Polarized RPE Secretome Preserves Photoreceptors in Retinal Dystrophic RCS Rats
Abstract
:1. Introduction
2. Materials and Methods
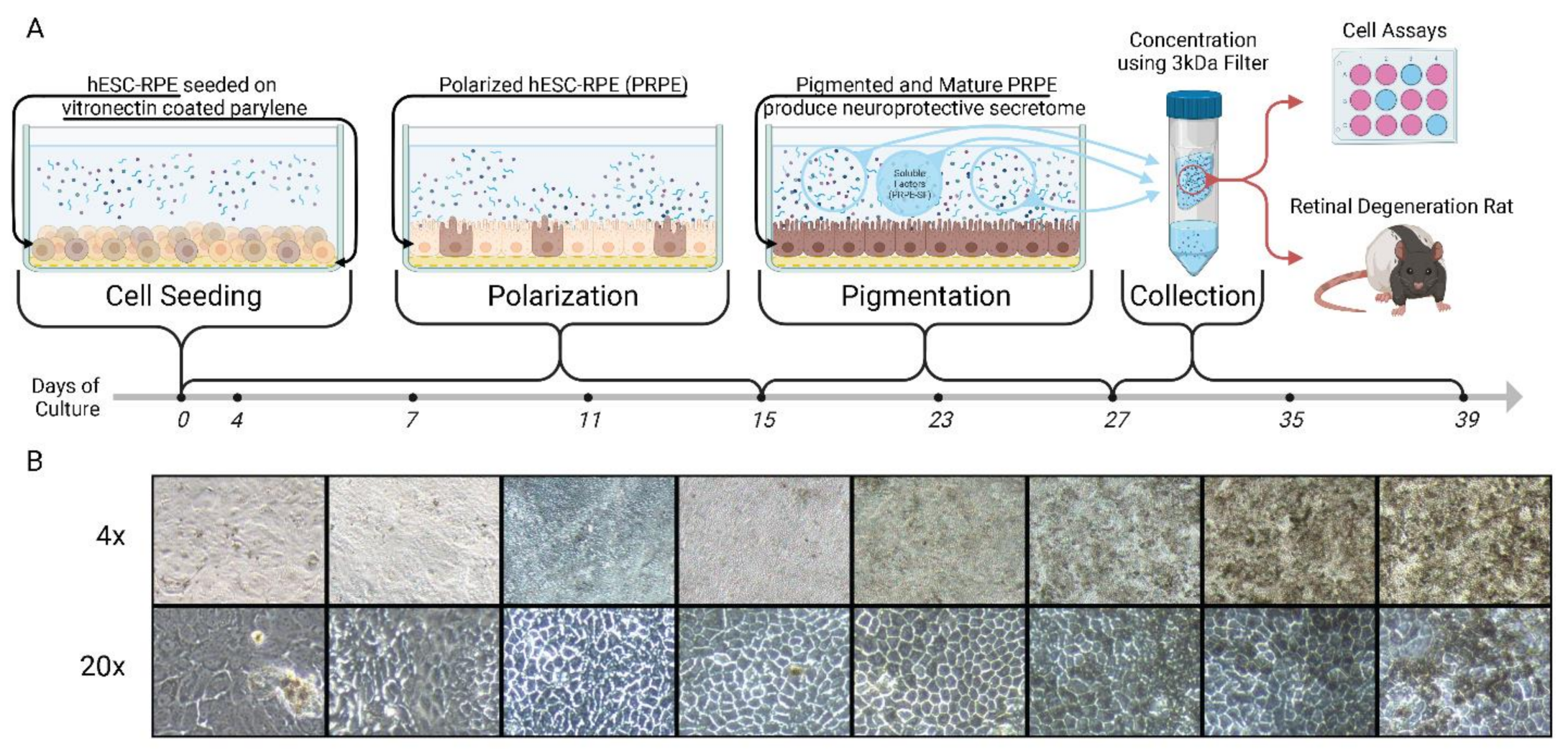
2.1. hESC-RPE Cell Culture and PRPE-SF1 Production
2.2. Fetal Retinal Progenitor Cell (RPC) and ARPE-19 Culture
2.3. ARPE-19 Cell Assays
2.4. Rhodopsin Staining
2.5. TUNEL Assay
2.6. RNA-Extraction and RT-qPCR
2.7. Animals and Experimental Design
2.8. Electroretinogram (ERG) Evaluation
2.9. Ocular Coherence Tomography (OCT) Evaluation
2.10. Intravitreal Injection
2.11. Euthanasia and Tissue Collection
2.12. Photoreceptor Counting
2.13. Immunofluorescence Staining
2.14. Statistical Analysis
2.15. Study Approval
3. Results
3.1. PRPE-SF Increases fRPC Cellular Viability and Rhodopsin Expression
3.2. PRPE-SF Induces Gene Expression in fRPC
3.3. PRPE-SF Preserves Retinal Structure and Function in RCS Rats
3.4. PRPE-SF Reduces Reactive Oxygen Species and Reactive Glial Activation
3.5. PRPE-SF Reduces NETosis Markers PAD4 and CitH3
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, W.L.; Su, X.; Li, X.; Cheung, C.M.G.; Klein, R.; Cheng, C.Y.; Wong, T.Y. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: A systematic review and meta-analysis. Lancet Glob. Health 2014, 2, e106–e116. [Google Scholar] [CrossRef] [Green Version]
- van Lookeren Campagne, M.; LeCouter, J.; Yaspan, B.L.; Ye, W. Mechanisms of age-related macular degeneration and therapeutic opportunities. J. Pathol. 2014, 232, 151–164. [Google Scholar] [CrossRef] [PubMed]
- Rozing, M.P.; Durhuus, J.A.; Krogh Nielsen, M.; Subhi, Y.; Kirkwood, T.B.L.; Westendorp, R.G.J.; Sørensen, T.L. Age-related macular degeneration: A two-level model hypothesis. Prog. Retin. Eye Res. 2020, 76, 100825. [Google Scholar] [CrossRef] [PubMed]
- Rein, D.B.; Wittenborn, J.S.; Burke-Conte, Z.; Gulia, R.; Robalik, T.; Ehrlich, J.R.; Lundeen, E.A.; Flaxman, A.D. Prevalence of Age-Related Macular Degeneration in the US in 2019. JAMA Ophthalmol. 2022, 140, 1202–1208. [Google Scholar] [CrossRef]
- Boyer, D.S.; Schmidt-Erfurth, U.; van Lookeren Campagne, M.; Henry, E.C.; Brittain, C. The Pathophysiology of Geographic Atrophy Secondary to Age-Related Macular Degeneration and the Complement Pathway as a Therapeutic Target. Retina 2017, 37, 819–835. [Google Scholar] [CrossRef]
- Chandramohan, A.; Stinnett, S.S.; Petrowski, J.T.; Schuman, S.G.; Toth, C.A.; Cousins, S.W.; Lad, E.M. Visual Function Measures in Early and Intermediate Age-Related Macular Degeneration. Retina 2016, 36, 1021–1031. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chew, E.Y.; Lindblad, A.S.; Clemons, T. Summary results and recommendations from the age-related eye disease study. Arch. Ophthalmol. 2009, 127, 1678–1679. [Google Scholar] [CrossRef] [PubMed]
- Aronow, M.; Chew, E. AREDS2: Perspectives, Recommendations, and Unanswered Questions. Curr. Opin. Ophthalmol. 2015, 25, 186–190. [Google Scholar] [CrossRef] [Green Version]
- Liao, D.S.; Grossi, F.V.; El Mehdi, D.; Gerber, M.R.; Brown, D.M.; Heier, J.S.; Wykoff, C.C.; Singerman, L.J.; Abraham, P.; Grassmann, F.; et al. Complement C3 Inhibitor Pegcetacoplan for Geographic Atrophy Secondary to Age-Related Macular Degeneration: A Randomized Phase 2 Trial. Ophthalmology 2020, 127, 186–195. [Google Scholar] [CrossRef] [Green Version]
- Meleth, A.D.; Wong, W.T.; Chew, E.Y. Treatment for atrophic macular degeneration. Curr. Opin. Ophthalmol. 2011, 22, 190–193. [Google Scholar] [CrossRef]
- Nazari, H.; Zhang, L.; Zhu, D.; Chader, G.J.; Falabella, P.; Stefanini, F.; Rowland, T.; Clegg, D.O.; Kashani, A.H.; Hinton, D.R.; et al. Stem cell based therapies for age-related macular degeneration: The promises and the challenges. Prog. Retin. Eye Res. 2015, 48, 1–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kashani, A.H.; Lebkowski, J.S.; Hinton, D.R.; Zhu, D.; Faynus, M.A.; Chen, S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chan, C.; et al. Survival of an HLA-mismatched, bioengineered RPE implant in dry age-related macular degeneration. Stem Cell Rep. 2022, 17, 448–458. [Google Scholar] [CrossRef] [PubMed]
- Mandai, M.; Kurimoto, Y.; Takahashi, M. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med. 2017, 377, 792–793. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, L.; Fynes, K.; Georgiadis, O.; Kerby, J.; Luo, Y.H.; Ahmado, A.; Vernon, A.; Daniels, J.T.; Nommiste, B.; Hasan, S.M.; et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat. Biotechnol. 2018, 36, 328–337. [Google Scholar] [CrossRef] [Green Version]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Chen, S.; Chan, C.; Palejwala, N.; Ingram, A.; Dang, W.; et al. One-Year Follow-Up in a Phase 1/2a Clinical Trial of an Allogeneic RPE Cell Bioengineered Implant for Advanced Dry Age-Related Macular Degeneration. Transl. Vis. Sci. Technol. 2021, 10, 13. [Google Scholar] [CrossRef] [PubMed]
- Kashani, A.H.; Lebkowski, J.S.; Rahhal, F.M.; Avery, R.L.; Salehi-Had, H.; Dang, W.; Lin, C.-M.M.; Mitra, D.; Zhu, D.; Thomas, B.B.; et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci. Transl. Med. 2018, 10, eaao4097. [Google Scholar] [CrossRef] [Green Version]
- Inanc Tekin, M.; Sekeroglu, M.A.; Demirtas, C.; Tekin, K.; Doguizi, S.; Bayraktar, S.; Yilmazbas, P. Brain-Derived Neurotrophic Factor in Patients With Age-Related Macular Degeneration and Its Correlation With Retinal Layer Thicknesses. Investig. Ophthalmol. Vis. Sci. 2018, 59, 2833–2840. [Google Scholar] [CrossRef] [Green Version]
- Kolomeyer, A.M.; Sugino, I.K.; Zarbin, M.A. Characterization of conditioned media collected from cultured adult versus fetal retinal pigment epithelial cells. Investig. Ophthalmol. Vis. Sci. 2011, 52, 5973–5986. [Google Scholar] [CrossRef] [Green Version]
- Mysona, B.A.; Shanab, A.Y.; Elshaer, S.L.; El-Remessy, A.B. Nerve growth factor in diabetic retinopathy: Beyond neurons. Expert Rev. Ophthalmol. 2014, 9, 99–107. [Google Scholar] [CrossRef]
- Meyer, J.G.; Garcia, T.Y.; Schilling, B.; Gibson, B.W.; Lamba, D.A. Proteome and Secretome Dynamics of Human Retinal Pigment Epithelium in Response to Reactive Oxygen Species. Sci. Rep. 2019, 9, 15440. [Google Scholar] [CrossRef] [Green Version]
- Yabe, T.; Kanemitsu, K.; Sanagi, T.; Schwartz, J.P.; Yamada, H. Pigment epithelium-derived factor induces pro-survival genes through cyclic AMP-responsive element binding protein and nuclear factor kappa B activation in rat cultured cerebellar granule cells: Implication for its neuroprotective effect. Neuroscience 2005, 133, 691–700. [Google Scholar] [CrossRef] [PubMed]
- LaVail, M.M.; Unoki, K.; Yasumura, D.; Matthes, M.T.; Yancopoulos, G.D.; Steinberg, R.H. Multiple growth factors, cytokines, and neurotrophins rescue photoreceptors from the damaging effects of constant light. Proc. Natl. Acad. Sci. USA 1992, 89, 11249–11253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, P.B.; Seiler, M.J.; Aramant, R.B.; Yan, F.; Mahoney, M.J.; Kitzes, L.M.; Keirstead, H.S. Trophic factors GDNF and BDNF improve function of retinal sheet transplants. Exp. Eye Res. 2010, 91, 727–738. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolomeyer, A.M.; Zarbin, M.A. Trophic factors in the pathogenesis and therapy for retinal degenerative diseases. Surv. Ophthalmol. 2014, 59, 134–165. [Google Scholar] [CrossRef]
- Kanu, L.N.; Ciolino, J.B. Nerve Growth Factor as an Ocular Therapy: Applications, Challenges, and Future Directions. Semin. Ophthalmol. 2021, 36, 224–231. [Google Scholar] [CrossRef]
- Diniz, B.; Thomas, P.; Thomas, B.; Ribeiro, R.; Hu, Y.; Brant, R.; Ahuja, A.; Zhu, D.; Liu, L.; Koss, M.; et al. Subretinal Implantation of Retinal Pigment Epithelial Cells Derived From Human Embryonic Stem Cells: Improved Survival When Implanted as a Monolayer. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5087–5096. [Google Scholar] [CrossRef] [Green Version]
- Sheedlo, H.J.; Li, L.; Turner, J.E. Effects of RPE-cell factors secreted from permselective fibers on retinal cells in vitro. Brain Res. 1992, 587, 327–337. [Google Scholar] [CrossRef]
- Sheedlo, H.J.; Li, L.; Turner, J.E. Effects of RPE age and culture conditions on support of photoreceptor cell survival in transplanted RCS dystrophic rats. Exp. Eye Res. 1993, 57, 753–761. [Google Scholar] [CrossRef]
- Sheedlo, H.J.; Fan, W.; Li, L.; Turner, J.E. Retinal pigment epithelial cell support of photoreceptor survival in vitro. Vitr. Cell. Dev. Biol.-Anim. J. Soc. Vitr. Biol. 1995, 31, 330–333. [Google Scholar] [CrossRef]
- Sheedlo, H.J.; Turner, J.E. Influence of a retinal pigment epithelial cell factor(s) on rat retinal progenitor cells. Dev. Brain Res. 1996, 93, 88–99. [Google Scholar] [CrossRef]
- Sheedlo, H.J.; Turner, J.E. Effects of retinal pigment epithelial cell-secreted factors on neonatal rat retinal explant progenitor cells. J. Neurosci. Res. 1996, 44, 519–531. [Google Scholar] [CrossRef]
- Sheedlo, H.J.; Wordinger, R.J.; Fan, W.; Turner, J.E. A transformed neonatal rat retinal pigment epithelial cell line: Secreted protein analysis and fibroblast growth factor and receptor expression. Curr. Eye Res. 1997, 16, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Sheedlo, H.J.; Nelson, T.H.; Lin, N.; Rogers, T.A.; Roque, R.S.; Turner, J.E. RPE secreted proteins and antibody influence photoreceptor cell survival and maturation. Dev. Brain Res. 1998, 107, 57–69. [Google Scholar] [CrossRef] [PubMed]
- Landowski, M.; Kelly, U.; Klingeborn, M.; Groelle, M.; Ding, J.D.; Grigsby, D.; Bowes Rickman, C. Human complement factor H Y402H polymorphism causes an age-related macular degeneration phenotype and lipoprotein dysregulation in mice. Proc. Natl. Acad. Sci. USA 2019, 116, 3703–3711. [Google Scholar] [CrossRef] [Green Version]
- Tong, Y.; Wang, S. Not All Stressors Are Equal: Mechanism of Stressors on RPE Cell Degeneration. Front. Cell Dev. Biol. 2020, 8, 591067. [Google Scholar] [CrossRef]
- Chen, L.; Perera, N.D.; Karoukis, A.J.; Feathers, K.L.; Ali, R.R.; Thompson, D.A.; Fahim, A.T. Oxidative stress differentially impacts apical and basolateral secretion of angiogenic factors from human iPSC-derived retinal pigment epithelium cells. Sci. Rep. 2022, 12, 12694. [Google Scholar] [CrossRef]
- Atienzar-Aroca, S.; Flores-Bellver, M.; Serrano-Heras, G.; Martinez-Gil, N.; Barcia, J.M.; Aparicio, S.; Perez-Cremades, D.; Garcia-Verdugo, J.M.; Diaz-Llopis, M.; Romero, F.J.; et al. Oxidative stress in retinal pigment epithelium cells increases exosome secretion and promotes angiogenesis in endothelial cells. J. Cell Mol. Med. 2016, 20, 1457–1466. [Google Scholar] [CrossRef]
- Sreekumar, P.G.; Kannan, R.; Kitamura, M.; Spee, C.; Barron, E.; Ryan, S.J.; Hinton, D.R. αB Crystallin Is Apically Secreted within Exosomes by Polarized Human Retinal Pigment Epithelium and Provides Neuroprotection to Adjacent Cells. PLoS ONE 2010, 5, e12578. [Google Scholar] [CrossRef] [Green Version]
- Rabin, D.M.; Rabin, R.L.; Blenkinsop, T.A.; Temple, S.; Stern, J.H. Chronic oxidative stress upregulates Drusen-related protein expression in adult human RPE stem cell-derived RPE cells: A novel culture model for dry AMD. Aging 2012, 5, 51–66. [Google Scholar] [CrossRef] [Green Version]
- Pennington, B.O.; Clegg, D.O.; Melkoumian, Z.K.; Hikita, S.T. Defined culture of human embryonic stem cells and xeno-free derivation of retinal pigmented epithelial cells on a novel, synthetic substrate. Stem Cells Transl. Med. 2015, 4, 165–177. [Google Scholar] [CrossRef]
- Hu, Y.; Liu, L.; Lu, B.; Zhu, D.; Ribeiro, R.; Diniz, B.; Thomas, P.B.; Ahuja, A.K.; Hinton, D.R.; Tai, Y.C.; et al. A novel approach for subretinal implantation of ultrathin substrates containing stem cell-derived retinal pigment epithelium monolayer. Ophthalmic. Res. 2012, 48, 186–191. [Google Scholar] [CrossRef] [PubMed]
- Rowland, T.J.; Blaschke, A.J.; Buchholz, D.E.; Hikita, S.T.; Johnson, L.V.; Clegg, D.O. Differentiation of human pluripotent stem cells to retinal pigmented epithelium in defined conditions using purified extracellular matrix proteins. J. Tissue Eng. Regen. Med. 2013, 7, 642–653. [Google Scholar] [CrossRef]
- Lu, B.; Zhu, D.; Hinton, D.; Humayun, M.S.; Tai, Y.C. Mesh-supported submicron parylene-C membranes for culturing retinal pigment epithelial cells. Biomed Microdevices 2012, 14, 659–667. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.B.; Zhu, D.; Lin, T.C.; Kim, Y.C.; Seiler, M.J.; Martinez-Camarillo, J.C.; Lin, B.; Shad, Y.; Hinton, D.R.; Humayun, M.S. A new immunodeficient retinal dystrophic rat model for transplantation studies using human-derived cells. Graefe’s Arch. Clin. Exp. Ophthalmol. 2018, 256, 2113–2125. [Google Scholar] [CrossRef] [Green Version]
- Gao, F.; Zhang, Y.; Wang, S.; Liu, Y.; Zheng, L.; Yang, J.; Huang, W.; Ye, Y.; Luo, W.; Xiao, D. Hes1 is involved in the self-renewal and tumourigenicity of stem-like cancer cells in colon cancer. Sci. Rep. 2014, 4, 3963. [Google Scholar] [CrossRef] [Green Version]
- Bras-Pereira, C.; Casares, F.; Janody, F. The retinal determination gene Dachshund restricts cell proliferation by limiting the activity of the Homothorax-Yorkie complex. Development 2015, 142, 1470–1479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tetreault, N.; Champagne, M.P.; Bernier, G. The LIM homeobox transcription factor Lhx2 is required to specify the retina field and synergistically cooperates with Pax6 for Six6 trans-activation. Dev. Biol. 2009, 327, 541–550. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yun, S.; Saijoh, Y.; Hirokawa, K.E.; Kopinke, D.; Murtaugh, L.C.; Monuki, E.S.; Levine, E.M. Lhx2 links the intrinsic and extrinsic factors that control optic cup formation. Development 2009, 136, 3895–3906. [Google Scholar] [CrossRef] [Green Version]
- Lee, J.H.; Park, H.S.; Shin, J.M.; Chun, M.H.; Oh, S.J. Nestin expressing progenitor cells during establishment of the neural retina and its vasculature. Anat. Cell Biol. 2012, 45, 38–46. [Google Scholar] [CrossRef] [Green Version]
- Fernández-López, B.; Romaus-Sanjurjo, D.; Senra-Martínez, P.; Anadón, R.; Barreiro-Iglesias, A.; Celina Rodicio, M. Spatiotemporal pattern of doublecortin expression in the retina of the sea lamprey. Front. Neuroanat. 2016, 10, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernardos, R.L.; Barthel, L.K.; Meyers, J.R.; Raymond, P.A. Late-stage neuronal progenitors in the retina are radial Muller glia that function as retinal stem cells. J. Neurosci. 2007, 27, 7028–7040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamba, D.A.; McUsic, A.; Hirata, R.K.; Wang, P.R.; Russell, D.; Reh, T.A. Generation, purification and transplantation of photoreceptors derived from human induced pluripotent stem cells. PLoS ONE 2010, 5, e8763. [Google Scholar] [CrossRef] [PubMed]
- Klassen, H.J.; Ng, T.F.; Kurimoto, Y.; Kirov, I.; Shatos, M.; Coffey, P.; Young, M.J. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Investig. Ophthalmol. Vis. Sci. 2004, 45, 4167–4173. [Google Scholar] [CrossRef]
- Schmitt, S.; Aftab, U.; Jiang, C.; Redenti, S.; Klassen, H.; Miljan, E.; Sinden, J.; Young, M. Molecular characterization of human retinal progenitor cells. Investig. Ophthalmol. Vis. Sci. 2009, 50, 5901–5908. [Google Scholar] [CrossRef]
- Salinas, M.; Diaz, R.; Abraham, N.G.; Ruiz de Galarreta, C.M.; Cuadrado, A. Nerve Growth Factor Protects against 6-Hydroxydopamine-induced Oxidative Stress by Increasing Expression of Heme Oxygenase-1 in a Phosphatidylinositol 3-Kinase-dependent Manner*. J. Biol. Chem. 2003, 278, 13898–13904. [Google Scholar] [CrossRef] [Green Version]
- Hacioglu, G.; Senturk, A.; Ince, I.; Alver, A. Assessment of oxidative stress parameters of brain-derived neurotrophic factor heterozygous mice in acute stress model. Iran. J. Basic Med. Sci. 2016, 19, 388–393. [Google Scholar]
- Dong, S.; Zhen, F.; Xu, H.; Li, Q.; Wang, J. Leukemia inhibitory factor protects photoreceptor cone cells against oxidative damage through activating JAK/STAT3 signaling. Ann. Transl. Med. 2021, 9, 152. [Google Scholar] [CrossRef]
- He, X.; Cheng, R.; Benyajati, S.; Ma, J.X. PEDF and its roles in physiological and pathological conditions: Implication in diabetic and hypoxia-induced angiogenic diseases. Clin. Sci. 2015, 128, 805–823. [Google Scholar] [CrossRef] [Green Version]
- Sardar Pasha, S.P.B.; Münch, R.; Schäfer, P.; Oertel, P.; Sykes, A.M.; Zhu, Y.; Karl, M.O. Retinal cell death dependent reactive proliferative gliosis in the mouse retina. Sci. Rep. 2017, 7, 9517. [Google Scholar] [CrossRef] [Green Version]
- Hollingsworth, T.J.; Gross, A.K. Innate and Autoimmunity in the Pathogenesis of Inherited Retinal Dystrophy. Cells 2020, 9, 630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rohrbach, A.S.; Slade, D.J.; Thompson, P.R.; Mowen, K.A. Activation of PAD4 in NET formation. Front. Immunol. 2012, 3, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, D.; Deng, X.; Spee, C.; Sonoda, S.; Hsieh, C.-L.L.; Barron, E.; Pera, M.; Hinton, D.R. Polarized Secretion of PEDF from Human Embryonic Stem Cell–Derived RPE Promotes Retinal Progenitor Cell Survival. Investig. Opthalmology Vis. Sci. 2011, 52, 1573–1575. [Google Scholar] [CrossRef] [PubMed]
- Danhong Zhu, J.H. Retinal Pigment Epithelial Cell Conditioned Medium Enhances the Yield of RPE Cells Differentiated from Human Embryonic Stem Cells. J. Clin. Exp. Pathol. 2015, 5, 216. [Google Scholar] [CrossRef]
- Gaur, V.P.; Liu, Y.; Turner, J.E. RPE conditioned medium stimulates photoreceptor cell survival, neurite outgrowth and differentiation in vitro. Exp. Eye Res. 1992, 54, 645–659. [Google Scholar] [CrossRef] [PubMed]
- Dutt, K.; Douglas, P.; Cao, Y. RPE-secreted factors: Influence differentiation in human retinal cell line in dose- and density-dependent manner. J. Ocul. Biol. Dis. Infor. 2010, 3, 144–160. [Google Scholar] [CrossRef] [Green Version]
- Kimura, A.; Namekata, K.; Guo, X.; Harada, C.; Harada, T. Neuroprotection, Growth Factors and BDNF-TrkB Signalling in Retinal Degeneration. Int. J. Mol. Sci. 2016, 17, 1584. [Google Scholar] [CrossRef] [Green Version]
- Raffaele, O.S.; Dompé Farmaceutici, S.p.A. Nerve Growth Factor Eye Drops Treatment in Patients With Retinitis Pigmentosa and Cystoid Macular Edema. 2015. Available online: https://clinicaltrials.gov/ct2/show/NCT02609165 (accessed on 6 March 2023).
- GenVec. Study of AdGVPEDF.11D in Neovascular Age-related Macular Degeneration (AMD). Available online: https://clinicaltrials.gov/ct2/show/NCT00109499 (accessed on 6 March 2023).
- Dompé Farmaceutici, S.p.A.; Cromsource. Study to Evaluate Safety and Efficacy of rhNGF Eye Drops Solution Versus Vehicle in Patients With Glaucoma. 2016. Available online: https://clinicaltrials.gov/ct2/show/NCT02855450 (accessed on 6 March 2023).
- Ye, F.; Kaneko, H.; Hayashi, Y.; Takayama, K.; Hwang, S.J.; Nishizawa, Y.; Kimoto, R.; Nagasaka, Y.; Tsunekawa, T.; Matsuura, T.; et al. Malondialdehyde induces autophagy dysfunction and VEGF secretion in the retinal pigment epithelium in age-related macular degeneration. Free. Radic. Biol. Med. 2016, 94, 121–134. [Google Scholar] [CrossRef]
- Papac-Milicevic, N.; Busch, C.J.; Binder, C.J. Malondialdehyde Epitopes as Targets of Immunity and the Implications for Atherosclerosis. Adv. Immunol. 2016, 131, 1–59. [Google Scholar] [CrossRef] [Green Version]
- Bonilha, V.L.; Shadrach, K.G.; Rayborn, M.E.; Li, Y.; Pauer, G.J.; Hagstrom, S.A.; Bhattacharya, S.K.; Hollyfield, J.G. Retinal deimination and PAD2 levels in retinas from donors with age-related macular degeneration (AMD). Exp. Eye Res. 2013, 111, 71–78. [Google Scholar] [CrossRef] [Green Version]
- Hinton, D.R.; Zhou, J.; He, S.; Zhang, N.; Spee, C.; Zhou, P.; Ryan, S.J.; Kannan, R. Neutrophils compromise retinal pigment epithelial barrier integrity. J. Biomed. Biotechnol. 2010, 2010, 289360. [Google Scholar] [CrossRef] [Green Version]
- Binet, F.; Cagnone, G.; Crespo-Garcia, S.; Hata, M.; Neault, M.; Dejda, A.; Wilson, A.M.; Buscarlet, M.; Mawambo, G.T.; Howard, J.P.; et al. Neutrophil extracellular traps target senescent vasculature for tissue remodeling in retinopathy. Science 2020, 369, eaay5356. [Google Scholar] [CrossRef] [PubMed]
- Dong, T.; Dave, P.; Yoo, E.; Ebright, B.; Ahluwalia, K.; Zhou, E.; Asante, I.; Salimova, M.; Pei, H.; Lin, T.; et al. NAP1051, a Lipoxin A4 Biomimetic Analogue, Demonstrates Antitumor Activity Against the Tumor Microenvironment. Mol. Cancer Ther. 2021, 20, 2384–2397. [Google Scholar] [CrossRef]
- Palko, S.I.; Saba, N.J.; Mullane, E.; Nicholas, B.D.; Nagasaka, Y.; Ambati, J.; Gelfand, B.D.; Ishigami, A.; Bargagna-Mohan, P.; Mohan, R. Compartmentalized citrullination in Muller glial endfeet during retinal degeneration. Proc. Natl. Acad. Sci. USA 2022, 119, e2121875119. [Google Scholar] [CrossRef]
- D’Cruz, P.M. Mutation of the receptor tyrosine kinase gene Mertk in the retinal dystrophic RCS rat. Hum. Mol. Genet. 2000, 9, 645–651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leenaars, C.H.C.; Kouwenaar, C.; Stafleu, F.R.; Bleich, A.; Ritskes-Hoitinga, M.; De Vries, R.B.M.; Meijboom, F.L.B. Animal to human translation: A systematic scoping review of reported concordance rates. J. Transl. Med. 2019, 17, 223. [Google Scholar] [CrossRef] [Green Version]









Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ahluwalia, K.; Martinez-Camarillo, J.-C.; Thomas, B.B.; Naik, A.; Gonzalez-Calle, A.; Pollalis, D.; Lebkowski, J.; Lee, S.Y.; Mitra, D.; Louie, S.G.; et al. Polarized RPE Secretome Preserves Photoreceptors in Retinal Dystrophic RCS Rats. Cells 2023, 12, 1689. https://doi.org/10.3390/cells12131689
Ahluwalia K, Martinez-Camarillo J-C, Thomas BB, Naik A, Gonzalez-Calle A, Pollalis D, Lebkowski J, Lee SY, Mitra D, Louie SG, et al. Polarized RPE Secretome Preserves Photoreceptors in Retinal Dystrophic RCS Rats. Cells. 2023; 12(13):1689. https://doi.org/10.3390/cells12131689
Chicago/Turabian StyleAhluwalia, Kabir, Juan-Carlos Martinez-Camarillo, Biju B. Thomas, Aditya Naik, Alejandra Gonzalez-Calle, Dimitrios Pollalis, Jane Lebkowski, Sun Young Lee, Debbie Mitra, Stan G. Louie, and et al. 2023. "Polarized RPE Secretome Preserves Photoreceptors in Retinal Dystrophic RCS Rats" Cells 12, no. 13: 1689. https://doi.org/10.3390/cells12131689
APA StyleAhluwalia, K., Martinez-Camarillo, J. -C., Thomas, B. B., Naik, A., Gonzalez-Calle, A., Pollalis, D., Lebkowski, J., Lee, S. Y., Mitra, D., Louie, S. G., & Humayun, M. S. (2023). Polarized RPE Secretome Preserves Photoreceptors in Retinal Dystrophic RCS Rats. Cells, 12(13), 1689. https://doi.org/10.3390/cells12131689





