Effect of Poria cocos Mushroom Polysaccharides (PCPs) on the Quality and DNA Methylation of Cryopreserved Shanghai White Pig Spermatozoa
Abstract
:1. Introduction
2. Materials and Methods
2.1. Semen Collection
2.2. Semen Cryopreservation
2.3. Semen Assessment after Thawing
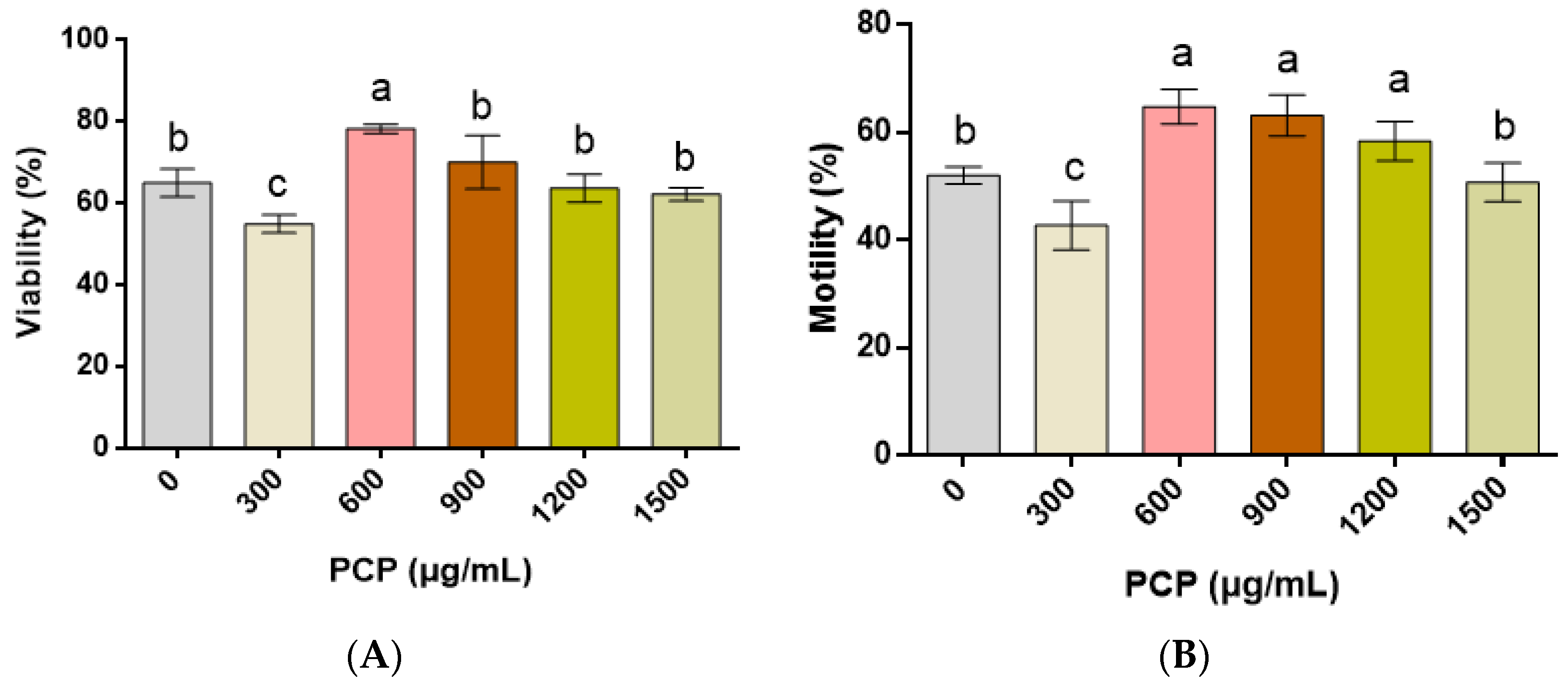
2.3.1. Viability and Motility of the Spermatozoa
2.3.2. Integrity of the Spermatozoa Acrosome
2.3.3. Integrity of the Spermatozoa Plasma Membranes
2.3.4. Mitochondrial Activity
2.3.5. Antioxidant Function
2.3.6. Spermatozoa DNA Methylation
2.4. Statistical Analysis
3. Results
3.1. Effect of PCPs on the Quality of Cryopreserved Spermatozoa
3.2. Effect of PCPs on the Antioxidant Function of Cryopreserved Spermatozoa
3.3. Effect of PCPs on the Spermatozoa DNA Methylation
4. Discussion
4.1. Effect of PCPs on the Quality of Cryopreserved Shanghai White Pig Spermatozoa
4.2. Effect of PCPs on the Antioxidant Function of Cryopreserved Shanghai White Pig Spermatozoa
4.3. Effect of PCPs on the DNA Methylation of Cryopreserved Shanghai White Pig Spermatozoa
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Roca, J.; Parrilla, I.; Bolarin, A.; Martinez, E.A.; Rodriguez-Martinez, H. Will AI in pigs become more efficient? Theriogenology 2016, 86, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Wu, C.; Xu, J.; Zhang, S.; Dai, J.; Zhang, D. Addition of butylated hydroxytoluene (BHT) in tris-based extender improves post-thaw quality and motion dynamics of dog spermatozoa. Cryobiology 2020, 97, 71–75. [Google Scholar] [CrossRef] [PubMed]
- Knox, R.V. The Fertility of Frozen Boar Sperm When used for Artificial Insemination. Reprod. Domest. Anim. 2015, 50, 90–97. [Google Scholar] [CrossRef] [PubMed]
- Reddy, N.S.S.; Mohanarao, G.J.; Atreja, S. Effects of adding taurine and trehalose to a tris-based egg yolk extender on buffalo (Bubalus bubalis) sperm quality following cryopreservation. Anim. Reprod. Sci. 2010, 119, 183–190. [Google Scholar] [CrossRef] [PubMed]
- Tunc, O.; Tremellen, K. Oxidative DNA damage impairs global sperm DNA methylation in infertile men. J. Assist. Reprod. Genet. 2009, 26, 537–544. [Google Scholar] [CrossRef]
- Li, X.; He, Y.; Zeng, P.; Liu, Y.; Zhang, M.; Hao, C.; Wang, H.; Lv, Z.; Zhang, L. Molecular basis for Poria cocos mushroom polysaccharide used as an antitumour drug in China. J. Cell. Mol. Med. 2019, 23, 4–20. [Google Scholar] [CrossRef]
- Tian, H.; Liu, Z.; Pu, Y.; Bao, Y. Immunomodulatory effects exerted by Poria Cocos polysaccharides via TLR4/TRAF6/NF-κB signaling in vitro and in vivo. Biomed. Pharmacother. 2019, 112, 108709. [Google Scholar] [CrossRef]
- Grieblová, A.; Pintus, E.; Ros-Santaella, J.L. Integrity of head and tail plasmalemma is associated with different kinetic variables in boar sperm. Anim. Reprod. Sci. 2017, 184, 218–227. [Google Scholar] [CrossRef]
- Jia, X.; Ma, L.; Li, P.; Chen, M.; He, C. Prospects of Poria cocos polysaccharides: Isolation process, structural features and bioactivities. Trends Food Sci. Technol. 2016, 54, 52–62. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, S.; Ren, Z. Effects of astragalus polysaccharides on the quality of frozen-thawed Tibetan boar spermatozoa and levels of genomic DNA methylation in the sperm. Thai J. Vet. Med. 2020, 50, 467–472. [Google Scholar]
- Pei, Y.; Yang, L.; Wu, L.; He, H.; Geng, G.; Xu, D.; Chen, H.; Li, Q. Combined effect of apigenin and ferulic acid on frozen-thawed boar sperm quality. Anim. Sci. J. 2018, 89, 956–965. [Google Scholar] [CrossRef]
- Zainuddin, Z.Z.; Tarmizi, M.R.M.; Yap, K.C.; Comizzoli, P.; Sipangkui, S. First Evaluations and Cryopreservation of Semen Samples from Sunda Clouded Leopards (Neofelis diardi). Animals 2020, 10, 1072. [Google Scholar] [CrossRef] [PubMed]
- Pursel, V.G.; Johnson, L.A. Freezing of Boar Spermatozoa: Fertilizing Capacity with Concentrated Semen and a New Thawing Procedure. J. Anim. Sci. 1975, 40, 99–102. [Google Scholar] [CrossRef] [PubMed]
- Shaoyong, W.; Li, Q.; Ren, Z.; Xiao, J.; Diao, Z.; Yang, G.; Pang, W. Effects of kojic acid on boar sperm quality and anti-bacterial activity during liquid preservation at 17 C. Theriogenology 2019, 140, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Flohe, L. Superoxide dismutase assays. In Methods in Enzymology; Academic press: Cambridge, MA, USA, 1984; Volume 105, pp. 93–104. [Google Scholar]
- Góth, L. A simple method for determination of serum catalase activity and revision of reference range. Clin. Chim. Acta 1991, 196, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Lawrence, R.A. Reprint of “Glutathione Peroxidase Activity in Selenium-Deficient Rat Liver”. Biochem. Biophys. Res. Commun. 2012, 425, 503–509. [Google Scholar] [CrossRef]
- Zhao, J.; Niu, X.; Yu, J.; Xiao, X.; Li, W.; Zang, L.; Hu, Z.; Ip, P.S.; Li, W. Poria cocos polysaccharides attenuated ox-LDL-induced inflammation and oxidative stress via ERK activated Nrf2/HO-1 signaling pathway and inhibited foam cell formation in VSMCs. Int. Immunopharmacol. 2020, 80, 106173. [Google Scholar] [CrossRef]
- Shen, T.; Jiang, Z.-L.; Liu, H.; Li, Q.-W. Effect of Salvia miltiorrhiza polysaccharides on boar spermatozoa during freezing–thawing. Anim. Reprod. Sci. 2015, 159, 25–30. [Google Scholar] [CrossRef]
- Yang, S.-M.; Wang, T.; Wen, D.-G.; Hou, J.-Q.; Li, H.-B. Protective effect of Rhodiola rosea polysaccharides on cryopreserved boar sperm. Carbohydr. Polym. 2016, 135, 44–47. [Google Scholar] [CrossRef]
- Yeste, M.; Estrada, E.; Pinart, E.; Bonet, S.; Miró, J.; Rodríguez-Gil, J.E. The improving effect of reduced glutathione on boar sperm cryotolerance is related with the intrinsic ejaculate freezability. Cryobiology 2014, 68, 251–261. [Google Scholar] [CrossRef]
- Zhang, S.; Li, Q.; Hu, J.; Jiang, Z.; Wu, C.; Zhang, T.; Zhang, D. Protective effect of rhodiola polysaccharides on freezing boar spermatozoa. J. Agric. Biotechnol. 2010, 18, 519–525. [Google Scholar]
- Sutkeviciene, N.; Riskeviciene, V.; Januskauskas, A.; Zilinskas, H.; Andersson, M. Assessment of sperm quality traits in relation to fertility in boar semen. Acta Vet. Scand. 2009, 51, 53. [Google Scholar] [CrossRef]
- Flores, E.; Fernández-Novell, J.; Peña, A.; Rigau, T.; Rodríguez-Gil, J. Cryopreservation-induced alterations in boar spermatozoa mitochondrial function are related to changes in the expression and location of midpiece mitofusin-2 and actin network. Theriogenology 2010, 74, 354–363. [Google Scholar] [CrossRef] [PubMed]
- Li, C.-Y.; Liu, L.; Zhao, Y.-W.; Chen, J.-Y.; Sun, X.-Y.; Ouyang, J.-M. Inhibition of Calcium Oxalate Formation and Antioxidant Activity of Carboxymethylated Poria cocos Polysaccharides. Oxidative Med. Cell. Longev. 2021, 2021, 6653593. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.; Shaoyong, W.; Li, Q.; Ma, L.; Xiao, J.; Jiao, J.; Yang, G.; Pang, W. Effects of Isatis root polysaccharide on boar sperm quality during liquid storage and in vitro fertilization. Anim. Reprod. Sci. 2019, 210, 106178. [Google Scholar] [CrossRef] [PubMed]
- Yan, B.; Zhang, X.; Wang, J.; Jia, S.; Zhou, Y.; Tian, J.; Wang, H.; Tang, Y. Inhibitory effect of Lycium barbarum polysaccharide on sperm damage during cryopreservation. Exp. Ther. Med. 2020, 20, 3051–3063. [Google Scholar] [CrossRef] [PubMed]
- Hu, J.-H.; Sun, X.-Z.; Li, Q.-W.; Zhang, T.; Hu, X.-C.; Hu, J.-H.; Wang, L.-Q. The effect of Laminaria japonic polysaccharide on sperm characteristics and biochemical parameters in cryopreserved boar sperm. Anim. Reprod. Sci. 2013, 139, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Satorre, M.; Breininger, E.; Beconi, M.; Beorlegui, N. α-Tocopherol modifies tyrosine phosphorylation and capacitation-like state of cryopreserved porcine sperm. Theriogenology 2007, 68, 958–965. [Google Scholar] [CrossRef]
- Xin, W.; Honghe, C.; Shemin, G.; Hongbin, L.; Yuehui, M.; Youmin, Z. Chinese miniature pigs^ genetic diversity by using micro-satellite marker. Xu Mu Shou Yi Xue Bao = Acta Vet. Et Zootech. Sin. 2002, 33, 530–532. [Google Scholar]
- Zamudio, N.; Barau, J.; Teissandier, A.; Walter, M.; Borsos, M.; Servant, N.; Bourc’His, D. DNA methylation restrains transposons from adopting a chromatin signature permissive for meiotic recombination. Genes Dev. 2015, 29, 1256–1270. [Google Scholar] [CrossRef]
- Capra, E.; Lazzari, B.; Turri, F.; Cremonesi, P.; Portela, A.M.R.; Ajmone-Marsan, P.; Stella, A.; Pizzi, F. Epigenetic analysis of high and low motile sperm populations reveals methylation variation in satellite regions within the pericentromeric position and in genes functionally related to sperm DNA organization and maintenance in Bos taurus. BMC Genom. 2019, 20, 940. [Google Scholar] [CrossRef] [PubMed]
- De Rycke, M.; Liebaers, I.; Van Steirteghem, A. Epigenetic risks related to assisted reproductive technologies: Risk analysis and epigenetic inheritance. Hum. Reprod. 2002, 17, 2487–2494. [Google Scholar] [CrossRef] [PubMed]
- Aurich, C.; Schreiner, B.; Ille, N.; Alvarenga, M.; Scarlet, D. Cytosine methylation of sperm DNA in horse semen after cryopreservation. Theriogenology 2016, 86, 1347–1352. [Google Scholar] [CrossRef] [PubMed]
- Yeste, M. Sperm cryopreservation update: Cryodamage, markers, and factors affecting the sperm freezability in pigs. Theriogenology 2016, 85, 47–64. [Google Scholar] [CrossRef] [PubMed]
- Ren, Z.L.; Song, T.Z.; Zhang, J.; Peng, W.C.; He, Z.Z.; Zhao, Y.L. Effect of rhodiola polysaccharide on frozen semen quality of Tibetan boar and levels of sperm genome DNA methylation. Chin. J. Vet. Sci. 2017, 37, 1385–1388. [Google Scholar]







| Target | Viability | Motility | Plasma Membrane Integrity | Acrosome Integrity | Mitochondrial Activity |
|---|---|---|---|---|---|
| Spermatozoa DNA methylation | −0.567 * | −0.553 * | −0.789 ** | −0.769 ** | −0.496 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, J.; Zhang, K.; Gao, J.; Xu, J.; Wu, C.; He, M.; Zhang, S.; Zhang, D.; Dai, J.; Sun, L. Effect of Poria cocos Mushroom Polysaccharides (PCPs) on the Quality and DNA Methylation of Cryopreserved Shanghai White Pig Spermatozoa. Cells 2023, 12, 1456. https://doi.org/10.3390/cells12111456
Zhou J, Zhang K, Gao J, Xu J, Wu C, He M, Zhang S, Zhang D, Dai J, Sun L. Effect of Poria cocos Mushroom Polysaccharides (PCPs) on the Quality and DNA Methylation of Cryopreserved Shanghai White Pig Spermatozoa. Cells. 2023; 12(11):1456. https://doi.org/10.3390/cells12111456
Chicago/Turabian StyleZhou, Jinyong, Keqin Zhang, Jun Gao, Jiehuan Xu, Caifeng Wu, Mengqian He, Shushan Zhang, Defu Zhang, Jianjun Dai, and Lingwei Sun. 2023. "Effect of Poria cocos Mushroom Polysaccharides (PCPs) on the Quality and DNA Methylation of Cryopreserved Shanghai White Pig Spermatozoa" Cells 12, no. 11: 1456. https://doi.org/10.3390/cells12111456





