Emerging Anti-Diabetic Drugs for Beta-Cell Protection in Type 1 Diabetes
Abstract
1. Introduction
2. Methods: A Description of Search Parameters, Results, and Choices Made for Exclusion or Inclusion
3. Investigational New Drugs with Effects on Beta Cells
3.1. Verapamil
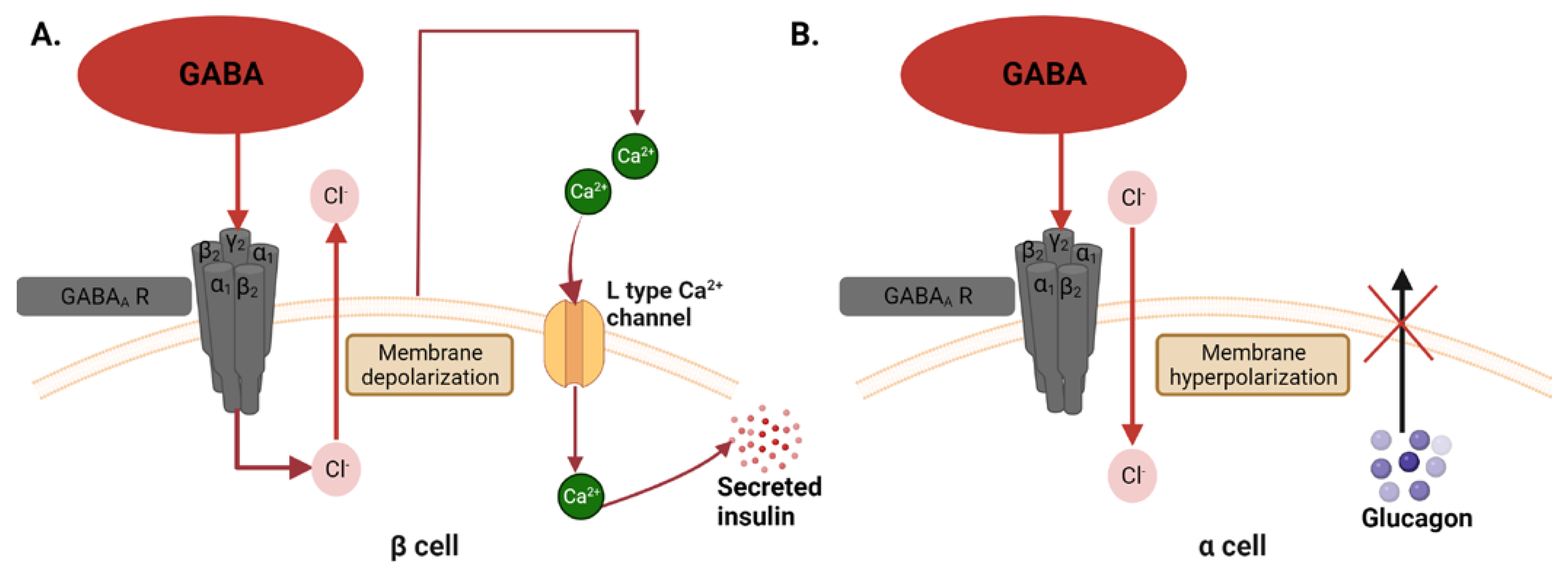
3.2. Gamma Aminobutyric Acid (GABA)
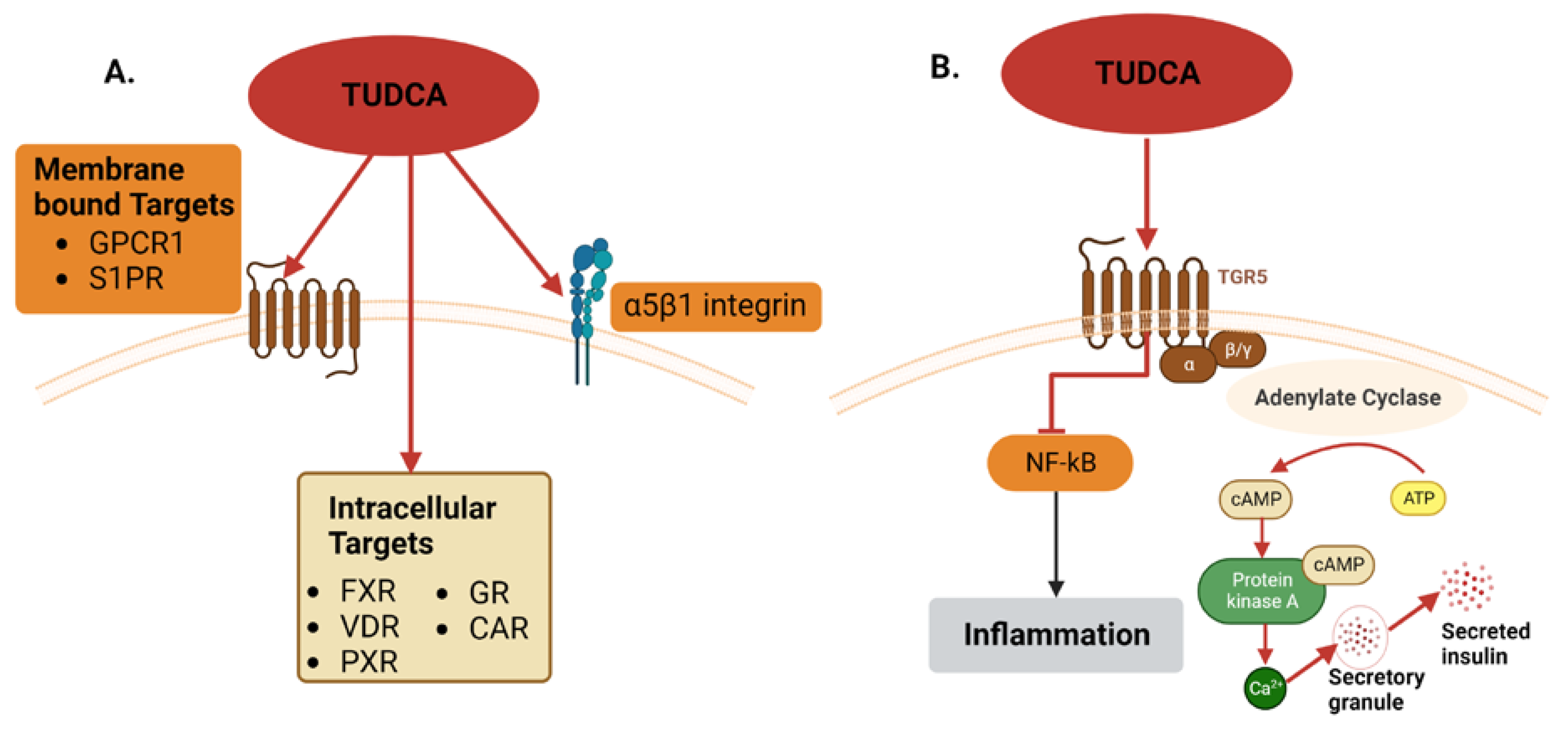
3.3. Tauroursodeoxycholic Acid (TUDCA)
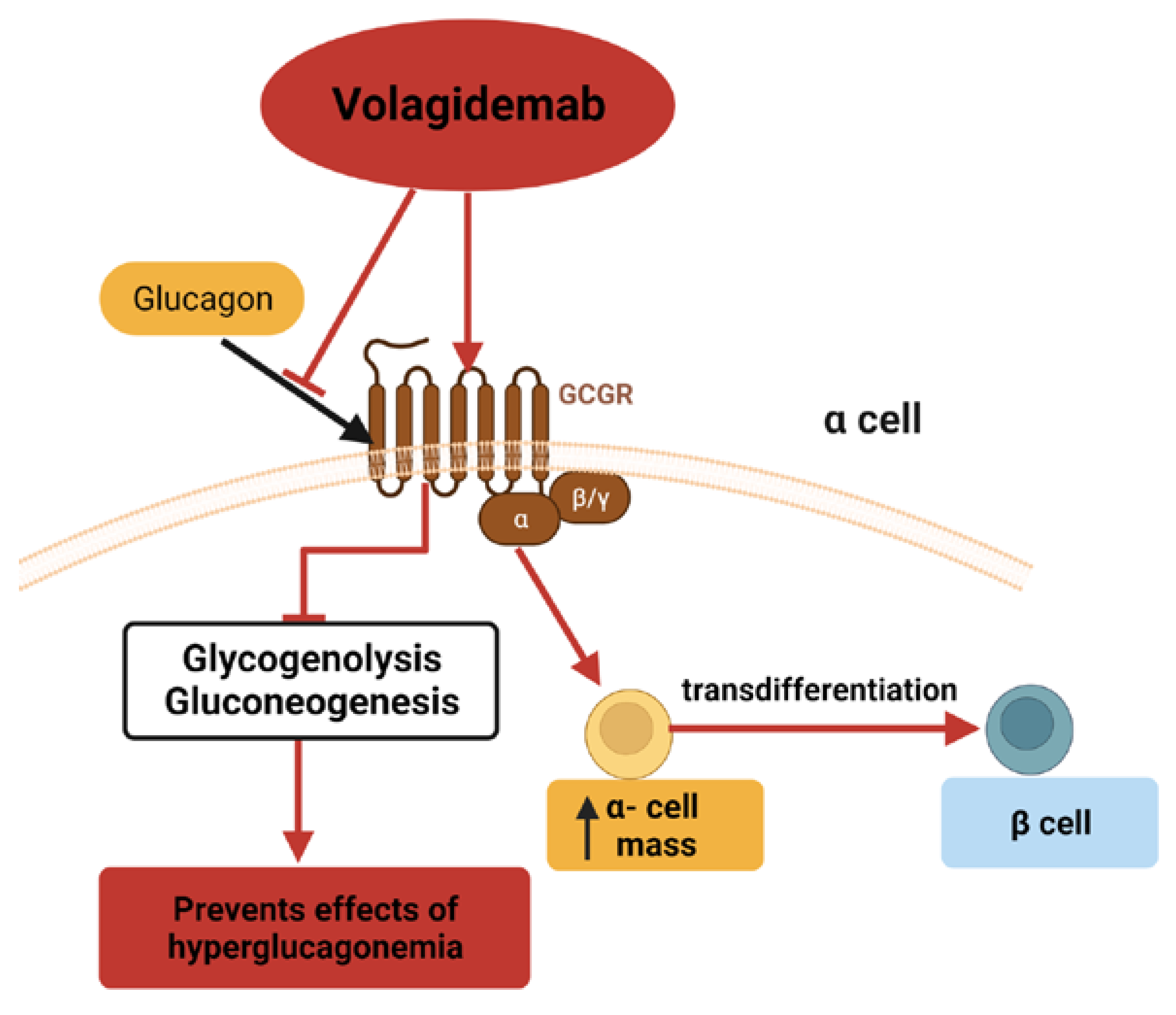
3.4. Volagidemab
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Draznin, B.; Aroda, V.R.; Bakris, G.; Benson, G.; Brown, F.M.; Freeman, R.; Green, J.; Huang, E.; Isaacs, D.; Kahan, S.; et al. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes—2022. Diabetes Care 2022, 45, S17–S38. [Google Scholar]
- Cai, Y.; Yan, J.; Gu, Y.; Chen, H.; Chen, Y.; Xu, X.; Zhang, M.; Yu, L.; Zheng, X.; Yang, T. Autoimmune Thyroid Disease Correlates to Islet Autoimmunity on Zinc Transporter 8 Autoantibody. Endocr. Connect. 2021, 10, 532–542. [Google Scholar] [CrossRef] [PubMed]
- Australia, D. Managing Type 1 Diabetes. 2021. Available online: https://www.diabetesaustralia.com.au/managing-diabetes/type-1/ (accessed on 19 May 2023).
- Haak, T.; Gölz, S.; Fritsche, A.; Füchtenbusch, M.; Siegmund, T.; Schnellbächer, E.; Klein, H.H.; Uebel, T.; Droßel, D. Therapy of Type 1 Diabetes. Exp. Clin. Endocrinol. Diabetes. 2019, 127 (Suppl. S1), S27–S38. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Orban, T.; Bundy, B.; Becker, D.J.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Greenbaum, C.J.; Marks, J.B.; Monzavi, R.; et al. Costimulation Modulation With Abatacept in Patients With Recent-Onset Type 1 Diabetes: Follow-up 1 Year After Cessation of Treatment. Diabetes Care 2014, 37, 1069–1075. [Google Scholar] [CrossRef][Green Version]
- Eizirik, D.L.; Colli, M.L.; Ortis, F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009, 5, 219–226. [Google Scholar] [CrossRef]
- Rewers, M.; Gottlieb, P. Immunotherapy for the prevention and treatment of type 1 diabetes: Human trials and a look into the future. Diabetes Care 2006, 32, 1769–1782. [Google Scholar] [CrossRef][Green Version]
- Bottazzo, G.F. Lawrence lecture. Death of a beta cell: Homicide or suicide? Diabet. Med. 1986, 3, 119–130. [Google Scholar] [CrossRef]
- Maganti, A.; Evans-Molina, C.; Mirmira, R. From immunobiology to β-cell biology: The changing perspective on type 1 diabetes. Islets 2014, 6, e28778. [Google Scholar] [CrossRef][Green Version]
- Chen, Y.-S.; Weng, S.-J.; Chang, S.-H.; Li, R.-Y.; Shane, G.-T.; Hsu, J.-P.; Yeh, S.-W.; Chang, A.-C.; Lee, M.-J.E. Evaluating the antidiabetic effects of R-verapamil in type 1 and type 2 diabetes mellitus mouse models. PLoS ONE 2021, 16, e0255405. [Google Scholar] [CrossRef]
- Klec, C.; Ziomek, G.; Pichler, M.; Malli, R.; Graier, W.F. Calcium Signaling in ß-cell Physiology and Pathology: A Revisit. Int. J. Mol. Sci. 2019, 20, 6110. [Google Scholar] [CrossRef][Green Version]
- Andersson, D.E.; Röjdmark, S. Blood glucose lowering effect of verapamil in fasted man. Horm. Metab. Res. 1984, 16 (Suppl. S1), 160–163. [Google Scholar] [CrossRef] [PubMed]
- Fadda, G.Z.; Hajjar, S.M.; Zhou, X.J.; Massry, S.G. Verapamil corrects abnormal metabolism of pancreatic islets and insulin secretion in phosphate depletion. Endocrinology 1992, 130, 193–202. [Google Scholar] [CrossRef] [PubMed]
- Jahan, I.; Corbin, K.L.; Bogart, A.M.; Whitticar, N.B.; Waters, C.D.; Schildmeyer, C.; Vann, N.W.; West, H.L.; Law, N.C.; Wiseman, J.S.; et al. Reducing Glucokinase Activity Restores Endogenous Pulsatility and Enhances Insulin Secretion in Islets From db/db Mice. Endocrinology 2018, 159, 3747–3760. [Google Scholar] [CrossRef][Green Version]
- Minn, A.H.; Pise-Masison, C.A.; Radonovich, M.; Brady, J.N.; Wang, P.; Kendziorski, C.; Shalev, A. Gene expression profiling in INS-1 cells overexpressing thioredoxin-interacting protein. Biochem. Biophys. Res. Commun. 2005, 336, 770–778. [Google Scholar] [CrossRef]
- Chen, J.; Fontes, G.; Saxena, G.; Poitout, V.; Shalev, A. Lack of TXNIP protects against mitochondria-mediated apoptosis but not against fatty acid-induced ER stress-mediated beta-cell death. Diabetes 2010, 59, 440–447. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chen, J.; Hui, S.T.; Couto, F.M.; Mungrue, I.N.; Davis, D.B.; Attie, A.D.; Lusis, A.J.; Davis, R.A.; Shalev, A. Thioredoxin-interacting protein deficiency induces Akt/Bcl-xL signaling and pancreatic beta-cell mass and protects against diabetes. FASEB J. 2008, 22, 3581–3594. [Google Scholar] [CrossRef][Green Version]
- Grunnet, L.G.; Aikin, R.; Tonnesen, M.F.; Paraskevas, S.; Blaabjerg, L.; Størling, J.; Rosenberg, L.; Billestrup, N.; Maysinger, D.; MandrupPoulsen, T. Proinflammatory cytokines activate the intrinsic apoptotic pathway in beta-cells. Diabetes 2009, 58, 1807–1815. [Google Scholar] [CrossRef][Green Version]
- Hong, K.; Xu, G.; Grayson, T.B.; Shalev, A. Cytokines Regulate β-Cell Thioredoxin-interacting Protein (TXNIP) via Distinct Mechanisms and Pathways. J. Biol. Chem. 2016, 291, 8428–8439. [Google Scholar] [CrossRef][Green Version]
- Xu, G.; Chen, J.; Jing, G.; Shalev, A. Preventing [Beta]-Cell Loss and Diabetes With Calcium Channel Blockers. Diabetes 2012, 61, 848. [Google Scholar] [CrossRef][Green Version]
- Ramadan, J.W.; Steiner, S.R.; O’Neill, C.M.; Nunemaker, C.S. The central role of calcium in the effects of cytokines on beta-cell function: Implications for type 1 and type 2 diabetes. Cell. Calcium. 2011, 50, 481–490. [Google Scholar] [CrossRef][Green Version]
- Bhosale, G.; Sharpe, J.A.; Sundier, S.Y.; Duchen, M.R. Calcium signaling as a mediator of cell energy demand and a trigger to cell death. Ann. N. Y. Acad. Sci. 2015, 1350, 107–116. [Google Scholar] [CrossRef]
- Zimmermann, P.; Aberer, F.; Eckstein, M.L.; Haupt, S.; Erlmann, M.P.; Moser, O. Verapamil and Its Role in Diabetes. Diabetology 2022, 3, 393–406. [Google Scholar] [CrossRef]
- Ovalle, F.; Grimes, T.; Xu, G.; Patel, A.J.; Grayson, T.B.; Thielen, L.A.; Li, P.; Shalev, A. Verapamil and beta cell function in adults with recent-onset type 1 diabetes. Nat. Med. 2018, 24, 1108–1112. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Grimes, T.D.; Grayson, T.B.; Chen, J.; Thielen, L.A.; Tse, H.M.; Li, P.; Kanke, M.; Lin, T.-T.; Schepmoes, A.A.; et al. Exploratory study reveals far reaching systemic and cellular effects of verapamil treatment in subjects with type 1 diabetes. Nat. Commun. 2022, 13, 1159. [Google Scholar] [CrossRef] [PubMed]
- Forlenza, G.P.; McVean, J.; Beck, R.W.; Bauza, C.; Bailey, R.; Buckingham, B.; DiMeglio, L.A.; Sherr, J.L.; Clements, M.; Neyman, A.; et al. Effect of Verapamil on Pancreatic Beta Cell Function in Newly Diagnosed Pediatric Type 1 Diabetes: A Randomized Clinical Trial. JAMA 2023, 329, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Boss, M.; Buitinga, M.; Jansen, T.J.P.; Brom, M.; Visser, E.P.; Gotthardt, M. PET-Based Human Dosimetry of 68Ga-NODAGA-Exendin-4, a Tracer for β-Cell Imaging. J. Nucl. Med. 2020, 61, 112–116. [Google Scholar] [CrossRef][Green Version]
- Choat, H.M.; Martin, A.; Mick, G.J.; Heath, K.E.; Hubert, M.T.; McGwin, G., Jr.; McCormick, K.L. Effect of gamma aminobutyric acid (GABA) or GABA with glutamic acid decarboxylase (GAD) on the progression of type 1 diabetes mellitus in children: Trial design and methodology. Contemp. Clin. Trials 2019, 82, 93–100. [Google Scholar] [CrossRef]
- Owens, D.F.; Kriegstein, A.R. Is there more to GABA than synaptic inhibition? Nat. Rev. Neurosci. 2002, 3, 715–727. [Google Scholar] [CrossRef]
- Represa, A.; Ben-Ari, Y. Trophic actions of GABA on neuronal development. Trends Neurosci. 2005, 28, 278–283. [Google Scholar] [CrossRef]
- Soltani, N.; Qiu, H.; Aleksic, M.; Glinka, Y.; Zhao, F.; Liu, R.; Li, Y.; Zhang, N.; Chakrabarti, R.; Ng, T.; et al. GABA exerts protective and regenerative effects on islet beta cells and reverses diabetes. Proc. Natl. Acad. Sci. USA 2011, 108, 11692–11697. [Google Scholar] [CrossRef][Green Version]
- Xu, E.; Kumar, M.; Zhang, Y.; Ju, W.; Obata, T.; Zhang, N.; Liu, S.; Wendt, A.; Deng, S.; Ebina, Y.; et al. Intra-islet insulin suppresses glucagon release via GABA-GABAA receptor system. Cell. Metab. 2006, 3, 47–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freese, J.; Al-Rawi, R.; Choat, H.; Martin, A.; Lunsford, A.; Tse, H.; Mick, G.; McCormick, K. Proinsulin to C-Peptide Ratio in the First Year After Diagnosis of Type 1 Diabetes. J. Clin. Endocrinol. Metab. 2021, 106, e4318–e4326. [Google Scholar] [CrossRef] [PubMed]
- Martin, A.; Mick, G.J.; Choat, H.M.; Lunsford, A.A.; Tse, H.M.; McGwin, G.G., Jr.; McCormick, K.L. A randomized trial of oral gamma aminobutyric acid (GABA) or the combination of GABA with glutamic acid decarboxylase (GAD) on pancreatic islet endocrine function in children with newly diagnosed type 1 diabetes. Nat. Commun. 2022, 13, 7928. [Google Scholar] [CrossRef] [PubMed]
- Espes, D.; Liljebäck, H.; Hill, H.; Elksnis, A.; Caballero-Corbalan, J.; Carlsson, P.-O. GABA induces a hormonal counter-regulatory response in subjects with long-standing type 1 diabetes. BMJ Open. Diabetes Res. Care 2021, 9, e002442. [Google Scholar] [CrossRef]
- Wherrett, D.K.; Bundy, B.; Becker, D.J.; DiMeglio, L.A.; Gitelman, S.E.; Goland, R.; Gottlieb, P.A.; Greenbaum, C.J.; Herold, K.C.; Marks, J.B.; et al. Antigen-based therapy with glutamic acid decarboxylase (GAD) vaccine in patients with recent-onset type 1 diabetes: A randomised double-blind trial. Lancet 2011, 378, 319–327. [Google Scholar] [CrossRef][Green Version]
- Vang, S.; Longley, K.; Steer, C.J.; Low, W.C. The Unexpected Uses of Urso- and Tauroursodeoxycholic Acid in the Treatment of Non-liver Diseases. Glob. Adv. Health Med. 2014, 3, 58–69. [Google Scholar] [CrossRef][Green Version]
- Kars, M.; Yang, L.; Gregor, M.F.; Mohammed, B.S.; Pietka, T.A.; Finck, B.N.; Patterson, B.W.; Horton, J.D.; Mittendorfer, B.; Hotamisligil, G.S.; et al. Tauroursodeoxycholic Acid May Improve Liver and Muscle but Not Adipose Tissue Insulin Sensitivity in Obese Men and Women. Diabetes 2010, 59, 1899–1905. [Google Scholar] [CrossRef][Green Version]
- Rosa, L.R.D.O.; Vettorazzi, J.F.; Zangerolamo, L.; Carneiro, E.M.; Barbosa, H.C.D.L. TUDCA receptors and their role on pancreatic beta cells. Prog. Biophys. Mol. Biol. 2021, 167, 26–31. [Google Scholar] [CrossRef]
- Zhu, Q.; Zhong, J.J.; Jin, J.F.; Yin, X.M. A chemical chaperone, prevents palmitate-induced apoptosis in pancreatic beta-cells by reducing ER stress. Clin. Endocrinol. Diabetes 2013, 121, 43–47. [Google Scholar]
- Vettorazzi, J.F.; Ribeiro, R.A.; Borck, P.C.; Branco, R.C.S.; Soriano, S.; Merino, B.; Boschero, A.C.; Nadal, A.; Quesada, I.; Carneiro, E.M. The bile acid TUDCA increases glucose-induced insulin secretion via the cAMP/PKA pathway in pancreatic beta cells. Metabolism 2016, 65, 54–63. [Google Scholar] [CrossRef]
- Lee, Y.Y.; Hong, S.H.; Lee, Y.J.; Chung, S.S.; Jung, H.S.; Park, S.G.; Park, K.S. Tauroursodeoxycholate (TUDCA), chemical chaperone, enhances function of islets by reducing ER stress. Biochem. Biophys. Res. Commun. 2010, 397, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Aung, M.H.; Prunty, M.C.; Hanif, A.M.; Hutson, L.M.; Boatright, J.H.; Pardue, M.T. Tauroursodeoxycholic Acid Protects Retinal and Visual Function in a Mouse Model of Type 1 Diabetes. Pharmaceutics 2021, 13, 1154. [Google Scholar] [CrossRef] [PubMed]
- McMillin, M.; DeMorrow, S. Effects of bile acids on neurological function and disease. FASEB J. 2016, 30, 3658–3668. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tersey, S.A.; Nishiki, Y.; Templin, A.T.; Cabrera, S.M.; Stull, N.D.; Colvin, S.C.; Evans-Molina, C.; Rickus, J.L.; Maier, B.; Mirmira, R.G. Islet β-Cell Endoplasmic Reticulum Stress Precedes the Onset of Type 1 Diabetes in the Nonobese Diabetic Mouse Model. Diabetes 2012, 61, 818–827. [Google Scholar] [CrossRef][Green Version]
- Engin, F.; Yermalovich, A.; Nguyen, T.; Hummasti, S.; Fu, W.; Eizirik, D.L.; Mathis, D.; Hotamisligil, G.S. Restoration of the Unfolded Protein Response in Pancreatic β Cells Protects Mice Against Type 1 Diabetes. Sci. Transl. Med. 2013, 5, 211ra156. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, J.; Zeng, C.; Gong, Q.-Y.; Yang, H.-B.; Li, X.-X.; Lei, G.-H.; Yang, T.-B. The association between dietary selenium intake and diabetes: A cross-sectional study among middle-aged and older adults. Nutr. J. 2015, 14, 18. [Google Scholar] [CrossRef][Green Version]
- Rayman, M.P. Selenium and human health. Lancet 2012, 379, 1256–1268. [Google Scholar] [CrossRef]
- Gul, F.; Ahmad, B.; Afzal, S.; Ullah, A.; Khan, S.; Aman, K.; Khan, M.T.; Hadi, F.; Kiran, K.; Zahra, M.; et al. Comparative analysis of various sources of selenium on the growth performance and antioxidant status in broilers under heat stress. Braz. J. Biol. 2021, 83, e251004. [Google Scholar] [CrossRef]
- Xing, D.; Zhou, Q.; Wang, Y.; Xu, J. Effects of Tauroursodeoxycholic Acid and 4-Phenylbutyric Acid on Selenium Distribution in Mice Model with Type 1 Diabetes. Biol. Trace Elem. Res. 2023, 201, 1205–1213. [Google Scholar] [CrossRef]
- Bronczek, G.A.; Vettorazzi, J.F.; Soares, G.M.; Kurauti, M.A.; dos Santos, C.; Bonfim, M.F.; Carneiro, E.M.; Balbo, S.L.; Boschero, A.C.; Júnior, J.M.C. The Bile Acid TUDCA Improves Beta-Cell Mass and Reduces Insulin Degradation in Mice With Early-Stage of Type-1 Diabetes. Front. Physiol. 2019, 10, 561. [Google Scholar] [CrossRef]
- Pearson, M.J.; Unger, R.H.; Holland, W.L. Clinical Trials, Triumphs, and Tribulations of Glucagon Receptor Antagonists. Diabetes Care 2016, 39, 1075–1077. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Scheen, A.J.; Paquot, N.; Lefèbvre, P.J. Investigational glucagon receptor antagonists in Phase I and II clinical trials for diabetes. Expert. Opin. Investig. Drugs 2017, 26, 1373–1389. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.-Y.; Yan, H.; Shi, Z.; Evans, M.R.; Yu, X.; Lee, Y.; Chen, S.; Williams, A.; Philippe, J.; Roth, M.G.; et al. Glucagon receptor antibody completely suppresses type 1 diabetes phenotype without insulin by disrupting a novel diabetogenic pathway. Proc. Natl. Acad. Sci. USA 2015, 112, 2503–2508. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lang, S.; Wei, R.; Wei, T.; Gu, L.; Feng, J.; Yan, H.; Yang, J.; Hong, T. Glucagon receptor antagonism promotes the production of gut proglucagon-derived peptides in diabetic mice. Peptides 2020, 131, 170349. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.; Yang, J.; Yang, K.; Gu, L.; Cui, X.; Wei, T.; Liu, J.; Le, Y.; Wang, H.; Wei, R.; et al. Glucagon receptor antagonist upregulates circulating GLP-1 level by promoting intestinal L-cell proliferation and GLP-1 production in type 2 diabetes. BMJ Open. Diabetes Res. Care 2020, 8, e001025. [Google Scholar] [CrossRef][Green Version]
- Sharma, A.X.; Quittner-Strom, E.B.; Lee, Y.; Johnson, J.A.; Martin, S.A.; Yu, X.; Li, J.; Lu, J.; Cai, Z.; Chen, S.; et al. Glucagon Receptor Antagonism Improves Glucose Metabolism and Cardiac Function by Promoting AMP-Mediated Protein Kinase in Diabetic Mice. Cell. Rep. 2018, 22, 1760–1773. [Google Scholar] [CrossRef][Green Version]
- Cui, X.; Feng, J.; Wei, T.; Gu, L.; Wang, D.; Lang, S.; Yang, K.; Yang, J.; Yan, H.; Wei, R.; et al. Pro-α-cell-derived β-cells contribute to β-cell neogenesis induced by antagonistic glucagon receptor antibody in type 2 diabetic mice. iScience 2022, 25, 104567. [Google Scholar] [CrossRef]
- Cui, X.; Feng, J.; Wei, T.; Zhang, L.; Lang, S.; Yang, K.; Yang, J.; Liu, J.; Sterr, M.; Lickert, H.; et al. Pancreatic alpha cell glucagon–liver FGF21 axis regulates beta cell regeneration in a mouse model of type 2 diabetes. Diabetologia 2023, 66, 535–550. [Google Scholar] [CrossRef]
- Gu, L.; Cui, X.; Lang, S.; Wang, H.; Hong, T.; Wei, R. Glucagon receptor antagonism increases mouse pancreatic δ-cell mass through cell proliferation and duct-derived neogenesis. Biochem. Biophys. Res. Commun. 2019, 512, 864–870. [Google Scholar] [CrossRef]
- Gu, L.; Wang, D.; Cui, X.; Wei, T.; Yang, K.; Yang, J.; Wei, R.; Hong, T. Combination of GLP-1 Receptor Activation and Glucagon Blockage Promotes Pancreatic β-Cell Regeneration In Situ in Type 1 Diabetic Mice. J. Diabetes Res. 2021, 2021, 7765623. [Google Scholar] [CrossRef]
- Wei, R.; Gu, L.; Yang, J.; Yang, K.; Liu, J.; Le, Y.; Lang, S.; Wang, H.; Thai, D.; Yan, H.; et al. Antagonistic Glucagon Receptor Antibody Promotes α-Cell Proliferation and Increases β-Cell Mass in Diabetic Mice. iScience 2019, 16, 326–339. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wei, T.; Cui, X.; Jiang, Y.; Wang, K.; Wang, D.; Li, F.; Lin, X.; Gu, L.; Yang, K.; Yang, J.; et al. Glucagon Acting at the GLP-1 Receptor Contributes to β-Cell Regeneration Induced by Glucagon Receptor Antagonism in Diabetic Mice. Diabetes 2023, 72, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Gao, C.; Ren, S.V.; Yu, J.; Baal, U.; Thai, D.; Lu, J.; Zeng, C.; Yan, H.; Wang, Y. Glucagon Receptor Antagonism Ameliorates Progression of Heart Failure. JACC Basic Transl. Sci. 2019, 4, 161–172. [Google Scholar] [CrossRef]
- Alessandro, P. Modulation of Glucagon Signaling. JACC Basic Transl. Sci. 2019, 4, 173–175. [Google Scholar]
- Okamoto, H.; Kim, J.; Aglione, J.; Lee, J.; Cavino, K.; Na, E.; Rafique, A.; Kim, J.H.; Harp, J.; Valenzuela, D.M.; et al. Glucagon Receptor Blockade With a Human Antibody Normalizes Blood Glucose in Diabetic Mice and Monkeys. Endocrinology 2015, 156, 2781–2794. [Google Scholar] [CrossRef][Green Version]
- Yan, H.; Gu, W.; Yang, J.; Bi, V.; Shen, Y.; Lee, E.; Winters, K.A.; Komorowski, R.; Zhang, C.; Patel, J.J.; et al. Fully Human Monoclonal Antibodies Antagonizing the Glucagon Receptor Improve Glucose Homeostasis in Mice and Monkeys. Experiment 2009, 329, 102–111. [Google Scholar] [CrossRef][Green Version]
- Pettus, J.; Reeds, D.; Cavaiola, T.S.; Boeder, S.; Levin, M.; Tobin, G.; Cava, E.; Thai, D.; Shi, J.; Yan, H.; et al. Effect of a glucagon receptor antibody (REMD-477) in type 1 diabetes: A randomized controlled trial. Diabetes, Obes. Metab. 2018, 20, 1302–1305. [Google Scholar] [CrossRef]
- Pettus, J.; Boeder, S.C.; Christiansen, M.P.; Denham, D.S.; Bailey, T.S.; Akturk, H.K.; Klaff, L.J.; Rosenstock, J.; Cheng, M.H.M.; Bode, B.W.; et al. Glucagon receptor antagonist volagidemab in type 1 diabetes: A 12-week, randomized, double-blind, phase 2 trial. Nat. Med. 2022, 28, 2092–2099. [Google Scholar] [CrossRef]

| Drug Name | Target | Key Effects | NCT Number (s) |
|---|---|---|---|
| Verapamil | Voltage-gated calcium channels |
| NCT02372253 NCT04545151 NCT04615910 NCT04233034 |
| Gamma aminobutyric acid (GABA) | GABA receptor |
| NCT01917760 NCT02002130 NCT03635437 NCT04375020 NCT00529399 NCT01561508 NCT01781884 NCT03721991 |
| Tauroursodeoxycholic acid (TUDCA) | Many receptors, TGR5possibly the main one |
| NCT00771901 (type 2 diabetes primarily) NCT02218619 |
| Volagidemab (REMD-477) | Antagonistic mono- clonal glucagon receptor antibody |
| NCT02715193 NCT03117998 NCT03919617 NCT04779645 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ajmal, N.; Bogart, M.C.; Khan, P.; Max-Harry, I.M.; Nunemaker, C.S. Emerging Anti-Diabetic Drugs for Beta-Cell Protection in Type 1 Diabetes. Cells 2023, 12, 1472. https://doi.org/10.3390/cells12111472
Ajmal N, Bogart MC, Khan P, Max-Harry IM, Nunemaker CS. Emerging Anti-Diabetic Drugs for Beta-Cell Protection in Type 1 Diabetes. Cells. 2023; 12(11):1472. https://doi.org/10.3390/cells12111472
Chicago/Turabian StyleAjmal, Nida, Maislin C. Bogart, Palwasha Khan, Ibiagbani M. Max-Harry, and Craig S. Nunemaker. 2023. "Emerging Anti-Diabetic Drugs for Beta-Cell Protection in Type 1 Diabetes" Cells 12, no. 11: 1472. https://doi.org/10.3390/cells12111472
APA StyleAjmal, N., Bogart, M. C., Khan, P., Max-Harry, I. M., & Nunemaker, C. S. (2023). Emerging Anti-Diabetic Drugs for Beta-Cell Protection in Type 1 Diabetes. Cells, 12(11), 1472. https://doi.org/10.3390/cells12111472








