Abstract
Iron and cobalt are micronutrients that play an important role in the regulation of cellular processes, being part of the centre of catalases, peroxidases, cytochromes and metalloproteins such as hemoglobin and myoglobin (Fe). Cobalt primarily functions as a component of hydroxycobalamin, which is essential for regulating red blood cell production. Maintaining normal levels of cobalt and iron in the human body is important, as a deficiency can lead to anaemia. These elements are also involved in reactions during which oxidative stress occurs and are therefore considered to be a cause of tumor formation. This paper will discuss aspects of the influence of cobalt and iron on mechanisms that may contribute to the growth of gynecological tumors, as well as other obstetric-gynecological disease entities, by altering the conditions of the microenvironment. In addition, the following review also highlights the role of cobalt and iron in the treatment of gynecological tumors.
1. Introduction
Iron and cobalt are among the micronutrients that have important functions for maintaining normal homeostasis in the human body [1,2]. Among other things, iron is involved in oxygen transport as a component of hemoglobin [3], and cobalt is embedded in the ring of hydroxycobalamin (vitamin B12), ref. [4] which is involved in erythropoiesis [5]. Iron and cobalt deficiency can lead to microcytic [6] and macrocytic [7] anaemia, respectively. During pregnancy, there is an increased iron requirement of approximately 1000–1200 mg, of which 1/3 of this iron pool is needed for placental-fetal tissues [8]. The causes and consequences of deficiencies of these micronutrients are well understood and described in the literature. The second equally important issue concerning cobalt and iron, is their excess. Their toxicity has been studied for several decades, as well as the links between cobalt and iron overload and tumorigenesis.
Cobalt and iron are among the elements that compete with each other, due to the fact that they most commonly occur on the same oxidation levels (2+/3+) [9] and the fact that they have similar properties [10]. Iron is a well-documented carcinogen in both animals and humans [11]. In contrast, there are no conclusive scientific reports on the potential carcinogenic effects of cobalt, and the evidence is more based on animal cell studies. However, cobalt metal and soluble cobalt(II) salts belong to the group of carcinogens for which there is evidence from studies with human cells and can be considered likely [12].
The most common gynecological malignancies are endometrial cancer, cervical cancer and ovarian cancer [13]. Ovarian cancer is the most common cause of death among patients with gynecological malignancies [14]. Very often its first symptoms appear already at an advanced stage [15]. In the prevention of cervical cancer, human papillomavirus (HPV) vaccines play a special role [16]. In contrast to ovarian cancer, endometrial cancer more often manifests itself at an early stage [17]. Symptoms of endometrial cancer mainly include abnormal uterine bleeding [18]. Hysteroscopy with histopathological examination allows initial diagnosis and initiation of treatment [19]. Diseases such as polycystic ovary syndrome and endometriosis, which increase the risk of endometrial cancer, or ovarian cancer, may also be associated with tumorigenesis [20].
The following literature review underline the role of cobalt and iron in gynecological diseases, folliculogenesis, placental function, as well as the effects of excess of these micronutrients on the development of gynecological cancers and the use of the radioisotope cobalt Co-60 in radiotherapy. We also discuss processes and reactions involving both elements, including ferroptosis as non-apoptotic cell death, which may prove useful in cancer therapy. The review of the work relates to the state of knowledge up to November 2022.
2. The Role of Iron
2.1. Iron
Iron is a micronutrient that belongs to the transition metals and has a number of important functions in the human body. First and foremost, it is essential for oxygen transport by hemoglobin, with which more iron is associated in the body [21]. Iron is used in a number of reactions due to its two degrees of oxidation: heme iron Fe2+ (ferrous cation) and non-heme iron Fe3+ (ferric cation) [22]. Reduction of Fe3+ to Fe2+ occurs by duodenal cytochrome b (Dcytb) and can then be transported into duodenal mucosal cells by the divalent metal transporter (DMT1) [23]. Fe2+ in the presence of superoxide and hydrogen peroxide (H2O2) can catalyse the formation of hydroxyl radical (*OH), and this, due to its oxidative properties, promotes lipid peroxidation, mutagenesis and DNA strand damage, oncogene activation and suppression of suppressor genes [24]. These two steps enable redox reactions to occur and thus the ability of various ligands to bind iron. Performing a number of biologically relevant functions, it also involves the generation of reactive oxygen species (ROS), which takes place during the Fenton reaction [25,26]. This occurs when superoxide (O2*-) releases ferric iron (Fe3+) from ferritin and hemosiderin in the cell [24]. Iron may affect the formation of lipofuscin, one of the better understood markers of ageing. Moreover, free radical formation via lipofuscin can be four times higher with iron than without it [27].
2.2. Transferrin
Transferrin (Tf), which is a glycoprotein that binds iron in the form of Fe3+ (ferric iron), is responsible for the binding and transport of iron absorbed from the gastrointestinal tract into tissues [28]. Thanks to transferrin receptors (TfR1) on the surface of cells, which are involved in iron uptake, there is no excessive transport of iron into the cells [29]. However, small amounts of iron can also be bound to ferritin as a hemopexin-hem complex and haptoglobin-hemoglobin, which is particularly important in cases of iron overload. Non-transferrin-bound (NTB) forms are directed to the liver, stored as ferritin or re-transported to plasma to bind to transferrin [30]. To maintain normal iron homeostasis, transferrin receptor 2 (TfR2) is responsible for the expression of hepcidin and regulates erythropoiesis, being located on erythroid precursors as a component of the erythropoietin (Epo)-Epo receptor (EpoR) complex [31]. Levels of iron and the molecules responsible for its transport, storage and metabolism should be tightly regulated, as increased iron stores as well as transferrin are associated with free radical formation [32].
2.3. Ferroportin
Ferroportin is a trans-membrane protein that acts as an iron transporter (iron efflux pump). It allows the absorption of iron in enterocytes (transcytosis). Ferroportin is also found in hepatocytes, spleen, kidney, Kupffer cells and macrophages [33]. Its activity can be regulated by transcription and also post-translationally. Iron deficiency, hypoxia, transition metals, hem and inflammatory cytokines regulate ferroportin transcription. Importantly, ferroportin is influenced by hepcidin, a peptide hormone that causes the internalisation and degradation of ferroportin [34].
2.4. Ferroptosis
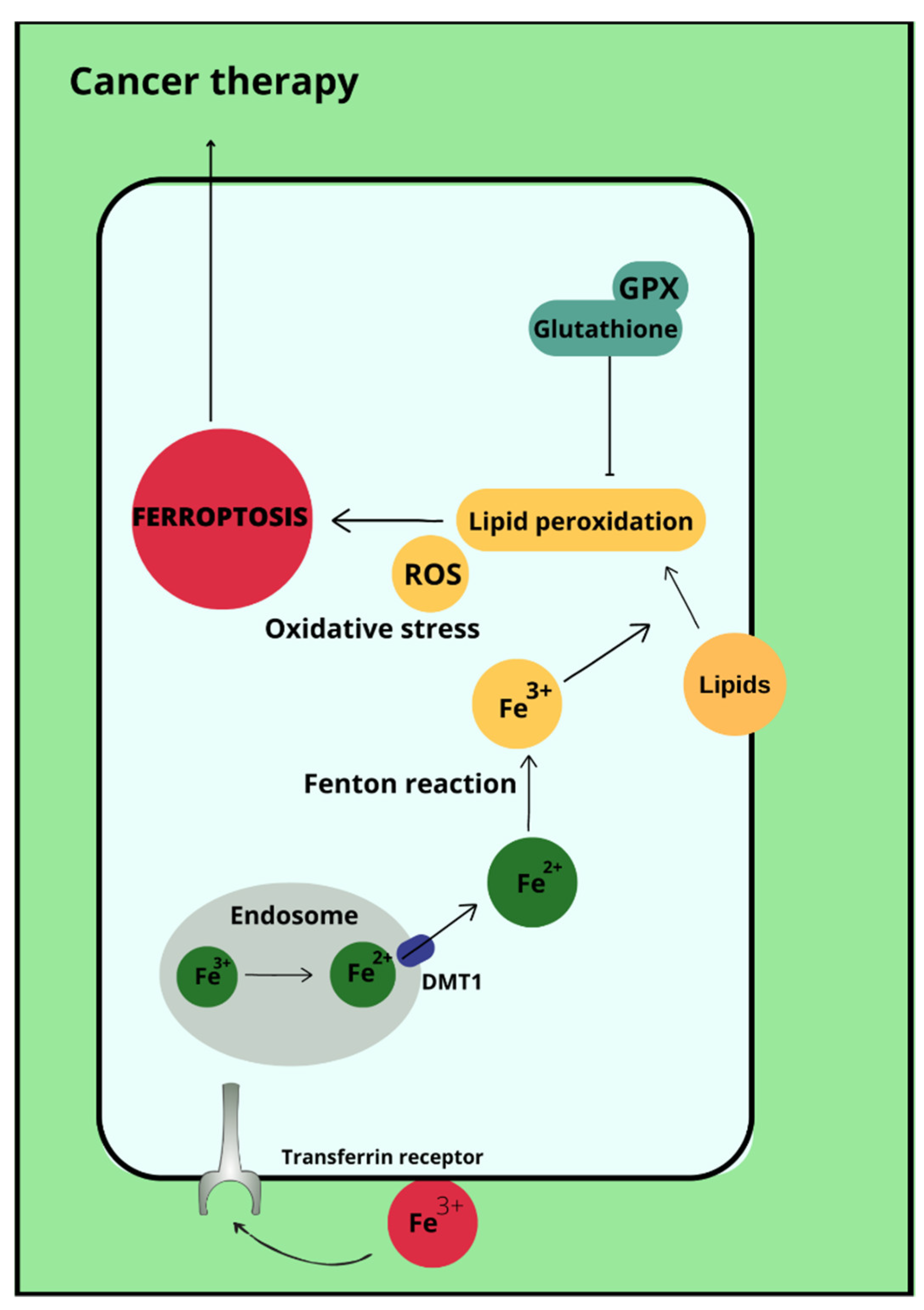
Ferroptosis is a type of cell death that occurs due to the accumulation of reactive oxygen species, which is iron-dependent [35]. Ferroptosis is not associated with either apoptosis or cell necrosis [36,37]. Doubts remain about autophagy, with which ferroptosis may have more in common. This is due to the accumulation of autophagosomes in response to ferroptosis-inducing factors. More specifically, the proteins involved in autophagy ATG3, ATG5, ATG4B, ATG7, ATG13 and BECN1 may also contribute to cell death by ferroptosis [38]. In its course, lipid hydroperoxides (LOOH) and iron ion (Fe2+) concentrations are increased in cells. Excessive amounts of reactive oxygen species initiate lipid peroxidation via Fenton reactions. This is followed by damage to the outer mitochondrial membrane, its rupture and condensation [37]. The process of ferroptosis can be initiated by biological or chemical agents, and the pathways leading to this cellular destruction are divided into extrinsic (transporter) and intrinsic (enzymamtic) [39,40]. The activating factor for ferroptosis may be signal transducer and activator of transcription 3 (STAT3), as demonstrated in pancreatic ductal adenocarcinoma cell lines [41]. Ferroptosis can also be promoted by ferritinophagy involving nuclear receptor coactivator 4 (NCOA4), Ras-related protein Rab-7a (RAB7A)-dependent lipophagy, inhibition of the xc system by Beclin 1 (BECN1) and the aforementioned autophagy together with chaperones [38]. Most importantly, the p53 protein reduces cystine uptake by inhibiting the expression of the cystine transporter Solute Carrier Family 7 Member 11 (SLC7A11) and sensitises cells to ferroptosis [42]. Enhancement of ferroptosis can also occur by increasing the expression of spermidine/spermine-N-acetyltransferase 1 (SAT1) and glutaminase (GLS1) [43]. However, p53 can also inhibit ferroptosis through direct inhibition of dipeptidyl peptidase 4 (DPP4) activity or through induction of cyclin-dependent kinase inhibitor 1A (CDKN1A/p21) [44]. In addition, it is possible that ferroptosis affects the immune properties of the tumor [45]. Ferroptosis therapies are being considered as a potential treatment for refractory triple-negative breast cancer, whose cells show sensitivity to ferroptosis-stimulating agents [46]. Ferroptosis-inducing drugs and iron chelating agents are being considered as effective cancer treatments. Used together with chemotherapy, radiotherapy and immunotherapy, but also after taking into account that different tumor cells may have different sensitivities to iron-induced death, they may have significant therapeutic value [47].
2.5. Iron and Carcinogenesis
Carcinogenesis is promoted by the formation of reactive oxygen species, which can lead to DNA and protein damage. This results in mutation of proto-oncogenes and tumor suppressor genes [48]. The effect of iron on tumorigenesis is well documented on animal cells, although there are no clear conclusions in all studies. Iron as an agent of carcinogenesis has been particularly studied on lung, kidney and peritoneal cells [49]. Some studies confirm that excess iron makes tumors grow faster [50]. Furthermore, it is possible that reduced iron concentrations in premenopausal women and blood donors, as well as hepatitis C patients who underwent phlebotomies, are associated with a lower risk of tumorigenesis [51]. Animal cell studies suggest that iron deficiency may also contribute to a higher incidence of neoplastic proliferation compared to groups with normal iron levels [52]. However, observations of lung adenocarcinoma proliferation in mice show that iron loading does not affect tumor development and that tumor size is also not dependent on iron concentration [53]. An explanation for the abnormal iron metabolism in tumour cells can be explained by the example of renal cell transformation, in which ferritin is downregulated by transforming protein p21 (H-Ras). This leads to increased levels of labile iron ions, and these lead to tumour cell proliferation [54].
3. The Role of Cobalt
3.1. Cobalt
For humans, the main source of cobalt is animal food [55], indicating a risk of vitamin B12 deficiency in vegetarians [56]. In serum, cobalt ions (Co(2+)) are bound to albumin and are subsequently incorporated into vitamin B12 [57]. The binding of cobalt to this vitamin is its key role in the human body [58]. Vitamin B12 is a hydroxycobalamin, and due to the hemin moiety into which cobalt is incorporated, this vitamin is not toxic to humans [59]. Cobalamin functions as a cofactor for two enzymes: methionine synthase and methylmalonyl-CoA mutase [60]. Vitamin B12 deficiency can result in neurological and hematological disorders and may also be a risk factor for coronary heart disease [61]. In contrast, excess hydroxycobalamin can lead to liver disease, malignancies, including hematological malignancies [62]. Controlling vitamin B12 levels is possible by determining serum vitamin B12, checking serum homocysteine, holotranscobalamin II or methylmalonic acid levels [56]. Cobalt compounds may be present and contribute to people’s daily lives. This is shown in a study by Cheong et. who showed that the release of cobalt from jewellery and metal clothing items is high [63]. The use of cobalt in industry carries a risk of developing occupational diseases, which occurs as a result of inhaling its compounds. In the past, there have been exposures to cobalt chloride in tablet form, which was used to treat anemia [64]. It was withdrawn due to its toxicity. Cobalt has also been used in the production of beers. The increase in the number of cardiomyopathies has been linked specifically to the consumption of cobalt, because shortly after it was removed from brewery production, no equal or greater incidence of heart disease was observed [65]. There are a number of methods that allow the use of biological indicators of cobalt exposure such as cobaltemia and cobalturia. This allows toxicological testing, which is particularly useful in groups exposed to cobalt-containing dusts [66].
In cells, cobalt exists as a divalent or trivalent cation, where it combines with other molecules. This results in the formation of oxides, chlorides or other cobalt compounds [67]. Through its similarity to iron (II) ions, cobalt can occupy the Fe2+ site in the heme porphyrin ring, resulting in a deoxygenated form of the protein. This results in hypoxia, whereby tissues are hypoxic, and the erythropoietin gene is activated in response [60]. It is possible that there is a synergistic toxicity of cobalt and zinc [68]. In addition to porphyrin, cobalt cations may enter enzyme sites instead of zinc, magnesium or manganese ions. This is associated with lower activity of these enzymes [69]. Moreover, they inhibit RNA polymerase [70]. Furthermore, excess cobalt can lead to hyperglycemia in animals, which happens through damage to pancreatic alpha cells [71]. The negative effects of cobalt on lung cells have also been described. Its toxicity may depend on the solubility of the cobalt compound. According to Smith et al. soluble cobalt compounds are more cytotoxic to human lung fibroblasts than insoluble ones [72]. There are also reports that cobalt may contribute to the development of pneumoconiosis and asthma [71]. Radioactive cobalt (60)Co and (58)Co can be a dangerous source of radiation for hospital workers [73].
3.2. Cobalt Chloride
Cobalt chloride is primarily used as an additive in feed and fertilisers [74,75]. The literature describes that cobalt salts contribute to the induction of mutagenic agents by inhibiting DNA repair [76]. Compared to other cobalt compounds, CoCl2 shows higher toxicity, and the toxicity of cobalt is linked to its solubility. More specifically, Co3O4 is a compound that is 99% excreted in faeces, is not soluble in water and is not a highly toxic compound compared to others. In contrast, 80% of cobalt (II) chloride excreted in feces is water soluble [77]. The relationship between the toxicity of cobalt and its solubility was also investigated by Smith et al. Conducting a study on human lung fibroblasts, it was noted that soluble cobalt may be more cytotoxic than solid cobalt. However, genotoxicity in this study was at comparable levels in both cobalt states. Furthermore, soluble cobalt contributes to cell cycle arrest at lower intracellular concentrations than cobalt oxide [72]. CoCl2 is known as a compound that can lead to hypoxia-like conditions in the cell. Such processes result from the production of reactive oxygen species [78] in a non-enzymatic, non-mitochondrial mechanism [79]. In the presence of CoCl2, hypoxia-inducible factor (HIF-1α), which has a role in angiogenesis, is activated [80]. Through increased cobalt-induced HIF-1α levels, increased erythropoietin production and thus erythropoiesis is also observed [79]. This means that the HIF-1α protein cooperates with genes encoding angiopoietin, erythropoietin and, moreover, also with genes encoding vascular endothelial growth factor, insulin growth factor and also proapoptotic proteins (for example, proetin encoded by the stress-responsive gene DNA-damage-inducible transcript 4 (RTP801) and protein related to the BH3-only family NIX) [81]. In addition to other factors, it is hypoxia conditions that may contribute to cancer metastasis, which particularly emphasises the importance of a thorough understanding of the properties of this compound. Under hypoxic conditions, HIF-1α binds to nuclear b-catenin, which enhances their action. It has been suggested that transcriptional associations with HIF-1α and NF-kB, β-catenin/p300 are able to alter tumor cell kinetics [82].
3.3. Cobalt and Cancerogenesis
For several decades, attempts have been made to resolve whether cobalt and its compounds are teratogenic (Table 1). It is known that cobalt is a potent initiator of oxidative stress in cells, thereby altering gene function and consequently may increase the risk of cancer [83]. 30 years ago, single experiments were based on the possibility that cobalt could cause cancer, but conclusions were made based on observations of the effect of cobalt on cells only at the site of injection. It was not possible to say unequivocally that cobalt was a factor in carcinogenesis [58]. For example, cobalt alloys are present in orthopedic implants. However, a meta-analysis testing whether they contribute to an increased risk of cancer did not show a link between the above [84,85]. A review of the literature on cobalt as a potential carcinogen shows that the evidence is mainly based on animal cell studies. Furthermore, when looking at cobalt, compounds of this metal should also be considered. Cobalt can exist as cobalt (II) and cobalt (III), with cobalt in the second oxidation state more commonly used in the chemical industry [10]. The properties of cobalt in ionic form differ from those of cobalt compounds. The International Agency for Research on Cancer have provided a classification of cobalt and its compounds into appropriate groups according to tumorigenic potential. It appears that cobalt without other metal alloys and soluble cobalt (II) salts can be classified as probably carcinogenic to humans, placing them in group 2A. Cobalt (II) oxide belongs to group 2B, indicating possibly carcinogenicity in humans. In contrast, cobalt (II,II) oxide, cobalt (II) sulfide and other cobalt (II) compounds belong to group 3 and are not classified as carcinogenic to humans [12]. A particular cobalt compound to look at is CoCl2. It induces hypoxia, which leads to inhibition of proliferation of a human ovarian cancer cell line [86].
3.4. The Role of Cobalt in Cancer Therapy
Treatment of cervical cancer stage IB2 and higher according to FIGO consists of chemo-radiotherapy with brachytherapy [87]. In the treatment of advanced cancers, radiotherapy in combination with cisplatin is used after surgery [88].
Radiotherapy can be distinguished by high-dose rate (HDR) brachytherapy [89]. Brachytherapy allows a high dose of radiation to be delivered to the tumor with limited radiation reaching normal tissues that are close to the tumor [90]. In the case of gynecological tumors, this will mainly be the bladder and rectum [89]. The main radioisotopes used in radiotherapy of cervical cancers are Ir-192 and Co-60, with iridium being the more commonly used isotope [91]. A compilation of results showing the toxicity and impact on patient survival depending on the radioisotope used is an important subject of research. Some sources show that Ir-192 and Co-60 do not differ in terms of survival or toxicity when used in brachytherapy [91,92]. A study importing the effects of iridium and cobalt radiation on the bladder and rectum showed no differences between the two isotopes [93]. Other work presents iridium as a radioisotope with better physical properties, allowing for more satisfactory brachytherapy effects [94]. It is likely that Ir-192 may contribute to greater tumor regression and will also damage nearby tissues to a lesser extent [95]. It is possible that cobalt-60 is most effective in low-grade tumors and those less than 2 cm in diameter [96]. An important consideration in the choice of radioisotope for the treatment of gynecological cancers is the cost of therapy. The cobalt isotope compares more favourably in economic terms [92]. This is related to the longer half-life of the cobalt isotope than that of iridium, 5.26 years, 73.8 days respectively, which contributes to the need to replace the iridium source 3–4 times per year [97]. Even the need for larger shielding when using cobalt than when using iridium brachytherapy does not outweigh the cost of using Ir-192 [98]. Therefore, brachytherapy with Co-60 is proving to be an important alternative for hospitals with lower financial resources [99]. Furthermore, according to the examples in the literature, it can be noted that cobalt compounds differ in their biochemical and biophysical properties. These characteristics and the lower toxicity of cobalt compared to other transition metals have sparked interest in cobalt as a new potential anticancer drug that could yield clinically relevant results [100]. Pasukonien created cobalt ferrite nanoparticles (Co-SPIONs) to study uptake, toxicity and effects on stem-like properties in the human ovarian cancer cell line A2780 and pancreatic cancer cell line MiaPaCa2. It was observed that both cell lines accumulated Co-SPIONs, but that A2780 cells (ovarian cancer cell line) were more sensitive to cobalt ferrite exposure. The nanoparticle could be used for diagnostics and targeted cancer therapy, but the safe concentration should be carefully assessed depending on the type of cancer cells [101]. Furthermore, it has been shown that three cobalt (II) complexes [Co(MQL)2Cl2] (CoCl2), [Co(MQL)2Br2] (CoBr) and [Co(MQL)2I2] (CoI), containing 8-methoxyquinoline (MQL), can probably exhibit greater antiproliferative activity than cisplatin for the treatment of cisplatin-resistant ovarian cancers [102]. The work by Law et al. tested whether six different cobalt tris(bipyridine) complexes, depending on a given concentration, could inhibit the proliferation of cancer cells in various tissues, which would occur by arresting cell cycle progression. In the case of cervical cancer cells (HeLa) to which complex 2 [CoIII(4,4′-Me2-bpy)3](PF6)3 was applied, only a high concentration stopped the HeLa cancer cell in S phase. To confirm this, the levels of cell cycle regulatory proteins from G1 to S phase were examined. An accumulation of cyclin A and E2F was observed, which could suggest that complex 2 prevents the proliferation of the cancer cell by arresting it in S phase. This study demonstrates the possible efficacy of alternative metal compounds in cancer cells in vivo in arresting their proliferation, which is important data, especially considering the limitations of cisplatin, carboplatin in cancer therapy due to the production of resistance against these compounds after the initial treatment [103].
4. Iron and Cobalt in Gynecological Diseases
4.1. Ovarian Cancer
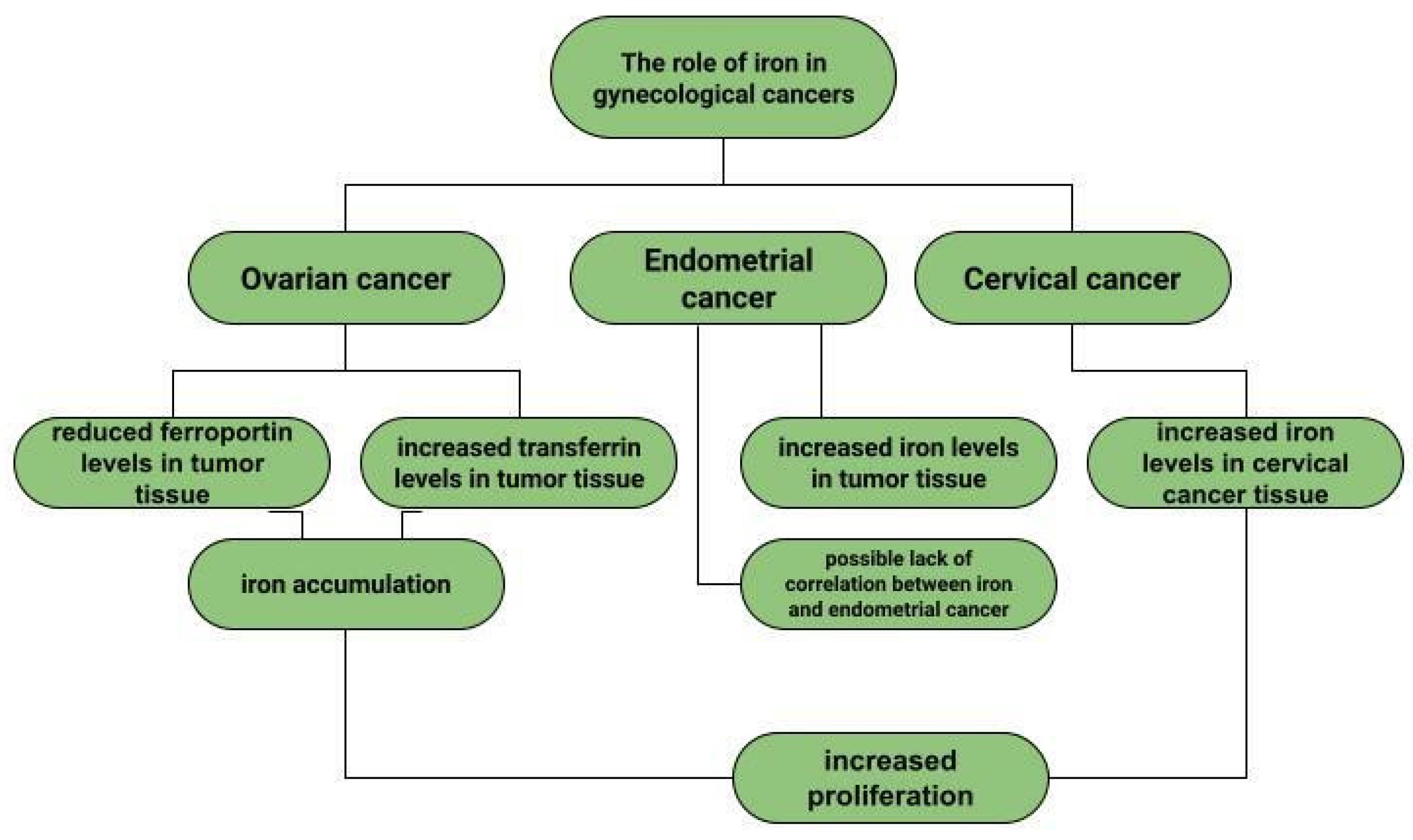
Ovarian cancer is a neoplasm that often manifests itself only at a late stage [104], and symptoms are often non-specific, sometimes resembling gastrointestinal symptoms [105]. Among other things, late diagnosis contributes to ovarian cancer being the most common cause of death among gynecological cancer patients [14]. Risk factors for ovarian cancer include early menarche, late menopause [106], BRCA1 and BRCA2 gene mutations [107], obesity [108], and asbestos [109] and talc [110] exposures. In addition to the above, the effect of metals on ovarian cancer risk is also being investigated. In ovarian cancer tissues compared to non-malignant tissues, a higher iron content in the second oxidation state is observed. In non-malignant lesions, such as in ovarian cyst fluid, the greater proportion of iron is Fe3+ [111]. These observations are not confirmed by the Yaman et al. study, in which no difference in iron concentrations was observed for cancerous and non-cancerous tissues [112]. Another study examined what effect a reduction in intracellular iron concentration has on tumor growth. The results led to the conclusion that ovarian cancer cells are sensitive to ferroptosis and iron chelators, which could be used in ovarian cancer therapy [113]. Basuli shows that ferroportin (FPN) levels are reduced in tumor tissue of high-grade serous ovarian cancer. In contrast, transferrin (TFR1), an iron importer, is increased in this tissue. This leads to iron accumulation in the tissue and increased proliferation (Figure 1) [113]. Dan Sun et al. examined the function of lidocaine in the process of ferroptosis of ovarian and breast cancer cells. They reported that lidocaine inhibited mRNA expression of solute carrier family 7 member 11 (SLC7A11) in a dose-dependent manner-3mM lidocaine produced the highest effect. Co-treatment of lidocaine and erastin was able to increase the effect of erastin on inhibiting ovarian cancer target line SKOV-3 and T47D. Total iron and ferrous iron (Fe2+) were analysed in the cells. Fe2+ levels were upregulated by lidocaine in SKOV-3 and T47D cells. This study also noted that lidocaine promoted lipid ROS accumulation in SKOV-3 and T47D cells. Expression of SLC7A11 and glutathione peroxidase 4 (GPX4) was repressed by lidocaine in SKOV-3 and T47D cells. Data suggest that lidocaine, through the accumulation of Fe2+, iron and lipid reactive oxygen species (ROS), induces ferroptosis of ovarian and breast cancer cells, which inhibits the proliferation of these cells [114]. Using the example of olaparib for the treatment of ovarian cancer, Ting Hong et. shows a correlation between PARP inhibitor action and ferroptosis, in which ferroptosis is thought to be responsible for olaparib’s efficacy. PARP inhibition with the pharmaceutical or genetic deletion of PARP reduces the expression of the cysteine transporter SLC7A11 in a p53-dependent manner. As a result, glutathione biosynthesis is reduced, which promotes lipid peroxidation and ferroptosis. This study leads to the conclusion that stimulation of the ferroptosis process by ferroptosis inducers (FINs) will sensitise ovarian cancer cells with a mutation of the BRCA gene to this process resulting in reduced tumor proliferation [115] (Figure 2).
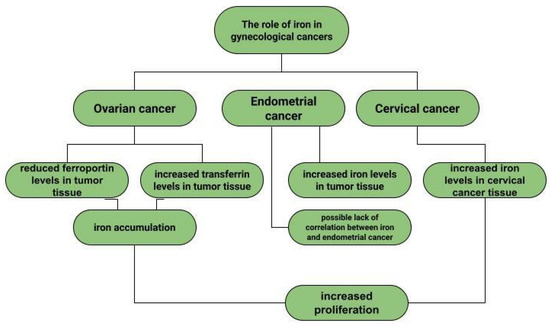
Figure 1.
The role of iron in gynecological diseases.
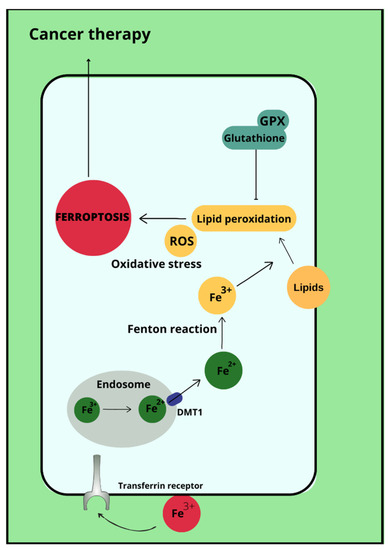
Figure 2.
Ferroptosis in cancer therapy.
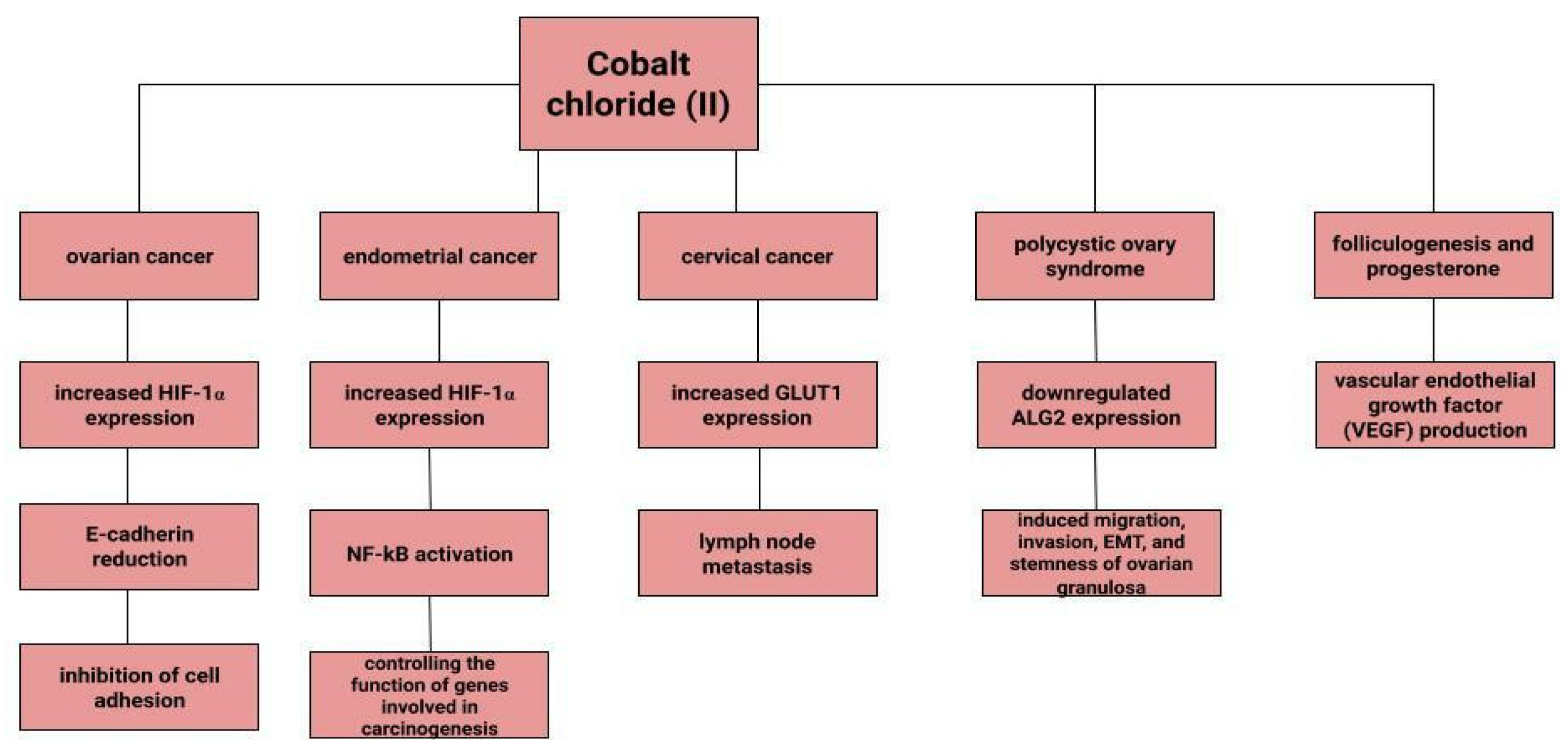
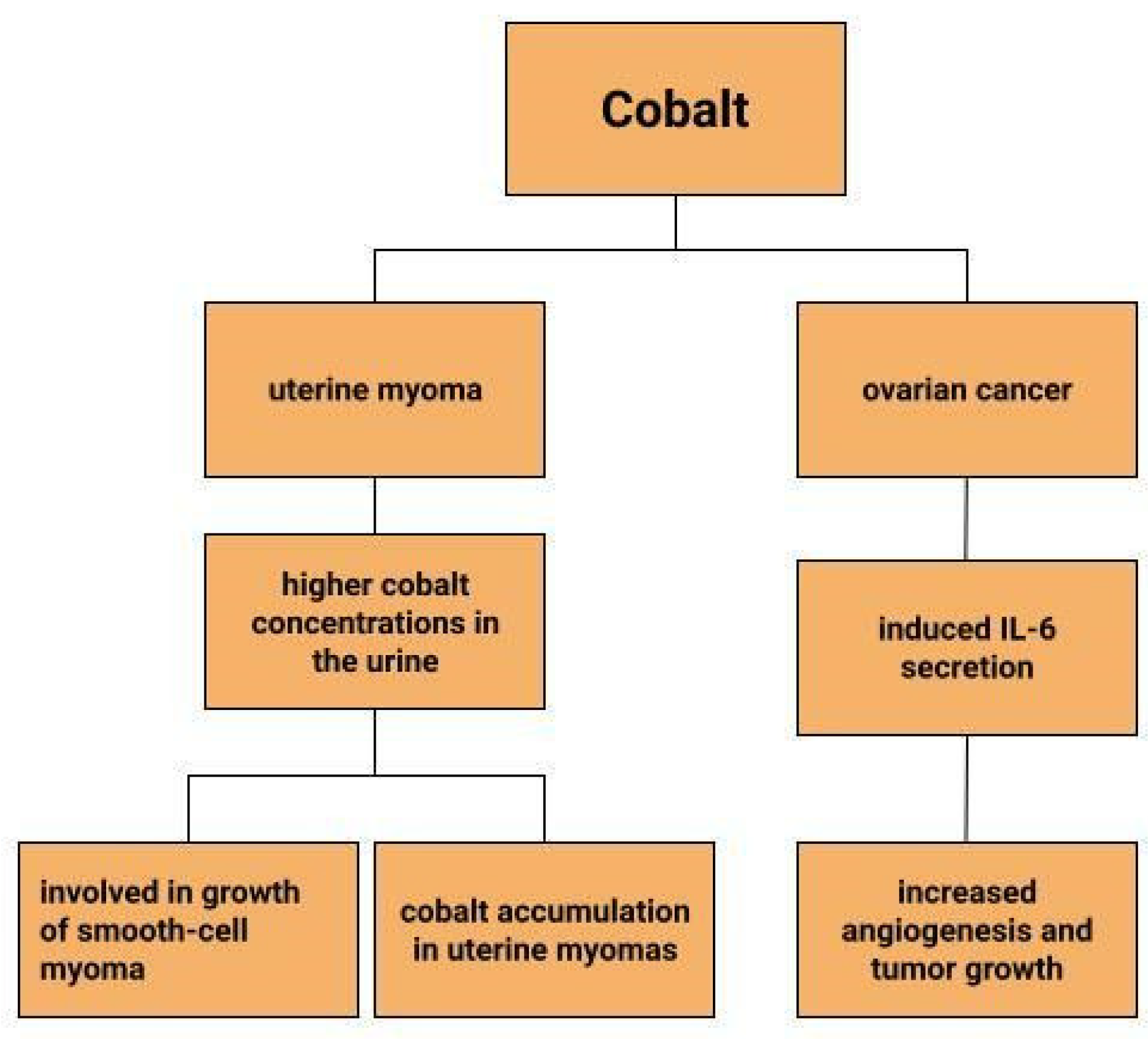
Dingx Li et. investigated the function of glutathione peroxidase 4 (GPX4) in ovarian cancer cells and mouse xenografts, as a critical regulator of ferroptosis. The study was conducted in which human ovarian cancer cells and healthy ovarian epithelial cells were administered deferoxamine (DFO) and ferrous ammonium citrate (FAC). DFO chelating intracellular iron impaired ovarian cancer cell survival. In contrast, FAC and blocking GPX4 resulted in inhibition of tumor growth and induction of ferroptosis. This accelerated cell apoptosis and reduced Fe3+ accumulation in mouse cells [116]. Some metals, including cobalt, can affect the human ovarian cancer G1 protein-coupled receptor (hOGR1) [117]. Cobalt, through activation of mammalian ovarian G-protein-coupled receptor 1 OGR1, can induce IL-6 cytokine secretion [118]. Human ovarian cancer G-protein-coupled receptor 1 (hOGR1) and GPR4 (hGPR4) can bind to extracellular protons, leading to activation of intracellular signalling pathways through G proteins. A reduction in angiogenesis and tumor growth was observed in GPR-null mice. It is possible that it is cobalt that stimulates the zOGR1-mediated SRE-promoter. Iron was also tested, and no such relationship was found for cobalt [117]. In contrast, CoCl2 inducing HIF-1alpha in a time- and dose-dependent manner may lead to inhibition of cell adhesion (Figure 3), and the relationship between proliferation and invasion of HO-8910PM human ovarian cancer cells is altered by induction of hypoxia conditions and subsequent reoxysgenation [86]. The role of HIF-1α, which cobalt chloride is used to induce, in ovarian cancer cells may involve a reduction in E-cadherin, as demonstrated in two human ovarian cancer cell lines (SKOV3 and OVCAR5) [119]. The role of cobalt in the development of ovarian cancer needs to be verified in further studies.
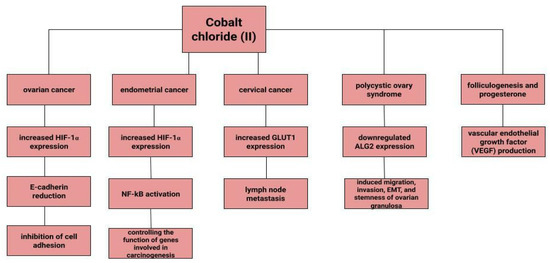
Figure 3.
The role of cobalt chloride (II) in gynecological diseases.
4.2. Endometrial Cancer
Endometrial cancer is a neoplasm that is more likely to manifest itself while still at an early stage than ovarian cancer [17]. Abnormal uterine bleeding, which includes prolonged menstrual bleeding and acyclic bleeding, is the most common symptom of endometrial cancer [18]. Risk factors for ovarian cancer include obesity, childlessness, smoking and diabetes. There are well-recognised protective factors such as pregnancy, hormonal contraception [120]. The expression of hypoxia-inducible factor (HIF-1α), the concentration of which increases in the presence of cobalt (II) chloride [80], may correlate with endometrial cancer malignancy. The classical nuclear transcription factor NF-kB pathway is thought to be activated in endometrial cancer cells via HIF-1α [82]. Nuclear transcription factor-kB is composed of subunits of Rel family proteins (p50, p52, p65 (RelA), c-Rel and RelB) [121]. NF-kB can control the function of many genes that are involved in inflammation, apoptosis and also carcinogenesis [122]. According to some sources, the use of iron supplements could be a potential marker of endometrial cancer rather than its aetiological factor [123]. Higher iron concentrations are observed in cancerous endometrial tissues than in non-cancerous tissues [112]. Some sources indicate a positive association between heme iron intake, total iron and liver intakes and endometrial cancer risk, with no association between intake of other red processed meat [124]. However, it is likely that dietary iron, as well as iron taken with red meat, or hem iron or non-heem iron do not affect the increased risk of endometrial cancer [125].
4.3. Cervical Cancer
Cervical cancer remains a significant oncological challenge, and particularly so in countries where human papillomavirus (HPV) vaccines are not used, which should be used before exposure to the virus [126,127,128]. However, HPV is not the only risk factor for cervical cancer development, and other variables may influence tumor progression. In a study by Cheng et al. under CoCl2-induced hypoxia, the expression of glucose transporter protein 1 (GLUT1) increases progressively according to the clinical stage of cervical cancer. Correlations of increased GLUT1 expression with lymph node metastasis are observed. In normal tissue, this expression is lowest, while it is higher in cervical intraepithelial neoplasia and highest in cervical cancer [129]. H. Cunzhi, on the basis of a study of 30 patients with myoma of the uterus, 40 patients with cervical cancer, and 50 healthy patients, showed that iron levels in cervical cancer tissue were higher than in unchanged tissue, and that serum iron levels were lower in patients with cervical cancer than in healthy patients. H. Cunzhi, based on a study of 30 patients with myoma, 40 patients with cervical cancer, and 50 healthy patients, showed that iron levels are higher in cervical cancer tissue than in myoma [130].
4.4. Uterine Myoma
Uterine myomas are among the most common benign uterine neoplasms [131]. Although the course is asymptomatic in most cases [132], they can sometimes cause prolonged, heavy, acyclic bleeding [133] resulting in the development of anaemia in patients with uterine myomas [134,135]. They also increase the risk of infertility, miscarriage, intrauterine growth retardation and preterm birth [136]. Depending on their location, they are divided into submucosal, intramural and subserosal [137]. A reflection of the lower serum iron concentrations in patients with myomas can be seen in measurements of iron concentrations in myometrial tissues. Compared to normal uterine tissues and cancerous uterine tissues, iron concentrations in myomas are significantly lower. Moreover, compared to other trace metals, it is the iron concentrations that show greater tissue-specific differences [135]. Similar observations are made by H. Cunzhi et al. who, on the basis of a study of 30 patients with myoma, 40 patients with cervical cancer, and 50 healthy patients, showed that iron concentrations were higher in cervical cancer tissue than in myoma [130]. There are attempts to look for a link between trace elements and uterine myomas. One study found higher cobalt concentrations in the urine of patients with uterine myomas, which is explained by the authors in two ways (Figure 4). One would be that higher cobalt exposure may contribute to the growth of smooth-cell myoma. A second reason considered for the increased cobalt levels in women with uterine myomas compared to women without this benign tumor could be that uterine myomas are a site of cobalt accumulation [138]. However, this relationship requires further research.
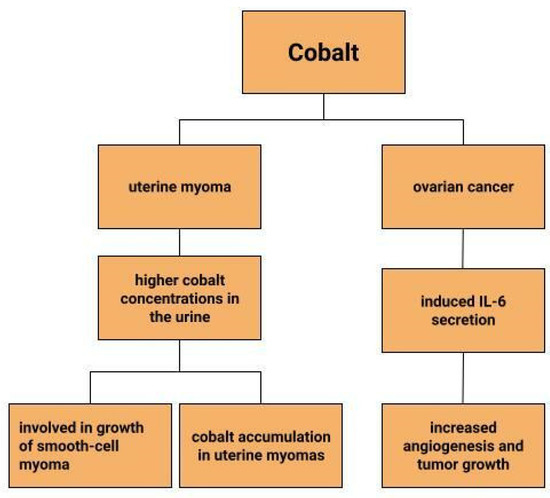
Figure 4.
The role of cobalt in gynecological diseases.
4.5. Endometriosis
Endometriosis is a chronic disease in which active endometrial tissue is found outside the uterine cavity [139]. Patients experience increased lower abdominal pain, heavy menstrual bleeding, and these women are also diagnosed with infertility [140]. Retrograde menstruation, chorionic metaplasia and residual Mϋllerian duct are mentioned in the pathogenesis. In addition, genetic factors as well as environmental factors may influence the development of endometriosis, resulting in the development of chronic inflammation [141]. In relation to the effect of iron on inflammation through the induction of oxidative stress in the cell, the role of iron in endometriosis is being investigated.
Through the high iron content of endometrial cysts, low-oxygen conditions may occur, leading to destabilisation of iron regulatory protein 2 (IRP2) resulting in too many iron (II) ions entering the stroma cells. Such a condition may be a potential factor for carcinogenesis in endometriosis [142]. In a study by Mori et al., it is suspected that ectopic endometrial tissue provides protection to ovarian epithelial cells by taking up excess iron. It was shown that the concentration of catalytic Fe (II) was higher in ectopic endometrial stromal cells than in normal eutopic endometrial stromal cells [143]. Endometriosis is among the factors in the development of ovarian clear cell adenocarcinoma and endometrioid carcinoma and are therefore endometriosis-associated ovarian cancers (EAOCs) [144]. Oxidative stress has been linked to the inflammatory response in endometriosis, and this may be iron-dependent [145,146]. Akashi et al. observed increased iron in ovarian endometrioma (OE) and clear cell carcinoma (CCC). They presented the localisation of iron in epithelial cells and in the ovarian stroma and that macrophages are involved in iron deposition in these cells, more specifically under the epithelium of ovarian endometrioma. These are the macrophage markers CD11c, CD163, CD206, and more CD163+ and CD206+ cells are observed in the deeper layer of the OE stroma than CD11c+ cells. Similarly, for CCC, in whose stroma CD163+ and CD206+ cells are found. The expression of iron transport proteins in OE and CCC, such as divalent metal transporter 1 (DMT1), transferrin receptor (TfR) and ferroportin FPN, is high, with TfR and FPN expression at a higher level in CCC than in OE [144].
4.6. Polycystic Ovary Syndrome
Polycystic ovary syndrome (PCOS) is one of the most common endocrinopathies among young women [147]. PCOS consists of hyperandrogenemia, ovulatory disorders, hyperinsulinism, and insulin resistance [148]. In addition, the syndrome raises the risk of endometrial cancer [20], as well as diabetes, cardiovascular disease [149] and contributes to reduced female fertility [150]. The role of iron in PCOS appears to be important, if only because iron may have a role as an oxidative factor in the development of insulin resistance in these patients [151]. Some studies suggest that this reduction in iron concentration may participate in the pathophysiology of polycystic ovary syndrome [152]. Other observations point to the possible efficacy of lowering iron levels in improving glucose tolerance in PCOS patients, as it has been noted that Fe is elevated in PCOS patients and especially when this syndrome is accompanied by abnormal glucose tolerance, suggesting that excess iron may be one of the causes of insulin resistance and diabetes in PCOS patients [153]. Insulin contributes to increased intestinal iron absorption and iron deposition in tissues. The findings of Luque-Ramírez et al. present that it is hyperinsulinemia and insulin resistance that cause increased tissue ferritin levels in women with PCOS [154]. Furthermore, it is possible that when insulin resistance is present, reduced hepcidin concentrations occur, resulting in increased iron levels [155]. The association of hepcidin and iron levels in PCOS patients has not been confirmed in another study, suggesting the need for expanded research [151]. Only one paper reports on the association between cobalt and PCOS. Serum concentrations of magnesium, cadmium and also cobalt in patients with PCOS were shown to differ between the study group and the control group [156]. The relationship between α-1,3/1,6 mannosyltransferase (ALG2) and ovulation is being investigated in patients with polycystic ovary syndrome. CoCl2 may downregulate ALG2 expression in PCOS patients, which may induce migration, invasion, EMT, and stemness of ovarian granulosa cells. In contrast, elevated ALG2 levels are observed in the serum of PCOS patients, which is being considered as a biomarker for the diagnosis of PCOS [157].
4.7. Folliculogenesis and Progesterone
Deficiency of vitamin B12, which contains cobalt, can affect oestrogen levels, stopping ovulation [158]. In animals, cobalt deficiency results in lower conception rates [159]. In addition, cobalt, as a probable factor affecting the secretory activity of ovarian granulosa cells, would be expected to affect progesterone and insulin-like growth factor 1 (IGF-1) concentrations [160]. Kolesarova et. Conducting a study on porcine ovarian granulosa cells, that the release of IGF-1 by these cells varies according to the concentration of cobalt administered. A similar effect was also observed on rat ovarian cells, in which the value of released IGF-I when administered 90 μg.mL of cobalt sulfate was 18.64 +/− ng.mL, against 8.85 +/− 2.99 ng.mL in the control group, i.e., without cobalt supply. In contrast, progesterone release by granulosa cells was inhibited by cobalt at a concentration of 0.09 mg/mL, but no such relationship was shown at higher cobalt concentrations [160]. Using rat ovarian cells as an example, it was noted that cobalt in the form of cobalt sulfate (CoSO4·7H2O) at doses of 170–500 µg mL can inhibit progesterone secretion [161]. In contrast, a study by Grasselli F. et al. does not link the inhibition of progesterone production to CoCl2 but is involved in vascular endothelial growth factor (VEGF) production in granulosa cells, thus controlling the folicular angiogenic process [162]. Bax and caspase-3 are among the factors involved in apoptosis. There are associations of cobalt with decreased expression of Bax and caspase-3 in rat ovarian cells [161]. The direct effect of ions including Co2+ on embryogenesis was tested by culturing mouse blastocysts in the presence of individual ions in vitro. It was observed that 100 microM cobalt ions reduced trophoblast size. Cobalt ions would also be expected to affect morphological changes including a reduction in adhesion capacity [163]. The above data suggest that cobalt or its compounds may affect folliculogenesis.
4.8. Placental Hypoxia
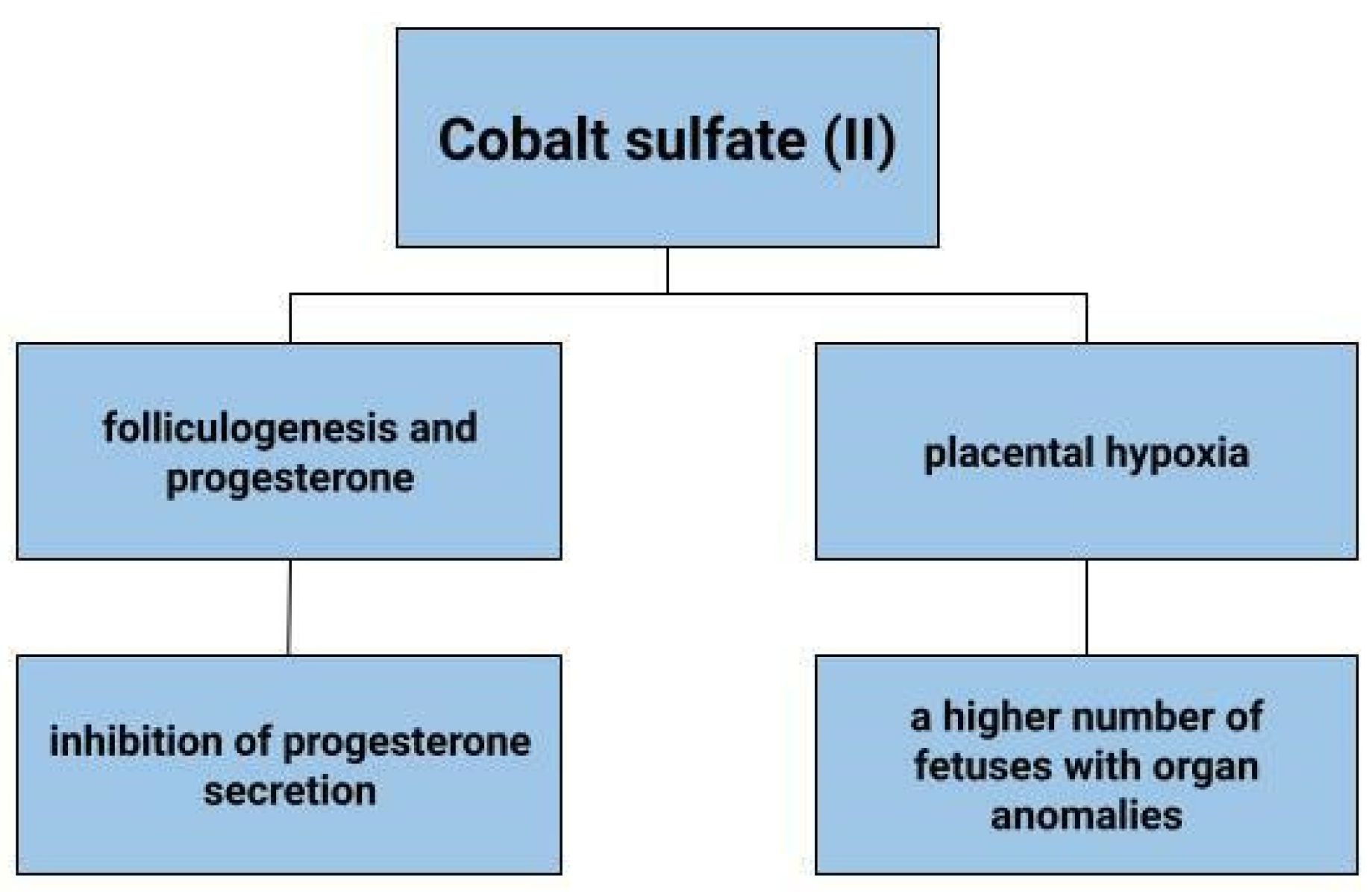
Hypoxia can adversely affect the development of the placenta. It can result in reduced fetal growth, as well as pre-eclampsia. In a state of cellular hypoxia, which in the study by Baumann et al. was induced by desferroxamine and cobalt chloride, there was an increase in the expression of the glucose transporter 1 GLUT1 in the human placental cell line that originates from a choriocarcinoma (BeWo) [164]. Furthermore, the increase in GLUT was shown to be due not only to low oxygen concentration, but also to other factors that induce HIF-1α. These results depict that hypoxia increases glucose transport into cells [164]. The effects of cobalt sulphate on pregnant mice and fetuses have also been tested (Figure 5). Cobalt can enter the blood and amniotic fluid of the foetus. Dose-dependent toxicity of this element was therefore observed in both mother and fetus. A higher number of fetuses with delayed skeletal development and weight were found, as well as organ anomalies, such as kidney, eye, genitourinary system [165]. In contrast, no toxic effects of cobalt on fetuses were observed in the work of Paternain et al. [166].
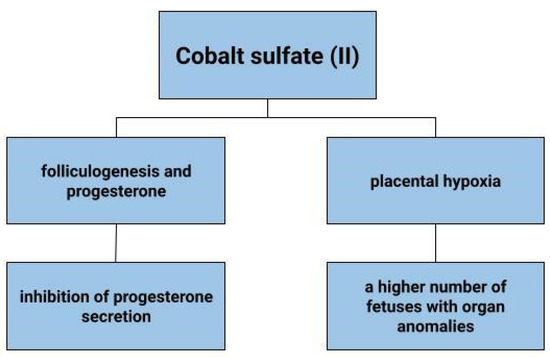
Figure 5.
The role of cobalt sulfate (II) in gynecological diseases.

Table 1.
Effects of cobalt and its compounds on specific tissues.
Table 1.
Effects of cobalt and its compounds on specific tissues.
| Tissues | Cobalt Form | Result | Reference |
|---|---|---|---|
| Ovary Animal tissue | Cobalt (II) chloride | Probably activated the zOGR1-mediated signaling pathway à increase in angiogenesis, increase in tumor size | Negishi et al. [117] |
| Ovary Gilts tissue | Cobalt (II) sulfate | Increase in released IGF-1, Inhibition of progesterone release | Kolesarova et al. [160] |
| Ovary Rat tissue | Cobalt (II) sulfate | Inhibition of progesterone release | Roychoudhury et al. [161] |
| Granulosa cells Human tissue | Cobalt (II) chloride | No effect of CoCl2 on progesterone, Involvement in the foliculalar angiogenic process through stimulation of VEGF production | Grasselli et al. [162] |
| Ovary Rat cells | Cobalt (II) sulfate | Decreased expression of Bax and caspase-3 à inhibition of apoptosis | Roychoudhury et al. [161] |
| Endometrial cancer | Cobalt (II) chloride | Effects on the classical nuclear pathway of the transcription factor NF-kB via HIF-1α; effects on carcinogenesis | Yoshida et al. [82] |
| Choriocarcinoma cells, a trophoblast cell model, and human placental villous tissue explants | Cobalt (II) chloride | Placental hypoxia | Baumann et al. [164] |
| Pregnant mice, fetuses | Cobalt (II) sulfate | Increased number of fetuses with delayed skeletal development and weight, organ anomalies-kidneys, eyes, genitourinary system | Szakmáry et al. [165] |
| Trophoblast, Mice | Cobalt (2+) | Reduction in trophoblost size and adhesion capacity | Paksy et al. [163] |
| Uterine myomas | Cobalt (2+) | Influence on smooth cell myxoma growth or myxoma as a site of cobalt accumulation | Johnstone et al. [138] |
| Polycystic ovary syndrome | Cobalt (2+) | No relation | Kurdoglu et al. [156] |
zOGR1-zebrafish ovarian cancer G-protein-coupled receptor 1, IGF-1-insulin-like growth factor 1, CoCl2-cobalt (II) chloride, VEGF-vascular endothelial growth factor, NF-kB-nuclear factor kappa-light-chain-enhancer of activated B cells, HIF-1α-hypoxia-inducible factor 1-alpha.
5. Conclusions
Cobalt and iron, through their properties, compound formation and participation in a number of chemical reactions in the human body, should not be interpreted without insight into these mechanisms. A review of the literature shows that cobalt compounds may be factors that increase the risk of cancer development and its faster progression. However, cobalt ions alone do not appear to pose such a risk. Most studies are based on animal cells, investigating the effects of cobalt (II) chloride. Cobalt salts can prevent DNA repair, contributing to the development of mutagenic agents and, through these processes, lead to cancer development. In addition, cobalt binding HIF-1α leads to the development of hypoxia-like conditions. However, due to its physical properties, it can be used in the radiotherapy of gynecological cancers, especially in countries with relatively lower budgets for cancer therapy. Most of the information relates to animal cells, which allows cobalt compounds to be considered as potentially carcinogenic and allocated to the appropriate group, as obtained in the IARC analysis. However, more evidence of the effect of cobalt on the development of gynecological diseases in humans is lacking. Iron may contribute to cancer and other disorders in gynecology. It has been observed at higher concentrations in cervical cancer cells, endometrial cancer, and ovarian cancer compared to healthy tissue. The role of iron in the development of polycystic ovary syndrome remains particularly unclear, and it is possible to observe lower iron concentrations in myomas than in non-transformed uterine tissues, but this may be related to heavy bleeding in these patients. Due to the participation of cobalt and iron in enzymatic, redox reactions, in which reactive oxygen species are formed, the existence of corresponding levels of these elements in the human body cannot be ruled out.
Author Contributions
Conceptualization, A.Ć., M.K. and A.C.-P.; methodology, validation, A.C.-P., M.K. and A.Ć.; formal analysis, M.K.; investigation, A.Ć.; resources, A.C.-P.; data curation, M.K.; writing—original draft preparation, A.Ć. and M.K.; writing—review and editing, A.Ć.; visualization, M.K.; supervision, A.C.-P.; project administration, M.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Sule, K.; Umbsaar, J.; Prenner, E.J. Mechanisms of Co, Ni, and Mn toxicity: From exposure and homeostasis to their interactions with and impact on lipids and biomembranes. Biochim. et Biophys. Acta (BBA) Biomembr. 2020, 1862, 183250. [Google Scholar] [CrossRef] [PubMed]
- Galaris, D.; Barbouti, A.; Pantopoulos, K. Iron homeostasis and oxidative stress: An intimate relationship. Biochim. et Biophys. Acta (BBA) Mol. Cell Res. 2019, 1866, 118535. [Google Scholar] [CrossRef] [PubMed]
- Makovec, T. Idea to explore: The structure of the oxygen and iron ion. Biochem. Mol. Biol. Educ. 2018, 46, 630–633. [Google Scholar] [CrossRef] [PubMed]
- Roth, J.R.; Lawrence, J.G.; Bobik, T.A. Cobalamin (coenzyme B12): Synthesis and biological significance. Annu. Rev. Microbiol. 1996, 50, 137–181. [Google Scholar] [CrossRef] [PubMed]
- Koury, M.J.; Ponka, P. New insights into erythropoiesis: The roles of folate, vitamin B12, and iron. Annu. Rev. Nutr. 2004, 24, 105–131. [Google Scholar] [CrossRef]
- Cappellini, M.D.; Musallam, K.M.; Taher, A.T. Iron deficiency anaemia revisited. J. Intern. Med. 2020, 287, 153–170. [Google Scholar] [CrossRef]
- Vallet, N.; Delaye, J.-B.; Ropert, M.; Foucault, A.; Ravalet, N.; Deriaz, S.; Chalopin, T.; Blasco, H.; Maillot, F.; Hérault, O.; et al. Megaloblastic anemia-related iron overload and erythroid regulators: A case report. J. Med. Case Rep. 2021, 15, 463. [Google Scholar] [CrossRef]
- Brannon, P.M.; Taylor, C.L. Iron Supplementation during Pregnancy and Infancy: Uncertainties and Implications for Research and Policy. Nutrients 2017, 9, 1327. [Google Scholar] [CrossRef]
- Okamoto, S.; Eltis, L.D. The biological occurrence and trafficking of cobalt. Metallomics 2011, 3, 963–970. [Google Scholar] [CrossRef]
- Barceloux, D.G. Cobalt. J. Toxicol. Clin. Toxicol. 1999, 37, 201–216. [Google Scholar] [CrossRef]
- Seidman, J.D.; Woodburn, R. Pseudoxanthomatous salpingitis as an ex vivo model of fallopian tube serous carcinogenesis: A clinicopathologic study of 49 cases. Int. J. Gynecol. Pathol. 2015, 34, 275–280. [Google Scholar] [CrossRef] [PubMed]
- Karagas, M.R.; Wang, A.; Dorman, D.C.; Hall, A.L.; Pi, J.; Sergi, C.M.; Symanski, E.; Ward, E.M.; Arrandale, V.H.; Azuma, K.; et al. Carcinogenicity of cobalt, antimony compounds, and weapons-grade tungsten alloy. Lancet Oncol. 2022, 23, 577–578. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.Q.; Li, L. A meta-analysis of XRCC1 single nucleotide polymorphism and susceptibility to gynecological malignancies. Medicine 2021, 100, E28030. [Google Scholar] [CrossRef]
- Kujawa, K.A.; Lisowska, K.M. Ovarian cancer—From biology to clinic. Postep. Hig. Med. Dosw. 2015, 69, 1275–1290. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Men, X.; Zhang, W.; Lei, P. Advances in tumor markers of ovarian cancer for early diagnosis. Indian J. Cancer 2014, 51 (Suppl. S3), e72–e76. [Google Scholar] [CrossRef] [PubMed]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri. Int. J. Gynecol. Obstet. 2018, 143 (Suppl. S2), 22–36. [Google Scholar] [CrossRef] [PubMed]
- Arend, R.C.; Jones, B.A.; Martinez, A.; Goodfellow, P. Endometrial cancer: Molecular markers and management of advanced stage disease. Gynecol. Oncol. 2018, 150, 569–580. [Google Scholar] [CrossRef]
- Clarke, M.A.; Long, B.J.; Morillo, A.d.; Arbyn, M.; Bakkum-Gamez, J.N.; Wentzensen, N. Association of Endometrial Cancer Risk with Postmenopausal Bleeding in Women: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2018, 178, 1201–1208. [Google Scholar] [CrossRef]
- Kietlińska, Z.; Stelmachów, J.; Antczak, A.; Timorek, A.; Sawicki, W.; Tymińska, B. Preoperative evaluation of cervical involvement in endometrial cancer. Ginekol. Polska 1998, 69, 247–251. Available online: https://pubmed.ncbi.nlm.nih.gov/9695321/ (accessed on 13 November 2022).
- Barry, J.A.; Azizia, M.M.; Hardiman, P.J. Risk of endometrial, ovarian and breast cancer in women with polycystic ovary syndrome: A systematic review and meta-analysis. Hum. Reprod. Updat. 2014, 20, 748–758. [Google Scholar] [CrossRef]
- Rockfield, S.; Raffel, J.; Mehta, R.; Rehman, N.; Nanjundan, M. Iron overload and altered iron metabolism in ovarian cancer. Biol. Chem. 2017, 398, 995–1007. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.Y.; Babitt, J.L. Liver iron sensing and body iron homeostasis. Blood 2019, 133, 18–29. [Google Scholar] [CrossRef] [PubMed]
- Fairweather-Tait, S.J. Iron. J. Nutr. 2001, 131, 1383S–1386S. [Google Scholar] [CrossRef] [PubMed]
- Kabat, G.C.; Rohan, T.E. Does excess iron play a role in breast carcinogenesis? An unresolved hypothesis. Cancer Causes Control 2007, 18, 1047–1053. [Google Scholar] [CrossRef] [PubMed]
- Torti, S.v.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [CrossRef] [PubMed]
- Winterbourn, C.C. Toxicity of iron and hydrogen peroxide: The Fenton reaction. Toxicol. Lett. 1995, 82–83, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Höhn, A.; Grune, T. Lipofuscin: Formation, effects and role of macroautophagy. Redox Biol. 2013, 1, 140–144. [Google Scholar] [CrossRef]
- Leitner, D.F.; Connor, J.R. Functional roles of transferrin in the brain. Biochim. et Biophys. Acta (BBA) Gen. Subj. 2012, 1820, 393–402. [Google Scholar] [CrossRef]
- Gammella, E.; Buratti, P.; Cairo, G.; Recalcati, S. The transferrin receptor: The cellular iron gate. Metallomics 2017, 9, 1367–1375. [Google Scholar] [CrossRef]
- Morgan, E.H. Cellular iron processing. J. Gastroenterol. Hepatol. 1996, 11, 1027–1030. [Google Scholar] [CrossRef]
- Parrow, N.L.; Li, Y.; Feola, M.; Guerra, A.; Casu, C.; Prasad, P.; Mammen, L.; Ali, F.; Vaicikauskas, E.; Rivella, S.; et al. Lobe specificity of iron binding to transferrin modulates murine erythropoiesis and iron homeostasis. Blood 2019, 134, 1373–1384. [Google Scholar] [CrossRef]
- Sikström, C.; Beckman, L.; Hallmans, G.; Asplund, K. Transferrin types, iron-binding capacity and body iron stores. Hum. Hered. 1993, 43, 337–341. [Google Scholar] [CrossRef] [PubMed]
- Ward, D.M.; Kaplan, J. Ferroportin-mediated iron transport: Expression and regulation. Biochim. Biophys. Acta (BBA) Mol. Cell Res. 2012, 1823, 1426–1433. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, E.; Ganz, T. Hepcidin-Ferroportin Interaction Controls Systemic Iron Homeostasis. Int. J. Mol. Sci. 2021, 22, 6493. [Google Scholar] [CrossRef] [PubMed]
- Dixon, S.J.; Lemberg, K.M.; Lamprecht, M.R.; Skouta, R.; Zaitsev, E.M.; Gleason, C.E.; Patel, D.N.; Bauer, A.J.; Cantley, A.M.; Yang, W.S.; et al. Ferroptosis: An iron-dependent form of nonapoptotic cell death. Cell 2012, 149, 1060–1072. [Google Scholar] [CrossRef]
- Zhou, X.Y.; Dai, H.Y.; Zhang, H.; Zhu, J.L.; Hu, H. Ferroptosis-Related lncRNA for the Establishment of Novel Prognostic Signature and Therapeutic Response Prediction to Endometrial Carcinoma. BioMed Res. Int. 2022, 2022, 2056913. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Jiang, W.; Wang, W.; Xiong, R.; Wu, X.; Geng, Q. Ferroptosis and its emerging roles in cardiovascular diseases. Pharmacol. Res. 2021, 166, 105466. [Google Scholar] [CrossRef]
- Zhou, B.; Liu, J.; Kang, R.; Klionsky, D.J.; Kroemer, G.; Tang, D. Ferroptosis is a type of autophagy-dependent cell death. Semin. Cancer Biol. 2020, 66, 89–100. [Google Scholar] [CrossRef]
- Chen, X.; Kang, R.; Kroemer, G.; Tang, D. Ferroptosis in infection, inflammation, and immunity. J. Exp. Med. 2021, 218, e20210518. [Google Scholar] [CrossRef]
- Tang, D.; Kroemer, G. Ferroptosis. Curr. Biol. 2020, 30, R1292–R1297. [Google Scholar] [CrossRef]
- Gao, H.; Bai, Y.; Jia, Y.; Zhao, Y.; Kang, R.; Tang, D.; Dai, E. Ferroptosis is a lysosomal cell death process. Biochem. Biophys. Res. Commun. 2018, 503, 1550–1556. [Google Scholar] [CrossRef] [PubMed]
- Jiang, L.; Kon, N.; Li, T.; Wang, S.-J.; Su, T.; Hibshoosh, H.; Baer, R.; Gu, W. Ferroptosis as a p53-mediated activity during tumour suppression. Nature 2015, 520, 57–62. [Google Scholar] [CrossRef] [PubMed]
- Kang, R.; Kroemer, G.; Tang, D. The tumor suppressor protein p53 and the ferroptosis network. Free. Radic. Biol. Med. 2019, 133, 162–168. [Google Scholar] [CrossRef]
- Xie, Y.; Zhu, S.; Song, X.; Sun, X.; Fan, Y.; Liu, J.; Zhong, M.; Yuan, H.; Zhang, L.; Billiar, T.R.; et al. The Tumor Suppressor p53 Limits Ferroptosis by Blocking DPP4 Activity. Cell Rep. 2017, 20, 1692–1704. [Google Scholar] [CrossRef] [PubMed]
- You, Y.; Fan, Q.; Huang, J.; Wu, Y.; Lin, H.; Zhang, Q. Ferroptosis-Related Gene Signature Promotes Ovarian Cancer by Influencing Immune Infiltration and Invasion. J. Oncol. 2021, 2021, 9915312. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.-L.; Linghu, D.-L.; Hung, M.-C. Ferroptosis: A promising target for cancer immunotherapy. Am. J. Cancer Res. 2021, 11, 5856–5863. Available online: https://pubmed.ncbi.nlm.nih.gov/35018229/ (accessed on 18 October 2022).
- Guo, Q.; Li, L.; Hou, S.; Yuan, Z.; Li, C.; Zhang, W.; Zheng, L.; Li, X. The Role of Iron in Cancer Progression. Front. Oncol. 2021, 11, 4571. [Google Scholar] [CrossRef]
- Chua, A.C.G.; Klopcic, B.; Lawrance, I.C.; Olynyk, J.K.; Trinder, D. Iron: An emerging factor in colorectal carcinogenesis. World J. Gastroenterol. WJG 2010, 16, 663. [Google Scholar] [CrossRef]
- Asia, B.P.; Toyokuni, S. Role of iron in carcinogenesis: Cancer as a ferrotoxic disease. Cancer Sci. 2009, 100, 9–16. [Google Scholar] [CrossRef]
- Iron Enhances Tumor Growth. Observation on Spontaneous Mammary Tumors in Mice—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/1657354/ (accessed on 6 November 2022).
- Zacharski, L.R.; Chow, B.K.; Howes, P.S.; Shamayeva, G.; Baron, J.A.; Dalman, R.L.; Malenka, D.J.; Ozaki, C.K.; Lavori, P.W. Decreased cancer risk after iron reduction in patients with peripheral arterial disease: Results from a randomized trial. J. Natl. Cancer Inst. 2008, 100, 996–1002. [Google Scholar] [CrossRef]
- Prime, S.S.; MacDonald, D.G.; Rennie, J.S. The effect of iron deficiency on experimental oral carcinogenesis in the rat. Br. J. Cancer 1983, 47, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Influence of Low Dietary Iron and Iron Overload on Urethan-Induced Lung Tumors in Mice—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/8358683/ (accessed on 6 November 2022).
- Salnikow, K. Role of iron in cancer. Semin. Cancer Biol. 2021, 76, 189–194. [Google Scholar] [CrossRef] [PubMed]
- Raeeszadeh, M.; Gravandi, H.; Akbari, A. Determination of some heavy metals levels in the meat of animal species (sheep, beef, turkey, and ostrich) and carcinogenic health risk assessment in Kurdistan province in the west of Iran. Environ. Sci. Pollut. Res. 2022, 29, 62248–62258. [Google Scholar] [CrossRef] [PubMed]
- Zeuschner, C.L.; Hokin, B.D.; Marsh, K.A.; Saunders, A.v.; Reid, M.A.; Ramsay, M.R. Vitamin B12 and vegetarian diets. Med. J. Aust. 2013, 199, S27–S32. [Google Scholar] [CrossRef] [PubMed]
- Simonsen, L.O.; Harbak, H.; Bennekou, P. Cobalt metabolism and toxicology—A brief update. Sci. Total. Environ. 2012, 432, 210–215. [Google Scholar] [CrossRef]
- Léonard, A.; Lauwerys, R. Mutagenicity, carcinogenicity and teratogenicity of cobalt metal and cobalt compounds. Mutat. Res./Rev. Genet. Toxicol. 1990, 239, 17–27. [Google Scholar] [CrossRef]
- Houeto, P.; Houzé, P.; Baud, F.J. Comparative study of the tissue distribution of equimolar repeated doses of hydroxocobalamin and cobalt chloride in the rats. Ann. Biol. Clin. 2018, 76, 179–184. [Google Scholar] [CrossRef]
- Yamada, K. Cobalt: Its role in health and disease. Met. Ions Life Sci. 2013, 13, 295–320. [Google Scholar] [CrossRef]
- Romain, M.; Sviri, S.; Linton, D.M.; Stav, I.; van Heerden, P.v. The role of Vitamin B12 in the critically ill—A review. Anaesth. Intensiv. Care 2016, 44, 447–452. [Google Scholar] [CrossRef]
- Serraj, K.; Mecili, M.; Housni, I.; Andrès, E. Hypervitaminemia B12 (high level of cobalamin): Physiopathology, role and interest in clinical practice. Presse Médicale 2011, 40, 1120–1127. [Google Scholar] [CrossRef]
- Cheong, S.H.; Choi, Y.W.; Choi, H.Y.; Byun, J.Y. Nickel and cobalt release from jewellery and metal clothing items in Korea. Contact Dermat. 2014, 70, 11–18. [Google Scholar] [CrossRef] [PubMed]
- Possible Cobalt Toxicity in Maintenance Hemodialysis Patients after Treatment with Cobaltous Chloride: A Study of Blood and Tissue Cobalt Concentrations in Normal Subjects and Patients with Terminal and Renal Failure—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/1253458/ (accessed on 4 November 2022).
- Kesteloot, H.; Roelandt, J.; Willems, J.; Claes, J.H.; Joossens, J.v. An enquiry into the role of cobalt in the heart disease of chronic beer drinkers. Circulation 1968, 37, 854–864. [Google Scholar] [CrossRef] [PubMed]
- Baruthio, F.; Pierre, F. Cobalt determination in serum and urine by electrothermal atomic absorption spectrometry. Biol. Trace Element Res. 1993, 39, 21–31. [Google Scholar] [CrossRef]
- Cheung, A.C.; Banerjee, S.; Cherian, J.J.; Wong, F.; Butany, J.; Gilbert, C.; Overgaard, C.; Syed, K.; Zywiel, M.G.; Jacobs, J.J.; et al. Systemic cobalt toxicity from total hip arthroplasties: Review of a rare condition Part 1—History, mechanism, measurements, and pathophysiology. Bone Jt. J. 2016, 98-B, 6–13. [Google Scholar] [CrossRef] [PubMed]
- Bresson, C.; Darolles, C.; Carmona, A.; Gautier, C.; Sage, N.; Roudeau, S.; Ortega, R.; Ansoborlo, E.; Malard, V. Cobalt chloride speciation, mechanisms of cytotoxicity on human pulmonary cells, and synergistic toxicity with zinc. Metallomics 2013, 5, 133–143. [Google Scholar] [CrossRef]
- Krzystek, J.; Swenson, D.C.; Zvyagin, S.A.; Smirnov, D.; Ozarowski, A.; Telser, J. Cobalt(II) ‘scorpionate’ complexes as models for cobalt-substituted zinc enzymes: Electronic structure investigation by high-frequency and -field electron paramagnetic resonance spectroscopy. J. Am. Chem. Soc. 2010, 132, 5241–5253. [Google Scholar] [CrossRef]
- Hokin, B.; Adams, M.; Ashton, J.; Louie, H. Analysis of the cobalt content in Australian foods. Asia Pac. J. Clin. Nutr. 2004, 13, 284–288. Available online: https://pubmed.ncbi.nlm.nih.gov/15331341/ (accessed on 22 October 2022).
- Domingo, J.L. Cobalt in the environment and its toxicological implications. Rev. Environ. Contam. Toxicol. 1989, 108, 105–132. [Google Scholar] [CrossRef]
- Smith, L.J.; Holmes, A.L.; Kandpal, S.K.; Mason, M.D.; Zheng, T.; Wise, J.P. The cytotoxicity and genotoxicity of soluble and particulate cobalt in human lung fibroblast cells. Toxicol. Appl. Pharmacol. 2014, 278, 259–265. [Google Scholar] [CrossRef]
- Holstein, H.; Ranebo, Y.; Rääf, C.L. Human metabolism of orally administered radioactive cobalt chloride. J. Environ. Radioact. 2015, 143, 152–158. [Google Scholar] [CrossRef]
- Casper, D.P.; Pretz, J.P.; Purvis, H.T. Supplementing additional cobalt as cobalt lactate in a high-forage total mixed ration fed to late-lactation dairy cows. J. Dairy Sci. 2021, 104, 10669–10677. [Google Scholar] [CrossRef] [PubMed]
- Webb, S.; Bartos, J.; Boles, R.; Hasty, E.; Thuotte, E.; Thiex, N.J. Simultaneous determination of arsenic, cadmium, calcium, chromium, cobalt, copper, iron, lead, magnesium, manganese, molybdenum, nickel, selenium, and zinc in fertilizers by microwave acid digestion and inductively coupled plasma-optical emission spectrometry detection: Single-laboratory validation of a modification and extension of AOAC 2006.03. J. AOAC Int. 2014, 97, 700–711. [Google Scholar] [CrossRef] [PubMed]
- Beyersmann, D.; Hartwig, A. The genetic toxicology of cobalt. Toxicol. Appl. Pharmacol. 1992, 115, 137–145. [Google Scholar] [CrossRef] [PubMed]
- Danzeisen, R.; Williams, D.L.; Viegas, V.; Dourson, M.; Verberckmoes, S.; Burzlaff, A. Bioelution, Bioavailability, and Toxicity of Cobalt Compounds Correlate. Toxicol. Sci. 2020, 174, 311–325. [Google Scholar] [CrossRef]
- Gault, N.; Sandre, C.; Poncy, J.L.; Moulin, C.; Lefaix, J.L.; Bresson, C. Cobalt toxicity: Chemical and radiological combined effects on HaCaT keratinocyte cell line. Toxicol. Vitr. 2010, 24, 92–98. [Google Scholar] [CrossRef]
- Lippi, G.; Franchini, M.; Guidi, G.C. Cobalt chloride administration in athletes: A new perspective in blood doping? Br. J. Sport. Med. 2005, 39, 872–873. [Google Scholar] [CrossRef][Green Version]
- Wang, G.L.; Semenza, G.L. General involvement of hypoxia-inducible factor 1 in transcriptional response to hypoxia. Proc. Natl. Acad. Sci. USA 1993, 90, 4304–4308. [Google Scholar] [CrossRef]
- Lippi, G.; Montagnana, M.; Guidi, G.C. Albumin cobalt binding and ischemia modified albumin generation: An endogenous response to ischemia? Int. J. Cardiol. 2006, 108, 410–411. [Google Scholar] [CrossRef]
- Yoshida, T.; Hashimura, M.; Mastumoto, T.; Tazo, Y.; Inoue, H.; Kuwata, T.; Saegusa, M. Transcriptional upregulation of HIF-1α by NF-κB/p65 and its associations with β-catenin/p300 complexes in endometrial carcinoma cells. Lab. Investig. 2013, 93, 1184–1193. [Google Scholar] [CrossRef]
- Jomova, K.; Valko, M. Advances in metal-induced oxidative stress and human disease. Toxicology 2011, 283, 65–87. [Google Scholar] [CrossRef]
- Zhang, S.; Holy, C.E.; Eichenbaum, G.; Perkins, L.E.; Hasgall, P.; Katz, L.B.; Brown, J.R.; Orlandini, L.; Fessel, G.; Nasseri-Aghbosh, B.; et al. Carcinogenic assessment of cobalt-containing alloys in medical devices or cobalt in occupational settings: A systematic review and meta-analysis of overall cancer risk from published epidemiologic studies. Regul. Toxicol. Pharmacol. 2021, 125, 104987. [Google Scholar] [CrossRef] [PubMed]
- Holy, C.E.; Zhang, S.; Perkins, L.E.; Hasgall, P.; Katz, L.B.; Brown, J.R.; Orlandini, L.; Fessel, G.; Nasseri-Aghbosh, B.; Eichenbaum, G.; et al. Site-specific cancer risk following cobalt exposure via orthopedic implants or in occupational settings: A systematic review and meta-analysis. Regul. Toxicol. Pharmacol. 2021, 129, 105096. [Google Scholar] [CrossRef] [PubMed]
- Effect of Hypoxia and Re-Oxygenation on Cell Invasion and Adhesion in Human Ovarian Carcinoma Cells—PubMed. Available online: https://pubmed.ncbi.nlm.nih.gov/18813821/ (accessed on 17 October 2022).
- Vulpe, H.; Asamoah, F.A.; Maganti, M.; Vanderpuye, V.; Fyles, A.; Yarney, J. External Beam Radiation Therapy and Brachytherapy for Cervical Cancer: The Experience of the National Centre for Radiotherapy in Accra, Ghana. Int. J. Radiat. Oncol. Biol. Phys. 2018, 100, 1246–1253. [Google Scholar] [CrossRef]
- Bhatla, N.; Aoki, D.; Sharma, D.N.; Sankaranarayanan, R. Cancer of the cervix uteri: 2021 update. Int. J. Gynecol. Obstet. 2021, 155 (Suppl. S1), 28–44. [Google Scholar] [CrossRef] [PubMed]
- Jamalludin, Z.; Malik, R.A.; Ung, N.M. Correlation analysis of CT-based rectal planning dosimetric parameters with in vivo dosimetry of MOSkin and PTW 9112 detectors in Co-60 source HDR intracavitary cervix brachytherapy. Phys. Eng. Sci. Med. 2021, 44, 773–783. [Google Scholar] [CrossRef]
- Mosalaei, A.; Mohammadianpanah, M.; Omidvari, S.; Ahmadloo, N. High-dose rate brachytherapy in the treatment of carcinoma of uterine cervix: Twenty-year experience with cobalt after-loading system. Int. J. Gynecol. Cancer 2006, 16, 1101–1105. [Google Scholar] [CrossRef]
- Abdollahi, S.; Dayyani, M.; Hoseinian-Azghadi, E.; Miri-Hakimabad, H.; Rafat-Motavalli, L. A revised dosimetric characterization of 60 Co BEBIG source: From single-source data to clinical dose distribution. Brachytherapy 2018, 17, 1011–1022. [Google Scholar] [CrossRef]
- Tantivatana, T.; Rongsriyam, K. Treatment outcomes of high-dose-rate intracavitary brachytherapy for cervical cancer: A comparison of Ir-192 versus Co-60 sources. J. Gynecol. Oncol. 2018, 29, e86. [Google Scholar] [CrossRef]
- Shukla, A.K.; Rana, B.S.; Singh, N.P.; Kumar, S. Dosimetric study of CO-60 source step size in uterine cervix intracavitary HDR brachytherapy. Brachytherapy 2019, 18, 180–185. [Google Scholar] [CrossRef]
- Yadav, S.; Singh, O.; Choudhary, S.; Saroj, D.; Yogi, V.; Goswami, B. Estimation and comparison of integral dose to target and organs at risk in three-dimensional computed tomography image-based treatment planning of carcinoma uterine cervix with two high-dose-rate brachytherapy sources: 60Co and 192Ir. J. Cancer Res. Ther. 2021, 17, 191–197. [Google Scholar] [CrossRef]
- Ivankova, V.S.; Domina, E.A.; Khrulenko, T.v.; Baranovska, L.M.; Hrinchenko, O.A. Iridium-192 radiotherapy benefits in the management of gynecological tumors. Probl. Radiatsiinoi Medytsyny Ta Radiobiolohii 2020, 2020, 569–578. [Google Scholar] [CrossRef] [PubMed]
- Pesee, M.; Krusun, S.; Padoongcharoen, P. High dose rate cobalt-60 after loading intracavitary therapy of the uterine cervical carcinoma in srinagarind hospital, analysis of residual disease. Asian Pac. J. Cancer Prev. 2012, 13, 4835–4837. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Mobit, P.N.; Packianathan, S.; He, R.; Yang, C.C. Comparison of Axxent-Xoft, 192Ir and 60Co high-dose-rate brachytherapy sources for image-guided brachytherapy treatment planning for cervical cancer. Br. J. Radiol. 2015, 88, 20150010. [Google Scholar] [CrossRef] [PubMed]
- Vega, R.B.M.; Barbee, D.; Talcott, W.; Duckworth, T.; Shah, B.A.; Ishaq, O.F.; Small, C.; Yeung, A.R.; Perez, C.A.; Schiff, P.B.; et al. Cost in perspective: Direct assessment of American market acceptability of Co-60 in gynecologic high-dose-rate brachytherapy and contrast with experience abroad. J. Contemp. Brachytherapy 2018, 10, 503–509. [Google Scholar] [CrossRef] [PubMed]
- Nikam, D.; Jagtap, A.; Vinothraj, R. Resolving the brachytherapy challenges with government funded hospital. Indian J. Cancer 2016, 53, 132–134. [Google Scholar] [CrossRef] [PubMed]
- Munteanu, C.R.; Suntharalingam, K. Advances in cobalt complexes as anticancer agents. Dalton Trans. 2015, 44, 13796–13808. [Google Scholar] [CrossRef]
- Pašukoniene, V.; Mlynska, A.; Steponkienė, S.; Poderys, V.; Matulionytė, M.; Karabanovas, V.; Statkutė, U.; Purvinienė, R.; Kraśko, J.A.; Jagminas, A.; et al. Accumulation and biological effects of cobalt ferrite nanoparticles in human pancreatic and ovarian cancer cells. Medicina 2014, 50, 237–244. [Google Scholar] [CrossRef]
- Wang, Y.F.; Tang, J.X.; Mo, Z.Y.; Li, J.; Liang, F.P.; Zou, H.H. The strong in vitro and vivo cytotoxicity of three new cobalt(II) complexes with 8-methoxyquinoline. Dalton Trans. 2022, 51, 8840–8847. [Google Scholar] [CrossRef]
- Law, B.Y.K.; Qu, Y.Q.; Mok, S.W.F.; Liu, H.; Zeng, W.; Han, Y.; Gordillo-Martinez, F.; Chan, W.-K.; Wong, K.M.-C.; Wong, V.K.W. New perspectives of cobalt tris(bipyridine) system: Anti-cancer effect and its collateral sensitivity towards multidrug-resistant (MDR) cancers. Oncotarget 2017, 8, 55003–55021. [Google Scholar] [CrossRef]
- Coughlan, A.Y.; Testa, G. Exploiting epigenetic dependencies in ovarian cancer therapy. Int. J. Cancer 2021, 149, 1732–1743. [Google Scholar] [CrossRef]
- Murphy, S.K. Targeting ovarian cancer-initiating cells. Anticancer Agents Med. Chem. 2010, 10, 157–163. [Google Scholar] [CrossRef] [PubMed]
- la Vecchia, C. Ovarian cancer: Epidemiology and risk factors. Eur. J. Cancer Prev. 2017, 26, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Ramus, S.J.; Gayther, S.A. The contribution of BRCA1 and BRCA2 to ovarian cancer. Mol. Oncol. 2009, 3, 138–150. [Google Scholar] [CrossRef] [PubMed]
- Dunneram, Y.; Greenwood, D.C.; Cade, J.E. Diet, menopause and the risk of ovarian, endometrial and breast cancer. Proc. Nutr. Soc. 2019, 78, 438–448. [Google Scholar] [CrossRef] [PubMed]
- Slomovitz, B.; de Haydu, C.; Taub, M.; Coleman, R.L.; Monk, B.J. Asbestos and ovarian cancer: Examining the historical evidence. Int. J. Gynecol. Cancer 2021, 31, 122–128. [Google Scholar] [CrossRef]
- Wentzensen, N.; O’Brien, K.M. Talc, body powder, and ovarian cancer: A summary of the epidemiologic evidence. Gynecol. Oncol. 2021, 163, 199–208. [Google Scholar] [CrossRef]
- Grzelak, M.M.; Chmura, Ł.; Wróbel, P.M.; Adamek, D.; Lankosz, M.; Jach, R.; Welter, E. Investigation of the role and chemical form of iron in the ovarian carcinogenesis process. J. Trace Elements Med. Biol. 2020, 60, 126500. [Google Scholar] [CrossRef]
- Yaman, M.; Kaya, G.; Simsek, M. Comparison of trace element concentrations in cancerous and noncancerous human endometrial and ovary tissues. Int. J. Gynecol. Cancer 2007, 17, 220–228. [Google Scholar] [CrossRef]
- Basuli, D.; Tesfay, L.; Deng, Z.; Paul, B.; Yamamoto, Y.; Ning, G.; Xian, W.; McKeon, F.; Lynch, M.; Crum, C.P.; et al. Iron addiction: A novel therapeutic target in ovarian cancer. Oncogene 2017, 36, 4089–4099. [Google Scholar] [CrossRef]
- Sun, D.; Li, Y.C.; Zhang, X.Y. Lidocaine Promoted Ferroptosis by Targeting miR-382-5p /SLC7A11 Axis in Ovarian and Breast Cancer. Front. Pharmacol. 2021, 12, 681223. [Google Scholar] [CrossRef]
- Hong, T.; Lei, G.; Chen, X.; Li, H.; Zhang, X.; Wu, N.; Zhao, Y.; Zhang, Y.; Wang, J. PARP inhibition promotes ferroptosis via repressing SLC7A11 and synergizes with ferroptosis inducers in BRCA-proficient ovarian cancer. Redox Biol. 2021, 42, 101928. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, M.; Chao, H. Significance of glutathione peroxidase 4 and intracellular iron level in ovarian cancer cells-“utilization” of ferroptosis mechanism. Inflamm. Res. 2021, 70, 1177–1189. [Google Scholar] [CrossRef] [PubMed]
- Negishi, J.; Omori, Y.; Shindo, M.; Takanashi, H.; Musha, S.; Nagayama, S.; Hirayama, J.; Nishina, H.; Nakakura, T.; Mogi, C.; et al. Manganese and cobalt activate zebrafish ovarian cancer G-protein-coupled receptor 1 but not GPR4. J. Recept. Signal Transduct. 2017, 37, 401–408. [Google Scholar] [CrossRef] [PubMed]
- Kadowaki, M.; Sato, K.; Kamio, H.; Kumagai, M.; Sato, R.; Nyui, T.; Umeda, Y.; Waseda, Y.; Anzai, M.; Aoki-Saito, H.; et al. Metal-Stimulated Interleukin-6 Production Through a Proton-Sensing Receptor, Ovarian Cancer G Protein-Coupled Receptor 1, in Human Bronchial Smooth Muscle Cells: A Response Inhibited by Dexamethasone. J. Inflamm. Res. 2021, 14, 7021–7034. [Google Scholar] [CrossRef] [PubMed]
- Cheng, J.C.; Klausen, C.; Leung, P.C.K. Hypoxia-inducible factor 1 alpha mediates epidermal growth factor-induced down-regulation of E-cadherin expression and cell invasion in human ovarian cancer cells. Cancer Lett. 2013, 329, 197–206. [Google Scholar] [CrossRef]
- Braun, M.M.; Overbeek-Wager, E.A.; Grumbo, R.J. Diagnosis and Management of Endometrial Cancer. Am. Fam. Physician 2016, 93, 468–474. Available online: https://pubmed.ncbi.nlm.nih.gov/26977831/ (accessed on 13 November 2022). [PubMed]
- Panwalkar, A.; Verstovsek, S.; Giles, F. Nuclear factor-kappaB modulation as a therapeutic approach in hematologic malignancies. Cancer 2004, 100, 1578–1589. [Google Scholar] [CrossRef]
- Sen, R.; Baltimore, D. Multiple nuclear factors interact with the immunoglobulin enhancer sequences. Cell 1986, 46, 705–716. [Google Scholar] [CrossRef]
- Terry, P.; Vainio, H.; Wolk, A.; Weiderpass, E. Dietary factors in relation to endometrial cancer: A nationwide case-control study in Sweden. Nutr. Cancer 2002, 42, 25–32. [Google Scholar] [CrossRef]
- Genkinger, J.M.; Friberg, E.; Goldbohm, R.A.; Wolk, A. Long-term dietary heme iron and red meat intake in relation to endometrial cancer risk. Am. J. Clin. Nutr. 2012, 96, 848–854. [Google Scholar] [CrossRef]
- Kabat, G.C.; Miller, A.B.; Jain, M.; Rohan, T.E. Dietary iron and haem iron intake and risk of endometrial cancer: A prospective cohort study. Br. J. Cancer 2008, 98, 194–198. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Reng, S.R.; Wang, L.; Lu, L.; Zhao, Z.H.; Zhang, Z.K.; Feng, X.D.; Ding, X.D.; Wang, J.; Feng, G.; et al. Overexpression of Y-box binding protein-1 in cervical cancer and its association with the pathological response rate to chemoradiotherapy. Med. Oncol. 2012, 29, 1992–1997. [Google Scholar] [CrossRef] [PubMed]
- Almonte, M.; Murillo, R.; Sanchez, G.I.; Jerónimo, J.; Salmerón, J.; Ferreccio, C.; Lazcano-Ponce, E.; Herrero, R. Nuevos paradigmas y desafíos en la prevención y control del cáncer de cuello uterino en América Latina. Salud Publica Mex. 2010, 52, 544–559. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Gabrielli, S.; Maggioni, E.; Fieschi, L. Cervical cancer prevention in senegal: An international cooperation project report. Acta Biomed. 2018, 89, 29–34. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Chen, G.; Hong, L.; Zhou, L.; Hu, M.; Li, B.; Huang, J.; Xia, L.; Li, C. How does hypoxia inducible factor-1α participate in enhancing the glycolysis activity in cervical cancer? Ann. Diagn. Pathol. 2013, 17, 305–311. [Google Scholar] [CrossRef]
- Cunzhi, H.; Jiexian, J.; Xianwen, Z.; Jingang, G.; Shumin, Z.; Lili, D. Serum and tissue levels of six trace elements and copper/zinc ratio in patients with cervical cancer and uterine myoma. Biol. Trace Element Res. 2003, 94, 113–122. [Google Scholar] [CrossRef]
- Tinelli, A.; Vinciguerra, M.; Malvasi, A.; Andjić, M.; Babović, I.; Sparić, R. Uterine fibroids and diet. Int. J. Environ. Res. Public Health 2021, 18, 1066. [Google Scholar] [CrossRef]
- Tanos, V.; Berry, K.E. Benign and malignant pathology of the uterus. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 46, 12–30. [Google Scholar] [CrossRef]
- Closon, F.; Tulandi, T. Uterine myomata: Organ-preserving surgery. Best Pract. Res. Clin. Obstet. Gynaecol. 2016, 35, 30–36. [Google Scholar] [CrossRef]
- Yang, J.H.; Chen, M.J.; der Chen, C.; Chen, C.L.; Ho, H.N.; Yang, Y.S. Impact of submucous myoma on the severity of anemia. Fertil. Steril. 2011, 95, 1769–1772.e1. [Google Scholar] [CrossRef]
- Nasiadek, M.; Krawczyk, T.; Sapota, A. Tissue levels of cadmium and trace elements in patients with myoma and uterine cancer. Hum. Exp. Toxicol. 2005, 24, 623–630. [Google Scholar] [CrossRef] [PubMed]
- González, V.G.; Moreta, A.H.; Triana, A.M.; Sierra, L.R.; García, I.C.; Méndez, N.I. Prolapsed cervical myoma during pregnancy. Eur. J. Obstet. Gynecol. Reprod. Biol. 2020, 252, 150–154. [Google Scholar] [CrossRef] [PubMed]
- Carbonnel, M.; Pirtea, P.; de Ziegler, D.; Ayoubi, J.M. Uterine factors in recurrent pregnancy losses. Fertil. Steril. 2021, 115, 538–545. [Google Scholar] [CrossRef] [PubMed]
- Johnstone, E.B.; Louis, G.M.B.; Parsons, P.J.; Steuerwald, A.J.; Palmer, C.D.; Chen, Z.; Sun, L.; Hammoud, A.O.; Dorais, J.; Peterson, C.M. Increased urinary cobalt and whole blood concentrations of cadmium and lead in women with uterine leiomyomata: Findings from the ENDO Study. Reprod. Toxicol. 2014, 49, 27–32. [Google Scholar] [CrossRef]
- Taylor, H.S.; Kotlyar, A.M.; Flores, V.A. Endometriosis is a chronic systemic disease: Clinical challenges and novel innovations. Lancet 2021, 397, 839–852. [Google Scholar] [CrossRef]
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [CrossRef]
- Vercellini, P.; Viganò, P.; Somigliana, E.; Fedele, L. Endometriosis: Pathogenesis and treatment. Nat. Rev. Endocrinol. 2014, 10, 261–275. [Google Scholar] [CrossRef]
- Takenaka, M.; Suzuki, N.; Mori, M.; Hirayama, T.; Nagasawa, H.; Morishige, K.i. Iron regulatory protein 2 in ovarian endometrial cysts. Biochem. Biophys. Res. Commun. 2017, 487, 789–794. [Google Scholar] [CrossRef]
- Mori, M.; Ito, F.; Shi, L.; Wang, Y.; Ishida, C.; Hattori, Y.; Niwa, M.; Hirayama, T.; Nagasawa, H.; Iwase, A.; et al. Ovarian endometriosis-associated stromal cells reveal persistently high affinity for iron. Redox Biol. 2015, 6, 578–586. [Google Scholar] [CrossRef]
- Akashi, K.; Nagashima, Y.; Tabata, T.; Oda, H. Immunochemical analysis of iron transporters and m2 macrophages in ovarian endometrioma and clear cell adenocarcinoma. Mol. Clin. Oncol. 2021, 15, 159. [Google Scholar] [CrossRef]
- Defrère, S.; Lousse, J.C.; González-Ramos, R.; Colette, S.; Donnez, J.; van Langendonckt, A. Potential involvement of iron in the pathogenesis of peritoneal endometriosis. Mol. Hum. Reprod. 2008, 14, 377–385. [Google Scholar] [CrossRef] [PubMed]
- Kondi-Pafiti, A.; Papakonstantinou, E.; Iavazzo, C.; Grigoriadis, C.; Salakos, N.; Gregoriou, O. Clinicopathological characteristics of ovarian carcinomas associated with endometriosis. Arch. Gynecol. Obstet. 2012, 285, 479–483. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Wang, F.; Li, D.; Yan, Y.; Wang, H. Transferrin receptor-mediated reactive oxygen species promotes ferroptosis of KGN cells via regulating NADPH oxidase 1/PTEN induced kinase 1/acyl-CoA synthetase long chain family member 4 signaling. Bioengineered 2021, 12, 4983–4994. [Google Scholar] [CrossRef] [PubMed]
- Patel, S. Polycystic ovary syndrome (PCOS), an inflammatory, systemic, lifestyle endocrinopathy. J. Steroid Biochem. Mol. Biol. 2018, 182, 27–36. [Google Scholar] [CrossRef]
- Bednarska, S.; Siejka, A. The pathogenesis and treatment of polycystic ovary syndrome: What’s new? Adv. Clin. Exp. Med. 2017, 26, 359–367. [Google Scholar] [CrossRef]
- Zehravi, M.; Maqbool, M.; Ara, I. Polycystic ovary syndrome and infertility: An update. Int. J. Adolesc. Med. Health 2021, 34, 1–9. [Google Scholar] [CrossRef]
- Rashidi, B.H.; Shams, S.; Shariat, M.; Jaliseh, H.K.; Mohebi, M.; Haghollahi, F. Evaluation of serum hepcidin and iron levels in patients with PCOS: A case-control study. J. Endocrinol. Investig. 2017, 40, 779–784. [Google Scholar] [CrossRef]
- Chen, T.S.; Chen, Y.T.; Liu, C.H.; Sun, C.C.; Mao, F.C. Effect of Chromium Supplementation on Element Distribution in a Mouse Model of Polycystic Ovary Syndrome. Biol. Trace Element Res. 2015, 168, 472–480. [Google Scholar] [CrossRef]
- Escobar-Morreale, H.F. Iron metabolism and the polycystic ovary syndrome. Trends Endocrinol. Metab. 2012, 23, 509–515. [Google Scholar] [CrossRef]
- Luque-Ramírez, M.; Álvarez-Blasco, F.; Botella-Carretero, J.I.; Sanchón, R.; Millán, J.L.S.; Escobar-Morreale, H.F. Increased body iron stores of obese women with polycystic ovary syndrome are a consequence of insulin resistance and hyperinsulinism and are not a result of reduced menstrual losses. Diabetes Care 2007, 30, 2309–2313. [Google Scholar] [CrossRef]
- Sam, A.H.; Busbridge, M.; Amin, A.; Webber, L.; White, D.; Franks, S.; Martin, N.M.; Sleeth, M.; Ismail, N.A.; Daud, N.M.; et al. Hepcidin levels in diabetes mellitus and polycystic ovary syndrome. Diabet. Med. 2013, 30, 1495–1499. [Google Scholar] [CrossRef] [PubMed]
- Kurdoglu, Z.; Kurdoglu, M.; Demir, H.; Sahin, H.G. Serum trace elements and heavy metals in polycystic ovary syndrome. Hum. Exp. Toxicol. 2012, 31, 452–456. [Google Scholar] [CrossRef] [PubMed]
- Zheng, Q.; Liu, M.; Fu, J. ALG2 inhibits the epithelial-to-mesenchymal transition and stemness of ovarian granulosa cells through the Wnt/β-catenin signaling pathway in polycystic ovary syndrome. Reprod. Biol. 2022, 22, 100706. [Google Scholar] [CrossRef] [PubMed]
- Mgongo, F.O.; Gombe, S.; Ogaa, J.S. The influence of cobalt/vitamin B12 deficiency as a ‘stressor’ affecting adrenal cortex and ovarian activities in goats. Reprod. Nutr. Dev. 1984, 24, 845–854. [Google Scholar] [CrossRef]
- Hidiroglou, M. Trace element deficiencies and fertility in ruminants: A review. J. Dairy Sci. 1979, 62, 1195–1206. [Google Scholar] [CrossRef]
- Kolesarova, A.; Capcarova, M.; Sirotkin, A.; Medvedova, M.; Kovacik, J. Cobalt-induced changes in the IGF-I and progesterone release, expression of proliferation- and apoptosis-related peptides in porcine ovarian granulosa cells in vitro. J. Environ. Sci. Health Part A 2010, 45, 810–817. [Google Scholar] [CrossRef]
- Roychoudhury, S.; Sirotkin, A.v.; Toman, R.; Kolesarova, A. Cobalt-induced hormonal and intracellular alterations in rat ovarian fragments in vitro. J. Environ. Sci. Health Part B 2014, 49, 971–977. [Google Scholar] [CrossRef]
- Grasselli, F.; Basini, G.; Bussolati, S.; Bianco, F. Cobalt chloride, a hypoxia-mimicking agent, modulates redox status and functional parameters of cultured swine granulosa cells. Reprod. Fertil. Dev. 2005, 17, 715–720. [Google Scholar] [CrossRef]
- Paksy, K.; Forgács, Z.; Gáti, I. In vitro comparative effect of Cd2+, Ni2+, and Co2+ on mouse postblastocyst development. Environ. Res. 1999, 80, 340–347. [Google Scholar] [CrossRef]
- Baumann, M.U.; Zamudio, S.; Illsley, N.P. Hypoxic upregulation of glucose transporters in BeWo choriocarcinoma cells is mediated by hypoxia-inducible factor-1. Am. J. Physiol. Physiol. 2007, 293, C477–C485. [Google Scholar] [CrossRef]
- Szakmáry, É.; Ungváry, G.; Hudák, A.; Tátrai, E.; Náray, M.; Morvai, V. Effects of cobalt sulfate on prenatal development of mice, rats, and rabbits, and on early postnatal development of rats. J. Toxicol. Environ. Health Part A 2001, 62, 367–386. [Google Scholar] [CrossRef] [PubMed]
- Paternain, J.L.; Domingo, J.L.; Corbella, J. Developmental toxicity of cobalt in the rat. J. Toxicol. Environ. Health Part A 1988, 24, 193–200. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).