miR-150-5p and let-7b-5p in Blood Myeloid Extracellular Vesicles Track Cognitive Symptoms in Patients with Multiple Sclerosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients Enrolled and Study Design
2.2. Serum Nf-L Determination
2.3. Total EVs and Myeloid EV Isolation
2.4. Analysis of EV Size and Concentration
2.5. Western Blotting
2.6. Selection of miRNAs with Synaptic Targets
2.7. Total RNA Isolation and Quantitative Real-Time PCR Analysis
2.8. Statistical Analysis
3. Results
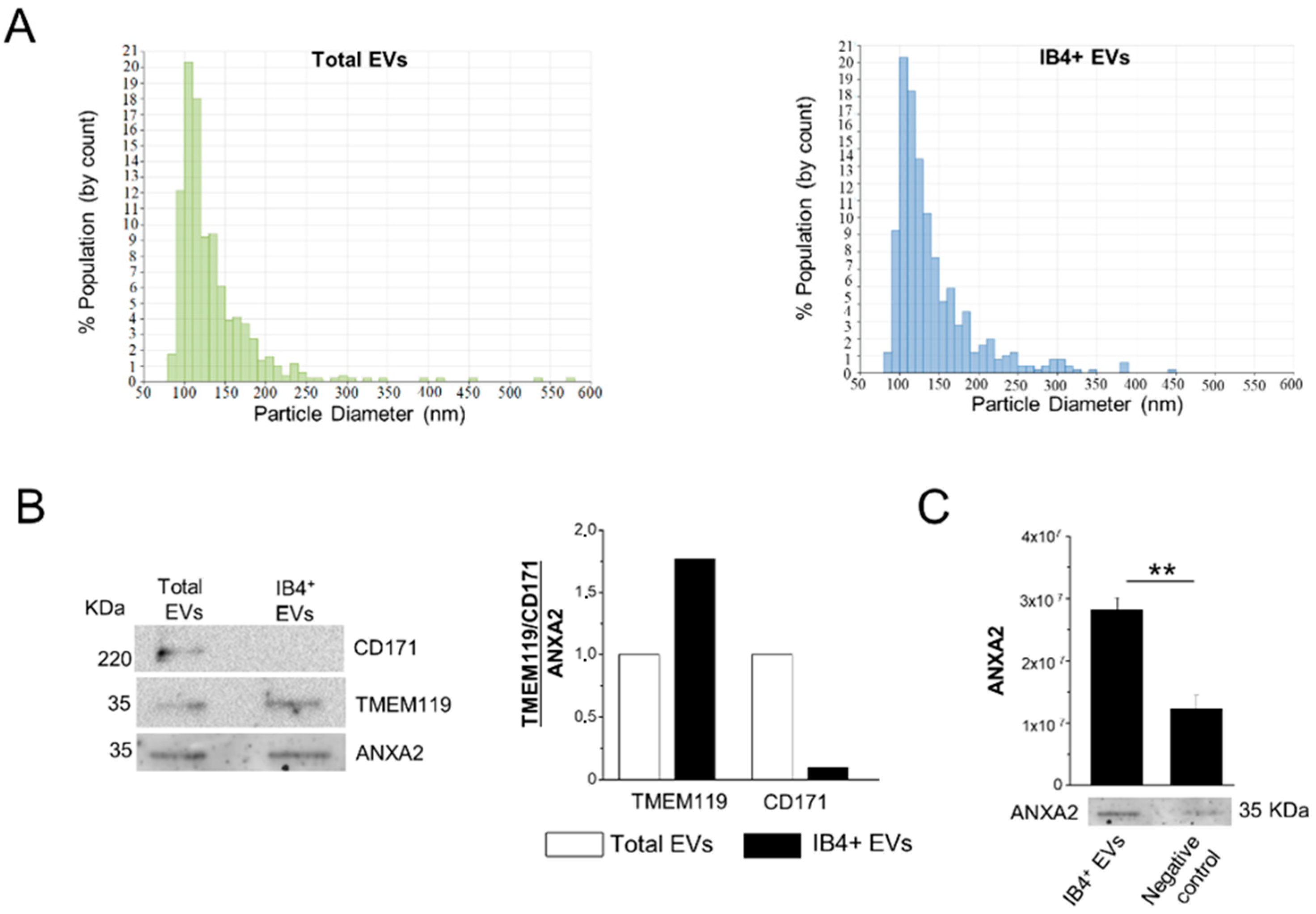
3.1. Isolation of Total and Myeloid EVs from Blood Plasma
3.2. Assessment of Myeloid EV Enrichment
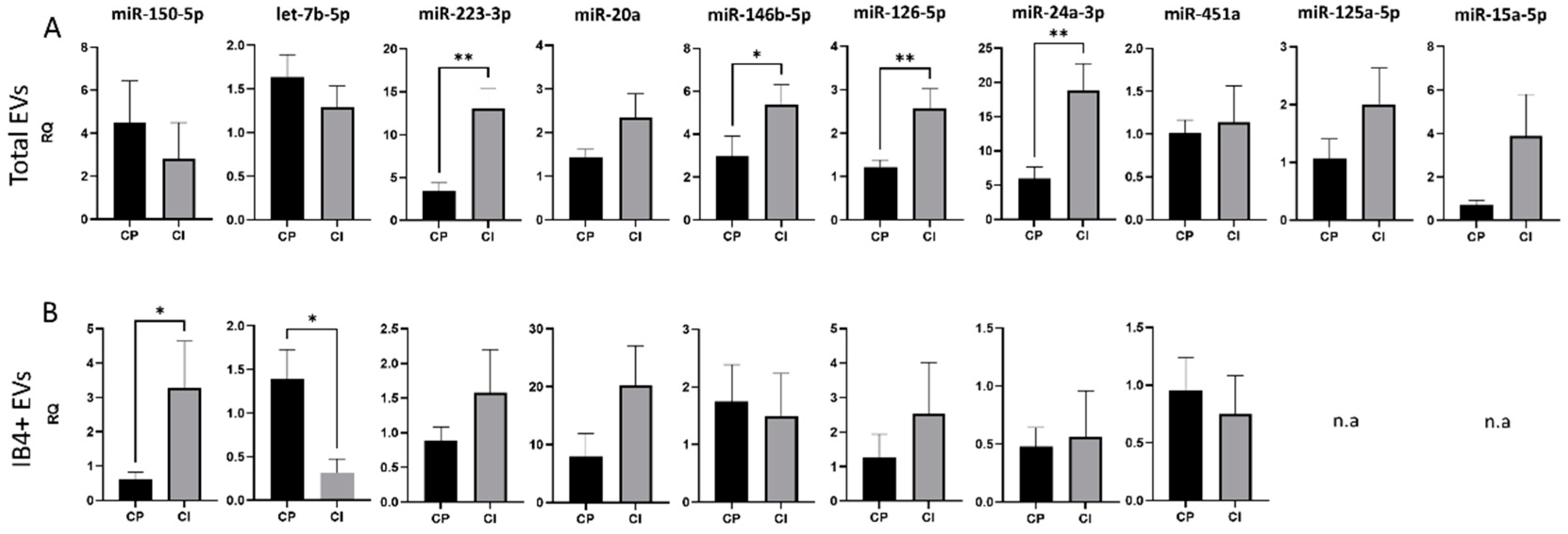
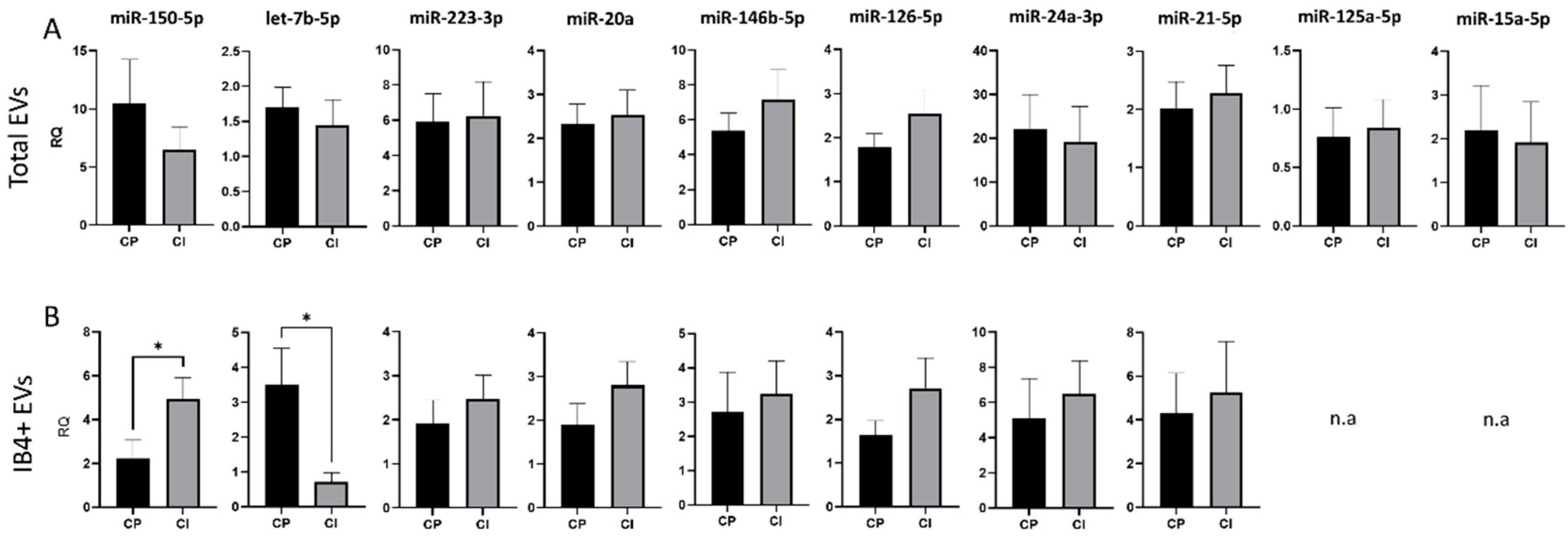
3.3. Expression of Selected miRNAs in Total and Myeloid EVs
3.4. Correlations between miRNA Levels and Clinical Data
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Draheim, T.; Liessem, A.; Scheld, M.; Wilms, F.; Weißflog, M.; Denecke, B.; Kensler, T.W.; Zendedel, A.; Beyer, C.; Kipp, M.; et al. Activation of the astrocytic Nrf2/ARE system ameliorates the formation of demyelinating lesions in a multiple sclerosis animal model. Glia 2016, 64, 2219–2230. [Google Scholar] [CrossRef] [PubMed]
- Kipp, M.; Nyamoya, S.; Hochstrasser, T.; Amor, S. Multiple sclerosis animal models: A clinical and histopathological perspective. Brain Pathol. 2017, 27, 123–137. [Google Scholar] [CrossRef] [PubMed]
- Geurts, J.J.; Barkhof, F. Grey matter pathology in multiple sclerosis. Lancet Neurol. 2008, 7, 841–851. [Google Scholar] [CrossRef]
- Fong, J.S.; Rae-Grant, A.; Huang, D.R. Neurodegeneration and neuroprotective agents in multiple sclerosis. Recent Pat. CNS Drug Discov. 2008, 3, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Benedict, R.H.B.; Amato, M.P.; DeLuca, J.; Geurts, J.J.G. Cognitive impairment in multiple sclerosis: Clinical management, MRI, and therapeutic avenues. Lancet Neurol. 2020, 19, 860–871. [Google Scholar] [CrossRef]
- Glanz, B.I.; Holland, C.M.; Gauthier, S.A.; Amunwa, E.L.; Liptak, Z.; Houtchens, M.K.; Sperling, R.A.; Khoury, S.J.; Guttmann, C.R.G.; Weiner, H.L. Cognitive dysfunction in patients with clinically isolated syndromes or newly diagnosed multiple sclerosis. Mult. Scler. 2007, 13, 1004–1010. [Google Scholar] [CrossRef]
- Nocentini, U.; Pasqualetti, P.; Bonavita, S.; Buccafusca, M.; De Caro, M.F.; Farina, D.; Girlanda, P.; Le Pira, F.; Lugaresi, A.; Quattrone, A.; et al. Cognitive dysfunction in patients with relapsing-remitting multiple sclerosis. Mult. Scler. 2006, 12, 77–87. [Google Scholar] [CrossRef]
- Zipoli, V.; Goretti, B.; Hakiki, B.; Siracusa, G.; Sorbi, S.; Portaccio, E.; Amato, M.P. Cognitive impairment predicts conversion to multiple sclerosis in clinically isolated syndromes. Mult. Scler. 2010, 16, 62–67. [Google Scholar] [CrossRef]
- Reuter, F.; Zaaraoui, W.; Crespy, L.; Faivre, A.; Rico, A.; Malikova, I.; Confort-Gouny, S.; Cozzone, P.J.; Ranjeva, J.P.; Pelletier, J.; et al. Cognitive impairment at the onset of multiple sclerosis: Relationship to lesion location. Mult. Scler. J. 2011, 17, 755–758. [Google Scholar] [CrossRef]
- Pitteri, M.; Romualdi, C.; Magliozzi, R.; Monaco, S.; Calabrese, M. Cognitive impairment predicts disability progression and cortical thinning in MS: An 8-year study. Mult. Scler. 2017, 23, 848–854. [Google Scholar] [CrossRef]
- Lazeron, R.H.C.; Rombouts, S.A.R.B.; Scheltens, P.; Polman, C.H.; Barkhof, F. An fMRI study of planning-related brain activity in patients with moderately advanced multiple sclerosis. Mult. Scler. 2004, 10, 549–555. [Google Scholar] [CrossRef]
- McCarthy, M.L.; MacKenzie, E.J.; Durbin, D.R.; Aitken, M.E.; Jaffe, K.M.; Paidas, C.N.; Slomine, B.S.; Dorsch, A.M.; Berk, R.A.; Christensen, J.R.; et al. The Pediatric Quality of Life Inventory: An evaluation of its reliability and validity for children with traumatic brain injury. Arch. Phys. Med. Rehabil. 2005, 86, 1901–1909. [Google Scholar] [CrossRef]
- Langdon, D.W. Cognition in multiple sclerosis. Curr. Opin. Neurol. 2011, 24, 244–249. [Google Scholar] [CrossRef]
- Kurtzke, J.F. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983, 33, 1444–1452. [Google Scholar] [CrossRef] [Green Version]
- Preziosa, P.; Rocca, M.A.; Pagani, E.; Stromillo, M.L.; Enzinger, C.; Gallo, A.; Hulst, H.E.; Atzori, M.; Pareto, D.; Riccitelli, G.C.; et al. Structural MRI correlates of cognitive impairment in patients with multiple sclerosis. Hum. Brain Mapp. 2016, 37, 1627–1644. [Google Scholar] [CrossRef]
- Houtchens, M.K.; Benedict, R.H.B.; Killiany, R.; Sharma, J.; Jaisani, Z.; Singh, B.; Weinstock-Guttman, B.; Guttmann, C.R.G.; Bakshi, R. Thalamic atrophy and cognition in multiple sclerosis. Neurology 2007, 69, 1213–1223. [Google Scholar] [CrossRef]
- Schoonheim, M.M.; Popescu, V.; Lopes, F.C.R.; Wiebenga, O.T.; Vrenken, H.; Douw, L.; Polman, C.H.; Geurts, J.J.G.; Barkhof, F. Subcortical atrophy and cognition. Neurology 2012, 79, 1754–1761. [Google Scholar] [CrossRef]
- Bergsland, N.; Pelizzari, L.; Laganá, M.M.; Di Tella, S.; Rossetto, F.; Nemni, R.; Clerici, M.; Baglio, F. Automated Assessment of the Substantia Nigra Pars Compacta in Parkinson’s Disease: A Diffusion Tensor Imaging Study. J. Pers. Med. 2021, 11, 1235. [Google Scholar] [CrossRef]
- Di Filippo, M.; De Iure, A.; Giampà, C.; Chiasserini, D.; Tozzi, A.; Orvietani, P.L.; Ghiglieri, V.; Tantucci, M.; Durante, V.; Quiroga-Varela, A.; et al. Persistent activation of microglia and NADPH drive hippocampal dysfunction in experimental multiple sclerosis. Sci. Rep. 2016, 6, 20926. [Google Scholar] [CrossRef]
- Hou, B.; Zhang, Y.; Liang, P.; He, Y.; Peng, B.; Liu, W.; Han, S.; Yin, J.; He, X. Inhibition of the NLRP3-inflammasome prevents cognitive deficits in experimental autoimmune encephalomyelitis mice via the alteration of astrocyte phenotype. Cell Death Dis. 2020, 11, 377. [Google Scholar] [CrossRef]
- Ramaglia, V.; Dubey, M.; Malpede, M.A.; Petersen, N.; de Vries, S.I.; Ahmed, S.M.; Lee, D.S.; Schenk, G.J.; Gold, S.M.; Huitinga, I.; et al. Complement-associated loss of CA2 inhibitory synapses in the demyelinated hippocampus impairs memory. Acta Neuropathol. 2021, 142, 643–667. [Google Scholar] [CrossRef]
- Verderio, C.; Muzio, L.; Turola, E.; Bergami, A.; Novellino, L.; Ruffini, F.; Riganti, L.; Corradini, I.; Francolini, M.; Garzetti, L.; et al. Myeloid microvesicles are a marker and therapeutic target for neuroinflammation. Ann. Neurol. 2012, 72, 610–624. [Google Scholar] [CrossRef] [Green Version]
- Prada, I.; Gabrielli, M.; Turola, E.; Iorio, A.; D’Arrigo, G.; Parolisi, R.; De Luca, M.; Pacifici, M.; Bastoni, M.; Lombardi, M.; et al. Glia-to-neuron transfer of miRNAs via extracellular vesicles: A new mechanism underlying inflammation-induced synaptic alterations. Acta Neuropathol. 2018, 135, 529–550. [Google Scholar] [CrossRef] [Green Version]
- Vella, L.J.; Hill, A.F.; Cheng, L. Focus on extracellular vesicles: Exosomes and their role in protein trafficking and biomarker potential in Alzheimer’s and Parkinson’s disease. Int. J. Mol. Sci. 2016, 17, 173. [Google Scholar] [CrossRef]
- Selmaj, I.; Cichalewska, M.; Namiecinska, M.; Galazka, G.; Horzelski, W.; Selmaj, K.W.; Mycko, M.P. Global exosome transcriptome profiling reveals biomarkers for multiple sclerosis. Ann. Neurol. 2017, 81, 703–717. [Google Scholar] [CrossRef]
- Polman, C.H.; Reingold, S.C.; Banwell, B.; Clanet, M.; Cohen, J.A.; Filippi, M.; Fujihara, K.; Havrdova, E.; Hutchinson, M.; Kappos, L.; et al. Diagnostic criteria for multiple sclerosis: 2010 Revisions to the McDonald criteria. Ann. Neurol. 2011, 69, 292–302. [Google Scholar] [CrossRef] [Green Version]
- Amato, M.P.; Portaccio, E.; Goretti, B.; Zipoli, V.; Ricchiuti, L.; De Caro, M.F.; Patti, F.; Vecchio, R.; Sorbi, S.; Trojano, M. The Rao’s Brief Repeatable Battery and Stroop Test: Normative values with age, education and gender corrections in an Italian population. Mult. Scler. 2006, 12, 787–793. [Google Scholar] [CrossRef]
- Benedict, R.H.; Cookfair, D.; Gavett, R.; Gunther, M.; Munschauer, F.; Garg, N.; Weinstock-Guttman, B. Validity of the minimal assessment of cognitive function in multiple sclerosis (MACFIMS). J. Int. Neuropsychol. Soc. 2006, 12, 549–558. [Google Scholar] [CrossRef]
- Nauta, I.M.; Bertens, D.; van Dam, M.; Huiskamp, M.; Driessen, S.; Geurts, J.J.; Uitdehaag, B.M.; Fasotti, L.; Hulst, H.E.; de Jong, B.A.; et al. Performance validity in outpatients with multiple sclerosis and cognitive complaints. Mult. Scler. 2021, 28, 642–653. [Google Scholar] [CrossRef]
- Jeppesen, D.K.; Fenix, A.M.; Franklin, J.L.; Higginbotham, J.N.; Zhang, Q.; Zimmerman, L.J.; Liebler, D.C.; Ping, J.; Liu, Q.; Evans, R.; et al. Reassessment of Exosome Composition. Cell 2019, 177, 428–445. [Google Scholar] [CrossRef] [Green Version]
- Serpente, M.; Fenoglio, C.; D’Anca, M.; Arcaro, M.; Sorrentino, F.; Visconte, C.; Arighi, A.; Fumagalli, G.G.; Porretti, L.; Cattaneo, A.; et al. MiRNA Profiling in Plasma Neural-Derived Small Extracellular Vesicles from Patients with Alzheimer’s Disease. Cells 2020, 9, 1443. [Google Scholar] [CrossRef] [PubMed]
- Zhao, F.; Cheng, L.; Shao, Q.; Chen, Z.; Lv, X.; Li, J.; He, L.; Sun, Y.; Ji, Q.; Lu, P.; et al. Characterization of serum small extracellular vesicles and their small RNA contents across humans, rats, and mice. Sci. Rep. 2020, 10, 4197. [Google Scholar] [CrossRef] [PubMed]
- Jovičić, A.; Roshan, R.; Moisoi, N.; Pradervand, S.; Moser, R.; Pillai, B.; Luthi-Carter, R. Comprehensive expression analyses of neural cell-type-specific miRNAs identify new determinants of the specification and maintenance of neuronal phenotypes. Ann. Intern. Med. 2013, 158, 5127–5137. [Google Scholar] [CrossRef] [Green Version]
- Ma, Z.; Shen, Y.; Zeng, Q.; Liu, J.; Yang, L.; Fu, R.; Hu, G. MiR-150-5p regulates EGR2 to promote the development of chronic rhinosinusitis via the DC-Th axis. Int. Immunopharmacol. 2018, 54, 188–197. [Google Scholar] [CrossRef]
- Rong, J.; Xu, L.; Hu, Y.; Liu, F.; Yu, Y.; Guo, H.; Ni, X.; Huang, Y.; Zhao, L.; Wang, Z. Inhibition of let-7b-5p contributes to an anti-tumorigenic macrophage phenotype through the SOCS1/STAT pathway in prostate cancer. Cancer Cell Int 2020, 20, 470. [Google Scholar] [CrossRef]
- Selimoglu-Buet, D.; Rivière, J.; Ghamlouch, H.; Bencheikh, L.; Lacout, C.; Morabito, M.; Diop, M.; Meurice, G.; Breckler, M.; Chauveau, A.; et al. A miR-150/TET3 pathway regulates the generation of mouse and human non-classical monocyte subset. Nat. Commun. 2018, 9, 5455. [Google Scholar] [CrossRef] [Green Version]
- Chen, C.; Zhou, Y.; Wang, J.; Yan, Y.; Peng, L.; Qiu, W. Dysregulated microRNA involvement in multiple sclerosis by induction of T helper 17 cell differentiation. Front. Immunol. 2018, 9, 1256. [Google Scholar] [CrossRef]
- Gaudet, A.D.; Fonken, L.K.; Watkins, L.R.; Nelson, R.J.; Popovich, P.G. MicroRNAs: Roles in Regulating Neuroinflammation. Neuroscientist 2018, 24, 221–245. [Google Scholar] [CrossRef] [Green Version]
- Marques-Rocha, J.L.; Garcia-Lacarte, M.; Samblas, M.; Bressan, J.; Martínez, J.A.; Milagro, F.I. Regulatory roles of miR-155 and let-7b on the expression of inflammation-related genes in THP-1 cells: Effects of fatty acids. J. Physiol. Biochem. 2018, 74, 579–589. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, M.; Zhong, M.; Suo, Q.; Lv, K. Expression profiles of miRNAs in polarized macrophages. Int. J. Mol. Med. 2013, 31, 797–802. [Google Scholar] [CrossRef] [Green Version]
- Hulsmans, M.; van Dooren, E.; Mathieu, C.; Holvoet, P. Decrease of miR-146b-5p in monocytes during obesity is associated with loss of the anti-inflammatory but not insulin signaling action of adiponectin. PLoS ONE 2012, 7, e32794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coleman, L.G.; Zou, J.; Crews, F.T. Microglial-derived miRNA let-7 and HMGB1 contribute to ethanol-induced neurotoxicity via TLR7. J. Neuroinflamm. 2017, 14, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Youn, Y.J.; Shrestha, S.; Lee, Y.B.; Kim, J.K.; Lee, J.H.; Hur, K.; Mali, N.M.; Nam, S.W.; Kim, S.H.; Lee, S.; et al. Neutrophil-derived trail is a proinflammatory subtype of neutrophil-derived extracellular vesicles. Theranostics 2021, 11, 2770–2787. [Google Scholar] [CrossRef] [PubMed]
- Amoruso, A.; Blonda, M.; Gironi, M.; Grasso, R.; Di Francescantonio, V.; Scaroni, F.; Furlan, R.; Verderio, C.; Avolio, C. Immune and central nervous system-related miRNAs expression profiling in monocytes of multiple sclerosis patients. Sci. Rep. 2020, 10, 6125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vistbakka, J.; Sumelahti, M.L.; Lehtimäki, T.; Elovaara, I.; Hagman, S. Evaluation of serum miR-191-5p, miR-24-3p, miR-128-3p, and miR-376c-3 in multiple sclerosis patients. Acta Neurol. Scand. 2018, 138, 130–136. [Google Scholar] [CrossRef] [PubMed]
- Lescher, J.; Paap, F.; Schultz, V.; Redenbach, L.; Scheidt, U.; Rosewich, H.; Nessler, S.; Fuchs, E.; Gärtner, J.; Brück, W.; et al. MicroRNA regulation in experimental autoimmune encephalomyelitis in mice and marmosets resembles regulation in human multiple sclerosis lesions. J. Neuroimmunol. 2012, 246, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Angelou, C.C.; Wells, A.C.; Vijayaraghavan, J.; Dougan, C.E.; Lawlor, R.; Iverson, E.; Lazarevic, V.; Kimura, M.Y.; Peyton, S.R.; Minter, L.M.; et al. Differentiation of Pathogenic Th17 Cells Is Negatively Regulated by Let-7 MicroRNAs in a Mouse Model of Multiple Sclerosis. Front. Immunol. 2020, 10, 3125. [Google Scholar] [CrossRef] [Green Version]
- Vistbakka, J.; Elovaara, I.; Lehtimäki, T.; Hagman, S. Circulating microRNAs as biomarkers in progressive multiple sclerosis. Mult. Scler. 2017, 23, 403–412. [Google Scholar] [CrossRef]
- Mandolesi, G.; Rizzo, F.R.; Balletta, S.; Stampanoni Bassi, M.; Gilio, L.; Guadalupi, L.; Nencini, M.; Moscatelli, A.; Ryan, C.P.; Licursi, V.; et al. The microRNA let-7b-5p Is Negatively Associated with Inflammation and Disease Severity in Multiple Sclerosis. Cells 2021, 10, 330. [Google Scholar] [CrossRef]
- Liguori, M.; Nuzziello, N.; Licciulli, F.; Consiglio, A.; Simone, M.; Viterbo, R.G.; Creanza, T.M.; Ancona, N.; Tortorella, C.; Margari, L.; et al. Combined microRNA and mRNA expression analysis in pediatric multiple sclerosis: An integrated approach to uncover novel pathogenic mechanisms of the disease. Hum. Mol. Genet. 2018, 27, 66–79. [Google Scholar] [CrossRef]
- Baulina, N.; Kulakova, O.; Kiselev, I.; Osmak, G.; Popova, E.; Boyko, A.; Favorova, O. Immune-related miRNA expression patterns in peripheral blood mononuclear cells differ in multiple sclerosis relapse and remission. J. Neuroimmunol. 2018, 317, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Pan, W.; Qian, L. Identification of the miRNA–mRNA regulatory network in multiple sclerosis. Neurol. Res. 2017, 39, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Bergman, P.; Piket, E.; Khademi, M.; James, T.; Brundin, L.; Olsson, T.; Piehl, F.; Jagodic, M. Circulating miR-150 in CSF is a novel candidate biomarker for multiple sclerosis. Neurol. Neuroimmunol. NeuroInflammation 2016, 3, e219. [Google Scholar] [CrossRef] [Green Version]
- Martinez, B.; Peplow, P. MicroRNAs as disease progression biomarkers and therapeutic targets in experimental autoimmune encephalomyelitis model of multiple sclerosis. Neural Regen. Res. 2020, 15, 1831–1837. [Google Scholar] [CrossRef] [PubMed]
- Angerstein, C.; Hecker, M.; Paap, B.K.; Koczan, D.; Thamilarasan, M.; Thiesen, H.J.; Zettl, U.K. Integration of MicroRNA databases to study MicroRNAs associated with multiple sclerosis. Mol. Neurobiol. 2012, 45, 520–535. [Google Scholar] [CrossRef]
- Juźwik, C.A.; Drake, S.S.; Zhang, Y.; Paradis-Isler, N.; Sylvester, A.; Amar-Zifkin, A.; Douglas, C.; Morquette, B.; Moore, C.S.; Fournier, A.E. microRNA dysregulation in neurodegenerative diseases: A systematic review. Prog. Neurobiol. 2019, 182, 101664. [Google Scholar] [CrossRef]
- Herrera-Espejo, S.; Santos-Zorrozua, B.; Álvarez-González, P.; Lopez-Lopez, E.; Garcia-Orad, Á. A Systematic Review of MicroRNA Expression as Biomarker of Late-Onset Alzheimer’s Disease. Mol. Neurobiol. 2019, 56, 8376–8391. [Google Scholar] [CrossRef]
- Lehmann, S.M.; Krüger, C.; Park, B.; Derkow, K.; Rosenberger, K.; Baumgart, J.; Trimbuch, T.; Eom, G.; Hinz, M.; Kaul, D.; et al. An unconventional role for miRNA: Let-7 activates Toll-like receptor 7 and causes neurodegeneration. Nat. Neurosci. 2012, 15, 827–835. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Théry, C.; Witwer, K.W.; Aikawa, E.; Alcaraz, M.J.; Anderson, J.D.; Andriantsitohaina, R.; Antoniou, A.; Arab, T.; Archer, F.; Atkin-Smith, G.K.; et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): A position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J. Extracell. Vesicles 2018, 7, 1535750. [Google Scholar] [CrossRef] [Green Version]
- Portaccio, E.; Goretti, B.; Zipoli, V.; Siracusa, G.; Sorbi, S.; Amato, M.P. A short version of Rao’s brief repeatable battery as a screening tool for cognitive impairment in multiple sclerosis. Clin. Neuropsychol. 2009, 23, 268–275. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Jiang, X.; Chen, J. The role of miR-150 in normal and malignant hematopoiesis. Oncogene 2014, 33, 3887–3893. [Google Scholar] [CrossRef]
- Wells, A.C.; Daniels, K.A.; Angelou, C.C.; Fagerberg, E.; Burnside, A.S.; Markstein, M.; Alfandari, D.; Welsh, R.M.; Pobezinskaya, E.L.; Pobezinsky, L.A. Modulation of let-7 miRNAs controls the differentiation of effector CD8 T cells. Elife 2017, 6, e26398. [Google Scholar] [CrossRef]
- Thebault, S.; Abdoli, M.; Fereshtehnejad, S.M.; Tessier, D.; Tabard-Cossa, V.; Freedman, M.S. Serum neurofilament light chain predicts long term clinical outcomes in multiple sclerosis. Sci. Rep. 2020, 10, 10381. [Google Scholar] [CrossRef] [PubMed]
- Aktas, O.; Renner, A.; Huss, A.; Filser, M.; Baetge, S.; Stute, N.; Gasis, M.; Lepka, K.; Goebels, N.; Senel, M.; et al. Serum neurofilament light chain. Neurol. Neuroimmunol. Neuroinflamm. 2020, 7, e885. [Google Scholar] [CrossRef]
- Gaetani, L.; Salvadori, N.; Lisetti, V.; Eusebi, P.; Mancini, A.; Gentili, L.; Borrelli, A.; Portaccio, E.; Sarchielli, P.; Blennow, K.; et al. Cerebrospinal fluid neurofilament light chain tracks cognitive impairment in multiple sclerosis. J. Neurol. 2019, 266, 2157–2163. [Google Scholar] [CrossRef]
- Friedova, L.; Motyl, J.; Srpova, B.; Oechtering, J.; Barro, C.; Vodehnalova, K.; Andelova, M.; Noskova, L.; Fialová, L.; Havrdova, E.K.; et al. The weak association between neurofilament levels at multiple sclerosis onset and cognitive performance after 9 years. Mult. Scler. Relat. Disord. 2020, 46, 102534. [Google Scholar] [CrossRef]
- Mattioli, F.; Bellomi, F.; Stampatori, C.; Mariotto, S.; Ferrari, S.; Monaco, S.; Mancinelli, C.; Capra, R. Longitudinal serum neurofilament light chain (sNfL) concentration relates to cognitive function in multiple sclerosis patients. J. Neurol. 2020, 267, 2245–2251. [Google Scholar] [CrossRef]
- Kuhle, J.; Barro, C.; Andreasson, U.; Derfuss, T.; Lindberg, R.; Sandelius, Å.; Liman, V.; Norgren, N.; Blennow, K.; Zetterberg, H. Comparison of three analytical platforms for quantification of the neurofilament light chain in blood samples: ELISA, electrochemiluminescence immunoassay and Simoa. Clin. Chem. Lab. Med. 2016, 54, 1655–1661. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.H.; Pandey, M.; Seigneur, E.M.; Panicker, L.M.; Koo, L.; Schwartz, O.M.; Chen, W.; Chen, C.K.; Simonds, W.F. Knockout of G protein β5 impairs brain development and causes multiple neurologic abnormalities in mice. J. Neurochem. 2011, 119, 544–554. [Google Scholar] [CrossRef] [Green Version]
- Thévenot, E.; Moreau, A.W.; Rousseau, V.; Combeau, G.; Domenichini, F.; Jacquet, C.; Goupille, O.; Amar, M.; Kreis, P.; Fossier, P.; et al. p21-activated kinase 3 (PAK3) protein regulates synaptic transmission through its interaction with the Nck2/Grb4 protein adaptor. J. Biol. Chem. 2011, 286, 40044–40059. [Google Scholar] [CrossRef] [Green Version]
- Qian, Y.; Wu, B.; Lu, Y.; Zhou, W.; Wang, S.; Wang, H. Novel PAK3 gene missense variant associated with two Chinese siblings with intellectual disability: A case report. BMC Med. Genet. 2020, 21, 31. [Google Scholar] [CrossRef] [Green Version]
- Chaudhry, A.; Noor, A.; Degagne, B.; Baker, K.; Bok, L.A.; Brady, A.F.; Chitayat, D.; Chung, B.H.; Cytrynbaum, C.; Dyment, D.; et al. Phenotypic spectrum associated with PTCHD1 deletions and truncating mutations includes intellectual disability and autism spectrum disorder. Clin. Genet. 2015, 88, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Ung, D.C.; Iacono, G.; Méziane, H.; Blanchard, E.; Papon, M.A.; Selten, M.; van Rhijn, J.R.; Montjean, R.; Rucci, J.; Martin, S.; et al. Ptchd1 deficiency induces excitatory synaptic and cognitive dysfunctions in mouse. Mol. Psychiatry 2018, 23, 1356–1367. [Google Scholar] [CrossRef] [Green Version]
- Guo, W.; Polich, E.D.; Su, J.; Gao, Y.; Christopher, D.M.; Allan, A.M.; Wang, M.; Wang, F.; Wang, G.; Zhao, X. Fragile X Proteins FMRP and FXR2P Control Synaptic GluA1 Expression and Neuronal Maturation via Distinct Mechanisms. Cell Rep. 2015, 11, 1651–1666. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beesley, P.W.; Herrera-Molina, R.; Smalla, K.H.; Seidenbecher, C. The Neuroplastin adhesion molecules: Key regulators of neuronal plasticity and synaptic function. J. Neurochem. 2014, 131, 268–283. [Google Scholar] [CrossRef] [PubMed]
- Keren-Shaul, H.; Spinrad, A.; Weiner, A.; Matcovitch-Natan, O.; Dvir-Szternfeld, R.; Ulland, T.K.; David, E.; Baruch, K.; Lara-Astaiso, D.; Toth, B.; et al. A Unique Microglia Type Associated with Restricting Development of Alzheimer’s Disease. Cell 2017, 169, 1276–1290.e17. [Google Scholar] [CrossRef]
- Krasemann, S.; Madore, C.; Cialic, R.; Baufeld, C.; Calcagno, N.; El Fatimy, R.; Beckers, L.; O’Loughlin, E.; Xu, Y.; Fanek, Z.; et al. The TREM2-APOE Pathway Drives the Transcriptional Phenotype of Dysfunctional Microglia in Neurodegenerative Diseases. Immunity 2017, 47, 566–581.e9. [Google Scholar] [CrossRef] [Green Version]
- Virgilio, E.; Vecchio, D.; Crespi, I.; Puricelli, C.; Barbero, P.; Galli, G.; Cantello, R.; Dianzani, U.; Comi, C. Cerebrospinal fluid biomarkers and cognitive functions at multiple sclerosis diagnosis. J. Neurol. 2022, 1, 1–9. [Google Scholar] [CrossRef] [PubMed]

| Italian Cohort | ||||
|---|---|---|---|---|
| Variable | All Patients | RRMS | PMS | |
| N | 21 | 17 | 4 | |
| Age, years (range) | 42 (21–69) | 39.1 (21–65) | 54.8 (34–69) | |
| Age at onset, years (range) | 36.2 (20–56) | 35.1 (20–56) | 46.2 (36–56) | |
| Gender F:M | 12:9 | 11:6 | 1:3 | |
| EDSS | 2.4 (0–7) | 1.8 (0–3.5) | 2.4 (5–7) | |
| Disease duration, years (range) | 6.8 (1–15) | 5.23 (1–10) | 13.5 (12–15) | |
| Amsterdam Cohort | ||||
| N | 28 | 23 | 5 | |
| Age, years (range) | 44.9 (29–68) | 40.4 (26–66) | 57.6 (49–68) | |
| Age at onset, years (range) | 31.5 (20–56) | 31 (23–44) | 35.7 (18–56) | |
| Gender F:M | 21:7 | 19:4 | 2:3 | |
| EDSS | 3.8 (2–6.5) | 4.75 (2–5.5) | 5.1 (3–6.5) | |
| Disease duration, years (range) | 14.9 (1.44–29.3) | 12.9 (1.4–31.6) | 28.1 (12.4–34.6) |
| miRNA | Highly Expressed/Present in Human Serum/Plasma EVs | Enriched in Microglia/Peripheral Myeloid Cells | Present in EVs from Brain/ Peripheral SMyeloid Cells | Putative MS Biomarkers | Dysregulated in Neurodegenerative Diseases |
|---|---|---|---|---|---|
| miR-146a-5p | Refs. [31,32] | Refs. [23,33] | Ref. [23] | Refs. [44,46] | Ref. [56] |
| miR-223-3p | Ref. [31] | Ref. [33] | Ref. [23] | Refs. [44,54] | Ref. [56] |
| miR-16-5p | Ref. [31] | Ref. [23] | Refs. [31,57] | ||
| miR-23a-3p | Ref. [31] | Ref. [23] | Ref. [44] | ||
| miR-20a | Ref. [31] | Ref. [23] | Refs. [52,55] | ||
| miR-21-5p | Ref. [31] | Refs. [37,38] | Refs. [46,51] | Ref. [56] | |
| miR-125a-5p | Refs. [31,32] | Ref. [50] | |||
| miR-146b-5p | Ref. [31] | Ref. [41] | Ref. [46] | ||
| miR-150-5p | Ref. [31] | Refs. [33,34,36] | Ref. [53] | ||
| miR-24-3p | Ref. [31] | Refs. [45,48] | |||
| miR-15a-5p | Ref. [31] | Ref. [23] | |||
| let-7b-5p | Refs. [31,32] | Refs. [35,39] | Ref. [42] | Refs. [47,49,50] | Refs. [42,58] |
| miR-126-5p | Ref. [31] | Ref. [33] | Ref. [43] | ||
| miR-451a | Refs. [31,32] | Refs. [36,40] | Ref. [43] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scaroni, F.; Visconte, C.; Serpente, M.; Golia, M.T.; Gabrielli, M.; Huiskamp, M.; Hulst, H.E.; Carandini, T.; De Riz, M.; Pietroboni, A.; et al. miR-150-5p and let-7b-5p in Blood Myeloid Extracellular Vesicles Track Cognitive Symptoms in Patients with Multiple Sclerosis. Cells 2022, 11, 1551. https://doi.org/10.3390/cells11091551
Scaroni F, Visconte C, Serpente M, Golia MT, Gabrielli M, Huiskamp M, Hulst HE, Carandini T, De Riz M, Pietroboni A, et al. miR-150-5p and let-7b-5p in Blood Myeloid Extracellular Vesicles Track Cognitive Symptoms in Patients with Multiple Sclerosis. Cells. 2022; 11(9):1551. https://doi.org/10.3390/cells11091551
Chicago/Turabian StyleScaroni, Federica, Caterina Visconte, Maria Serpente, Maria Teresa Golia, Martina Gabrielli, Marijn Huiskamp, Hanneke E. Hulst, Tiziana Carandini, Milena De Riz, Anna Pietroboni, and et al. 2022. "miR-150-5p and let-7b-5p in Blood Myeloid Extracellular Vesicles Track Cognitive Symptoms in Patients with Multiple Sclerosis" Cells 11, no. 9: 1551. https://doi.org/10.3390/cells11091551
APA StyleScaroni, F., Visconte, C., Serpente, M., Golia, M. T., Gabrielli, M., Huiskamp, M., Hulst, H. E., Carandini, T., De Riz, M., Pietroboni, A., Rotondo, E., Scarpini, E., Galimberti, D., Teunissen, C. E., van Dam, M., de Jong, B. A., Fenoglio, C., & Verderio, C. (2022). miR-150-5p and let-7b-5p in Blood Myeloid Extracellular Vesicles Track Cognitive Symptoms in Patients with Multiple Sclerosis. Cells, 11(9), 1551. https://doi.org/10.3390/cells11091551








