Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart
Abstract
:1. Introduction
2. The PI3K/Akt Pathway and Its Impact on the Peri-Infarct Processes
2.1. Components and Mechanisms of PI3K/Akt Activation
2.2. Regulation of the PI3K/Akt Signaling
2.3. Impact of PI3K/Akt on Peri-Infarct Processes
3. Activity of the PI3K/Akt Pathway in Myocardial Infarction
3.1. Necrosis
3.2. Programmed Type of Cell Death
3.2.1. Apoptosis
3.2.2. Necroptosis
3.2.3. Ferroptosis
3.2.4. Pyroptosis
3.3. Ischemic Conditioning
4. The Role of the PI3K/Akt Pathway in Post-Infarction Left Ventricular Remodeling
4.1. Inflammation
4.2. Autophagy
4.3. Fibrosis
4.4. Cardiac Hypertrophy
4.5. Angiogenesis
4.6. Conduction Disturbances
5. Diabetes
6. MicroRNA
| miRNA | DM2 | Ref. | The Potential Regulatory Mechanism |
|---|---|---|---|
| miR-19 | - | - | MiR-19a protects H9C2 cardiomyocytes against H/R-induced apoptosis by inhibiting PTEN [292]; MiR-19b promotes NRCFs proliferation and migration by targeting PTEN [293]. |
| miR-21 | up * | [294,295,296,297] | Targets PTEN expression and promotes adverse ventricular remodeling by induction of MMP-2 in cardiac fibroblasts [269], alleviates cardiomyocytes apoptosis and reduces infarct size through decreasing Bax/Bcl-2 ratio and caspase-3 expression [271], decreases cardiomyocytes autophagy [273]. |
| miR-34 | up | [294] | Inhibition of miR-34a attenuates MI-induced LV remodeling in mice and induces Akt phosphorylation [298]; activates PI3K/AKT pathways via up-regulating ZEB1 in cardiomyocytes and attenuates hypoxia-induced injury [299]; protects H9C2 cardiomyocytes from high-glucose-induced injury [300]. |
| miR-122 | up | [295,301] | Aggravates oxygen–glucose deprivation and reperfusion apoptosis of H9C2 cardiomyocytes inhibiting AKT/GSK-3β/β-catenin and AKT/mTOR pathway signaling [302,303]; |
| miR-126 | down | [295,304,305,306,307,308] | Targets PIK3R2 and SPRED1 expression, resulting in elevated activity of the PI3K/Akt signal and improved angiogenesis, left ventricle function after MI, and alleviated apoptosis of both endothelial cells and cardiomyocytes [278,279,280,281,282,283,284] |
| miR-130 | up | [295,309,310] | Attenuates LV dysfunction and remodeling after MI targeting PTEN and increasing activity of Akt [311] |
| miR-145 | down | [312] | Inhibits cardiac cells apoptosis and ROC activity by enhancing the PI3K/Akt and SGK1 activity [285], promotes autophagy and reduces myocardial infarct size targeting FRS2 and inducing PI3K/Akt/mTOR activity [286,287], attenuates fibrosis via activation of the Akt/CREB and suppression of the AKT/GSK-3β/β-catenin pathways [288,289]. |
| miR-155 | up | [313,314] | Targets IKKi expression decreasing its cardioprotective role in activating Akt and NF-κB, independent of the PI3K, and enhances cardiac hypertrophy [315], inhibits the AKT/CREB pathway signal and impairs the left ventricle function [316]. |
| miR-223 | up | [310,317,318] | Inhibits angiogenic function of CMECs by decreasing the PI3K/Akt signal activity [319], regulates cardiac hypertrophy by modulating the p-Akt activation [320], and mediates cardiac fibrosis after MI targeting RASA1 expression, which promotes MEK1/2, ERK1/2 and AKT phosphorylation [321]. |
| miR-320 | up | [295,306] | Increases vulnerability of cardiomyocytes to hypoxia/reoxygenation injury targeting expression of Akt3 [322]. |
| miR-375 | up | [323,324] | Exacerbates inflammation and cardiomyocyte apoptosis, decreases angiogenesis and impairs the LV function after MI by a reduction in PDK-1 expression, which results in decreased Akt Thr-308 phosphorylation [325,326]. |
7. Further Perspectives
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleh, M.; Ambrose, J.A. Understanding myocardial infarction. F1000Research 2018, 7, 1378. [Google Scholar] [CrossRef] [PubMed]
- Boateng, S.; Sanborn, T. Acute myocardial infarction. Dis. Mon. 2013, 59, 83–96. [Google Scholar] [CrossRef] [PubMed]
- Reddy, K.; Khaliq, A.; Henning, R.J. Recent advances in the diagnosis and treatment of acute myocardial infarction. World J. Cardiol. 2015, 7, 243–276. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Buring, J.E.; Badimon, L.; Hansson, G.K.; Deanfield, J.; Bittencourt, M.S.; Tokgozoglu, L.; Lewis, E.F. Atherosclerosis. Nat. Rev. Dis. Primers 2019, 5, 56. [Google Scholar] [CrossRef]
- Anderson, J.L.; Morrow, D.A. Acute Myocardial Infarction. N. Engl. J. Med. 2017, 376, 2053–2064. [Google Scholar] [CrossRef] [Green Version]
- Alabas, O.A.; Jernberg, T.; Pujades-Rodriguez, M.; Rutherford, M.J.; West, R.M.; Hall, M.; Timmis, A.; Lindahl, B.; Fox, K.A.A.; Hemingway, H.; et al. Statistics on mortality following acute myocardial infarction in 842 897 Europeans. Cardiovasc. Res. 2020, 116, 149–157. [Google Scholar] [CrossRef]
- Lu, L.; Liu, M.; Sun, R.; Zheng, Y.; Zhang, P. Myocardial Infarction: Symptoms and Treatments. Cell Biochem. Biophys. 2015, 72, 865–867. [Google Scholar] [CrossRef]
- Schumacher, B.; Pecher, P.; von Specht, B.U.; Stegmann, T. Induction of neoangiogenesis in ischemic myocardium by human growth factors: First clinical results of a new treatment of coronary heart disease. Circulation 1998, 97, 645–650. [Google Scholar] [CrossRef]
- Ezekowitz, J.A.; Kaul, P.; Bakal, J.A.; Armstrong, P.W.; Welsh, R.C.; McAlister, F.A. Declining in-hospital mortality and increasing heart failure incidence in elderly patients with first myocardial infarction. J. Am. Coll. Cardiol. 2009, 53, 13–20. [Google Scholar] [CrossRef] [Green Version]
- Mela, A.; Rdzanek, E.; Poniatowski, L.A.; Jaroszynski, J.; Furtak-Niczyporuk, M.; Galazka-Sobotka, M.; Olejniczak, D.; Niewada, M.; Staniszewska, A. Economic Costs of Cardiovascular Diseases in Poland Estimates for 2015–2017 Years. Front. Pharmacol. 2020, 11, 1231. [Google Scholar] [CrossRef]
- Kologrivova, I.; Shtatolkina, M.; Suslova, T.; Ryabov, V. Cells of the Immune System in Cardiac Remodeling: Main Players in Resolution of Inflammation and Repair after Myocardial Infarction. Front. Immunol. 2021, 12, 664457. [Google Scholar] [CrossRef] [PubMed]
- Gibb, A.A.; Hill, B.G. Metabolic Coordination of Physiological and Pathological Cardiac Remodeling. Circ. Res. 2018, 123, 107–128. [Google Scholar] [CrossRef] [PubMed]
- Bellis, A.; Di Gioia, G.; Mauro, C.; Mancusi, C.; Barbato, E.; Izzo, R.; Trimarco, B.; Morisco, C. Reducing Cardiac Injury during ST-Elevation Myocardial Infarction: A Reasoned Approach to a Multitarget Therapeutic Strategy. J. Clin. Med. 2021, 10, 2968. [Google Scholar] [CrossRef]
- Ibanez, B.; James, S.; Agewall, S.; Antunes, M.J.; Bucciarelli-Ducci, C.; Bueno, H.; Caforio, A.L.P.; Crea, F.; Goudevenos, J.A.; Halvorsen, S.; et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: The Task Force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur. Heart J. 2018, 39, 119–177. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Collet, J.P.; Thiele, H.; Barbato, E.; Barthelemy, O.; Bauersachs, J.; Bhatt, D.L.; Dendale, P.; Dorobantu, M.; Edvardsen, T.; Folliguet, T.; et al. 2020 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation. Eur. Heart J. 2021, 42, 1289–1367. [Google Scholar] [CrossRef] [PubMed]
- Cohn, J.N.; Ferrari, R.; Sharpe, N. Cardiac remodeling—Concepts and clinical implications: A consensus paper from an international forum on cardiac remodeling. J. Am. Coll. Cardiol. 2000, 35, 569–582. [Google Scholar] [CrossRef] [Green Version]
- Anand, I.S.; Florea, V.G.; Solomon, S.D.; Konstam, M.A.; Udelson, J.E. Noninvasive assessment of left ventricular remodeling: Concepts, techniques, and implications for clinical trials. J. Card Fail. 2002, 8, S452–S464. [Google Scholar] [CrossRef]
- Han, B.; Trew, M.L.; Zgierski-Johnston, C.M. Cardiac Conduction Velocity, Remodeling and Arrhythmogenesis. Cells 2021, 10, 2923. [Google Scholar] [CrossRef]
- Bahit, M.C.; Kochar, A.; Granger, C.B. Post-Myocardial Infarction Heart Failure. JACC Heart Fail. 2018, 6, 179–186. [Google Scholar] [CrossRef]
- Jenča, D.; Melenovský, V.; Stehlik, J.; Staněk, V.; Kettner, J.; Kautzner, J.; Adámková, V.; Wohlfahrt, P. Heart failure after myocardial infarction: Incidence and predictors. ESC Heart Fail. 2021, 8, 222–237. [Google Scholar] [CrossRef]
- Whitman, M.; Downes, C.P.; Keeler, M.; Keller, T.; Cantley, L. Type I phosphatidylinositol kinase makes a novel inositol phospholipid, phosphatidylinositol-3-phosphate. Nature 1988, 332, 644–646. [Google Scholar] [CrossRef] [PubMed]
- Staal, S.P. Molecular cloning of the akt oncogene and its human homologues AKT1 and AKT2: Amplification of AKT1 in a primary human gastric adenocarcinoma. Proc. Natl. Acad. Sci. USA 1987, 84, 5034–5037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bi, L.; Okabe, I.; Bernard, D.J.; Nussbaum, R.L. Early embryonic lethality in mice deficient in the p110beta catalytic subunit of PI 3-kinase. Mamm. Genome 2002, 13, 169–172. [Google Scholar] [CrossRef] [PubMed]
- Marques, M.; Kumar, A.; Cortes, I.; Gonzalez-Garcia, A.; Hernandez, C.; Moreno-Ortiz, M.C.; Carrera, A.C. Phosphoinositide 3-kinases p110alpha and p110beta regulate cell cycle entry, exhibiting distinct activation kinetics in G1 phase. Mol. Cell. Biol. 2008, 28, 2803–2814. [Google Scholar] [CrossRef] [Green Version]
- Huang, X.; Liu, G.; Guo, J.; Su, Z. The PI3K/AKT pathway in obesity and type 2 diabetes. Int. J. Biol. Sci. 2018, 14, 1483–1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rai, S.N.; Dilnashin, H.; Birla, H.; Singh, S.S.; Zahra, W.; Rathore, A.S.; Singh, B.K.; Singh, S.P. The Role of PI3K/Akt and ERK in Neurodegenerative Disorders. Neurotox. Res. 2019, 35, 775–795. [Google Scholar] [CrossRef]
- Zeng, B.; Liu, L.; Liao, X.; Zhang, C.; Ruan, H. Thyroid hormone protects cardiomyocytes from H(2)O(2)-induced oxidative stress via the PI3K-AKT signaling pathway. Exp. Cell Res. 2019, 380, 205–215. [Google Scholar] [CrossRef]
- Fruman, D.A.; Chiu, H.; Hopkins, B.D.; Bagrodia, S.; Cantley, L.C.; Abraham, R.T. The PI3K Pathway in Human Disease. Cell 2017, 170, 605–635. [Google Scholar] [CrossRef] [Green Version]
- Guo, H.; German, P.; Bai, S.; Barnes, S.; Guo, W.; Qi, X.; Lou, H.; Liang, J.; Jonasch, E.; Mills, G.B.; et al. The PI3K/AKT Pathway and Renal Cell Carcinoma. J. Genet. Genom. 2015, 42, 343–353. [Google Scholar] [CrossRef] [Green Version]
- Vanhaesebroeck, B.; Guillermet-Guibert, J.; Graupera, M.; Bilanges, B. The emerging mechanisms of isoform-specific PI3K signalling. Nat. Rev. Mol. Cell Biol. 2010, 11, 329–341. [Google Scholar] [CrossRef]
- Cantley, L.C. The phosphoinositide 3-kinase pathway. Science 2002, 296, 1655–1657. [Google Scholar] [CrossRef] [PubMed]
- Leslie, N.R.; Biondi, R.M.; Alessi, D.R. Phosphoinositide-regulated kinases and phosphoinositide phosphatases. Chem. Rev. 2001, 101, 2365–2380. [Google Scholar] [CrossRef] [PubMed]
- Alessi, D.R.; James, S.R.; Downes, C.P.; Holmes, A.B.; Gaffney, P.R.J.; Reese, C.B.; Cohen, P. Characterization of a 3-phosphoinositide-dependent protein kinase which phosphorylates and activates protein kinase Bα. Curr. Biol. 1997, 7, 261–269. [Google Scholar] [CrossRef] [Green Version]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [Green Version]
- Sciarretta, S.; Zhai, P.; Maejima, Y.; del Re, D.P.; Nagarajan, N.; Yee, D.; Liu, T.; Magnuson, M.A.; Volpe, M.; Frati, G.; et al. mTORC2 regulates cardiac response to stress by inhibiting MST1. Cell Rep. 2015, 11, 125–136. [Google Scholar] [CrossRef] [Green Version]
- Feng, J.; Park, J.; Cron, P.; Hess, D.; Hemmings, B.A. Identification of a PKB/Akt hydrophobic motif Ser-473 kinase as DNA-dependent protein kinase. J. Biol. Chem. 2004, 279, 41189–41196. [Google Scholar] [CrossRef] [Green Version]
- Brazil, D.P.; Hemmings, B.A. Ten years of protein kinase B signalling: A hard Akt to follow. Trends Biochem. Sci. 2001, 26, 657–664. [Google Scholar] [CrossRef]
- Bellacosa, A.; Testa, J.R.; Moore, R.; Larue, L. A portrait of AKT kinases: Human cancer and animal models depict a family with strong individualities. Cancer Biol. Ther. 2004, 3, 268–275. [Google Scholar] [CrossRef] [Green Version]
- Toulany, M.; Maier, J.; Iida, M.; Rebholz, S.; Holler, M.; Grottke, A.; Juker, M.; Wheeler, D.L.; Rothbauer, U.; Rodemann, H.P. Akt1 and Akt3 but not Akt2 through interaction with DNA-PKcs stimulate proliferation and post-irradiation cell survival of K-RAS-mutated cancer cells. Cell Death Discov. 2017, 3, 17072. [Google Scholar] [CrossRef]
- Shen, Y.H.; Zhang, L.; Ren, P.; Nguyen, M.T.; Zou, S.; Wu, D.; Wang, X.L.; Coselli, J.S.; LeMaire, S.A. AKT2 confers protection against aortic aneurysms and dissections. Circ. Res. 2013, 112, 618–632. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.Y.; Luciano, A.K.; Ackah, E.; Rodriguez-Vita, J.; Bancroft, T.A.; Eichmann, A.; Simons, M.; Kyriakides, T.R.; Morales-Ruiz, M.; Sessa, W.C. Endothelial Akt1 mediates angiogenesis by phosphorylating multiple angiogenic substrates. Proc. Natl. Acad. Sci. USA 2014, 111, 12865–12870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sansal, I.; Sellers, W.R. The biology and clinical relevance of the PTEN tumor suppressor pathway. J. Clin. Oncol. 2004, 22, 2954–2963. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Chen, M.; Pandolfi, P.P. The functions and regulation of the PTEN tumour suppressor: New modes and prospects. Nat. Rev. Mol. Cell Biol. 2018, 19, 547–562. [Google Scholar] [CrossRef]
- Yang, J.; Nie, J.; Ma, X.; Wei, Y.; Peng, Y.; Wei, X. Targeting PI3K in cancer: Mechanisms and advances in clinical trials. Mol. Cancer 2019, 18, 26. [Google Scholar] [CrossRef] [Green Version]
- Grunwald, V.; DeGraffenried, L.; Russel, D.; Friedrichs, W.E.; Ray, R.B.; Hidalgo, M. Inhibitors of mTOR reverse doxorubicin resistance conferred by PTEN status in prostate cancer cells. Cancer Res. 2002, 62, 6141–6145. [Google Scholar] [PubMed]
- Carracedo, A.; Pandolfi, P.P. The PTEN-PI3K pathway: Of feedbacks and cross-talks. Oncogene 2008, 27, 5527–5541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Krijnen, P.A.; Nijmeijer, R.; Meijer, C.J.; Visser, C.A.; Hack, C.E.; Niessen, H.W. Apoptosis in myocardial ischaemia and infarction. J. Clin. Pathol. 2002, 55, 801–811. [Google Scholar] [CrossRef] [Green Version]
- Chiong, M.; Wang, Z.V.; Pedrozo, Z.; Cao, D.J.; Troncoso, R.; Ibacache, M.; Criollo, A.; Nemchenko, A.; Hill, J.A.; Lavandero, S. Cardiomyocyte death: Mechanisms and translational implications. Cell Death Dis. 2011, 2, e244. [Google Scholar] [CrossRef]
- Xie, Y.; Hou, W.; Song, X.; Yu, Y.; Huang, J.; Sun, X.; Kang, R.; Tang, D. Ferroptosis: Process and function. Cell Death Differ. 2016, 23, 369–379. [Google Scholar] [CrossRef] [Green Version]
- Vanden Berghe, T.; Linkermann, A.; Jouan-Lanhouet, S.; Walczak, H.; Vandenabeele, P. Regulated necrosis: The expanding network of non-apoptotic cell death pathways. Nat. Rev. Mol. Cell Biol. 2014, 15, 135–147. [Google Scholar] [CrossRef]
- Conrad, M.; Angeli, J.P.; Vandenabeele, P.; Stockwell, B.R. Regulated necrosis: Disease relevance and therapeutic opportunities. Nat. Rev. Drug Discov. 2016, 15, 348–366. [Google Scholar] [CrossRef] [PubMed]
- Durham, K.K.; Chathely, K.M.; Trigatti, B.L. High-density lipoprotein protects cardiomyocytes against necrosis induced by oxygen and glucose deprivation through SR-B1, PI3K, and AKT1 and 2. Biochem. J. 2018, 475, 1253–1265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Murphy, M.P. How mitochondria produce reactive oxygen species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatterjee, S.; Browning, E.A.; Hong, N.; DeBolt, K.; Sorokina, E.M.; Liu, W.; Birnbaum, M.J.; Fisher, A.B. Membrane depolarization is the trigger for PI3K/Akt activation and leads to the generation of ROS. Am. J. Physiol. Heart Circ. Physiol. 2012, 302, H105–H114. [Google Scholar] [CrossRef] [Green Version]
- Festjens, N.; Vanden Berghe, T.; Vandenabeele, P. Necrosis, a well-orchestrated form of cell demise: Signalling cascades, important mediators and concomitant immune response. Biochim. Biophys. Acta 2006, 1757, 1371–1387. [Google Scholar] [CrossRef]
- Bijur, G.N.; Jope, R.S. Rapid accumulation of Akt in mitochondria following phosphatidylinositol 3-kinase activation. J. Neurochem. 2003, 87, 1427–1435. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Vazquez, E.J.; Moghaddas, S.; Hoppel, C.L.; Lesnefsky, E.J. Production of reactive oxygen species by mitochondria: Central role of complex III. J. Biol. Chem. 2003, 278, 36027–36031. [Google Scholar] [CrossRef] [Green Version]
- Kukreja, R.C.; Kontos, H.A.; Hess, M.L.; Ellis, E.F. PGH synthase and lipoxygenase generate superoxide in the presence of NADH or NADPH. Circ. Res. 1986, 59, 612–619. [Google Scholar] [CrossRef] [Green Version]
- Mailloux, R.J.; Gardiner, D.; O’Brien, M. 2-Oxoglutarate dehydrogenase is a more significant source of O2−/H2O2 than pyruvate dehydrogenase in cardiac and liver tissue. Free Radic. Biol. Med. 2016, 97, 501–512. [Google Scholar] [CrossRef]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef]
- Cao, W.; Xie, Y.H.; Li, X.Q.; Zhang, X.K.; Chen, Y.T.; Kang, R.; Chen, X.; Miao, S.; Wang, S.W. Burn-induced apoptosis of cardiomyocytes is survivin dependent and regulated by PI3K/Akt, p38 MAPK and ERK pathways. Basic Res. Cardiol. 2011, 106, 1207–1220. [Google Scholar] [CrossRef] [PubMed]
- Tang, X.L.; Liu, J.X.; Dong, W.; Li, P.; Li, L.; Lin, C.R.; Zheng, Y.Q.; Cong, W.H.; Hou, J.C. Cardioprotective effect of protocatechuic acid on myocardial ischemia/reperfusion injury. J. Pharmacol. Sci. 2014, 125, 176–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duronio, V. The life of a cell: Apoptosis regulation by the PI3K/PKB pathway. Biochem. J. 2008, 415, 333–344. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Manning, B.D.; Cantley, L.C. AKT/PKB signaling: Navigating downstream. Cell 2007, 129, 1261–1274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Q.; Li, X.; Qin, X.; Yu, C.; Jin, Y.; Qian, X. PTEN inhibitor improves vascular remodeling and cardiac function after myocardial infarction through PI3k/Akt/VEGF signaling pathway. Mol. Med. 2020, 26, 111. [Google Scholar] [CrossRef] [PubMed]
- Ruan, Y.; Jin, Q.; Zeng, J.; Ren, F.; Xie, Z.; Ji, K.; Wu, L.; Wu, J.; Li, L. Grape Seed Proanthocyanidin Extract Ameliorates Cardiac Remodelling after Myocardial Infarction Through PI3K/AKT Pathway in Mice. Front. Pharmacol. 2020, 11, 585984. [Google Scholar] [CrossRef]
- Kim, A.H.; Khursigara, G.; Sun, X.; Franke, T.F.; Chao, M.V. Akt phosphorylates and negatively regulates apoptosis signal-regulating kinase 1. Mol. Cell. Biol. 2001, 21, 893–901. [Google Scholar] [CrossRef] [Green Version]
- Takeda, K.; Noguchi, T.; Naguro, I.; Ichijo, H. Apoptosis signal-regulating kinase 1 in stress and immune response. Annu. Rev. Pharmacol. Toxicol. 2008, 48, 199–225. [Google Scholar] [CrossRef]
- Linseman, D.A.; Butts, B.D.; Precht, T.A.; Phelps, R.A.; Le, S.S.; Laessig, T.A.; Bouchard, R.J.; Florez-McClure, M.L.; Heidenreich, K.A. Glycogen synthase kinase-3beta phosphorylates Bax and promotes its mitochondrial localization during neuronal apoptosis. J. Neurosci. 2004, 24, 9993–10002. [Google Scholar] [CrossRef]
- Blume-Jensen, P.; Janknecht, R.; Hunter, T. The Kit receptor promotes cell survival via activation of PI 3-kinase and subsequent Akt-mediated phosphorylation of Bad on Ser136. Curr. Biol. 1998, 8, 779–785. [Google Scholar] [CrossRef]
- Polzien, L.; Baljuls, A.; Rennefahrt, U.E.E.; Fischer, A.; Schmitz, W.; Zahedi, R.P.; Sickmann, A.; Metz, R.; Albert, S.; Benz, R.; et al. Identification of novel in vivo phosphorylation sites of the human proapoptotic protein BAD: Pore-forming activity of BAD is regulated by phosphorylation. J. Biol. Chem. 2009, 284, 28004–28020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Putcha, G.V.; Le, S.; Frank, S.; Besirli, C.G.; Clark, K.; Chu, B.; Alix, S.; Youle, R.J.; LaMarche, A.; Maroney, A.C.; et al. JNK-Mediated BIM Phosphorylation Potentiates BAX-Dependent Apoptosis. Neuron 2003, 38, 899–914. [Google Scholar] [CrossRef] [Green Version]
- Kotrasova, V.; Keresztesova, B.; Ondrovicova, G.; Bauer, J.A.; Havalova, H.; Pevala, V.; Kutejova, E.; Kunova, N. Mitochondrial Kinases and the Role of Mitochondrial Protein Phosphorylation in Health and Disease. Life 2021, 11, 82. [Google Scholar] [CrossRef] [PubMed]
- Bergmann, M.W.; Rechner, C.; Freund, C.; Baurand, A.; El Jamali, A.; Dietz, R. Statins inhibit reoxygenation-induced cardiomyocyte apoptosis: Role for glycogen synthase kinase 3beta and transcription factor beta-catenin. J. Mol. Cell. Cardiol. 2004, 37, 681–690. [Google Scholar] [CrossRef] [PubMed]
- Zhai, P.; Sciarretta, S.; Galeotti, J.; Volpe, M.; Sadoshima, J. Differential roles of GSK-3beta during myocardial ischemia and ischemia/reperfusion. Circ. Res. 2011, 109, 502–511. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Ding, C.; Zhang, J.; Xie, M.; Park, D.; Ding, Y.; Chen, G.; Zhang, G.; Gilbert-Ross, M.; Zhou, W.; et al. Modulation of Bax and mTOR for Cancer Therapeutics. Cancer Res. 2017, 77, 3001–3012. [Google Scholar] [CrossRef] [Green Version]
- Kale, J.; Kutuk, O.; Brito, G.C.; Andrews, T.S.; Leber, B.; Letai, A.; Andrews, D.W. Phosphorylation switches Bax from promoting to inhibiting apoptosis thereby increasing drug resistance. EMBO Rep. 2018, 19, e45235. [Google Scholar] [CrossRef]
- Nagaoka, K.; Matoba, T.; Mao, Y.; Nakano, Y.; Ikeda, G.; Egusa, S.; Tokutome, M.; Nagahama, R.; Nakano, K.; Sunagawa, K.; et al. A New Therapeutic Modality for Acute Myocardial Infarction: Nanoparticle-Mediated Delivery of Pitavastatin Induces Cardioprotection from Ischemia-Reperfusion Injury via Activation of PI3K/Akt Pathway and Anti-Inflammation in a Rat Model. PLoS ONE 2015, 10, e0132451. [Google Scholar] [CrossRef]
- Pan, Q.; Xie, X.; Guo, Y.; Wang, H. Simvastatin promotes cardiac microvascular endothelial cells proliferation, migration and survival by phosphorylation of p70 S6K and FoxO3a. Cell Biol. Int. 2014, 38, 599–609. [Google Scholar] [CrossRef]
- Zhan, L.; Wang, X.; Zhang, Y.; Zhu, G.; Ding, Y.; Chen, X.; Jiang, W.; Wu, S. Benazepril hydrochloride protects against doxorubicin cardiotoxicity by regulating the PI3K/Akt pathway. Exp. Ther. Med. 2021, 22, 1082. [Google Scholar] [CrossRef]
- Mahajan, U.B.; Patil, P.D.; Chandrayan, G.; Patil, C.R.; Agrawal, Y.O.; Ojha, S.; Goyal, S.N. Eplerenone pretreatment protects the myocardium against ischaemia/reperfusion injury through the phosphatidylinositol 3-kinase/Akt-dependent pathway in diabetic rats. Mol. Cell. Biochem. 2018, 446, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, J.; Xie, L.; Cai, X.; Ma, X.; Gong, J. Bisoprolol, a β(1) antagonist, protects myocardial cells from ischemia-reperfusion injury via PI3K/AKT/GSK3β pathway. Fundam. Clin. Pharmacol. 2020, 34, 708–720. [Google Scholar] [CrossRef] [PubMed]
- Baraka, S.A.; Tolba, M.F.; Elsherbini, D.A.; El-Naga, R.N.; Awad, A.S.; El-Demerdash, E. Rosuvastatin and low-dose carvedilol combination protects against isoprenaline-induced myocardial infarction in rats: Role of PI3K/Akt/Nrf2/HO-1 signalling. Clin. Exp. Pharmacol. Physiol. 2021, 48, 1358–1370. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.X.; Zhang, Y.; Wang, Y.F.; Li, X.C.; Xiang, M.X.; Bian, C.; Chen, P. Upregulation of heme oxygenase-1 expression by hydroxysafflor yellow A conferring protection from anoxia/reoxygenation-induced apoptosis in H9c2 cardiomyocytes. Int. J. Cardiol. 2012, 160, 95–101. [Google Scholar] [CrossRef]
- Liu, Y.N.; Zhou, Z.M.; Chen, P. Evidence that hydroxysafflor yellow A protects the heart against ischaemia-reperfusion injury by inhibiting mitochondrial permeability transition pore opening. Clin. Exp. Pharmacol. Physiol. 2008, 35, 211–216. [Google Scholar] [CrossRef]
- Ji, D.B.; Zhu, M.C.; Zhu, B.; Zhu, Y.Z.; Li, C.L.; Ye, J.; Zhu, H.B. Hydroxysafflor yellow A enhances survival of vascular endothelial cells under hypoxia via upregulation of the HIF-1 alpha-VEGF pathway and regulation of Bcl-2/Bax. J. Cardiovasc. Pharmacol. 2008, 52, 191–202. [Google Scholar] [CrossRef]
- Wang, J.; Chen, H.; Zhou, Y.; Su, Q.; Liu, T.; Wang, X.T.; Li, L. Atorvastatin Inhibits Myocardial Apoptosis in a Swine Model of Coronary Microembolization by Regulating PTEN/PI3K/Akt Signaling Pathway. Cell. Physiol. Biochem. 2016, 38, 207–219. [Google Scholar] [CrossRef]
- Galluzzi, L.; Vanden Berghe, T.; Vanlangenakker, N.; Buettner, S.; Eisenberg, T.; Vandenabeele, P.; Madeo, F.; Kroemer, G. Programmed necrosis from molecules to health and disease. Int. Rev. Cell Mol. Biol. 2011, 289, 1–35. [Google Scholar] [CrossRef]
- Piamsiri, C.; Maneechote, C.; Siri-Angkul, N.; Chattipakorn, S.C.; Chattipakorn, N. Targeting necroptosis as therapeutic potential in chronic myocardial infarction. J. Biomed. Sci. 2021, 28, 25. [Google Scholar] [CrossRef]
- Heusch, G. Myocardial ischaemia-reperfusion injury and cardioprotection in perspective. Nat. Rev. Cardiol. 2020, 17, 773–789. [Google Scholar] [CrossRef]
- Van Herreweghe, F.; Festjens, N.; Declercq, W.; Vandenabeele, P. Tumor necrosis factor-mediated cell death: To break or to burst, that’s the question. Cell. Mol. Life Sci. 2010, 67, 1567–1579. [Google Scholar] [CrossRef] [PubMed]
- Zhang, D.W.; Zheng, M.; Zhao, J.; Li, Y.Y.; Huang, Z.; Li, Z.; Han, J. Multiple death pathways in TNF-treated fibroblasts: RIP3- and RIP1-dependent and independent routes. Cell Res. 2011, 21, 368–371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cai, Z.; Jitkaew, S.; Zhao, J.; Chiang, H.C.; Choksi, S.; Liu, J.; Ward, Y.; Wu, L.G.; Liu, Z.G. Plasma membrane translocation of trimerized MLKL protein is required for TNF-induced necroptosis. Nat. Cell. Biol. 2014, 16, 55–65. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, H.; Wang, Z.; He, S.; Chen, S.; Liao, D.; Wang, L.; Yan, J.; Liu, W.; Lei, X.; et al. Mixed lineage kinase domain-like protein mediates necrosis signaling downstream of RIP3 kinase. Cell 2012, 148, 213–227. [Google Scholar] [CrossRef] [Green Version]
- Hu, S.; Chang, X.; Zhu, H.; Wang, D.; Chen, G. PI3K mediates tumor necrosis factor induced-necroptosis through initiating RIP1-RIP3-MLKL signaling pathway activation. Cytokine 2020, 129, 155046. [Google Scholar] [CrossRef]
- Tuuminen, R.; Holmstrom, E.; Raissadati, A.; Saharinen, P.; Rouvinen, E.; Krebs, R.; Lemstrom, K.B. Simvastatin pretreatment reduces caspase-9 and RIPK1 protein activity in rat cardiac allograft ischemia-reperfusion. Transpl. Immunol. 2016, 37, 40–45. [Google Scholar] [CrossRef] [Green Version]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef] [Green Version]
- Hu, H.; Chen, Y.; Jing, L.; Zhai, C.; Shen, L. The Link Between Ferroptosis and Cardiovascular Diseases: A Novel Target for Treatment. Front. Cardiovasc. Med. 2021, 8, 710963. [Google Scholar] [CrossRef]
- Wu, X.; Li, Y.; Zhang, S.; Zhou, X. Ferroptosis as a novel therapeutic target for cardiovascular disease. Theranostics 2021, 11, 3052–3059. [Google Scholar] [CrossRef]
- Zhao, W.K.; Zhou, Y.; Xu, T.T.; Wu, Q. Ferroptosis: Opportunities and Challenges in Myocardial Ischemia-Reperfusion Injury. Oxid Med. Cell Longev. 2021, 2021, 9929687. [Google Scholar] [CrossRef]
- Zhao, S.; Li, P.; Wu, W.; Wang, Q.; Qian, B.; Li, X.; Shen, M. Roles of ferroptosis in urologic malignancies. Cancer Cell Int. 2021, 21, 676. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wang, H.; Xu, D.; Yu, S.; Zhang, L.; Li, X. Lapatinib induces mitochondrial dysfunction to enhance oxidative stress and ferroptosis in doxorubicin-induced cardiomyocytes via inhibition of PI3K/AKT signaling pathway. Bioengineered 2022, 13, 48–60. [Google Scholar] [CrossRef] [PubMed]
- D’Amato, V.; Raimondo, L.; Formisano, L.; Giuliano, M.; De Placido, S.; Rosa, R.; Bianco, R. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer Treat. Rev. 2015, 41, 877–883. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Li, F.; Du, C.; Wang, H.; Mahato, R.I.; Huang, Y. Doxorubicin and lapatinib combination nanomedicine for treating resistant breast cancer. Mol. Pharm. 2014, 11, 2600–2611. [Google Scholar] [CrossRef]
- Mei, S.; Hong, L.; Cai, X.; Xiao, B.; Zhang, P.; Shao, L. Oxidative stress injury in doxorubicin-induced cardiotoxicity. Toxicol. Lett. 2019, 307, 41–48. [Google Scholar] [CrossRef]
- Segredo, M.P.; Salvadori, D.M.; Rocha, N.S.; Moretto, F.C.; Correa, C.R.; Camargo, E.A.; de Almeida, D.C.; Reis, R.A.; Freire, C.M.; Braz, M.G.; et al. Oxidative stress on cardiotoxicity after treatment with single and multiple doses of doxorubicin. Hum. Exp. Toxicol. 2014, 33, 748–760. [Google Scholar] [CrossRef]
- Yi, J.; Zhu, J.; Wu, J.; Thompson, C.B.; Jiang, X. Oncogenic activation of PI3K-AKT-mTOR signaling suppresses ferroptosis via SREBP-mediated lipogenesis. Proc. Natl. Acad. Sci. USA 2020, 117, 31189–31197. [Google Scholar] [CrossRef]
- Li, G.; Yang, J.; Zhao, G.; Shen, Z.; Yang, K.; Tian, L.; Zhou, Q.; Chen, Y.; Huang, Y. Dysregulation of ferroptosis may involve in the development of non-small-cell lung cancer in Xuanwei area. J. Cell. Mol. Med. 2021, 25, 2872–2884. [Google Scholar] [CrossRef]
- Jiang, Y.; Chen, L.; Chao, Z.; Chen, T.; Zhou, Y. Ferroptosis Related Genes in Ischemic and Idiopathic Cardiomyopathy: Screening for Potential Pharmacological Targets. Front. Cell Dev. Biol. 2022, 10, 817819. [Google Scholar] [CrossRef]
- Yu, P.; Zhang, X.; Liu, N.; Tang, L.; Peng, C.; Chen, X. Pyroptosis: Mechanisms and diseases. Signal Transduct. Target. Ther. 2021, 6, 128. [Google Scholar] [CrossRef]
- Guo, X.; Hu, S.; Liu, J.J.; Huang, L.; Zhong, P.; Fan, Z.X.; Ye, P.; Chen, M.H. Piperine protects against pyroptosis in myocardial ischaemia/reperfusion injury by regulating the miR-383/RP105/AKT signalling pathway. J. Cell. Mol. Med. 2021, 25, 244–258. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Shen, J.; Li, Y.; Wu, J.; Luo, X.; Yu, Y.; Zhang, Y.; Gu, L.; Zhang, X.; Jiang, C.; et al. Pyroptosis inhibition improves the symptom of acute myocardial infarction. Cell Death Dis. 2021, 12, 852. [Google Scholar] [CrossRef] [PubMed]
- Do Carmo, H.; Arjun, S.; Petrucci, O.; Yellon, D.M.; Davidson, S.M. The Caspase 1 Inhibitor VX-765 Protects the Isolated Rat Heart via the RISK Pathway. Cardiovasc. Drugs Ther. 2018, 32, 165–168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reed, G.W.; Rossi, J.E.; Cannon, C.P. Acute myocardial infarction. Lancet 2017, 389, 197–210. [Google Scholar] [CrossRef]
- Guo, X.; Jiang, H.; Chen, J. RP105-PI3K-Akt axis: A potential therapeutic approach for ameliorating myocardial ischemia/reperfusion injury. Int. J. Cardiol. 2016, 206, 95–96. [Google Scholar] [CrossRef]
- Qin, Q.; Cui, L.; Zhou, Z.; Zhang, Z.; Wang, Y.; Zhou, C. Inhibition of microRNA-141-3p Reduces Hypoxia-Induced Apoptosis in H9c2 Rat Cardiomyocytes by Activating the RP105-Dependent PI3K/AKT Signaling Pathway. Med. Sci. Monit. 2019, 25, 7016–7025. [Google Scholar] [CrossRef]
- Guo, X.; Li, X.-Y.; Hu, S.; Wu, G.; Chen, Z.; Liu, J.-J.; Ye, P.; Chen, M.-H. SH2B1 protects cardiomyocytes from ischemia/reperfusion injury via the activation of the PI3K/AKT pathway. Int. Immunopharmacol. 2020, 83, 105910. [Google Scholar] [CrossRef]
- Yellon, D.M.; Downey, J.M. Preconditioning the myocardium: From cellular physiology to clinical cardiology. Physiol. Rev. 2003, 83, 1113–1151. [Google Scholar] [CrossRef]
- Rezkalla, S.H.; Kloner, R.A. Ischemic preconditioning and preinfarction angina in the clinical arena. Nat. Clin. Pract. Cardiovasc. Med. 2004, 1, 96–102. [Google Scholar] [CrossRef]
- Hausenloy, D.J.; Yellon, D.M. Preconditioning and postconditioning: United at reperfusion. Pharmacol. Ther. 2007, 116, 173–191. [Google Scholar] [CrossRef]
- Su, F.; Zhao, L.; Zhang, S.; Wang, J.; Chen, N.; Gong, Q.; Tang, J.; Wang, H.; Yao, J.; Wang, Q.; et al. Cardioprotection by PI3K-mediated signaling is required for anti-arrhythmia and myocardial repair in response to ischemic preconditioning in infarcted pig hearts. Lab. Investig. 2015, 95, 860–871. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rossello, X.; Riquelme, J.A.; Davidson, S.M.; Yellon, D.M. Role of PI3K in myocardial ischaemic preconditioning: Mapping pro-survival cascades at the trigger phase and at reperfusion. J. Cell. Mol. Med. 2017, 22, 926–935. [Google Scholar] [CrossRef] [Green Version]
- Cai, Z.P.; Parajuli, N.; Zheng, X.; Becker, L. Remote ischemic preconditioning confers late protection against myocardial ischemia-reperfusion injury in mice by upregulating interleukin-10. Basic Res. Cardiol. 2012, 107, 277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, X.; Cohen, M.V.; Downey, J.M. Mechanism of cardioprotection by early ischemic preconditioning. Cardiovasc. Drugs Ther. 2010, 24, 225–234. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barsukevich, V.; Basalay, M.; Sanchez, J.; Mrochek, A.; Whittle, J.; Ackland, G.L.; Gourine, A.V.; Gourine, A. Distinct cardioprotective mechanisms of immediate, early and delayed ischaemic postconditioning. Basic Res. Cardiol. 2015, 110, 452. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Wang, J.; Tu, T.; Iyan, Z.; Mungun, D.; Yang, Z.; Guo, Y. Remote Ischemic Postconditioning Protects against Myocardial Ischemia-Reperfusion Injury by Inhibition of the RAGE-HMGB1 Pathway. Biomed. Res. Int. 2018, 2018, 4565630. [Google Scholar] [CrossRef] [PubMed]
- Li, C.M.; Shen, S.W.; Wang, T.; Zhang, X.H. Myocardial ischemic post-conditioning attenuates ischemia reperfusion injury via PTEN/Akt signal pathway. Int. J. Clin. Exp. Med. 2015, 8, 15801–15807. [Google Scholar]
- Milano, G.; Abruzzo, P.M.; Bolotta, A.; Marini, M.; Terraneo, L.; Ravara, B.; Gorza, L.; Vitadello, M.; Burattini, S.; Curzi, D.; et al. Impact of the phosphatidylinositide 3-kinase signaling pathway on the cardioprotection induced by intermittent hypoxia. PLoS ONE 2013, 8, e76659. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhang, X. Ischaemic preconditioning-induced serum exosomes protect against myocardial ischaemia/reperfusion injury in rats by activating the PI3K/AKT signalling pathway. Cell Biochem. Funct. 2020, 39, 287–295. [Google Scholar] [CrossRef]
- Lassen, T.R.; Just, J.; Hjortbak, M.V.; Jespersen, N.R.; Stenz, K.T.; Gu, T.; Yan, Y.; Su, J.; Hansen, J.; Bæk, R.; et al. Cardioprotection by remote ischemic conditioning is transferable by plasma and mediated by extracellular vesicles. Basic Res. Cardiol. 2021, 116, 16. [Google Scholar] [CrossRef]
- Swiatkiewicz, I.; Kozinski, M.; Magielski, P.; Fabiszak, T.; Sukiennik, A.; Navarese, E.P.; Odrowaz-Sypniewska, G.; Kubica, J. Value of C-reactive protein in predicting left ventricular remodelling in patients with a first ST-segment elevation myocardial infarction. Mediat. Inflamm. 2012, 2012, 250867. [Google Scholar] [CrossRef] [PubMed]
- Bolognese, L.; Neskovic, A.N.; Parodi, G.; Cerisano, G.; Buonamici, P.; Santoro, G.M.; Antoniucci, D. Left ventricular remodeling after primary coronary angioplasty: Patterns of left ventricular dilation and long-term prognostic implications. Circulation 2002, 106, 2351–2357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, A.S.; Ambrosy, A.P.; Velazquez, E.J. Adverse Remodeling and Reverse Remodeling After Myocardial Infarction. Curr. Cardiol. Rep. 2017, 19, 71. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Braunwald, E. Ventricular remodeling after myocardial infarction. Experimental observations and clinical implications. Circulation 1990, 81, 1161–1172. [Google Scholar] [CrossRef] [Green Version]
- Carrick, D.; Haig, C.; Rauhalammi, S.; Ahmed, N.; Mordi, I.; McEntegart, M.; Petrie, M.C.; Eteiba, H.; Lindsay, M.; Watkins, S.; et al. Pathophysiology of LV Remodeling in Survivors of STEMI: Inflammation, Remote Myocardium, and Prognosis. JACC Cardiovasc. Imaging 2015, 8, 779–789. [Google Scholar] [CrossRef] [Green Version]
- Dutka, M.; Bobinski, R.; Korbecki, J. The relevance of microRNA in post-infarction left ventricular remodelling and heart failure. Heart Fail. Rev. 2019, 24, 575–586. [Google Scholar] [CrossRef] [Green Version]
- Stone, G.W.; Selker, H.P.; Thiele, H.; Patel, M.R.; Udelson, J.E.; Ohman, E.M.; Maehara, A.; Eitel, I.; Granger, C.B.; Jenkins, P.L.; et al. Relationship Between Infarct Size and Outcomes Following Primary PCI: Patient-Level Analysis From 10 Randomized Trials. J. Am. Coll. Cardiol. 2016, 67, 1674–1683. [Google Scholar] [CrossRef]
- Thomas, T.P.; Grisanti, L.A. The Dynamic Interplay Between Cardiac Inflammation and Fibrosis. Front. Physiol. 2020, 11, 529075. [Google Scholar] [CrossRef]
- Savoye, C.; Equine, O.; Tricot, O.; Nugue, O.; Segrestin, B.; Sautiere, K.; Elkohen, M.; Pretorian, E.M.; Taghipour, K.; Philias, A.; et al. Left ventricular remodeling after anterior wall acute myocardial infarction in modern clinical practice (from the REmodelage VEntriculaire [REVE] study group). Am. J. Cardiol. 2006, 98, 1144–1149. [Google Scholar] [CrossRef]
- Funaro, S.; La Torre, G.; Madonna, M.; Galiuto, L.; Scara, A.; Labbadia, A.; Canali, E.; Mattatelli, A.; Fedele, F.; Alessandrini, F.; et al. Incidence, determinants, and prognostic value of reverse left ventricular remodelling after primary percutaneous coronary intervention: Results of the Acute Myocardial Infarction Contrast Imaging (AMICI) multicenter study. Eur. Heart J. 2009, 30, 566–575. [Google Scholar] [CrossRef] [Green Version]
- Meng, H.; Zhang, Y.; An, S.T.; Chen, Y. Annexin A3 gene silencing promotes myocardial cell repair through activation of the PI3K/Akt signaling pathway in rats with acute myocardial infarction. J. Cell. Physiol. 2019, 234, 10535–10546. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhabyeyev, P.; Azad, A.K.; Vanhaesebroeck, B.; Grueter, C.E.; Murray, A.G.; Kassiri, Z.; Oudit, G.Y. Pharmacological and cell-specific genetic PI3Kalpha inhibition worsens cardiac remodeling after myocardial infarction. J. Mol. Cell. Cardiol. 2021, 157, 17–30. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Regulation of the inflammatory response in cardiac repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Nian, M.; Lee, P.; Khaper, N.; Liu, P. Inflammatory cytokines and postmyocardial infarction remodeling. Circ. Res. 2004, 94, 1543–1553. [Google Scholar] [CrossRef]
- Takahashi, T.; Anzai, T.; Kaneko, H.; Mano, Y.; Anzai, A.; Nagai, T.; Kohno, T.; Maekawa, Y.; Yoshikawa, T.; Fukuda, K.; et al. Increased C-reactive protein expression exacerbates left ventricular dysfunction and remodeling after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, H1795–H1804. [Google Scholar] [CrossRef] [Green Version]
- Suleiman, M.; Khatib, R.; Agmon, Y.; Mahamid, R.; Boulos, M.; Kapeliovich, M.; Levy, Y.; Beyar, R.; Markiewicz, W.; Hammerman, H.; et al. Early inflammation and risk of long-term development of heart failure and mortality in survivors of acute myocardial infarction predictive role of C-reactive protein. J. Am. Coll. Cardiol. 2006, 47, 962–968. [Google Scholar] [CrossRef]
- Swiatkiewicz, I.; Magielski, P.; Kubica, J.; Zadourian, A.; DeMaria, A.N.; Taub, P.R. Enhanced Inflammation is a Marker for Risk of Post-Infarct Ventricular Dysfunction and Heart Failure. Int. J. Mol. Sci. 2020, 21, 807. [Google Scholar] [CrossRef] [Green Version]
- Swiatkiewicz, I.; Magielski, P.; Kubica, J. C-Reactive Protein as a Risk Marker for Post-Infarct Heart Failure over a Multi-Year Period. Int. J. Mol. Sci. 2021, 22, 3169. [Google Scholar] [CrossRef]
- Westman, P.C.; Lipinski, M.J.; Luger, D.; Waksman, R.; Bonow, R.O.; Wu, E.; Epstein, S.E. Inflammation as a Driver of Adverse Left Ventricular Remodeling After Acute Myocardial Infarction. J. Am. Coll. Cardiol. 2016, 67, 2050–2060. [Google Scholar] [CrossRef]
- Sproston, N.R.; Ashworth, J.J. Role of C-Reactive Protein at Sites of Inflammation and Infection. Front. Immunol. 2018, 9, 754. [Google Scholar] [CrossRef]
- Fu, Y.; Wu, Y.; Liu, E. C-reactive protein and cardiovascular disease: From animal studies to the clinic (Review). Exp. Ther. Med. 2020, 20, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Bisoendial, R.J.; Boekholdt, S.M.; Vergeer, M.; Stroes, E.S.; Kastelein, J.J. C-reactive protein is a mediator of cardiovascular disease. Eur. Heart J. 2010, 31, 2087–2091. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.W.; Lee, K.H.; Kim, S.H.; Jin, T.; Lee, B.S.; Oh, J.; Won, H.Y.; Kim, S.Y.; Kang, S.M.; Chung, J.H. C-reactive protein induces p53-mediated cell cycle arrest in H9c2 cardiac myocytes. Biochem. Biophys. Res. Commun. 2011, 410, 525–530. [Google Scholar] [CrossRef] [PubMed]
- Orn, S.; Manhenke, C.; Ueland, T.; Damas, J.K.; Mollnes, T.E.; Edvardsen, T.; Aukrust, P.; Dickstein, K. C-reactive protein, infarct size, microvascular obstruction, and left-ventricular remodelling following acute myocardial infarction. Eur. Heart J. 2009, 30, 1180–1186. [Google Scholar] [CrossRef] [Green Version]
- Mather, A.N.; Fairbairn, T.A.; Artis, N.J.; Greenwood, J.P.; Plein, S. Relationship of cardiac biomarkers and reversible and irreversible myocardial injury following acute myocardial infarction as determined by cardiovascular magnetic resonance. Int. J. Cardiol. 2013, 166, 458–464. [Google Scholar] [CrossRef]
- Fertin, M.; Hennache, B.; Hamon, M.; Ennezat, P.V.; Biausque, F.; Elkohen, M.; Nugue, O.; Tricot, O.; Lamblin, N.; Pinet, F.; et al. Usefulness of serial assessment of B-type natriuretic peptide, troponin I, and C-reactive protein to predict left ventricular remodeling after acute myocardial infarction (from the REVE-2 study). Am. J. Cardiol. 2010, 106, 1410–1416. [Google Scholar] [CrossRef]
- Pepys, M.B.; Hirschfield, G.M.; Tennent, G.A.; Gallimore, J.R.; Kahan, M.C.; Bellotti, V.; Hawkins, P.N.; Myers, R.M.; Smith, M.D.; Polara, A.; et al. Targeting C-reactive protein for the treatment of cardiovascular disease. Nature 2006, 440, 1217–1221. [Google Scholar] [CrossRef] [Green Version]
- Abbate, A.; Biondi-Zoccai, G.G.L.; Bussani, R.; Dobrina, A.; Camilot, D.; Feroce, F.; Rossiello, R.; Baldi, F.; Silvestri, F.; Biasucci, L.M.; et al. Increased myocardial apoptosis in patients with unfavorable left ventricular remodeling and early symptomatic post-infarction heart failure. J. Am. Coll. Cardiol. 2003, 41, 753–760. [Google Scholar] [CrossRef] [Green Version]
- Boras, E.; Slevin, M.; Alexander, M.Y.; Aljohi, A.; Gilmore, W.; Ashworth, J.; Krupinski, J.; Potempa, L.A.; Al Abdulkareem, I.; Elobeid, A.; et al. Monomeric C-reactive protein and Notch-3 co-operatively increase angiogenesis through PI3K signalling pathway. Cytokine 2014, 69, 165–179. [Google Scholar] [CrossRef]
- Chen, J.; Gu, Z.; Wu, M.; Yang, Y.; Zhang, J.; Ou, J.; Zuo, Z.; Wang, J.; Chen, Y. C-reactive protein can upregulate VEGF expression to promote ADSC-induced angiogenesis by activating HIF-1alpha via CD64/PI3k/Akt and MAPK/ERK signaling pathways. Stem Cell Res. Ther. 2016, 7, 114. [Google Scholar] [CrossRef] [Green Version]
- Tanigaki, K.; Mineo, C.; Yuhanna, I.S.; Chambliss, K.L.; Quon, M.J.; Bonvini, E.; Shaul, P.W. C-reactive protein inhibits insulin activation of endothelial nitric oxide synthase via the immunoreceptor tyrosine-based inhibition motif of FcgammaRIIB and SHIP-1. Circ. Res. 2009, 104, 1275–1282. [Google Scholar] [CrossRef] [PubMed]
- Muniyappa, R.; Montagnani, M.; Koh, K.K.; Quon, M.J. Cardiovascular actions of insulin. Endocr. Rev. 2007, 28, 463–491. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.S.; Kim, S.H.; Oh, J.; Jin, T.; Choi, E.Y.; Park, S.; Lee, S.H.; Chung, J.H.; Kang, S.M. C-reactive protein inhibits survivin expression via Akt/mTOR pathway downregulation by PTEN expression in cardiac myocytes. PLoS ONE 2014, 9, e98113. [Google Scholar] [CrossRef] [PubMed]
- Parajuli, N.; Yuan, Y.; Zheng, X.; Bedja, D.; Cai, Z.P. Phosphatase PTEN is critically involved in post-myocardial infarction remodeling through the Akt/interleukin-10 signaling pathway. Basic Res. Cardiol. 2012, 107, 248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Chen, Z.; Zou, Y.; Ge, J. Atorvastatin represses the angiotensin 2-induced oxidative stress and inflammatory response in dendritic cells via the PI3K/Akt/Nrf 2 pathway. Oxid. Med. Cell. Longev. 2014, 2014, 148798. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, S.; Xue, J.; Wei, Z.; Ao, P.; Shen, B.; Ding, L. CGRP Reduces Apoptosis of DRG Cells Induced by High-Glucose Oxidative Stress Injury through PI3K/AKT Induction of Heme Oxygenase-1 and Nrf-2 Expression. Oxid. Med. Cell. Longev. 2019, 2019, 2053149. [Google Scholar] [CrossRef] [PubMed]
- Zhao, S.M.; Gao, H.L.; Wang, Y.L.; Xu, Q.; Guo, C.Y. Attenuation of High Glucose-Induced Rat Cardiomyocyte Apoptosis by Exendin-4 via Intervention of HO-1/Nrf-2 and the PI3K/AKT Signaling Pathway. Chin. J. Physiol. 2017, 60, 89–96. [Google Scholar] [CrossRef] [Green Version]
- Xiao, Z.; Peng, J.; Gan, N.; Arafat, A.; Yin, F. Interleukin-1beta Plays a Pivotal Role via the PI3K/Akt/mTOR Signaling Pathway in the Chronicity of Mesial Temporal Lobe Epilepsy. Neuroimmunomodulation 2016, 23, 332–344. [Google Scholar] [CrossRef]
- Li, B.; Smith, T.J. PI3K/AKT pathway mediates induction of IL-1RA by TSH in fibrocytes: Modulation by PTEN. J. Clin. Endocrinol. Metab. 2014, 99, 3363–3372. [Google Scholar] [CrossRef] [Green Version]
- Teshima, S.; Nakanishi, H.; Nishizawa, M.; Kitagawa, K.; Kaibori, M.; Yamada, M.; Habara, K.; Kwon, A.H.; Kamiyama, Y.; Ito, S.; et al. Up-regulation of IL-1 receptor through PI3K/Akt is essential for the induction of iNOS gene expression in hepatocytes. J. Hepatol. 2004, 40, 616–623. [Google Scholar] [CrossRef]
- Abbate, A.; Toldo, S.; Marchetti, C.; Kron, J.; Van Tassell, B.W.; Dinarello, C.A. Interleukin-1 and the Inflammasome as Therapeutic Targets in Cardiovascular Disease. Circ. Res. 2020, 126, 1260–1280. [Google Scholar] [CrossRef] [PubMed]
- Abbate, A.; Van Tassell, B.W.; Biondi-Zoccai, G.; Kontos, M.C.; Grizzard, J.D.; Spillman, D.W.; Oddi, C.; Roberts, C.S.; Melchior, R.D.; Mueller, G.H.; et al. Effects of interleukin-1 blockade with anakinra on adverse cardiac remodeling and heart failure after acute myocardial infarction [from the Virginia Commonwealth University-Anakinra Remodeling Trial (2) (VCU-ART2) pilot study]. Am. J. Cardiol. 2013, 111, 1394–1400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Everett, B.M.; Cornel, J.H.; Lainscak, M.; Anker, S.D.; Abbate, A.; Thuren, T.; Libby, P.; Glynn, R.J.; Ridker, P.M. Anti-Inflammatory Therapy With Canakinumab for the Prevention of Hospitalization for Heart Failure. Circulation 2019, 139, 1289–1299. [Google Scholar] [CrossRef]
- Choi, H.W.; Shin, P.G.; Lee, J.H.; Choi, W.S.; Kang, M.J.; Kong, W.S.; Oh, M.J.; Seo, Y.B.; Kim, G.D. Anti-inflammatory effect of lovastatin is mediated via the modulation of NF-kappaB and inhibition of HDAC1 and the PI3K/Akt/mTOR pathway in RAW264.7 macrophages. Int. J. Mol. Med. 2018, 41, 1103–1109. [Google Scholar] [CrossRef]
- Xie, S.; Chen, M.; Yan, B.; He, X.; Chen, X.; Li, D. Identification of a role for the PI3K/AKT/mTOR signaling pathway in innate immune cells. PLoS ONE 2014, 9, e94496. [Google Scholar] [CrossRef] [PubMed]
- Chartoumpekis, D.; Ziros, P.G.; Psyrogiannis, A.; Kyriazopoulou, V.; Papavassiliou, A.G.; Habeos, I.G. Simvastatin lowers reactive oxygen species level by Nrf2 activation via PI3K/Akt pathway. Biochem. Biophys. Res. Commun. 2010, 396, 463–466. [Google Scholar] [CrossRef] [PubMed]
- Mizushima, N.; Komatsu, M. Autophagy: Renovation of cells and tissues. Cell 2011, 147, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Choi, A.M.; Ryter, S.W.; Levine, B. Autophagy in human health and disease. N. Engl. J. Med. 2013, 368, 651–662. [Google Scholar] [CrossRef]
- Levine, B.; Kroemer, G. Autophagy in the pathogenesis of disease. Cell 2008, 132, 27–42. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, S. Choose Delicately and Reuse Adequately: The Newly Revealed Process of Autophagy. Biol. Pharm. Bull. 2015, 38, 1098–1103. [Google Scholar] [CrossRef] [Green Version]
- Marambio, P.; Toro, B.; Sanhueza, C.; Troncoso, R.; Parra, V.; Verdejo, H.; Garcia, L.; Quiroga, C.; Munafo, D.; Diaz-Elizondo, J.; et al. Glucose deprivation causes oxidative stress and stimulates aggresome formation and autophagy in cultured cardiac myocytes. Biochim. Biophys. Acta 2010, 1802, 509–518. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, L.; Vatner, D.E.; Kim, S.J.; Ge, H.; Masurekar, M.; Massover, W.H.; Yang, G.; Matsui, Y.; Sadoshima, J.; Vatner, S.F. Autophagy in chronically ischemic myocardium. Proc. Natl. Acad. Sci. USA 2005, 102, 13807–13812. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sciarretta, S.; Maejima, Y.; Zablocki, D.; Sadoshima, J. The Role of Autophagy in the Heart. Annu. Rev. Physiol. 2018, 80, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Sciarretta, S.; Zhai, P.; Shao, D.; Maejima, Y.; Robbins, J.; Volpe, M.; Condorelli, G.; Sadoshima, J. Rheb is a critical regulator of autophagy during myocardial ischemia: Pathophysiological implications in obesity and metabolic syndrome. Circulation 2012, 125, 1134–1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matsui, Y.; Takagi, H.; Qu, X.; Abdellatif, M.; Sakoda, H.; Asano, T.; Levine, B.; Sadoshima, J. Distinct roles of autophagy in the heart during ischemia and reperfusion: Roles of AMP-activated protein kinase and Beclin 1 in mediating autophagy. Circ. Res. 2007, 100, 914–922. [Google Scholar] [CrossRef]
- Kim, J.; Kundu, M.; Viollet, B.; Guan, K.L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nat. Cell Biol. 2011, 13, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Martina, J.A.; Chen, Y.; Gucek, M.; Puertollano, R. MTORC1 functions as a transcriptional regulator of autophagy by preventing nuclear transport of TFEB. Autophagy 2012, 8, 903–914. [Google Scholar] [CrossRef] [Green Version]
- Sciarretta, S.; Volpe, M.; Sadoshima, J. Mammalian target of rapamycin signaling in cardiac physiology and disease. Circ. Res. 2014, 114, 549–564. [Google Scholar] [CrossRef] [Green Version]
- Mendoza, M.C.; Er, E.E.; Blenis, J. The Ras-ERK and PI3K-mTOR pathways: Cross-talk and compensation. Trends Biochem. Sci. 2011, 36, 320–328. [Google Scholar] [CrossRef] [Green Version]
- Kwak, D.; Choi, S.; Jeong, H.; Jang, J.H.; Lee, Y.; Jeon, H.; Lee, M.N.; Noh, J.; Cho, K.; Yoo, J.S.; et al. Osmotic stress regulates mammalian target of rapamycin (mTOR) complex 1 via c-Jun N-terminal Kinase (JNK)-mediated Raptor protein phosphorylation. J. Biol. Chem. 2012, 287, 18398–18407. [Google Scholar] [CrossRef] [Green Version]
- Majid, S.; Saini, S.; Dahiya, R. Wnt signaling pathways in urological cancers: Past decades and still growing. Mol. Cancer 2012, 11, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, Y.; Kang, R.; Tang, D. Role of the Beclin 1 Network in the Cross-Regulation Between Autophagy and Apoptosis. In Autophagy: Cancer, Other Pathologies, Inflammation, Immunity, Infection, and Aging; Academic Press: Cambridge, MA, USA, 2016; pp. 75–88. [Google Scholar]
- Ma, X.; Liu, H.; Foyil, S.R.; Godar, R.J.; Weinheimer, C.J.; Hill, J.A.; Diwan, A. Impaired autophagosome clearance contributes to cardiomyocyte death in ischemia/reperfusion injury. Circulation 2012, 125, 3170–3181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhattacharya, D.; Mukhopadhyay, M.; Bhattacharyya, M.; Karmakar, P. Is autophagy associated with diabetes mellitus and its complications? A review. EXCLI J. 2018, 17, 709–720. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Hua, Y.; Li, X.; Arslan, I.M.; Zhang, W.; Meng, G. Distinct Types of Cell Death and the Implication in Diabetic Cardiomyopathy. Front. Pharmacol. 2020, 11, 42. [Google Scholar] [CrossRef]
- Buss, S.J.; Muenz, S.; Riffel, J.H.; Malekar, P.; Hagenmueller, M.; Weiss, C.S.; Bea, F.; Bekeredjian, R.; Schinke-Braun, M.; Izumo, S.; et al. Beneficial effects of Mammalian target of rapamycin inhibition on left ventricular remodeling after myocardial infarction. J. Am. Coll. Cardiol. 2009, 54, 2435–2446. [Google Scholar] [CrossRef] [Green Version]
- Zhang, D.; Contu, R.; Latronico, M.V.; Zhang, J.; Rizzi, R.; Catalucci, D.; Miyamoto, S.; Huang, K.; Ceci, M.; Gu, Y.; et al. MTORC1 regulates cardiac function and myocyte survival through 4E-BP1 inhibition in mice. J. Clin. Investig. 2010, 120, 2805–2816. [Google Scholar] [CrossRef]
- Shioi, T.; McMullen, J.R.; Tarnavski, O.; Converso, K.; Sherwood, M.C.; Manning, W.J.; Izumo, S. Rapamycin attenuates load-induced cardiac hypertrophy in mice. Circulation 2003, 107, 1664–1670. [Google Scholar] [CrossRef] [Green Version]
- Maejima, Y.; Kyoi, S.; Zhai, P.; Liu, T.; Li, H.; Ivessa, A.; Sciarretta, S.; Del Re, D.P.; Zablocki, D.K.; Hsu, C.P.; et al. Mst1 inhibits autophagy by promoting the interaction between Beclin1 and Bcl-2. Nat. Med. 2013, 19, 1478–1488. [Google Scholar] [CrossRef] [Green Version]
- Krenning, G.; Zeisberg, E.M.; Kalluri, R. The origin of fibroblasts and mechanism of cardiac fibrosis. J. Cell. Physiol. 2010, 225, 631–637. [Google Scholar] [CrossRef] [Green Version]
- Gyongyosi, M.; Winkler, J.; Ramos, I.; Do, Q.T.; Firat, H.; McDonald, K.; Gonzalez, A.; Thum, T.; Diez, J.; Jaisser, F.; et al. Myocardial fibrosis: Biomedical research from bench to bedside. Eur. J. Heart Fail. 2017, 19, 177–191. [Google Scholar] [CrossRef]
- Yu, P.; Ma, S.; Dai, X.; Cao, F. Elabela alleviates myocardial ischemia reperfusion-induced apoptosis, fibrosis and mitochondrial dysfunction through PI3K/AKT signaling. Am. J. Transl. Res. 2020, 12, 4467–4477. [Google Scholar] [PubMed]
- Wang, L.; Tian, X.; Cao, Y.; Ma, X.; Shang, L.; Li, H.; Zhang, X.; Deng, F.; Li, S.; Guo, T.; et al. Cardiac Shock Wave Therapy Improves Ventricular Function by Relieving Fibrosis Through PI3K/Akt Signaling Pathway: Evidence From a Rat Model of Post-infarction Heart Failure. Front. Cardiovasc. Med. 2021, 8, 693875. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Cao, L.; Massey, I.Y. Role of PI3K/Akt signaling pathway in cardiac fibrosis. Mol. Cell. Biochem. 2021, 476, 4045–4059. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ren, Y.; Ren, H.; Wu, Y.; Liu, X.; Chen, H.; Ying, C. The mechanism of myocardial fibrosis is ameliorated by myocardial infarction-associated transcript through the PI3K/Akt signaling pathway to relieve heart failure. J. Int. Med. Res. 2021, 49, 3000605211031433. [Google Scholar] [CrossRef]
- Colombo, F.; Noel, J.; Mayers, P.; Mercier, I.; Calderone, A. beta-Adrenergic stimulation of rat cardiac fibroblasts promotes protein synthesis via the activation of phosphatidylinositol 3-kinase. J. Mol. Cell. Cardiol. 2001, 33, 1091–1106. [Google Scholar] [CrossRef]
- Mann, D.L.; Bogaev, R.; Buckberg, G.D. Cardiac remodelling and myocardial recovery: Lost in translation? Eur. J. Heart Fail. 2010, 12, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.M.; Solomon, S.D. A unified view of ventricular remodelling. Eur. J. Heart Fail. 2010, 12, 779–781. [Google Scholar] [CrossRef] [Green Version]
- Shimizu, I.; Minamino, T. Physiological and pathological cardiac hypertrophy. J. Mol. Cell. Cardiol. 2016, 97, 245–262. [Google Scholar] [CrossRef]
- Chaanine, A.H.; Hajjar, R.J. AKT signalling in the failing heart. Eur. J. Heart Fail. 2011, 13, 825–829. [Google Scholar] [CrossRef]
- Shiojima, I.; Yefremashvili, M.; Luo, Z.; Kureishi, Y.; Takahashi, A.; Tao, J.; Rosenzweig, A.; Kahn, C.R.; Abel, E.D.; Walsh, K. Akt signaling mediates postnatal heart growth in response to insulin and nutritional status. J. Biol. Chem. 2002, 277, 37670–37677. [Google Scholar] [CrossRef] [Green Version]
- Phung, T.L.; Ziv, K.; Dabydeen, D.; Eyiah-Mensah, G.; Riveros, M.; Perruzzi, C.; Sun, J.; Monahan-Earley, R.A.; Shiojima, I.; Nagy, J.A.; et al. Pathological angiogenesis is induced by sustained Akt signaling and inhibited by rapamycin. Cancer Cell 2006, 10, 159–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kemi, O.J.; Ceci, M.; Wisloff, U.; Grimaldi, S.; Gallo, P.; Smith, G.L.; Condorelli, G.; Ellingsen, O. Activation or inactivation of cardiac Akt/mTOR signaling diverges physiological from pathological hypertrophy. J. Cell. Physiol. 2008, 214, 316–321. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Cui, J.; He, G. Bcl-2 Is Involved in Cardiac Hypertrophy through PI3K-Akt Pathway. Biomed. Res. Int. 2021, 2021, 6615502. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Zhao, L.; Shen, L.; Xu, D.; Huang, B.; Wang, Q.; Lin, J.; Zou, Y.; Ge, J. Comparison of various niches for endothelial progenitor cell therapy on ischemic myocardial repair: Coexistence of host collateralization and Akt-mediated angiogenesis produces a superior microenvironment. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 910–923. [Google Scholar] [CrossRef] [Green Version]
- Ma, J.; Sawai, H.; Ochi, N.; Matsuo, Y.; Xu, D.; Yasuda, A.; Takahashi, H.; Wakasugi, T.; Takeyama, H. PTEN regulates angiogenesis through PI3K/Akt/VEGF signaling pathway in human pancreatic cancer cells. Mol. Cell. Biochem. 2009, 331, 161–171. [Google Scholar] [CrossRef]
- Cochain, C.; Channon, K.M.; Silvestre, J.S. Angiogenesis in the infarcted myocardium. Antioxid. Redox. Signal. 2013, 18, 1100–1113. [Google Scholar] [CrossRef]
- Cheng, S.; Zhang, X.; Feng, Q.; Chen, J.; Shen, L.; Yu, P.; Yang, L.; Chen, D.; Zhang, H.; Sun, W.; et al. Astragaloside IV exerts angiogenesis and cardioprotection after myocardial infarction via regulating PTEN/PI3K/Akt signaling pathway. Life Sci. 2019, 227, 82–93. [Google Scholar] [CrossRef]
- Kuo, H.M.; Lin, C.Y.; Lam, H.C.; Lin, P.R.; Chan, H.H.; Tseng, J.C.; Sun, C.K.; Hsu, T.F.; Wu, C.C.; Yang, C.Y.; et al. PTEN overexpression attenuates angiogenic processes of endothelial cells by blockade of endothelin-1/endothelin B receptor signaling. Atherosclerosis 2012, 221, 341–349. [Google Scholar] [CrossRef]
- Jiang, B.H.; Zheng, J.Z.; Aoki, M.; Vogt, P.K. Phosphatidylinositol 3-kinase signaling mediates angiogenesis and expression of vascular endothelial growth factor in endothelial cells. Proc. Natl. Acad. Sci. USA 2000, 97, 1749–1753. [Google Scholar] [CrossRef] [Green Version]
- Skinner, H.D.; Zheng, J.Z.; Fang, J.; Agani, F.; Jiang, B.H. Vascular endothelial growth factor transcriptional activation is mediated by hypoxia-inducible factor 1alpha, HDM2, and p70S6K1 in response to phosphatidylinositol 3-kinase/AKT signaling. J. Biol. Chem. 2004, 279, 45643–45651. [Google Scholar] [CrossRef] [Green Version]
- Robich, M.P.; Chu, L.M.; Oyamada, S.; Sodha, N.R.; Sellke, F.W. Myocardial therapeutic angiogenesis: A review of the state of development and future obstacles. Expert Rev. Cardiovasc. Ther. 2011, 9, 1469–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gorenek, B.; Blomstrom Lundqvist, C.; Brugada Terradellas, J.; Camm, A.J.; Hindricks, G.; Huber, K.; Kirchhof, P.; Kuck, K.H.; Kudaiberdieva, G.; Lin, T.; et al. Cardiac arrhythmias in acute coronary syndromes: Position paper from the joint EHRA, ACCA, and EAPCI task force. EuroIntervention 2015, 10, 1095–1108. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.S.; Ge, L.Z.; Cao, J.M. Research Advances in Sympathetic Remodeling after Myocardial Infarction and Its Significance in Forensic Science. Fa Yi Xue Za Zhi 2019, 35, 68–73. [Google Scholar] [CrossRef] [PubMed]
- Gardner, R.T.; Ripplinger, C.M.; Myles, R.C.; Habecker, B.A. Molecular Mechanisms of Sympathetic Remodeling and Arrhythmias. Circ. Arrhythm. Electrophysiol. 2016, 9, e001359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, J.M.; Fishbein, M.C.; Han, J.B.; Lai, W.W.; Lai, A.C.; Wu, T.J.; Czer, L.; Wolf, P.L.; Denton, T.A.; Shintaku, I.P.; et al. Relationship between regional cardiac hyperinnervation and ventricular arrhythmia. Circulation 2000, 101, 1960–1969. [Google Scholar] [CrossRef] [PubMed]
- Allen, S.J.; Watson, J.J.; Shoemark, D.K.; Barua, N.U.; Patel, N.K. GDNF, NGF and BDNF as therapeutic options for neurodegeneration. Pharmacol. Ther. 2013, 138, 155–175. [Google Scholar] [CrossRef]
- Wei, K.; Liu, L.; Xie, F.; Hao, X.; Luo, J.; Min, S. Nerve growth factor protects the ischemic heart via attenuation of the endoplasmic reticulum stress induced apoptosis by activation of phosphatidylinositol 3-kinase. Int. J. Med. Sci. 2015, 12, 83–91. [Google Scholar] [CrossRef] [Green Version]
- Li, C.Y.; Li, Y.G. Cardiac Sympathetic Nerve Sprouting and Susceptibility to Ventricular Arrhythmias after Myocardial Infarction. Cardiol. Res. Pract. 2015, 2015, 698368. [Google Scholar] [CrossRef] [Green Version]
- Li, S.S.; Kang, N.; Li, X.L.; Yuan, J.; Ling, R.; Li, P.; Li, J.L. LianXia Formula Granule Attenuates Cardiac Sympathetic Remodeling in Rats with Myocardial Infarction via the NGF/TrKA/PI3K/AKT Signaling Pathway. Evid.-Based Complement. Altern. Med. 2021, 2021, 5536406. [Google Scholar] [CrossRef]
- Miki, T.; Itoh, T.; Sunaga, D.; Miura, T. Effects of diabetes on myocardial infarct size and cardioprotection by preconditioning and postconditioning. Cardiovasc. Diabetol. 2012, 11, 67. [Google Scholar] [CrossRef] [Green Version]
- Donahoe, S.M.; Stewart, G.C.; McCabe, C.H.; Mohanavelu, S.; Murphy, S.A.; Cannon, C.P.; Antman, E.M. Diabetes and mortality following acute coronary syndromes. JAMA 2007, 298, 765–775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alegria, J.R.; Miller, T.D.; Gibbons, R.J.; Yi, Q.L.; Yusuf, S.; Collaborative Organization of RheothRx Evaluation (CORE) Trial Investigators. Infarct size, ejection fraction, and mortality in diabetic patients with acute myocardial infarction treated with thrombolytic therapy. Am. Heart J. 2007, 154, 743–750. [Google Scholar] [CrossRef] [PubMed]
- Marso, S.P.; Miller, T.; Rutherford, B.D.; Gibbons, R.J.; Qureshi, M.; Kalynych, A.; Turco, M.; Schultheiss, H.P.; Mehran, R.; Krucoff, M.W.; et al. Comparison of myocardial reperfusion in patients undergoing percutaneous coronary intervention in ST-segment elevation acute myocardial infarction with versus without diabetes mellitus (from the EMERALD Trial). Am. J. Cardiol. 2007, 100, 206–210. [Google Scholar] [CrossRef] [PubMed]
- Mocanu, M.M.; Yellon, D.M. PTEN, the Achilles’ heel of myocardial ischaemia/reperfusion injury? Br. J. Pharmacol. 2007, 150, 833–838. [Google Scholar] [CrossRef]
- Kui, L.; Weiwei, Z.; Ling, L.; Daikun, H.; Guoming, Z.; Linuo, Z.; Renming, H. Ghrelin inhibits apoptosis induced by high glucose and sodium palmitate in adult rat cardiomyocytes through the PI3K-Akt signaling pathway. Regul. Pept. 2009, 155, 62–69. [Google Scholar] [CrossRef]
- Sun, D.; Huang, J.; Zhang, Z.; Gao, H.; Li, J.; Shen, M.; Cao, F.; Wang, H. Luteolin limits infarct size and improves cardiac function after myocardium ischemia/reperfusion injury in diabetic rats. PLoS ONE 2012, 7, e33491. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Xu, T.; Li, D.; Pan, D.; Wu, P.; Luo, Y.; Ma, Y.; Liu, Y. JNK/PI3K/Akt signaling pathway is involved in myocardial ischemia/reperfusion injury in diabetic rats: Effects of salvianolic acid A intervention. Am. J. Transl. Res. 2016, 8, 2534–2548. [Google Scholar]
- An, S.; Wang, X.; Shi, H.; Zhang, X.; Meng, H.; Li, W.; Chen, D.; Ge, J. Apelin protects against ischemia-reperfusion injury in diabetic myocardium via inhibiting apoptosis and oxidative stress through PI3K and p38-MAPK signaling pathways. Aging 2020, 12, 25120–25137. [Google Scholar] [CrossRef]
- Yu, L.; Li, Z.; Dong, X.; Xue, X.; Liu, Y.; Xu, S.; Zhang, J.; Han, J.; Yang, Y.; Wang, H. Polydatin Protects Diabetic Heart against Ischemia-Reperfusion Injury via Notch1/Hes1-Mediated Activation of Pten/Akt Signaling. Oxid. Med. Cell. Longev. 2018, 2018, 2750695. [Google Scholar] [CrossRef] [Green Version]
- Tsang, A.; Hausenloy, D.J.; Mocanu, M.M.; Carr, R.D.; Yellon, D.M. Preconditioning the diabetic heart: The importance of Akt phosphorylation. Diabetes 2005, 54, 2360–2364. [Google Scholar] [CrossRef] [Green Version]
- Yin, X.; Zheng, Y.; Zhai, X.; Zhao, X.; Cai, L. Diabetic inhibition of preconditioning- and postconditioning-mediated myocardial protection against ischemia/reperfusion injury. Exp. Diabetes Res. 2012, 2012, 198048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Drenger, B.; Ostrovsky, I.A.; Barak, M.; Nechemia-Arbely, Y.; Ziv, E.; Axelrod, J.H. Diabetes blockade of sevoflurane postconditioning is not restored by insulin in the rat heart: Phosphorylated signal transducer and activator of transcription 3- and phosphatidylinositol 3-kinase-mediated inhibition. Anesthesiology 2011, 114, 1364–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, S.; Su, W.; Xia, Z.Y.; Wang, Y.; Zhou, L.; Qiao, S.; Zhao, B.; Xia, Z.; Irwin, M.G. Hyperglycemia-Induced Oxidative Stress Abrogates Remifentanil Preconditioning-Mediated Cardioprotection in Diabetic Rats by Impairing Caveolin-3-Modulated PI3K/Akt and JAK2/STAT3 Signaling. Oxid. Med. Cell. Longev. 2019, 2019, 9836302. [Google Scholar] [CrossRef] [PubMed]
- Gao, S.; Wang, R.; Dong, S.; Wu, J.; Perek, B.; Xia, Z.; Yao, S.; Wang, T. Inactivation of TOPK Caused by Hyperglycemia Blocks Diabetic Heart Sensitivity to Sevoflurane Postconditioning by Impairing the PTEN/PI3K/Akt Signaling. Oxid. Med. Cell. Longev. 2021, 2021, 6657529. [Google Scholar] [CrossRef]
- Xue, R.; Lei, S.; Xia, Z.Y.; Wu, Y.; Meng, Q.; Zhan, L.; Su, W.; Liu, H.; Xu, J.; Liu, Z.; et al. Selective inhibition of PTEN preserves ischaemic post-conditioning cardioprotection in STZ-induced Type 1 diabetic rats: Role of the PI3K/Akt and JAK2/STAT3 pathways. Clin. Sci. 2016, 130, 377–392. [Google Scholar] [CrossRef]
- Cheng, X.; Hu, J.; Wang, Y.; Ye, H.; Li, X.; Gao, Q.; Li, Z. Effects of Dexmedetomidine Postconditioning on Myocardial Ischemia/Reperfusion Injury in Diabetic Rats: Role of the PI3K/Akt-Dependent Signaling Pathway. J. Diabetes Res. 2018, 2018, 3071959. [Google Scholar] [CrossRef]
- Tai, W.; Shi, E.; Yan, L.; Jiang, X.; Ma, H.; Ai, C. Diabetes abolishes the cardioprotection induced by sevoflurane postconditioning in the rat heart in vivo: Roles of glycogen synthase kinase-3β and its upstream pathways. J. Surg. Res. 2012, 178, 96–104. [Google Scholar] [CrossRef]
- Lamblin, N.; Fertin, M.; de Groote, P.; Bauters, C. Cardiac remodeling and heart failure after a first anterior myocardial infarction in patients with diabetes mellitus. J. Cardiovasc. Med. 2012, 13, 353–359. [Google Scholar] [CrossRef]
- Shah, A.M.; Hung, C.L.; Shin, S.H.; Skali, H.; Verma, A.; Ghali, J.K.; Kober, L.; Velazquez, E.J.; Rouleau, J.L.; McMurray, J.J.; et al. Cardiac structure and function, remodeling, and clinical outcomes among patients with diabetes after myocardial infarction complicated by left ventricular systolic dysfunction, heart failure, or both. Am. Heart J. 2011, 162, 685–691. [Google Scholar] [CrossRef]
- Nakatani, D.; Sakata, Y.; Mizuno, H.; Shimizu, M.; Suna, S.; Usami, M.; Ito, H.; Yasumura, Y.; Hirayama, A.; Takeda, H.; et al. Impact of Diabetes Mellitus on Rehospitalization for Heart Failure Among Survivors of Acute Myocardial Infarction in the Percutaneous Coronary Intervention Era. Circ. J. 2009, 73, 662–666. [Google Scholar] [CrossRef] [Green Version]
- Akashi, N.; Tsukui, T.; Yamamoto, K.; Seguchi, M.; Taniguchi, Y.; Sakakura, K.; Wada, H.; Momomura, S.-I.; Fujita, H. Comparison of clinical outcomes and left ventricular remodeling after ST-elevation myocardial infarction between patients with and without diabetes mellitus. Heart Vessel. 2021, 36, 1445–1456. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.D.; Shen, Y.; Lu, L.; Ding, F.H.; Yang, Z.K.; Zhang, R.Y.; Shen, W.F.; Jin, W.; Wang, X.Q. Insulin resistance and dysglycemia are associated with left ventricular remodeling after myocardial infarction in non-diabetic patients. Cardiovasc. Diabetol. 2019, 18, 6657529. [Google Scholar] [CrossRef] [PubMed]
- Shiomi, T.; Tsutsui, H.; Ikeuchi, M.; Matsusaka, H.; Hayashidani, S.; Suematsu, N.; Wen, J.; Kubota, T.; Takeshita, A. Streptozotocin-induced hyperglycemia exacerbates left ventricular remodeling and failure after experimental myocardial infarction. J. Am. Coll. Cardiol. 2003, 42, 165–172. [Google Scholar] [CrossRef] [Green Version]
- Backlund, T.; Palojoki, E.; Saraste, A.; Eriksson, A.; Finckenberg, P.; Kyto, V.; Lakkisto, P.; Mervaala, E.; Voipio-Pulkki, L.M.; Laine, M.; et al. Sustained cardiomyocyte apoptosis and left ventricular remodelling after myocardial infarction in experimental diabetes. Diabetologia 2004, 47, 325–330. [Google Scholar] [CrossRef]
- Thakker, G.D.; Frangogiannis, N.G.; Bujak, M.; Zymek, P.; Gaubatz, J.W.; Reddy, A.K.; Taffet, G.; Michael, L.H.; Entman, M.L.; Ballantyne, C.M. Effects of diet-induced obesity on inflammation and remodeling after myocardial infarction. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H2504–H2514. [Google Scholar] [CrossRef] [Green Version]
- Vahtola, E.; Louhelainen, M.; Forstén, H.; Merasto, S.; Raivio, J.; Kaheinen, P.; Kytö, V.; Tikkanen, I.; Levijoki, J.; Mervaala, E. Sirtuin1-p53, forkhead box O3a, p38 and post-infarct cardiac remodeling in the spontaneously diabetic Goto-Kakizaki rat. Cardiovasc. Diabetol. 2010, 9, 5. [Google Scholar] [CrossRef] [Green Version]
- Liang, D.; Zhong, P.; Hu, J.; Lin, F.; Qian, Y.; Xu, Z.; Wang, J.; Zeng, C.; Li, X.; Liang, G. EGFR inhibition protects cardiac damage and remodeling through attenuating oxidative stress in STZ-induced diabetic mouse model. J. Mol. Cell. Cardiol. 2015, 82, 63–74. [Google Scholar] [CrossRef]
- Bhamra, G.S.; Hausenloy, D.J.; Davidson, S.M.; Carr, R.D.; Paiva, M.; Wynne, A.M.; Mocanu, M.M.; Yellon, D.M. Metformin protects the ischemic heart by the Akt-mediated inhibition of mitochondrial permeability transition pore opening. Basic Res. Cardiol. 2008, 103, 274–284. [Google Scholar] [CrossRef]
- Yang, F.; Qin, Y.; Wang, Y.; Meng, S.; Xian, H.; Che, H.; Lv, J.; Li, Y.; Yu, Y.; Bai, Y.; et al. Metformin Inhibits the NLRP3 Inflammasome via AMPK/mTOR-dependent Effects in Diabetic Cardiomyopathy. Int. J. Biol. Sci. 2019, 15, 1010–1019. [Google Scholar] [CrossRef] [Green Version]
- Ji, L.; Fu, F.; Zhang, L.; Liu, W.; Cai, X.; Zhang, L.; Zheng, Q.; Zhang, H.; Gao, F. Insulin attenuates myocardial ischemia/reperfusion injury via reducing oxidative/nitrative stress. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E871–E880. [Google Scholar] [CrossRef] [Green Version]
- Teshima, Y.; Takahashi, N.; Thuc, L.C.; Nishio, S.; Nagano-Torigoe, Y.; Miyazaki, H.; Ezaki, K.; Yufu, K.; Hara, M.; Nakagawa, M.; et al. High-glucose condition reduces cardioprotective effects of insulin against mechanical stress-induced cell injury. Life Sci. 2010, 87, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Aoyagi, T.; Kusakari, Y.; Xiao, C.Y.; Inouye, B.T.; Takahashi, M.; Scherrer-Crosbie, M.; Rosenzweig, A.; Hara, K.; Matsui, T. Cardiac mTOR protects the heart against ischemia-reperfusion injury. Am. J. Physiol. Heart Circ. Physiol. 2012, 303, H75–H85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cardoso, R.; Graffunder, F.P.; Ternes, C.M.P.; Fernandes, A.; Rocha, A.V.; Fernandes, G.; Bhatt, D.L. SGLT2 inhibitors decrease cardiovascular death and heart failure hospitalizations in patients with heart failure: A systematic review and meta-analysis. EClinicalMedicine 2021, 36, 100933. [Google Scholar] [CrossRef] [PubMed]
- Hasan, R.; Lasker, S.; Hasan, A.; Zerin, F.; Zamila, M.; Chowdhury, F.I.; Nayan, S.I.; Rahman, M.M.; Khan, F.; Subhan, N.; et al. Canagliflozin attenuates isoprenaline-induced cardiac oxidative stress by stimulating multiple antioxidant and anti-inflammatory signaling pathways. Sci. Rep. 2020, 10, 14459. [Google Scholar] [CrossRef] [PubMed]
- Robinson, E.; Cassidy, R.S.; Tate, M.; Zhao, Y.; Lockhart, S.; Calderwood, D.; Church, R.; Mcgahon, M.K.; Brazil, D.P.; Mcdermott, B.J.; et al. Exendin-4 protects against post-myocardial infarction remodelling via specific actions on inflammation and the extracellular matrix. Basic Res. Cardiol. 2015, 110, 20. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.; Hayder, H.; Zayed, Y.; Peng, C. Overview of MicroRNA Biogenesis, Mechanisms of Actions, and Circulation. Front. Endocrinol. 2018, 9, 402. [Google Scholar] [CrossRef] [Green Version]
- Majka, M.; Kleibert, M.; Wojciechowska, M. Impact of the Main Cardiovascular Risk Factors on Plasma Extracellular Vesicles and Their Influence on the Heart’s Vulnerability to Ischemia-Reperfusion Injury. Cells 2021, 10, 3331. [Google Scholar] [CrossRef]
- Roy, S.; Khanna, S.; Hussain, S.-R.A.; Biswas, S.; Azad, A.; Rink, C.; Gnyawali, S.; Shilo, S.; Nuovo, G.J.; Sen, C.K. MicroRNA expression in response to murine myocardial infarction: miR-21 regulates fibroblast metalloprotease-2 via phosphatase and tensin homologue. Cardiovasc. Res. 2009, 82, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Bergman, M.; Nguyen, A.; Turcato, S.; Swigart, P.; Rodrigo, M.; Simpson, P.; Karliner, J.; Lovett, D.; Baker, A. Cardiac transgenic matrix metalloproteinase-2 expression directly induces impaired contractility. Cardiovasc. Res. 2006, 69, 688–696. [Google Scholar] [CrossRef] [Green Version]
- Yang, Q.; Yang, K.; Li, A.Y. Trimetazidine protects against hypoxia-reperfusion-induced cardiomyocyte apoptosis by increasing microRNA-21 expression. Int. J. Clin. Exp. Pathol. 2015, 8, 3735–3741. [Google Scholar]
- Ma, N.; Bai, J.; Zhang, W.; Luo, H.; Zhang, X.; Liu, D.; Qiao, C. Trimetazidine protects against cardiac ischemia/reperfusion injury via effects on cardiac miRNA-21 expression, Akt and the Bcl-2/Bax pathway. Mol. Med. Rep. 2016, 14, 4216–4222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Z.; Wu, S.; Kong, F.; Cai, X.; Ye, B.; Shan, P.; Huang, W. MicroRNA-21 protects against cardiac hypoxia/reoxygenation injury by inhibiting excessive autophagy in H9c2 cells via the Akt/mTOR pathway. J. Cell. Mol. Med. 2017, 21, 467–474. [Google Scholar] [CrossRef] [PubMed]
- Tu, Y.; Wan, L.; Fan, Y.; Wang, K.; Bu, L.; Huang, T.; Cheng, Z.; Shen, B. Ischemic Postconditioning-Mediated miRNA-21 Protects against Cardiac ischemia/reperfusion Injury via PTEN/Akt Pathway. PLoS ONE 2013, 8, e75872. [Google Scholar] [CrossRef] [Green Version]
- Qiao, S.; Olson, J.M.; Paterson, M.; Yan, Y.; Zaja, I.; Liu, Y.; Riess, M.L.; Kersten, J.R.; Liang, M.; Warltier, D.C.; et al. MicroRNA-21 Mediates Isoflurane-induced Cardioprotection against Ischemia–Reperfusion Injury via Akt/Nitric Oxide Synthase/Mitochondrial Permeability Transition Pore Pathway. Anesthesiology 2015, 123, 786–798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayourian, J.; Ceholski, D.K.; Gorski, P.A.; Mathiyalagan, P.; Murphy, J.F.; Salazar, S.I.; Stillitano, F.; Hare, J.M.; Sahoo, S.; Hajjar, R.J.; et al. Exosomal microRNA-21-5p Mediates Mesenchymal Stem Cell Paracrine Effects on Human Cardiac Tissue Contractility. Circ. Res. 2018, 122, 933–944. [Google Scholar] [CrossRef] [PubMed]
- Qiao, L.; Hu, S.; Liu, S.; Zhang, H.; Ma, H.; Huang, K.; Li, Z.; Su, T.; Vandergriff, A.; Tang, J.; et al. microRNA-21-5p dysregulation in exosomes derived from heart failure patients impairs regenerative potential. J. Clin. Investig. 2019, 129, 2237–2250. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Liang, Q.; Cai, M.; Tian, Z. HIF-1α-induced up-regulation of microRNA-126 contributes to the effectiveness of exercise training on myocardial angiogenesis in myocardial infarction rats. J. Cell. Mol. Med. 2020, 24, 12970–12979. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Xu, Y.; Zhou, K.; Kao, G.; Xiao, J. MicroRNA-126 and VEGF enhance the function of endothelial progenitor cells in acute myocardial infarction. Exp. Ther. Med. 2022, 23, 142. [Google Scholar] [CrossRef]
- Gomes, J.L.; Fernandes, T.; Soci, U.P.; Silveira, A.C.; Barretti, D.L.; Negrão, C.E.; Oliveira, E.M. Obesity Downregulates MicroRNA-126 Inducing Capillary Rarefaction in Skeletal Muscle: Effects of Aerobic Exercise Training. Oxid. Med. Cell. Longev. 2017, 2017, 2415246. [Google Scholar] [CrossRef]
- Meng, S.; Cao, J.T.; Zhang, B.; Zhou, Q.; Shen, C.X.; Wang, C.Q. Downregulation of microRNA-126 in endothelial progenitor cells from diabetes patients, impairs their functional properties, via target gene Spred-1. J. Mol. Cell. Cardiol. 2012, 53, 64–72. [Google Scholar] [CrossRef]
- Chen, L.; Wang, J.; Wang, B.; Yang, J.; Gong, Z.; Zhao, X.; Zhang, C.; Du, K. MiR-126 inhibits vascular endothelial cell apoptosis through targeting PI3K/Akt signaling. Ann. Hematol. 2016, 95, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Li, S.H.; Wang, M.S.; Ke, W.L.; Wang, M.R. Naringenin alleviates myocardial ischemia reperfusion injury by enhancing the myocardial miR-126-PI3K/AKT axis in streptozotocin-induced diabetic rats. Exp. Ther. Med. 2021, 22, 810. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ouyang, P.; He, G.; Wang, X.; Song, D.; Yang, Y.; He, X. Exosomes from microRNA-126 overexpressing mesenchymal stem cells promote angiogenesis by targeting the PIK3R2-mediated PI3K/Akt signalling pathway. J. Cell. Mol. Med. 2021, 25, 2148–2162. [Google Scholar] [CrossRef] [PubMed]
- Sun, N.; Meng, F.; Xue, N.; Pang, G.; Wang, Q.; Ma, H. Inducible miR-145 expression by HIF-1α protects cardiomyocytes against apoptosis via regulating SGK1 in simulated myocardial infarction hypoxic microenvironment. Cardiol. J. 2013, 25, 268–278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashi, K.; Yamada, Y.; Minatoguchi, S.; Baba, S.; Iwasa, M.; Kanamori, H.; Kawasaki, M.; Nishigaki, K.; Takemura, G.; Kumazaki, M.; et al. MicroRNA-145 repairs infarcted myocardium by accelerating cardiomyocyte autophagy. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1813–H1826. [Google Scholar] [CrossRef] [Green Version]
- Yan, L.; Guo, N.; Cao, Y.; Zeng, S.; Wang, J.; Lv, F.; Wang, Y.; Cao, X. miRNA145 inhibits myocardial infarctioninduced apoptosis through autophagy via Akt3/mTOR signaling pathway in vitro and in vivo. Int. J. Mol. Med. 2018, 42, 1537–1547. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Tao, B.; Fan, S.; Cui, S.; Pu, Y.; Qiu, L.; Xia, H.; Xu, L. Over-expression of microRNA-145 drives alterations in β-adrenergic signaling and attenuates cardiac remodeling in heart failure post myocardial infarction. Aging 2020, 12, 11603–11622. [Google Scholar] [CrossRef]
- Cui, S.; Liu, Z.; Tao, B.; Fan, S.; Pu, Y.; Meng, X.; Li, D.; Xia, H.; Xu, L. miR-145 attenuates cardiac fibrosis through the AKT/GSK-3beta/beta-catenin signaling pathway by directly targeting SOX9 in fibroblasts. J. Cell. Biochem. 2021, 122, 209–221. [Google Scholar] [CrossRef]
- Dong, Y.M.; Liu, X.X.; Wei, G.Q.; Da, Y.N.; Cha, L.; Ma, C.S. Prediction of long-term outcome after acute myocardial infarction using circulating miR-145. Scand. J. Clin. Lab. Investig. 2015, 75, 85–91. [Google Scholar] [CrossRef]
- Zhang, M.; Cheng, Y.J.; Sara, J.D.; Liu, L.J.; Liu, L.P.; Zhao, X.; Gao, H. Circulating MicroRNA-145 is Associated with Acute Myocardial Infarction and Heart Failure. Chin. Med. J. 2017, 130, 51–56. [Google Scholar] [CrossRef]
- Sun, G.; Lu, Y.; Li, Y.; Mao, J.; Zhang, J.; Jin, Y.; Li, Y.; Sun, Y.; Liu, L.; Li, L. miR-19a protects cardiomyocytes from hypoxia/reoxygenation-induced apoptosis via PTEN/PI3K/p-Akt pathway. Biosci. Rep. 2017, 37, BSR20170899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhong, C.; Wang, K.; Liu, Y.; Lv, D.; Zheng, B.; Zhou, Q.; Sun, Q.; Chen, P.; Ding, S.; Xu, Y.; et al. miR-19b controls cardiac fibroblast proliferation and migration. J. Cell. Mol. Med. 2016, 20, 1191–1197. [Google Scholar] [CrossRef] [PubMed]
- Dehaini, H.; Awada, H.; El-Yazbi, A.; Zouein, F.A.; Issa, K.; Eid, A.A.; Ibrahim, M.; Badran, A.; Baydoun, E.; Pintus, G.; et al. MicroRNAs as Potential Pharmaco-targets in Ischemia-Reperfusion Injury Compounded by Diabetes. Cells 2019, 8, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, H.; Bae, Y.-U.; Lee, H.; Kim, H.; Jeon, J.S.; Noh, H.; Han, D.C.; Byun, D.W.; Kim, S.H.; Park, H.K.; et al. Effect of diabetes on exosomal miRNA profile in patients with obesity. BMJ Open Diabetes Res. Care 2020, 8, e001403. [Google Scholar] [CrossRef]
- Chien, H.-Y.; Lee, T.-P.; Chen, C.-Y.; Chiu, Y.-H.; Lin, Y.-C.; Lee, L.-S.; Li, W.-C. Circulating microRNA as a diagnostic marker in populations with type 2 diabetes mellitus and diabetic complications. J. Chin. Med. Assoc. 2015, 78, 204–211. [Google Scholar] [CrossRef] [Green Version]
- Sekar, D.; Venugopal, B.; Sekar, P.; Ramalingam, K. Role of microRNA 21 in diabetes and associated/related diseases. Gene 2016, 582, 14–18. [Google Scholar] [CrossRef]
- Bernardo, B.C.; Gao, X.-M.; Winbanks, C.E.; Boey, E.J.H.; Tham, Y.K.; Kiriazis, H.; Gregorevic, P.; Obad, S.; Kauppinen, S.; Du, X.-J.; et al. Therapeutic inhibition of the miR-34 family attenuates pathological cardiac remodeling and improves heart function. Proc. Natl. Acad. Sci. USA 2012, 109, 17615–17620. [Google Scholar] [CrossRef] [Green Version]
- Shi, K.; Sun, H.; Zhang, H.; Xie, D.; Yu, B. miR-34a-5p aggravates hypoxia-induced apoptosis by targeting ZEB1 in cardiomyocytes. Biol. Chem. 2019, 400, 227–236. [Google Scholar] [CrossRef]
- Zhu, Y.; Qian, X.; Li, J.; Lin, X.; Luo, J.; Huang, J.; Jin, Z. Astragaloside-IV protects H9C2(2-1) cardiomyocytes from high glucose-induced injury via miR-34a-mediated autophagy pathway. Artif. Cells Nanomed. Biotechnol. 2019, 47, 4172–4181. [Google Scholar] [CrossRef] [Green Version]
- Wang, R.; Hong, J.; Cao, Y.; Shi, J.; Gu, W.; Ning, G.; Zhang, Y.; Wang, W. Elevated circulating microRNA-122 is associated with obesity and insulin resistance in young adults. Eur. J. Endocrinol. 2015, 172, 291–300. [Google Scholar] [CrossRef]
- Gong, L.; Chang, H.; Xu, H. LncRNA MALAT1 knockdown alleviates oxygen-glucose deprivation and reperfusion induced cardiomyocyte apoptotic death by regulating miR-122. Exp. Mol. Pathol. 2019, 111, 104325. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, H.; Cui, Z.; Zhou, Z.; Chen, S.; Ma, J.; Hou, L.; Pan, X.; Li, Q. Long non-coding RNA UCA1 relieves cardiomyocytes H9c2 injury aroused by oxygen-glucose deprivation via declining miR-122. Artif. Cells Nanomed. Biotechnol. 2019, 47, 3492–3499. [Google Scholar] [CrossRef] [PubMed]
- Jansen, F.; Wang, H.; Przybilla, D.; Franklin, B.S.; Dolf, A.; Pfeifer, P.; Schmitz, T.; Flender, A.; Endl, E.; Nickenig, G.; et al. Vascular endothelial microparticles-incorporated microRNAs are altered in patients with diabetes mellitus. Cardiovasc. Diabetol. 2016, 15, 49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, K.; Yang, Y.; Zhong, Y.; Ammar, H.M.; Zhang, P.; Guo, R.; Liu, H.; Cheng, C.; Koroscil, T.M.; Chen, Y.; et al. The effects of microvesicles on endothelial progenitor cells are compromised in type 2 diabetic patients via downregulation of the miR-126/VEGFR2 pathway. Am. J. Physiol. Endocrinol. Metab. 2016, 310, E828–E837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Villard, A.; Marchand, L. Diagnostic Value of Cell-free Circulating Micrornas for Obesity and Type 2 Diabetes: A Meta-analysis. J. Mol. Biomark. Diagn. 2015, 6, 251. [Google Scholar] [CrossRef] [Green Version]
- Ortega, F.J.; Mercader, J.M.; Moreno-Navarrete, J.M.; Rovira, O.; Guerra, E.; Esteve, E.; Xifra, G.; Martinez, C.; Ricart, W.; Rieusset, J.; et al. Profiling of circulating microRNAs reveals common microRNAs linked to type 2 diabetes that change with insulin sensitization. Diabetes Care 2014, 37, 1375–1383. [Google Scholar] [CrossRef] [Green Version]
- Zampetaki, A.; Kiechl, S.; Drozdov, I.; Willeit, P.; Mayr, U.; Prokopi, M.; Mayr, A.; Weger, S.; Oberhollenzer, F.; Bonora, E.; et al. Plasma MicroRNA Profiling Reveals Loss of Endothelial MiR-126 and Other MicroRNAs in Type 2 Diabetes. Circ. Res. 2010, 107, 810–817. [Google Scholar] [CrossRef]
- Gan, L.; Xie, D.; Liu, J.; Lau, W.B.; Christopher, T.A.; Lopez, B.; Zhang, L.; Gao, E.; Koch, W.; Ma, X.L.; et al. Small Extracellular Microvesicles Mediated Pathological Communications between Dysfunctional Adipocytes and Cardiomyocytes as a Novel Mechanisms Exacerbating Ischemia/Reperfusion Injury in Diabetic Mice. Circulation 2020, 141, 968–983. [Google Scholar] [CrossRef]
- Liang, Y.Z.; Li, J.J.H.; Xiao, H.B.; He, Y.; Zhang, L.; Yan, Y.X. Identification of stress-related microRNA biomarkers in type 2 diabetes mellitus: A systematic review and meta-analysis. J. Diabetes 2020, 12, 633–644. [Google Scholar] [CrossRef] [Green Version]
- Lu, C.; Wang, X.; Ha, T.; Hu, Y.; Liu, L.; Zhang, X.; Yu, H.; Miao, J.; Kao, R.; Kalbfleisch, J.; et al. Attenuation of cardiac dysfunction and remodeling of myocardial infarction by microRNA-130a are mediated by suppression of PTEN and activation of PI3K dependent signaling. J. Mol. Cell. Cardiol. 2015, 89, 87–97. [Google Scholar] [CrossRef] [Green Version]
- Shahrokhi, S.Z.; Saeidi, L.; Sadatamini, M.; Jafarzadeh, M.; Rahimipour, A.; Kazerouni, F. Can miR-145-5p be used as a marker in diabetic patients? Arch. Physiol. Biochem. 2020. [Google Scholar] [CrossRef] [PubMed]
- Deng, Z.B.; Poliakov, A.; Hardy, R.W.; Clements, R.; Liu, C.; Liu, Y.; Wang, J.; Xiang, X.; Zhang, S.; Zhuang, X.; et al. Adipose tissue exosome-like vesicles mediate activation of macrophage-induced insulin resistance. Diabetes 2009, 58, 2498–2505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, S.; Wei, J.; Zhang, C.; Li, X.; Meng, W.; Mo, X.; Zhang, Q.; Liu, Q.; Ren, K.; Du, R.; et al. Cell-Derived Microparticles in Patients with Type 2 Diabetes Mellitus: A Systematic Review and Meta-Analysis. Cell. Physiol. Biochem. 2016, 39, 2439–2450. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Y.; Wang, J.; Chen, Q.; Wu, Q.; Deng, W.; Zhou, H.; Shen, D. Long non-coding RNA cytoskeleton regulator RNA (CYTOR) modulates pathological cardiac hypertrophy through miR-155-mediated IKKi signaling. Biochim. Biophys. Acta Mol. Basis Dis. 2019, 1865, 1421–1427. [Google Scholar] [CrossRef] [PubMed]
- Gao, L.; Li, T.; Li, S.; Song, Z.; Chang, Y.; Yuan, L. Schisandrin A protects against isoproterenol-induced chronic heart failure via miR-155. Mol. Med. Rep. 2021, 25, 24. [Google Scholar] [CrossRef]
- Sanchez-Ceinos, J.; Rangel-Zuniga, O.A.; Clemente-Postigo, M.; Podadera-Herreros, A.; Camargo, A.; Alcala-Diaz, J.F.; Guzman-Ruiz, R.; Lopez-Miranda, J.; Malagon, M.M. miR-223-3p as a potential biomarker and player for adipose tissue dysfunction preceding type 2 diabetes onset. Mol. Ther. Nucleic Acids 2021, 23, 1035–1052. [Google Scholar] [CrossRef]
- Yan, L.-N.; Zhang, X.; Xu, F.; Fan, Y.-Y.; Ge, B.; Guo, H.; Li, Z.-L. Four-microRNA signature for detection of type 2 diabetes. World J. Clin. Cases 2020, 8, 1923–1931. [Google Scholar] [CrossRef]
- Dai, G.-H.; Liu, N.; Zhu, J.-W.; Yao, J.; Yang, C.; Ma, P.-Z.; Song, X.-B. Qi-Shen-Yi-Qi Dripping Pills Promote Angiogenesis of Ischemic Cardiac Microvascular Endothelial Cells by Regulating MicroRNA-223-3p Expression. Evid.-Based Complement. Altern. Med. 2016, 2016, 5057328. [Google Scholar] [CrossRef] [Green Version]
- Yang, L.; Li, Y.; Wang, X.; Mu, X.; Qin, D.; Huang, W.; Alshahrani, S.; Nieman, M.; Peng, J.; Essandoh, K.; et al. Overexpression of miR-223 Tips the Balance of Pro- and Anti-hypertrophic Signaling Cascades toward Physiologic Cardiac Hypertrophy. J. Biol. Chem. 2016, 291, 15700–15713. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Xu, Y.; Deng, Y.; Li, H. MicroRNA-223 Regulates Cardiac Fibrosis After Myocardial Infarction by Targeting RASA1. Cell. Physiol. Biochem. 2018, 46, 1439–1454. [Google Scholar] [CrossRef]
- Cao, L.; Chai, S. miR-320-3p is involved in morphine pre-conditioning to protect rat cardiomyocytes from ischemia/reperfusion injury through targeting Akt3. Mol. Med. Rep. 2020, 22, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Sun, K.; Chang, X.; Yin, L.; Li, J.; Zhou, T.; Zhang, C.; Chen, X. Expression and DNA methylation status of microRNA-375 in patients with type 2 diabetes mellitus. Mol. Med. Rep. 2014, 9, 967–972. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, X.; Li, Y.; Man, B.; Li, D. Assessing MicroRNA-375 Levels in Type 2 Diabetes Mellitus (T2DM) Patients and Their First-Degree Relatives with T2DM. Diabetes Metab. Syndr. Obes. Targets Ther. 2021, 14, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Garikipati, V.N.S.; Verma, S.K.; Jolardarashi, D.; Cheng, Z.; Ibetti, J.; Cimini, M.; Tang, Y.; Khan, M.; Yue, Y.; Benedict, C.; et al. Therapeutic inhibition of miR-375 attenuates post-myocardial infarction inflammatory response and left ventricular dysfunction via PDK-1-AKT signalling axis. Cardiovasc. Res. 2017, 113, 938–949. [Google Scholar] [CrossRef] [Green Version]
- Yan, M.; Liu, Q.; Jiang, Y.; Wang, B.; Ji, Y.; Liu, H.; Xie, Y. Long Noncoding RNA LNC_000898 Alleviates Cardiomyocyte Apoptosis and Promotes Cardiac Repair After Myocardial Infarction Through Modulating the miR-375/PDK1 Axis. J. Cardiovasc. Pharmacol. 2020, 76, 77–85. [Google Scholar] [CrossRef]
- Zheng, K.; Zhang, Q.; Lin, G.; Li, Y.; Sheng, Z.; Wang, J.; Chen, L.; Lu, H.H. Activation of Akt by SC79 protects myocardiocytes from oxygen and glucose deprivation (OGD)/re-oxygenation. Oncotarget 2017, 8, 14978–14987. [Google Scholar] [CrossRef] [Green Version]
- Moreira, J.B.; Wohlwend, M.; Alves, M.N.; Wisloff, U.; Bye, A. A small molecule activator of AKT does not reduce ischemic injury of the rat heart. J. Transl. Med. 2015, 13, 76. [Google Scholar] [CrossRef] [Green Version]
- Matsui, T.; Tao, J.; del Monte, F.; Lee, K.H.; Li, L.; Picard, M.; Force, T.L.; Franke, T.F.; Hajjar, R.J.; Rosenzweig, A. Akt activation preserves cardiac function and prevents injury after transient cardiac ischemia in vivo. Circulation 2001, 104, 330–335. [Google Scholar] [CrossRef] [Green Version]
- Peng, Y.; Wang, Y.; Zhou, C.; Mei, W.; Zeng, C. PI3K/Akt/mTOR Pathway and Its Role in Cancer Therapeutics: Are We Making Headway? Front. Oncol. 2022, 12, 819128. [Google Scholar] [CrossRef]

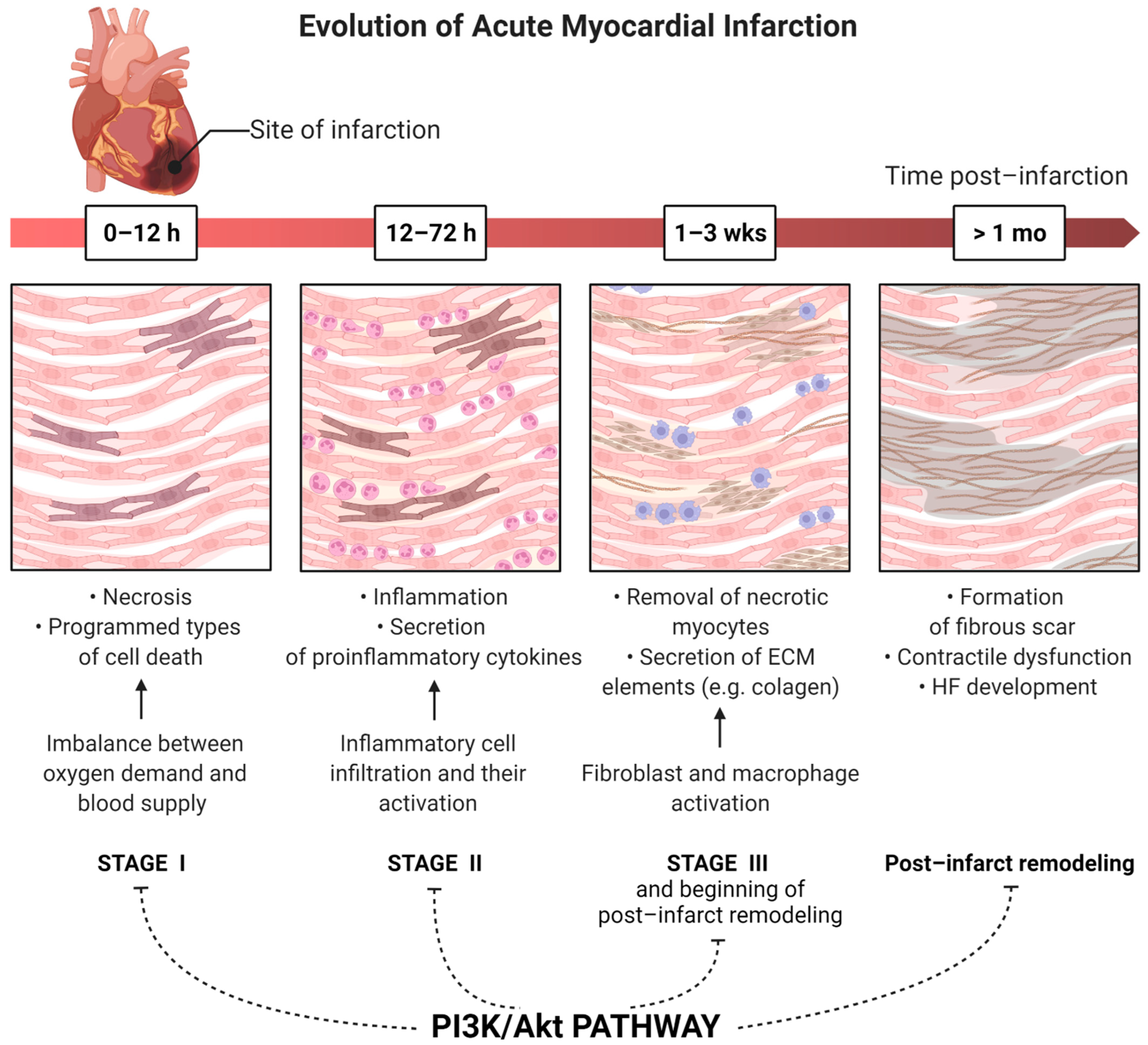
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Walkowski, B.; Kleibert, M.; Majka, M.; Wojciechowska, M. Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells 2022, 11, 1553. https://doi.org/10.3390/cells11091553
Walkowski B, Kleibert M, Majka M, Wojciechowska M. Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells. 2022; 11(9):1553. https://doi.org/10.3390/cells11091553
Chicago/Turabian StyleWalkowski, Bartosz, Marcin Kleibert, Miłosz Majka, and Małgorzata Wojciechowska. 2022. "Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart" Cells 11, no. 9: 1553. https://doi.org/10.3390/cells11091553
APA StyleWalkowski, B., Kleibert, M., Majka, M., & Wojciechowska, M. (2022). Insight into the Role of the PI3K/Akt Pathway in Ischemic Injury and Post-Infarct Left Ventricular Remodeling in Normal and Diabetic Heart. Cells, 11(9), 1553. https://doi.org/10.3390/cells11091553








