabetic mouse aorta reveals major changes in fatty acid biosynthesis as potential hallmark in diabetes mellitus-associated vascular disease. Circ. Cardiovasc. Genet. 2014, 7, 161–170. [Google Scholar] [CrossRef]
- Jin, Q.; Ma, R.C.W. Metabolomics in Diabetes and Diabetic Complications: Insights from Epidemiological Studies. Cells 2021, 10, 2832. [Google Scholar] [CrossRef]
- Drabkova, P.; Sanderova, J.; Kovarik, J.; Kandar, R. An Assay of Selected Serum Amino Acids in Patients with Type 2 Diabetes Mellitus. Adv. Clin. Exp. Med. 2015, 24, 447–451. [Google Scholar] [CrossRef]
- Yuan, W.; Zhang, J.; Li, S.; Edwards, J.L. Amine metabolomics of hyperglycemic endothelial cells using capillary LC-MS with isobaric tagging. J. Proteome Res. 2011, 10, 5242–5250. [Google Scholar] [CrossRef]
- Kovamees, O.; Shemyakin, A.; Pernow, J. Amino acid metabolism reflecting arginase activity is increased in patients with type 2 diabetes and associated with endothelial dysfunction. Diab. Vasc. Dis. Res. 2016, 13, 354–360. [Google Scholar] [CrossRef]
- Welsh, P.; Rankin, N.; Li, Q.; Mark, P.B.; Wurtz, P.; Ala-Korpela, M.; Marre, M.; Poulter, N.; Hamet, P.; Chalmers, J.; et al. Circulating amino acids and the risk of macrovascular, microvascular and mortality outcomes in individuals with type 2 diabetes: Results from the ADVANCE trial. Diabetologia 2018, 61, 1581–1591. [Google Scholar] [CrossRef] [PubMed]
- Pawlak, D.; Tankiewicz, A.; Matys, T.; Buczko, W. Peripheral distribution of kynurenine metabolites and activity of kynurenine pathway enzymes in renal failure. J. Physiol. Pharm. 2003, 54, 175–189. [Google Scholar]
- Colombo, M.; Valo, E.; McGurnaghan, S.J.; Sandholm, N.; Blackbourn, L.A.K.; Dalton, R.N.; Dunger, D.; Groop, P.H.; McKeigue, P.M.; Forsblom, C.; et al. Biomarker panels associated with progression of renal disease in type 1 diabetes. Diabetologia 2019, 62, 1616–1627. [Google Scholar] [CrossRef] [PubMed]
- Gambardella, J.; Khondkar, W.; Morelli, M.B.; Wang, X.; Santulli, G.; Trimarco, V. Arginine and Endothelial Function. Biomedicines 2020, 8, 277. [Google Scholar] [CrossRef] [PubMed]
- Niewczas, M.A.; Sirich, T.L.; Mathew, A.V.; Skupien, J.; Mohney, R.P.; Warram, J.H.; Smiles, A.; Huang, X.; Walker, W.; Byun, J.; et al. Uremic solutes and risk of end-stage renal disease in type 2 diabetes: Metabolomic study. Kidney Int. 2014, 85, 1214–1224. [Google Scholar] [CrossRef]
- Vasdev, S.; Stuckless, J. Antihypertensive effects of dietary protein and its mechanism. Int. J. Angiol. 2010, 19, e7–e20. [Google Scholar] [CrossRef]
- Aluko, R.E. Antihypertensive peptides from food proteins. Annu. Rev. Food Sci. Technol. 2015, 6, 235–262. [Google Scholar] [CrossRef]
- Wang, F.; Wang, B.; Chen, X.; Liu, W.; Wang, G.; Li, X.; Liu, X.; Li, N.; Zhang, J.; Yin, T.; et al. Association Between Blood Pressure and Branched-Chain/Aromatic Amino Acid Excretion Rate in 24-Hour Urine Samples from Elderly Hypertension Patients. Diabetes Metab. Syndr. Obes. 2021, 14, 3965–3973. [Google Scholar] [CrossRef]
- Mirmiran, P.; Teymoori, F.; Asghari, G.; Azizi, F. Dietary Intakes of Branched Chain Amino Acids and the Incidence of Hypertension: A Population-Based Prospective Cohort Study. Arch. Iran. Med. 2019, 22, 182–188. [Google Scholar]
- Yamaguchi, N.; Mahbub, M.H.; Takahashi, H.; Hase, R.; Ishimaru, Y.; Sunagawa, H.; Amano, H.; Kobayashi-Miura, M.; Kanda, H.; Fujita, Y.; et al. Plasma free amino acid profiles evaluate risk of metabolic syndrome, diabetes, dyslipidemia, and hypertension in a large Asian population. Env. Health Prev. Med. 2017, 22, 35. [Google Scholar] [CrossRef]
- Teymoori, F.; Asghari, G.; Farhadnejad, H.; Mirmiran, P.; Azizi, F. Do dietary amino acid ratios predict risk of incident hypertension among adults? Int. J. Food Sci. Nutr. 2019, 70, 387–395. [Google Scholar] [CrossRef]
- Du Plessis, J.P.; Nienaber-Rousseau, C.; Lammertyn, L.; Schutte, A.E.; Pieters, M.; Kruger, H.S. The Relationship of Circulating Homocysteine with Fibrinogen, Blood Pressure, and Other Cardiovascular Measures in African Adolescents. J. Pediatr. 2021, 234, 158–163.e152. [Google Scholar] [CrossRef]
- Sved, A.F.; Fernstrom, J.D.; Wurtman, R.J. Tyrosine administration reduces blood pressure and enhances brain norepinephrine release in spontaneously hypertensive rats. Proc. Natl. Acad. Sci. USA 1979, 76, 3511–3514. [Google Scholar] [CrossRef]
- Yamori, Y.; Fujiwara, M.; Horie, R.; Lovenberg, W. The hypotensive effect of centrally administered tyrosine. Eur. J. Pharm. 1980, 68, 201–204. [Google Scholar] [CrossRef]
- Stamler, J.; Brown, I.J.; Daviglus, M.L.; Chan, Q.; Kesteloot, H.; Ueshima, H.; Zhao, L.; Elliott, P.; Group, I.R. Glutamic acid, the main dietary amino acid, and blood pressure: The INTERMAP Study (International Collaborative Study of Macronutrients, Micronutrients and Blood Pressure). Circulation 2009, 120, 221–228. [Google Scholar] [CrossRef]
- Zhao, G. Inherited metabolic aberration of phenylalanine in the family members of patients with essential hypertension and stroke. Zhonghua Yi Xue Za Zhi 1991, 71, 388–390. [Google Scholar]
- Altorf-van der Kuil, W.; Engberink, M.F.; Brink, E.J.; van Baak, M.A.; Bakker, S.J.; Navis, G.; van ‘t Veer, P.; Geleijnse, J.M. Dietary protein and blood pressure: A systematic review. PLoS ONE 2010, 5, e12102. [Google Scholar] [CrossRef]
- Watts, S.W.; Morrison, S.F.; Davis, R.P.; Barman, S.M. Serotonin and blood pressure regulation. Pharm. Rev. 2012, 64, 359–388. [Google Scholar] [CrossRef]
- Wang, Y.; Liu, H.; McKenzie, G.; Witting, P.K.; Stasch, J.P.; Hahn, M.; Changsirivathanathamrong, D.; Wu, B.J.; Ball, H.J.; Thomas, S.R.; et al. Kynurenine is an endothelium-derived relaxing factor produced during inflammation. Nat. Med. 2010, 16, 279–285. [Google Scholar] [CrossRef]
- Vasdev, S.; Gill, V.D.; Singal, P.K. Modulation of oxidative stress-induced changes in hypertension and atherosclerosis by antioxidants. Exp. Clin. Cardiol. 2006, 11, 206–216. [Google Scholar]
- Vasdev, S.; Singal, P.; Gill, V. The antihypertensive effect of cysteine. Int. J. Angiol. 2009, 18, 7–21. [Google Scholar] [CrossRef]
- Hsu, C.N.; Tain, Y.L. Amino Acids and Developmental Origins of Hypertension. Nutrients 2020, 12, 1763. [Google Scholar] [CrossRef]
- Chen, Y.; Wu, X.; Zhang, J.; Pan, G.; Wang, X.; Guo, X.; Wang, J.; Cui, X.; Gao, H.; Cheng, M.; et al. Amino acid starvation-induced LDLR trafficking accelerates lipoprotein endocytosis and LDL clearance. EMBO Rep. 2022, 23, e53373. [Google Scholar] [CrossRef]
- Trinder, M.; Francis, G.A.; Brunham, L.R. Association of Monogenic vs. Polygenic Hypercholesterolemia With Risk of Atherosclerotic Cardiovascular Disease. JAMA Cardiol. 2020, 5, 390–399. [Google Scholar] [CrossRef]
- Sharifi, M.; Futema, M.; Nair, D.; Humphries, S.E. Polygenic Hypercholesterolemia and Cardiovascular Disease Risk. Curr. Cardiol. Rep. 2019, 21, 43. [Google Scholar] [CrossRef]
- Laufs, U.; Banach, M.; Mancini, G.B.J.; Gaudet, D.; Bloedon, L.T.; Sterling, L.R.; Kelly, S.; Stroes, E.S.G. Efficacy and Safety of Bempedoic Acid in Patients With Hypercholesterolemia and Statin Intolerance. J. Am. Heart Assoc. 2019, 8, e011662. [Google Scholar] [CrossRef]
- Rocha, V.Z.; Santos, R.D. Past, Present, and Future of Familial Hypercholesterolemia Management. Methodist Debakey Cardiovasc. J. 2021, 17, 28–35. [Google Scholar] [CrossRef]
- Teymoori, F.; Asghari, G.; Salehi, P.; Sadeghian, S.; Mirmiran, P.; Azizi, F. Are dietary amino acids prospectively predicts changes in serum lipid profile? Diabetes Metab. Syndr. 2019, 13, 1837–1843. [Google Scholar] [CrossRef]
- Gonzalez-Pena, D.; Dudzik, D.; Garcia, A.; Ancos, B.; Barbas, C.; Sanchez-Moreno, C. Metabolomic Fingerprinting in the Comprehensive Study of Liver Changes Associated with Onion Supplementation in Hypercholesterolemic Wistar Rats. Int. J. Mol. Sci. 2017, 18, 267. [Google Scholar] [CrossRef]
- Gonzalez-Pena, D.; Dudzik, D.; Colina-Coca, C.; de Ancos, B.; Garcia, A.; Barbas, C.; Sanchez-Moreno, C. Multiplatform metabolomic fingerprinting as a tool for understanding hypercholesterolemia in Wistar rats. Eur. J. Nutr. 2016, 55, 997–1010. [Google Scholar] [CrossRef]
- Popovic, P.J.; Zeh, H.J., 3rd; Ochoa, J.B. Arginine and immunity. J. Nutr. 2007, 137, 1681S–1686S. [Google Scholar] [CrossRef] [PubMed]
- Shen, D.; Ma, N.; Yang, Y.; Liu, X.; Qin, Z.; Li, S.; Jiao, Z.; Kong, X.; Li, J. UPLC-Q-TOF/MS-Based Plasma Metabolomics to Evaluate the Effects of Aspirin Eugenol Ester on Blood Stasis in Rats. Molecules 2019, 24, 2380. [Google Scholar] [CrossRef] [PubMed]
- Zou, Z.J.; Liu, Z.H.; Gong, M.J.; Han, B.; Wang, S.M.; Liang, S.W. Intervention effects of puerarin on blood stasis in rats revealed by a (1)H NMR-based metabonomic approach. Phytomedicine 2015, 22, 333–343. [Google Scholar] [CrossRef] [PubMed]
- Ma, N.; Karam, I.; Liu, X.W.; Kong, X.J.; Qin, Z.; Li, S.H.; Jiao, Z.H.; Dong, P.C.; Yang, Y.J.; Li, J.Y. UPLC-Q-TOF/MS-based urine and plasma metabonomics study on the ameliorative effects of aspirin eugenol ester in hyperlipidemia rats. Toxicol. Appl. Pharm. 2017, 332, 40–51. [Google Scholar] [CrossRef]
- Ma, N.; Yang, Y.; Liu, X.; Kong, X.; Li, S.; Qin, Z.; Jiao, Z.; Li, J. UPLC-Q-TOF/MS-based metabonomic studies on the intervention effects of aspirin eugenol ester in atherosclerosis hamsters. Sci. Rep. 2017, 7, 10544. [Google Scholar] [CrossRef]
- Xiao-Rong, L.; Ning, M.; Xi-Wang, L.; Shi-Hong, L.; Zhe, Q.; Li-Xia, B.; Ya-Jun, Y.; Jian-Yong, L. Untargeted and Targeted Metabolomics Reveal the Underlying Mechanism of Aspirin Eugenol Ester Ameliorating Rat Hyperlipidemia via Inhibiting FXR to Induce CYP7A1. Front. Pharm. 2021, 12, 733789. [Google Scholar] [CrossRef]
- Huang, M.Z.; Lu, X.R.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, J.Y. Cellular Metabolomics Reveal the Mechanism Underlying the Anti-Atherosclerotic Effects of Aspirin Eugenol Ester on Vascular Endothelial Dysfunction. Int. J. Mol. Sci. 2019, 20, 3165. [Google Scholar] [CrossRef]
- Munshi, R.; Clanachan, A.S.; Baer, H.P. 5’-Deoxy-5’-methylthioadenosine: A nucleoside which differentiates between adenosine receptor types. Biochem. Pharm. 1988, 37, 2085–2089. [Google Scholar] [CrossRef]
- Dou, L.; Bertrand, E.; Cerini, C.; Faure, V.; Sampol, J.; Vanholder, R.; Berland, Y.; Brunet, P. The uremic solutes p-cresol and indoxyl sulfate inhibit endothelial proliferation and wound repair. Kidney Int. 2004, 65, 442–451. [Google Scholar] [CrossRef]
- Chen, J.; Wu, F.; Long, Y.; Yu, W. Glutathione Supplementation Attenuates Oxidative Stress and Improves Vascular Hyporesponsiveness in Experimental Obstructive Jaundice. Oxid. Med. Cell Longev. 2015, 2015, 486148. [Google Scholar] [CrossRef]
- Martin-Lorenzo, M.; Gonzalez-Calero, L.; Maroto, A.S.; Martinez, P.J.; Zubiri, I.; de la Cuesta, F.; Mourino-Alvarez, L.; Barderas, M.G.; Heredero, A.; Aldamiz-Echevarria, G.; et al. Cytoskeleton deregulation and impairment in amino acids and energy metabolism in early atherosclerosis at aortic tissue with reflection in plasma. Biochim. Biophys. Acta 2016, 1862, 725–732. [Google Scholar] [CrossRef]
- Huang, M.Z.; Yang, Y.J.; Liu, X.W.; Qin, Z.; Li, J.Y. Aspirin eugenol ester attenuates oxidative injury of vascular endothelial cells by regulating NOS and Nrf2 signalling pathways. Br. J. Pharm. 2019, 176, 906–918. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, T.; Wang, H.; Tang, J.; Hou, A.; Yan, X.; Yu, B.; Ran, S.; Luo, M.; Tang, Y.; et al. Effects of individualized administration of folic acid on prothrombotic state and vascular endothelial function with H-type hypertension: A double-blinded, randomized clinical cohort study. Medicine 2022, 101, e28628. [Google Scholar] [CrossRef]
- Zhang, B.Q.; Hu, S.J.; Qiu, L.H.; Zhu, J.H.; Xie, X.J.; Sun, J.; Zhu, Z.H.; Xia, Q.; Bian, K. Effects of Astragalus membranaceus and its main components on the acute phase endothelial dysfunction induced by homocysteine. Vasc. Pharm. 2007, 46, 278–285. [Google Scholar] [CrossRef]
- Yu, X.; Guo, L.; Deng, X.; Yang, F.; Tian, Y.; Liu, P.; Xu, F.; Zhang, Z.; Huang, Y. Attenuation of doxorubicin-induced oxidative damage in rat brain by regulating amino acid homeostasis with Astragali Radix. Amino Acids 2021, 53, 893–901. [Google Scholar] [CrossRef]
- Chen, L.T.; Xu, T.T.; Qiu, Y.Q.; Liu, N.Y.; Ke, X.Y.; Fang, L.; Yan, J.P.; Zhu, D.Y. Homocysteine induced a calcium-mediated disruption of mitochondrial function and dynamics in endothelial cells. J. Biochem. Mol. Toxicol. 2021, 35, e22737. [Google Scholar] [CrossRef]
- Balint, B.; Jepchumba, V.K.; Gueant, J.L.; Gueant-Rodriguez, R.M. Mechanisms of homocysteine-induced damage to the endothelial, medial and adventitial layers of the arterial wall. Biochimie 2020, 173, 100–106. [Google Scholar] [CrossRef]
- Pan, L.; Yu, G.; Huang, J.; Zheng, X.; Xu, Y. Homocysteine inhibits angiogenesis through cytoskeleton remodeling. Biosci. Rep. 2017, 37, BSR20170860. [Google Scholar] [CrossRef]
- Graham, I.M.; O’Callaghan, P. The role of folic acid in the prevention of cardiovascular disease. Curr. Opin. Lipidol. 2000, 11, 577–587. [Google Scholar] [CrossRef]
- Stanhewicz, A.E.; Kenney, W.L. Role of folic acid in nitric oxide bioavailability and vascular endothelial function. Nutr. Rev. 2017, 75, 61–70. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, W.; Xiong, Y.; Du, G.; Qin, X. Evaluations of the effect of HuangQi against heart failure based on comprehensive echocardiography index and metabonomics. Phytomedicine 2018, 50, 205–212. [Google Scholar] [CrossRef]
- Fu, J.; Wang, Z.; Huang, L.; Zheng, S.; Wang, D.; Chen, S.; Zhang, H.; Yang, S. Review of the botanical characteristics, phytochemistry, and pharmacology of Astragalus membranaceus (Huangqi). Phytother. Res. 2014, 28, 1275–1283. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.J.; Hufnagl, P.; Binder, B.R.; Wojta, J. Antiinflammatory activity of astragaloside IV is mediated by inhibition of NF-kappaB activation and adhesion molecule expression. Thromb. Haemost. 2003, 90, 904–914. [Google Scholar] [CrossRef] [PubMed]
- Li, H.B.; Ge, Y.K.; Zhang, L.; Zheng, X.X. Astragaloside IV improved barrier dysfunction induced by acute high glucose in human umbilical vein endothelial cells. Life Sci. 2006, 79, 1186–1193. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, X.H.; Zhong, M.F.; Liu, R.H.; Li, H.L.; Zhang, W.D.; Chen, H. Mechanisms underlying vasorelaxant action of astragaloside IV in isolated rat aortic rings. Clin. Exp. Pharm. Physiol. 2007, 34, 387–392. [Google Scholar] [CrossRef]
- Tang, D.; He, B.; Zheng, Z.G.; Wang, R.S.; Gu, F.; Duan, T.T.; Cheng, H.Q.; Zhu, Q. Inhibitory effects of two major isoflavonoids in Radix Astragali on high glucose-induced mesangial cells proliferation and AGEs-induced endothelial cells apoptosis. Planta Med. 2011, 77, 729–732. [Google Scholar] [CrossRef]
- Zhang, Y.; Hu, G.; Lin, H.C.; Hong, S.J.; Deng, Y.H.; Tang, J.Y.; Seto, S.W.; Kwan, Y.W.; Waye, M.M.; Wang, Y.T.; et al. Radix Astragali extract promotes angiogenesis involving vascular endothelial growth factor receptor-related phosphatidylinositol 3-kinase/Akt-dependent pathway in human endothelial cells. Phytother. Res. 2009, 23, 1205–1213. [Google Scholar] [CrossRef]
- Abushouk, A.I.; Ismail, A.; Salem, A.M.A.; Afifi, A.M.; Abdel-Daim, M.M. Cardioprotective mechanisms of phytochemicals against doxorubicin-induced cardiotoxicity. Biomed. Pharm. 2017, 90, 935–946. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Fang, L.; Li, H.; Li, Z.; Lyu, L.; Wang, H.; Xiao, J. Astragaloside IV alleviates doxorubicin induced cardiomyopathy by inhibiting NADPH oxidase derived oxidative stress. Eur. J. Pharm. 2019, 859, 172490. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.N.; Yang, L.; He, S.S.; Qin, X.M.; Li, A.P. Metabolomics coupled with integrative pharmacology reveal the protective effect of FangjiHuangqi Decoction against adriamycin-induced rat nephropathy model. J. Pharm. Biomed. Anal. 2019, 174, 525–533. [Google Scholar] [CrossRef] [PubMed]
- Penninger, J.M.; Grant, M.B.; Sung, J.J.Y. The Role of Angiotensin Converting Enzyme 2 in Modulating Gut Microbiota, Intestinal Inflammation, and Coronavirus Infection. Gastroenterology 2021, 160, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, T.; Perlot, T.; Rehman, A.; Trichereau, J.; Ishiguro, H.; Paolino, M.; Sigl, V.; Hanada, T.; Hanada, R.; Lipinski, S.; et al. ACE2 links amino acid malnutrition to microbial ecology and intestinal inflammation. Nature 2012, 487, 477–481. [Google Scholar] [CrossRef]
- Hayashi, Y.; Seino, Y. Regulation of amino acid metabolism and alpha-cell proliferation by glucagon. J. Diabetes Investig. 2018, 9, 464–472. [Google Scholar] [CrossRef]
- Van der Velden, W.J.C.; Lindquist, P.; Madsen, J.S.; Stassen, R.; Wewer Albrechtsen, N.J.; Holst, J.J.; Hauser, A.S.; Rosenkilde, M.M. Molecular and in vivo phenotyping of missense variants of the human glucagon receptor. J. Biol. Chem. 2021, 298, 101413. [Google Scholar] [CrossRef]
- Yin, W.H.; Chen, J.W.; Tsai, C.; Chiang, M.C.; Young, M.S.; Lin, S.J. L-arginine improves endothelial function and reduces LDL oxidation in patients with stable coronary artery disease. Clin. Nutr. 2005, 24, 988–997. [Google Scholar] [CrossRef]
- Siasos, G.; Tousoulis, D.; Vlachopoulos, C.; Antoniades, C.; Stefanadi, E.; Ioakeimidis, N.; Zisimos, K.; Siasou, Z.; Papavassiliou, A.G.; Stefanadis, C. The impact of oral L-arginine supplementation on acute smoking-induced endothelial injury and arterial performance. Am. J. Hypertens. 2009, 22, 586–592. [Google Scholar] [CrossRef][Green Version]
- Nerla, R.; Di Monaco, A.; Sestito, A.; Lamendola, P.; Di Stasio, E.; Romitelli, F.; Lanza, G.A.; Crea, F. Transient endothelial dysfunction following flow-mediated dilation assessment. Heart Vessel. 2011, 26, 524–529. [Google Scholar] [CrossRef]
- Deveaux, A.; Pham, I.; West, S.G.; Andre, E.; Lantoine-Adam, F.; Bunouf, P.; Sadi, S.; Hermier, D.; Mathe, V.; Fouillet, H.; et al. l-Arginine Supplementation Alleviates Postprandial Endothelial Dysfunction When Baseline Fasting Plasma Arginine Concentration Is Low: A Randomized Controlled Trial in Healthy Overweight Adults with Cardiometabolic Risk Factors. J. Nutr. 2016, 146, 1330–1340. [Google Scholar] [CrossRef]
- Kashyap, V.S.; Lakin, R.O.; Campos, P.; Allemang, M.; Kim, A.; Sarac, T.P.; Hausladen, A.; Stamler, J.S. The LargPAD Trial: Phase IIA evaluation of l-arginine infusion in patients with peripheral arterial disease. J. Vasc. Surg. 2017, 66, 187–194. [Google Scholar] [CrossRef]
- Chin-Dusting, J.P.; Kaye, D.M.; Lefkovits, J.; Wong, J.; Bergin, P.; Jennings, G.L. Dietary supplementation with L-arginine fails to restore endothelial function in forearm resistance arteries of patients with severe heart failure. J. Am. Coll. Cardiol. 1996, 27, 1207–1213. [Google Scholar] [CrossRef]
- Chin-Dusting, J.P.; Alexander, C.T.; Arnold, P.J.; Hodgson, W.C.; Lux, A.S.; Jennings, G.L. Effects of in vivo and in vitro L-arginine supplementation on healthy human vessels. J. Cardiovasc. Pharm. 1996, 28, 158–166. [Google Scholar] [CrossRef]
- Mariotti, F.; Huneau, J.F.; Szezepanski, I.; Petzke, K.J.; Aggoun, Y.; Tome, D.; Bonnet, D. Meal amino acids with varied levels of arginine do not affect postprandial vascular endothelial function in healthy young men. J. Nutr. 2007, 137, 1383–1389. [Google Scholar] [CrossRef][Green Version]
- Wilson, A.M.; Harada, R.; Nair, N.; Balasubramanian, N.; Cooke, J.P. L-arginine supplementation in peripheral arterial disease: No benefit and possible harm. Circulation 2007, 116, 188–195. [Google Scholar] [CrossRef]
- Yeo, T.W.; Lampah, D.A.; Rooslamiati, I.; Gitawati, R.; Tjitra, E.; Kenangalem, E.; Price, R.N.; Duffull, S.B.; Anstey, N.M. A randomized pilot study of L-arginine infusion in severe falciparum malaria: Preliminary safety, efficacy and pharmacokinetics. PLoS ONE 2013, 8, e69587. [Google Scholar] [CrossRef]
- Monti, L.D.; Galluccio, E.; Villa, V.; Fontana, B.; Spadoni, S.; Piatti, P.M. Decreased diabetes risk over 9 year after 18-month oral L-arginine treatment in middle-aged subjects with impaired glucose tolerance and metabolic syndrome (extension evaluation of L-arginine study). Eur. J. Nutr. 2018, 57, 2805–2817. [Google Scholar] [CrossRef]
- Piatti, P.; Fragasso, G.; Monti, L.D.; Setola, E.; Lucotti, P.; Fermo, I.; Paroni, R.; Galluccio, E.; Pozza, G.; Chierchia, S.; et al. Acute intravenous L-arginine infusion decreases endothelin-1 levels and improves endothelial function in patients with angina pectoris and normal coronary arteriograms: Correlation with asymmetric dimethylarginine levels. Circulation 2003, 107, 429–436. [Google Scholar] [CrossRef]
- Boger, G.I.; Rudolph, T.K.; Maas, R.; Schwedhelm, E.; Dumbadze, E.; Bierend, A.; Benndorf, R.A.; Boger, R.H. Asymmetric dimethylarginine determines the improvement of endothelium-dependent vasodilation by simvastatin: Effect of combination with oral L-arginine. J. Am. Coll. Cardiol. 2007, 49, 2274–2282. [Google Scholar] [CrossRef]
- Lucotti, P.; Monti, L.; Setola, E.; La Canna, G.; Castiglioni, A.; Rossodivita, A.; Pala, M.G.; Formica, F.; Paolini, G.; Catapano, A.L.; et al. Oral L-arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass. Metabolism 2009, 58, 1270–1276. [Google Scholar] [CrossRef]
- Sun, Q.; Wang, B.; Li, Y.; Sun, F.; Li, P.; Xia, W.; Zhou, X.; Li, Q.; Wang, X.; Chen, J.; et al. Taurine Supplementation Lowers Blood Pressure and Improves Vascular Function in Prehypertension: Randomized, Double-Blind, Placebo-Controlled Study. Hypertension 2016, 67, 541–549. [Google Scholar] [CrossRef]
- Moloney, M.A.; Casey, R.G.; O’Donnell, D.H.; Fitzgerald, P.; Thompson, C.; Bouchier-Hayes, D.J. Two weeks taurine supplementation reverses endothelial dysfunction in young male type 1 diabetics. Diab. Vasc. Dis. Res. 2010, 7, 300–310. [Google Scholar] [CrossRef]
- Katakawa, M.; Fukuda, N.; Tsunemi, A.; Mori, M.; Maruyama, T.; Matsumoto, T.; Abe, M.; Yamori, Y. Taurine and magnesium supplementation enhances the function of endothelial progenitor cells through antioxidation in healthy men and spontaneously hypertensive rats. Hypertens Res. 2016, 39, 848–856. [Google Scholar] [CrossRef]
- Ra, S.G.; Choi, Y.; Akazawa, N.; Kawanaka, K.; Ohmori, H.; Maeda, S. Effects of Taurine Supplementation on Vascular Endothelial Function at Rest and After Resistance Exercise. Adv. Exp. Med. Biol. 2019, 1155, 407–414. [Google Scholar] [CrossRef] [PubMed]
- Lang, J.A.; Krajek, A.C.; Schwartz, K.S.; Rand, J.E. Oral L-Tyrosine Supplementation Improves Core Temperature Maintenance in Older Adults. Med. Sci. Sports Exerc. 2020, 52, 928–934. [Google Scholar] [CrossRef] [PubMed]
- Schwedhelm, E.; Maas, R.; Freese, R.; Jung, D.; Lukacs, Z.; Jambrecina, A.; Spickler, W.; Schulze, F.; Boger, R.H. Pharmacokinetic and pharmacodynamic properties of oral L-citrulline and L-arginine: Impact on nitric oxide metabolism. Br. J. Clin. Pharm. 2008, 65, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Figueroa, A.; Sanchez-Gonzalez, M.A.; Perkins-Veazie, P.M.; Arjmandi, B.H. Effects of watermelon supplementation on aortic blood pressure and wave reflection in individuals with prehypertension: A pilot study. Am. J. Hypertens. 2011, 24, 40–44. [Google Scholar] [CrossRef]
- Vincellette, C.M.; Losso, J.; Early, K.; Spielmann, G.; Irving, B.A.; Allerton, T.D. Supplemental Watermelon Juice Attenuates Acute Hyperglycemia-Induced Macro-and Microvascular Dysfunction in Healthy Adults. J. Nutr. 2021, 151, 3450–3458. [Google Scholar] [CrossRef] [PubMed]
- Qaradakhi, T.; Gadanec, L.K.; McSweeney, K.R.; Abraham, J.R.; Apostolopoulos, V.; Zulli, A. The Anti-Inflammatory Effect of Taurine on Cardiovascular Disease. Nutrients 2020, 12, 2847. [Google Scholar] [CrossRef]
- Wojcik, O.P.; Koenig, K.L.; Zeleniuch-Jacquotte, A.; Costa, M.; Chen, Y. The potential protective effects of taurine on coronary heart disease. Atherosclerosis 2010, 208, 19–25. [Google Scholar] [CrossRef]
- Lang, J.A.; Smaller, K.A. Oral l-tyrosine supplementation augments the vasoconstriction response to whole-body cooling in older adults. Exp. Physiol. 2017, 102, 835–844. [Google Scholar] [CrossRef]
- Perland, E.; Fredriksson, R. Classification Systems of Secondary Active Transporters. Trends Pharm. Sci. 2017, 38, 305–315. [Google Scholar] [CrossRef]
- Broer, S.; Broer, A. Amino acid homeostasis and signalling in mammalian cells and organisms. Biochem. J. 2017, 474, 1935–1963. [Google Scholar] [CrossRef]
- Bergstrom, J.; Furst, P.; Noree, L.O.; Vinnars, E. Intracellular free amino acid concentration in human muscle tissue. J. Appl. Physiol. 1974, 36, 693–697. [Google Scholar] [CrossRef]
- Hoffmann, E.K.; Lambert, I.H. Amino acid transport and cell volume regulation in Ehrlich ascites tumour cells. J. Physiol. 1983, 338, 613–625. [Google Scholar] [CrossRef]
- Ye, L.; Swingen, C.; Zhang, J. Induced pluripotent stem cells and their potential for basic and clinical sciences. Curr. Cardiol. Rev. 2013, 9, 63–72. [Google Scholar] [CrossRef]
- Su, L.; Kong, X.; Loo, S.; Gao, Y.; Liu, B.; Su, X.; Dalan, R.; Ma, J.; Ye, L. Thymosin beta-4 improves endothelial function and reparative potency of diabetic endothelial cells differentiated from patient induced pluripotent stem cells. Stem Cell Res. 2022, 13, 13. [Google Scholar] [CrossRef]
| Risk Factors | Experimental Model | Amino Acids | Findings | Reference |
|---|---|---|---|---|
| Diabetes | Patients with T2Ds | Serum AAs | Significantly decreased levels of arginine, asparagine, glycine, serine, threonine, and significantly increased levels of alanine, isoleucine, leucine, and valine in diabetics. | [118] |
| Hyperglycemic human aortic ECs | AAs metabolism | ECs exposed to short-term hyperglycemia showed increased levels of alanine, proline, glycine, serine, and glutamine. AAs oxidative stress metabolites significantly increased when ECs exposed to glucose for 7 days. | [119] | |
| HiPSC lines from patients with T2Ds | Glycine | Dia-hiPSC-ECs had disrupted glycine homeostasis, increased senescence, and impaired mitochondrial function and angiogenic potential as compared with healthy hiPSC-ECs. | [16] | |
| Patients with T2Ds and healthy controls | Plasma AAs | The ratios of ornithine/citrulline and proline/citrulline were 60% and 95% higher, respectively, in patients with diabetes than in controls. The plasma ornithine/arginine ratio was 36% higher in patients with diabetes, indicating increased arginase activity. | [120] | |
| 3587 men and women(a case-cohort study) | Plasma AAs (phenylalanine, isoleucine, glutamine, leucine, alanine, tyrosine, histidine, and valine) | Phenylalanine was positively associated with the risk of macrovascular disease, while histidine was inversely associated; higher tyrosine and alanine levels were associated with decreased risk of microvascular disease. | [121] | |
| Rats with experimental chronic renal failure | L-tryptophan levels and plasma concentrations in kidney, liver, lung, intestine, and spleen homogenates. | In animals with renal insufficiency, the plasma concentration and the content of l-tryptophan in homogenates of the kidney, liver, lung, intestine, and spleen were significantly decreased, while the plasma concentration and tissue levels of l-tryptophan metabolites in the kidney, liver, lung, intestine, spleen, and muscles were increased. | [122] | |
| 859 patients with type 1 diabetes (baseline eGFR 30–75 mL/min/1.73 m2) | Plasma AAs | The patients showed decreased tryptophan/kynurenine, threonine, methionine, and tryptophan levels. | [123] |
| Risk Factor | Experimental Model | Amino Acids | Findings | Reference |
|---|---|---|---|---|
| Hypertension | 4288 participants aged 20–70 years without hypertension (3-year follow-up) | Dietary intakes of BCAAs (valine, leucine, and isoleucine) | Higher BCAA intake, particularly valine, is associated with a higher risk of incident hypertension. | [129] |
| 8589 Japanese subjects | Plasma AAs | Higher intake of aromatic AAs is associated with s significantly higher risk of developing hypertension. | [130] | |
| 4287 adults (41.9% men), aged 20–70 years. | Dietary intake of AAs | High dietary intake of Leu.Ser/Thr.Trp ratio is associated with a higher risk of incident hypertension. | [131] | |
| 172 South African adolescents (105 girls, ages 13 to <18 years) | Circulating HCY concentrations | Of these adolescents, 40% had elevated BP, of whom 37% fell in the lowest and 38% in the highest HCY tertiles. | [132] | |
| Normotensive or spontaneously hypertensive rats | L-Tyrosine, Tryptophan, Leucine, Isoleucine, Valine, Alanine, Arginine, and Aspartate | In spontaneously hypertensive rats, tyrosine (50 mg/kg) reduced BP by about 12 mmHg, while 200 mg/kg reduced BP by about 40 mmHg. Tryptophan injection (225 mg/kg) reduced BP in spontaneously hypertensive rats, but only by about half as much as an equivalent dose of tyrosine. Other AAs have no effect on BP. | [133] | |
| Spontaneously hypertensive rat | L-tyrosine | Intraventricular injection of 15 micrograms of l-tyrosine results in a significantly lower BP in the spontaneously hypertensive rat. | [134] | |
| 4680 persons aged 40–59 years from China, Japan, the United Kingdom, and the United States | Dietary AA (glutamic, proline, phenylalanine, serine, and cystine) | Dietary glutamic acid (percentage of total protein intake) was inversely related to BP. | [135] |
| Risk Factors | Experimental Model | Amino Acids | Findings | Reference |
|---|---|---|---|---|
| Hypercholes terolemia | Hypercholesterolemic Wistar Rats | Liver AAs | A hypercholesterolemic diet resulted in decreased levels of glycine, serine, threonine, and histidine, and increased concentrations of asparagine and valine. | [149] |
| Hypercholesterolemic Wistar Rats | Plasma AAs | A hypercholesterolemic diet led to a decrease in spermidine level and an increase in the level of the spermidine metabolites such as ornithine and spermidine. | [150] |
| Medication | Experimental Model | Amino Acids | Findings | Reference |
|---|---|---|---|---|
| Aspirin eugenol ester | Blood stasis in rat | Plasma AAs | AEE treatment showed a favorable inhibition of the increase of phenylalanine, isoleucine, valine, and tryptophan. | [152] |
| Hyperlipidemic rat | Plasma and urine AAs | AEE inhibits hyperlipidemia by inhibiting the production of tyrosine metabolite, hydroxyphenyllactic acid, and tryptophan metabolite, xanthurenic acid. | [154] | |
| Atherosclerotic hamster | Plasma and urine AAs | AEE promotes the TCA cycle and attenuates energy metabolism impairment by ameliorating blood lipid profile, reducing GLU and citric acid, as well as elevating the level of valine and leucine. | [155] | |
| Hyperlipidemia hamster | Liver and feces | AEE may improve lipid and bile metabolism, and reduce oxidative stress and inflammation, which were all beneficial for hyperlipidemia treatment. | [156] | |
| Folic acid | 126 patients with H-type hypertension | Serum HCY | After 3 months’ treatment with an FA dose adjusted according to methylene tetrahydrofolate reductase C677T genotype, HCY and ET-1/NO levels were significantly decreased in the intervention group and were lower than those after the first treatment phase and lower than in the control group (p < 0.01). | [163] |
| Astragali Radix | Acute phase endothelial dysfunction induced by HCY | HCY | AR and ASP protected endothelium-dependent relaxation against acute injury from HCY through NO regulatory pathways, in which antioxidation played a key role. | [164] |
| Low-dose DOX-induced toxicity rat model | Rat brain AAs | The levels of six AAs, including glutamate, glycine, serine, alanine, citrulline, and ornithine, correlated with brain oxidative damage caused by DOX and rescued by AR. | [165] |
| Amino Acid | Experimental Model | Dose | TreatmentTime | Findings | Reference |
|---|---|---|---|---|---|
| Arginine | Stable CAD patients | 2 times/d (10 g/d) | 4 weeks | Oral l-arginine supplement improved EF and reduced LDL oxidation in stable CAD patients. | [185] |
| Healthy young smokers | 3 times/d (21 g/d) | 3 days | Oral l-arginine improves EF and vascular elastic properties of the arterial tree during the acute phase of smoking. | [186] | |
| Healthy male subjects | Intravenous l-arginine (10 g) | 20 min | FMD assessment leads to impairment of EF by inducing an increase in ADMA, which is reversed by l-arginine administration. | [187] | |
| Healthy overweight adults with the HTW | 3 times/d (4.5 g/d) | 4 weeks | Supplementation with low-dose SR-arginine alleviates postprandial ED in healthy HTW adults when the baseline plasma arginine concentration is relatively low. | [188] | |
| Patients with peripheral arterial disease | 50/100/500 mg l-arginine intra-arterially | once | Infusion of l-arginine increases blood flow and enhances the EF in diseased lower extremity human arteries. | [189] | |
| Patients with heart failure | 20 g/day | 28 days | Oral administration with l-arginine was ineffective in influencing EF in these patients with heart failure. | [190] | |
| Healthy males | 20 g/day | 28 days | Oral supplementation with l-arginine does not affect EF in normal healthy adults. | [191] | |
| Healthy young males | 3 g | once | In healthy men, meal arginine only slightly enters the NO pathway and has no effect on basal EF. | [192] | |
| Patients with intermittent claudication due to PAD | 3 g/day | 6 months | In patients with intermittent claudication and PAD, oral l-arginine was less effective. | [193] | |
| Patients with severe malaria | 12 g | once | L-arginine infused at 12 g over 8 h was safe but did not improve lactate clearance or endothelial NO bioavailability | [194] | |
| Patients with impaired glucose tolerance and metabolic syndrome | 6.4 g/day | 18 months | L-arginine increased the levels of EPCs and ADMA in subjects, suggesting that l-arginine can increase the expression levels of genes involved in metabolic and EF. | [195] | |
| Patients with CSX | 0.125 g/min | 120 min | Acute l-arginine infusion increases NO availability, decreases endothelin-1 levels, and improves EF in CSX patients. | [196] | |
| Clinically asymptomatic elderly subjects | 3 g/day | 3 weeks | Simvastatin does not enhance EF in subjects with elevated ADMA, but its combination with oral l-arginine improves EF in subjects with high ADMA. | [197] | |
| Patients with cardiovascular disease previously submitted to an aortocoronary bypass | 6.4 g/day | 6 months | Long-term oral l-arginine improves EF, decrease ADMA levels, and ameliorates insulin sensitivity and glucose tolerance. | [34,198] | |
| Taurine | Prehypertensive individuals | 1.6 g/day | 12 weeks | Long-term taurine supplementation exerts antihypertensive effects by improving vascular function. | [199] |
| Asymptomatic male diabetics | 3 times/d (1.5 g/d) | 2 weeks | Taurine supplementation reverses early, detectable conduit vessel abnormalities in young male diabetics. | [200] | |
| Healthy men | 3 g/day | 2 weeks | Taurine and Mg supplementation significantly increased EPC colony numbers and significantly decreased free radical levels in healthy men. | [201] | |
| Healthy men | 6 g/day | 2 weeks | 2 weeks of taurine supplementation significantly increased vascular EF at rest. | [202] | |
| Tyrosine | Young (25 ± 3 year) and older (72 ± 8 year) | 150 mg/kg | once | Tyrosine supplementation was found to improve the contractile response of skin vessels to cold stimuli. | [203] |
| Citrulline | Healthy volunteers | 2 times/d (0.75/1.5/3 g) | 1 week | Oral l-citrulline supplementation raises plasma l-arginine concentration and augments NO-dependent signaling. | [204] |
| Subjects with prehypertension | 2 times/d (l-citrulline/l-arginine: 1.35 g/0.65 g) | 6 weeks | WMJ supplementation improved aortic hemodynamics in middle-aged adults with prehypertension. | [205] | |
| Acute hyperglycemia in healthy adults | WMJ (500 mL/day) | 2 weeks | WMJ supplementation improved FMD and microvascular function during acute hyperglycemia in healthy adults. | [206] | |
| Leucine | Male volunteers | 25 g | once | Leucine administration prevents hyperglycaemia-mediated ED probably due to enhanced insulin secretion. | [110] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Wu, Y.; Ye, L. The Role of Amino Acids in Endothelial Biology and Function. Cells 2022, 11, 1372. https://doi.org/10.3390/cells11081372
Li M, Wu Y, Ye L. The Role of Amino Acids in Endothelial Biology and Function. Cells. 2022; 11(8):1372. https://doi.org/10.3390/cells11081372
Chicago/Turabian StyleLi, Meng, Yanqing Wu, and Lei Ye. 2022. "The Role of Amino Acids in Endothelial Biology and Function" Cells 11, no. 8: 1372. https://doi.org/10.3390/cells11081372
APA StyleLi, M., Wu, Y., & Ye, L. (2022). The Role of Amino Acids in Endothelial Biology and Function. Cells, 11(8), 1372. https://doi.org/10.3390/cells11081372







