The Major Stilbene Compound Accumulated in the Roots of a Resistant Variety of Phoenix dactylifera L. Activates Proteasome for a Path in Anti-Aging Strategy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Sample
2.2. Preparation of the Extracts
2.3. Biological Activities
2.3.1. Phenolic Content and Antioxidant Activities
2.3.2. FoA Mycelium Growth Inhibitory Assay
2.3.3. Cell Viability and 20S Proteasome Activity
2.3.4. Inhibitory Effect on Cell-Free Mushroom Tyrosinase
2.4. Analyses, Isolation and Structure Elucidation Procedures
2.5. Statistical Analysis
3. Results and Discussion
3.1. Total Phenolic and Flavonoid Contents of Methanolic Extracts
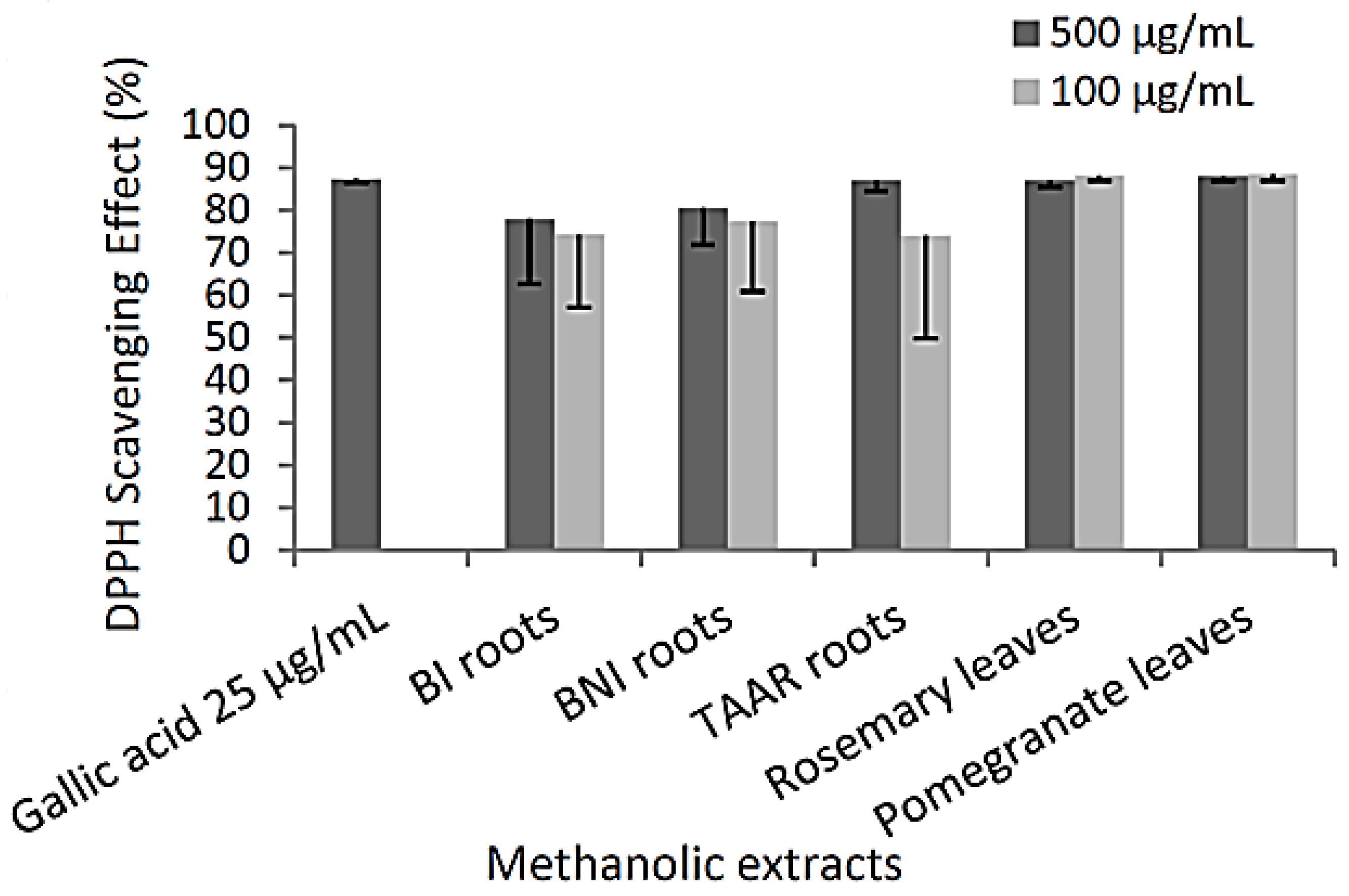
3.2. Antioxidant Potential of Methanolic Extracts
3.3. Mushroom Tyrosinase Activity
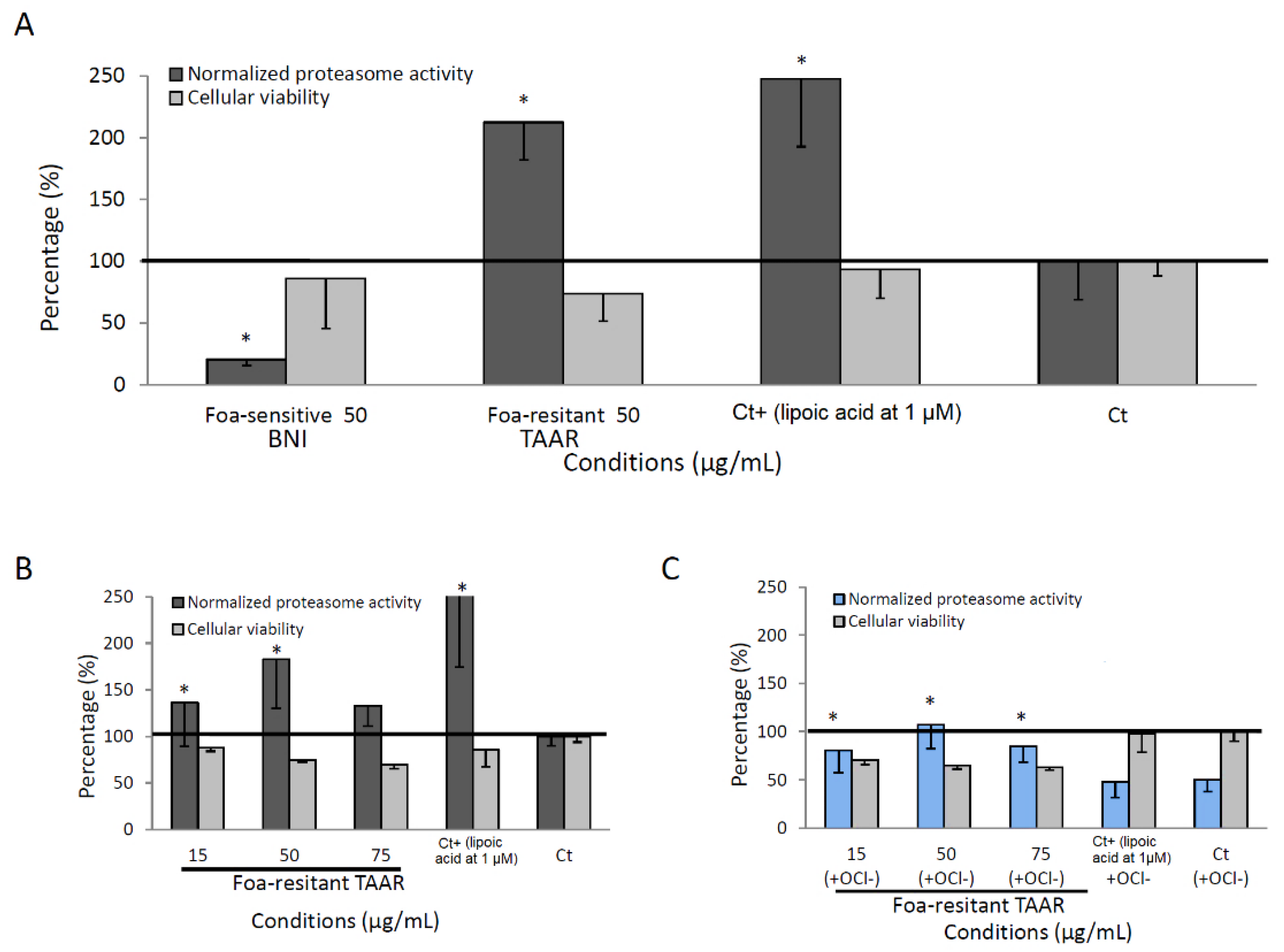
3.4. Proteasome Activity of Methanolic Root Date Palm Extracts
3.5. Spectrometric Analyses
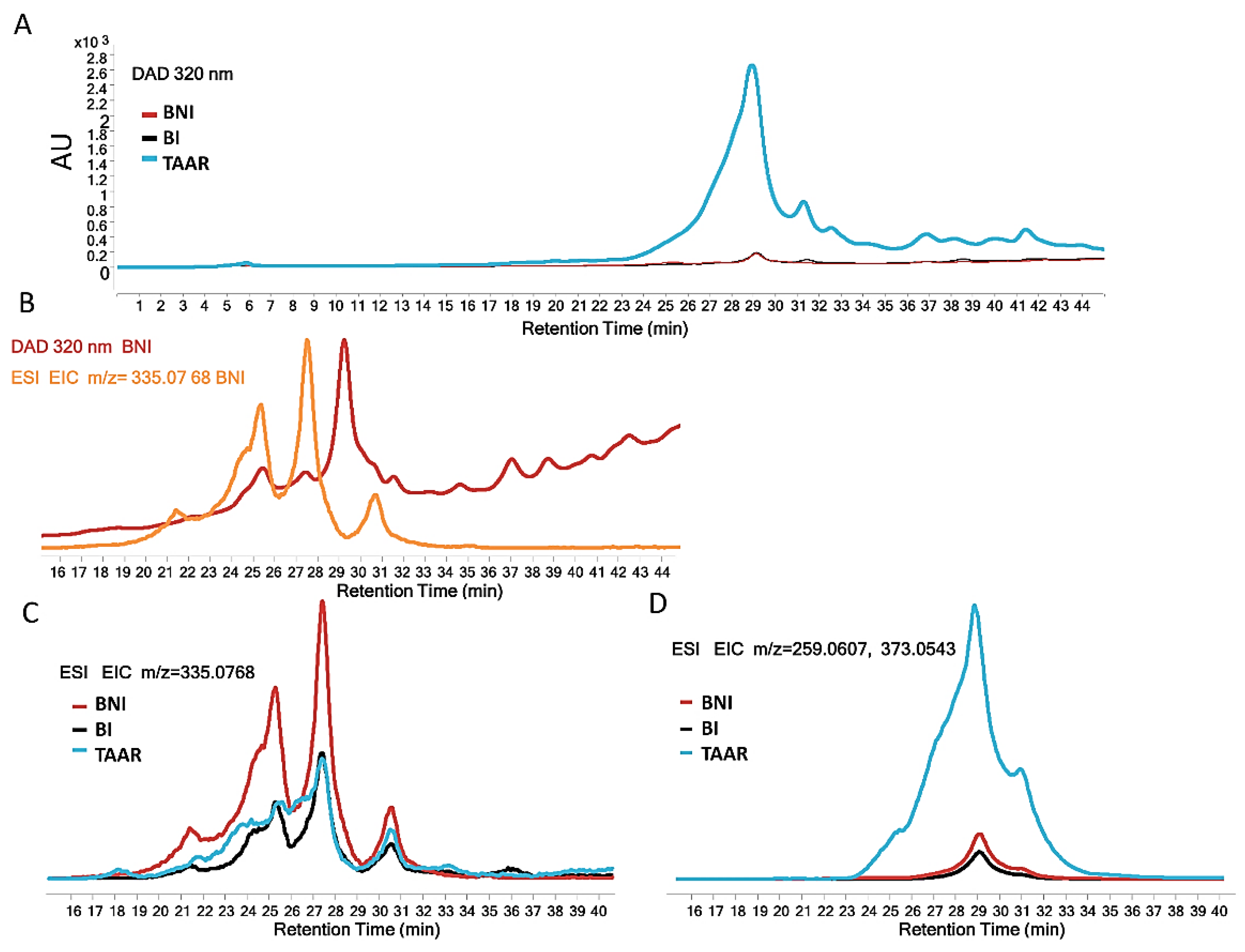
3.5.1. LC-DAD-MS Analysis of Methanolic Extracts
3.5.2. Determination by MS/MS and NMR of the Major Differential Compound in Date Palm TAAR Resistant Date Palm
3.6. The Stilbene Inhibition of FoA Mycelium Growth Is Associated with Date Palm Resistance to FoA
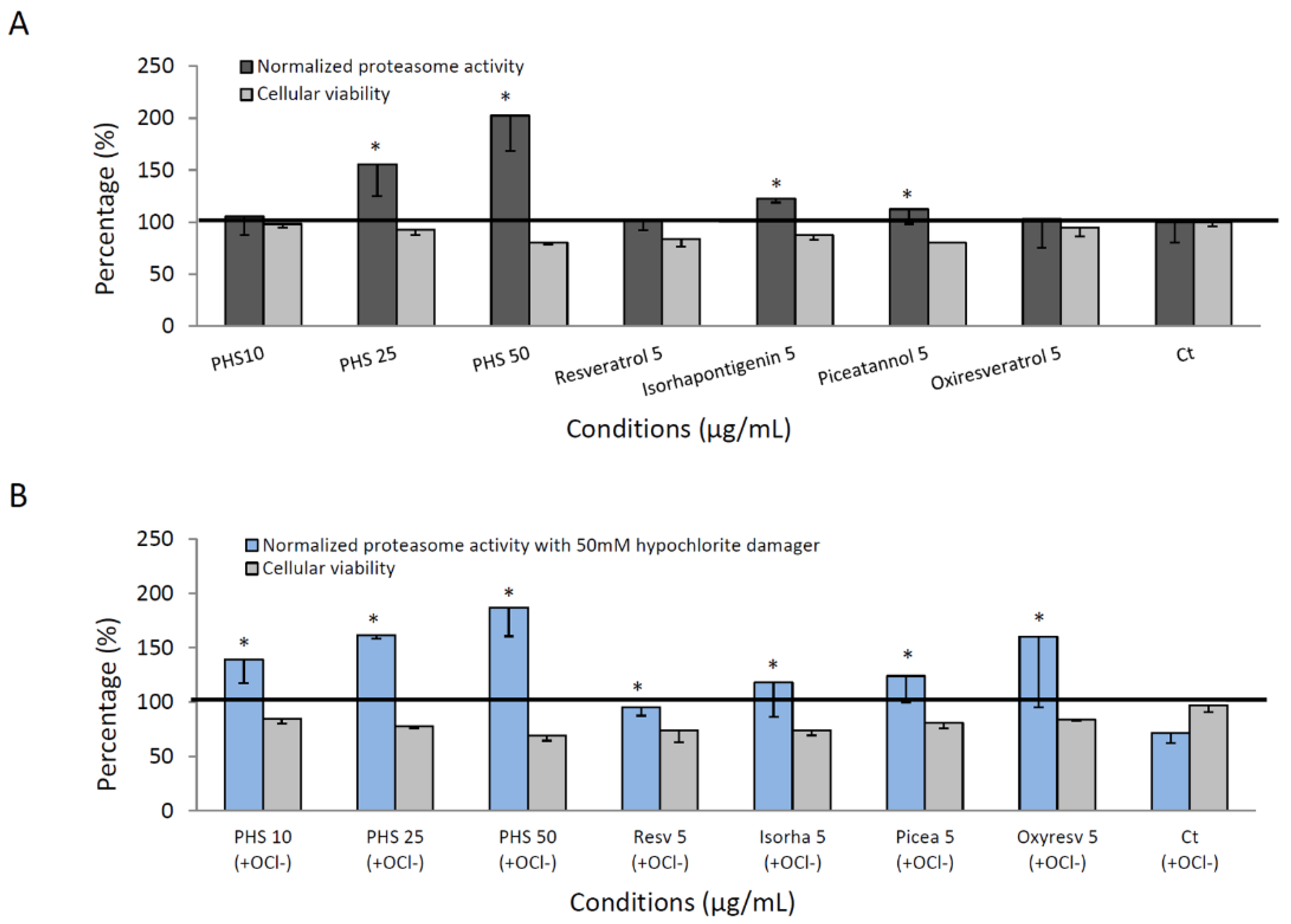
3.7. Proteasome Activity of Pure Stilbene Compounds
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Djerbi, M. Bayoud disease in North Africa: History, distribution, diagnosis and control. Date Palm J. 1982, 1, 153–197. [Google Scholar]
- Boumedjout, H. Morocco Markets Bayoud-Resistant Strains of Date Palm; Nature Publishing Group: Berlin, Germany, 2010. [Google Scholar]
- El Hadrami, A.; El Idrissi-Tourane, A.; El Hassni, M.; Daayf, F.; El Hadrami, I. Toxin-based in-vitro selection and its potential application to date palm for resistance to the bayoud Fusarium wilt. Comptes Rendus Biol. 2005, 328, 732–744. [Google Scholar] [CrossRef] [PubMed]
- Boulenouar, N.; Marouf, A.; Cheriti, A. Antifungal activity and phytochemical screening of extracts from Phoenix dactylifera L. cultivars. Nat. Prod. Res. 2011, 25, 1999–2002. [Google Scholar] [CrossRef] [PubMed]
- Ziouti, A.; El Modafar, C.; Fleuriet, A.; El Boustani, S.; Macheix, J. Phenolic compounds in date palm cultivars sensitive and resistant to Fusarium oxysporum. Biol. Plant. 1996, 38, 451–457. [Google Scholar] [CrossRef]
- El Modafar, C.; Tantaoui, A.; El Boustani, E. Effect of caffeoylshikimic acid of date palm roots on activity and production of Fusarium oxysporum f. sp. albedinis cell wall degrading enzymes. J. Phytopathol. 2000, 148, 101–108. [Google Scholar]
- Ouahhoud, S.; Bencheikh, N.; Khoulati, A.; Kadda, S.; Mamri, S.; Ziani, A.; Baddaoui, S.; Eddabbeh, F.-E.; Elassri, S.; Lahmass, I. Crocus sativus L. Stigmas, Tepals and Leaves Ameliorate Gentamicin-Induced Renal Toxicity: A Biochemical and Histopathological Study. Evid.-Based Complement. Altern. Med. 2022, 2022, 7127037. [Google Scholar] [CrossRef]
- Ouahhoud, S.; Khoulati, A.; Kadda, S.; Bencheikh, N.; Mamri, S.; Ziani, A.; Baddaoui, S.; Eddabbeh, F.-E.; Lahmass, I.; Benabbes, R. Antioxidant Activity, Metal Chelating Ability and DNA Protective Effect of the Hydroethanolic Extracts of Crocus sativus Stigmas, Tepals and Leaves. Antioxidants 2022, 11, 932. [Google Scholar] [CrossRef]
- Ouahhoud, S.; Touiss, I.; Khoulati, A.; Lahmass, I.; Mamri, S.; Meziane, M.; Elassri, S.; Bencheikh, N.; Benabbas, R.; Asehraou, A. Hepatoprotective effects of hydroethanolic extracts of Crocus sativus tepals, stigmas and leaves on carbon tetrachloride induced acute liver injury in rats. J. Physiol. Pharmacol. 2021, 25, 178–188. [Google Scholar] [CrossRef]
- Ouahhoud, S.; Lahmass, I.; Bouhrim, M.; Khoulati, A.; Sabouni, A.; Benabbes, R.; Asehraou, A.; Choukri, M.; Bnouham, M.; Saalaoui, E. Antidiabetic effect of hydroethanolic extract of Crocus sativus stigmas, tepals and leaves in streptozotocin-induced diabetic rats. J. Physiol. Pharmacol. 2019, 23, 9–20. [Google Scholar]
- Halliwell, B.; Gutteridge, J.M. Free Radicals in Biology and Medicine; Clarendon Press: Oxford, UK, 1989. [Google Scholar]
- Pandey, K.B.; Rizvi, S.I. Plant polyphenols as dietary antioxidants in human health and disease. Oxid. Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [Green Version]
- Ciechanover, A. The ubiquitin-proteasome pathway: On protein death and cell life. EMBO J. 1998, 17, 7151–7160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chondrogianni, N.; Gonos, E.S. Proteasome activation as a novel antiaging strategy. IUBMB Life 2008, 60, 651–655. [Google Scholar] [CrossRef] [PubMed]
- Hakkou, A.; Bouakka, M. In Vitro Inhibitory Effect of the Extract Powder of Rosemary (Rosmarinus officinalis), Oleander (Nerium oleander), Grenadier (Punica granatum) on the Growth of Fusarium oxysporum fs Albidinis and In Vivo Test Antagonist Fungi on the Incidence and the Control of Vascular wilt Disease of Date Palm in Palm Grove in Figuig South of Morocco. Adv. Environ. Biol. 2015, 9, 126–132. [Google Scholar]
- MAPM. Stratégies d’Intervention de la DPA de Figuig; Direction Provinciale De l’Agriculture De Figuig: Figuig, Morocco, 2009; p. 25. [Google Scholar]
- Nacoulma, A.P.; Compaoré, M.; De Lorenzi, M.; Kiendrebeogo, M.; Nacoulma, O.G. In vitro Antioxidant and Anti-inflammatory Activities of Extracts from Nicotiana tabacum L. (Solanaceae) Leafy Galls Induced by Rhodococcus fascians. J. Phytopathol. 2012, 160, 617–621. [Google Scholar] [CrossRef]
- Neri, F.; Mari, M.; Brigati, S. Control of Penicillium expansum by plant volatile compounds. Plant Pathol. 2006, 55, 100–105. [Google Scholar] [CrossRef]
- Reinheckel, T.; Sitte, N.; Ullrich, O.; Kuckelkorn, U.; Davies, K.J.; Grune, T. Comparative resistance of the 20S and 26S proteasome to oxidative stress. Biochem. J. 1998, 335, 637–642. [Google Scholar] [CrossRef]
- Chan, Y.; Kim, K.; Cheah, S. Inhibitory effects of Sargassum polycystum on tyrosinase activity and melanin formation in B16F10 murine melanoma cells. J. Ethnopharmacol. 2011, 137, 1183–1188. [Google Scholar] [CrossRef]
- Basile, A.; Ferrara, L.; Del Pezzo, M.; Mele, G.; Sorbo, S.; Bassi, P.; Montesano, D. Antibacterial and antioxidant activities of ethanol extract from Paullinia cupana Mart. J. Ethnopharmacol. 2005, 102, 32–36. [Google Scholar] [CrossRef]
- Mensor, L.L.; Menezes, F.S.; Leitão, G.G.; Reis, A.S.; dos Santos, T.C.; Coube, C.S.; Leitão, S.G. Screening of Brazilian plant extracts for antioxidant activity by the use of DPPH free radical method. Phytother. Res. 2001, 15, 127–130. [Google Scholar] [CrossRef]
- Rice-evans, C.A.; Miller, N.J.; Bolwell, P.G.; Bramley, P.M.; Pridham, J.B. The relative antioxidant activities of plant-derived polyphenolic flavonoids. Free Radic. Res. 1995, 22, 375–383. [Google Scholar] [CrossRef]
- Makris, D.; Kefalas, P. Association between in vitro antiradical activity and ferric reducing power in aged red wines: A mechanistic approach. Food Sci. Technol. Int. 2005, 11, 11–18. [Google Scholar] [CrossRef]
- Asanuma, M.; Miyazaki, I.; Ogawa, N. Dopamine-or L-DOPA-induced neurotoxicity: The role of dopamine quinone formation and tyrosinase in a model of Parkinson’s disease. Neurotox. Res. 2003, 5, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Meyskens, F.L.; Van Chau, H.; Tohidian, N.; Buckmeier, J. Luminol-enhanced chemiluminescent response of human melanocytes and melanoma cells to hydrogen peroxide stress. Pigment Cell Res. 1997, 10, 184–189. [Google Scholar] [CrossRef] [Green Version]
- Fais, A.; Corda, M.; Era, B.; Fadda, M.B.; Matos, M.J.; Quezada, E.; Santana, L.; Picciau, C.; Podda, G.; Delogu, G. Tyrosinase inhibitor activity of coumarin-resveratrol hybrids. Molecules 2009, 14, 2514–2520. [Google Scholar] [CrossRef]
- Ohguchi, K.; Tanaka, T.; Kido, T.; Baba, K.; Iinuma, M.; Matsumoto, K.; Akao, Y.; Nozawa, Y. Effects of hydroxystilbene derivatives on tyrosinase activity. Biochem. Biophys. Res. Commun. 2003, 307, 861–863. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.S.; Hwang, J.S.; Chang, I.; Kim, S. Age-associated decrease in proteasome content and activities in human dermal fibroblasts: Restoration of normal level of proteasome subunits reduces aging markers in fibroblasts from elderly persons. J. Gerontol. Ser. A 2007, 62, 490–499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stella, L.; De Rosso, M.; Panighel, A.; Vedova, A.D.; Flamini, R.; Traldi, P. Collisionally induced fragmentation of [M–H]− species of resveratrol and piceatannol investigated by deuterium labelling and accurate mass measurements. Rapid Commun. Mass Spectrom. 2008, 22, 3867–3872. [Google Scholar] [CrossRef]
- Abbas, G.M.; Abdel Bar, F.M.; Baraka, H.N.; Gohar, A.A.; Lahloub, M.-F. A new antioxidant stilbene and other constituents from the stem bark of Morus nigra L. Nat. Prod. Res. 2014, 28, 952–959. [Google Scholar] [CrossRef]
- Wang, M.; Jin, Y.; Ho, C.-T. Evaluation of resveratrol derivatives as potential antioxidants and identification of a reaction product of resveratrol and 2, 2-diphenyl-1-picryhydrazyl radical. J. Agric. Food Chem. 1999, 47, 3974–3977. [Google Scholar] [CrossRef]
- Jian, W.; He, D.; Xi, P.; Li, X. Synthesis and biological evaluation of novel fluorine-containing stilbene derivatives as fungicidal agents against phytopathogenic fungi. J. Agric. Food Chem. 2015, 63, 9963–9969. [Google Scholar] [CrossRef]
- Lam, S.-H.; Lee, S.-S. Unusual stilbenoids and a stilbenolignan from seeds of Syagrus romanzoffiana. Phytochemistry 2010, 71, 792–797. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Cuendet, M.; Vigo, J.S.; Graham, J.G.; Cabieses, F.; Fong, H.H.; Pezzuto, J.M.; Kinghorn, A.D. A novel cyclooxygenase-inhibitory stilbenolignan from the seeds of Aiphanes aculeata. Org. Lett. 2001, 3, 2169–2171. [Google Scholar] [CrossRef] [PubMed]
- Fernández, M.I.; Pedro, J.R.; Seoane, E. Two polyhydroxystilbenes from stems of Phoenix dactylifera. Phytochemistry 1983, 22, 2819–2821. [Google Scholar] [CrossRef]
- Langcake, P.; Pryce, R. The production of resveratrol by Vitis vinifera and other members of the Vitaceae as a response to infection or injury. Physiol. Plant Pathol. 1976, 9, 77–86. [Google Scholar] [CrossRef]
- Lam, S.-H.; Chen, J.-M.; Kang, C.-J.; Chen, C.-H.; Lee, S.-S. α-Glucosidase inhibitors from the seeds of Syagrus romanzoffiana. Phytochemistry 2008, 69, 1173–1178. [Google Scholar] [CrossRef]
- Pflieger, A.; Waffo Teguo, P.; Papastamoulis, Y.; Chaignepain, S.; Subra, F.; Munir, S.; Delelis, O.; Lesbats, P.; Calmels, C.; Andreola, M.-L. Natural stilbenoids isolated from grapevine exhibiting inhibitory effects against HIV-1 integrase and eukaryote MOS1 transposase in vitro activities. PLoS ONE 2013, 8, e81184. [Google Scholar] [CrossRef]
- Simmler, C.; Antheaume, C.; Lobstein, A. Antioxidant biomarkers from Vanda coerulea stems reduce irradiated HaCaT PGE-2 production as a result of COX-2 inhibition. PLoS ONE 2010, 5, e13713. [Google Scholar] [CrossRef] [Green Version]
- Cheng, T.-C.; Lai, C.-S.; Chung, M.-C.; Kalyanam, N.; Majeed, M.; Ho, C.-T.; Ho, Y.-S.; Pan, M.-H. Potent anti-cancer effect of 3′-hydroxypterostilbene in human colon xenograft tumors. PLoS ONE 2014, 9, e111814. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.W.; Kang, N.J.; Rogozin, E.A.; Oh, S.M.; Heo, Y.S.; Pugliese, A.; Bode, A.M.; Lee, H.J.; Dong, Z. The resveratrol analogue 3, 5, 3′, 4′, 5′-pentahydroxy-trans-stilbene inhibits cell transformation via MEK. Int. J. Cancer 2008, 123, 2487–2496. [Google Scholar] [CrossRef] [Green Version]
- Watjen, W.; Michels, G.; Steffan, B.; Niering, P.; Chovolou, Y.; Kampkotter, A.; Tran-Thi, Q.-H.; Proksch, P.; Kahl, R. Low concentrations of flavonoids are protective in rat H4IIE cells whereas high concentrations cause DNA damage and apoptosis. J. Nutr. 2005, 135, 525–531. [Google Scholar] [CrossRef] [Green Version]
- Li, H.; Wu, W.K.K.; Zheng, Z.; Che, C.T.; Li, Z.J.; Xu, D.D.; Wong, C.C.M.; Ye, C.G.; Sung, J.J.Y.; Cho, C.H. 3, 3′, 4, 5, 5′-pentahydroxy-trans-stilbene, a resveratrol derivative, induces apoptosis in colorectal carcinoma cells via oxidative stress. Eur. J. Pharmacol. 2010, 637, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Bekhet, O.H.; Eid, M.E. The interplay between reactive oxygen species and antioxidants in cancer progression and therapy: A narrative review. Transl. Cancer Res. 2021, 10, 4196. [Google Scholar] [CrossRef] [PubMed]
- Olaf, H.; Waltraud, K.-M.; Kerstin, E.; Frank, J.; Claudia, J.; Anke, K.; Ursula, E.; Marianne, W.-L.; Anemone, T.; Soraya, H. Cellular rejuvenation compounds. Henkel. EP2005941A2; European Patent Office, 29 May 2008. [Google Scholar]
- Kasiotis, K.M.; Pratsinis, H.; Kletsas, D.; Haroutounian, S.A. Resveratrol and related stilbenes: Their anti-aging and anti-angiogenic properties. Food Chem. Toxicol. 2013, 61, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Reinisalo, M.; Kårlund, A.; Koskela, A.; Kaarniranta, K.; Karjalainen, R.O. Polyphenol Stilbenes: Molecular Mechanisms of Defence against Oxidative Stress and Aging-Related Diseases. Oxid. Med. Cell. Longev. 2015, 2015, 340520. [Google Scholar] [CrossRef] [PubMed]

| Methanolic Extract (500 µg/mL) | Polyphenols Contents (mg of GAE/g) | Flavonoid Contents (mg of QE/g) | ||||
|---|---|---|---|---|---|---|
| BI roots | 171 | ± | 14 | 245 | ± | 104 |
| BNI roots | 253 | ± | 47 | 406 * | ± | 66 |
| TAAR roots | 204 | ± | 14 | 240 * | ± | 25 |
| Rosemary leaves | 117 | ± | 22 | 207 | ± | 62 |
| Pomegranate leaves | 175 | ± | 0 | 207 | ± | 4 |
| Methanolic Extract (500 µg/mL) | Reducing Power Assay (mg of AAE/g) | ||
|---|---|---|---|
| BI roots | 43 | ± | 5 |
| BNI roots | 27 * | ± | 2 |
| TAAR roots | 95 * | ± | 1 |
| Rosemary leaves | 73 | ± | 2 |
| Pomegranate leaves | 165 | ± | 15 |
| Gallic acid (25 µg/mL) | 1026 | ± | 144 |
| Methanolic Extract (500 µg/mL) | Inhibition of Tyrosinase Activity (mg of GAE/g) | ||
|---|---|---|---|
| BI roots | 27 | ± | 11 |
| BNI roots | 11 | ± | 4 |
| TAAR roots | 87 | ± | 26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benabbes, R.; Ouahhoud, S.; Moueqqit, M.; Addi, M.; Hano, C.; Delporte, C.; Nacoulma, A.P.; Megalizzi, V. The Major Stilbene Compound Accumulated in the Roots of a Resistant Variety of Phoenix dactylifera L. Activates Proteasome for a Path in Anti-Aging Strategy. Cells 2023, 12, 71. https://doi.org/10.3390/cells12010071
Benabbes R, Ouahhoud S, Moueqqit M, Addi M, Hano C, Delporte C, Nacoulma AP, Megalizzi V. The Major Stilbene Compound Accumulated in the Roots of a Resistant Variety of Phoenix dactylifera L. Activates Proteasome for a Path in Anti-Aging Strategy. Cells. 2023; 12(1):71. https://doi.org/10.3390/cells12010071
Chicago/Turabian StyleBenabbes, Redouane, Sabir Ouahhoud, Mohammed Moueqqit, Mohamed Addi, Christophe Hano, Cédric Delporte, Aminata P. Nacoulma, and Véronique Megalizzi. 2023. "The Major Stilbene Compound Accumulated in the Roots of a Resistant Variety of Phoenix dactylifera L. Activates Proteasome for a Path in Anti-Aging Strategy" Cells 12, no. 1: 71. https://doi.org/10.3390/cells12010071






