Sex-Based Disparities in Leukocyte Migration and Activation in Response to Inhalation Lung Injury: Role of SDF-1/CXCR4 Signaling
Abstract
:1. Introduction
2. Methods
2.1. Mouse Model
2.2. Cl2 Exposure
2.3. Isolation of Bronchoalveolar Lavage Fluid (BALF) and Blood Leukocytes
2.4. Isolating Single Cell Suspension from Whole Lung
2.5. SDF-1 Protein Quantification
2.6. Evaluation of Surface Levels of CXCR4 in Lung Cells Using Flow Cytometry
2.7. Leukocyte Migration Assay
2.8. Myeloperoxidase Activity Assay
2.9. Elastase Activity Assay
2.10. Statistics
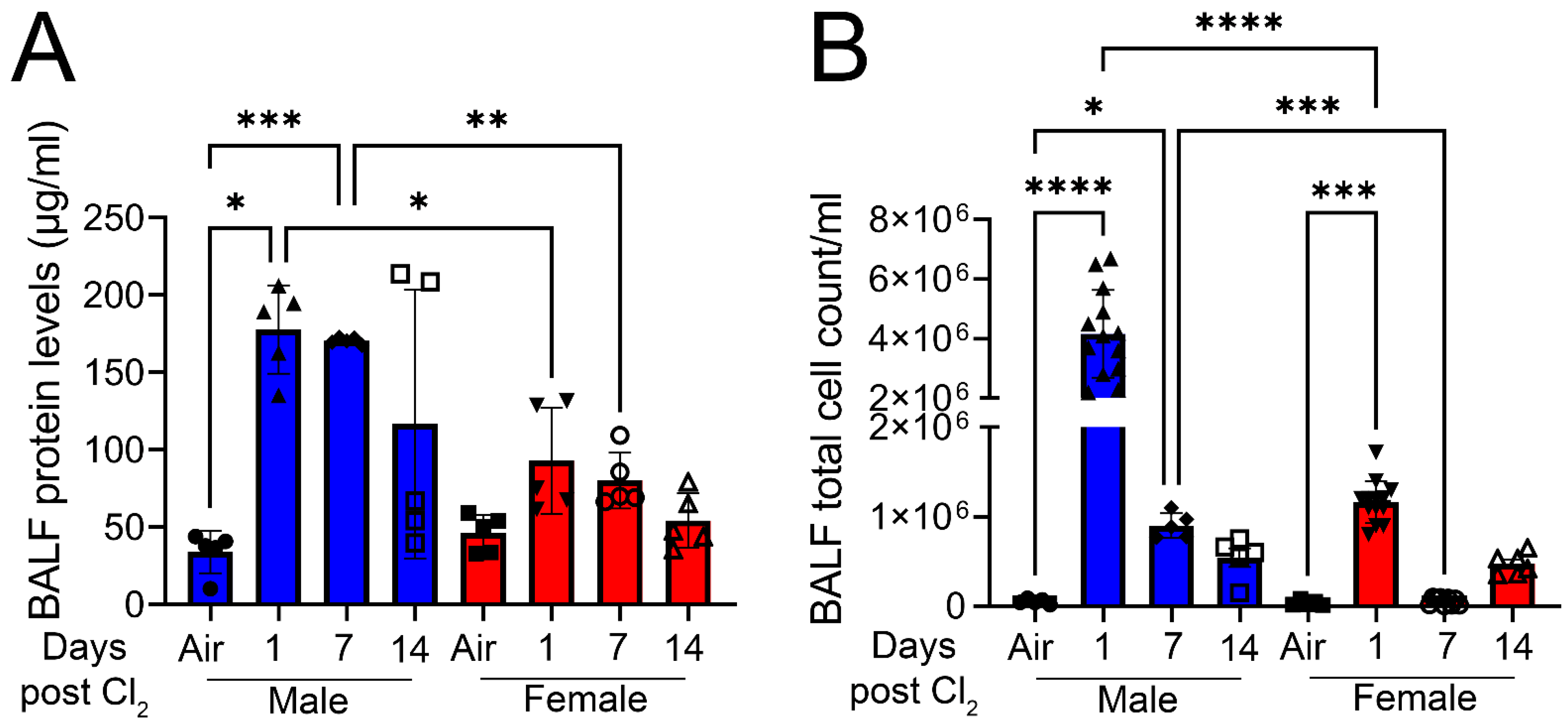
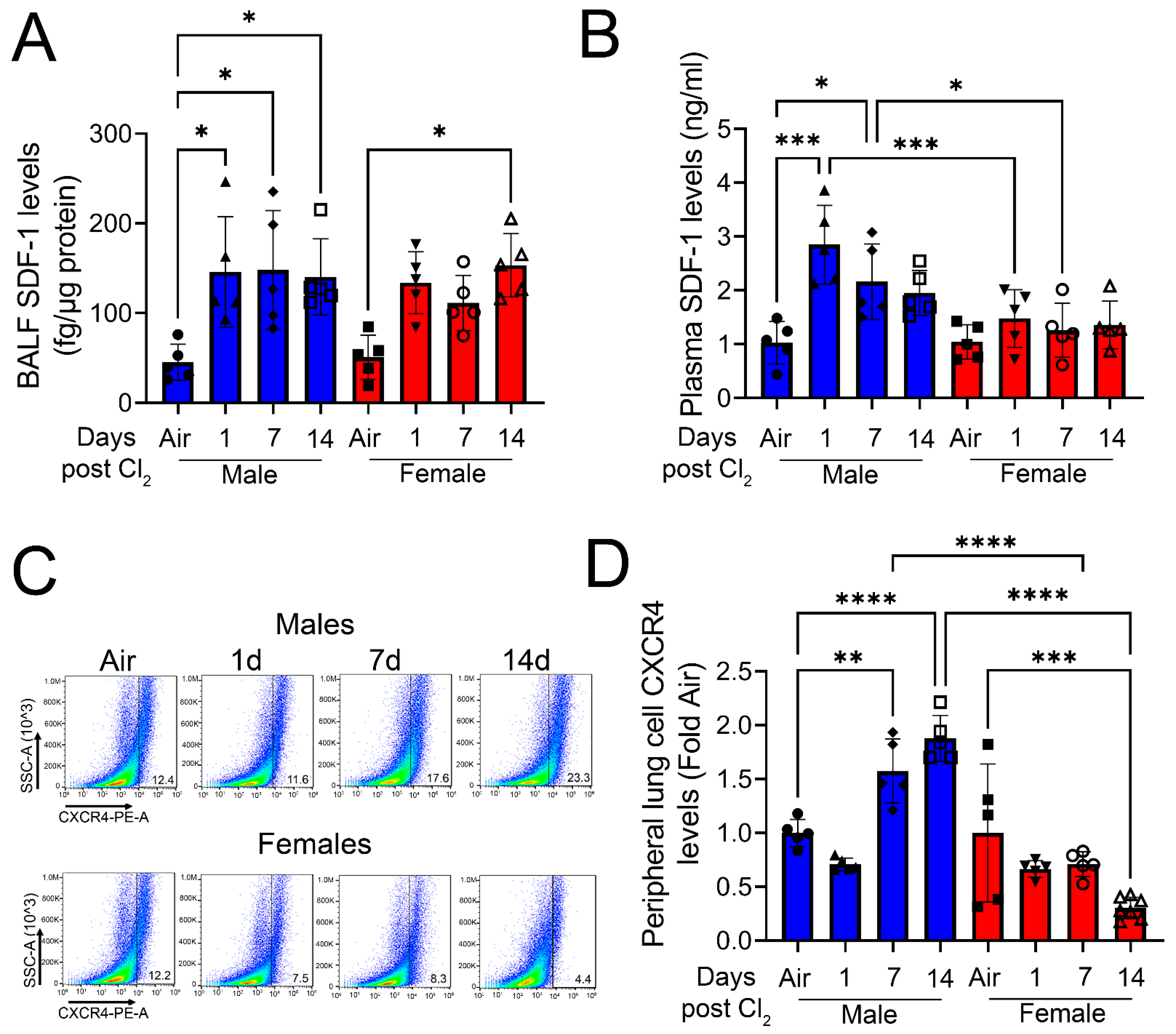
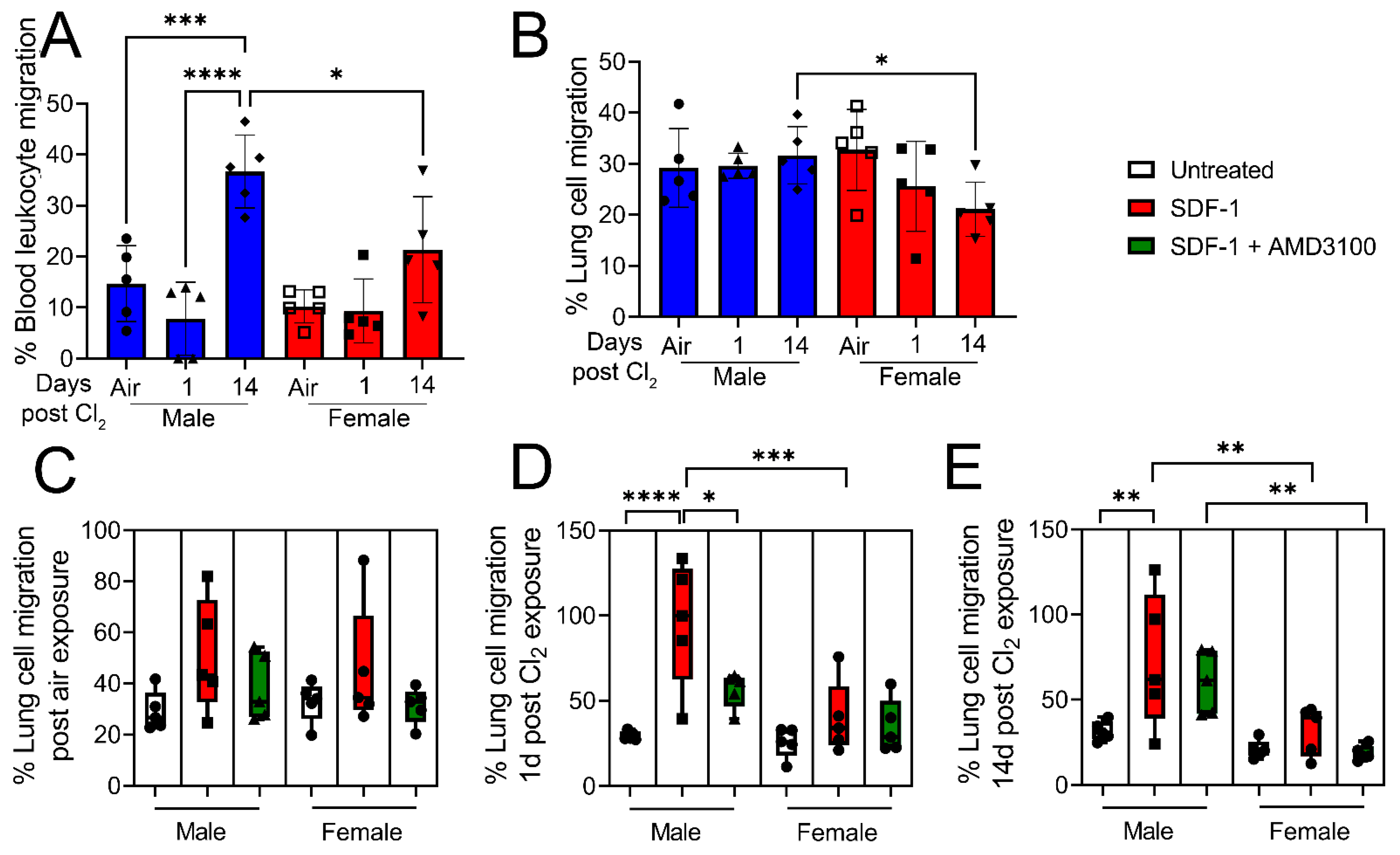
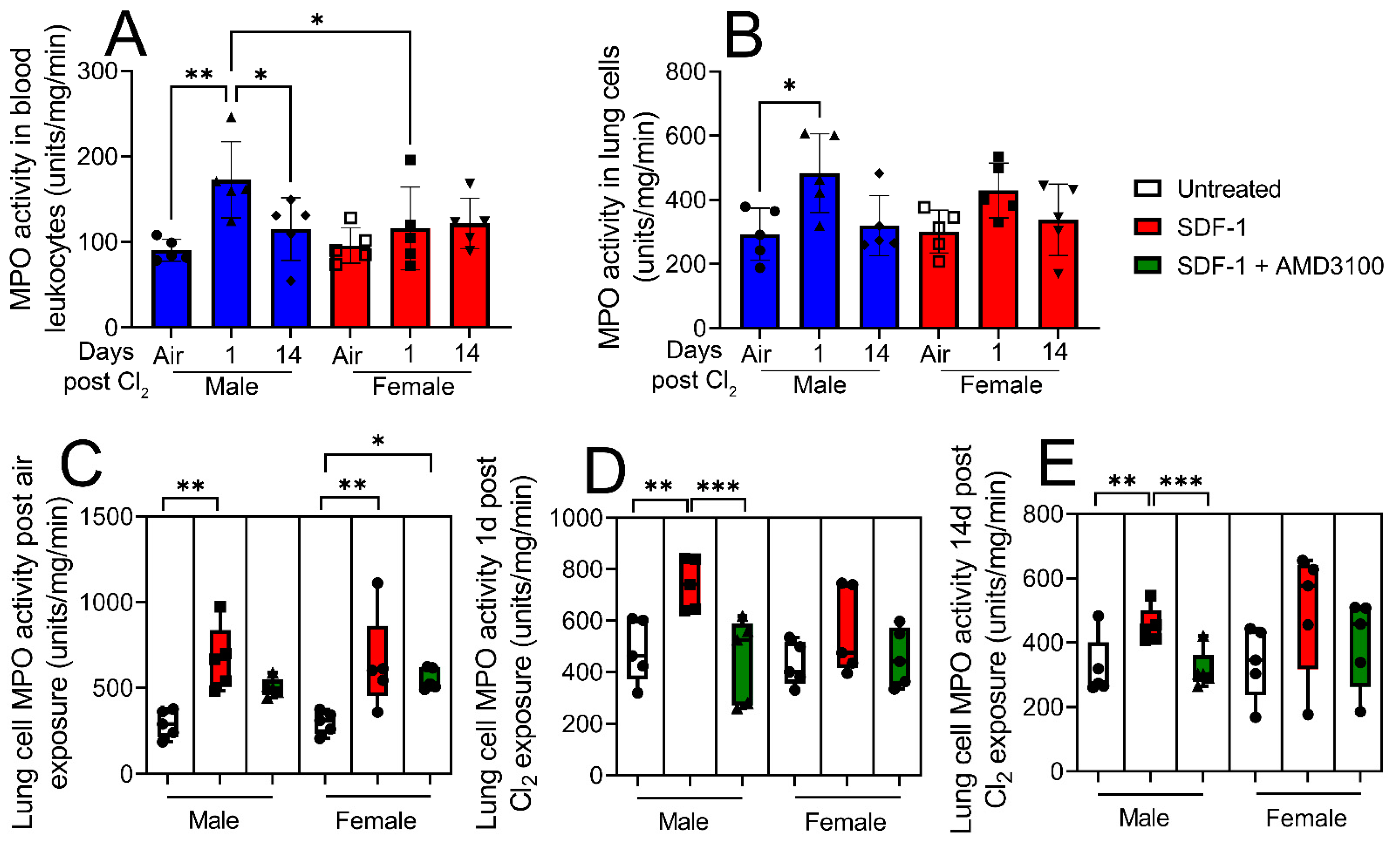
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jacobson, D.L.; Gange, S.J.; Rose, N.R.; Graham, N.M. Epidemiology and Estimated Population Burden of Selected Autoimmune Diseases in the United States. Clin. Immunol. Immunopathol. 1997, 84, 223–243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cook, M.B.; Dawsey, S.M.; Freedman, N.D.; Inskip, P.D.; Wichner, S.M.; Quraishi, S.M.; Devesa, S.S.; McGlynn, K.A. Sex Disparities in Cancer Incidence by Period and Age. Cancer Epidemiol. Biomark. Prev. 2009, 18, 1174–1182. [Google Scholar] [CrossRef] [Green Version]
- Cook, M.B.; McGlynn, K.A.; Devesa, S.S.; Freedman, N.D.; Anderson, W.F. Sex Disparities in Cancer Mortality and Survival. Cancer Epidemiol. Biomark. Prev. 2011, 20, 1629–1637. [Google Scholar] [CrossRef] [Green Version]
- Vom Steeg, L.G.; Klein, S.L. SeXX Matters in Infectious Disease Pathogenesis. PLoS Pathog. 2016, 12, e1005374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Engler, R.J.M.; Nelson, M.R.; Klote, M.M.; VanRaden, M.J.; Huang, C.-Y.; Cox, N.J.; Klimov, A.; Keitel, W.A.; Nichol, K.L.; Carr, W.W.; et al. Half- vs Full-Dose Trivalent Inactivated Influenza Vaccine (2004–2005): Age, dose, and sex effects on immune responses. Arch. Intern. Med. 2008, 168, 2405–2414. [Google Scholar] [CrossRef]
- Benten, W.P.M.; Wunderlich, F.; Mossmann, H. Testosterone-induced suppression of self-healing Plasmodium chabaudi malaria: An effect not mediated by androgen receptors? J. Endocrinol. 1992, 135, 407–413. [Google Scholar] [CrossRef]
- Takahashi, T.; Ellingson, M.K.; Wong, P.; Israelow, B.; Lucas, C.; Klein, J.; Silva, J.; Mao, T.; Oh, J.E.; Tokuyama, M.; et al. Sex differences in immune responses that underlie COVID-19 disease outcomes. Nature 2020, 588, 315–320. [Google Scholar] [CrossRef]
- Gummin, D.D.; Mowry, J.B.; Spyker, D.A.; Brooks, D.E.; Fraser, M.O.; Banner, W. 2016 Annual Report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 34th Annual Report. Clin. Toxicol. 2017, 55, 1072–1252. [Google Scholar] [CrossRef]
- Aggarwal, S.; Lazrak, A.; Ahmad, I.; Yu, Z.; Bryant, A.; Mobley, J.A.; Ford, D.A.; Matalon, S. Reactive species generated by heme impair alveolar epithelial sodium channel function in acute respiratory distress syndrome. Redox Biol. 2020, 36, 101592. [Google Scholar] [CrossRef]
- Carlisle, M.; Lam, A.; Svendsen, E.R.; Aggarwal, S.; Matalon, S. Chlorine-induced cardiopulmonary injury. Ann. N. Y. Acad. Sci. 2016, 1374, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Honavar, J.; Doran, S.; Oh, J.-Y.; Steele, C.; Matalon, S.; Patel, R.P. Nitrite therapy improves survival postexposure to chlorine gas. Am. J. Physiol. Lung Cell. Mol. Physiol. 2014, 307, L888–L894. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Honavar, J.; Doran, S.; Ricart, K.; Matalon, S.; Patel, R.P. Nitrite therapy prevents chlorine gas toxicity in rabbits. Toxicol. Lett. 2017, 271, 20–25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jurkuvenaite, A.; Benavides, G.A.; Komarova, S.; Doran, S.F.; Johnson, M.; Aggarwal, S.; Zhang, J.; Darley-Usmar, V.M.; Matalon, S. Upregulation of autophagy decreases chlorine-induced mitochondrial injury and lung inflammation. Free. Radic. Biol. Med. 2015, 85, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Lazrak, A.; Creighton, J.; Yu, Z.; Komarova, S.; Doran, S.F.; Aggarwal, S.; Sr, C.W.E.; Stober, V.P.; Trempus, C.S.; Garantziotis, S.; et al. Hyaluronan mediates airway hyperresponsiveness in oxidative lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2015, 308, L891–L903. [Google Scholar] [CrossRef]
- Lazrak, A.; Song, W.; Zhou, T.; Aggarwal, S.; Jilling, T.; Garantziotis, S.; Matalon, S. Hyaluronan and halogen-induced airway hyperresponsiveness and lung injury. Ann. N. Y. Acad. Sci. 2020, 1479, 29–43. [Google Scholar] [CrossRef] [PubMed]
- Kubo, H.; Graham, L.; Doyle, N.A.; Quinlan, W.M.; Hogg, J.C.; Doerschuk, C.M. Complement fragment-induced release of neutrophils from bone marrow and sequestration within pulmonary capillaries in rabbits. Blood 1998, 92, 283–290. [Google Scholar]
- Kubo, H.; Morgenstern, D.; Quinian, W.M.; Ward, P.A.; Dinauer, M.C.; Doerschuk, C.M. Preservation of complement-induced lung injury in mice with deficiency of NADPH oxidase. J. Clin. Investig. 1996, 97, 2680–2684. [Google Scholar] [CrossRef]
- Bleul, C.C.; Farzan, M.; Choe, H.; Parolin, C.; Clark-Lewis, I.; Sodroski, J.; Springer, T.A. The lymphocyte chemoattractant SDF-1 is a ligand for LESTR/fusin and blocks HIV-1 entry. Nature 1996, 382, 829–833. [Google Scholar] [CrossRef]
- Nagasawa, T.; Hirota, S.; Tachibana, K.; Takakura, N.; Nishikawa, S.-I.; Kitamura, Y.; Yoshida, N.; Kikutani, H.; Kishimoto, T. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature 1996, 382, 635–638. [Google Scholar] [CrossRef]
- Oberlin, E.; Amara, A.; Bachelerie, F.; Bessia, C.; Virelizier, J.-L.; Arenzana-Seisdedos, F.; Schwartz, O.; Heard, J.-M.; Clark-Lewis, I.; Legler, D.F.; et al. The CXC chemokine SDF-1 is the ligand for LESTR/fusin and prevents infection by T-cell-line-adapted HIV-1. Nature 1996, 382, 833–835. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.; Burdon, P.C.; Bridger, G.; Gutierrez-Ramos, J.-C.; Williams, T.J.; Rankin, S.M. Chemokines Acting via CXCR2 and CXCR4 Control the Release of Neutrophils from the Bone Marrow and Their Return following Senescence. Immunity 2003, 19, 583–593. [Google Scholar] [CrossRef] [Green Version]
- Eash, K.J.; Means, J.M.; White, D.W.; Link, D.C. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood 2009, 113, 4711–4719. [Google Scholar] [CrossRef] [Green Version]
- Liles, W.C.; Broxmeyer, H.E.; Rodger, E.; Wood, B.; Hubel, K.; Cooper, S.; Hangoc, G.; Bridger, G.J.; Henson, G.W.; Calandra, G.; et al. Mobilization of hematopoietic progenitor cells in healthy volunteers by AMD3100, a CXCR4 antagonist. Blood 2003, 102, 2728–2730. [Google Scholar] [CrossRef]
- Suratt, B.T.; Petty, J.M.; Young, S.K.; Malcolm, K.C.; Lieber, J.G.; Nick, J.A.; Gonzalo, J.-A.; Henson, P.M.; Worthen, G.S. Role of the CXCR4/SDF-1 chemokine axis in circulating neutrophil homeostasis. Blood 2004, 104, 565–571. [Google Scholar] [CrossRef] [Green Version]
- Yamada, M.; Kubo, H.; Kobayashi, S.; Ishizawa, K.; He, M.; Suzuki, T.; Fujino, N.; Kunishima, H.; Hatta, M.; Nishimaki, K.; et al. The increase in surface CXCR4 expression on lung extravascular neutrophils and its effects on neutrophils during endotoxin-induced lung injury. Cell. Mol. Immunol. 2011, 8, 305–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nanki, T.; Hayashida, K.; El-Gabalawy, H.S.; Suson, S.; Shi, K.; Girschick, H.J.; Yavuz, S.; Lipsky, P.E. Stromal Cell-Derived Factor-1-CXC Chemokine Receptor 4 Interactions Play a Central Role in CD4+ T Cell Accumulation in Rheumatoid Arthritis Synovium. J. Immunol. 2000, 165, 6590–6598. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matthys, P.; Hatse, S.; Vermeire, K.; Wuyts, A.; Bridger, G.; Henson, G.W.; De Clercq, E.; Billiau, A.; Schols, D. AMD3100, a Potent and Specific Antagonist of the Stromal Cell-Derived Factor-1 Chemokine Receptor CXCR4, Inhibits Autoimmune Joint Inflammation in IFN-γ Receptor-Deficient Mice. J. Immunol. 2001, 167, 4686–4692. [Google Scholar] [CrossRef] [Green Version]
- Pablos, J.L.; Santiago, B.; Galindo, M.; Torres, C.; Brehmer, M.T.; Blanco, F.J.; García-Lázaro, F.J. Synoviocyte-Derived CXCL12 Is Displayed on Endothelium and Induces Angiogenesis in Rheumatoid Arthritis. J. Immunol. 2003, 170, 2147–2152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradfield, P.F.; Amft, N.; Vernon-Wilson, E.; Exley, A.E.; Parsonage, G.; Rainger, G.E.; Nash, G.B.; Thomas, A.M.C.; Simmons, D.L.; Salmon, M.; et al. Rheumatoid fibroblast-like synoviocytes overexpress the chemokine stromal cell-derived factor 1 (CXCL12), which supports distinct patterns and rates of CD4+ and CD8+ T cell migration within synovial tissue. Arthritis Rheum. 2003, 48, 2472–2482. [Google Scholar] [CrossRef]
- Struyf, S.; Gouwy, M.; Dillen, C.; Proost, P.; Opdenakker, G.; Van Damme, J. Chemokines synergize in the recruitment of circulating neutrophils into inflamed tissue. Eur. J. Immunol. 2005, 35, 1583–1591. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, S.; DeBerry, J.J.; Ahmad, I.; Lynn, P.; Dewitte, C.; Malik, S.; Merlin, J.S.; Goodin, B.R.; Heath, S.L.; Matalon, S. Heme attenuates beta-endorphin levels in leukocytes of HIV positive individuals with chronic widespread pain. Redox Biol. 2020, 36, 101684. [Google Scholar] [CrossRef] [PubMed]
- Bellemare, F.; Jeanneret, A.; Couture, J. Sex Differences in Thoracic Dimensions and Configuration. Am. J. Respir. Crit. Care Med. 2003, 168, 305–312. [Google Scholar] [CrossRef]
- Torres-Tamayo, N.; García-Martínez, D.; Zlolniski, S.L.; Torres-Sánchez, I.; Garcia-Rio, F.; Bastir, M. 3D analysis of sexual dimorphism in size, shape and breathing kinematics of human lungs. J. Anat. 2018, 232, 227–237. [Google Scholar] [CrossRef] [Green Version]
- Thurlbeck, W.M. Postnatal human lung growth. Thorax 1982, 37, 564–571. [Google Scholar] [CrossRef] [Green Version]
- Colebatch, H.J.; Greaves, I.A.; Ng, C.K. Exponential analysis of elastic recoil and aging in healthy males and females. J. Appl. Physiol. Respir. Environ. Exerc. Physiol. 1979, 47, 683–691. [Google Scholar] [CrossRef]
- Lingappan, K.; Jiang, W.; Wang, L.; Couroucli, X.I.; Barrios, R.; Moorthy, B. Sex-specific differences in hyperoxic lung injury in mice: Implications for acute and chronic lung disease in humans. Toxicol. Appl. Pharmacol. 2013, 272, 281–290. [Google Scholar] [CrossRef] [Green Version]
- Cabello, N.; Mishra, V.; Sinha, U.; DiAngelo, S.L.; Chroneos, Z.C.; Ekpa, N.A.; Cooper, T.K.; Caruso, C.R.; Silveyra, P. Sex differences in the expression of lung inflammatory mediators in response to ozone. Am. J. Physiol. Cell. Mol. Physiol. 2015, 309, L1150–L1163. [Google Scholar] [CrossRef]
- Gonzalo, J.-A.; Lloyd, C.M.; Peled, A.; Delaney, T.; Coyle, A.J.; Gutierrez-Ramos, J.-C. Critical Involvement of the Chemotactic Axis CXCR4/Stromal Cell-Derived Factor-1α in the Inflammatory Component of Allergic Airway Disease. J. Immunol. 2000, 165, 499–508. [Google Scholar] [CrossRef] [Green Version]
- Lukacs, N.W.; Berlin, A.; Schols, D.; Skerlj, R.T.; Bridger, G.J. AMD3100, a CxCR4 Antagonist, Attenuates Allergic Lung Inflammation and Airway Hyperreactivity. Am. J. Pathol. 2002, 160, 1353–1360. [Google Scholar] [CrossRef] [Green Version]
- Phillips, R.J.; Burdick, M.D.; Hong, K.; Lutz, M.A.; Murray, L.A.; Xue, Y.Y.; Belperio, J.A.; Keane, M.P.; Strieter, R.M. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J. Clin. Investig. 2004, 114, 438–446. [Google Scholar] [CrossRef] [Green Version]
- Hashimoto, N.; Jin, H.; Liu, T.; Chensue, S.W.; Phan, S.H. Bone marrow–derived progenitor cells in pulmonary fibrosis. J. Clin. Investig. 2004, 113, 243–252. [Google Scholar] [CrossRef]
- Li, F.; Xu, X.; Geng, J.; Wan, X.; Dai, H. The autocrine CXCR4/CXCL12 axis contributes to lung fibrosis through modulation of lung fibroblast activity. Exp. Ther. Med. 2020, 19, 1844–1854. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, J.S.; Kang, C.M.; Kang, H.H.; Yoon, H.K.; Kim, Y.K.; Kim, K.H.; Moon, H.S.; Park, S.H. Inhibitory effect of CXC chemokine receptor 4 antagonist AMD3100 on bleomycin induced murine pulmonary fibrosis. Exp. Mol. Med. 2010, 42, 465–476. [Google Scholar] [CrossRef]
- Müller, A.; Homey, B.; Soto, H.; Ge, N.; Catron, D.; Buchanan, M.E.; McClanahan, T.; Murphy, E.; Yuan, W.; Wagner, S.N.; et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 2001, 410, 50–56. [Google Scholar] [CrossRef]
- Murakami, T.; Maki, W.; Cardones, A.R.; Fang, H.; Kyi, A.T.; Nestle, F.O.; Hwang, S.T. Expression of CXC chemokine receptor-4 enhances the pulmonary metastatic potential of murine B16 melanoma cells. Cancer Res. 2002, 62, 7328–7334. [Google Scholar]
- Phillips, R.J.; Burdick, M.D.; Lutz, M.; Belperio, J.A.; Keane, M.P.; Strieter, R.M. The Stromal Derived Factor–1/CXCL12–CXC Chemokine Receptor 4 Biological Axis in Non–Small Cell Lung Cancer Metastases. Am. J. Respir. Crit. Care Med. 2003, 167, 1676–1686. [Google Scholar] [CrossRef] [Green Version]
- Petty, J.M.; Sueblinvong, V.; Lenox, C.C.; Jones, C.C.; Cosgrove, G.P.; Cool, C.D.; Rai, P.R.; Brown, K.K.; Weiss, D.J.; Poynter, M.E.; et al. Pulmonary Stromal-Derived Factor-1 Expression and Effect on Neutrophil Recruitment during Acute Lung Injury. J. Immunol. 2007, 178, 8148–8157. [Google Scholar] [CrossRef] [Green Version]
- Schioppa, T.; Uranchimeg, B.; Saccani, A.; Biswas, S.K.; Doni, A.; Rapisarda, A.; Bernasconi, S.; Saccani, S.; Nebuloni, M.; Vago, L.; et al. Regulation of the Chemokine Receptor CXCR4 by Hypoxia. J. Exp. Med. 2003, 198, 1391–1402. [Google Scholar] [CrossRef] [Green Version]
- Santiago, B.; Calonge, E.; Del Rey, M.J.; Gutierrez-Cañas, I.; Izquierdo, E.; Usategui, A.; Galindo, M.; Alcamí, J.; Pablos, J.L. CXCL12 gene expression is upregulated by hypoxia and growth arrest but not by inflammatory cytokines in rheumatoid synovial fibroblasts. Cytokine 2011, 53, 184–190. [Google Scholar] [CrossRef]
- De Falco, E.; Porcelli, D.; Torella, A.R.; Straino, S.; Iachininoto, M.G.; Orlandi, A.; Truffa, S.; Biglioli, P.; Napolitano, M.; Capogrossi, M.C.; et al. SDF-1 involvement in endothelial phenotype and ischemia-induced recruitment of bone marrow progenitor cells. Blood 2004, 104, 3472–3482. [Google Scholar] [CrossRef] [Green Version]
- Sieveking, D.P.; Lim, P.; Chow, R.W.; Dunn, L.L.; Bao, S.; McGrath, K.C.; Heather, A.K.; Handelsman, D.J.; Celermajer, D.S.; Ng, M.K. A sex-specific role for androgens in angiogenesis. J. Exp. Med. 2010, 207, 345–352. [Google Scholar] [CrossRef] [PubMed]
- Okponyia, O.C.; McGraw, M.D.; Dysart, M.M.; Garlick, R.B.; Rioux, J.S.; Murphy, A.L.; Roe, G.B.; White, C.W.; Veress, L.A. Oxygen Administration Improves Survival but Worsens Cardiopulmonary Functions in Chlorine-exposed Rats. Am. J. Respir. Cell Mol. Biol. 2018, 58, 107–116. [Google Scholar] [CrossRef] [PubMed]
- Mabjeesh, N.J.; Willard, M.T.; Frederickson, C.E.; Zhong, H.; Simons, J.W. Androgens stimulate hypoxia-inducible factor 1 activation via autocrine loop of tyrosine kinase receptor/phosphatidylinositol 3′-kinase/protein kinase B in prostate cancer cells. Clin. Cancer Res. 2003, 9, 2416–2425. [Google Scholar] [PubMed]
- Rodriguez-Lara, V.; Peña-Mirabal, E.; Baez-Saldaña, R.; Esparza-Silva, A.L.; García-Zepeda, E.; Cervantes, M.A.C.; Diaz, D.; Fortoul, T.I. Estrogen Receptor Beta and CXCR4/CXCL12 Expression: Differences by Sex and Hormonal Status in Lung Adenocarcinoma. Arch. Med. Res. 2014, 45, 158–169. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Lara, V.; Ignacio, G.-S.; Cervantes, M.A.C. Estrogen induces CXCR4 overexpression and CXCR4/CXL12 pathway activation in lung adenocarcinoma cells in vitro. Endocr. Res. 2017, 42, 219–231. [Google Scholar] [CrossRef]
- Wagner, P.L.; Hyjek, E.; Vazquez, M.F.; Meherally, D.; Liu, Y.F.; Chadwick, P.A.; Rengifo, T.; Sica, G.L.; Port, J.L.; Lee, P.C.; et al. CXCL12 and CXCR4 in adenocarcinoma of the lung: Association with metastasis and survival. J. Thorac. Cardiovasc. Surg. 2009, 137, 615–621. [Google Scholar] [CrossRef] [Green Version]
- Lin, W.-C.; Fessler, M.B. Regulatory mechanisms of neutrophil migration from the circulation to the airspace. Cell. Mol. Life Sci. 2021, 78, 4095–4124. [Google Scholar] [CrossRef]
- Balamayooran, G.; Batra, S.; Fessler, M.B.; Happel, K.I.; Jeyaseelan, S. Mechanisms of Neutrophil Accumulation in the Lungs Against Bacteria. Am. J. Respir. Cell Mol. Biol. 2010, 43, 5–16. [Google Scholar] [CrossRef]
- Bhatia, M.; Zemans, R.L.; Jeyaseelan, S. Role of Chemokines in the Pathogenesis of Acute Lung Injury. Am. J. Respir. Cell Mol. Biol. 2012, 46, 566–572. [Google Scholar] [CrossRef] [Green Version]
- Alcamo, E.; Mizgerd, J.P.; Horwitz, B.H.; Bronson, R.; Beg, A.A.; Scott, M.; Doerschuk, C.M.; Hynes, R.O.; Baltimore, D. Targeted Mutation of TNF Receptor I Rescues the RelA-Deficient Mouse and Reveals a Critical Role for NF-κB in Leukocyte Recruitment. J. Immunol. 2001, 167, 1592–1600. [Google Scholar] [CrossRef] [Green Version]
- Poynter, M.E.; Irvin, C.G.; Janssen-Heininger, Y.M.W. A Prominent Role for Airway Epithelial NF-κB Activation in Lipopolysaccharide-Induced Airway Inflammation. J. Immunol. 2003, 170, 6257–6265. [Google Scholar] [CrossRef] [Green Version]
- Reutershan, J.; Morris, M.A.; Burcin, T.L.; Smith, D.F.; Chang, D.; Saprito, M.S.; Ley, K. Critical role of endothelial CXCR2 in LPS-induced neutrophil migration into the lung. J. Clin. Investig. 2006, 116, 695–702. [Google Scholar] [CrossRef]
- Weathington, N.M.; Van Houwelingen, A.H.; Noerager, B.D.; Jackson, P.L.; Kraneveld, A.D.; Galin, F.S.; Folkerts, G.; Nijkamp, F.P.; Blalock, J.E. A novel peptide CXCR ligand derived from extracellular matrix degradation during airway inflammation. Nat. Med. 2006, 12, 317–323. [Google Scholar] [CrossRef]
- Gaggar, A.; Jackson, P.L.; Noerager, B.D.; O’reilly, P.J.; McQuaid, D.B.; Rowe, S.M.; Clancy, J.P.; Blalock, J.E. A Novel Proteolytic Cascade Generates an Extracellular Matrix-Derived Chemoattractant in Chronic Neutrophilic Inflammation. J. Immunol. 2008, 180, 5662–5669. [Google Scholar] [CrossRef] [Green Version]
- Gelfand, E.W. Importance of the leukotriene B4-BLT1 and LTB4-BLT2 pathways in asthma. Semin. Immunol. 2017, 33, 44–51. [Google Scholar] [CrossRef]
- Batra, S.; Cai, S.; Balamayooran, G.; Jeyaseelan, S. Intrapulmonary Administration of Leukotriene B4 Augments Neutrophil Accumulation and Responses in the Lung to Klebsiella Infection in CXCL1 Knockout Mice. J. Immunol. 2012, 188, 3458–3468. [Google Scholar] [CrossRef] [Green Version]
- Hicks, A.; Goodnow, R.; Cavallo, G.; Tannu, S.A.; Ventre, J.D.; Lavelle, D.; Lora, J.M.; Satjawatcharaphong, J.; Brovarney, M.; Dabbagh, K.; et al. Effects of LTB4 receptor antagonism on pulmonary inflammation in rodents and non-human primates. Prostaglandins Other Lipid Mediat. 2010, 92, 33–43. [Google Scholar] [CrossRef]
- Okuno, T.; Yokomizo, T.; Hori, T.; Miyano, M.; Shimizu, T. Leukotriene B4 Receptor and the Function of Its Helix 8. J. Biol. Chem. 2005, 280, 32049–32052. [Google Scholar] [CrossRef] [Green Version]
- Chillingworth, N.L.; Morham, S.G.; Donaldson, L.F. Sex differences in inflammation and inflammatory pain in cyclooxygenase-deficient mice. Am. J. Physiol. Integr. Comp. Physiol. 2006, 291, R327–R334. [Google Scholar] [CrossRef]
- Chistyakov, D.V.; Azbukina, N.V.; Astakhova, A.A.; Goriainov, S.V.; Chistyakov, V.V.; Sergeeva, M.G. Sex-Mediated Differences in LPS Induced Alterations of TNFα, IL-10 Expression, and Prostaglandin Synthesis in Primary Astrocytes. Int. J. Mol. Sci. 2018, 19, 2793. [Google Scholar] [CrossRef] [Green Version]
- Yaeger, M.J.; Reece, S.W.; Kilburg-Basnyat, B.; Hodge, M.X.; Pal, A.; Dunigan-Russell, K.; Luo, B.; You, D.J.; Bonner, J.C.; Spangenburg, E.E.; et al. Sex Differences in Pulmonary Eicosanoids and Specialized Pro-Resolving Mediators in Response to Ozone Exposure. Toxicol. Sci. 2021, 183, 170–183. [Google Scholar] [CrossRef] [PubMed]
- Schultz, J.; Kaminker, K. Myeloperoxidase of the leucocyte of normal human blood. I. Content and localization. Arch. Biochem. Biophys. 1962, 96, 465–467. [Google Scholar] [CrossRef] [PubMed]
- Heinecke, J.W. Mechanisms of oxidative damage by myeloperoxidase in atherosclerosis and other inflammatory disorders. J. Lab. Clin. Med. 1999, 133, 321–325. [Google Scholar] [CrossRef]
- Zhang, R.; Brennan, M.L.; Fu, X.; Aviles, R.J.; Pearce, G.L.; Penn, M.S.; Topol, E.J.; Sprecher, D.L.; Hazen, S.L. Association Between Myeloperoxidase Levels and Risk of Coronary Artery Disease. JAMA 2001, 286, 2136–2142. [Google Scholar] [CrossRef] [Green Version]
- Gray, E.; Thomas, T.L.; Betmouni, S.; Scolding, N.; Love, S. Elevated myeloperoxidase activity in white matter in multiple sclerosis. Neurosci. Lett. 2008, 444, 195–198. [Google Scholar] [CrossRef]
- Emokpae, M.A.; Mrakpor, B.A. Do Sex Differences in Respiratory Burst Enzyme Activities Exist in Human Immunodeficiency Virus-1 Infection? Med. Sci. 2016, 4, 19. [Google Scholar] [CrossRef] [Green Version]
- Gupta, S.; Nakabo, S.; Blanco, L.P.; O’Neil, L.J.; Wigerblad, G.; Goel, R.R.; Mistry, P.; Jiang, K.; Carmona-Rivera, C.; Chan, D.W.; et al. Sex differences in neutrophil biology modulate response to type I interferons and immunometabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 16481–16491. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chatterjee, T.; Lewis, T.L.; Arora, I.; Gryshyna, A.E.; Underwood, L.; Masjoan Juncos, J.X.; Aggarwal, S. Sex-Based Disparities in Leukocyte Migration and Activation in Response to Inhalation Lung Injury: Role of SDF-1/CXCR4 Signaling. Cells 2023, 12, 1719. https://doi.org/10.3390/cells12131719
Chatterjee T, Lewis TL, Arora I, Gryshyna AE, Underwood L, Masjoan Juncos JX, Aggarwal S. Sex-Based Disparities in Leukocyte Migration and Activation in Response to Inhalation Lung Injury: Role of SDF-1/CXCR4 Signaling. Cells. 2023; 12(13):1719. https://doi.org/10.3390/cells12131719
Chicago/Turabian StyleChatterjee, Tanima, Terry L. Lewis, Itika Arora, Anastasiia E. Gryshyna, Lilly Underwood, Juan Xavier Masjoan Juncos, and Saurabh Aggarwal. 2023. "Sex-Based Disparities in Leukocyte Migration and Activation in Response to Inhalation Lung Injury: Role of SDF-1/CXCR4 Signaling" Cells 12, no. 13: 1719. https://doi.org/10.3390/cells12131719
APA StyleChatterjee, T., Lewis, T. L., Arora, I., Gryshyna, A. E., Underwood, L., Masjoan Juncos, J. X., & Aggarwal, S. (2023). Sex-Based Disparities in Leukocyte Migration and Activation in Response to Inhalation Lung Injury: Role of SDF-1/CXCR4 Signaling. Cells, 12(13), 1719. https://doi.org/10.3390/cells12131719







