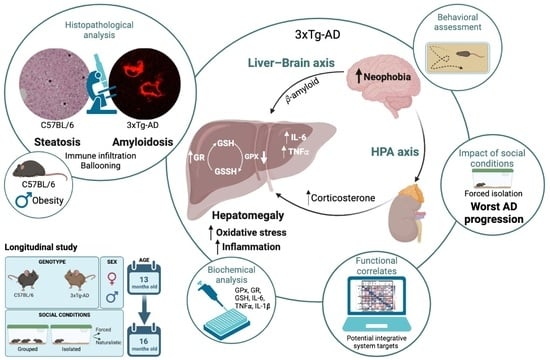
Hepatic Oxi-Inflammation and Neophobia as Potential Liver–Brain Axis Targets for Alzheimer’s Disease and Aging, with Strong Sensitivity to Sex, Isolation, and Obesity
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
2.2. Extrinsic Factors: Naturalistic and Forced Social Isolation
2.3. Longitudinal Behavioral Assessment
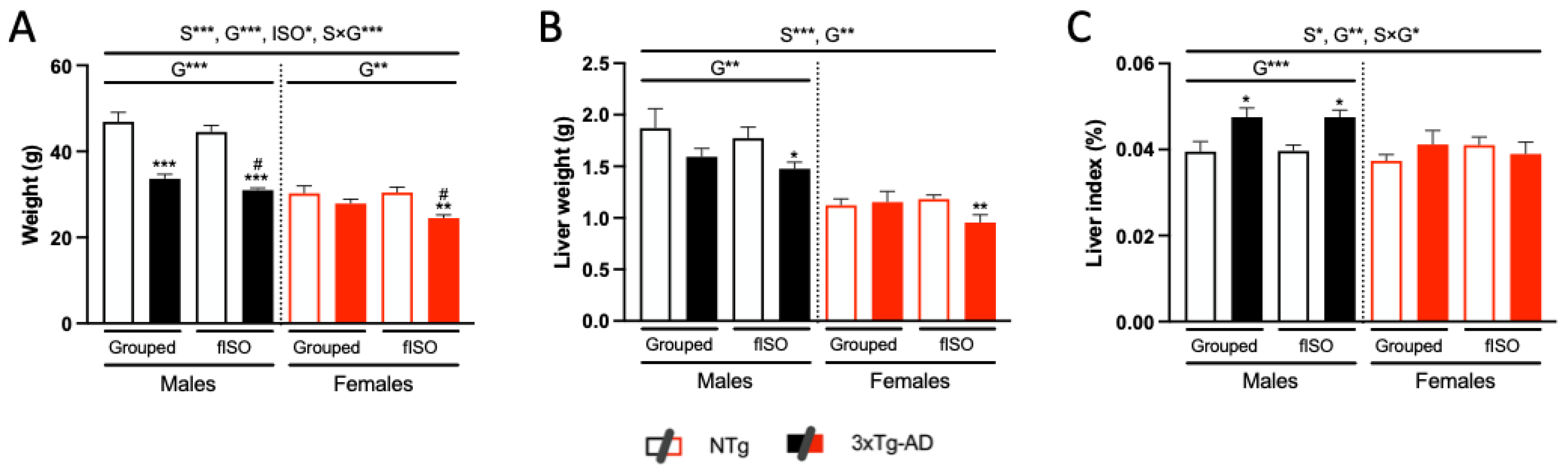
2.4. Physical Status: Body Weight and Liver Index
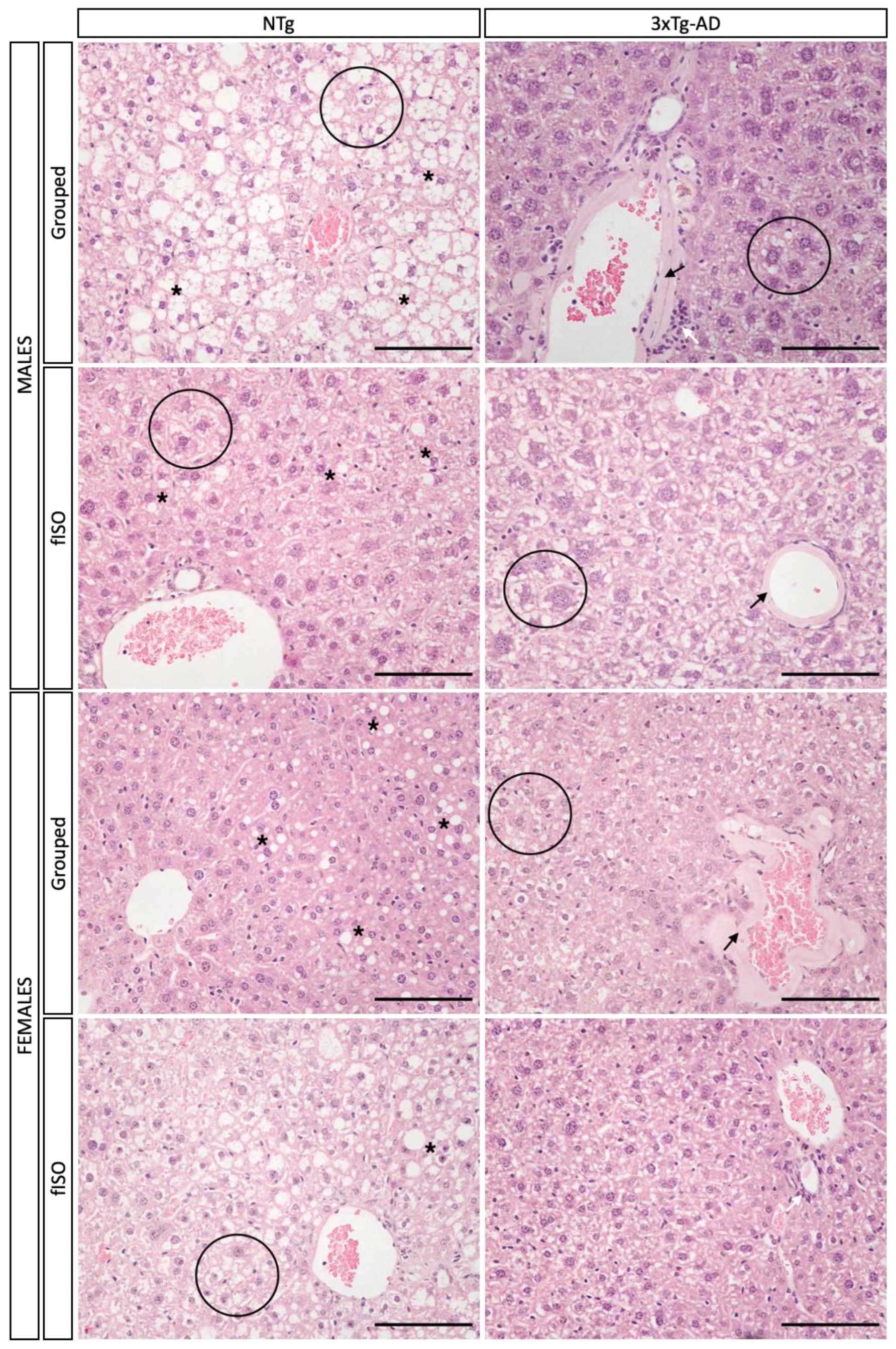
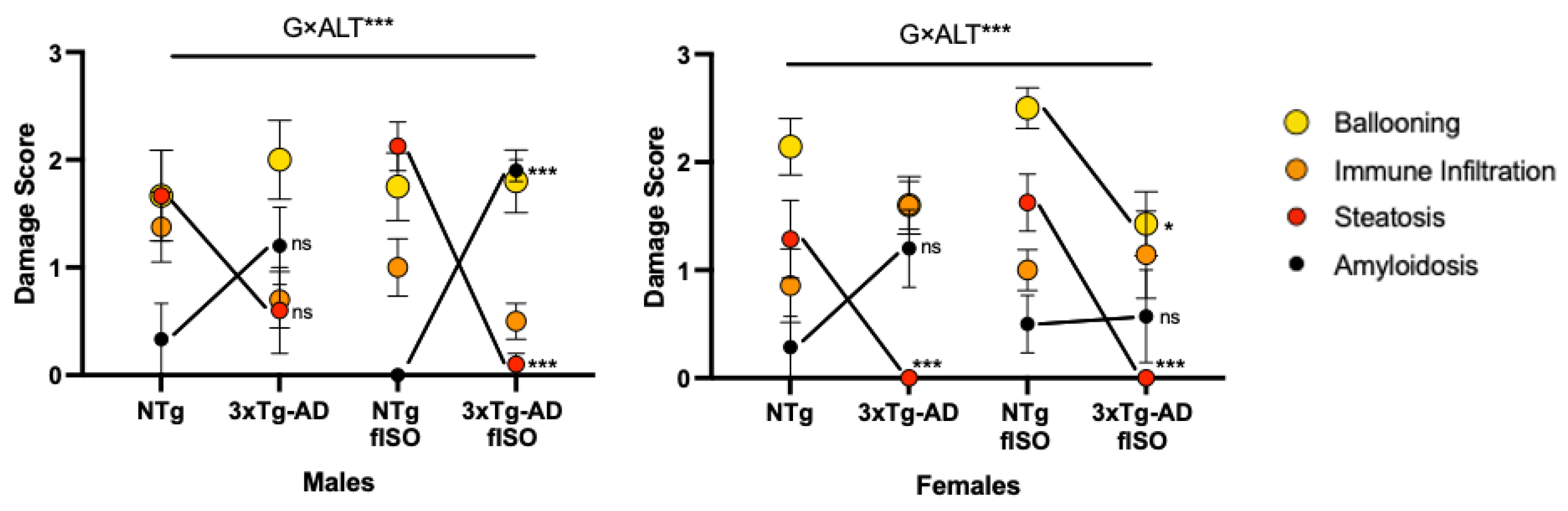
2.5. Histopathological Assessment
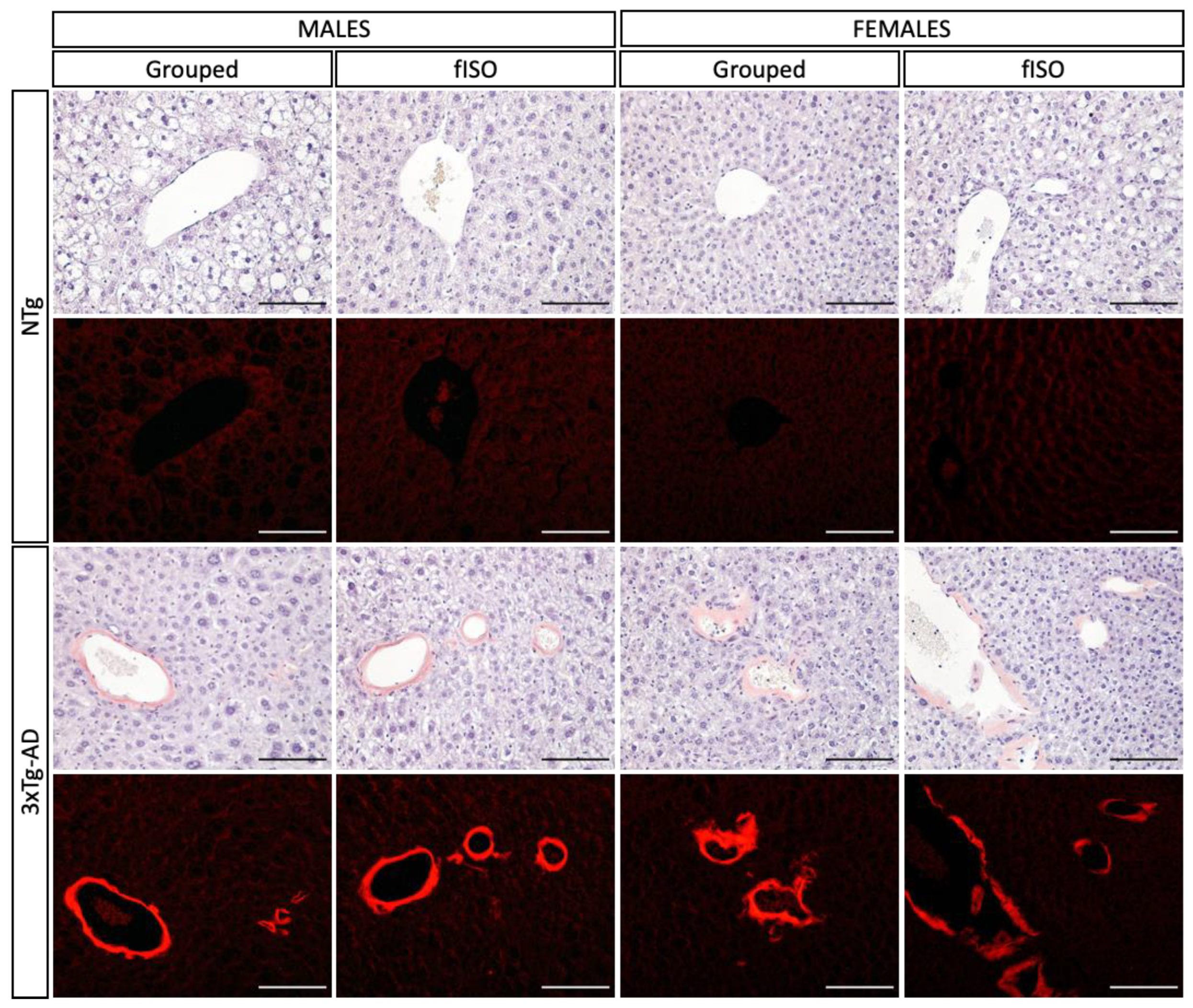
2.5.1. Morphological and Structural Analysis with H&E
2.5.2. Congo Red Staining for Amyloidosis
2.6. Biochemical Analysis
2.6.1. HPA Axis: Glucocorticoids Measurements
2.6.2. Hepatic Oxidative Stress
2.6.3. Hepatic Inflammation
2.7. Functional Correlates
2.8. Statistics
3. Results
3.1. Longitudinal Behavioral Assessment
3.2. Physical Status: Body Weight and Liver Index
3.3. Histopathological Assessment: H&E and Congo Red Staining
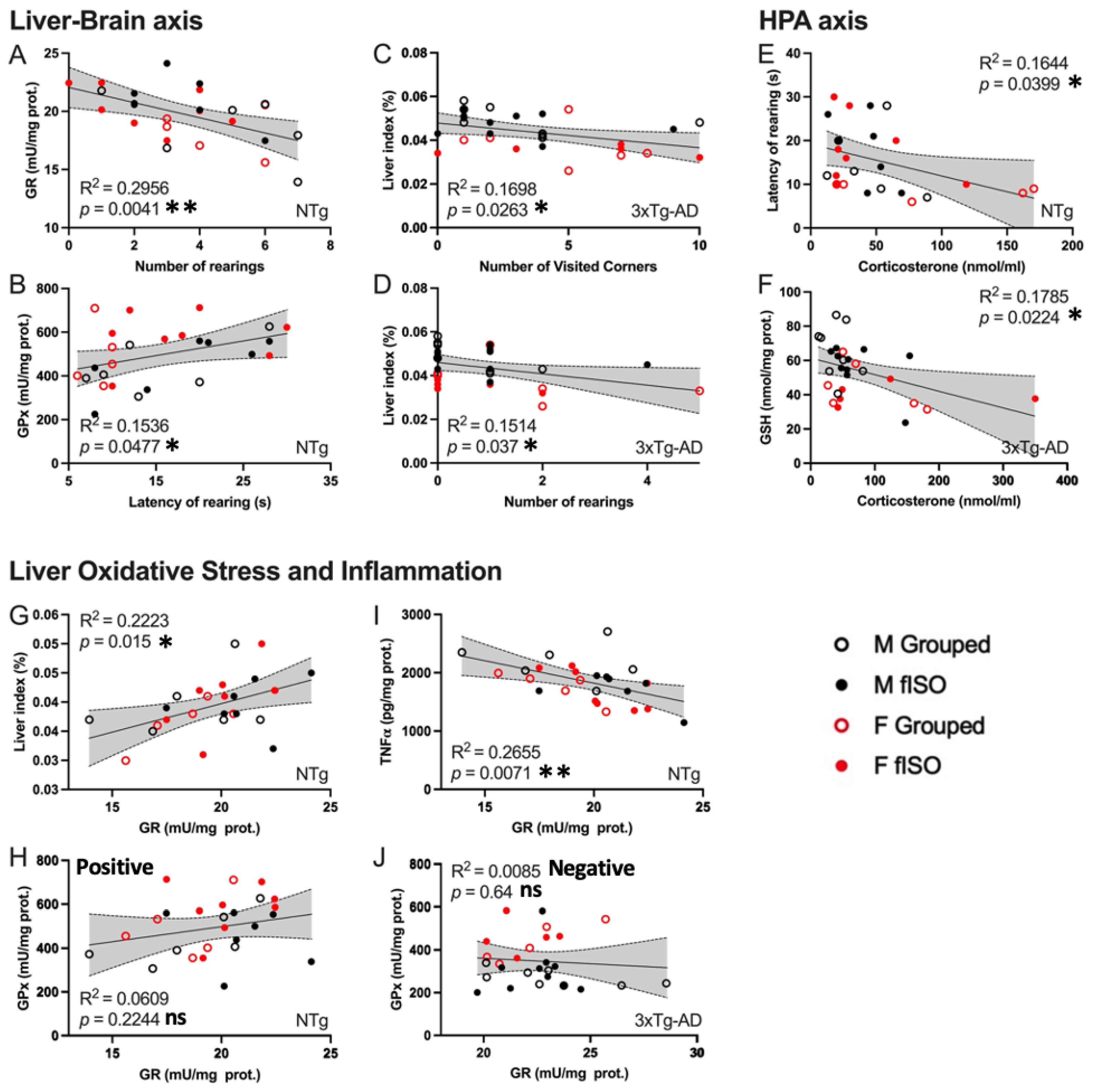
3.4. Biochemical Status of the Liver–Brain and HPA Axis
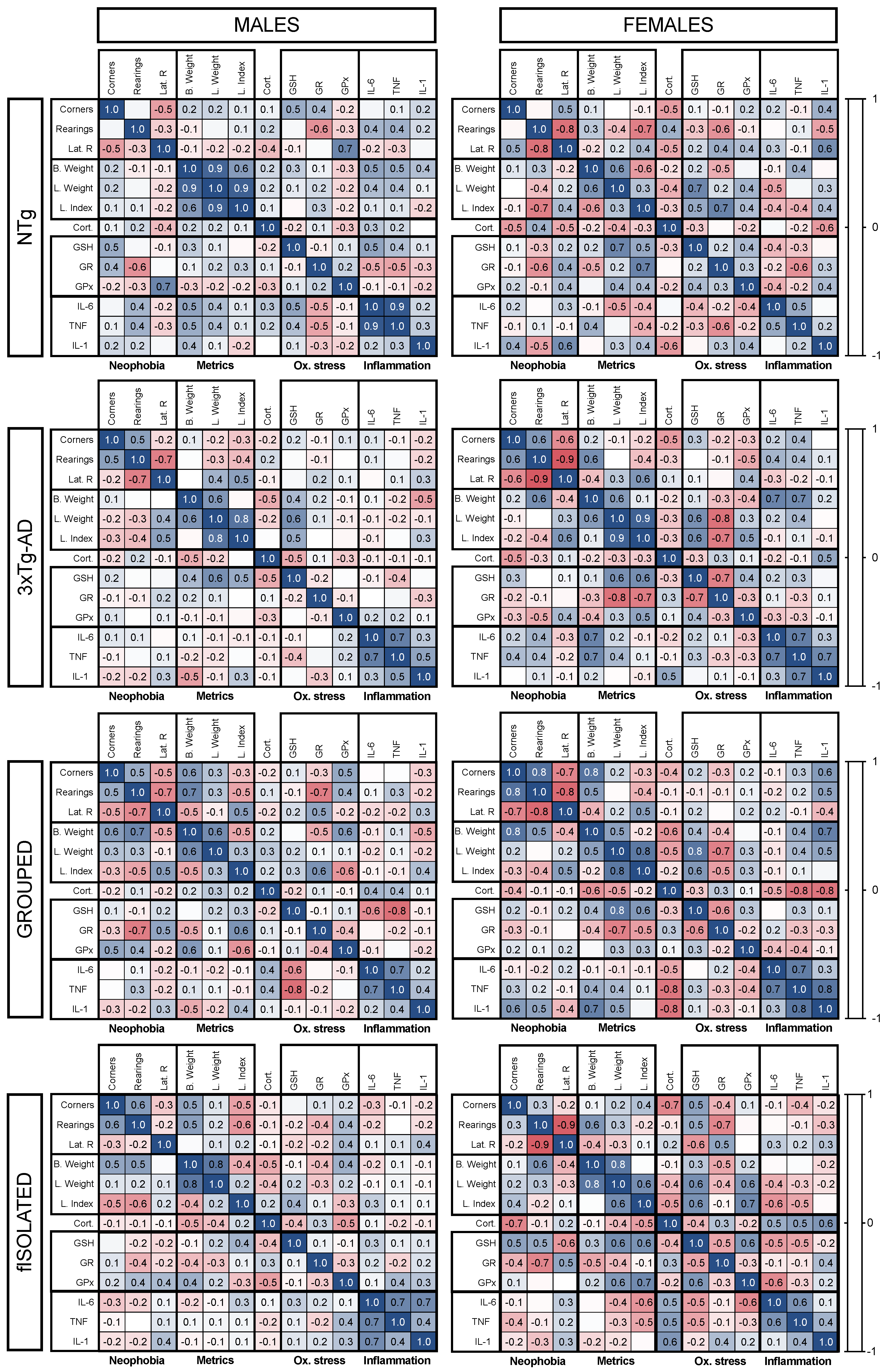
3.5. Meaningful Functional Correlates between Behavioral, Physical Metrics, Liver Oxidative Stress, and Inflammation Variables
4. Discussion
4.1. Hepatic Pathology Changes according to Genotype
4.2. Hepatic Oxi-Inflammation Metabolic Disruption
4.3. HPA Axis Disruption, Oxidative Stress, and Inflammation in the Liver
4.4. Meaningful Functional Correlates between Behavioral, Physical Metrics, Liver Oxidative Stress, and Inflammation Variables
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- García-Mesa, Y.; Colie, S.; Corpas, R.; Cristòfol, R.; Comellas, F.; Nebreda, A.R.; Giménez-Llort, L.; Sanfeliu, C. Oxidative Stress Is a Central Target for Physical Exercise Neuroprotection Against Pathological Brain Aging. J. Gerontol. A Biol. Sci. Med. Sci. 2016, 71, 40–49. [Google Scholar] [CrossRef] [PubMed]
- Tiwari, S.; Atluri, V.; Kaushik, A.; Yndart, A.; Nair, M. Alzheimer’s Disease: Pathogenesis, Diagnostics, and Therapeutics. Int. J. Nanomed. 2019, 14, 5541–5554. [Google Scholar] [CrossRef] [PubMed]
- Scheltens, P.; De Strooper, B.; Kivipelto, M.; Holstege, H.; Chételat, G.; Teunissen, C.E.; Cummings, J.; van der Flier, W.M. Alzheimer’s Disease. Lancet 2021, 397, 1577–1590. [Google Scholar] [CrossRef]
- Alzheimer’s Association. 2021 Alzheimer’s Disease Facts and Figures. Alzheimer’s Dement. 2021, 17, 327–406. [Google Scholar] [CrossRef] [PubMed]
- Hasegawa, M. Molecular Mechanisms in the Pathogenesis of Alzheimer’s Disease and Tauopathies-Prion-Like Seeded Aggregation and Phosphorylation. Biomolecules 2016, 6, 24. [Google Scholar] [CrossRef]
- Vida, C.; Martinez de Toda, I.; Garrido, A.; Carro, E.; Molina, J.A.; De la Fuente, M. Impairment of Several Immune Functions and Redox State in Blood Cells of Alzheimer’s Disease Patients. Relevant Role of Neutrophils in Oxidative Stress. Front. Immunol. 2018, 8, 1974. [Google Scholar] [CrossRef]
- Morris, G.; Berk, M.; Maes, M.; Puri, B.K. Could Alzheimer’s Disease Originate in the Periphery and If So How So? Mol. Neurobiol. 2019, 56, 406–434. [Google Scholar] [CrossRef]
- Gibson, G.E.; Huang, H.-M. Oxidative Stress in Alzheimer’s Disease. Neurobiol. Aging 2005, 26, 575–578. [Google Scholar] [CrossRef]
- Pugazhenthi, S.; Qin, L.; Reddy, P.H. Common Neurodegenerative Pathways in Obesity, Diabetes, and Alzheimer’s Disease. Biochim. Biophys. Acta (BBA)—Mol. Basis Dis. 2017, 1863, 1037–1045. [Google Scholar] [CrossRef]
- Muntsant, A.; Giménez-Llort, L. Crosstalk of Alzheimer’s Disease-Phenotype, HPA Axis, Splenic Oxidative Stress and Frailty in Late-Stages of Dementia, with Special Concerns on the Effects of Social Isolation: A Translational Neuroscience Approach. Front. Aging Neurosci. 2022, 14, 969381. [Google Scholar] [CrossRef]
- Baeta-Corral, R.; De la Fuente, M.; Giménez-Llort, L. Sex-Dependent Worsening of NMDA-Induced Responses, Anxiety, Hypercortisolemia, and Organometry of Early Peripheral Immunoendocrine Impairment in Adult 3xTg-AD Mice and Their Long-Lasting Ontogenic Modulation by Neonatal Handling. Behav. Brain Res. 2023, 438, 114189. [Google Scholar] [CrossRef]
- Angiulli, F.; Conti, E.; Zoia, C.P.; Da Re, F.; Appollonio, I.; Ferrarese, C.; Tremolizzo, L. Blood-Based Biomarkers of Neuroinflammation in Alzheimer’s Disease: A Central Role for Periphery? Diagnostics 2021, 11, 1525. [Google Scholar] [CrossRef] [PubMed]
- Racanelli, V.; Rehermann, B. The Liver as an Immunological Organ. Hepatology 2006, 43, S54–S62. [Google Scholar] [CrossRef] [PubMed]
- Sarkar, S.R.; Dutta, R. Role of Liver in Alzheimer’s Disease. ARC J. Neurosci. 2020, 4, 20–25. [Google Scholar] [CrossRef]
- Li, S.; Hong, M.; Tan, H.-Y.; Wang, N.; Feng, Y. Insights into the Role and Interdependence of Oxidative Stress and Inflammation in Liver Diseases. Oxidative Med. Cell. Longev. 2016, 2016, 4234061. [Google Scholar] [CrossRef]
- Nho, K.; Kueider-Paisley, A.; Ahmad, S.; MahmoudianDehkordi, S.; Arnold, M.; Risacher, S.L.; Louie, G.; Blach, C.; Baillie, R.; Han, X.; et al. Association of Altered Liver Enzymes With Alzheimer Disease Diagnosis, Cognition, Neuroimaging Measures, and Cerebrospinal Fluid Biomarkers. JAMA Netw. Open 2019, 2, e197978. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Lin, H.W.; Zhang, Q.; Zong, X. Targeting Alzheimer’s Disease: The Critical Crosstalk between the Liver and Brain. Nutrients 2022, 14, 4298. [Google Scholar] [CrossRef]
- Estrada, L.D.; Ahumada, P.; Cabrera, D.; Arab, J.P. Liver Dysfunction as a Novel Player in Alzheimer’s Progression: Looking Outside the Brain. Front. Aging Neurosci. 2019, 11, 174. [Google Scholar] [CrossRef]
- Gimenez-Llort, L.; De Las Casas-Engel, M.; Laferla, F.M.; De La Fuente, M. P4-245: Decrease of the Antioxidant Defenses in 3xTg-AD Triple-transgenic Adult Mice for Alzheimer’s Disease. Alzheimer’s Dement. 2009, 5, P502. [Google Scholar] [CrossRef]
- Hernanz, A.; Vida, C.; Manassra, R.; Gimenez-Llort, L.; De la Fuente, M. P2-063: Early Peripheral Oxidative Stress Status In Male And Female Triple-Transgenic Mice For Alzheimer’s Disease. Alzheimer’s Dement. 2014, 10, P492–P493. [Google Scholar] [CrossRef]
- Cummings, J.L. The Neuropsychiatric Inventory: Assessing Psychopathology in Dementia Patients. Neurology 1997, 48, 10S–16S. [Google Scholar] [CrossRef] [PubMed]
- Oddo, S.; Caccamo, A.; Shepherd, J.D.; Murphy, M.P.; Golde, T.E.; Kayed, R.; Metherate, R.; Mattson, M.P.; Akbari, Y.; LaFerla, F.M. Triple-Transgenic Model of Alzheimer’s Disease with Plaques and Tangles. Neuron 2003, 39, 409–421. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Arranz, L.; Maté, I.; De la Fuente, M. Gender-Specific Neuroimmunoendocrine Aging in a Triple-Transgenic 3×Tg-AD Mouse Model for Alzheimer’s Disease and Its Relation with Longevity. Neuroimmunomodulation 2008, 15, 331–343. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Blázquez, G.; Cañete, T.; Johansson, B.; Oddo, S.; Tobeña, A.; LaFerla, F.M.; Fernández-Teruel, A. Modeling Behavioral and Neuronal Symptoms of Alzheimer’s Disease in Mice: A Role for Intraneuronal Amyloid. Neurosci. Biobehav. Rev. 2007, 31, 125–147. [Google Scholar] [CrossRef]
- Ahmad, M.H.; Fatima, M.; Mondal, A.C. Role of Hypothalamic-Pituitary-Adrenal Axis, Hypothalamic-Pituitary-Gonadal Axis and Insulin Signaling in the Pathophysiology of Alzheimer’s Disease. Neuropsychobiology 2019, 77, 197–205. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Llort, L.; Maté, I.; Manassra, R.; Vida, C.; De la Fuente, M. Peripheral Immune System and Neuroimmune Communication Impairment in a Mouse Model of Alzheimer’s Disease. Ann. N. Y. Acad. Sci. 2012, 1262, 74–84. [Google Scholar] [CrossRef]
- De la Fuente, M.; Manassra, R.; Mate, I.; Vida, C.; Hernanz, A.; Gimenez-Llort, L. Early Oxidation and Inflammation State of the Immune System in Male and Female Triple Transgenic Mice for Alzheimer’s Disease (3xTgAD). Free Radic. Biol. Med. 2012, 53, S165. [Google Scholar] [CrossRef]
- Maté, I.; Cruces, J.; Giménez-Llort, L.; De la Fuente, M. Function and Redox State of Peritoneal Leukocytes as Preclinical and Prodromic Markers in a Longitudinal Study of Triple-Transgenic Mice for Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 43, 213–226. [Google Scholar] [CrossRef]
- Blázquez, G.; Cañete, T.; Tobeña, A.; Giménez-Llort, L.; Fernández-Teruel, A. Cognitive and Emotional Profiles of Aged Alzheimer’s Disease (3×TgAD) Mice: Effects of Environmental Enrichment and Sexual Dimorphism. Behav. Brain Res. 2014, 268, 185–201. [Google Scholar] [CrossRef]
- Esmaeili, M.H.; Bahari, B.; Salari, A.-A. ATP-Sensitive Potassium-Channel Inhibitor Glibenclamide Attenuates HPA Axis Hyperactivity, Depression- and Anxiety-Related Symptoms in a Rat Model of Alzheimer’s Disease. Brain Res. Bull. 2018, 137, 265–276. [Google Scholar] [CrossRef]
- Feng, Y.; Hu, Y.; Hou, Z.; Sun, Q.; Jia, Y.; Zhao, R. Chronic Corticosterone Exposure Induces Liver Inflammation and Fibrosis in Association with M6A-Linked Post-Transcriptional Suppression of Heat Shock Proteins in Chicken. Cell Stress Chaperones 2020, 25, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Berkman, L.F.; Glass, T.; Brissette, I.; Seeman, T.E. From Social Integration to Health: Durkheim in the New Millennium. Soc. Sci. Med. 2000, 51, 843–857. [Google Scholar] [CrossRef] [PubMed]
- Muntsant, A.; Giménez-Llort, L. Impact of Social Isolation on the Behavioral, Functional Profiles, and Hippocampal Atrophy Asymmetry in Dementia in Times of Coronavirus Pandemic (COVID-19): A Translational Neuroscience Approach. Front. Psychiatry 2020, 11, 572583. [Google Scholar] [CrossRef]
- Gimenez-Llort, L.; Alveal-Mellado, D. Digging Signatures in 13-Month-Old 3xTg-AD Mice for Alzheimer’s Disease and Its Disruption by Isolation Despite Social Life Since They Were Born. Front. Behav. Neurosci. 2021, 14, 611384. [Google Scholar] [CrossRef]
- Baeza, I.; De Castro, N.M.; Giménez-Llort, L.; De la Fuente, M. Ovariectomy, a Model of Menopause in Rodents, Causes a Premature Aging of the Nervous and Immune Systems. J. Neuroimmunol. 2010, 219, 90–99. [Google Scholar] [CrossRef] [PubMed]
- Torres-Lista, V.; De la Fuente, M.; Giménez-Llort, L. Survival Curves and Behavioral Profiles of Female 3xTg-AD Mice Surviving to 18-Months of Age as Compared to Mice with Normal Aging. J. Alzheimers Dis. Rep. 2017, 1, 47–57. [Google Scholar] [CrossRef]
- Jack, C.R.; Lowe, V.J.; Weigand, S.D.; Wiste, H.J.; Senjem, M.L.; Knopman, D.S.; Shiung, M.M.; Gunter, J.L.; Boeve, B.F.; Kemp, B.J.; et al. Serial PIB and MRI in Normal, Mild Cognitive Impairment and Alzheimer’s Disease: Implications for Sequence of Pathological Events in Alzheimer’s Disease. Brain 2009, 132, 1355–1365. [Google Scholar] [CrossRef] [PubMed]
- Baeta-Corral, R.; Giménez-Llort, L. Persistent Hyperactivity and Distinctive Strategy Features in the Morris Water Maze in 3xTg-AD Mice at Advanced Stages of Disease. Behav. Neurosci. 2015, 129, 129–137. [Google Scholar] [CrossRef]
- Kilkenny, C.; Browne, W.J.; Cuthill, I.C.; Emerson, M.; Altman, D.G. Improving Bioscience Research Reporting: The ARRIVE Guidelines for Reporting Animal Research. PLoS Biol. 2010, 8, e1000412. [Google Scholar] [CrossRef]
- Tietze, F. Enzymic Method for Quantitative Determination of Nanogram Amounts of Total and Oxidized Glutathione: Applications to Mammalian Blood and Other Tissues. Anal. Biochem. 1969, 27, 502–522. [Google Scholar] [CrossRef]
- Rahman, I.; Kode, A.; Biswas, S.K. Assay for Quantitative Determination of Glutathione and Glutathione Disulfide Levels Using Enzymatic Recycling Method. Nat. Protoc. 2006, 1, 3159–3165. [Google Scholar] [CrossRef] [PubMed]
- Massey, V.; Williams, C.H. On the Reaction Mechanism of Yeast Glutathione Reductase. J. Biol. Chem. 1965, 240, 4470–4480. [Google Scholar] [CrossRef]
- Lawrence, R.A.; Burk, R.F. Glutathione Peroxidase Activity in Selenium-Deficient Rat Liver. Biochem. Biophys. Res. Commun. 1976, 71, 952–958. [Google Scholar] [CrossRef]
- Alvarado, C.; Álvarez, P.; Jiménez, L.; De la Fuente, M. Oxidative Stress in Leukocytes from Young Prematurely Aging Mice Is Reversed by Supplementation with Biscuits Rich in Antioxidants. Dev. Comp. Immunol. 2006, 30, 1168–1180. [Google Scholar] [CrossRef]
- Trefts, E.; Gannon, M.; Wasserman, D.H. The Liver. Curr. Biol. 2017, 27, R1147–R1151. [Google Scholar] [CrossRef]
- Muntsant, A.; Giménez-Llort, L. Genotype Load Modulates Amyloid Burden and Anxiety-Like Patterns in Male 3xTg-AD Survivors despite Similar Neuro-Immunoendocrine, Synaptic and Cognitive Impairments. Biomedicines 2021, 9, 715. [Google Scholar] [CrossRef]
- De la Fuente, M.; Gimenez-Llort, L. Models of Aging of Neuroimmunomodulation: Strategies for Its Improvement. Neuroimmunomodulation 2010, 17, 213–216. [Google Scholar] [CrossRef]
- Siersbæk, M.S.; Ditzel, N.; Hejbøl, E.K.; Præstholm, S.M.; Markussen, L.K.; Avolio, F.; Li, L.; Lehtonen, L.; Hansen, A.K.; Schrøder, H.D.; et al. C57BL/6J Substrain Differences in Response to High-Fat Diet Intervention. Sci. Rep. 2020, 10, 14052. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Wu, H.; Liu, Y.; Yang, L. High Fat Diet Induced Obesity Model Using Four Strains of Mice: Kunming, C57BL/6, BALB/c and ICR. Exp. Anim. 2020, 69, 326–335. [Google Scholar] [CrossRef] [PubMed]
- Friedman, S.L.; Neuschwander-Tetri, B.A.; Rinella, M.; Sanyal, A.J. Mechanisms of NAFLD Development and Therapeutic Strategies. Nat. Med. 2018, 24, 908–922. [Google Scholar] [CrossRef]
- Ipsen, D.H.; Lykkesfeldt, J.; Tveden-Nyborg, P. Molecular Mechanisms of Hepatic Lipid Accumulation in Non-Alcoholic Fatty Liver Disease. Cell. Mol. Life Sci. 2018, 75, 3313–3327. [Google Scholar] [CrossRef]
- Simon, G.; Heckmann, V.; Tóth, D.; Pauka, D.; Petrus, K.; Molnár, T.F. The Effect of Hepatic Steatosis and Fibrosis on Liver Weight and Dimensions. Leg. Med. 2020, 47, 101781. [Google Scholar] [CrossRef]
- Sarkar, S.; Raymick, J.; Cuevas, E.; Rosas-Hernandez, H.; Hanig, J. Modification of Methods to Use Congo-Red Stain to Simultaneously Visualize Amyloid Plaques and Tangles in Human and Rodent Brain Tissue Sections. Metab. Brain Dis. 2020, 35, 1371–1383. [Google Scholar] [CrossRef] [PubMed]
- Clement, C.G.; Truong, L.D. An Evaluation of Congo Red Fluorescence for the Diagnosis of Amyloidosis. Hum. Pathol. 2014, 45, 1766–1772. [Google Scholar] [CrossRef] [PubMed]
- Gioeva, Z.; Kieninger, B.; Röcken, C. Amyloidose in Leberbiopsien. Pathologe 2009, 30, 240–245. [Google Scholar] [CrossRef]
- Muchtar, E.; Dispenzieri, A.; Magen, H.; Grogan, M.; Mauermann, M.; McPhail, E.D.; Kurtin, P.J.; Leung, N.; Buadi, F.K.; Dingli, D.; et al. Systemic Amyloidosis from A (AA) to T (ATTR): A Review. J. Intern. Med. 2021, 289, 268–292. [Google Scholar] [CrossRef]
- Wiȩckowska-Gacek, A.; Mietelska-Porowska, A.; Chutorański, D.; Wydrych, M.; Długosz, J.; Wojda, U. Western Diet Induces Impairment of Liver-Brain Axis Accelerating Neuroinflammation and Amyloid Pathology in Alzheimer’s Disease. Front. Aging Neurosci. 2021, 13, 654509. [Google Scholar] [CrossRef] [PubMed]
- Cheng, Y.; Tian, D.-Y.; Wang, Y.-J. Peripheral Clearance of Brain-Derived Aβ in Alzheimer’s Disease: Pathophysiology and Therapeutic Perspectives. Transl. Neurodegener. 2020, 9, 16. [Google Scholar] [CrossRef]
- González-Marrero, I.; Giménez-Llort, L.; Johanson, C.E.; Carmona-Calero, E.M.; Castañeyra-Ruiz, L.; Brito-Armas, J.M.; Castañeyra-Perdomo, A.; Castro-Fuentes, R. Choroid Plexus Dysfunction Impairs Beta-Amyloid Clearance in a Triple Transgenic Mouse Model of Alzheimer’s Disease. Front. Cell. Neurosci. 2015, 9, 17. [Google Scholar] [CrossRef]
- Marchese, M.; Cowan, D.; Head, E.; Ma, D.; Karimi, K.; Ashthorpe, V.; Kapadia, M.; Zhao, H.; Davis, P.; Sakic, B. Autoimmune Manifestations in the 3xTg-AD Model of Alzheimer’s Disease. J. Alzheimer’s Dis. 2014, 39, 191–210. [Google Scholar] [CrossRef] [PubMed]
- Machado, M.V.; Cortez-Pinto, H. Cell Death and Nonalcoholic Steatohepatitis: Where Is Ballooning Relevant? Expert Rev. Gastroenterol. Hepatol. 2011, 5, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Lackner, C. Hepatocellular Ballooning in Nonalcoholic Steatohepatitis: The Pathologist’s Perspective. Expert Rev. Gastroenterol. Hepatol. 2011, 5, 223–231. [Google Scholar] [CrossRef] [PubMed]
- Presnell, E.C.; Bhatti, G.; Numan, L.S.; Lerche, M.; Alkhateeb, S.K.; Ghalib, M.; Shammaa, M.; Kavdia, M. Computational Insights into the Role of Glutathione in Oxidative Stress. Curr. Neurovascular Res. 2013, 10, 185–194. [Google Scholar] [CrossRef]
- Wu, G.; Lupton, J.R.; Turner, N.D.; Fang, Y.-Z.; Yang, S. Glutathione Metabolism and Its Implications for Health. J. Nutr. 2004, 134, 489–492. [Google Scholar] [CrossRef] [PubMed]
- Sentellas, S.; Morales-Ibanez, O.; Zanuy, M.; Albertí, J.J. GSSG/GSH Ratios in Cryopreserved Rat and Human Hepatocytes as a Biomarker for Drug Induced Oxidative Stress. Toxicol. Vitr. 2014, 28, 1006–1015. [Google Scholar] [CrossRef] [PubMed]
- Zitka, O.; Skalickova, S.; Gumulec, J.; Masarik, M.; Adam, V.; Hubalek, J.; Trnkova, L.; Kruseova, J.; Eckschlager, T.; Kizek, R. Redox Status Expressed as GSH:GSSG Ratio as a Marker for Oxidative Stress in Paediatric Tumour Patients. Oncol. Lett. 2012, 4, 1247–1253. [Google Scholar] [CrossRef]
- Han, D.; Hanawa, N.; Saberi, B.; Kaplowitz, N. Mechanisms of Liver Injury. III. Role of Glutathione Redox Status in Liver Injury. Am. J. Physiol.-Gastrointest. Liver Physiol. 2006, 291, G1–G7. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Bhandar, B.; Kavdia, M. Interaction of ROS and RNS with GSH and GSH/GPX Systems. FASEB J. 2015, 29, 636.7. [Google Scholar] [CrossRef]
- Ferre, N.; Claria, J. New Insights into the Regulation of Liver Inflammation and Oxidative Stress. Mini-Rev. Med. Chem. 2006, 6, 1321–1330. [Google Scholar] [CrossRef] [PubMed]
- Lyketsos, C.G.; Carrillo, M.C.; Ryan, J.M.; Khachaturian, A.S.; Trzepacz, P.; Amatniek, J.; Cedarbaum, J.; Brashear, R.; Miller, D.S. Neuropsychiatric Symptoms in Alzheimer’s Disease. Alzheimer’s Dement. 2011, 7, 532–539. [Google Scholar] [CrossRef]
- Canet, G.; Hernandez, C.; Zussy, C.; Chevallier, N.; Desrumaux, C.; Givalois, L. Is AD a Stress-Related Disorder? Focus on the HPA Axis and Its Promising Therapeutic Targets. Front. Aging Neurosci. 2019, 11, 269. [Google Scholar] [CrossRef]
- Kosel, F.; Pelley, J.M.S.; Franklin, T.B. Behavioural and Psychological Symptoms of Dementia in Mouse Models of Alzheimer’s Disease-Related Pathology. Neurosci. Biobehav. Rev. 2020, 112, 634–647. [Google Scholar] [CrossRef]
- Nguyen, E.T.; Selmanovic, D.; Maltry, M.; Morano, R.; Franco-Villanueva, A.; Estrada, C.M.; Solomon, M.B. Endocrine Stress Responsivity and Social Memory in 3xTg-AD Female and Male Mice: A Tale of Two Experiments. Horm. Behav. 2020, 126, 104852. [Google Scholar] [CrossRef]
- Xie, X.; Shen, Q.; Yu, C.; Xiao, Q.; Zhou, J.; Xiong, Z.; Li, Z.; Fu, Z. Depression-like Behaviors Are Accompanied by Disrupted Mitochondrial Energy Metabolism in Chronic Corticosterone-Induced Mice. J. Steroid Biochem. Mol. Biol. 2020, 200, 105607. [Google Scholar] [CrossRef] [PubMed]
- Liao, J.; Keiser, J.A.; Scales, W.E.; Kunkel, S.L.; Kluger, M.J. Role of Corticosterone in TNF and IL-6 Production in Isolated Perfused Rat Liver. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1995, 268, R699–R706. [Google Scholar] [CrossRef]
- Battaglia, S.; Thayer, J.F. Functional Interplay between Central and Autonomic Nervous Systems in Human Fear Conditioning. Trends Neurosci. 2022, 45, 504–506. [Google Scholar] [CrossRef] [PubMed]
- Battaglia, S.; Orsolini, S.; Borgomaneri, S.; Barbieri, R.; Diciotti, S.; di Pellegrino, G. Characterizing Cardiac Autonomic Dynamics of Fear Learning in Humans. Psychophysiology 2022, 59, e14122. [Google Scholar] [CrossRef]
- Villagarcía, H.G.; Sabugo, V.; Castro, M.C.; Schinella, G.; Castrogiovanni, D.; Spinedi, E.; Massa, M.L.; Francini, F. Chronic Glucocorticoid-Rich Milieu and Liver Dysfunction. Int. J. Endocrinol. 2016, 2016, 7838290. [Google Scholar] [CrossRef] [PubMed]
- Baliño, P.; Romero-Cano, R.; Sánchez-Andrés, J.V.; Valls, V.; Aragón, C.G.; Muriach, M. Effects of Acute Ethanol Administration on Brain Oxidative Status: The Role of Acetaldehyde. Alcohol. Clin. Exp. Res. 2019, 43, 1672–1681. [Google Scholar] [CrossRef]
- Tao, W.; Sun, W.; Liu, L.; Wang, G.; Xiao, Z.; Pei, X.; Wang, M. Chitosan Oligosaccharide Attenuates Nonalcoholic Fatty Liver Disease Induced by High Fat Diet through Reducing Lipid Accumulation, Inflammation and Oxidative Stress in C57BL/6 Mice. Mar. Drugs 2019, 17, 645. [Google Scholar] [CrossRef] [PubMed]
- Ke, Z.; Zhao, Y.; Tan, S.; Chen, H.; Li, Y.; Zhou, Z.; Huang, C. Citrus Reticulata Blanco Peel Extract Ameliorates Hepatic Steatosis, Oxidative Stress and Inflammation in HF and MCD Diet-Induced NASH C57BL/6 J Mice. J. Nutr. Biochem. 2020, 83, 108426. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.-X.; Yang, Y.-X.; Kuo, K.; Li, H.-Q.; Chen, S.-D.; Chen, K.-L.; Dong, Q.; Tan, L.; Yu, J.-T. Social Isolation, Social Interaction, and Alzheimer’s Disease: A Mendelian Randomization Study. J. Alzheimer’s Dis. 2021, 80, 665–672. [Google Scholar] [CrossRef] [PubMed]


| 3xTg-AD, Male Grouped (n = 8) Mean ± SEM | 3xTg-AD, Male ISO (n = 15) Mean ± SEM | 3xTg-AD, Male fISO (n = 10) Mean ± SEM | 3xTg-AD, Male nISO (n = 5) Mean ± SEM | |
|---|---|---|---|---|
| Visited corners | 3.38 ± 1.07 | 3.00 ± 0.59 | 2.70 ± 0.82 | 3.60 ± 0.75 |
| Rearings | 0.50 ± 0.27 | 1.13 ± 0.34 | 0.90 ± 0.38 | 1.60 ± 0.68 |
| Lat. Rearing (s) | 22.75 ± 3.70 | 16.93 ± 2.83 | 18.20 ± 3.31 | 14.40 ± 5.70 |
| Body weight (g) | 33.60 ± 1.08 | 31.27 ± 0.49 # | 30.97 ± 0.51 # | 31.88 ± 1.12 |
| Liver weight (g) | 1.59 ± 0.08 | 1.43 ± 0.05 | 1.48 ± 0.07 | 1.33 ± 0.09 |
| Liver index (%) | 0.05 ± 0.002 | 0.05 ± 0.001 | 0.05 ± 0.002 | 0.4 ± 0.002 |
| GSH (nmol/mg prot.) | 66.13 ± 23.19 | 55.19 ± 3.46 | 57.12 ± 4.06 | 51.34 ± 6.76 |
| GR (mU/mg prot.) | 23.19 ± 0.86 | 22.85 ± 0.51 | 22.49 ± 0.46 | 23.57 ± 1.24 |
| GPx (mU/mg prot.) | 291.46 ± 17.49 | 300.90 ± 26.93 | 303.58 ± 35.02 | 295.55 ± 45.67 |
| IL-6 (pg/ mg prot.) | 8742.12 ± 847.56 | 7125.19 ± 696.54 | 6735.39 ± 689.15 | 7904.79 ± 1652.04 |
| TNF𝛼 (pg/ mg prot.) | 1930.90 ± 169.66 | 1769.08 ± 101.78 | 1727.44 ± 79.16 | 1852.35 ± 279.25 |
| IL-1β (pg/ mg prot.) | 19.63 ± 1.20 | 18.92 ± 1.22 | 18.98 ± 1.65 | 18.81 ± 1.81 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fraile-Ramos, J.; Garrit, A.; Reig-Vilallonga, J.; Giménez-Llort, L. Hepatic Oxi-Inflammation and Neophobia as Potential Liver–Brain Axis Targets for Alzheimer’s Disease and Aging, with Strong Sensitivity to Sex, Isolation, and Obesity. Cells 2023, 12, 1517. https://doi.org/10.3390/cells12111517
Fraile-Ramos J, Garrit A, Reig-Vilallonga J, Giménez-Llort L. Hepatic Oxi-Inflammation and Neophobia as Potential Liver–Brain Axis Targets for Alzheimer’s Disease and Aging, with Strong Sensitivity to Sex, Isolation, and Obesity. Cells. 2023; 12(11):1517. https://doi.org/10.3390/cells12111517
Chicago/Turabian StyleFraile-Ramos, Juan, Anna Garrit, Josep Reig-Vilallonga, and Lydia Giménez-Llort. 2023. "Hepatic Oxi-Inflammation and Neophobia as Potential Liver–Brain Axis Targets for Alzheimer’s Disease and Aging, with Strong Sensitivity to Sex, Isolation, and Obesity" Cells 12, no. 11: 1517. https://doi.org/10.3390/cells12111517
APA StyleFraile-Ramos, J., Garrit, A., Reig-Vilallonga, J., & Giménez-Llort, L. (2023). Hepatic Oxi-Inflammation and Neophobia as Potential Liver–Brain Axis Targets for Alzheimer’s Disease and Aging, with Strong Sensitivity to Sex, Isolation, and Obesity. Cells, 12(11), 1517. https://doi.org/10.3390/cells12111517