Jamming Transitions in Astrocytes and Glioblastoma Are Induced by Cell Density and Tension
Abstract
:1. Introduction
2. Methods
2.1. Cell Culture
2.2. Measuring Properties of Collective Cell Migration
2.3. Measuring Properties of Single Cell Migration
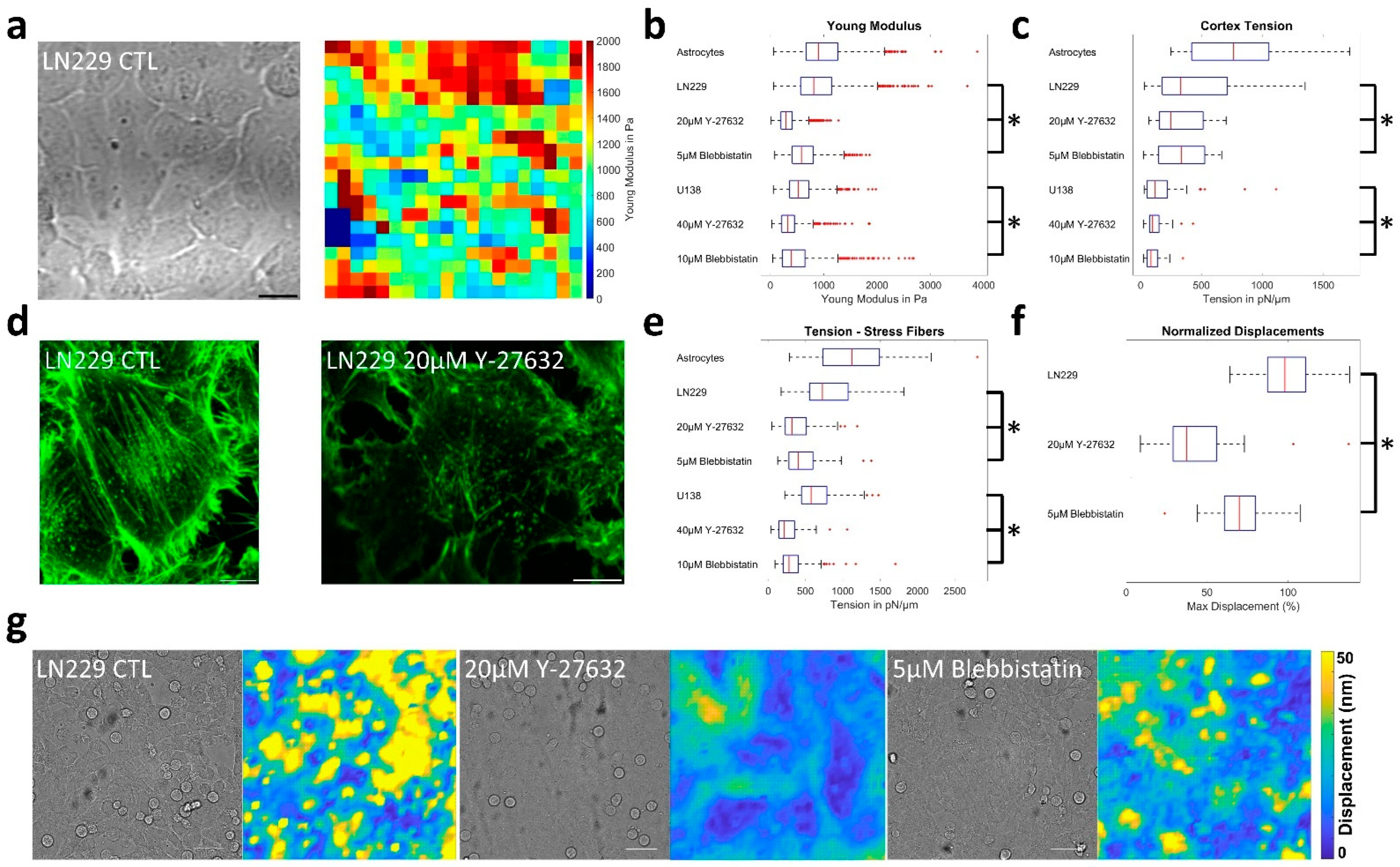
2.4. Determination of Cortex Tension by Atomic Force Microscopy
2.5. Determination of Elastic Modulus by Atomic Force Microscopy
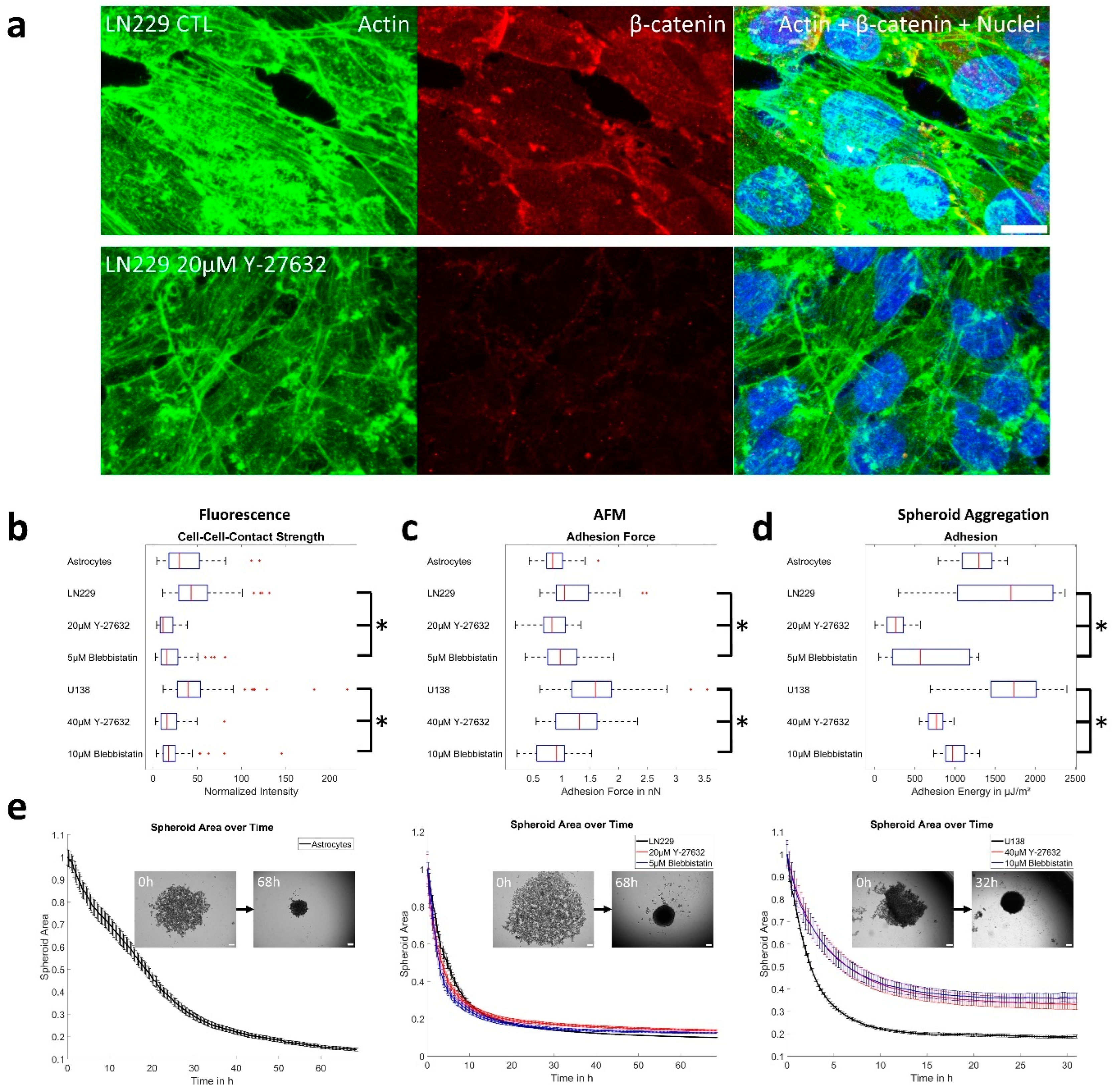
2.6. Determination of Adhesion Energies by Atomic Force Microscopy
2.7. 3D Tumor Aggregate Formation Assay
2.8. Fluorescence Staining
2.9. Estimation of Stress Fiber Tension
2.10. Traction Force Microscopy
2.11. Statistics
3. Results
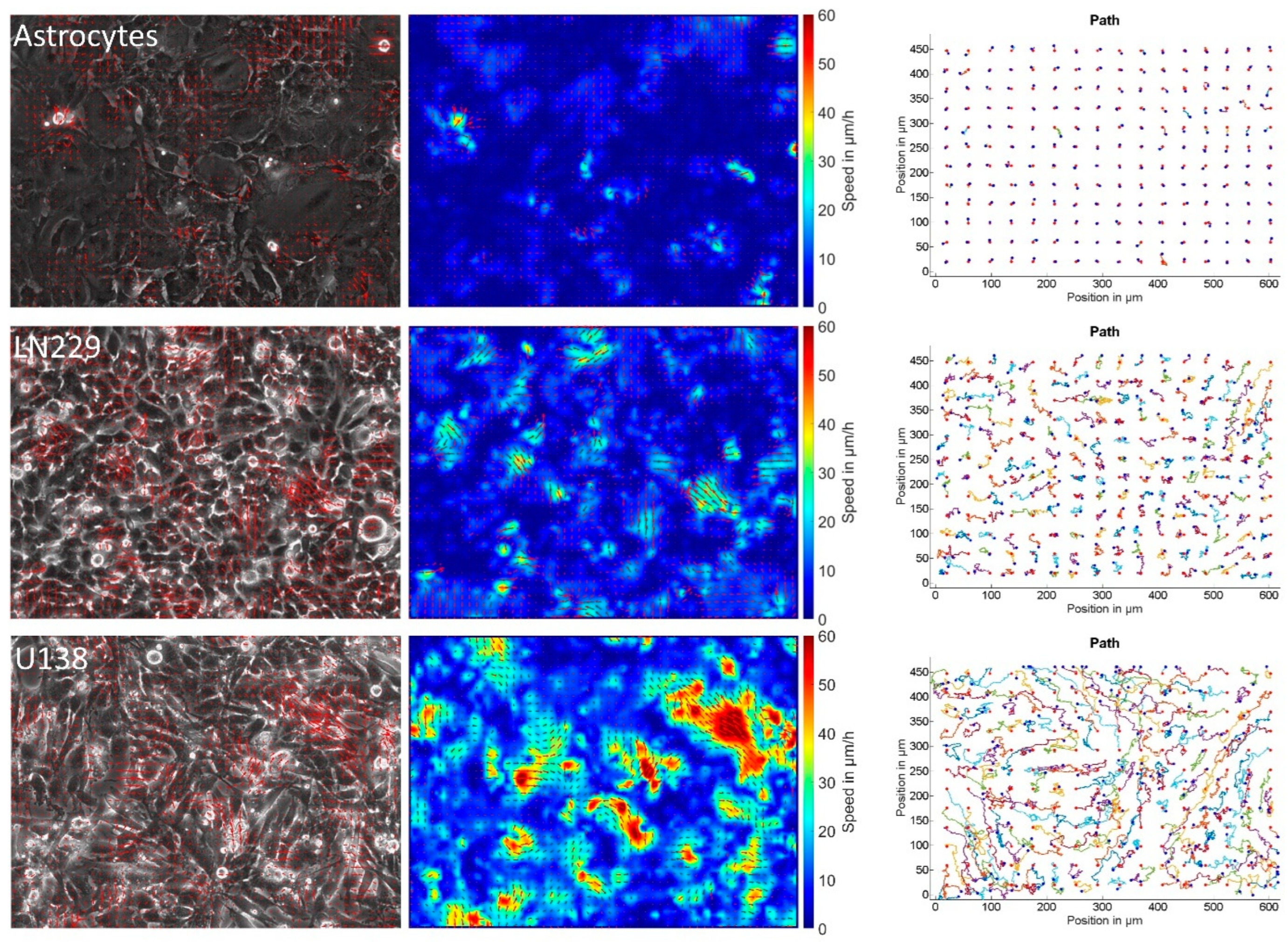
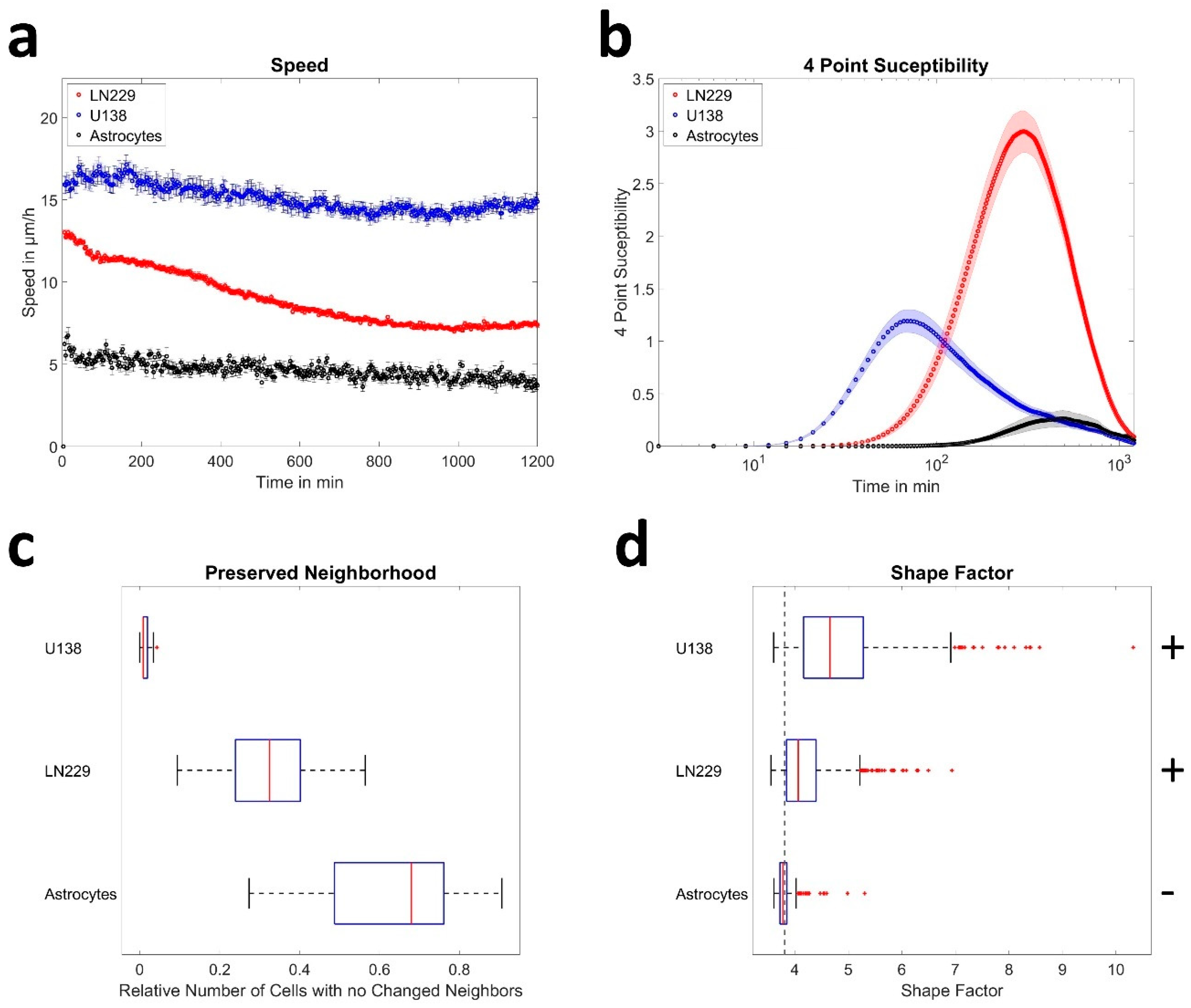
3.1. Astrocytes Are Non-Migratory and Jammed but Not Glioblastoma
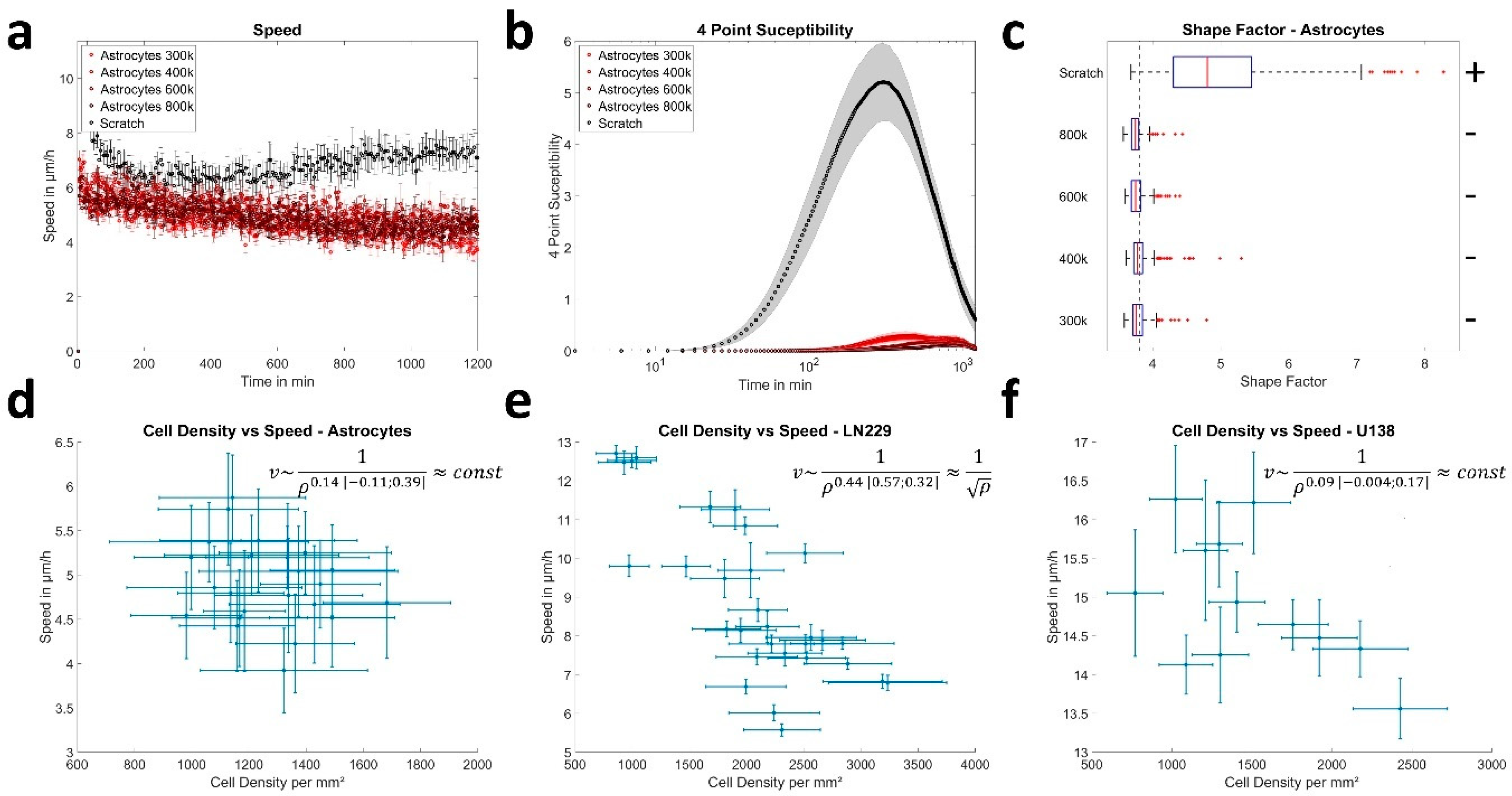
3.2. Cell Density Affects Jamming in a Cell Line Dependent Manner
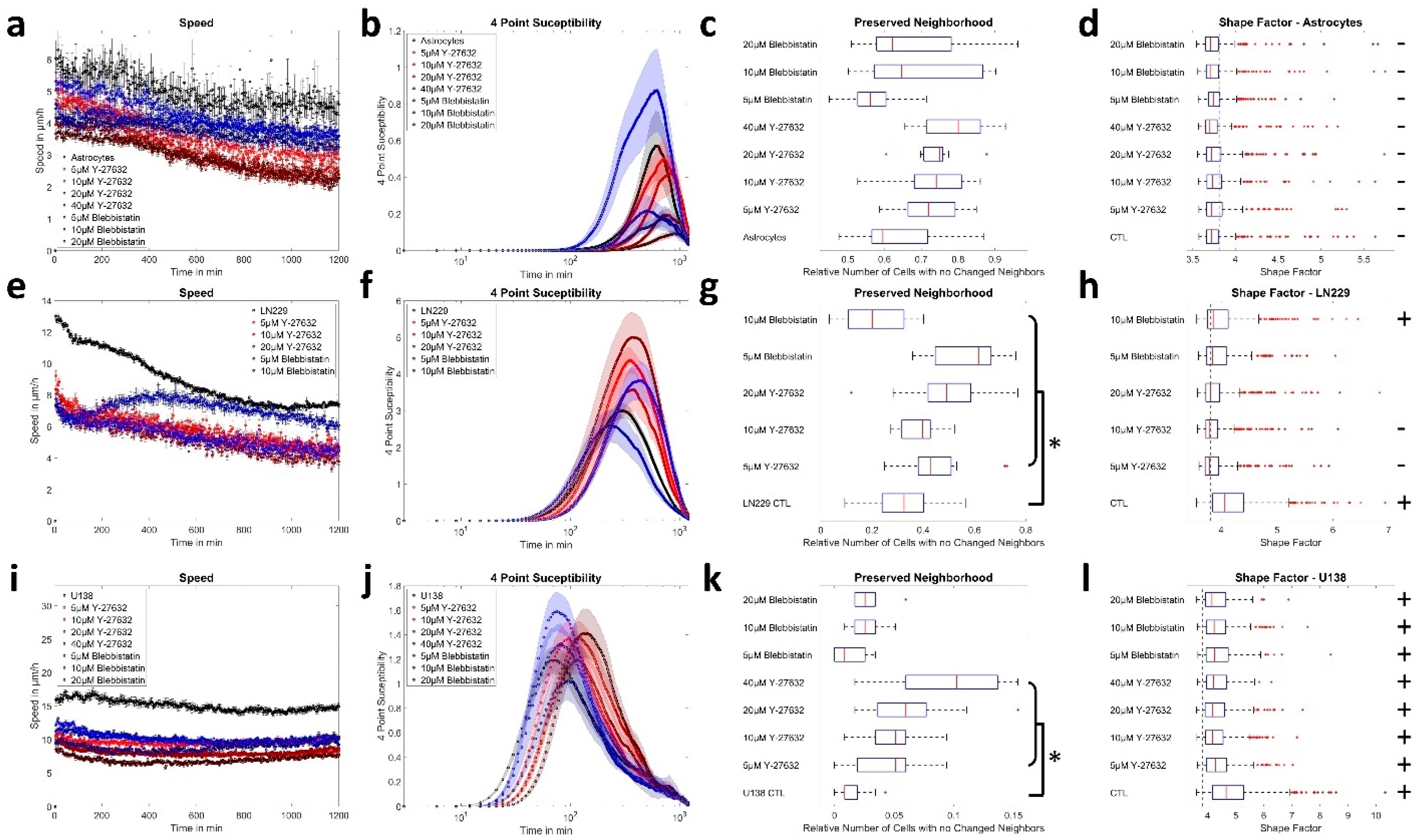
3.3. ROCK and Myosin II Inhibition Causes Jamming in Glioblastoma Cells
3.4. (Un-)Jamming Is Determined by the Ratio of Adhesion and Tension
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cabezas, R.; Ávila, M.; Gonzalez, J.; El-Bachá, R.S.; Báez, E.; García-Segura, L.M.; Coronel, J.C.J.; Capani, F.; Cardona-Gomez, G.P.; Barreto, G.E. Astrocytic Modulation of Blood Brain Barrier: Perspectives on Parkinson’s Disease. Front. Cell. Neurosci. 2014, 8, 211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, K.; Lee, S.J. Deciphering the Star Codings: Astrocyte Manipulation Alters Mouse Behavior. Exp. Mol. Med. 2020, 52, 1028–1038. [Google Scholar] [CrossRef] [PubMed]
- Kıray, H.; Lindsay, S.L.; Hosseinzadeh, S.; Barnett, S.C. The Multifaceted Role of Astrocytes in Regulating Myelination. Exp. Neurol. 2016, 283, 541–549. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Xu, S.; Xin, Y.; Yu, S.; Ping, Y.; Chen, L.; Xiao, H.; Wang, B.; Yi, L.; Wang, Q.; et al. Tumor-Associated Microglia/Macrophages Enhance the Invasion of Glioma Stem-like Cells via TGF-Β1 Signaling Pathway. J. Immunol. 2012, 189, 444–453. [Google Scholar] [CrossRef] [Green Version]
- Johnson, D.R.; O’Neill, B.P. Glioblastoma Survival in the United States before and during the Temozolomide Era. J. Neurooncol. 2012, 107, 359–364. [Google Scholar] [CrossRef]
- Burger, P.C.; Kleihues, P. Cytologic Composition of the Untreated Glioblastoma with Implications for Evaluation of Needle Biopsies. Cancer 1989, 63, 2014–2023. [Google Scholar] [CrossRef]
- Hoelzinger, D.B.; Demuth, T.; Berens, M.E. Autocrine Factors That Sustain Glioma Invasion and Paracrine Biology in the Brain Microenvironment. J. Natl. Cancer Inst. 2007, 99, 1583–1593. [Google Scholar] [CrossRef]
- Lim, D.A.; Cha, S.; Mayo, M.C.; Chen, M.-H.; Keles, E.; VandenBerg, S.; Berger, M.S. Relationship of Glioblastoma Multiforme to Neural Stem Cell Regions Predicts Invasive and Multifocal Tumor Phenotype. Neuro Oncol. 2007, 9, 424–429. [Google Scholar] [CrossRef]
- Serres, E.; Debarbieux, F.; Stanchi, F.; Maggiorella, L.; Grall, D.; Turchi, L.; Burel-Vandenbos, F.; Figarella-Branger, D.; Virolle, T.; Rougon, G.; et al. Fibronectin Expression in Glioblastomas Promotes Cell Cohesion, Collective Invasion of Basement Membrane in Vitro and Orthotopic Tumor Growth in Mice. Oncogene 2014, 33, 3451–3462. [Google Scholar] [CrossRef]
- Gritsenko, P.; Leenders, W.; Friedl, P. Recapitulating in Vivo-like Plasticity of Glioma Cell Invasion along Blood Vessels and in Astrocyte-Rich Stroma. Histochem. Cell Biol. 2017, 148, 395–406. [Google Scholar] [CrossRef]
- Volovetz, J.; Berezovsky, A.D.; Alban, T.; Chen, Y.; Lauko, A.; Aranjuez, G.F.; Burtscher, A.; Shibuya, K.; Silver, D.J.; Peterson, J.; et al. Identifying Conserved Molecular Targets Required for Cell Migration of Glioblastoma Cancer Stem Cells. Cell Death Dis. 2020, 11, 152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vollmann-Zwerenz, A.; Leidgens, V.; Feliciello, G.; Klein, C.A.; Hau, P. Tumor Cell Invasion in Glioblastoma. Int. J. Mol. Sci. 2020, 21, 1932. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gritsenko, P.G.; Atlasy, N.; Dieteren, C.E.J.; Navis, A.C.; Venhuizen, J.H.; Veelken, C.; Schubert, D.; Acker-Palmer, A.; Westerman, B.A.; Wurdinger, T.; et al. P120-Catenin-Dependent Collective Brain Infiltration By Glioma Cell Networks. Nat. Cell Biol. 2020, 22, 97–107. [Google Scholar] [CrossRef] [PubMed]
- Van Helvert, S.; Storm, C.; Friedl, P. Mechanoreciprocity in Cell Migration. Nat. Cell Biol. 2018, 20, 8–20. [Google Scholar] [CrossRef]
- Ilina, O.; Friedl, P. Mechanisms of Collective Cell Migration at a Glance. J. Cell Sci. 2009, 122, 3203–3208. [Google Scholar] [CrossRef] [Green Version]
- Hohmann, T.; Dehghani, F. The Cytoskeleton—A Complex Interacting Meshwork. Cells 2019, 8, 362. [Google Scholar] [CrossRef] [Green Version]
- Angelini, T.E.; Hannezo, E.; Trepatc, X.; Marquez, M.; Fredberg, J.J.; Weitz, D.A. Glass-like Dynamics of Collective Cell Migration. Proc. Natl. Acad. Sci. USA 2011, 108, 4714–4719. [Google Scholar] [CrossRef] [Green Version]
- Nnetu, K.D.; Knorr, M.; Pawlizak, S.; Fuhs, T.; Käs, J.A. Slow and Anomalous Dynamics of an MCF-10A Epithelial Cell Monolayer. Soft Matter 2013, 9, 9335–9341. [Google Scholar] [CrossRef]
- Park, J.A.; Kim, J.H.; Bi, D.; Mitchel, J.A.; Qazvini, N.T.; Tantisira, K.; Park, C.Y.; McGill, M.; Kim, S.H.; Gweon, B.; et al. Unjamming and Cell Shape in the Asthmatic Airway Epithelium. Nat. Mater. 2015, 14, 1040–1048. [Google Scholar] [CrossRef]
- Garcia, S.; Hannezo, E.; Elgeti, J.; Joanny, J.F.; Silberzan, P.; Gov, N.S. Physics of Active Jamming during Collective Cellular Motion in a Monolayer. Proc. Natl. Acad. Sci. USA 2015, 112, 15314–15319. [Google Scholar] [CrossRef]
- Atia, L.; Bi, D.; Sharma, Y.; Mitchel, J.A.; Gweon, B.; Koehler, S.A.; Decamp, S.J.; Lan, B.; Kim, J.H.; Hirsch, R.; et al. Geometric Constraints during Epithelial Jamming. Nat. Phys. 2018, 14, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Ilina, O.; Gritsenko, P.G.; Syga, S.; Lippoldt, J.; La Porta, C.A.M.; Chepizhko, O.; Grosser, S.; Vullings, M.; Bakker, G.J.; Starruß, J.; et al. Cell–Cell Adhesion and 3D Matrix Confinement Determine Jamming Transitions in Breast Cancer Invasion. Nat. Cell Biol. 2020, 22, 1103–1115. [Google Scholar] [CrossRef] [PubMed]
- Tambe, D.T.; Corey Hardin, C.; Angelini, T.E.; Rajendran, K.; Park, C.Y.; Serra-Picamal, X.; Zhou, E.H.; Zaman, M.H.; Butler, J.P.; Weitz, D.A.; et al. Collective Cell Guidance by Cooperative Intercellular Forces. Nat. Mater. 2011, 10, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Doxzen, K.; Vedula, S.R.K.; Leong, M.C.; Hirata, H.; Gov, N.S.; Kabla, A.J.; Ladoux, B.; Lim, C.T. Guidance of Collective Cell Migration by Substrate Geometry. Integr. Biol. 2013, 5, 1026–1035. [Google Scholar] [CrossRef] [PubMed]
- Saraswathibhatla, A.; Notbohm, J. Tractions and Stress Fibers Control Cell Shape and Rearrangements in Collective Cell Migration. Phys. Rev. X 2020, 10, 11016. [Google Scholar] [CrossRef] [Green Version]
- Bi, D.; Lopez, J.H.; Schwarz, J.M.; Manning, M.L. A Density-Independent Rigidity Transition in Biological Tissues. Nat. Phys. 2015, 11, 1074–1079. [Google Scholar] [CrossRef] [Green Version]
- Bi, D.; Yang, X.; Marchetti, M.C.; Manning, M.L. Motility-Driven Glass and Jamming Transitions in Biological Tissues. Phys. Rev. X 2016, 6, 021011. [Google Scholar] [CrossRef] [Green Version]
- Cai, G.; Nguyen, A.; Bashirzadeh, Y.; Lin, S.-S.; Bi, D.; Liu, A.P. Compressive Stress Drives Adhesion-Dependent Unjamming Transitions in Breast Cancer Cell Migration. bioRxiv 2022. [Google Scholar] [CrossRef]
- Gamboa Castro, M.; Leggett, S.E.; Wong, I.Y. Clustering and Jamming in Epithelial-Mesenchymal Co-Cultures. Soft Matter 2016, 12, 8327–8337. [Google Scholar] [CrossRef] [Green Version]
- Käs, J.; Lippoldt, J.; Grosser, S.; Tschodu, D.; Renner, F.; Kubitschke, H.; Poßögel, A.-K.; Wegscheider, A.-S.; Ulm, B.; Friedrichs, K.; et al. Cancer Cell Motility through Unjamming Impacts Metastatic Risk 2022; Research Square: Durham, NC, USA, 2022. [Google Scholar]
- Grabiec, U.; Koch, M.; Kallendrusch, S.; Kraft, R.; Hill, K.; Merkwitz, C.; Ghadban, C.; Lutz, B.; Straiker, A.; Dehghani, F. The Endocannabinoid N-Arachidonoyldopamine (NADA) Exerts Neuroprotective Effects after Excitotoxic Neuronal Damage via Cannabinoid Receptor 1 (CB 1). Neuropharmacology 2012, 62, 1797–1807. [Google Scholar] [CrossRef]
- von Widdern, J.C.; Hohmann, T.; Dehghani, F. Abnormal Cannabidiol Affects Production of Pro-Inflammatory Mediators and Astrocyte Wound Closure in Primary Astrocytic-Microglial Cocultures. Molecules 2020, 25, 496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dabov, K.; Foi, A.; Katkovnik, V.; Egiazarian, K. Image Denoising by Sparse 3-D Transform-Domain Collaborative Filtering. IEEE Trans. Image Process. 2007, 16, 2080–2095. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, U.; Walsleben, C.; Ghadban, C.; Kirchhoff, F.; Dehghani, F.; Hohmann, T. Interaction of Glia Cells with Glioblastoma and Melanoma Cells under the Influence of Phytocannabinoids. Cells 2022, 11, 147. [Google Scholar] [CrossRef] [PubMed]
- Hohmann, T.; Hohmann, U.; Dahlmann, M.; Kobelt, D.; Stein, U.; Dehghani, F. MACC1-Induced Collective Migration Is Promoted by Proliferation Rather Than Single Cell Biomechanics. Cancers 2022, 14, 2857. [Google Scholar] [CrossRef] [PubMed]
- Abate, A.R.; Durian, D.J. Topological Persistence and Dynamical Heterogeneities near Jamming. Phys. Rev. E—Stat. Nonlinear Soft Matter Phys. 2007, 76, 021306. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cartagena-Rivera, A.X.; Logue, J.S.; Waterman, C.M.; Chadwick, R.S. Actomyosin Cortical Mechanical Properties in Nonadherent Cells Determined by Atomic Force Microscopy. Biophys. J. 2016, 110, 2528–2539. [Google Scholar] [CrossRef] [Green Version]
- Logue, J.S.; Cartagena-Rivera, A.X.; Baird, M.A.; Davidson, M.W.; Chadwick, R.S.; Waterman, C.M. Erk Regulation of Actin Capping and Bundling by Eps8 Promotes Cortex Tension and Leader Bleb- Based Migration. eLife 2015, 4, e08314. [Google Scholar] [CrossRef]
- Song, B.; Chan, T. A Fast Algorithm for Level Set Based Optimization. Cam-Ucla 2002, 68, 1–20. [Google Scholar] [CrossRef]
- Hohmann, T.; Hohmann, U.; Kolbe, M.R.; Dahlmann, M.; Kobelt, D.; Stein, U. MACC1 Driven Alterations in Cellular Biomechanics Facilitate Cell Motility in Glioblastoma. Cell Commun. Signal. 2020, 18, 85. [Google Scholar] [CrossRef]
- Frasca, G.; Du, V.; Bacri, J.-C.; Gazeau, F.; Gay, C.; Wilhelm, C. Magnetically Shaped Cell Aggregates: From Granular to Contractile Materials. Soft Matter 2014, 10, 5045. [Google Scholar] [CrossRef]
- Thoresen, T.; Lenz, M.; Gardel, M.L. Thick Filament Length and Isoform Composition Determine Self-Organized Contractile Units in Actomyosin Bundles. Biophys. J. 2013, 104, 655–665. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoresen, T.; Lenz, M.; Gardel, M. Reconstitution of Contractile Actomyosin Arrays. Methods Enzymol. 2011, 540, 265–282. [Google Scholar] [CrossRef] [Green Version]
- Kim, T. Determinants of Contractile Forces Generated in Disorganized Actomyosin Bundles. Biomech. Model. Mechanobiol. 2015, 14, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Soiné, J.R.D.; Brand, C.A.; Stricker, J.; Oakes, P.W.; Gardel, M.L.; Schwarz, U.S. Model-Based Traction Force Microscopy Reveals Differential Tension in Cellular Actin Bundles. PLoS Comput. Biol. 2015, 11, e1004076. [Google Scholar] [CrossRef] [Green Version]
- Tanner, K.; Boudreau, A.; Bissell, M.J.; Kumar, S. Dissecting Regional Variations in Stress Fiber Mechanics in Living Cells with Laser Nanosurgery. Biophys. J. 2010, 99, 2775–2783. [Google Scholar] [CrossRef] [Green Version]
- Kassianidou, E.; Brand, C.A.; Schwarz, U.S.; Kumar, S. Geometry and Network Connectivity Govern the Mechanics of Stress Fibers. Proc. Natl. Acad. Sci. USA 2017, 114, 2622–2627. [Google Scholar] [CrossRef] [Green Version]
- Lowe, D.G. Distinctive Image Features from Scale-Invariant Keypoints. Int. J. Comput. Vis. 2004, 60, 91–110. [Google Scholar] [CrossRef]
- Szabó, B.; Szöllösi, G.J.; Gönci, B.; Jurányi, Z.; Selmeczi, D.; Vicsek, T. Phase Transition in the Collective Migration of Tissue Cells: Experiment and Model. Phys. Rev. E—Stat. Nonlinear Soft Matter Phys. 2006, 74, 061908. [Google Scholar] [CrossRef] [Green Version]
- Li, B.; Sun, S.X. Coherent Motions in Confluent Cell Monolayer Sheets. Biophys. J. 2014, 107, 1532–1541. [Google Scholar] [CrossRef] [Green Version]
- Parsons, J.T.; Horwitz, A.R.; Schwartz, M.A. Cell Adhesion: Integrating Cytoskeletal Dynamics and Cellular Tension. Nat. Rev. Mol. Cell Biol. 2010, 11, 633–643. [Google Scholar] [CrossRef]
- Pasapera, A.M.; Schneider, I.C.; Rericha, E.; Schlaepfer, D.D.; Waterman, C.M. Myosin II Activity Regulates Vinculin Recruitment to Focal Adhesions through FAK-Mediated Paxillin Phosphorylation. J. Cell Biol. 2010, 188, 877–890. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chepizhko, O.; Lionetti, M.C.; Malinverno, C.; Giampietro, C.; Scita, G.; Zapperi, S.; La Porta, C.A.M. From Jamming to Collective Cell Migration through a Boundary Induced Transition. Soft Matter 2018, 14, 3774–3782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vishwakarma, M.; Di Russo, J.; Probst, D.; Schwarz, U.S.; Das, T.; Spatz, J.P. Mechanical Interactions among Followers Determine the Emergence of Leaders in Migrating Epithelial Cell Collectives. Nat. Commun. 2018, 9, 3469. [Google Scholar] [CrossRef] [PubMed]
- Nnetu, K.D.; Knorr, M.; Käs, J.; Zink, M. The Impact of Jamming on Boundaries of Collectively Moving Weak-Interacting Cells. New J. Phys. 2012, 14, 115012. [Google Scholar] [CrossRef]
- Devany, J.; Sussman, D.M.; Yamamoto, T.; Manning, M.L.; Gardel, M.L. Cell Cycle–Dependent Active Stress Drives Epithelia Remodeling. Proc. Natl. Acad. Sci. USA 2021, 118, e1917853118. [Google Scholar] [CrossRef]
- Rossen, N.S.; Tarp, J.M.; Mathiesen, J.; Jensen, M.H.; Oddershede, L.B. Long-Range Ordered Vorticity Patterns in Living Tissue Induced by Cell Division. Nat. Commun. 2014, 5, 5720. [Google Scholar] [CrossRef] [Green Version]
- Doostmohammadi, A.; Thampi, S.P.; Saw, T.B.; Lim, C.T.; Ladoux, B.; Yeomans, J.M. Celebrating Soft Matter’s 10th Anniversary: Cell Division: A Source of Active Stress in Cellular Monolayers. Soft Matter 2015, 11, 7328–7336. [Google Scholar] [CrossRef] [Green Version]
- Czajkowski, M.; Sussman, D.M.; Marchetti, M.C.; Manning, M.L. Glassy Dynamics in Models of Confluent Tissue with Mitosis and Apoptosis. Soft Matter 2019, 15, 9133–9149. [Google Scholar] [CrossRef] [Green Version]
- Matoz-Fernandez, D.A.; Martens, K.; Sknepnek, R.; Barrat, J.L.; Henkes, S. Cell Division and Death Inhibit Glassy Behaviour of Confluent Tissues. Soft Matter 2017, 13, 3205–3212. [Google Scholar] [CrossRef] [Green Version]
- Ranft, J.; Basan, M.; Elgeti, J.; Joanny, J.F.; Prost, J.; Jülicher, F. Fluidization of Tissues by Cell Division and Apoptosis. Proc. Natl. Acad. Sci. USA 2010, 107, 20863–20868. [Google Scholar] [CrossRef]
- Agnihotri, S.; Zadeh, G. Metabolic Reprogramming in Glioblastoma: The Influence of Cancer Metabolism on Epigenetics and Unanswered Questions. Neuro Oncol. 2016, 18, 160–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galland, F.; Seady, M.; Taday, J.; Smaili, S.S.; Gonçalves, C.A.; Leite, M.C. Astrocyte Culture Models: Molecular and Function Characterization of Primary Culture, Immortalized Astrocytes and C6 Glioma Cells. Neurochem. Int. 2019, 131, 104538. [Google Scholar] [CrossRef] [PubMed]
- DeCamp, S.J.; Tsuda, V.M.K.; Ferruzzi, J.; Koehler, S.A.; Giblin, J.T.; Roblyer, D.; Zaman, M.H.; Weiss, S.T.; Kılıç, A.; De Marzio, M.; et al. Epithelial Layer Unjamming Shifts Energy Metabolism toward Glycolysis. Sci. Rep. 2020, 10, 18302. [Google Scholar] [CrossRef] [PubMed]
- De Marzio, M.; Kiliç, A.; Maiorino, E.; Mitchel, J.A.; Mwase, C.; O’Sullivan, M.J.; McGill, M.; Chase, R.; Fredberg, J.J.; Park, J.A.; et al. Genomic Signatures of the Unjamming Transition in Compressed Human Bronchial Epithelial Cells. Sci. Adv. 2021, 7, eabf1088. [Google Scholar] [CrossRef]
- Blauth, E.; Kubitschke, H.; Gottheil, P.; Grosser, S.; Käs, J.A. Jamming in Embryogenesis and Cancer Progression. Front. Phys. 2021, 9, 666709. [Google Scholar] [CrossRef]
- Ivkovic, S.; Beadle, C.; Noticewala, S.; Massey, S.C.; Swanson, K.R.; Toro, L.N.; Bresnick, A.R.; Canoll, P.; Rosenfeld, S.S. Direct Inhibition of Myosin II Effectively Blocks Glioma Invasion in the Presence of Multiple Motogens. Mol. Biol. Cell 2012, 23, 533–542. [Google Scholar] [CrossRef]
- Zohrabian, V.M.; Forzani, B.; Chau, Z.; Murali, R.; Jhanwar-Uniyal, M. Rho/ROCK and MAPK Signaling Pathways Are Involved in Glioblastoma Cell Migration and Proliferation. Anticancer Res. 2009, 29, 119–123. [Google Scholar]
- Huang, Y.; Tong, L.; Yi, L.; Zhang, C.; Hai, L.; Li, T.; Yu, S.; Wang, W.; Tao, Z.; Ma, H.; et al. Three-Dimensional Hydrogel Is Suitable for Targeted Investigation of Amoeboid Migration of Glioma Cells. Mol. Med. Rep. 2018, 17, 250–256. [Google Scholar] [CrossRef]
- Kumar, S.; Maxwell, I.Z.; Heisterkamp, A.; Polte, T.R.; Lele, T.P.; Salanga, M.; Mazur, E.; Ingber, D.E. Viscoelastic Retraction of Single Living Stress Fibers and Its Impact on Cell Shape, Cytoskeletal Organization, and Extracellular Matrix Mechanics. Biophys. J. 2006, 90, 3762–3773. [Google Scholar] [CrossRef] [Green Version]
- Cheng, S.; Castillo, V.; Welty, M.; Eliaz, I.; Sliva, D. Honokiol Inhibits Migration of Renal Cell Carcinoma through Activation of RhoA/ROCK/MLC Signaling Pathway. Int. J. Oncol. 2016, 49, 1525–1530. [Google Scholar] [CrossRef] [Green Version]
- Totsukawa, G.; Wu, Y.; Sasaki, Y.; Hartshorne, D.J.; Yamakita, Y.; Yamashiro, S.; Matsumura, F. Distinct Roles of MLCK and ROCK in the Regulation of Membrane Protrusions and Focal Adhesion Dynamics during Cell Migration of Fibroblasts. J. Cell Biol. 2004, 164, 427–439. [Google Scholar] [CrossRef] [PubMed]
- Aifuwa, I.; Giri, A.; Longe, N.; Lee, S.H.; An, S.S.; Wirtz, D. Senescent Stromal Cells Induce Cancer Cell Migration via Inhibition of RhoA/ROCK/Myosin-Based Cell Contractility. Oncotarget 2015, 6, 30516–30531. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohec, P.; Tailleur, J.; Van Wijland, F.; Richert, A.; Gallet, F. Distribution of Active Forces in the Cell Cortex. Soft Matter 2019, 15, 6952–6966. [Google Scholar] [CrossRef]
- Salbreux, G.; Charras, G.; Paluch, E. Actin Cortex Mechanics and Cellular Morphogenesis. Trends Cell Biol. 2012, 22, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Gavara, N.; Chadwick, R.S. Relationship between Cell Stiffness and Stress Fiber Amount, Assessed by Simultaneous Atomic Force Microscopy and Live-Cell Fluorescence Imaging. Biomech. Model. Mechanobiol. 2016, 15, 511–523. [Google Scholar] [CrossRef] [Green Version]
- Lowery, J.; Kuczmarski, E.R.; Herrmann, H.; Goldma, R.D. Intermediate Filaments Play a Pivotal Role in Regulating Cell Architecture and Function. J. Biol. Chem. 2015, 290, 17145–17153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fortier, P.; Suei, S.; Kreplak, L. Nanoscale Strain-Hardening of Keratin Fibres. PLoS ONE 2012, 7, e41814. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Tseng, Y.; Wirtz, D. Strain Hardening of Actin Filament Networks: Regulation by the Dynamic Cross-Linking Protein α-Actinin. J. Biol. Chem. 2000, 275, 35886–35892. [Google Scholar] [CrossRef] [Green Version]
- Schmoller, K.M.; Fernández, P.; Arevalo, R.C.; Blair, D.L.; Bausch, A.R. Cyclic Hardening in Bundled Actin Networks. Nat. Commun. 2010, 1, 134. [Google Scholar] [CrossRef]

| Treatment | Tension to CTL | Adhesion to CTL # |
|---|---|---|
| LN229 + Y-27632 | 38% | 26%/16% |
| LN229 + Blebbistatin | 71% | 35%/33% |
| U138 + Y-27632 | 38% * | 40%/44% |
| U138 + Blebbistatin | 49% * | 44%/56% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hohmann, U.; von Widdern, J.C.; Ghadban, C.; Giudice, M.C.L.; Lemahieu, G.; Cavalcanti-Adam, E.A.; Dehghani, F.; Hohmann, T. Jamming Transitions in Astrocytes and Glioblastoma Are Induced by Cell Density and Tension. Cells 2023, 12, 29. https://doi.org/10.3390/cells12010029
Hohmann U, von Widdern JC, Ghadban C, Giudice MCL, Lemahieu G, Cavalcanti-Adam EA, Dehghani F, Hohmann T. Jamming Transitions in Astrocytes and Glioblastoma Are Induced by Cell Density and Tension. Cells. 2023; 12(1):29. https://doi.org/10.3390/cells12010029
Chicago/Turabian StyleHohmann, Urszula, Julian Cardinal von Widdern, Chalid Ghadban, Maria Cristina Lo Giudice, Grégoire Lemahieu, Elisabetta Ada Cavalcanti-Adam, Faramarz Dehghani, and Tim Hohmann. 2023. "Jamming Transitions in Astrocytes and Glioblastoma Are Induced by Cell Density and Tension" Cells 12, no. 1: 29. https://doi.org/10.3390/cells12010029





