Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease
Abstract
:1. Introduction
2. Relationship between Oxidative Stress and Mitochondria in Kidney Diseases
2.1. Mitochondrial Homeostasis
2.2. Mitochondrial Antioxidant System
2.3. Sustaining Mitochondrial Energy Metabolism
2.4. Improvement of Mitochondrial Biogenesis
2.5. Maintaining Mitochondrial Dynamics (Fusion/Fission and Mitophagy)
2.6. Others
2.6.1. Cardiolipin
2.6.2. Mitochondria-Mediated Apoptotic Pathway
3. Causes and Risk Factors of Oxidative Stress-Related Mitochondrial Dysfunction in CKD and ESRD
3.1. Environmental Renal Injury
3.1.1. Air Pollution
3.1.2. Heavy Metals
3.1.3. Fungicides, Herbicides, and Insecticides
3.1.4. Plasticizer Compounds/Organic Pollutants
3.1.5. Nanoparticles
3.1.6. Food Contamination
3.2. Lifestyle-Related Renal Injury
3.2.1. Chronic Alcohol/Ethanol Consumption
3.2.2. High Fat Diet (HFD)/Obesity/Metabolic Syndrome
3.2.3. Smoking
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| 5/6Nx | five-sixths nephrectomy |
| Atg | autophagy-related protein |
| ATP | adenosine triphosphate |
| BNIP3 | BCL2 interacting protein 3 |
| BNIP3L | BCL2 interacting protein 3 like |
| Cd | cadmium |
| CKD | chronic kidney disease |
| Cr | chromium |
| cyt c | cytochrome c |
| ESRD | end-stage renal disease |
| ETC | electron transport chain |
| FIS | mitochondrial fission 1 |
| FUNDC1 | FUN14 domain containing protein 1 |
| GSK3β | glycogen synthase kinase 3 beta |
| H2O2 | hydrogen peroxide |
| HFD | high fat diet |
| IMM | inner mitochondrial membrane |
| LC3 | light chain 3 |
| Mfn1/2 | mitofusin 1/2 |
| MitoQ | mitoquinone (10-(6′-ubiquinonyl)decyltriphenylphosphonium bromide) |
| MMP | mitochondrial membrane potential |
| MnSOD | manganese-superoxide dismutase |
| Mo | molybdenum |
| mPTP | mitochondrial permeability transition pore |
| mtDNA | mitochondrial DNA |
| mtROS | mitochondrial ROS |
| MWCNTs | multi-walled carbon nanotubes |
| NAC | N-acetyl-cysteine |
| NOX | NADPH oxidase |
| Nrf1/2 | nuclear factor erythroid 2-related factor 1/2 |
| O2˙− | superoxide |
| OMM | outer mitochondrial membranes |
| OPA1 | optic atrophy 1 |
| OXPHOS | oxidative phosphorylation |
| Pb | lead |
| PGC-1α | peroxisome proliferator-activated receptor-gamma coactivator-1alpha |
| ROS | reactive oxygen species |
| SGK1 | serum- and glucocorticoid-induced kinase 1 |
| Sirt1/3 | silent mating type information regulation 2 homolog 1/3, sirtuin 1/3 |
| SOD | superoxide dismutase |
| TFAM | mitochondrial transcription factor A |
| U | uranium |
| UCP2 | uncoupling protein 2 |
| UUO | unilateral ureteral obstruction |
| W | tungsten |
References
- Wang, Z.M.; Yang, Z.L.; Bosy-westphal, A.; Zhang, J.Y.; Schautz, B.; Later, W.; Heymsfield, S.B.; Muller, M.J. Specific meta-bolic rates of major organs and tissues across adulthood: Evaluation by mechanistic model of resting energy expenditure. Am. J. Clin. Nutr. 2010, 92, 1369–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pagliarini, D.J.; Calvo, S.E.; Chang, B.; Sheth, S.A.; Vafai, S.B.; Ong, S.-E.; Walford, G.A.; Sugiana, C.; Boneh, A.; Chen, W.K.; et al. A Mitochondrial Protein Compendium Elucidates Complex I Disease Biology. Cell 2008, 134, 112–123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhargava, P.; Schnellmann, R.G. Mitochondrial energetics in the kidney. Nat. Rev. Nephrol. 2017, 13, 629–646. [Google Scholar] [CrossRef]
- Fredericks, W.J.; Yin, H.; Lal, P.; Puthiyaveettil, R.; Malkowicz, S.B.; Fredericks, N.J.; Tomaszewski, J.; Rauscher, F.J., 3rd; Malkowicz, S.B. Ectopic expression of the TERE1 (UBIAD1) protein inhibits growth of renal clear cell carcinoma cells: Altered metabolic phenotype associated with reactive oxygen species, nitric oxide and SXR target genes involved in cholesterol and lipid metabolism. Int. J. Oncol. 2013, 43, 638–652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [CrossRef] [PubMed]
- Zorov, D.B.; Juhaszova, M.; Sollott, S.J. Mitochondrial Reactive Oxygen Species (ROS) and ROS-Induced ROS Release. Physiol. Rev. 2014, 94, 909–950. [Google Scholar] [CrossRef] [Green Version]
- Jiang, M.; Bai, M.; Lei, J.; Xie, Y.; Xu, S.; Jia, Z.; Zhang, A. Mitochondrial dysfunction and the AKI-to-CKD transition. Am. J. Physiol. Physiol. 2020, 319, F1105–F1116. [Google Scholar] [CrossRef]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef] [PubMed]
- Krata, N.; Zagożdżon, R.; Foroncewicz, B.; Mucha, K. Oxidative Stress in Kidney Diseases: The Cause or the Consequence? Arch. Immunol. Ther. Exp. 2017, 66, 211–220. [Google Scholar] [CrossRef] [Green Version]
- Gyurászová, M.; Gurecká, R.; Bábíčková, J.; Tóthová, L. Oxidative Stress in the Pathophysiology of Kidney Disease: Implications for Noninvasive Monitoring and Identification of Biomarkers. Oxidative Med. Cell Longev. 2020, 2020, 5478708. [Google Scholar] [CrossRef]
- Yang, H.-C.; Zuo, Y.; Fogo, A.B. Models of chronic kidney disease. Drug Discov. Today Dis. Model. 2010, 7, 13–19. [Google Scholar] [CrossRef] [Green Version]
- Ueda, S.; Ozawa, S.; Mori, K.; Asanuma, K.; Yanagita, M.; Uchida, S.; Nakagawa, T. ENOS deficiency causes podocyte injury with mitochondrial abnormality. Free Radic. Biol. Med. 2015, 87, 181–192. [Google Scholar] [CrossRef]
- Khan, M.A.; Wang, X.; Giuliani, K.T.; Nag, P.; Grivei, A.; Ungerer, J.; Hoy, W.; Healy, H.; Gobe, G.; Kassianos, A.J. Underlying Histopathology Determines Response to Oxidative Stress in Cultured Human Primary Proximal Tubular Epithelial Cells. Int. J. Mol. Sci. 2020, 21, 560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeong, J.Y.; Na, K.R.; Shin, J.A.; Suh, K.-S.; Kim, J.-J.; Lee, K.W.; Choi, D.E. Collecting Duct-Specific CR6-Interacting Factor-1-Deletion Aggravates Renal Inflammation and Fibrosis Induced by Unilateral Ureteral Obstruction. Int. J. Mol. Sci. 2021, 22, 11699. [Google Scholar] [CrossRef] [PubMed]
- Ruiz-Ortega, M.; Rayego-Mateos, S.; Lamas, S.; Ortiz, A.; Rodrigues-Diez, R.R. Targeting the progression of chronic kidney disease. Nat. Rev. Nephrol. 2020, 16, 269–288. [Google Scholar] [CrossRef] [PubMed]
- Cachofeiro, V.; Goicochea, M.; de Vinuesa, S.G.; Oubiña, P.; Lahera, V.; Luño, J. Oxidative stress and inflammation, a link between chronic kidney disease and cardiovascular disease. Kidney Int. 2008, 74, S4–S9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Irazabal, M.V.; Torres, V.E. Reactive Oxygen Species and Redox Signaling in Chronic Kidney Disease. Cells 2020, 9, 1342. [Google Scholar] [CrossRef]
- Eddy, A.A.; López-Guisa, J.M.; Okamura, D.M.; Yamaguchi, I. Investigating mechanisms of chronic kidney disease in mouse models. Pediatr. Nephrol. 2011, 27, 1233–1247. [Google Scholar] [CrossRef] [Green Version]
- Dos Santos, I.F.; Sheriff, S.; Amlal, S.; Ahmed, R.P.H.; Thakar, C.V.; Amlal, H. Adenine acts in the kidney as a signaling factor and causes salt- and water-losing nephropathy: Early mechanism of adenine-induced renal injury. Am. J. Physiol. Physiol. 2019, 316, F743–F757. [Google Scholar] [CrossRef]
- Diwan, V.; Brown, L.; Gobe, G.C. Adenine-induced chronic kidney disease in rats. Nephrology 2017, 23, 5–11. [Google Scholar] [CrossRef]
- Krishnaraj, P.; Ravindran, S.; Kurian, G.A. The renal mitochondrial dysfunction in patients with vascular calcification is prevented by sodium thiosulfate. Int. Urol. Nephrol. 2016, 48, 1927–1935. [Google Scholar] [CrossRef]
- Zhao, H.; Liu, Y.-J.; Liu, Z.-R.; Tang, D.-D.; Chen, X.-W.; Chen, Y.-H.; Zhou, R.-N.; Chen, S.-Q.; Niu, H.-X. Role of mitochondrial dysfunction in renal fibrosis promoted by hypochlorite-modified albumin in a remnant kidney model and protective effects of antioxidant peptide SS-31. Eur. J. Pharmacol. 2017, 804, 57–67. [Google Scholar] [CrossRef]
- Chung, S.D.; Lai, T.Y.; Chien, C.T.; Yu, H.J. Activating Nrf-2 Signaling Depresses Unilateral Ureteral Obstruction-Evoked Mitochondrial Stress-Related Autophagy, Apoptosis and Pyroptosis in Kidney. PLoS ONE 2012, 7, e47299. [Google Scholar] [CrossRef]
- Wang, R.; Kairen, C.; Li, L.; Zhang, L.; Gong, H.; Huang, X. Overexpression of NDUFV1 alleviates renal damage by improving mitochondrial function in unilateral ureteral obstruction model mice. Cell Biol. Int. 2022, 46, 381–390. [Google Scholar] [CrossRef]
- Halestrap, A.P. What is the mitochondrial permeability transition pore? J. Mol. Cell Cardiol. 2009, 46, 821–831. [Google Scholar] [CrossRef]
- Bernardi, P.; Rasola, A.; Forte, M.; Lippe, G. The Mitochondrial Permeability Transition Pore: Channel Formation by F-ATP Synthase, Integration in Signal Transduction, and Role in Pathophysiology. Physiol. Rev. 2015, 95, 1111–1155. [Google Scholar] [CrossRef] [Green Version]
- Kurian, G.A.; Mohan, D.; Balasubramanian, E.D.; Ravindran, S. Renal mitochondria can withstand hypoxic/ischemic injury secondary to renal failure in uremic rats pretreated with sodium thiosulfate. Indian J. Pharmacol. 2017, 49, 317–321. [Google Scholar] [CrossRef]
- Mitchell, T.; Rotaru, D.; Saba, H.; Smith, R.A.J.; Murphy, M.P.; MacMillan-Crow, L.A. The Mitochondria-Targeted Antioxidant Mitoquinone Protects against Cold Storage Injury of Renal Tubular Cells and Rat Kidneys. J. Pharmacol. Exp. Ther. 2010, 336, 682–692. [Google Scholar] [CrossRef] [Green Version]
- Parajuli, N.; Campbell, L.H.; Marine, A.; Brockbank, K.G.M.; MacMillan-Crow, L.A. MitoQ Blunts Mitochondrial and Renal Damage during Cold Preservation of Porcine Kidneys. PLoS ONE 2012, 7, e48590. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Xu, X.; Zhang, F.; Wang, M.; Xu, Y.; Tang, D.; Wang, J.; Qin, Y.; Liu, Y.; Tang, C.; et al. The mitochondria-targeted antioxidant MitoQ ameliorated tubular injury mediated by mitophagy in diabetic kidney disease via Nrf2/PINK1. Redox Biol. 2017, 11, 297–311. [Google Scholar] [CrossRef]
- Pokrzywinski, K.L.; Biel, T.G.; Kryndushkin, D.; Rao, V.A. Therapeutic Targeting of the Mitochondria Initiates Excessive Superoxide Production and Mitochondrial Depolarization Causing Decreased mtDNA Integrity. PLoS ONE 2016, 11, e0168283. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, C.; Liu, X.; Di, C.; Wang, Z.; Mi, X.; Liu, Y.; Zhao, Q.; Mao, A.; Chen, W.; Gan, L.; et al. MitoQ regulates autophagy by inducing a pseudo-mitochondrial membrane potential. Autophagy 2017, 13, 730–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gottwald, E.M.; Duss, M.; Bugarski, M.; Haenni, D.; Schuh, C.D.; Landau, E.M.; Hall, A.M. The targeted anti-oxidant MitoQ causes mitochondrial swelling and depolarization in kidney tissue. Physiol. Rep. 2018, 6, e13667. [Google Scholar] [CrossRef] [PubMed]
- Holmström, K.M.; Finkel, T. Cellular mechanisms and physiological consequences of redox-dependent signalling. Nat. Rev. Mol. Cell Biol. 2014, 15, 411–421. [Google Scholar] [CrossRef]
- Stöcker, S.; Van Laer, K.; Mijuskovic, A.; Dick, T.P. The Conundrum of Hydrogen Peroxide Signaling and the Emerging Role of Peroxiredoxins as Redox Relay Hubs. Antioxidants Redox Signal. 2018, 28, 558–573. [Google Scholar] [CrossRef]
- Mailloux, R.J. An Update on Mitochondrial Reactive Oxygen Species Production. Antioxidants 2020, 9, 472. [Google Scholar] [CrossRef]
- Muñoz, M.; López-Oliva, M.E.; Rodríguez, C.; Martínez, M.P.; Sáenz-Medina, J.; Sánchez, A.; Climent, B.; Benedito, S.; García-Sacristán, A.; Rivera, L.; et al. Differential contribution of Nox1, Nox2 and Nox4 to kidney vascular oxidative stress and endothelial dysfunction in obesity. Redox Biol. 2019, 28, 101330. [Google Scholar] [CrossRef]
- Lee, D.-Y.; Wauquier, F.; Eid, A.A.; Roman, L.J.; Ghosh-Choudhury, G.; Khazim, K.; Block, K.; Gorin, Y. Nox4 NADPH Oxidase Mediates Peroxynitrite-dependent Uncoupling of Endothelial Nitric-oxide Synthase and Fibronectin Expression in Response to Angiotensin II: Role of mitochondrial reactive oxygen species. J. Biol. Chem. 2013, 288, 28668–28686. [Google Scholar] [CrossRef] [Green Version]
- Østergaard, M.; Christensen, M.; Nilsson, L.; Carlsen, I.; Frøkiær, J.; Nørregaard, R. ROS dependence of cyclooxygenase-2 induction in rats subjected to unilateral ureteral obstruction. Am. J. Physiol. Physiol. 2014, 306, F259–F270. [Google Scholar] [CrossRef]
- Liu, E.-S.; Chen, N.-C.; Jao, T.-M.; Chen, C.-L. Dextromethorphan Reduces Oxidative Stress and Inhibits Uremic Artery Calcification. Int. J. Mol. Sci. 2021, 22, 12277. [Google Scholar] [CrossRef]
- Pierelli, G.; Stanzione, R.; Forte, M.; Migliarino, S.; Perelli, M.; Volpe, M.; Rubattu, S. Uncoupling Protein 2: A Key Player and a Potential Therapeutic Target in Vascular Diseases. Oxidative Med. Cell Longev. 2017, 2017, 7348372. [Google Scholar] [CrossRef]
- Nigro, M.; De Sanctis, C.; Formisano, P.; Stanzione, R.; Forte, M.; Capasso, G.; Gigliotti, G.; Rubattu, S.; Viggiano, D. Cellular and subcellular localization of uncoupling protein 2 in the human kidney. Histochem. J. 2018, 49, 437–445. [Google Scholar] [CrossRef]
- Friederich-Persson, M.; Aslam, S.; Nordquist, L.; Welch, W.J.; Wilcox, C.S.; Palm, F. Acute Knockdown of Uncoupling Protein-2 Increases Uncoupling via the Adenine Nucleotide Transporter and Decreases Oxidative Stress in Diabetic Kidneys. PLoS ONE 2012, 7, e39635. [Google Scholar] [CrossRef]
- Ye, J.; Li, J.; Xia, R.; Zhou, M.; Yu, L. Prohibitin protects proximal tubule epithelial cells against oxidative injury through mitochondrial pathways. Free Radic. Res. 2015, 49, 1393–1403. [Google Scholar] [CrossRef]
- Su, J.; Liu, J.; Yan, X.-Y.; Zhang, Y.; Zhang, J.-J.; Zhang, L.-C.; Sun, L.-K. Cytoprotective Effect of the UCP2-SIRT3 Signaling Pathway by Decreasing Mitochondrial Oxidative Stress on Cerebral Ischemia–Reperfusion Injury. Int. J. Mol. Sci. 2017, 18, 1599. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Wang, Y.; Ding, W.; Wang, Y. Mito-TEMPO Alleviates Renal Fibrosis by Reducing Inflammation, Mitochondrial Dysfunction, and Endoplasmic Reticulum Stress. Oxidative Med. Cell Longev. 2018, 2018, 5828120. [Google Scholar] [CrossRef] [Green Version]
- Kirkman, D.; Muth, B.; Ramick, M.G.; Townsend, R.R.; Edwards, D.G. Role of mitochondria-derived reactive oxygen species in microvascular dysfunction in chronic kidney disease. Am. J. Physiol. Physiol. 2018, 314, F423–F429. [Google Scholar] [CrossRef]
- Cui, L.; Zhou, Q.; Zheng, X.; Sun, B.; Zhao, S. Mitoquinone attenuates vascular calcification by suppressing oxidative stress and reducing apoptosis of vascular smooth muscle cells via the Keap1/Nrf2 pathway. Free Radic. Biol. Med. 2020, 161, 23–31. [Google Scholar] [CrossRef]
- Kirkman, D.L.; Shenouda, N.; Stock, J.M.; Muth, B.J.; Chouramanis, N.; Townsend, R.R.; Edwards, D.G. Abstract 16926: The Effects of a Mitochondrial Targeted Ubiquinone (MitoQ) on Vascular Function in Chronic Kidney Disease. Circulation 2020, 142, A16926. [Google Scholar] [CrossRef]
- Wallace, D.C.; Fan, W.; Procaccio, V. Mitochondrial Energetics and Therapeutics. Annu. Rev. Pathol. Mech. Dis. 2010, 5, 297–348. [Google Scholar] [CrossRef]
- Martínez-Reyes, I.; Chandel, N.S. Mitochondrial TCA cycle metabolites control physiology and disease. Nat. Commun. 2020, 11, 102. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schaub, J.A.; Venkatachalam, M.A.; Weinberg, J.M. Proximal Tubular Oxidative Metabolism in Acute Kidney Injury and the Transition to CKD. Kidney360 2020, 2, 355–364. [Google Scholar] [CrossRef] [PubMed]
- Ahn, B.-H.; Kim, H.-S.; Song, S.; Lee, I.H.; Liu, J.; Vassilopoulos, A.; Deng, C.-X.; Finkel, T. A role for the mitochondrial deacetylase Sirt3 in regulating energy homeostasis. Proc. Natl. Acad. Sci. USA 2008, 105, 14447–14452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aparicio-Trejo, O.E.; Rojas-Morales, P.; Avila-Rojas, S.H.; León-Contreras, J.C.; Hernández-Pando, R.; Jiménez-Uribe, A.P.; Prieto-Carrasco, R.; Sánchez-Lozada, L.G.; Pedraza-Chaverri, J.; Tapia, E. Temporal Alterations in Mitochondrial β-Oxidation and Oxidative Stress Aggravate Chronic Kidney Disease Development in 5/6 Nephrectomy Induced Renal Damage. Int. J. Mol. Sci. 2020, 21, 6512. [Google Scholar] [CrossRef]
- Chen, J.-F.; Liu, H.; Ni, H.-F.; Lv, L.-L.; Zhang, M.-H.; Zhang, A.-H.; Tang, R.-N.; Chen, P.-S.; Liu, B.-C. Improved Mitochondrial Function Underlies the Protective Effect of Pirfenidone against Tubulointerstitial Fibrosis in 5/6 Nephrectomized Rats. PLoS ONE 2013, 8, e83593. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, T.; Yamaguchi, H.; Kikusato, M.; Matsuhashi, T.; Matsuo, A.; Sato, T.; Oba, Y.; Watanabe, S.; Minaki, D.; Saigusa, D.; et al. Mitochonic Acid 5 (MA-5), a Derivative of the Plant Hormone Indole-3-Acetic Acid, Improves Survival of Fibroblasts from Patients with Mitochondrial Diseases. Tohoku J. Exp. Med. 2015, 236, 225–232. [Google Scholar] [CrossRef] [Green Version]
- Matsuhashi, T.; Sato, T.; Kanno, S.-I.; Suzuki, T.; Matsuo, A.; Oba, Y.; Kikusato, M.; Ogasawara, E.; Kudo, T.; Suzuki, K.; et al. Mitochonic Acid 5 (MA-5) Facilitates ATP Synthase Oligomerization and Cell Survival in Various Mitochondrial Diseases. Ebiomedicine 2017, 20, 27–38. [Google Scholar] [CrossRef] [Green Version]
- Suzuki, T.; Yamaguchi, H.; Kikusato, M.; Hashizume, O.; Nagatoishi, S.; Matsuo, A.; Sato, T.; Kudo, T.; Matsuhashi, T.; Murayama, K.; et al. Mitochonic Acid 5 Binds Mitochondria and Ameliorates Renal Tubular and Cardiac Myocyte Damage. J. Am. Soc. Nephrol. 2015, 27, 1925–1932. [Google Scholar] [CrossRef] [Green Version]
- Lee, H.-C.; Wei, Y.-H. Mitochondrial biogenesis and mitochondrial DNA maintenance of mammalian cells under oxidative stress. Int. J. Biochem. Cell Biol. 2005, 37, 822–834. [Google Scholar] [CrossRef]
- Li, P.A.; Hou, X.; Hao, S. Mitochondrial biogenesis in neurodegeneration. J. Neurosci. Res. 2017, 95, 2025–2029. [Google Scholar] [CrossRef]
- Gureev, A.P.; Shaforostova, E.A.; Popov, V.N. Regulation of Mitochondrial Biogenesis as a Way for Active Longevity: Interaction Between the Nrf2 and PGC-1α Signaling Pathways. Front. Genet. 2019, 10, 435. [Google Scholar] [CrossRef] [Green Version]
- Popov, L. Mitochondrial biogenesis: An update. J. Cell Mol. Med. 2020, 24, 4892–4899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, M.R.; Tran, M.T.; Parikh, S.M. PGC1α in the kidney. Am. J. Physiol. Physiol. 2018, 314, F1–F8. [Google Scholar] [CrossRef] [PubMed]
- Fontecha-Barriuso, M.; Martin-Sanchez, D.; Martinez-Moreno, J.; Monsalve, M.; Ramos, A.; Sanchez-Niño, M.; Ruiz-Ortega, M.; Ortiz, A.; Sanz, A. The Role of PGC-1α and Mitochondrial Biogenesis in Kidney Diseases. Biomolecules 2020, 10, 347. [Google Scholar] [CrossRef] [Green Version]
- Choi, H.-I.; Kim, H.-J.; Park, J.-S.; Kim, I.-J.; Bae, E.H.; Ma, S.K.; Kim, S.W. PGC-1α attenuates hydrogen peroxide-induced apoptotic cell death by upregulating Nrf-2 via GSK3β inactivation mediated by activated p38 in HK-2 Cells. Sci. Rep. 2017, 7, 4319. [Google Scholar] [CrossRef] [Green Version]
- Ciotti, S.; Iuliano, L.; Cefalù, S.; Comelli, M.; Mavelli, I.; Di Giorgio, E.; Brancolini, C. GSK3β is a key regulator of the ROS-dependent necrotic death induced by the quinone DMNQ. Cell Death Dis. 2020, 11, 2. [Google Scholar] [CrossRef] [Green Version]
- Jiang, D.; Fu, C.; Xiao, J.; Zhang, Z.; Zou, J.; Ye, Z.; Zhang, X. SGK1 Attenuates Oxidative Stress-Induced Renal Tubular Epithelial Cell Injury by Regulating Mitochondrial Function. Oxidative Med. Cell Longev. 2019, 2019, 2013594. [Google Scholar] [CrossRef]
- Bao, L.; Cai, X.; Zhang, Z.; Li, Y. Grape seed procyanidin B2 ameliorates mitochondrial dysfunction and inhibits apoptosis via the AMP-activated protein kinase–silent mating type information regulation 2 homologue 1–PPARγ co-activator-1α axis in rat mesangial cells under high-dose glucosamine. Br. J. Nutr. 2014, 113, 35–44. [Google Scholar] [CrossRef] [Green Version]
- Ghosh, S.S.; Gehr, T.W.B.; Ghosh, S. Curcumin and Chronic Kidney Disease (CKD): Major Mode of Action through Stimulating Endogenous Intestinal Alkaline Phosphatase. Molecules 2014, 19, 20139–20156. [Google Scholar] [CrossRef] [Green Version]
- Aparicio-Trejo, O.E.; Tapia, E.; Molina-Jijón, E.; Medina-Campos, O.N.; Macías-Ruvalcaba, N.A.; León-Contreras, J.C.; Hernández-Pando, R.; García-Arroyo, F.E.; Cristóbal, M.; Sánchez-Lozada, L.G.; et al. Curcumin prevents mitochondrial dynamics disturbances in early 5/6 nephrectomy: Relation to oxidative stress and mitochondrial bioenergetics. BioFactors 2016, 43, 293–310. [Google Scholar] [CrossRef]
- Liao, X.; Lv, X.; Zhang, Y.; Han, Y.; Li, J.; Zeng, J.; Tang, D.; Meng, J.; Yuan, X.; Peng, Z.; et al. Fluorofenidone Inhibits UUO/IRI-Induced Renal Fibrosis by Reducing Mitochondrial Damage. Oxidative Med. Cell Longev. 2022, 2022, 2453617. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Aparicio-Trejo, O.E.; Tapia, E.; Sánchez-Lozada, L.G.; García-Arroyo, F.E.; Amador-Martínez, I.; Orozco-Ibarra, M.; Fernández-Valverde, F.; Pedraza-Chaverri, J. Sulforaphane Protects against Unilateral Ureteral Obstruction-Induced Renal Damage in Rats by Alleviating Mitochondrial and Lipid Metabolism Impairment. Antioxidants 2022, 11, 1854. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Zhou, F.; Zhang, Z.; Xing, D. Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. FEBS J. 2011, 278, 941–954. [Google Scholar] [CrossRef] [PubMed]
- Youle, R.J.; van der Bliek, A.M. Mitochondrial Fission, Fusion, and Stress. Science 2012, 337, 1062–1065. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Twig, G.; Shirihai, O.S. The Interplay Between Mitochondrial Dynamics and Mitophagy. Antioxid. Redox Signal. 2011, 14, 1939–1951. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Picard, M.; Shirihai, O.S.; Gentil, B.J.; Burelle, Y. Mitochondrial morphology transitions and functions: Implications for retrograde signaling? Am. J. Physiol. Integr. Comp. Physiol. 2013, 304, R393–R406. [Google Scholar] [CrossRef] [Green Version]
- Gall, J.M.; Wang, Z.; Liesa, M.; Molina, A.; Havasi, A.; Schwartz, J.H.; Shirihai, O.; Borkan, S.C.; Bonegio, R.G.B. Role of Mitofusin 2 in the Renal Stress Response. PLoS ONE 2012, 7, e31074. [Google Scholar] [CrossRef] [Green Version]
- Tang, W.X.; Wu, W.H.; Zeng, X.X.; Bo, H.; Huang, S.M. Early protective effect of mitofusion 2 overexpression in STZ-induced diabetic rat kidney. Endocrine 2011, 41, 236–247. [Google Scholar] [CrossRef]
- Sun, H.; Li, X.; Chen, X.; Xiong, Y.; Cao, Y.; Wang, Z. Drp1 activates ROS/HIF-1α/EZH2 and triggers mitochondrial fragmentation to deteriorate hypercalcemia-associated neuronal injury in mouse model of chronic kidney disease. J. Neuroinflamm. 2022, 19, 213. [Google Scholar] [CrossRef]
- Westermann, B. Mitochondrial fusion and fission in cell life and death. Nat. Rev. Mol. Cell Biol. 2010, 11, 872–884. [Google Scholar] [CrossRef]
- Yoo, S.-M.; Jung, Y.-K. A Molecular Approach to Mitophagy and Mitochondrial Dynamics. Mol. Cells 2018, 41, 18–26. [Google Scholar] [PubMed]
- Tanida, I.; Minematsu-Ikeguchi, N.; Ueno, T.; Kominami, E. Lysosomal Turnover, but Not a Cellular Level, of Endogenous LC3 is a Marker for Autophagy. Autophagy 2005, 1, 84–91. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoshii, S.R.; Mizushima, N. Monitoring and Measuring Autophagy. Int. J. Mol. Sci. 2017, 18, 1865. [Google Scholar] [CrossRef] [PubMed]
- Lampert, M.A.; Orogo, A.M.; Najor, R.H.; Hammerling, B.C.; Leon, L.J.; Wang, B.J.; Kim, T.; Sussman, M.A.; Gustafsson, B. BNIP3L/NIX and FUNDC1-mediated mitophagy is required for mitochondrial network remodeling during cardiac progenitor cell differentiation. Autophagy 2019, 15, 1182–1198. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Cai, J.; Tang, C.; Dong, Z. Mitophagy in Acute Kidney Injury and Kidney Repair. Cells 2020, 9, 338. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, C.; Cai, J.; Yin, X.-M.; Weinberg, J.M.; Venkatachalam, M.A.; Dong, Z. Mitochondrial quality control in kidney injury and repair. Nat. Rev. Nephrol. 2020, 17, 299–318. [Google Scholar] [CrossRef] [PubMed]
- Dagar, N.; Kale, A.; Steiger, S.; Anders, H.-J.; Gaikwad, A.B. Receptor-mediated mitophagy: An emerging therapeutic target in acute kidney injury. Mitochondrion 2022, 66, 82–91. [Google Scholar] [CrossRef]
- Bhatia, D.; Chung, K.-P.; Nakahira, K.; Patino, E.; Rice, M.C.; Torres, L.K.; Muthukumar, T.; Choi, A.M.; Akchurin, O.M.; Choi, M.E. Mitophagy-dependent macrophage reprogramming protects against kidney fibrosis. J. Clin. Investig. 2019, 4, e132826. [Google Scholar] [CrossRef] [Green Version]
- Jia, Q.; Han, L.; Zhang, X.; Yang, W.; Gao, Y.; Shen, Y.; Li, B.; Wang, S.; Qin, M.; Lowe, S.; et al. Tongluo Yishen Decoction Ameliorates Renal Fibrosis via Regulating Mitochondrial Dysfunction Induced by Oxidative Stress in Unilateral Ureteral Obstruction Rats. Front. Pharmacol. 2021, 12, 762756. [Google Scholar] [CrossRef]
- Xu, Y.; Ruan, S.; Wu, X.; Chen, H.; Zheng, K.; Fu, B. Autophagy and apoptosis in tubular cells following unilateral ureteral obstruction are associated with mitochondrial oxidative stress. Int. J. Mol. Med. 2013, 31, 628–636. [Google Scholar] [CrossRef]
- Small, D.M.; Morais, C.; Coombes, J.; Bennett, N.C.; Johnson, D.W.; Gobe, G.C. Oxidative stress-induced alterations in PPAR-γ and associated mitochondrial destabilization contribute to kidney cell apoptosis. Am. J. Physiol. Physiol. 2014, 307, F814–F822. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, D.; Capili, A.; Choi, M.E. Mitochondrial dysfunction in kidney injury, inflammation, and disease: Potential therapeutic approaches. Kidney Res. Clin. Pract. 2020, 39, 244–258. [Google Scholar] [CrossRef] [PubMed]
- LeCocq, J.; Ballou, C.E. On the Structure of Cardiolipin*. Biochemistry 1964, 3, 976–980. [Google Scholar] [CrossRef] [PubMed]
- Paradies, G.; Paradies, V.; De Benedictis, V.; Ruggiero, F.M.; Petrosillo, G. Functional role of cardiolipin in mitochondrial bioenergetics. Biochim. Biophys. Acta (BBA)—Bioenerg. 2014, 1837, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Paradies, G.; Paradies, V.; Ruggiero, F.M.; Petrosillo, G. Role of Cardiolipin in Mitochondrial Function and Dynamics in Health and Disease: Molecular and Pharmacological Aspects. Cells 2019, 8, 728. [Google Scholar] [CrossRef] [Green Version]
- Birk, A.V.; Liu, S.; Soong, Y.; Mills, W.; Singh, P.; Warren, J.D.; Seshan, S.V.; Pardee, J.D.; Szeto, H.H. The Mitochondrial-Targeted Compound SS-31 Re-Energizes Ischemic Mitochondria by Interacting with Cardiolipin. J. Am. Soc. Nephrol. 2013, 24, 1250–1261. [Google Scholar] [CrossRef] [Green Version]
- Paradies, G.; Petrosillo, G.; Paradies, V.; Ruggiero, F.M. Role of cardiolipin peroxidation and Ca2+ in mitochondrial dysfunction and disease. Cell Calcium 2009, 45, 643–650. [Google Scholar] [CrossRef]
- Miranda-Díaz, A.G.; Cardona-Muñoz, E.G.; Pacheco-Moisés, F.P. The Role of Cardiolipin and Mitochondrial Damage in Kidney Transplant. Oxidative Med. Cell Longev. 2019, 2019, 3836186. [Google Scholar] [CrossRef]
- Birk, A.V.; Chao, W.M.; Bracken, C.; Warren, J.D.; Szeto, H.H. Targeting mitochondrial cardiolipin and the cytochrome c/cardiolipin complex to promote electron transport and optimize mitochondrial ATP synthesis. Br. J. Pharmacol. 2014, 171, 2017–2028. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.; Soong, Y.; Seshan, S.V.; Szeto, H.H. Novel cardiolipin therapeutic protects endothelial mitochondria during renal ischemia and mitigates microvascular rarefaction, inflammation, and fibrosis. Am. J. Physiol. Physiol. 2014, 306, F970–F980. [Google Scholar] [CrossRef]
- Shi, Y. A structural view of mitochondria-mediated apoptosis. Nat. Struct. Biol. 2001, 8, 394–401. [Google Scholar] [CrossRef] [PubMed]
- Xiong, S.; Mu, T.; Wang, G.; Jiang, X. Mitochondria-mediated apoptosis in mammals. Protein Cell 2014, 5, 737–749. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Lu, Y.; Hou, J.; Liu, C.; Sun, Y. A Polysaccharide Purified from Morchella conica Pers. Prevents Oxidative Stress Induced by H2O2 in Human Embryonic Kidney (HEK) 293T Cells. Int. J. Mol. Sci. 2018, 19, 4027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cao, Y.; Xu, J.; Cui, D.; Liu, L.; Zhang, S.; Shen, B.; Wu, Y.; Zhang, Q. Protective effect of carnosine on hydrogen peroxide–induced oxidative stress in human kidney tubular epithelial cells. Biochem. Biophys. Res. Commun. 2020, 534, 576–582. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-H.; Chuang, H.-C.; Lee, Y.-H.; Lin, Y.-F.; Chen, Y.-J.; Hsiao, T.-C.; Wu, M.-Y.; Chiu, H.-W. Traffic-related particulate matter exposure induces nephrotoxicity in vitro and in vivo. Free Radic. Biol. Med. 2019, 135, 235–244. [Google Scholar] [CrossRef]
- Kochi, C.; Pokkunuri, I.; Salvi, A.; Asghar, M.; Salim, S. Simulated vehicle exhaust exposure (SVEE) in rats impairs renal mitochondrial function. Clin. Exp. Hypertens. 2020, 42, 571–579. [Google Scholar] [CrossRef]
- Mao, W.P.; Zhang, N.N.; Zhou, F.Y.; Li, W.X.; Liu, H.Y.; Feng, J.; Zhou, L.; Wei, C.J.; Pan, Y.B.; He, Z.J. Cadmium directly induced mitochondrial dysfunction of human embryonic kidney cells. Hum. Exp. Toxicol. 2010, 30, 920–929. [Google Scholar] [CrossRef]
- Chen, S.; Liu, G.; Long, M.; Zou, H.; Cui, H. Alpha lipoic acid attenuates cadmium-induced nephrotoxicity via the mitochondrial apoptotic pathways in rat. J. Inorg. Biochem. 2018, 184, 19–26. [Google Scholar] [CrossRef]
- Sahu, B.D.; Koneru, M.; Bijargi, S.R.; Kota, A.; Sistla, R. Chromium-induced nephrotoxicity and ameliorative effect of carvedilol in rats: Involvement of oxidative stress, apoptosis and inflammation. Chem. Interact. 2014, 223, 69–79. [Google Scholar] [CrossRef]
- Liu, C.-M.; Ma, J.-Q.; Sun, Y.-Z. Puerarin protects rat kidney from lead-induced apoptosis by modulating the PI3K/Akt/eNOS pathway. Toxicol. Appl. Pharmacol. 2012, 258, 330–342. [Google Scholar] [CrossRef]
- Wang, C.; Nie, G.; Yang, F.; Chen, J.; Zhuang, Y.; Dai, X.; Liao, Z.; Yang, Z.; Cao, H.; Xing, C.; et al. Molybdenum and cadmium co-induce oxidative stress and apoptosis through mitochondria-mediated pathway in duck renal tubular epithelial cells. J. Hazard. Mater. 2019, 383, 121157. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, J.; Nie, G.; Hu, R.; Wang, C.; Xing, C.; Li, G.; Hu, G.; Yang, F.; Zhang, C. Inhibition of autophagy aggravates molybdenum-induced mitochondrial dysfunction by aggravating oxidative stress in duck renal tubular epithelial cells. Ecotoxicol. Environ. Saf. 2020, 209, 111771. [Google Scholar] [CrossRef] [PubMed]
- Shaki, F.; Hosseini, M.-J.; Ghazi-Khansari, M.; Pourahmad, J. Toxicity of depleted uranium on isolated rat kidney mitochondria. Biochim. Biophys. Acta (BBA)—Gen. Subj. 2012, 1820, 1940–1950. [Google Scholar] [CrossRef]
- Yu, L.; Li, W.; Chu, J.; Chen, C.; Li, X.; Tang, W.; Xia, B.; Xiong, Z. Uranium inhibits mammalian mitochondrial cytochrome c oxidase and ATP synthase. Environ. Pollut. 2020, 271, 116377. [Google Scholar] [CrossRef]
- Cheraghi, G.; Hajiabedi, E.; Niaghi, B.; Nazari, F.; Naserzadeh, P.; Hosseini, M.-J. High doses of sodium tungstate can promote mitochondrial dysfunction and oxidative stress in isolated mitochondria. J. Biochem. Mol. Toxicol. 2018, 33, e22266. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Guo, C.; Cui, Y.; Zhang, X.; Xiao, B.; Liu, M.; Song, M.; Li, Y. Activation of PINK1/Parkin-mediated mitophagy protects against apoptosis in kidney damage caused by aluminum. J. Inorg. Biochem. 2022, 230, 21–27. [Google Scholar] [CrossRef]
- Kobroob, A.; Chattipakorn, N.; Wongmekiat, O. Caffeic acid phenethyl ester ameliorates cadmium-induced kidney mitochondrial injury. Chem. Interact. 2012, 200, 21–27. [Google Scholar] [CrossRef]
- Navaneethan, D.; Rasool, M.K. An experimental study to investigate the impact of p-coumaric acid, a common dietary polyphenol, on cadmium chloride-induced renal toxicity. Food Funct. 2014, 5, 2438–2445. [Google Scholar] [CrossRef]
- Shen, R.; Liu, D.; Hou, C.; Liu, D.; Zhao, L.; Cheng, J.; Wang, D.; Bai, D. Protective effect of Potentilla anserina polysaccharide on cadmium-induced nephrotoxicity in vitro and in vivo. Food Funct. 2017, 8, 3636–3646. [Google Scholar] [CrossRef]
- Fan, R.-F.; Li, Z.-F.; Zhang, D.; Wang, Z.-Y. Involvement of Nrf2 and mitochondrial apoptotic signaling in trehalose protection against cadmium-induced kidney injury. Metallomics 2020, 12, 2098–2107. [Google Scholar] [CrossRef]
- Wongmekiat, O.; Peerapanyasut, W.; Kobroob, A. Catechin supplementation prevents kidney damage in rats repeatedly exposed to cadmium through mitochondrial protection. Naunyn-Schmiedeberg’s Arch. Pharmacol. 2018, 391, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zhang, C.; Ge, J.; Lv, M.-W.; Talukder, M.; Guo, K.; Li, Y.-H.; Li, J.-L. Ameliorative effects of resveratrol against cadmium-induced nephrotoxicity via modulating nuclear xenobiotic receptor response and PINK1/Parkin-mediated Mitophagy. Food Funct. 2020, 11, 1856–1868. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wu, Y.; Luo, K.; Liu, Y.; Zhou, M.; Yan, S.; Shi, H.; Cai, Y. The protective effects of selenium on cadmium-induced oxidative stress and apoptosis via mitochondria pathway in mice kidney. Food Chem. Toxicol. 2013, 58, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Lai, H.; Nie, T.; Zhang, Y.; Chen, Y.; Tao, J.; Lin, T.; Ge, T.; Li, F.; Li, H. Selenium Deficiency-Induced Damage and Altered Expression of Mitochondrial Biogenesis Markers in the Kidneys of Mice. Biol. Trace Elem. Res. 2020, 199, 185–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Li, Z.; Wang, J.; Wang, H.; Wang, Z.; Wang, L. Puerarin protects against lead-induced cytotoxicity in cultured primary rat proximal tubular cells. Hum. Exp. Toxicol. 2014, 33, 1071–1080. [Google Scholar] [CrossRef]
- Li, W.; Yu, L.; Fu, B.; Chu, J.; Chen, C.; Li, X.; Ma, J.; Tang, W. Protective effects of Polygonatum kingianum polysaccharides and aqueous extract on uranium-induced toxicity in human kidney (HK-2) cells. Int. J. Biol. Macromol. 2022, 202, 68–79. [Google Scholar] [CrossRef]
- Flampouri, E.; Mavrikou, S.; Mouzaki-Paxinou, A.-C.; Kintzios, S. Alterations of cellular redox homeostasis in cultured fibroblast-like renal cells upon exposure to low doses of cytochrome bc1 complex inhibitor kresoxim-methyl. Biochem. Pharmacol. 2016, 113, 97–109. [Google Scholar] [CrossRef]
- Keshk, W.A.; Zahran, S.M. Mechanistic role of cAMP and hepatocyte growth factor signaling in thioacetamide-induced nephrotoxicity: Unraveling the role of platelet rich plasma. Biomed. Pharmacother. 2018, 109, 1078–1084. [Google Scholar] [CrossRef]
- Zhang, C.; Qin, L.; Dou, D.-C.; Li, X.-N.; Ge, J.; Li, J.-L. Atrazine induced oxidative stress and mitochondrial dysfunction in quail (Coturnix C. coturnix) kidney via modulating Nrf2 signaling pathway. Chemosphere 2018, 212, 974–982. [Google Scholar] [CrossRef]
- Ding, R.; Cao, Z.; Wang, Y.; Gao, X.; Luo, H.; Zhang, C.; Ma, S.; Ma, X.; Jin, H.; Lu, C. The implication of p66shc in oxidative stress induced by deltamethrin. Chem. Interact. 2017, 278, 162–169. [Google Scholar] [CrossRef]
- Aoiadni, N.; Jdidi, H.; El Feki, A.; Fetoui, H.; Koubaa, F.G. Mitochondrial bioenergetics and redox dysfunction in nephrotoxicity induced by pyrethroid permethrin are ameliorated by flavonoid-rich fraction. Environ. Sci. Pollut. Res. 2022, 29, 63973–63987. [Google Scholar] [CrossRef] [PubMed]
- Bosch-Panadero, E.; Mas, S.; Civantos, E.; Abaigar, P.; Camarero, V.; Ruiz-Priego, A.; Ortiz, A.; Egido, J.; González-Parra, E. Bisphenol A is an exogenous toxin that promotes mitochondrial injury and death in tubular cells. Environ. Toxicol. 2017, 33, 325–332. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Jiang, L.; Ge, L.; Chen, M.; Yang, G.; Ji, F.; Zhong, L.; Guan, Y.; Liu, X. Oxidative DNA damage induced by di-(2-ethylhexyl) phthalate in HEK-293 cell line. Environ. Toxicol. Pharmacol. 2015, 39, 1099–1106. [Google Scholar] [CrossRef] [PubMed]
- Ashari, S.; Karami, M.; Shokrzadeh, M.; Ghandadi, M.; Ghassemi-Barghi, N.; Dashti, A.; Ranaee, M.; Mohammadi, H. The implication of mitochondrial dysfunction and mitochondrial oxidative damage in di (2-ethylhexyl) phthalate induced nephrotoxicity in both in vivo and in vitro models. Toxicol. Mech. Methods 2020, 30, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Amara, I.; Timoumi, R.; Graiet, I.; Ben Salem, I.; Adelou, K.; Abid-Essefi, S. Di (2-ethylhexyl) phthalate induces cytotoxicity in HEK-293 cell line, implication of the Nrf-2/HO-1 antioxidant pathway. Environ. Toxicol. 2019, 34, 1034–1042. [Google Scholar] [CrossRef]
- Peerapanyasut, W.; Kobroob, A.; Palee, S.; Chattipakorn, N.; Wongmekiat, O. Bisphenol A aggravates renal ischemia–reperfusion injury by disrupting mitochondrial homeostasis and N-acetylcysteine mitigates the injurious outcomes. IUBMB Life 2019, 72, 758–770. [Google Scholar] [CrossRef]
- Peerapanyasut, W.; Kobroob, A.; Palee, S.; Chattipakorn, N.; Wongmekiat, O. Activation of Sirtuin 3 and Maintenance of Mitochondrial Integrity by N-Acetylcysteine Protects Against Bisphenol A-Induced Kidney and Liver Toxicity in Rats. Int. J. Mol. Sci. 2019, 20, 267. [Google Scholar] [CrossRef] [Green Version]
- Kobroob, A.; Peerapanyasut, W.; Kumfu, S.; Chattipakorn, N.; Wongmekiat, O. Effectiveness of N-Acetylcysteine in the Treatment of Renal Deterioration Caused by Long-Term Exposure to Bisphenol A. Biomolecules 2021, 11, 655. [Google Scholar] [CrossRef]
- Kobroob, A.; Peerapanyasut, W.; Chattipakorn, N.; Wongmekiat, O. Damaging Effects of Bisphenol A on the Kidney and the Protection by Melatonin: Emerging Evidences from In Vivo and In Vitro Studies. Oxidative Med. Cell Longev. 2018, 2018, 3082438. [Google Scholar] [CrossRef] [Green Version]
- Shirani, M.; Alizadeh, S.; Mahdavinia, M.; Dehghani, M.A. The ameliorative effect of quercetin on bisphenol A-induced toxicity in mitochondria isolated from rats. Environ. Sci. Pollut. Res. 2019, 26, 7688–7696. [Google Scholar] [CrossRef]
- Jiang, W.; Zhao, H.; Zhang, L.; Wu, B.; Zha, Z. Maintenance of mitochondrial function by astaxanthin protects against bisphenol A-induced kidney toxicity in rats. Biomed. Pharmacother. 2019, 121, 109629. [Google Scholar] [CrossRef] [PubMed]
- Vedi, M.; Rasool, M.; Sabina, E.P. Protective effect of administration of Withania somifera against bromobenzene induced nephrotoxicity and mitochondrial oxidative stress in rats. Ren. Fail. 2014, 36, 1095–1103. [Google Scholar] [CrossRef] [PubMed]
- Vedi, M.; Sabina, E.P. Assessment of hepatoprotective and nephroprotective potential of withaferin A on bromobenzene-induced injury in Swiss albino mice: Possible involvement of mitochondrial dysfunction and inflammation. Cell Biol. Toxicol. 2016, 32, 373–390. [Google Scholar] [CrossRef] [PubMed]
- Reddy, A.R.N.; Reddy, Y.N.; Krishna, D.R.; Himabindu, V. Multi wall carbon nanotubes induce oxidative stress and cytotoxicity in human embryonic kidney (HEK293) cells. Toxicology 2010, 272, 11–16. [Google Scholar] [CrossRef] [PubMed]
- Zamani, F.; Samiei, F.; Mousavi, Z.; Azari, M.R.; Seydi, E.; Pourahmad, J. Apigenin ameliorates oxidative stress and mitochondrial damage induced by multiwall carbon nanotubes in rat kidney mitochondria. J. Biochem. Mol. Toxicol. 2021, 35, 1–7. [Google Scholar] [CrossRef]
- Singh, J.; Dutta, T.; Kim, K.-H.; Rawat, M.; Samddar, P.; Kumar, P. ‘Green’ synthesis of metals and their oxide nanoparticles: Applications for environmental remediation. J. Nanobiotechnol. 2018, 16, 84. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, applications and toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Ferreira, G.K.; Cardoso, E.; Vuolo, F.S.; Michels, M.; Zanoni, E.T.; Carvalho-Silva, M.; Gomes, L.M.; Dal-Pizzol, F.; Rezin, G.T.; Streck, E.L.; et al. Gold nanoparticles alter parameters of oxidative stress and energy metabolism in organs of adult rats. Biochem. Cell Biol. 2015, 93, 548–557. [Google Scholar] [CrossRef]
- Vasanth, S.B.; Kurian, G.A. Toxicity evaluation of silver nanoparticles synthesized by chemical and green route in different experimental models. Artif. Cells Nanomed. Biotechnol. 2017, 45, 1721–1727. [Google Scholar] [CrossRef] [Green Version]
- Sarkar, A.; Das, J.; Manna, P.; Sil, P.C. Nano-copper induces oxidative stress and apoptosis in kidney via both extrinsic and intrinsic pathways. Toxicology 2011, 290, 208–217. [Google Scholar] [CrossRef]
- Almeer, R.S.; Ali, D.; Alarifi, S.; Alkahtani, S.; Almansour, M. Green Platinum Nanoparticles Interaction With HEK293 Cells: Cellular Toxicity, Apoptosis, and Genetic Damage. Dose-Response 2018, 1, 15593258188073826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daems, N.; Penninckx, S.; Nelissen, I.; Van Hoecke, K.; Cardinaels, T.; Baatout, S.; Michiels, C.; Lucas, S.; Aerts, A. Gold nanoparticles affect the antioxidant status in selected normal human cells. Int. J. Nanomed. 2019, 14, 4991–5015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baratli, Y.; Charles, A.-L.; Wolff, V.; Ben Tahar, L.; Smiri, L.; Bouitbir, J.; Zoll, J.; Piquard, F.; Tebourbi, O.; Sakly, M.; et al. Impact of iron oxide nanoparticles on brain, heart, lung, liver and kidneys mitochondrial respiratory chain complexes activities and coupling. Toxicol. Vitr. 2013, 27, 2142–2148. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.; Kurian, G.A. Evaluating the effect of green synthesised copper oxide nanoparticles on oxidative stress and mitochondrial function using murine model. IET Nanobiotechnol. 2018, 12, 669–672. [Google Scholar] [CrossRef]
- Betzer, O.; Shilo, M.; Opochinsky, R.; Barnoy, E.; Motiei, M.; Okun, E.; Yadid, G.; Popovtzer, R. The effect of nanoparticle size on the ability to cross the blood–brain barrier: An in vivo study. Nanomedicine 2017, 12, 1533–1546. [Google Scholar] [CrossRef]
- Yang, H.; Du, L.; Tian, X.; Fan, Z.; Sun, C.; Liu, Y.; Keelan, J.A.; Nie, G. Effects of nanoparticle size and gestational age on maternal biodistribution and toxicity of gold nanoparticles in pregnant mice. Toxicol. Lett. 2014, 230, 10–18. [Google Scholar] [CrossRef]
- Enea, M.; Pereira, E.; De Almeida, M.P.; Araújo, A.M.; Bastos, M.D.L.; Carmo, H. Gold Nanoparticles Induce Oxidative Stress and Apoptosis in Human Kidney Cells. Nanomaterials 2020, 10, 995. [Google Scholar] [CrossRef]
- Ibrahim, K.E.; Al-Mutary, M.G.; Bakhiet, A.O.; Khan, H.A. Histopathology of the Liver, Kidney, and Spleen of Mice Exposed to Gold Nanoparticles. Molecules 2018, 23, 1848. [Google Scholar] [CrossRef] [Green Version]
- Khan, H.A.; Abdelhalim, M.A.K.; Alhomida, A.S.; Al-Ayed, M.S. Effects of Naked Gold Nanoparticles on Proinflammatory Cytokines mRNA Expression in Rat Liver and Kidney. BioMed Res. Int. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Er, R.; Aydın, B.; Şekeroğlu, V.; Şekeroğlu, Z.A. Protective effect of Argan oil on mitochondrial function and oxidative stress against acrylamide-induced liver and kidney injury in rats. Biomarkers 2020, 25, 458–467. [Google Scholar] [CrossRef]
- Nazari, F.; Naserzadeh, P.; Dizaji, R.; Manjili, H.K.; Bahrami, H.; Soleimani, M.; Sharafi, A.; Hosseini, M. Toxicological assessment of 3-monochloropropane-1,2-diol (3-MCPD) as a main contaminant of foodstuff in three different in vitro models: Involvement of oxidative stress and cell death signaling pathway. J. Food Sci. 2020, 85, 4061–4069. [Google Scholar] [CrossRef]
- Khosrokhavar, R.; Dizaji, R.; Nazari, F.; Sharafi, A.; Tajkey, J.; Hosseini, M. The role of PGC-1α and metabolic signaling pathway in kidney injury following chronic administration with 3-MCPD as a food processing contaminant. J. Food Biochem. 2021, 45, e13744. [Google Scholar] [CrossRef]
- Wang, Y.; Song, M.; Wang, Q.; Guo, C.; Zhang, J.; Zhang, X.; Cui, Y.; Cao, Z.; Li, Y. PINK1/Parkin-mediated mitophagy is activated to protect against AFB1-induced kidney damage in mice. Chem. Interact. 2022, 358, 109884. [Google Scholar] [CrossRef]
- Vettorazzi, A.; Pastor, L.; Guruceaga, E.; de Cerain, A.L. Sex-dependent gene expression after ochratoxin A insult in F344 rat kidney. Food Chem. Toxicol. 2018, 123, 337–348. [Google Scholar] [CrossRef]
- Liu, J.; Liu, Q.; Han, J.; Feng, J.; Guo, T.; Li, Z.; Min, F.; Jin, R.; Peng, X. N-Acetylcysteine Inhibits Patulin-Induced Apoptosis by Affecting ROS-Mediated Oxidative Damage Pathway. Toxins 2021, 13, 595. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, S.; Zhang, M.; Yang, L.; Cheng, B.; Li, J.; Shan, A. Individual and combined effects of Fusarium toxins on apoptosis in PK15 cells and the protective role of N -acetylcysteine. Food Chem. Toxicol. 2018, 111, 27–43. [Google Scholar] [CrossRef]
- Ma, K.; Bai, Y.; Li, J.; Ren, Z.; Li, J.; Zhang, J.; Shan, A. Lactobacillus rhamnosus GG ameliorates deoxynivalenol-induced kidney oxidative damage and mitochondrial injury in weaned piglets. Food Funct. 2022, 13, 3905–3916. [Google Scholar] [CrossRef]
- Harris, P.S.; Roy, S.R.; Coughlan, C.; Orlicky, D.J.; Liang, Y.; Shearn, C.T.; Roede, J.R.; Fritz, K.S. Chronic ethanol consumption induces mitochondrial protein acetylation and oxidative stress in the kidney. Redox Biol. 2015, 6, 33–40. [Google Scholar] [CrossRef] [Green Version]
- Ruggiero, C.; Ehrenshaft, M.; Cleland, E.; Stadler, K. High-fat diet induces an initial adaptation of mitochondrial bioenergetics in the kidney despite evident oxidative stress and mitochondrial ROS production. Am. J. Physiol. Metab. 2011, 300, E1047–E1058. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; He, L.; Yang, Y.; Chen, Y.; Song, Y.; Lu, X.; Liang, Y. The inhibition of Nrf2 accelerates renal lipid deposition through suppressing the ACSL1 expression in obesity-related nephropathy. Ren. Fail. 2019, 41, 821–831. [Google Scholar] [CrossRef]
- Sun, Y.; Ge, X.; Li, X.; He, J.; Wei, X.; Du, J.; Sun, J.; Li, X.; Xun, Z.; Liu, W.; et al. High-fat diet promotes renal injury by inducing oxidative stress and mitochondrial dysfunction. Cell Death Dis. 2020, 11, 914. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Morúa, A.; Soto-Urquieta, M.G.; Robles, E.F.; Zúñiga-Trujillo, I.; Campos-Cervantes, A.; Perez-Vazquez, V.; Ramírez-Emiliano, J. Curcumin decreases oxidative stress in mitochondria isolated from liver and kidneys of high-fat diet-induced obese mice. J. Asian Nat. Prod. Res. 2013, 15, 905–915. [Google Scholar] [CrossRef] [PubMed]
- Feng, B.; Meng, R.; Huang, B.; Bi, Y.; Shen, S.; Zhu, D. Silymarin protects against renal injury through normalization of lipid metabolism and mitochondrial biogenesis in high fat-fed mice. Free Radic. Biol. Med. 2017, 110, 240–249. [Google Scholar] [CrossRef] [PubMed]
- Andres-Hernando, A.; Lanaspa, M.A.; Kuwabara, M.; Orlicky, D.J.; Cicerchi, C.; Bales, E.; Garcia, G.E.; Roncal-Jimenez, C.A.; Sato, Y.; Johnson, R.J. Obesity causes renal mitochondrial dysfunction and energy imbalance and accelerates chronic kidney disease in mice. Am. J. Physiol. Physiol. 2019, 317, F941–F948. [Google Scholar] [CrossRef] [PubMed]
- Eirin, A.; Woollard, J.R.; Ferguson, C.M.; Jordan, K.L.; Tang, H.; Textor, S.C.; Lerman, A.; Lerman, L.O. The metabolic syndrome induces early changes in the swine renal medullary mitochondria. Transl. Res. 2017, 184, 45–56.e9. [Google Scholar] [CrossRef]
- Arany, I.; Clark, J.S.; Reed, D.K.; Juncos, L.A.; Dixit, M. Role of p66shc in Renal Toxicity of Oleic Acid. Am. J. Nephrol. 2013, 38, 226–232. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.-Q.; Jian, T.-Y.; Gai, Y.-N.; Niu, G.-T.; Liu, Y.; Meng, X.-H.; Li, J.; Lyu, H.; Ren, B.-R.; Chen, J. Chicoric Acid Attenuated Renal Tubular Injury in HFD-Induced Chronic Kidney Disease Mice through the Promotion of Mitophagy via the Nrf2/PINK/Parkin Pathway. J. Agric. Food Chem. 2022, 70, 2923–2935. [Google Scholar] [CrossRef]
- Cui, J.; Shi, S.; Sun, X.; Cai, G.; Cui, S.; Hong, Q.; Chen, X.; Bai, X.-Y. Mitochondrial Autophagy Involving Renal Injury and Aging Is Modulated by Caloric Intake in Aged Rat Kidneys. PLoS ONE 2013, 8, e69720. [Google Scholar] [CrossRef] [Green Version]
- Nguyen, L.T.; Stangenberg, S.; Chen, H.; Al-Odat, I.; Chan, Y.L.; Gosnell, M.E.; Anwer, A.G.; Goldys, E.M.; Pollock, C.A.; Saad, S. l-Carnitine reverses maternal cigarette smoke exposure-induced renal oxidative stress and mitochondrial dysfunction in mouse offspring. Am. J. Physiol. Physiol. 2015, 308, F689–F696. [Google Scholar] [CrossRef] [Green Version]
- Stangenberg, S.; Nguyen, L.T.; Chen, H.; Al-Odat, I.; Killingsworth, M.C.; Gosnell, M.E.; Anwer, A.G.; Goldys, E.M.; Pollock, C.A.; Saad, S. Oxidative stress, mitochondrial perturbations and fetal programming of renal disease induced by maternal smoking. Int. J. Biochem. Cell Biol. 2015, 64, 81–90. [Google Scholar] [CrossRef]
- Arany, I.; Carter, A.; Hall, S.; Fulop, T.; Dixit, M. Coenzyme Q10 protects renal proximal tubule cells against nicotine-induced apoptosis through induction of p66shc-dependent antioxidant responses. Apoptosis 2016, 22, 220–228. [Google Scholar] [CrossRef]
- Patergnani, S.; Bouhamida, E.; Leo, S.; Pinton, P.; Rimessi, A. Mitochondrial Oxidative Stress and “Mito-Inflammation”: Actors in the Diseases. Biomedicines 2021, 9, 216. [Google Scholar] [CrossRef]
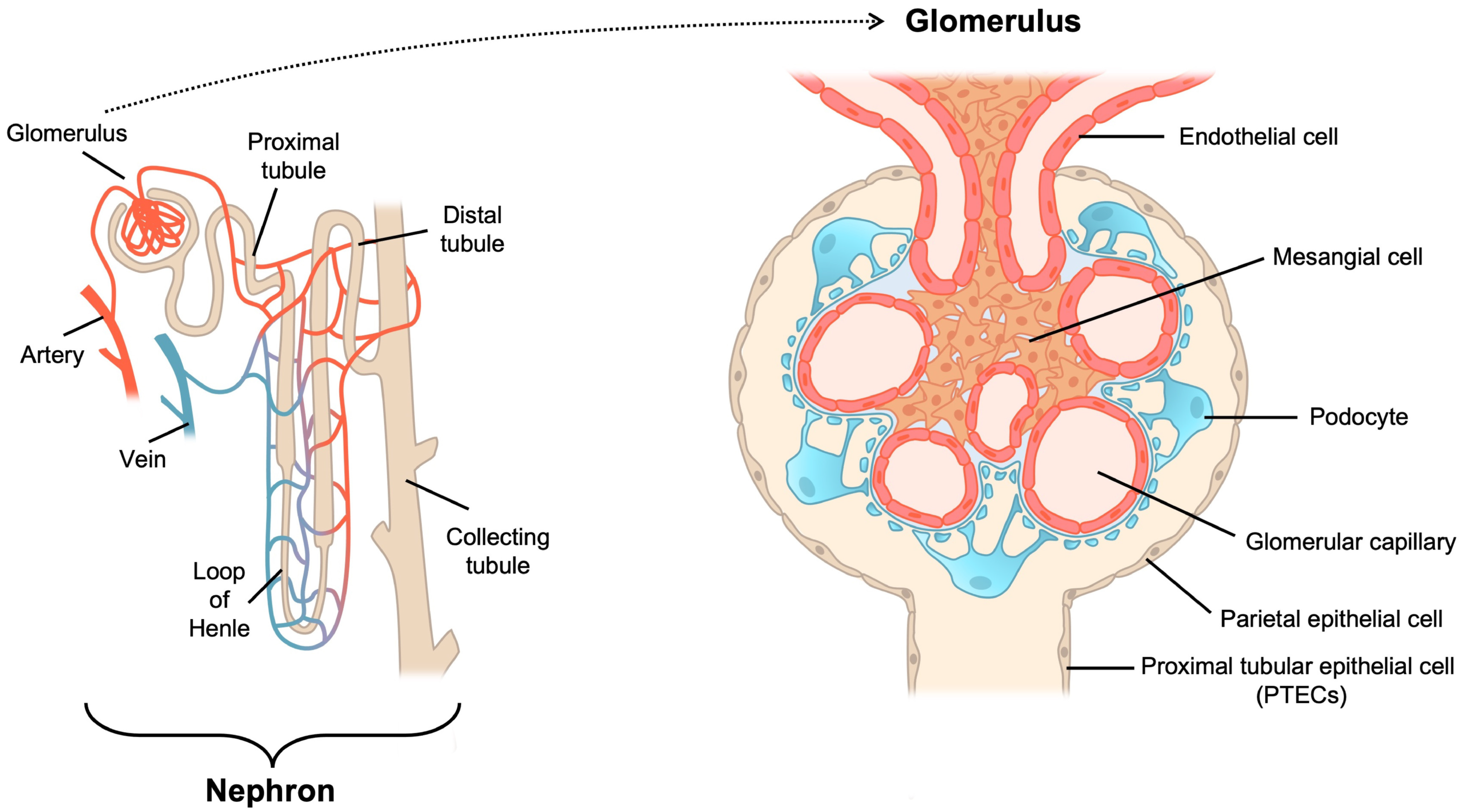


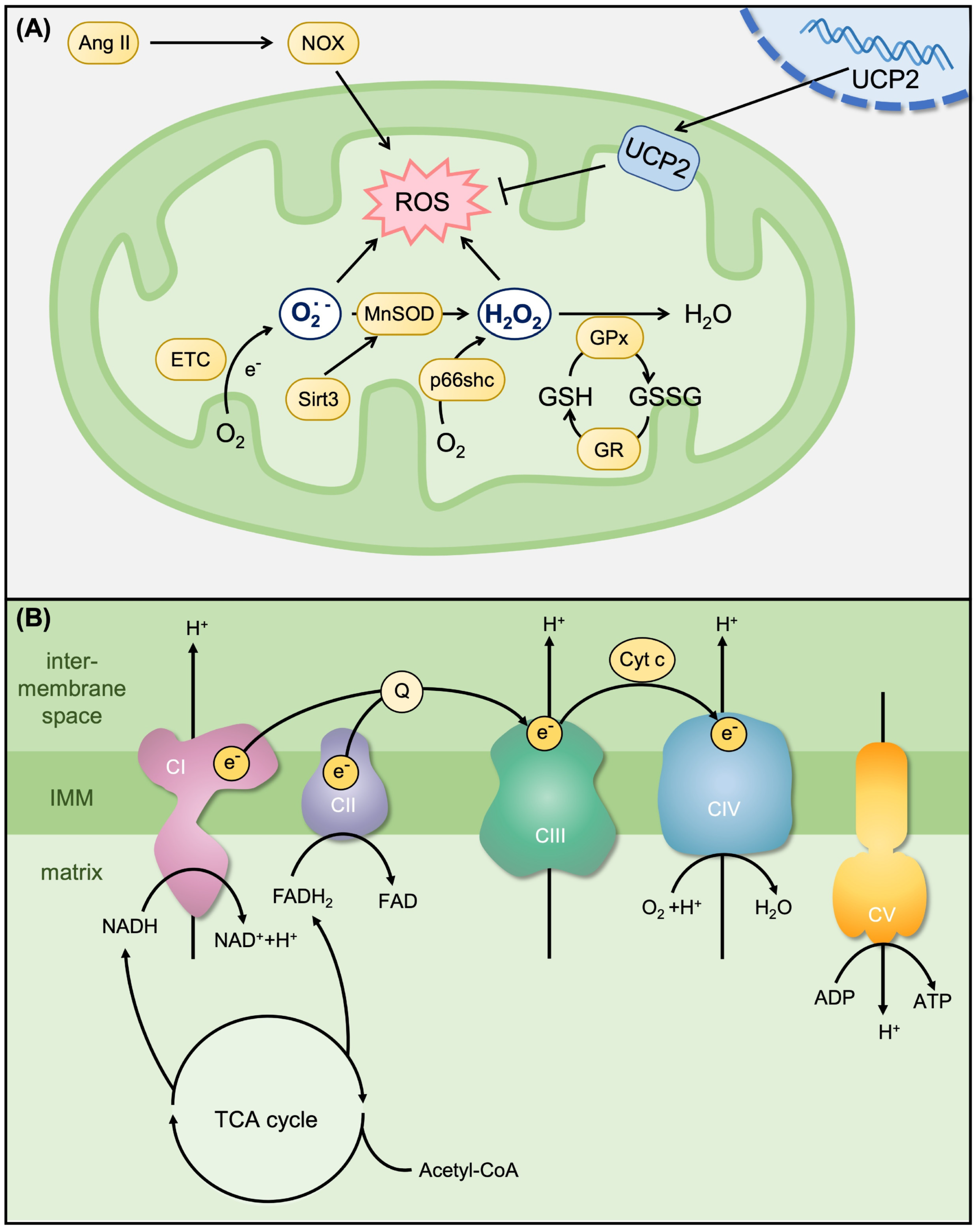
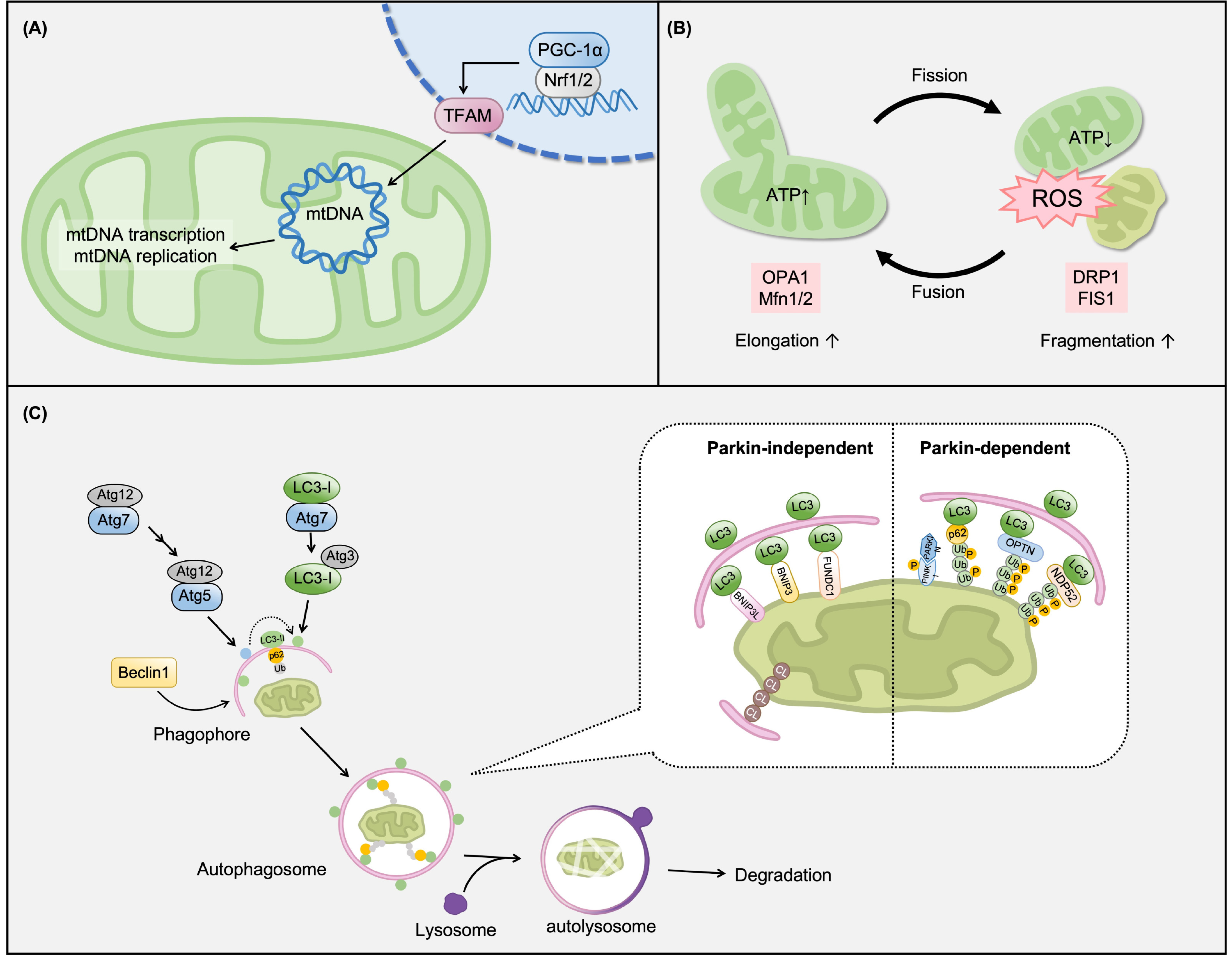
| Causes and Risk Factors | Mechanisms | Model [Ref.] | Treatment [Ref.] | Effects on Mitochondria |
|---|---|---|---|---|
| Environmental renal injury | ||||
| Air pollution | ||||
| particulate matter | mtROS↑, MMP↓, autophagy↑, mitochondrial-related apoptosis↑ | Sprague Dawley (SD) rats [105]; human kidney proximal tubular (HK-2) cells [105] | − | − |
| gaseous mixtures | MMP and ATP↓, mitochondrial respiration and fusion↓, mitophagy↑ | SD rats [106] | − | − |
| Heavy metals | ||||
| cadmium (Cd) | mitochondrial swelling, MMP and ATPase↓, PGC-1α/Nrf2-related pathway↑, mitochondrial-related apoptosis↑ | human embryonic kidney (HEK293) cells [107,119]; SD rats [108,120]; Wistar rats [117,118,121]; BALB/c mice [119]; ICR mice [123]; Hy-Line Variety White chickens [122] | α-lipoic acid [108] | improved mitochondrial swelling; inhibited mitochondrial-related apoptosis |
| caffeic acid phenethyl ester [117] | improved mitochondrial swelling and dysfunction | |||
| p-coumaric acid [118] | regulated gluconeogenic and glycolytic enzyme activities;enhanced TCA cycle and ETC enzyme activities | |||
| Potentilla anserina [119] | regulated PGC-1α/Nrf2-related pathway; inhibited mitochondrial-related apoptosis | |||
| trehalose [120] | inhibited mitochondrial-related apoptosis | |||
| catechin [121] | improved renal function and mitochondrial antioxidant status | |||
| resveratrol [122] | improved renal mitochondrial injury via regulation of mitochondrial biogenesis and dynamics | |||
| selenium [123] | inhibited mitochondrial-related apoptosis | |||
| chromium (Cr) | ETC and antioxidative enzymes activities↓, mitochondrial-related apoptosis↑ | SD rats [109] | carvedilol [109] | enhanced antioxidants and ETC enzyme activities and inhibited mitochondrial-related apoptosis |
| lead (Pb) | MMP↓, PI3K/Akt/eNOS pathway↓mitochondrial-related apoptosis↑ | primary rat proximal tubular cells [125]; Wistar rats [110] | Puerarin [110,125] | improved mitochondrial injury and inhibited mitochondrial-related apoptosis |
| molybdenum (Mo) | intracellular [Ca2+]↑, MMP↓, ATPase activity↓, mitochondrial content↓, mitochondrial-related apoptosis↑ | primary duck renal tubular epithelial cells [111,112] | 3-methyladenine [111] | an autophagy inhibitor;aggravated Mo-induced mitochondrial dysfunction by regulating oxidative stress |
| uranium (U) | mitochondrial swelling, mtROS and mtMDA↑, mtGSH, MMP, and ATP↓, ETC and ATPase activities↓, mitochondrial-related apoptosis↑ | HK-2 cells [114,126]; Wistar rats [113] | Polygonatum kingianum [126] | improved mitochondrial injury and inhibited mitochondrial-related apoptosis via regulating the GSK3β/Nrf2-related pathway |
| tungsten (W) | mitochondrial swelling, mtROS and mtMDA↑, mtGSH, MMP, and ATP↓, mitochondrial-related apoptosis↑ | Wistar rats [115] | - | - |
| aluminum (Al) | MMP↓, mitophagy↑, mitochondrial-related apoptosis↑ | C57BL/6 mice [116] | - | Parkin deficiency aggravated Al-induced oxidative stress and mitochondrial damage |
| Fungicides, herbicides, and insecticides | ||||
| Kresoxim-methyl | intracellular [Ca2+] and mtROS↑, MMP↓ | monkey kidney Vero CCL-81 cells [127] | − | − |
| thioacetamide | mitochondrial biogenesis↓, autophagy↑, mitochondrial-related apoptosis↑ | albino rats [128] | platelet-rich plasma [128] | improved mitochondrial injury via regulating the PGC1α-related pathway; inhibited autophagy and mitochondrial-related apoptosis |
| Atrazine | mitochondria content↓ and damage↑,mitochondrial-related apoptosis↑ | quail [129] | − | − |
| Deltamethrin | kidney and other organs failure | SD rats [130] | − | − |
| Permethrin | mitochondrial swelling, ETC activities↓ | albino Wistar rats [131] | Fumaria officinalis extract [131] | improved renal injury, antioxidant status and mitochondrial bioenergetics |
| Plasticizer compounds/organic pollutants | ||||
| bisphenol A | intracellular [Ca2+] and mtROS↑, MMP and ATP↓, AMPK-PGC-1α-SIRT3-pathway↓, mitochondrial fission↑, mitochondrial-related apoptosis↑ | HK-2 cells [132]; Wistar rats [136,137,138,139,140,141] | NAC [136,137,138] | inhibited mitochondrial fission; regulated AMPK-PGC-1α-SIRT3 signaling |
| melatonin [139] | improved renal mitochondrial swelling and injury | |||
| quercetin [140] | improved renal mitochondrial injury | |||
| astaxanthin [141] | regulated ETC activities; inhibited mitochondrial-related apoptosis | |||
| di-(2-ethylhexyl) phthalate | mitochondrial swelling, MMP↓, mitochondrial content↓, mitochondrial oxidative stress↑, mitochondrial-related apoptosis↑ | HEK293 cells [133,134,135]; Wistar rats [134] | NAC [133,135] | improved renal mitochondrial injury |
| bromobenzene | mtGSH↓, TCA cycle enzymes and ETC activities ↓, mitochondrial-related apoptosis↑ | albino Wistar rats [142]; albino Swiss mice [143] | Withaferin A [142,143] | improved renal mitochondrial injury and mitochondrial enzymes activities; inhibited mitochondrial-related apoptosis |
| Nanoparticles | ||||
| multi-walled carbon nanotubes | mitochondrial swelling, SDH activity and MMP↓; mtROS↑, mitochondrial-related apoptosis↑ | HEK293 cells [144]; Wistar rats [145] | Apigenin [145] | improved renal mitochondrial injury and mitochondrial enzymes activities; inhibited mitochondrial-related apoptosis |
| AuNPs | energy metabolism (ETC activity) impairment, MMP and ATP↓, mitochondrial-related apoptosis↑ | HK-2 cells [152,157]; Wistar rats [148] | NAC [152] | improved mitochondrial injury and energy metabolism;inhibited mitochondrial-related apoptosis |
| AgNPs | mitochondrial swelling, mitochondrial enzyme activities↓ | albino Wistar rats [149]; pig kidney epithelial LLC PK1 cells [149] | − | − |
| CuNPs | MMP↓, mitochondrial-related apoptosis↑ | albino Swiss mice [150] | − | − |
| PtNPs | MMP↓, mitochondrial-related apoptosis↑ | HEK293 cells [151] | − | − |
| Food contamination | ||||
| Acrylamide | ATP↓, mitochondrial enzyme activities↓ | SD rats [160] | Argan oil [160] | improved mitochondrial enzymes activities |
| 3-monochloropropane-1,2-diol | MMP and mtDNA↓, mitochondrial biogenesis↓, mitochondrial-related apoptosis↑ | HEK293 cells [161]; C57 mice [162]; Wistar rats [161] | − | − |
| Aflatoxin B1 | MMP and ATP↓, mitophagy↑, mitochondrial-related apoptosis↑ | C57BL/6N mice [163] | − | − |
| ochratoxin A | males: cell damage, fibrosis, cell signaling, and metabolism↑;females: renal safety biomarkers and mitochondrial biogenesis↑ | Fischer 344 rats [164] | − | − |
| patulin | ATP and MMP↓, ETC impairmentmitochondrial-related apoptosis↑ | HEK293 cells [165] | NAC [165] | inhibited mitochondrial-related apoptosis modulated ETC activity, and maintaining mitochondrial function |
| deoxynivalenol, zearalenone, and fumonisin B1 | mitochondrial swelling, mitochondrial biogenesis↓, fusion↓/fission↑, mitophagy↑, mitochondrial-related apoptosis↑ | porcine kidney PK15 cells [166]; piglets (Duroc × Landrace × Yorkshire) [167] | NAC [166] | inhibited mitochondrial-related apoptosis |
| Lactobacillus rhamnosus GG [167] | increased Sirt3 to maintain redox balance, regulated mitochondrial fusion/fission, and prevented mitophagy | |||
| Lifestyle-related renal injury | ||||
| Chronic alcohol | ||||
| mitochondrial proteins acetylation↑ | C57BL/6J mice [168] | − | − | |
| HFD/obesity | ||||
| HFD-induced obesity/fatty acid-induced lipotoxicity | mitochondrial swelling, mitochondrial bioenergetic adaptation: PGC-1β, NRF2, TFAM, and ERRα ↑, mitophagy↑, ATP and MMP↓, oxygen consumption↓ | HK-2 cells [170,171,173]; mesangial SV40 MES 13 cells [171]; mouse kidney proximal tubular TKPTS cells [176]; C57BL mice [169,171,172,173]; aged Fischer 344 rats [178]; | silymarin [173] | regulated β-oxidation, and mitochondrial biogenesis |
| NAC [171,176] | p66shc↓, MMP↓, mitochondrial fission↓mitochondrial-related apoptosis↓ | |||
| curcumin [172] | increased oxygen consumption; decrease lipid and protein peroxidation | |||
| calorie restriction [178] | aggravated and mitophagy was markedly decreased in aging HFD kidneys, whereas they were markedly ameliorated | |||
| obesity patients/genic obesity animals | renal biopsy: ACSL1 and Nrf2↓ob/ob mice: ACSL1, Nrf2, and SOD↓; ROS and MDA↑ | obesity-related nephropathy patients [170]; C57BL/6 J ob/ob mice [170]; | − | − |
| Metabolic syndrome | ||||
| genic model | (with adenine diet) mtDNA and ATP↓, mitochondrial genes↓ | POUND mice [174] | − | − |
| high-cholesterol/carbohydrate diet | cardiolipin content↓, cardiolipin remodeling↓, ATP↓, mitochondrial-related apoptosis↑ | farm pig [175] | SS-31 [175] | improved renal mitochondrial cardiolipin content and ATP level; inhibited mitochondrial-related apoptosis |
| Smoking | ||||
| mother/offspring | mitochondrial density and mtDNA↑, p66shc↑, mtROS↑, ETC↓,mitochondrial-related apoptosis↑ | rat kidney proximal tubular NRK52E2 cells [181]; Balb/c mice and offspring [179,180] | L-Carnitine [179] | improved renal mitochondrial respiration and reduced mtROS |
| CoQ10 [181] | reduced ROS production via regulating p66shc-related pathway; inhibited apoptosis | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ho, H.-J.; Shirakawa, H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells 2023, 12, 88. https://doi.org/10.3390/cells12010088
Ho H-J, Shirakawa H. Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells. 2023; 12(1):88. https://doi.org/10.3390/cells12010088
Chicago/Turabian StyleHo, Hsin-Jung, and Hitoshi Shirakawa. 2023. "Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease" Cells 12, no. 1: 88. https://doi.org/10.3390/cells12010088
APA StyleHo, H.-J., & Shirakawa, H. (2023). Oxidative Stress and Mitochondrial Dysfunction in Chronic Kidney Disease. Cells, 12(1), 88. https://doi.org/10.3390/cells12010088







