In Situ Identification of Both IL-4 and IL-10 Cytokine–Receptor Interactions during Tissue Regeneration
Abstract
:1. Introduction
2. Materials and Methods
2.1. Tissue Samples
2.2. Histological Staining
2.3. Immunolabeling
2.4. Proximity Ligation Assay (PLA)
2.5. Quantification and Statistical Analysis
3. Results
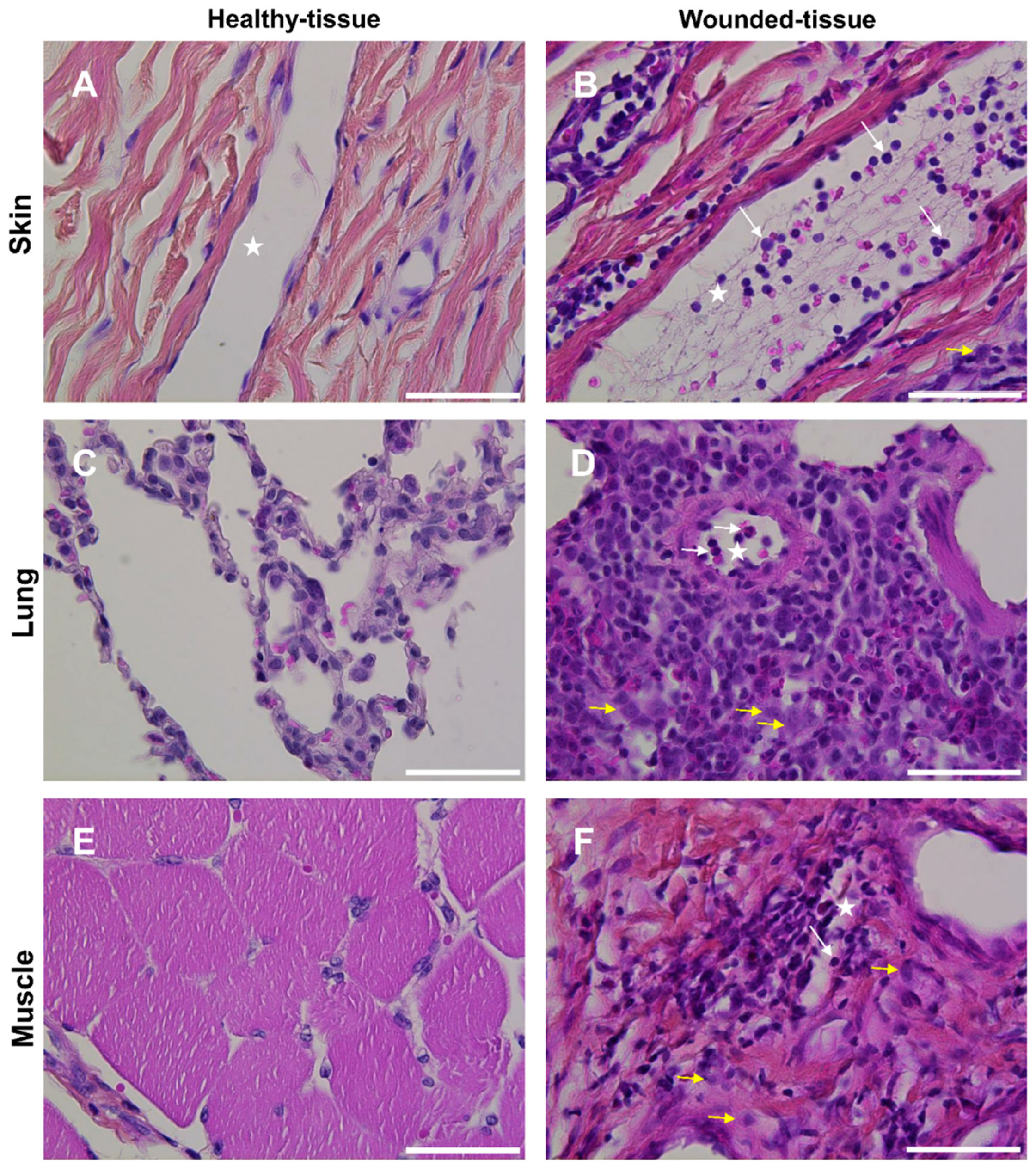
3.1. Tissue Wounds Induce Endothelial Cell Activation and Immune Cell Infiltration
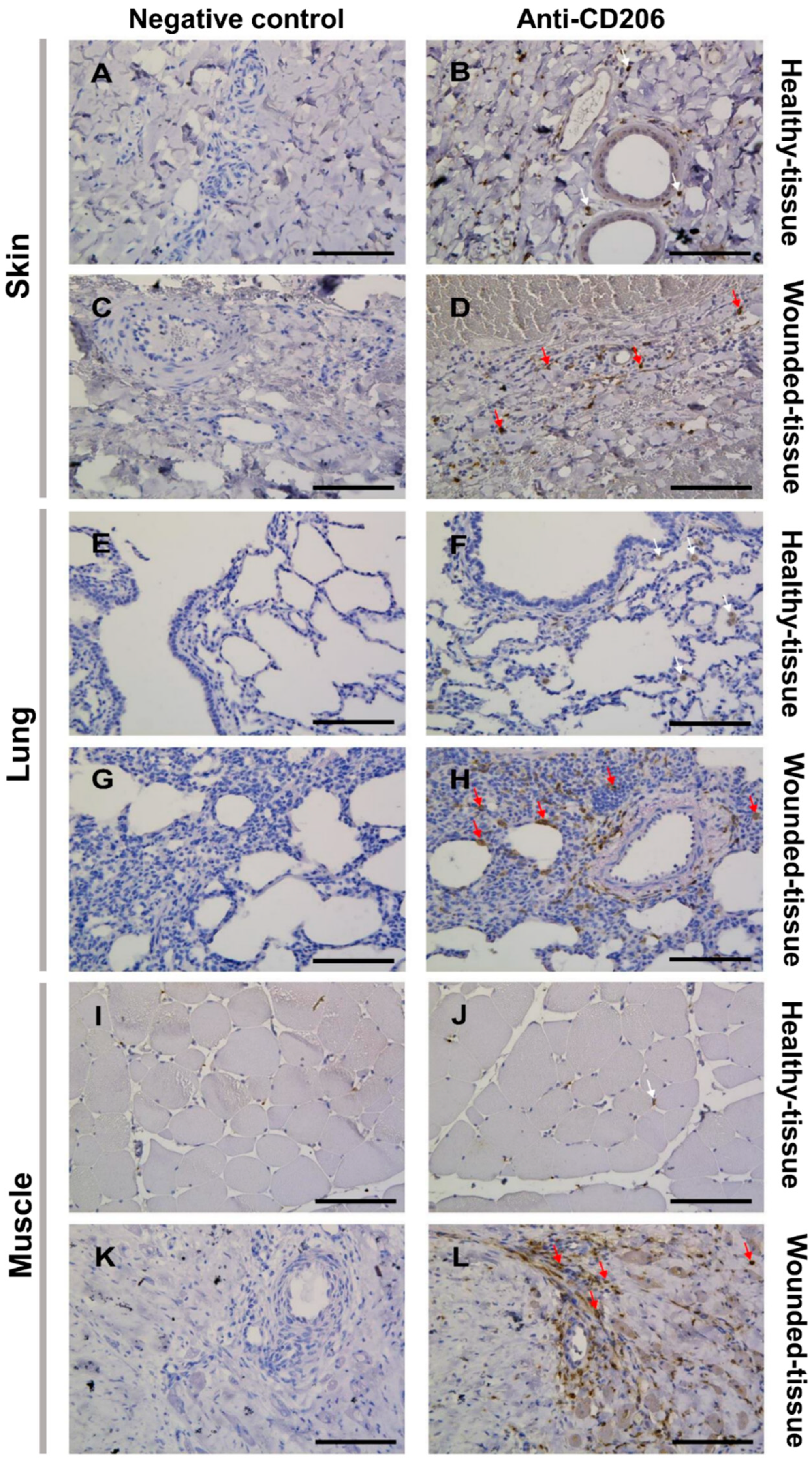
3.2. Tissue Damage Results in the Accumulation of M2 Macrophages around the Blood Vessel
3.3. Tissue Injury Activates IL-4R Expression on the Surface of Macrophages
3.4. Injury in Muscle Tissue Displayed High IL-10R Expression
3.5. Granulocytes Show High IL-4 Expression
3.6. Expression of IL-10 Cytokine in Different Tissues
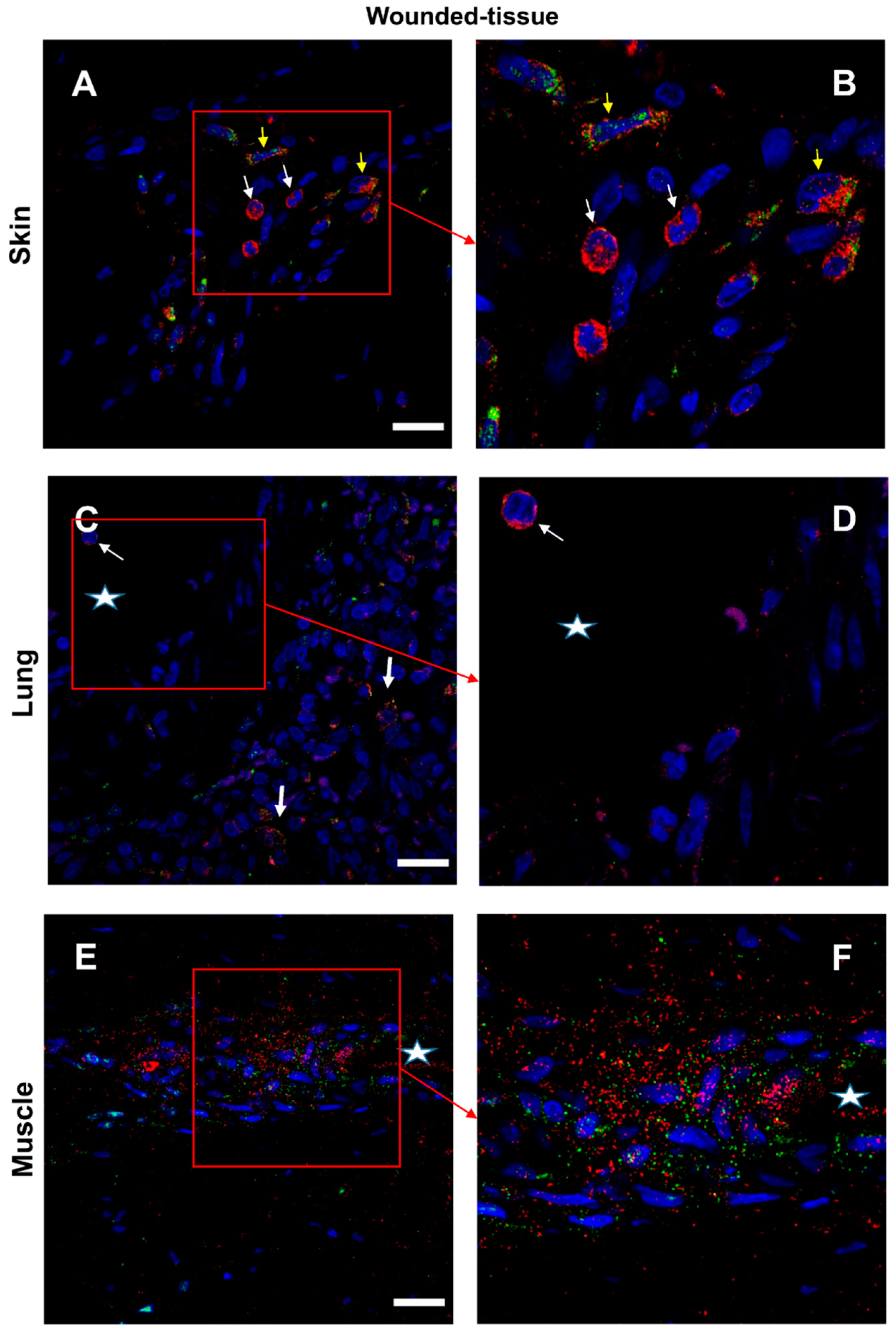
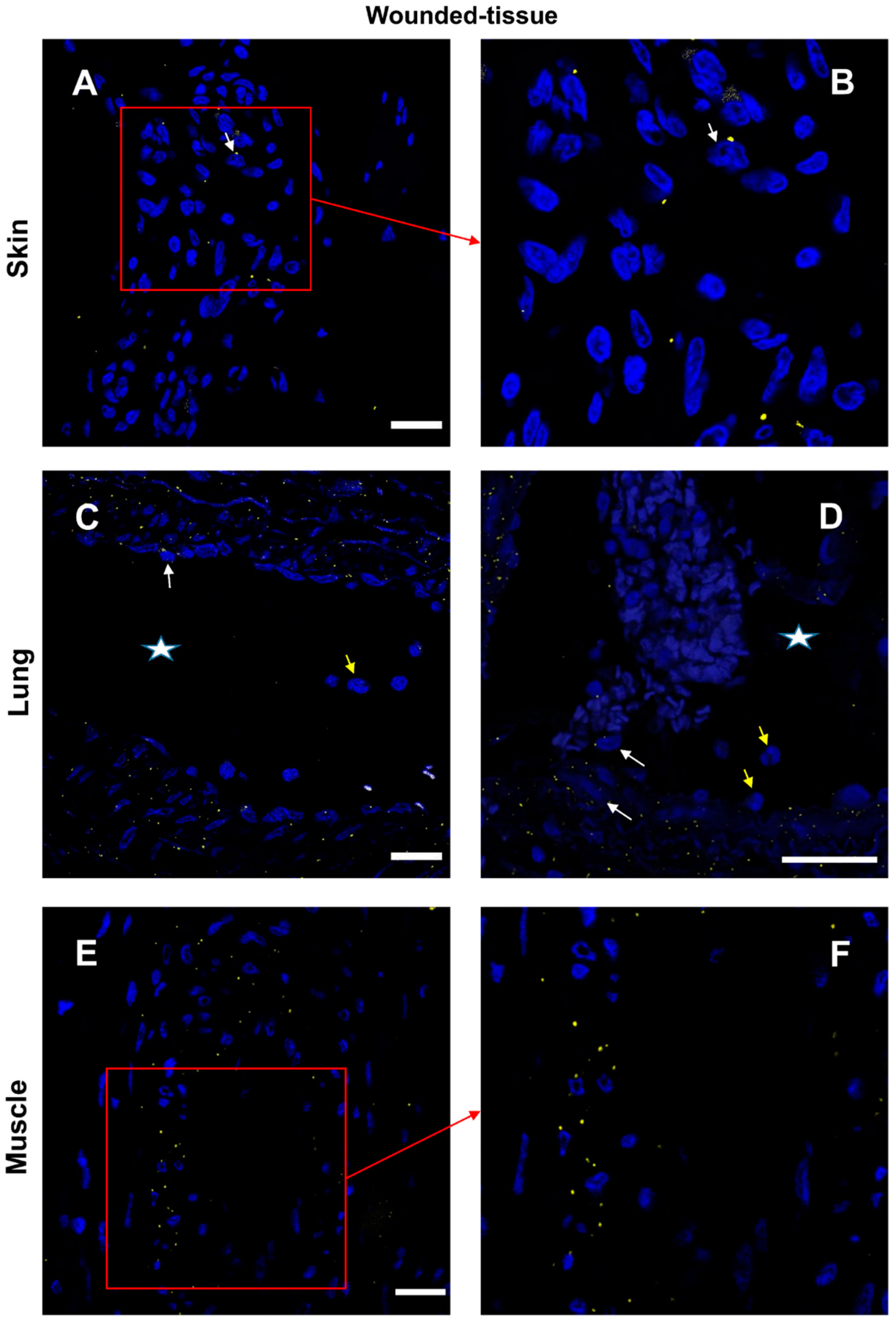
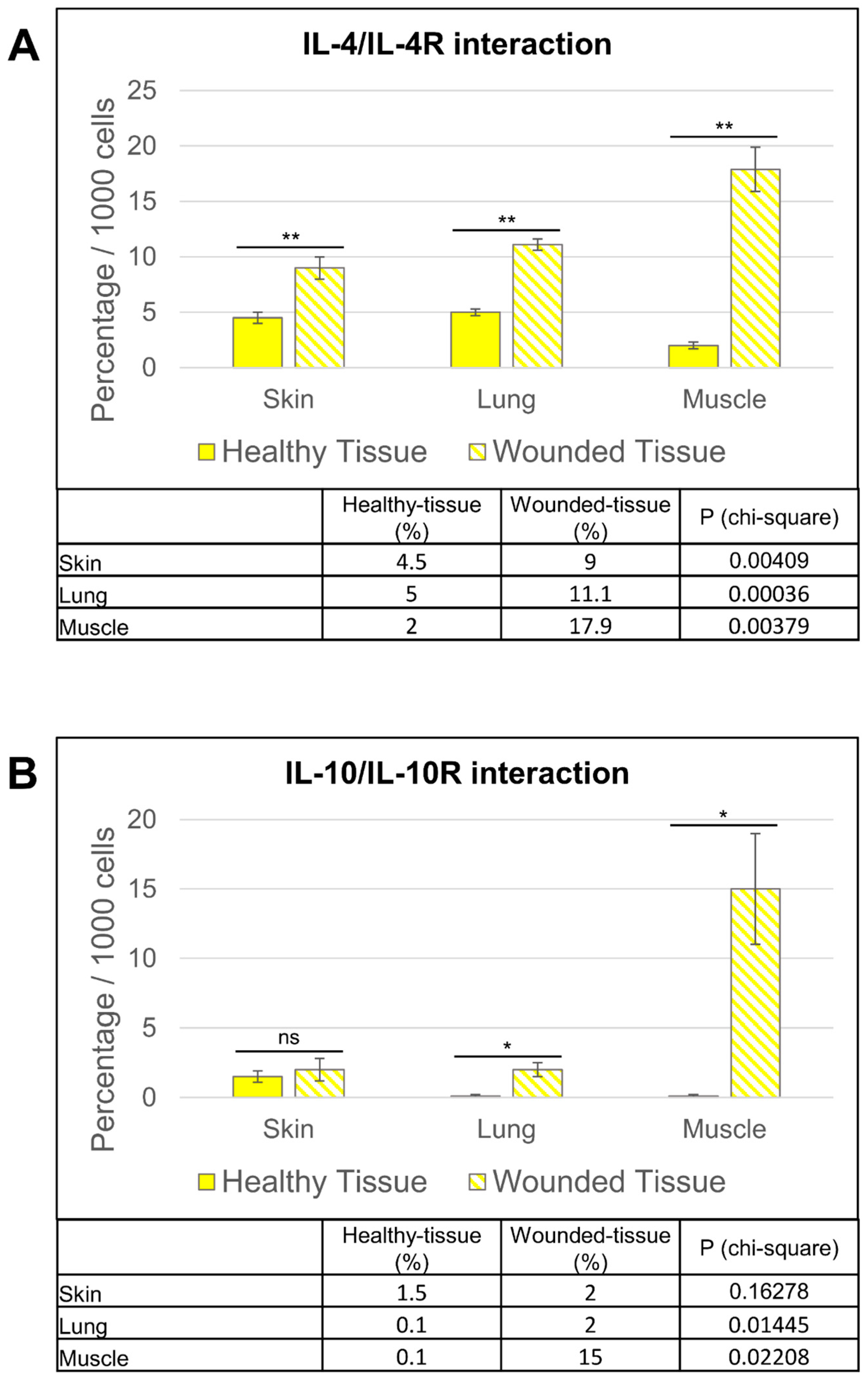
3.7. IL-4/IL-4R and IL-10/IL-10R Interaction In Situ
4. Discussion
5. Conclusions
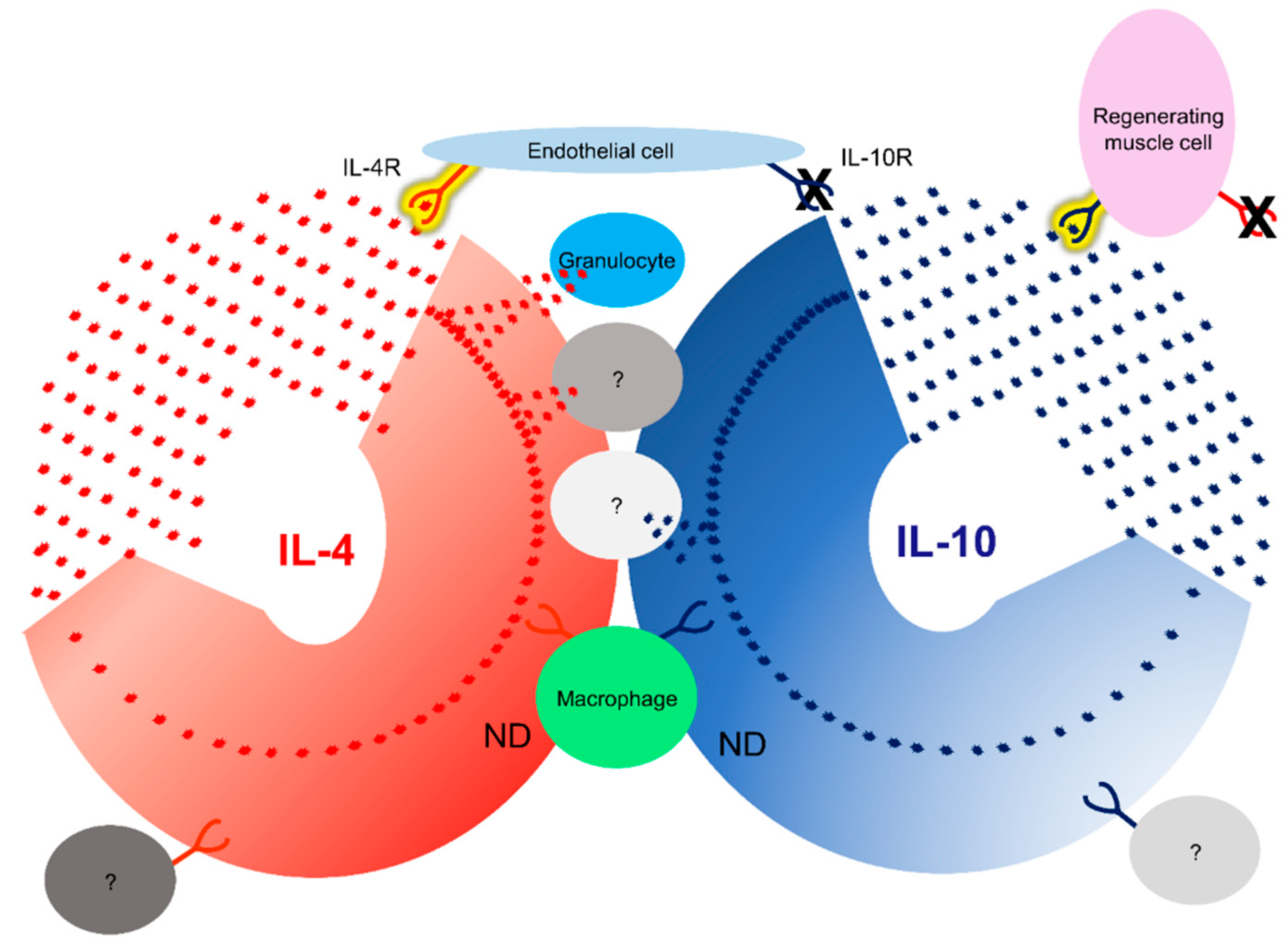
- Using immunolabeling, M2-like macrophages were identified in regenerating tissues.
- Cut-infection-irradiation injuries induced the expression of IL-4 and IL-10 receptors in M2-like macrophages in all three tissues.
- Cytokine receptor induction was not limited to macrophages, as other cells also showed receptor induction.
- The two different cytokine receptors showed different localization. IL-4R expression was mainly expressed in endothelial cells and localized around the vasculature. IL-10R expression was particularly intense in the regenerating irradiated muscle cells.
- The PLA method provided valuable information for a better understanding of tissue regeneration. Using this technique, we have shown that IL-4 can interact with its receptor around the vasculature and IL-10 interacts with its receptor in regenerating muscle.
- Granulocytes do not have an IL-4 cytokine/receptor interaction, suggesting that these cells are not targeted but are a source of cytokine.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Martinez, F.O.; Gordon, S. The M1 and M2 Paradigm of Macrophage Activation: Time for Reassessment. F1000Prime Rep. 2014, 6, 13. [Google Scholar] [CrossRef] [PubMed]
- Snyder, R.J.; Lantis, J.; Kirsner, R.S.; Shah, V.; Molyneaux, M.; Carter, M.J. Macrophages: A Review of Their Role in Wound Healing and Their Therapeutic Use. Wound Repair. Regen. 2016, 24, 613–629. [Google Scholar] [CrossRef]
- Abdelaziz, M.H.; Abdelwahab, S.F.; Wan, J.; Cai, W.; Huixuan, W.; Jianjun, C.; Kumar, K.D.; Vasudevan, A.; Sadek, A.; Su, Z.; et al. Alternatively Activated Macrophages; a Double-Edged Sword in Allergic Asthma. J. Transl. Med. 2020, 18, 58. [Google Scholar] [CrossRef]
- Xu, H.-T.; Lee, C.-W.; Li, M.-Y.; Wang, Y.-F.; Yung, P.S.-H.; Lee, O.K.-S. The Shift in Macrophages Polarisation after Tendon Injury: A Systematic Review. J. Orthop. Translat 2020, 21, 24–34. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.; Dev, K.; Agarwal, B.; Das, P.; Syed, M.A. Macrophages: Their Role, Activation, and Polarization in Pulmonary Diseases. Immunobiology 2018, 223, 383–396. [Google Scholar] [CrossRef] [PubMed]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.-A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage Plasticity, Polarization, and Function in Health and Disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Gharib, S.A.; McMahan, R.S.; Eddy, W.E.; Long, M.E.; Parks, W.C.; Aitken, M.L.; Manicone, A.M. Transcriptional and Functional Diversity of Human Macrophage Repolarization. J. Allergy Clin. Immunol. 2019, 143, 1536–1548. [Google Scholar] [CrossRef]
- Tidball, J.G.; Dorshkind, K.; Wehling-Henricks, M. Shared Signaling Systems in Myeloid Cell-Mediated Muscle Regeneration. Development 2014, 141, 1184–1196. [Google Scholar] [CrossRef]
- Kieler, M.; Hofmann, M.; Schabbauer, G. More than Just Protein Building Blocks: How Amino Acids and Related Metabolic Pathways Fuel Macrophage Polarization. FEBS J. 2021, 288, 3694–3714. [Google Scholar] [CrossRef]
- Hoeksema, M.A.; Shen, Z.; Holtman, I.R.; Zheng, A.; Spann, N.J.; Cobo, I.; Gymrek, M.; Glass, C.K. Mechanisms Underlying Divergent Responses of Genetically Distinct Macrophages to IL-4. Sci. Adv. 2021, 7, eabf9808. [Google Scholar] [CrossRef]
- Howard, M.; Farrar, J.; Hilfiker, M.; Johnson, B.; Takatsu, K.; Hamaoka, T.; Paul, W.E. Identification of a T Cell-Derived b Cell Growth Factor Distinct from Interleukin 2. J. Exp. Med. 1982, 155, 914–923. [Google Scholar] [CrossRef]
- Isakson, P.C.; Puré, E.; Vitetta, E.S.; Krammer, P.H. T Cell-Derived B Cell Differentiation Factor(s). Effect on the Isotype Switch of Murine B Cells. J. Exp. Med. 1982, 155, 734–748. [Google Scholar] [CrossRef] [PubMed]
- Mosmann, T.R.; Cherwinski, H.; Bond, M.W.; Giedlin, M.A.; Coffman, R.L. Two Types of Murine Helper T Cell Clone. I. Definition According to Profiles of Lymphokine Activities and Secreted Proteins. J. Immunol. 1986, 136, 2348–2357. [Google Scholar] [CrossRef]
- Brown, A.M. A Spreadsheet Template Compatible with Microsoft Excel and IWork Numbers That Returns the Simultaneous Confidence Intervals for All Pairwise Differences between Multiple Sample Means. Comput. Methods Programs Biomed. 2010, 98, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Bendelac, A.; Watson, C.; Hu-Li, J.; Paul, W.E. Role of NK1.1+ T Cells in a TH2 Response and in Immunoglobulin E Production. Science 1995, 270, 1845–1847. [Google Scholar] [CrossRef] [PubMed]
- Nonaka, M.; Nonaka, R.; Woolley, K.; Adelroth, E.; Miura, K.; Okhawara, Y.; Glibetic, M.; Nakano, K.; O’Byrne, P.; Dolovich, J. Distinct Immunohistochemical Localization of IL-4 in Human Inflamed Airway Tissues. IL-4 Is Localized to Eosinophils in Vivo and Is Released by Peripheral Blood Eosinophils. J. Immunol. 1995, 155, 3234–3244. [Google Scholar] [CrossRef] [PubMed]
- Moro, K.; Yamada, T.; Tanabe, M.; Takeuchi, T.; Ikawa, T.; Kawamoto, H.; Furusawa, J.-I.; Ohtani, M.; Fujii, H.; Koyasu, S. Innate Production of T(H)2 Cytokines by Adipose Tissue-Associated c-Kit(+)Sca-1(+) Lymphoid Cells. Nature 2010, 463, 540–544. [Google Scholar] [CrossRef]
- Saenz, S.A.; Siracusa, M.C.; Perrigoue, J.G.; Spencer, S.P.; Urban, J.F.J.; Tocker, J.E.; Budelsky, A.L.; Kleinschek, M.A.; Kastelein, R.A.; Kambayashi, T.; et al. IL25 Elicits a Multipotent Progenitor Cell Population That Promotes T(H)2 Cytokine Responses. Nature 2010, 464, 1362–1366. [Google Scholar] [CrossRef]
- Paul, W.E. History of Interleukin-4. Cytokine 2015, 75, 3–7. [Google Scholar] [CrossRef]
- Junttila, I.S. Tuning the Cytokine Responses: An Update on Interleukin (IL)-4 and IL-13 Receptor Complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- Iwaszko, M.; Biały, S.; Bogunia-Kubik, K. Significance of Interleukin (IL)-4 and IL-13 in Inflammatory Arthritis. Cells 2021, 10, 3000. [Google Scholar] [CrossRef] [PubMed]
- Song, X.; Traub, B.; Shi, J.; Kornmann, M. Possible Roles of Interleukin-4 and -13 and Their Receptors in Gastric and Colon Cancer. Int. J. Mol. Sci. 2021, 22, 727. [Google Scholar] [CrossRef] [PubMed]
- Fiorentino, D.F.; Bond, M.W.; Mosmann, T.R. Two Types of Mouse T Helper Cell. IV. Th2 Clones Secrete a Factor That Inhibits Cytokine Production by Th1 Clones. J. Exp. Med. 1989, 170, 2081–2095. [Google Scholar] [CrossRef]
- Yssel, H.; De Waal Malefyt, R.; Roncarolo, M.G.; Abrams, J.S.; Lahesmaa, R.; Spits, H.; de Vries, J.E. IL-10 Is Produced by Subsets of Human CD4+ T Cell Clones and Peripheral Blood T Cells. J. Immunol. 1992, 149, 2378–2384. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.W.; de Waal Malefyt, R.; Coffman, R.L.; O’Garra, A. Interleukin-10 and the Interleukin-10 Receptor. Annu. Rev. Immunol. 2001, 19, 683–765. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.-V.V.; Frye, J.B.; Zbesko, J.C.; Stepanovic, K.; Hayes, M.; Urzua, A.; Serrano, G.; Beach, T.G.; Doyle, K.P. Multiplex Immunoassay Characterization and Species Comparison of Inflammation in Acute and Non-Acute Ischemic Infarcts in Human and Mouse Brain Tissue. Acta Neuropathol. Commun. 2016, 4, 100. [Google Scholar] [CrossRef]
- Kucuksezer, U.C.; Aktas Cetin, E.; Esen, F.; Tahrali, I.; Akdeniz, N.; Gelmez, M.Y.; Deniz, G. The Role of Natural Killer Cells in Autoimmune Diseases. Front. Immunol. 2021, 12, 622306. [Google Scholar] [CrossRef]
- Nagata, K.; Nishiyama, C. IL-10 in Mast Cell-Mediated Immune Responses: Anti-Inflammatory and Proinflammatory Roles. Int. J. Mol. Sci. 2021, 22, 4972. [Google Scholar] [CrossRef]
- Jarry, A.; Bossard, C.; Bou-Hanna, C.; Masson, D.; Espaze, E.; Denis, M.G.; Laboisse, C.L. Mucosal IL-10 and TGF-Beta Play Crucial Roles in Preventing LPS-Driven, IFN-Gamma-Mediated Epithelial Damage in Human Colon Explants. J. Clin. Investig. 2008, 118, 1132–1142. [Google Scholar] [CrossRef]
- Saraiva, M.; Vieira, P.; O’Garra, A. Biology and Therapeutic Potential of Interleukin-10. J. Exp. Med. 2020, 217. [Google Scholar] [CrossRef]
- Howes, A.; Taubert, C.; Blankley, S.; Spink, N.; Wu, X.; Graham, C.M.; Zhao, J.; Saraiva, M.; Ricciardi-Castagnoli, P.; Bancroft, G.J.; et al. Differential Production of Type I IFN Determines the Reciprocal Levels of IL-10 and Proinflammatory Cytokines Produced by C57BL/6 and BALB/c Macrophages. J. Immunol. 2016, 197, 2838–2853. [Google Scholar] [CrossRef] [PubMed]
- Frangogiannis, N.G. Regulation of the Inflammatory Response in Cardiac Repair. Circ. Res. 2012, 110, 159–173. [Google Scholar] [CrossRef] [PubMed]
- Davies, L.C.; Jenkins, S.J.; Allen, J.E.; Taylor, P.R. Tissue-Resident Macrophages. Nat. Immunol. 2013, 14, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, I.; Imaizumi, K.; Hashimoto, N.; Furukawa, H.; Noda, Y.; Kawabe, T.; Honda, T.; Ogawa, T.; Matsuo, M.; Imai, N.; et al. Aqueous Fraction of Sauropus Androgynus Might Be Responsible for Bronchiolitis Obliterans. Respirology 2013, 18, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Qian, B.-Z.; Pollard, J.W. Macrophage Diversity Enhances Tumor Progression and Metastasis. Cell 2010, 141, 39–51. [Google Scholar] [CrossRef]
- Stein, M.; Keshav, S.; Harris, N.; Gordon, S. Interleukin 4 Potently Enhances Murine Macrophage Mannose Receptor Activity: A Marker of Alternative Immunologic Macrophage Activation. J. Exp. Med. 1992, 176, 287–292. [Google Scholar] [CrossRef]
- Gordon, S. Alternative Activation of Macrophages. Nat. Rev. Immunol. 2003, 3, 23–35. [Google Scholar] [CrossRef]
- Gordon, S.; Lawson, L.; Rabinowitz, S.; Crocker, P.R.; Morris, L.; Perry, V.H. Antigen Markers of Macrophage Differentiation in Murine Tissues. Curr. Top. Microbiol. Immunol. 1992, 181, 1–37. [Google Scholar] [CrossRef]
- McKenzie, G.J.; Bancroft, A.; Grencis, R.K.; McKenzie, A.N. A Distinct Role for Interleukin-13 in Th2-Cell-Mediated Immune Responses. Curr. Biol. 1998, 8, 339–342. [Google Scholar] [CrossRef]
- Sica, A.; Mantovani, A. Macrophage Plasticity and Polarization: In Vivo Veritas. J. Clin. Investig. 2012, 122, 787–795. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage Activation and Polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef] [PubMed]
- Nikovics, K.; Favier, A.-L. Macrophage Identification In Situ. Biomedicines 2021, 9, 1393. [Google Scholar] [CrossRef] [PubMed]
- Wynn, T.A.; Chawla, A.; Pollard, J.W. Macrophage Biology in Development, Homeostasis and Disease. Nature 2013, 496, 445–455. [Google Scholar] [CrossRef]
- Wang, L.-X.; Zhang, S.-X.; Wu, H.-J.; Rong, X.-L.; Guo, J. M2b Macrophage Polarization and Its Roles in Diseases. J. Leukoc. Biol. 2019, 106, 345–358. [Google Scholar] [CrossRef]
- Lin, J.-S.; Lai, E.-M. Protein-Protein Interactions: Yeast Two-Hybrid System. Methods Mol. Biol. 2017, 1615, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, N. FRET and Mechanobiology. Integr. Biol. 2009, 1, 565–573. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.E.; Kim, Y.; Huh, W.-K.; Park, H.-O. Bimolecular Fluorescence Complementation (BiFC) Analysis: Advances and Recent Applications for Genome-Wide Interaction Studies. J. Mol. Biol. 2015, 427, 2039–2055. [Google Scholar] [CrossRef]
- Fredriksson, S.; Gullberg, M.; Jarvius, J.; Olsson, C.; Pietras, K.; Gústafsdóttir, S.M.; Ostman, A.; Landegren, U. Protein Detection Using Proximity-Dependent DNA Ligation Assays. Nat. Biotechnol. 2002, 20, 473–477. [Google Scholar] [CrossRef]
- Gullberg, M.; Gústafsdóttir, S.M.; Schallmeiner, E.; Jarvius, J.; Bjarnegård, M.; Betsholtz, C.; Landegren, U.; Fredriksson, S. Cytokine Detection by Antibody-Based Proximity Ligation. Proc. Natl. Acad. Sci. USA 2004, 101, 8420–8424. [Google Scholar] [CrossRef]
- Bagchi, S.; Fredriksson, R.; Wallén-Mackenzie, Å. In Situ Proximity Ligation Assay (PLA). Methods Mol. Biol. 2015, 1318, 149–159. [Google Scholar] [CrossRef]
- Alam, M.S. Proximity Ligation Assay (PLA). Curr. Protoc. Immunol. 2018, 123, e58. [Google Scholar] [CrossRef]
- Wang, P.; Yang, Y.; Hong, T.; Zhu, G. Proximity Ligation Assay: An Ultrasensitive Method for Protein Quantification and Its Applications in Pathogen Detection. Appl. Microbiol. Biotechnol. 2021, 105, 923–935. [Google Scholar] [CrossRef] [PubMed]
- Ezzelarab, M.B.; Cooper, D.K.C. Systemic Inflammation in Xenograft Recipients (SIXR): A New Paradigm in Pig-to-Primate Xenotransplantation? Int. J. Surg. 2015, 23, 301–305. [Google Scholar] [CrossRef] [PubMed]
- Lunney, J.K.; Van Goor, A.; Walker, K.E.; Hailstock, T.; Franklin, J.; Dai, C. Importance of the Pig as a Human Biomedical Model. Sci. Transl. Med. 2021, 13, eabd5758. [Google Scholar] [CrossRef] [PubMed]
- Sullivan, T.P.; Eaglstein, W.H.; Davis, S.C.; Mertz, P. The Pig as a Model for Human Wound Healing. Wound Repair. Regen. 2001, 9, 66–76. [Google Scholar] [CrossRef]
- Riccobono, D.; Nikovics, K.; François, S.; Favier, A.-L.; Jullien, N.; Schrock, G.; Scherthan, H.; Drouet, M. First Insights Into the M2 Inflammatory Response After Adipose-Tissue-Derived Stem Cell Injections in Radiation-Injured Muscles. Health Physics 2018, 115, 37–48. [Google Scholar] [CrossRef]
- Nikovics, K.; Durand, M.; Castellarin, C.; Burger, J.; Sicherre, E.; Collombet, J.-M.; Oger, M.; Holy, X.; Favier, A.-L. Macrophages Characterization in an Injured Bone Tissue. Biomedicines 2022, 10, 1385. [Google Scholar] [CrossRef]
- Midway, S.; Robertson, M.; Flinn, S.; Kaller, M. Comparing Multiple Comparisons: Practical Guidance for Choosing the Best Multiple Comparisons Test. PeerJ 2020, 8, e10387. [Google Scholar] [CrossRef]
- Xu, J.; Yu, L.; Guo, J.; Xiang, J.; Zheng, Z.; Gao, D.; Shi, B.; Hao, H.; Jiao, D.; Zhong, L.; et al. Generation of Pig Induced Pluripotent Stem Cells Using an Extended Pluripotent Stem Cell Culture System. Stem Cell. Res. Ther. 2019, 10, 193. [Google Scholar] [CrossRef]
- Minutti, C.M.; Knipper, J.A.; Allen, J.E.; Zaiss, D.M.W. Tissue-Specific Contribution of Macrophages to Wound Healing. Semin. Cell. Dev. Biol. 2017, 61, 3–11. [Google Scholar] [CrossRef]
- Pan, D.; Schellhardt, L.; Acevedo-Cintron, J.A.; Hunter, D.; Snyder-Warwick, A.K.; Mackinnon, S.E.; Wood, M.D. IL-4 Expressing Cells Are Recruited to Nerve after Injury and Promote Regeneration. Exp. Neurol. 2022, 347, 113909. [Google Scholar] [CrossRef] [PubMed]
- Orecchioni, M.; Ghosheh, Y.; Pramod, A.B.; Ley, K. Macrophage Polarization: Different Gene Signatures in M1(LPS+) vs. Classically and M2(LPS-) vs. Alternatively Activated Macrophages. Front. Immunol. 2019, 10, 1084. [Google Scholar] [CrossRef]
- Zizzo, G.; Hilliard, B.A.; Monestier, M.; Cohen, P.L. Efficient Clearance of Early Apoptotic Cells by Human Macrophages Requires M2c Polarization and MerTK Induction. J. Immunol. 2012, 189, 3508–3520. [Google Scholar] [CrossRef]
- Xu, W.; Zhao, X.; Daha, M.R.; van Kooten, C. Reversible Differentiation of Pro- and Anti-Inflammatory Macrophages. Mol. Immunol. 2013, 53, 179–186. [Google Scholar] [CrossRef] [PubMed]
- Arnold, D.E.; Heimall, J.R. A Review of Chronic Granulomatous Disease. Adv. Ther. 2017, 34, 2543–2557. [Google Scholar] [CrossRef]
- Jenkins, S.J.; Ruckerl, D.; Thomas, G.D.; Hewitson, J.P.; Duncan, S.; Brombacher, F.; Maizels, R.M.; Hume, D.A.; Allen, J.E. IL-4 Directly Signals Tissue-Resident Macrophages to Proliferate beyond Homeostatic Levels Controlled by CSF-1. J. Exp. Med. 2013, 210, 2477–2491. [Google Scholar] [CrossRef] [PubMed]
- Shintani, Y.; Ito, T.; Fields, L.; Shiraishi, M.; Ichihara, Y.; Sato, N.; Podaru, M.; Kainuma, S.; Tanaka, H.; Suzuki, K. IL-4 as a Repurposed Biological Drug for Myocardial Infarction through Augmentation of Reparative Cardiac Macrophages: Proof-of-Concept Data in Mice. Sci. Rep. 2017, 7, 6877. [Google Scholar] [CrossRef]
- Huynh, T.; Reed, C.; Blackwell, Z.; Phelps, P.; Herrera, L.C.P.; Almodovar, J.; Zaharoff, D.A.; Wolchok, J. Local IL-10 Delivery Modulates the Immune Response and Enhances Repair of Volumetric Muscle Loss Muscle Injury. Sci. Rep. 2023, 13, 1983. [Google Scholar] [CrossRef]
- De Waal Malefyt, R.; Abrams, J.; Bennett, B.; Figdor, C.G.; de Vries, J.E. Interleukin 10(IL-10) Inhibits Cytokine Synthesis by Human Monocytes: An Autoregulatory Role of IL-10 Produced by Monocytes. J. Exp. Med. 1991, 174, 1209–1220. [Google Scholar] [CrossRef]
- O’Farrell, A.M.; Liu, Y.; Moore, K.W.; Mui, A.L. IL-10 Inhibits Macrophage Activation and Proliferation by Distinct Signaling Mechanisms: Evidence for Stat3-Dependent and -Independent Pathways. EMBO J. 1998, 17, 1006–1018. [Google Scholar] [CrossRef]
- Dagdeviren, S.; Jung, D.Y.; Lee, E.; Friedline, R.H.; Noh, H.L.; Kim, J.H.; Patel, P.R.; Tsitsilianos, N.; Tsitsilianos, A.V.; Tran, D.A.; et al. Altered Interleukin-10 Signaling in Skeletal Muscle Regulates Obesity-Mediated Inflammation and Insulin Resistance. Mol. Cell. Biol. 2016, 36, 2956–2966. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.-J.; Wei, R.; Li, F.; Liao, S.-Y.; Tse, H.-F. Mesenchymal Stromal Cell-Derived Exosomes in Cardiac Regeneration and Repair. Stem Cell. Rep. 2021, 16, 1662–1673. [Google Scholar] [CrossRef]
- Jorda, A.; Campos-Campos, J.; Aldasoro, C.; Colmena, C.; Aldasoro, M.; Alvarez, K.; Valles, S.L. Protective Action of Ultrasound-Guided Electrolysis Technique on the Muscle Damage Induced by Notexin in Rats. PLoS ONE 2022, 17, e0276634. [Google Scholar] [CrossRef] [PubMed]
- Hong, E.-G.; Ko, H.J.; Cho, Y.-R.; Kim, H.-J.; Ma, Z.; Yu, T.Y.; Friedline, R.H.; Kurt-Jones, E.; Finberg, R.; Fischer, M.A.; et al. Interleukin-10 Prevents Diet-Induced Insulin Resistance by Attenuating Macrophage and Cytokine Response in Skeletal Muscle. Diabetes 2009, 58, 2525–2535. [Google Scholar] [CrossRef] [PubMed]
- Chang, C.-M.; Lam, H.Y.P.; Hsu, H.-J.; Jiang, S.-J. Interleukin-10: A Double-Edged Sword in Breast Cancer. Tzu Chi Med. J. 2021, 33, 203–211. [Google Scholar] [CrossRef] [PubMed]
- Silk, A.W.; Margolin, K. Cytokine Therapy. Hematol. Oncol. Clin. N. Am. 2019, 33, 261–274. [Google Scholar] [CrossRef]
- Ho, I.-C.; Miaw, S.-C. Regulation of IL-4 Expression in Immunity and Diseases. Adv. Exp. Med. Biol. 2016, 941, 31–77. [Google Scholar] [CrossRef]
- Ohara, J.; Paul, W.E. Receptors for B-Cell Stimulatory Factor-1 Expressed on Cells of Haematopoietic Lineage. Nature 1987, 325, 537–540. [Google Scholar] [CrossRef]
- Von Haehling, S.; Wolk, K.; Höflich, C.; Kunz, S.; Grünberg, B.H.; Döcke, W.-D.; Reineke, U.; Asadullah, K.; Sterry, W.; Volk, H.-D.; et al. Interleukin-10 Receptor-1 Expression in Monocyte-Derived Antigen-Presenting Cell Populations: Dendritic Cells Partially Escape from IL-10’s Inhibitory Mechanisms. Genes Immun. 2015, 16, 8–14. [Google Scholar] [CrossRef]
- Weidemann, T.; Worch, R.; Kurgonaite, K.; Hintersteiner, M.; Bökel, C.; Schwille, P. Single Cell Analysis of Ligand Binding and Complex Formation of Interleukin-4 Receptor Subunits. Biophys. J. 2011, 101, 2360–2369. [Google Scholar] [CrossRef]
- Moretti, L.; Stalfort, J.; Barker, T.H.; Abebayehu, D. The Interplay of Fibroblasts, the Extracellular Matrix, and Inflammation in Scar Formation. J. Biol. Chem. 2022, 298, 101530. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Ding, X.; Zhang, H.; Ding, Y.; Tan, Q. IL-25 Improves Diabetic Wound Healing through Stimulating M2 Macrophage Polarization and Fibroblast Activation. Int. Immunopharmacol. 2022, 106, 108605. [Google Scholar] [CrossRef] [PubMed]
- Ronca, R.; Van Ginderachter, J.A.; Turtoi, A. Paracrine Interactions of Cancer-Associated Fibroblasts, Macrophages and Endothelial Cells: Tumor Allies and Foes. Curr. Opin. Oncol. 2018, 30, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Künze, G.; Gehrcke, J.-P.; Pisabarro, M.T.; Huster, D. NMR Characterization of the Binding Properties and Conformation of Glycosaminoglycans Interacting with Interleukin-10. Glycobiology 2014, 24, 1036–1049. [Google Scholar] [CrossRef] [PubMed]
- Gandhi, H.; Worch, R.; Kurgonaite, K.; Hintersteiner, M.; Schwille, P.; Bökel, C.; Weidemann, T. Dynamics and Interaction of Interleukin-4 Receptor Subunits in Living Cells. Biophys. J. 2014, 107, 2515–2527. [Google Scholar] [CrossRef]
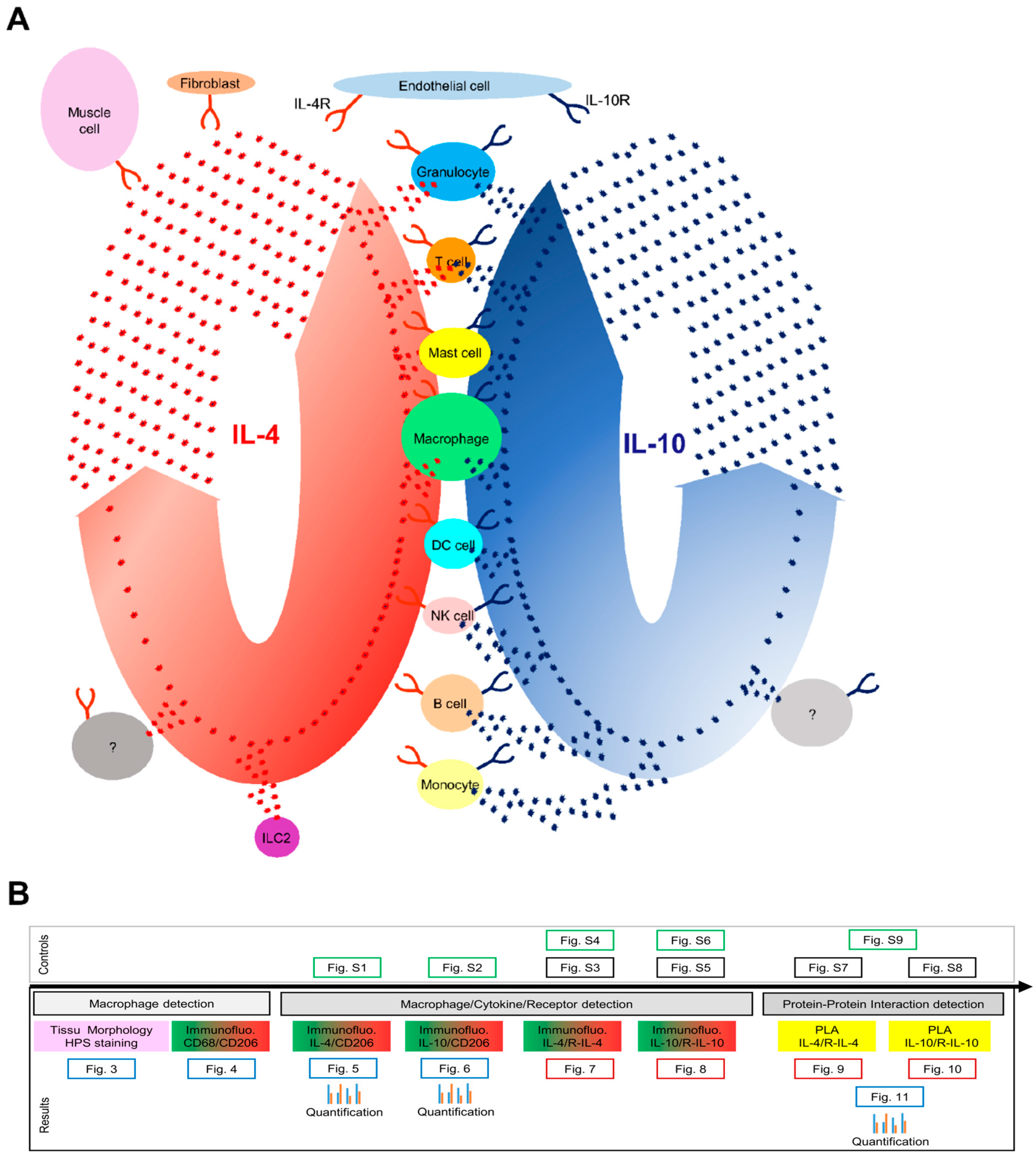





| Expression | IL-4 | IL-10 | ||
|---|---|---|---|---|
| Cytokine | Receptor | Cytokine | Receptor | |
| Granulocyte | + | + | + | + |
| T cell | + | + | + | + |
| Mast cell | + | + | + | + |
| Macrophage | + | + | + | + |
| Monocyte | − | + | + | + |
| B cell | − | + | + | + |
| DC cell | − | + | + | + |
| NK cell | − | + | + | + |
| ILC2 | − | − | − | − |
| Endothelial cell | − | + | − | + |
| Fibroblast | − | + | − | − |
| Muscle cell | − | + | − | − |
| (A) | ||||
| Expression | IL-4 | IL-10 | ||
| Skin/Lung/Muscle | Cytokine | Receptor | Cytokine | Receptor |
| Granulocyte | + * | − | − | − |
| Macrophage | ND | + | ND | + |
| Endothelial cell | − | + | − | - |
| Regenerating muscle cell | − | − | − | + |
| Uncharacterized cell | + | + | − | − |
| Uncharacterized cell | − | − | + | + |
| (B) | ||||
| Cytokine/Receptor Interaction | IL-4/IL-4R | IL-10/IL-10R | ||
| Endothelial cell | + | − | ||
| Regenerating muscle cell | − | + | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nikovics, K.; Favier, A.-L.; Rocher, M.; Mayinga, C.; Gomez, J.; Dufour-Gaume, F.; Riccobono, D. In Situ Identification of Both IL-4 and IL-10 Cytokine–Receptor Interactions during Tissue Regeneration. Cells 2023, 12, 1522. https://doi.org/10.3390/cells12111522
Nikovics K, Favier A-L, Rocher M, Mayinga C, Gomez J, Dufour-Gaume F, Riccobono D. In Situ Identification of Both IL-4 and IL-10 Cytokine–Receptor Interactions during Tissue Regeneration. Cells. 2023; 12(11):1522. https://doi.org/10.3390/cells12111522
Chicago/Turabian StyleNikovics, Krisztina, Anne-Laure Favier, Mathilde Rocher, Céline Mayinga, Johanna Gomez, Frédérique Dufour-Gaume, and Diane Riccobono. 2023. "In Situ Identification of Both IL-4 and IL-10 Cytokine–Receptor Interactions during Tissue Regeneration" Cells 12, no. 11: 1522. https://doi.org/10.3390/cells12111522





