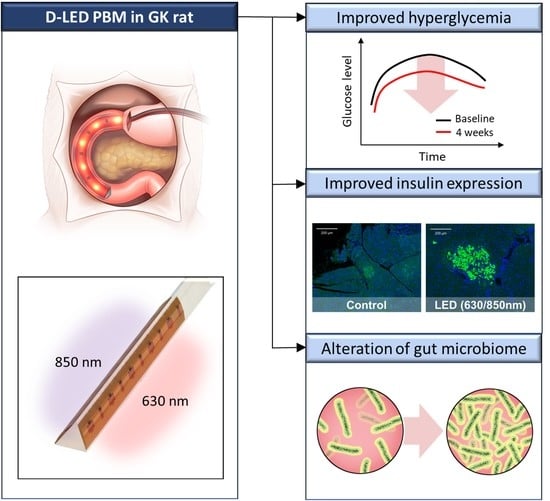
Duodenal Dual-Wavelength Photobiomodulation Improves Hyperglycemia and Hepatic Parameters with Alteration of Gut Microbiome in Type 2 Diabetes Animal Model
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Oral Glucose Tolerance Test (OGTT)
2.3. Biochemical Analysis
2.4. Histology
2.5. Microbiome Analysis
2.6. Statistical Analysis
3. Results
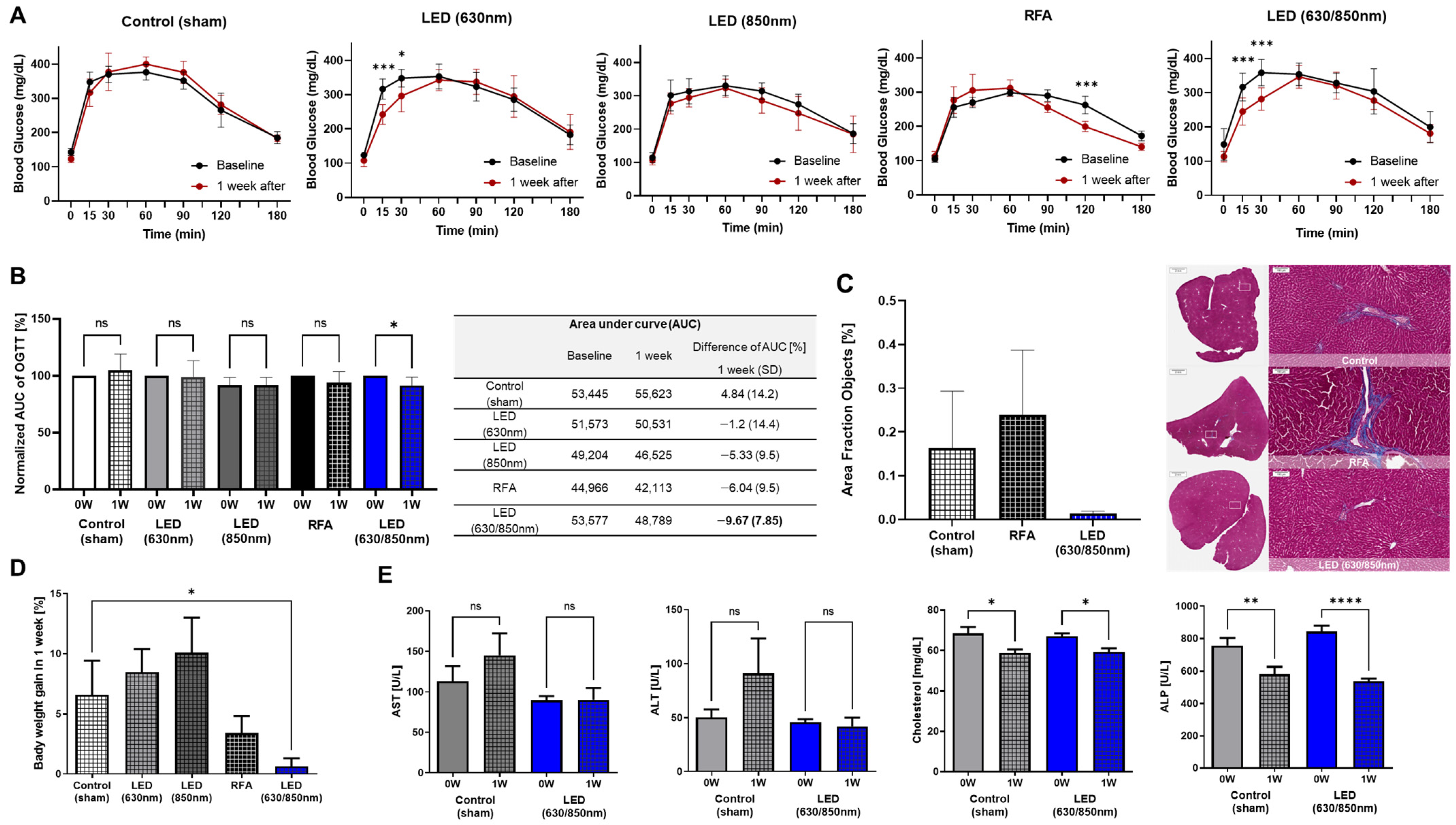
3.1. Baseline Characteristics of Duodenal PBM and Other Treatment Groups
3.2. Effects on Metabolic Parameters in the Pilot Study
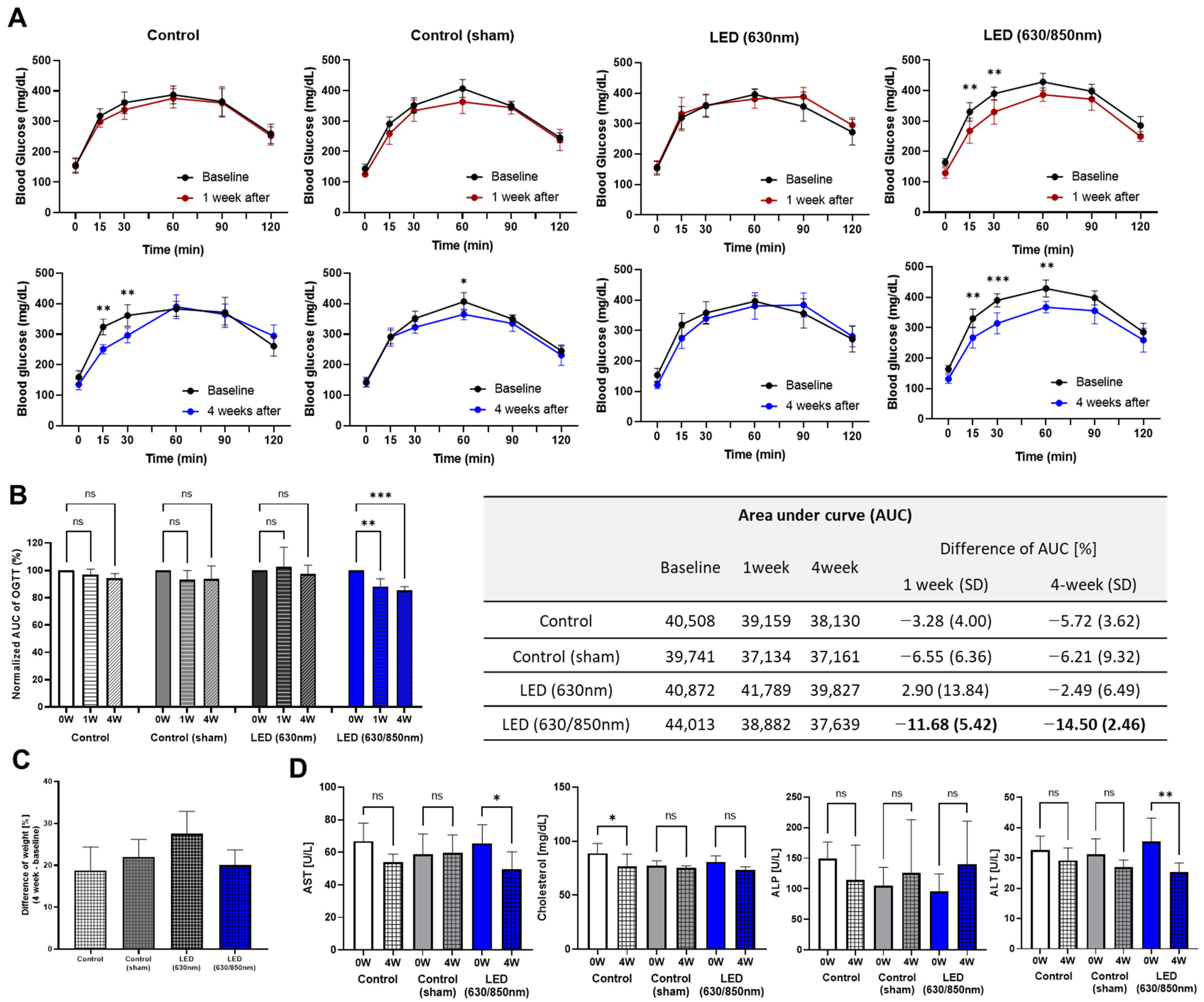
3.3. Effects on Metabolic Parameters in the 4-Week Follow-Up Study
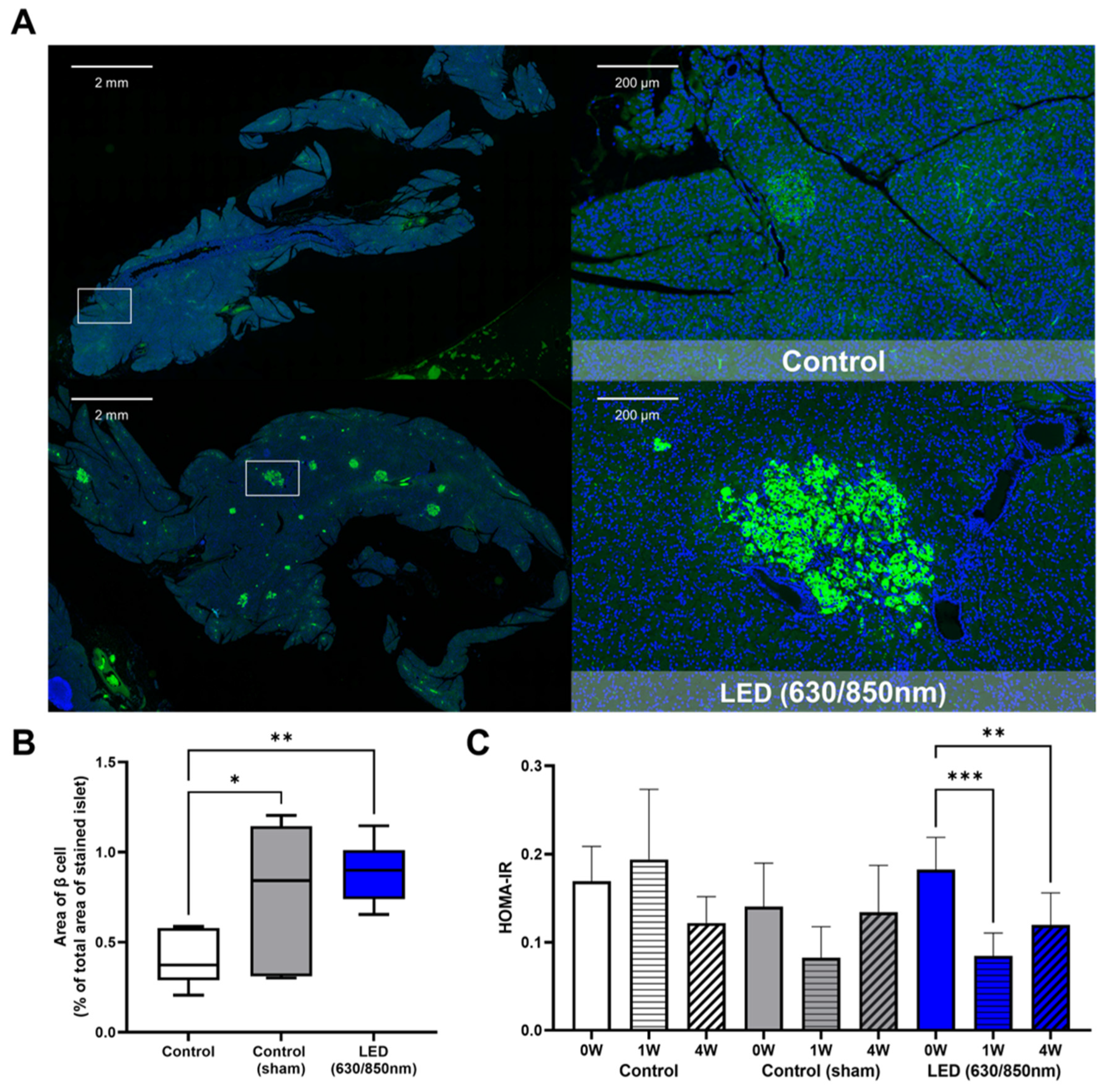
3.4. Effects on the Pancreatic Islets, Liver Fibrosis, and Duodenum in the 4-Week Follow-Up Study
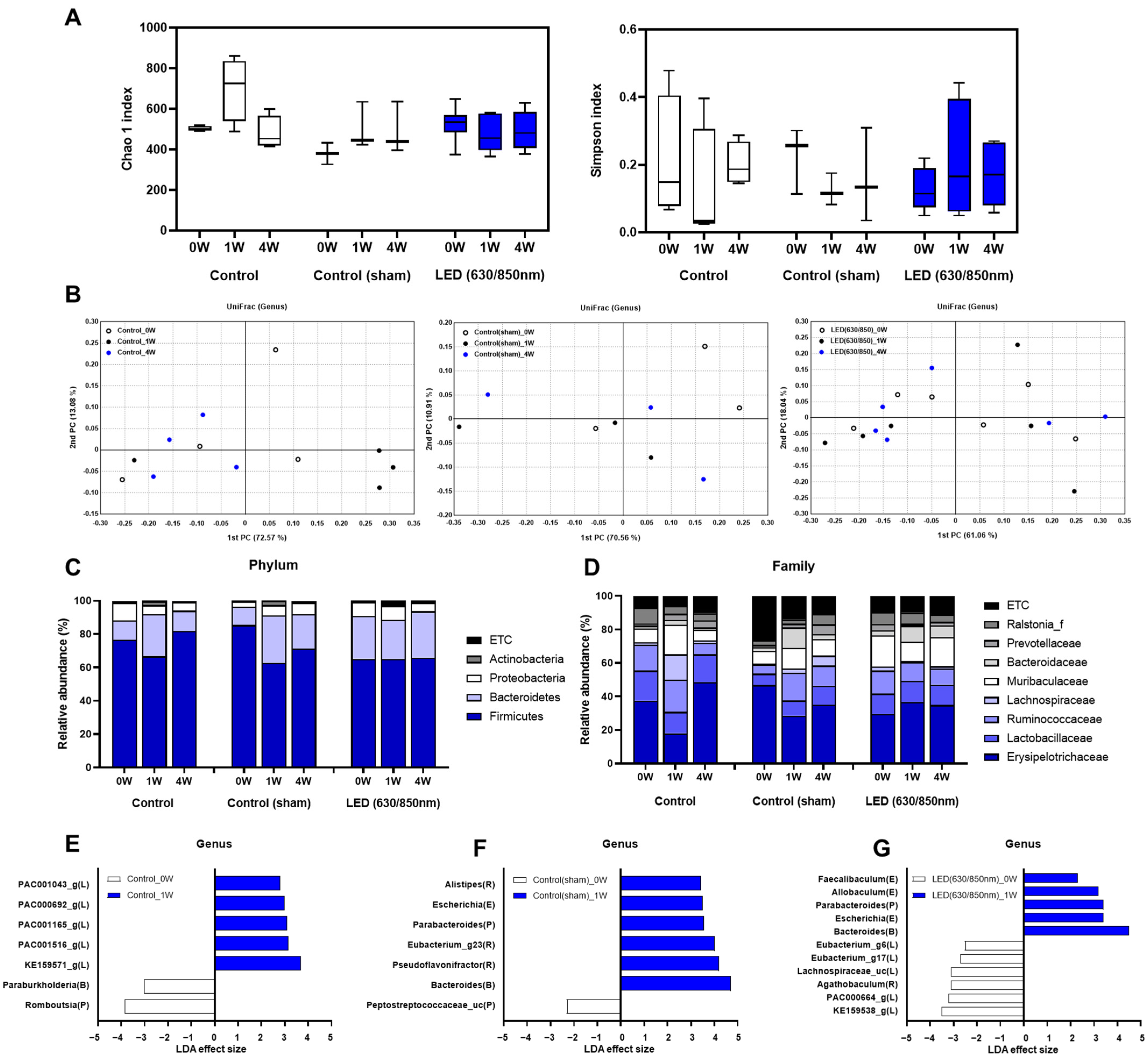
3.5. Composition of the Gut Microbiome in the 4-Week Follow-Up Study
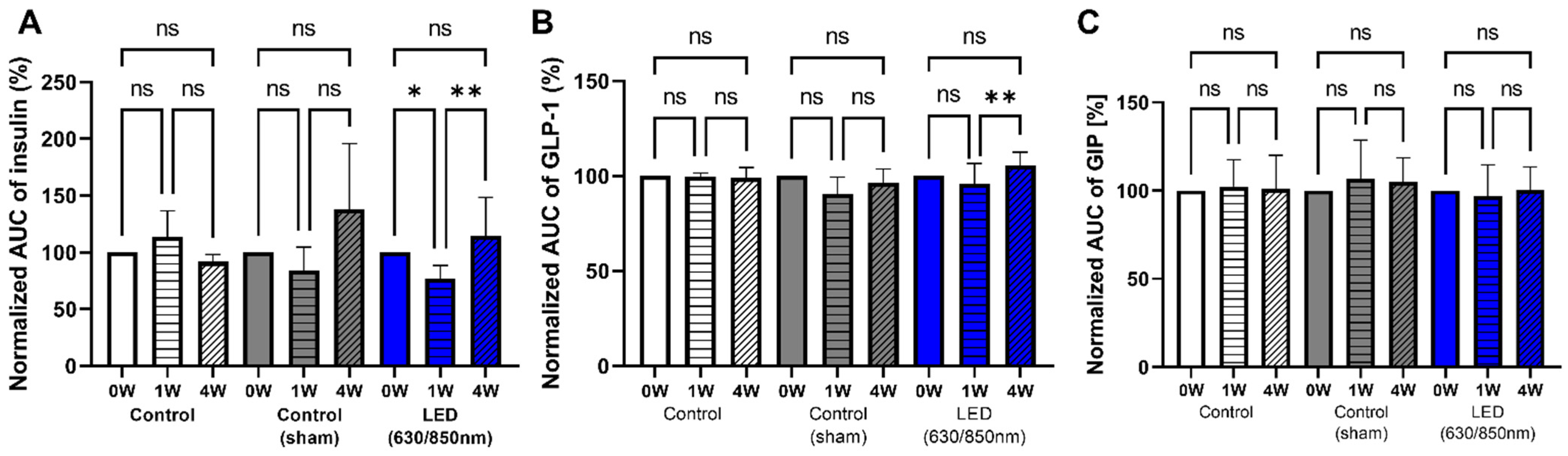
3.6. Association of Altered Gut Microbiome and GIP, GLP-1, Insulin Resistance in Weeks
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edelman, S.V.; Polonsky, W.H. Type 2 Diabetes in the Real World: The Elusive Nature of Glycemic Control. Diabetes Care 2017, 40, 1425–1432. [Google Scholar] [CrossRef] [PubMed]
- Carpio, G.R.A.; Fonseca, V.A. Update on Safety Issues Related to Antihyperglycemic Therapy. Diabetes Spectr. 2014, 27, 92–100. [Google Scholar] [CrossRef] [PubMed]
- Miras, A.D.; Le Roux, C.W. Mechanisms underlying weight loss after bariatric surgery. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 575–584. [Google Scholar] [CrossRef] [PubMed]
- American Diabetes Association. Professional Practice Committee: Standards of Medical Care in Diabetes—2022. Diabetes Care 2021, 45, S3. [Google Scholar]
- Sjöström, L.; Peltonen, M.; Jacobson, P.; Ahlin, S.; Andersson-Assarsson, J.; Anveden, Å.; Bouchard, C.; Carlsson, B.; Karason, K.; Lönroth, H.; et al. Association of bariatric surgery with long-term remission of type 2 diabetes and with microvascular and macrovascular complications. JAMA 2014, 311, 2297–2304. [Google Scholar] [CrossRef]
- Gniuli, D.; Calcagno, A.; Libera, L.D.; Calvani, R.; Leccesi, L.; Caristo, M.E.; Vettor, R.; Castagneto, M.; Ghirlanda, G.; Mingrone, G. High-fat feeding stimulates endocrine, glucose-dependent insulinotropic polypeptide (GIP)-expressing cell hyperplasia in the duodenum of Wistar rats. Diabetologia 2010, 53, 2233–2240. [Google Scholar] [CrossRef]
- Silva, G.; Ferraresi, C.; De Almeida, R.T.; Motta, M.L.; Paixão, T.; Ottone, V.O.; Fonseca, I.A.; Oliveira, M.X.; Rocha-Vieira, E.; Dias-Peixoto, M.F.; et al. Insulin resistance is improved in high-fat fed mice by photobiomodulation therapy at 630 nm. J. Biophotonics 2019, 13, e201960140. [Google Scholar] [CrossRef]
- Liebman, C.; Loya, S.; Lawrence, M.; Bashoo, N.; Cho, M. Stimulatory responses in α- and β-cells by near-infrared (810 nm) photobiomodulation. J. Biophotonics 2022, 15, e202100257. [Google Scholar] [CrossRef]
- Huang, H.-H.; Stillman, T.J.; Branham, L.A.; Williams, S.C. The Effects of Photobiomodulation Therapy on Porcine Islet Insulin Secretion. Photobiomodul. Photomed. Laser Surg. 2022, 40, 395–401. [Google Scholar] [CrossRef]
- Bonifacio, M.; Benfato, I.D.; Cruz, M.D.A.; de Sales, D.C.; Pandolfo, I.L.; Quintana, H.T.; Carvalho, C.P.D.F.; de Oliveira, C.A.M.; Renno, A.C.M. Effects of photobiomodulation on glucose homeostasis and morphometric parameters in pancreatic islets of diabetic mice. Lasers Med. Sci. 2022, 37, 1799–1809. [Google Scholar] [CrossRef]
- Tatmatsu-Rocha, J.C.; de Castro, C.A.; Sene-Fiorese, M.; Parizotto, N.A. Light-emitting diode modulates carbohydrate metabolism by pancreatic duct regeneration. Lasers Med. Sci. 2017, 32, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y.; Cho, E.A.; Bae, S.M.; Kim, S.Y.; Park, D.H. The effect of a newly developed mini-light-emitting diode catheter for interstitial photodynamic therapy in pancreatic cancer xenografts. J. Transl. Med. 2021, 19, 248. [Google Scholar] [PubMed]
- Kirino, I.; Fujita, K.; Sakanoue, K.; Sugita, R.; Yamagishi, K.; Takeoka, S.; Fujie, T.; Uemoto, S.; Morimoto, Y. Metronomic photodynamic therapy using an implantable LED device and orally administered 5-aminolevulinic acid. Sci. Rep. 2020, 10, 22017. [Google Scholar] [CrossRef] [PubMed]
- Fekrazad, R.; Mirmoezzi, A.; Kalhori, K.A.; Arany, P. The effect of red, green, and blue lasers on healing of oral wounds in diabetic rats. J. Photochem. Photobiol. B Biol. 2015, 148, 242–245. [Google Scholar]
- Dompe, C.; Moncrieff, L.; Matys, J.; Grzech-Leśniak, K.; Kocherova, I.; Bryja, A.; Bruska, M.; Dominiak, M.; Mozdziak, P.; Skiba, T.H.I.; et al. Photobiomodulation-Underlying Mechanism and Clinical Applications. J. Clin. Med. 2020, 9, 1724. [Google Scholar]
- Tao, L.; Liu, Q.; Zhang, F.; Fu, Y.; Zhu, X.; Weng, X.; Han, H.; Huang, Y.; Suo, Y.; Chen, L.; et al. Microglia modulation with 1070-nm light attenuates Aβ burden and cognitive impairment in Alzheimer’s disease mouse model. Light Sci. Appl. 2021, 10, 179. [Google Scholar] [PubMed]
- de Oliveira Francisco, C.; Beltrame, T.; Hughson, R.L.; Milan-Mattos, J.C.; Ferroli-Fabricio, A.M.; Benze, B.G.; Ferraresi, C.; Parizotto, N.A.; Bagnato, V.S.; Borghi-Silva, A.; et al. Effects of light-emitting diode therapy (LEDT) on cardiopulmonary and hemodynamic adjustments during aerobic exercise and glucose levels in patients with diabetes mellitus: A randomized, crossover, double-blind and placebo-controlled clinical trial. Complement. Ther. Med. 2019, 42, 178–183. [Google Scholar]
- Huang, Y.-Y.; Sharma, S.K.; Carroll, J.; Hamblin, M.R. Biphasic dose response in low level light therapy—An update. Dose Response 2011, 9, 602–618. [Google Scholar]
- Castro, K.M.R.; de Paiva Carvalho, R.L.; Junior, G.M.R.; Tavares, B.A.; Simionato, L.H.; Bortoluci, C.H.F.; Soto, C.A.T.; Ferraresi, C. Can photobiomodulation therapy (PBMT) control blood glucose levels and alter muscle glycogen synthesis? J. Photochem. Photobiol. B Biol. 2020, 207, 111877. [Google Scholar]
- Flores Luna, G.L.; de Andrade, A.L.M.; Brassolatti, P.; Bossini, P.S.; Anibal, F.D.F.; Parizotto, N.A.; Leal, Â.M.D.O. Biphasic Dose/Response of Photobiomodulation Therapy on Culture of Human Fibroblasts. Photobiomodul. Photomed. Laser Surg. 2020, 38, 413–418. [Google Scholar]
- Haidry, R.J.; van Baar, A.C.; Neto, M.P.G.; Rajagopalan, H.; Caplan, J.; Levin, P.S.; Bergman, J.J.; Rodriguez, L.; Deviere, J.; Thompson, C.C. Duodenal mucosal resurfacing: Proof-of-concept, procedural development, and initial implementation in the clinical setting. Gastrointest. Endosc. 2019, 90, 673–681.e2. [Google Scholar] [CrossRef]
- Van Baar, A.C.; Holleman, F.; Crenier, L.; Haidry, R.; Magee, C.; Hopkins, D.; Grunert, L.R.; Neto, M.G.; Vignolo, P.; Hayee, B.H.; et al. Endoscopic duodenal mucosal resurfacing for the treatment of type 2 diabetes mellitus: One year results from the first international, open-label, prospective, multicentre study. Gut 2020, 69, 295–303. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Zhang, Y.; Ye, Y.; Yao, W.; Xu, Z.; Xia, Z.; Wang, S.; Zhou, C. Clinical efficacy of low-temperature radiofrequency ablation of pharyngolaryngeal cyst in 84 Chinese infants. Medicine 2017, 96, e8237. [Google Scholar] [CrossRef]
- Koyama, M.; Wada, R.I.; Sakuraba, H.; Mizukami, H.; Yagihashi, S. Accelerated loss of islet beta cells in sucrose-fed Goto-Kakizaki rats, a genetic model of non-insulin-dependent diabetes mellitus. Am. J. Pathol. 1998, 153, 537–545. [Google Scholar] [CrossRef]
- Portha, B.; Giroix, M.-H.; Tourrel-Cuzin, C.; Le-Stunff, H.; Movassat, J. The GK rat: A prototype for the study of non-overweight type 2 diabetes. Methods Mol. Biol. 2012, 933, 125–159. [Google Scholar] [PubMed]
- Klausner, G.; Troussier, I.; Canova, C.-H.; Bensadoun, R.-J. Clinical use of photobiomodulation as a supportive care during radiation therapy. Support. Care Cancer 2022, 30, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Cancer.Net Editorial Board. Upper Endoscopy. 2019. Available online: https://www.cancer.net/navigating-cancer-care/diagnosing-cancer/tests-and-procedures/upper-endoscopy (accessed on 1 September 2022).
- Instruction Manual, FastDNATM SPIN Kit for Feces. MP Biomedicals. 2019. Available online: https://media.mpbio.com/document/file/manual/dest/f/a/s/t/d/FastDNA_SPIN_Kit_for_Feces_Kit_Manual.pdf (accessed on 1 September 2022).
- Segata, N.; Izard, J.; Waldron, L.; Gevers, D.; Miropolsky, L.; Garrett, W.S.; Huttenhower, C. Metagenomic biomarker discovery and explanation. Genome Biol. 2011, 12, R60. [Google Scholar] [CrossRef]
- Miskelly, M.G.; Shcherbina, L.; Fischer, A.-H.T.; Abels, M.; Lindqvist, A.; Wierup, N. GK-rats respond to gastric bypass surgery with improved glycemia despite unaffected insulin secretion and beta cell mass. Peptides 2021, 136, 170445. [Google Scholar] [CrossRef]
- Magalhães, F.D.C.; Ferraresi, C. Photobiomodulation Therapy on the Treatment of Insulin Resistance: A Narrative Review. Photobiomodul. Photomed. Laser Surg. 2022, 40, 597–603. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, B.; Liebert, A.; Johnstone, D.; Kiat, H. Photobiomodulation of the microbiome: Implications for metabolic and inflammatory diseases. Lasers Med. Sci. 2019, 34, 317–327. [Google Scholar] [CrossRef]
- Chen, Q.; Wu, J.; Dong, X.; Yin, H.; Shi, X.; Su, S.; Che, B.; Li, Y.; Yang, J. Gut flora-targeted photobiomodulation therapy improves senile dementia in an Aß-induced Alzheimer’s disease animal model. J. Photochem. Photobiol. B Biol. 2021, 216, 112152. [Google Scholar] [CrossRef] [PubMed]
- Oh, T.J.; Ahn, C.H.; Cho, Y.M. Contribution of the distal small intestine to metabolic improvement after bariatric/metabolic surgery: Lessons from ileal transposition surgery. J. Diabetes Investig. 2016, 7 (Suppl. 1), 94–101. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Qin, J.; Li, Y.; Cai, Z.; Li, S.; Zhu, J.; Zhang, F.; Liang, S.; Zhang, W.; Guan, Y.; Shen, D.; et al. A metagenome-wide association study of gut microbiota in type 2 diabetes. Nature 2012, 490, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Bicknell, B.; Laakso, E.-L.; Liebert, A.; Kiat, H. Modifying the Microbiome as a Potential Mechanism of Photobiomodulation: A Case Report. Photobiomodul. Photomed. Laser Surg. 2022, 40, 88–97. [Google Scholar] [CrossRef]
- Yang, J.-Y.; Lee, Y.-S.; Kim, Y.; Lee, S.-H.; Ryu, S.; Fukuda, S.; Hase, K.; Yang, C.-S.; Lim, H.S.; Kim, M.-S.; et al. Gut commensal Bacteroides acidifaciens prevents obesity and improves insulin sensitivity in mice. Mucosal Immunol. 2017, 10, 104–116. [Google Scholar] [CrossRef] [PubMed]
- Arora, T.; Seyfried, F.; Docherty, N.G.; Tremaroli, V.; Le Roux, C.W.; Perkins, R.; Bäckhed, F. Diabetes-associated microbiota in fa/fa rats is modified by Roux-en-Y gastric bypass. ISME J. 2017, 11, 2035–2046. [Google Scholar] [CrossRef] [PubMed]
- Kang, X.; Zhan, L.-B.; Lu, X.-G.; Song, J.-B.; Zhong, Y.; Wang, Y.; Yang, Y.-L.; Fan, Z.-W.; Jiang, X.Z.; Sun, R. Characteristics of Gastric Microbiota in GK Rats with Spontaneous Diabetes: A Comparative Study. Diabetes Metab. Syndr. Obes. 2020, 13, 1435–1447. [Google Scholar] [CrossRef]
- Gastaldelli, A.; Cusi, K. From NASH to diabetes and from diabetes to NASH: Mechanisms and treatment options. JHEP Rep. 2019, 1, 312–328. [Google Scholar] [CrossRef]
- Joo, J.; Choi, G.-M.; Lee, C.; Eom, Y.-S.; Kye, I.-S.; Jang, K.-S.; Hwang, S.T.; Kim, J.D.; Choi, K.-S. Mini LED array transferred onto a flexible substrate using Simultaneous Transfer and Bonding (SITRAB) process and Anisotropic Solder Film (ASF). In Proceedings of the 2022 IEEE 72nd Electronic Components and Technology Conference (ECTC), Grande Lakes, FL, USA, 30 May–2 June 2022. [Google Scholar]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Min, S.H.; Kwon, J.; Do, E.-J.; Kim, S.H.; Kim, E.S.; Jeong, J.-Y.; Bae, S.M.; Kim, S.-Y.; Park, D.H. Duodenal Dual-Wavelength Photobiomodulation Improves Hyperglycemia and Hepatic Parameters with Alteration of Gut Microbiome in Type 2 Diabetes Animal Model. Cells 2022, 11, 3490. https://doi.org/10.3390/cells11213490
Min SH, Kwon J, Do E-J, Kim SH, Kim ES, Jeong J-Y, Bae SM, Kim S-Y, Park DH. Duodenal Dual-Wavelength Photobiomodulation Improves Hyperglycemia and Hepatic Parameters with Alteration of Gut Microbiome in Type 2 Diabetes Animal Model. Cells. 2022; 11(21):3490. https://doi.org/10.3390/cells11213490
Chicago/Turabian StyleMin, Se Hee, Jinhee Kwon, Eun-Ju Do, So Hee Kim, Eun Sil Kim, Jin-Yong Jeong, Sang Mun Bae, Sang-Yeob Kim, and Do Hyun Park. 2022. "Duodenal Dual-Wavelength Photobiomodulation Improves Hyperglycemia and Hepatic Parameters with Alteration of Gut Microbiome in Type 2 Diabetes Animal Model" Cells 11, no. 21: 3490. https://doi.org/10.3390/cells11213490
APA StyleMin, S. H., Kwon, J., Do, E.-J., Kim, S. H., Kim, E. S., Jeong, J.-Y., Bae, S. M., Kim, S.-Y., & Park, D. H. (2022). Duodenal Dual-Wavelength Photobiomodulation Improves Hyperglycemia and Hepatic Parameters with Alteration of Gut Microbiome in Type 2 Diabetes Animal Model. Cells, 11(21), 3490. https://doi.org/10.3390/cells11213490