Characterization of the Zebrafish Elastin a (elnasa12235) Mutant: A New Model of Elastinopathy Leading to Heart Valve Defects
Abstract
:1. Introduction
2. Materials and Methods
2.1. Zebrafish Husbandry
2.2. Genotyping
2.3. Survival and Weight
2.4. Histology
2.4.1. Elastic Fiber Staining—Orcein Acid (According to Shikata’s Method)
2.4.2. Collagen Fiber Staining—Picrosirius Red
2.4.3. Lipid Staining—Oil Red O
2.4.4. Immunohistochemistry
2.5. Statistical Analysis
3. Results
3.1. The ARMS-PCR Genotyping Technique
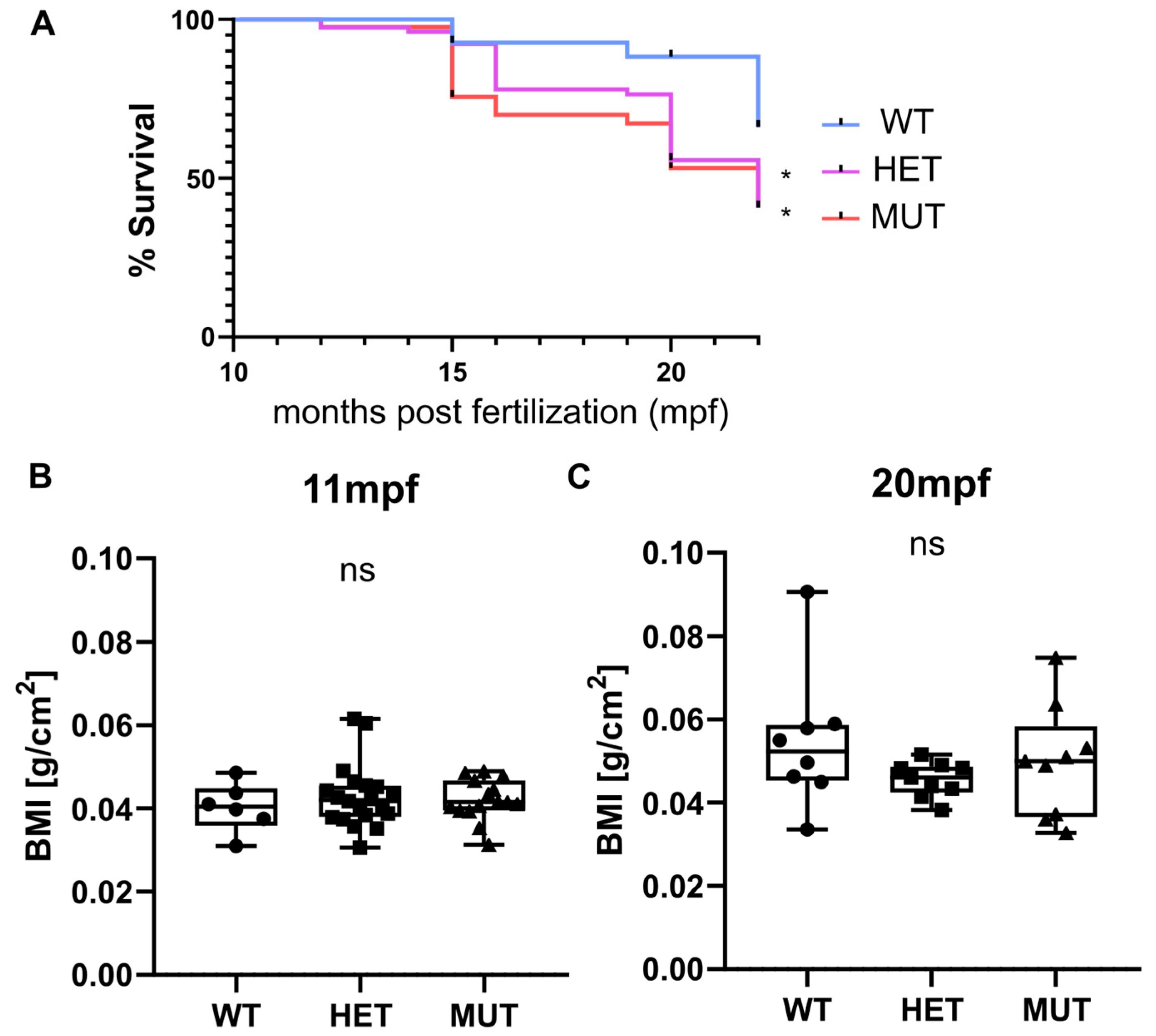
3.2. Survival and Weight
3.3. Severe but Highly Heterogenous Complications
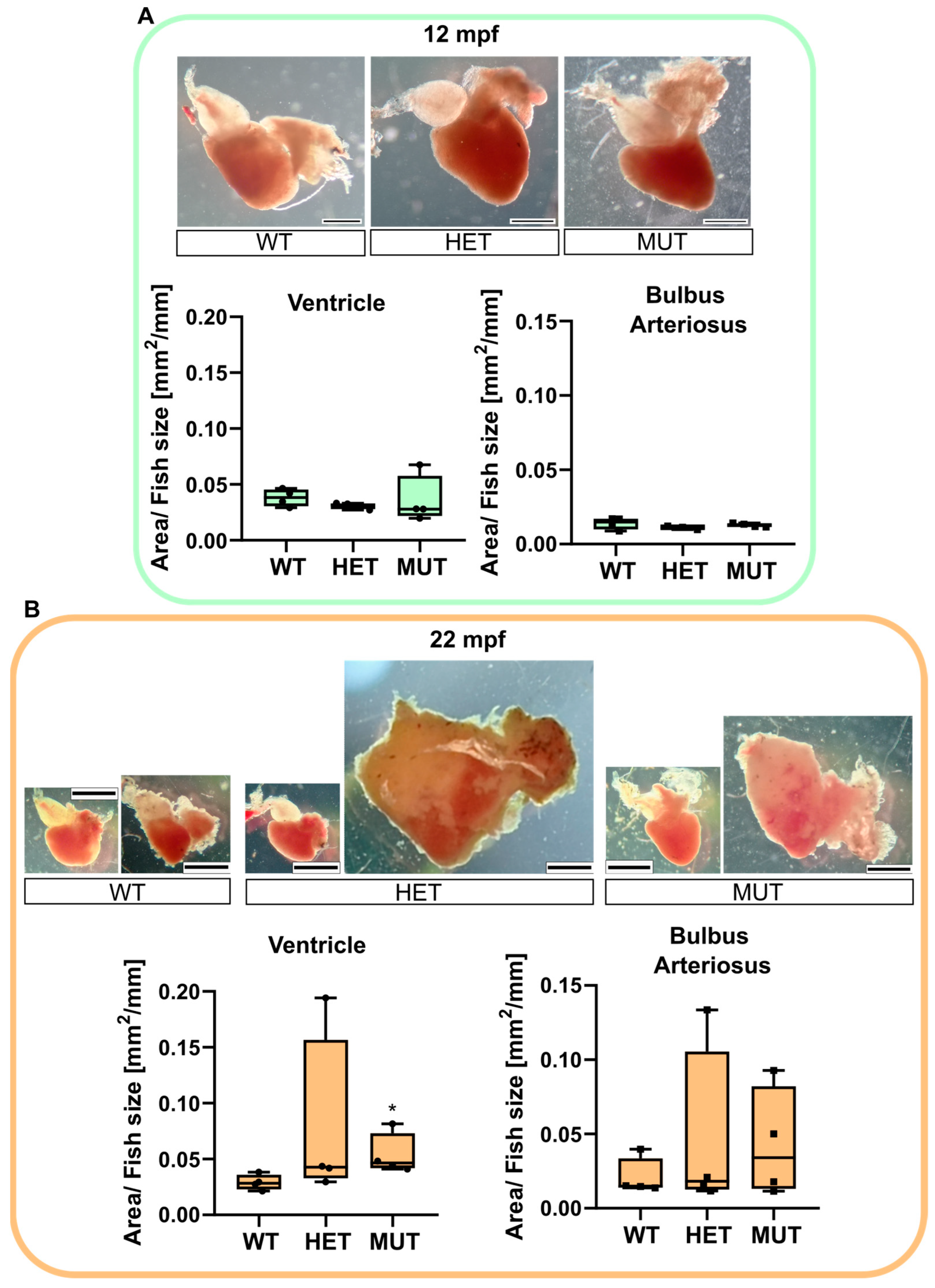
3.4. Age-Associated Cardiomegaly
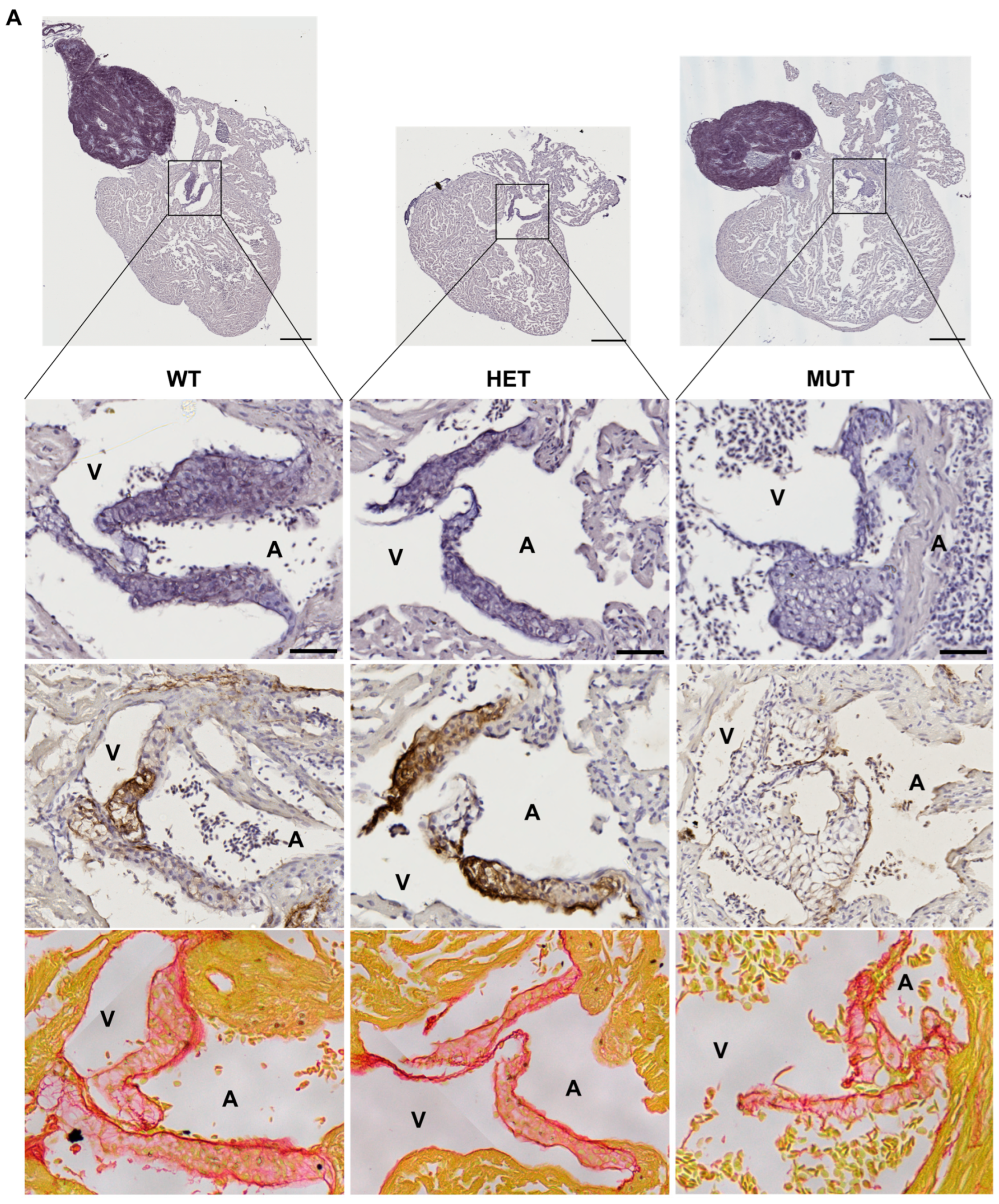
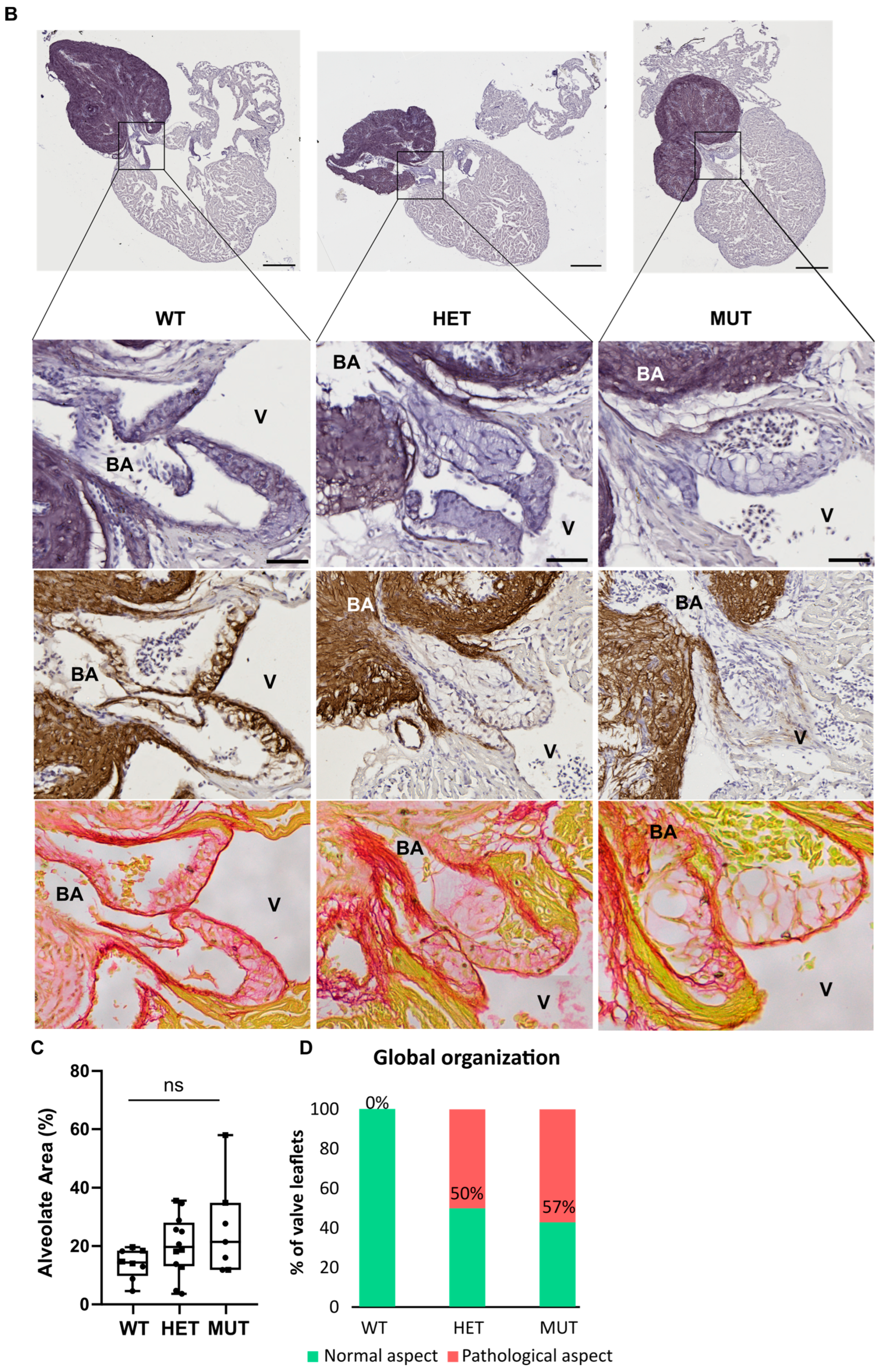
3.5. Cardiac Valve Morphology and Composition
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wise, S.G.; Weiss, A.S. Tropoelastin. Int. J. Biochem. Cell Biol. 2009, 41, 494–497. [Google Scholar] [CrossRef] [PubMed]
- Wagenseil, J.E.; Mecham, R.P. Vascular Extracellular Matrix and Arterial Mechanics. Physiol. Rev. 2009, 89, 957–989. [Google Scholar] [CrossRef] [PubMed]
- Cocciolone, A.J.; Hawes, J.Z.; Staiculescu, M.C.; Johnson, E.O.; Murshed, M.; Wagenseil, J.E. Elastin, Arterial Mechanics, and Cardiovascular Disease. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H189–H205. [Google Scholar] [CrossRef] [PubMed]
- Baldwin, A.K.; Simpson, A.; Steer, R.; Cain, S.A.; Kielty, C.M. Elastic Fibres in Health and Disease. Expert Rev. Mol. Med. 2013, 15, e8. [Google Scholar] [CrossRef]
- Sherratt, M.J. Tissue Elasticity and the Ageing Elastic Fibre. Age 2009, 31, 305–325. [Google Scholar] [CrossRef]
- Duque Lasio, M.L.; Kozel, B.A. Elastin-Driven Genetic Diseases. Matrix Biol. 2018, 71–72, 144–160. [Google Scholar] [CrossRef]
- Kozel, B.A.; Barak, B.; Ae Kim, C.; Mervis, C.B.; Osborne, L.R.; Porter, M.; Pober, B.R. Williams Syndrome. Nat. Rev. Dis. Primers 2021, 7, 42. [Google Scholar] [CrossRef]
- Graul-Neumann, L.M.; Hausser, I.; Essayie, M.; Rauch, A.; Kraus, C. Highly Variable Cutis Laxa Resulting from a Dominant Splicing Mutation of the Elastin Gene. Am. J. Med. Genet. 2008, 146A, 977–983. [Google Scholar] [CrossRef]
- Detrich, H.W.; Westerfield, M.; Zon, L.I. Overview of the Zebrafish System. Methods Cell Biol. 1999, 59, 3–10. [Google Scholar] [CrossRef]
- Choi, T.-Y.; Choi, T.-I.; Lee, Y.-R.; Choe, S.-K.; Kim, C.-H. Zebrafish as an Animal Model for Biomedical Research. Exp. Mol. Med. 2021, 53, 310–317. [Google Scholar] [CrossRef]
- González-Rosa, J.M. Zebrafish Models of Cardiac Disease: From Fortuitous Mutants to Precision Medicine. Circ. Res. 2022, 130, 1803–1826. [Google Scholar] [CrossRef]
- Gut, P.; Reischauer, S.; Stainier, D.Y.R.; Arnaout, R. Little fish, big data: Zebrafish as a model for cardiovascular and metabolic disease. Physiol. Rev. 2017, 97, 889–938. [Google Scholar] [CrossRef] [PubMed]
- Bowley, G.; Kugler, E.; Wilkinson, R.; Lawrie, A.; Eeden, F.; Chico, T.J.A.; Evans, P.C.; Noël, E.S.; Serbanovic-Canic, J. Zebrafish as a Tractable Model of Human Cardiovascular Disease. Br. J. Pharmacol. 2022, 179, 900–917. [Google Scholar] [CrossRef]
- Howe, K.; Clark, M.D.; Torroja, C.F.; Torrance, J.; Berthelot, C.; Muffato, M.; Collins, J.E.; Humphray, S.; McLaren, K.; Matthews, L.; et al. The Zebrafish Reference Genome Sequence and Its Relationship to the Human Genome. Nature 2013, 496, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Glasauer, S.M.K.; Neuhauss, S.C.F. Whole-Genome Duplication in Teleost Fishes and Its Evolutionary Consequences. Mol. Genet. Genom. 2014, 289, 1045–1060. [Google Scholar] [CrossRef]
- Pasquier, J.; Cabau, C.; Nguyen, T.; Jouanno, E.; Severac, D.; Braasch, I.; Journot, L.; Pontarotti, P.; Klopp, C.; Postlethwait, J.H.; et al. Gene Evolution and Gene Expression after Whole Genome Duplication in Fish: The PhyloFish Database. BMC Genom. 2016, 17, 368. [Google Scholar] [CrossRef]
- Moriyama, Y.; Ito, F.; Takeda, H.; Yano, T.; Okabe, M.; Kuraku, S.; Keeley, F.W.; Koshiba-Takeuchi, K. Evolution of the Fish Heart by Sub/Neofunctionalization of an Elastin Gene. Nat. Commun. 2016, 7, 10397. [Google Scholar] [CrossRef]
- Hoareau, M.; El Kholti, N.; Debret, R.; Lambert, E. Zebrafish as a Model to Study Vascular Elastic Fibers and Associated Pathologies. Int. J. Mol. Sci. 2022, 23, 2102. [Google Scholar] [CrossRef] [PubMed]
- Miao, M.; Bruce, A.E.E.; Bhanji, T.; Davis, E.C.; Keeley, F.W. Differential Expression of Two Tropoelastin Genes in Zebrafish. Matrix Biol. 2007, 26, 115–124. [Google Scholar] [CrossRef] [PubMed]
- Kettleborough, R.N.W.; Busch-Nentwich, E.M.; Harvey, S.A.; Dooley, C.M.; de Bruijn, E.; van Eeden, F.; Sealy, I.; White, R.J.; Herd, C.; Nijman, I.J.; et al. A Systematic Genome-Wide Analysis of Zebrafish Protein-Coding Gene Function. Nature 2013, 496, 494–497. [Google Scholar] [CrossRef]
- Westerfield, M. The Zebrafish Book: A Guide for the Laboratory Use of Zebrafish (Danio rerio); University of Oregon Press: Eugene, OR, USA, 2000. [Google Scholar]
- Shikata, T.; Uzawa, T.; Yoshiwara, N.; Akatsuka, T.; Yamazaki, S. Staining Methods of Australia Antigen in Paraffin Section--Detection of Cytoplasmic Inclusion Bodies. Jpn. J. Exp. Med. 1974, 44, 25–36. [Google Scholar]
- Henwood, A.F. Shikata’s Orcein Stain—A Routine Stain for Liver Biopsies. Aust. J. Med. Lab. Sci. 1983, 4, 76–80. [Google Scholar]
- Waters, P.J. Degradation of Mutant Proteins, Underlying “Loss of Function” Phenotypes, Plays a Major Role in Genetic Disease. Curr. Issues Mol. Biol. 2001, 3, 57–65. [Google Scholar] [PubMed]
- Brogna, S.; Wen, J. Nonsense-Mediated MRNA Decay (NMD) Mechanisms. Nat. Struct. Mol. Biol. 2009, 16, 107–113. [Google Scholar] [CrossRef]
- Little, S. Amplification-Refractory Mutation System (ARMS) Analysis of Point Mutations. Curr. Protoc. Hum. Genet. 2001, 9, 9.8. [Google Scholar] [CrossRef]
- Peng, B.; Wang, Q.; Luo, Y.; He, J.; Tan, T.; Zhu, H. A Novel and Quick PCR-Based Method to Genotype Mice with a Leptin Receptor Mutation (Db/Db Mice). Acta Pharmacol. Sin. 2018, 39, 117–123. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.M.; Arnheim, N.; Goodman, M.F. Extension of Base Mispairs by Taq DNA Polymerase: Implications for Single Nucleotide Discrimination in PCR. Nucleic Acids Res. 1992, 20, 4567–4573. [Google Scholar] [CrossRef]
- Vamvakopoulos, J.E. Multiplex Universal Genotyping Using a Modified ARMS-PCR Protocol. Biotechniques 2002, 33, 1110–1112. [Google Scholar] [CrossRef]
- Ye, S.; Dhillon, S.; Ke, X.; Collins, A.R.; Day, I.N. An Efficient Procedure for Genotyping Single Nucleotide Polymorphisms. Nucleic Acids Res. 2001, 29, E88. [Google Scholar] [CrossRef] [PubMed]
- Schoen, F.J. Evolving Concepts of Cardiac Valve Dynamics: The Continuum of Development, Functional Structure, Pathobiology, and Tissue Engineering. Circulation 2008, 118, 1864–1880. [Google Scholar] [CrossRef]
- Hinton, R.B.; Yutzey, K.E. Heart Valve Structure and Function in Development and Disease. Annu. Rev. Physiol. 2011, 73, 29–46. [Google Scholar] [CrossRef]
- Bekeredjian, R.; Grayburn, P.A. Valvular Heart Disease: Aortic Regurgitation. Circulation 2005, 112, 125–134. [Google Scholar] [CrossRef] [PubMed]
- Hoppe, U.C. How to detect impending cardiac insufficiency. MMW Fortschr. Med. 2005, 147, 30–33. [Google Scholar] [PubMed]
- Gorton, A.J.; Keshavamurthy, S.; Saha, S.P. Diagnosis and Management of Aortic Valvular Disease in the Elderly. Int. J. Angiol. 2022, 31, 232–243. [Google Scholar] [CrossRef]
- Kallikourdis, M.; Martini, E.; Carullo, P.; Sardi, C.; Roselli, G.; Greco, C.M.; Vignali, D.; Riva, F.; Ormbostad Berre, A.M.; Stølen, T.O.; et al. T Cell Costimulation Blockade Blunts Pressure Overload-Induced Heart Failure. Nat. Commun. 2017, 8, 14680. [Google Scholar] [CrossRef] [PubMed]
- Cooper, T.K.; Spitsbergen, J.M. Valvular and Mural Endocardiosis in Aging Zebrafish (Danio rerio). Vet. Pathol. 2016, 53, 504–509. [Google Scholar] [CrossRef]
- Sephel, G.C.; Byers, P.H.; Holbrook, K.A.; Davidson, J.M. Heterogeneity of Elastin Expression in Cutis Laxa Fibroblast Strains. J. Investig. Dermatol. 1989, 93, 147–153. [Google Scholar] [CrossRef]
- Marchase, P.; Holbrook, K.; Pinnell, S.R. A Familial Cutis Laxa Syndrome with Ultrastructural Abnormalities of Collagen and Elastin. J. Investig. Dermatol. 1980, 75, 399–403. [Google Scholar] [CrossRef]
- Gistelinck, C.; Kwon, R.Y.; Malfait, F.; Symoens, S.; Harris, M.P.; Henke, K.; Hawkins, M.B.; Fisher, S.; Sips, P.; Guillemyn, B.; et al. Zebrafish Type I Collagen Mutants Faithfully Recapitulate Human Type I Collagenopathies. Proc. Natl. Acad. Sci. USA 2018, 115, E8037–E8046. [Google Scholar] [CrossRef]
- Hinton, R.B.; Adelman-Brown, J.; Witt, S.; Krishnamurthy, V.K.; Osinska, H.; Sakthivel, B.; James, J.F.; Li, D.Y.; Narmoneva, D.A.; Mecham, R.P.; et al. Elastin Haploinsufficiency Results in Progressive Aortic Valve Malformation and Latent Valve Disease in a Mouse Model. Circ. Res. 2010, 107, 549–557. [Google Scholar] [CrossRef]
- Poss, K.D.; Wilson, L.G.; Keating, M.T. Heart Regeneration in Zebrafish. Science 2002, 298, 2188–2190. [Google Scholar] [CrossRef]
- Bensimon-Brito, A.; Ramkumar, S.; Boezio, G.L.M.; Guenther, S.; Kuenne, C.; Helker, C.S.M.; Sánchez-Iranzo, H.; Iloska, D.; Piesker, J.; Pullamsetti, S.; et al. TGF-β Signaling Promotes Tissue Formation during Cardiac Valve Regeneration in Adult Zebrafish. Dev. Cell 2020, 52, 9–20.e7. [Google Scholar] [CrossRef] [PubMed]
- Marques, I.J.; Lupi, E.; Mercader, N. Model Systems for Regeneration: Zebrafish. Development 2019, 146, dev167692. [Google Scholar] [CrossRef]
- Hadi, H.A.; Alsheikh-Ali, A.A.; Mahmeed, W.A.; Suwaidi, J.M.A. Inflammatory Cytokines and Atrial Fibrillation: Current and Prospective Views. J. Inflamm. Res. 2010, 3, 75–97. [Google Scholar] [CrossRef] [PubMed]
- Aghajanian, H.; Kimura, T.; Rurik, J.G.; Hancock, A.S.; Leibowitz, M.S.; Li, L.; Scholler, J.; Monslow, J.; Lo, A.; Han, W.; et al. Targeting Cardiac Fibrosis with Engineered T Cells. Nature 2019, 573, 430–433. [Google Scholar] [CrossRef]
- Ibáñez-Fonseca, A.; Ramos, T.L.; González de Torre, I.; Sánchez-Abarca, L.I.; Muntión, S.; Arias, F.J.; del Cañizo, M.C.; Alonso, M.; Sánchez-Guijo, F.; Rodríguez-Cabello, J.C. Biocompatibility of Two Model Elastin-like Recombinamer-Based Hydrogels Formed through Physical or Chemical Cross-Linking for Various Applications in Tissue Engineering and Regenerative Medicine. J. Tissue Eng. Regen. Med. 2018, 12, e1450–e1460. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez de Torre, I.; Alonso, M.; Rodriguez-Cabello, J.-C. Elastin-Based Materials: Promising Candidates for Cardiac Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 657. [Google Scholar] [CrossRef] [PubMed]
- Coquand-Gandit, M.; Jacob, M.-P.; Fhayli, W.; Romero, B.; Georgieva, M.; Bouillot, S.; Estève, E.; Andrieu, J.-P.; Brasseur, S.; Bouyon, S.; et al. Chronic Treatment with Minoxidil Induces Elastic Fiber Neosynthesis and Functional Improvement in the Aorta of Aged Mice. Rejuvenation Res. 2017, 20, 218–230. [Google Scholar] [CrossRef]
- Knutsen, R.H.; Beeman, S.C.; Broekelmann, T.J.; Liu, D.; Tsang, K.M.; Kovacs, A.; Ye, L.; Danback, J.R.; Watson, A.; Wardlaw, A.; et al. Minoxidil Improves Vascular Compliance, Restores Cerebral Blood Flow, and Alters Extracellular Matrix Gene Expression in a Model of Chronic Vascular Stiffness. Am. J. Physiol. Heart Circ. Physiol. 2018, 315, H18–H32. [Google Scholar] [CrossRef]
- Fhayli, W.; Boëté, Q.; Harki, O.; Briançon-Marjollet, A.; Jacob, M.-P.; Faury, G. Rise and Fall of Elastic Fibers from Development to Aging. Consequences on Arterial Structure-Function and Therapeutical Perspectives. Matrix Biol. 2019, 84, 41–56. [Google Scholar] [CrossRef]
- Fhayli, W.; Boëté, Q.; Kihal, N.; Cenizo, V.; Sommer, P.; Boyle, W.A.; Jacob, M.-P.; Faury, G. Dill Extract Induces Elastic Fiber Neosynthesis and Functional Improvement in the Ascending Aorta of Aged Mice with Reversal of Age-Dependent Cardiac Hypertrophy and Involvement of Lysyl Oxidase-Like-1. Biomolecules 2020, 10, 173. [Google Scholar] [CrossRef] [PubMed]
- Cenizo, V.; André, V.; Reymermier, C.; Sommer, P.; Damour, O.; Perrier, E. LOXL as a Target to Increase the Elastin Content in Adult Skin: A Dill Extract Induces the LOXL Gene Expression. Exp. Dermatol. 2006, 15, 574–581. [Google Scholar] [CrossRef]
- Sohm, B.; Cenizo, V.; André, V.; Zahouani, H.; Pailler-Mattei, C.; Vogelgesang, B. Evaluation of the Efficacy of a Dill Extract in Vitro and in Vivo. Int. J. Cosmet. Sci. 2011, 33, 157–163. [Google Scholar] [CrossRef]
- Tsoporis, J.; Keeley, F.W.; Lee, R.M.; Leenen, F.H. Arterial Vasodilation and Vascular Connective Tissue Changes in Spontaneously Hypertensive Rats. J. Cardiovasc. Pharmacol. 1998, 31, 960–962. [Google Scholar] [CrossRef]
- Slove, S.; Lannoy, M.; Behmoaras, J.; Pezet, M.; Sloboda, N.; Lacolley, P.; Escoubet, B.; Buján, J.; Jacob, M.-P. Potassium Channel Openers Increase Aortic Elastic Fiber Formation and Reverse the Genetically Determined Elastin Deficit in the BN Rat. Hypertension 2013, 62, 794–801. [Google Scholar] [CrossRef] [PubMed]
- Li, D.Y.; Brooke, B.; Davis, E.C.; Mecham, R.P.; Sorensen, L.K.; Boak, B.B.; Eichwald, E.; Keating, M.T. Elastin Is an Essential Determinant of Arterial Morphogenesis. Nature 1998, 393, 276–280. [Google Scholar] [CrossRef]
- Halabi, C.M.; Broekelmann, T.J.; Knutsen, R.H.; Ye, L.; Mecham, R.P.; Kozel, B.A. Chronic Antihypertensive Treatment Improves Pulse Pressure but Not Large Artery Mechanics in a Mouse Model of Congenital Vascular Stiffness. Am. J. Physiol. Heart Circ. Physiol. 2015, 309, H1008–H1016. [Google Scholar] [CrossRef]
- Faury, G.; Pezet, M.; Knutsen, R.H.; Boyle, W.A.; Heximer, S.P.; McLean, S.E.; Minkes, R.K.; Blumer, K.J.; Kovacs, A.; Kelly, D.P.; et al. Developmental Adaptation of the Mouse Cardiovascular System to Elastin Haploinsufficiency. J. Clin. Investig. 2003, 112, 1419–1428. [Google Scholar] [CrossRef]
- Wagenseil, J.E.; Nerurkar, N.L.; Knutsen, R.H.; Okamoto, R.J.; Li, D.Y.; Mecham, R.P. Effects of Elastin Haploinsufficiency on the Mechanical Behavior of Mouse Arteries. Am. J. Physiol. Heart Circ. Physiol. 2005, 289, H1209–H1217. [Google Scholar] [CrossRef] [PubMed]
- Pezet, M.; Jacob, M.-P.; Escoubet, B.; Gheduzzi, D.; Tillet, E.; Perret, P.; Huber, P.; Quaglino, D.; Vranckx, R.; Li, D.Y.; et al. Elastin Haploinsufficiency Induces Alternative Aging Processes in the Aorta. Rejuvenation Res. 2008, 11, 97–112. [Google Scholar] [CrossRef]
- Milan, D.J.; Jones, I.L.; Ellinor, P.T.; MacRae, C.A. In Vivo Recording of Adult Zebrafish Electrocardiogram and Assessment of Drug-Induced QT Prolongation. Am. J. Physiol. Heart Circ. Physiol. 2006, 291, H269–H273. [Google Scholar] [CrossRef] [PubMed]
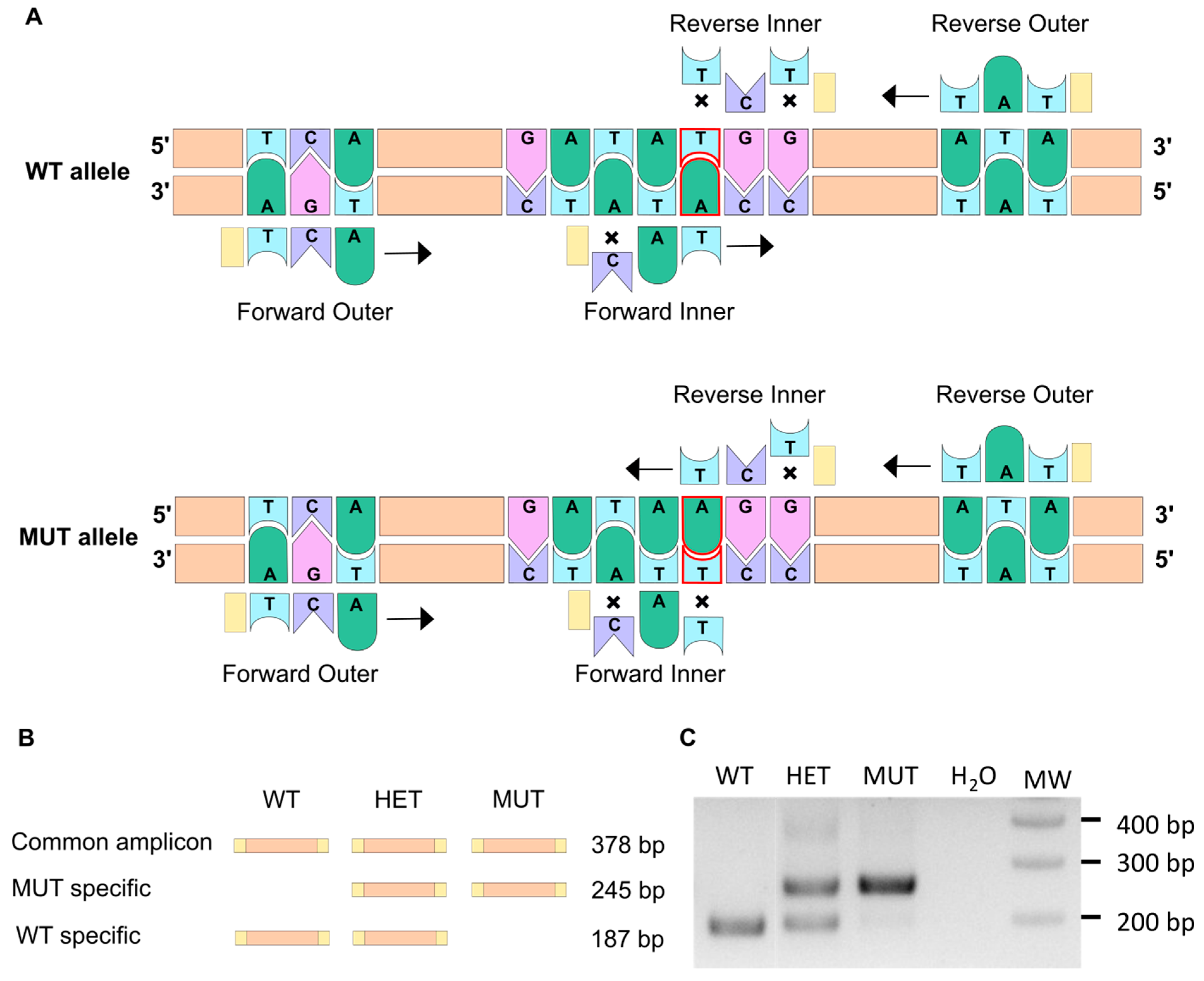

| Primer Sequence | Tm | |
|---|---|---|
| Forward outer | AATAATTCTCAATGGAGCCTAACTCCTCA | 65 |
| Reverse outer | TCCTAGAGGGAAACATGAATGCTCATAT | 65 |
| Forward inner | ACATATCTCTCTGGCTGTAGGTGGACAT | 65 |
| Reverse inner | CACCACCATATCCACCATAACCCTCT | 66 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hoareau, M.; El Kholti, N.; Debret, R.; Lambert, E. Characterization of the Zebrafish Elastin a (elnasa12235) Mutant: A New Model of Elastinopathy Leading to Heart Valve Defects. Cells 2023, 12, 1436. https://doi.org/10.3390/cells12101436
Hoareau M, El Kholti N, Debret R, Lambert E. Characterization of the Zebrafish Elastin a (elnasa12235) Mutant: A New Model of Elastinopathy Leading to Heart Valve Defects. Cells. 2023; 12(10):1436. https://doi.org/10.3390/cells12101436
Chicago/Turabian StyleHoareau, Marie, Naïma El Kholti, Romain Debret, and Elise Lambert. 2023. "Characterization of the Zebrafish Elastin a (elnasa12235) Mutant: A New Model of Elastinopathy Leading to Heart Valve Defects" Cells 12, no. 10: 1436. https://doi.org/10.3390/cells12101436
APA StyleHoareau, M., El Kholti, N., Debret, R., & Lambert, E. (2023). Characterization of the Zebrafish Elastin a (elnasa12235) Mutant: A New Model of Elastinopathy Leading to Heart Valve Defects. Cells, 12(10), 1436. https://doi.org/10.3390/cells12101436







