ICAMs in Immunity, Intercellular Adhesion and Communication
Abstract
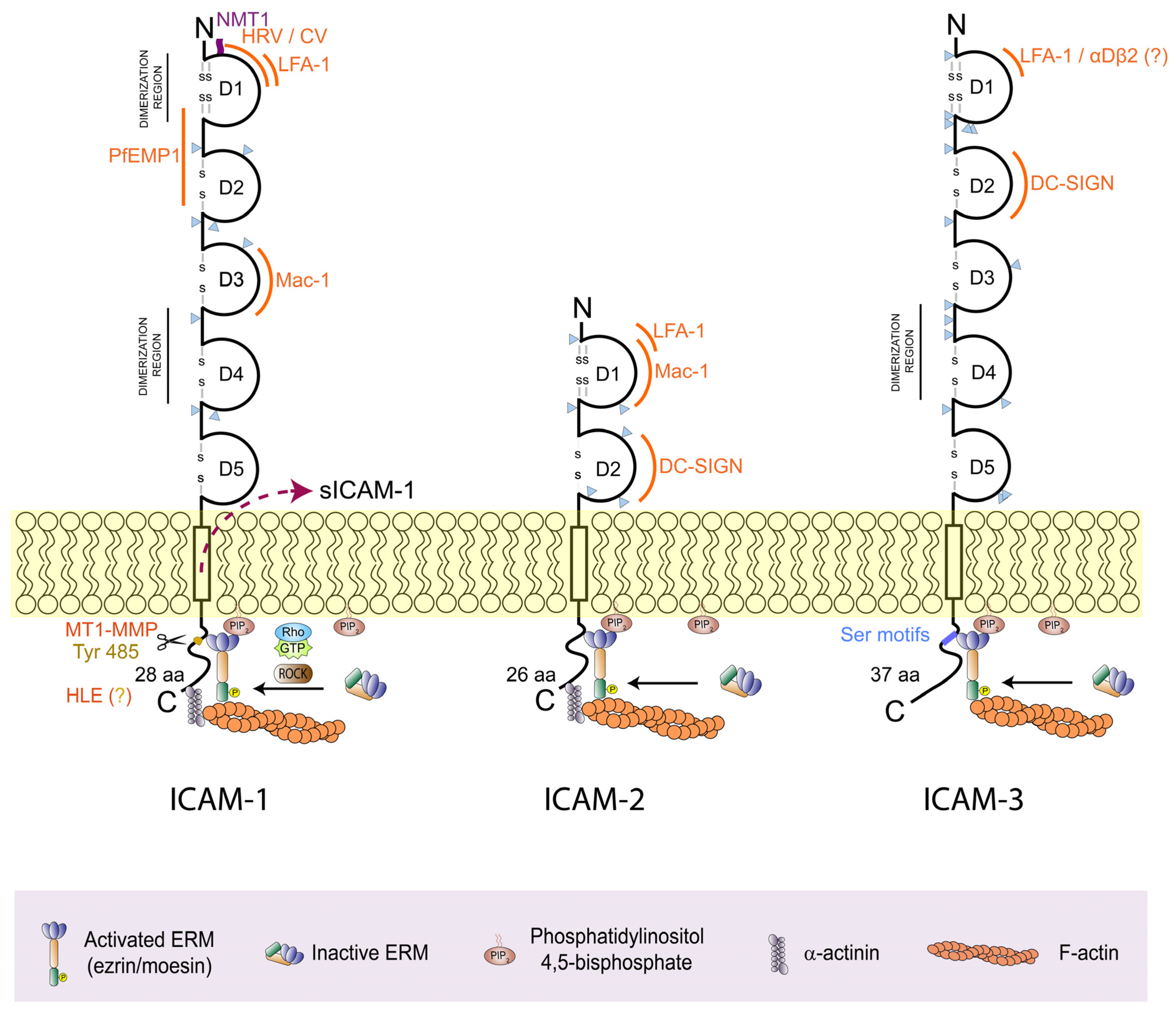
:1. Introduction
2. ICAMs in the Immune System
2.1. ICAM-1 (CD54)
2.2. ICAM-2 (CD102)
2.3. ICAM-3 (CD50)
3. ICAMs Anchor to the Actin Cortex
4. ICAMs as Bidirectional Signalling Receptors
4.1. LFA-1 Signalling by ICAM Binding
4.2. Reciprocal Signalling by ICAMs
5. ICAMs in Disease
5.1. Dry Eye Disease
5.2. Cardiovascular Disease
5.3. Intestinal Inflammation
5.4. Pulmonary Fibrosis
5.5. Autoimmunity
5.6. Hematopoietic Stem Cell Transplantation (HSCT)
5.7. Cancer
6. Conclusions and Future Perspectives
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Springer, T.A. Adhesion receptors of the immune system. Nature 1990, 346, 425–434. [Google Scholar] [CrossRef]
- Barreiro, O.; de la Fuente, H.; Mittelbrunn, M.; Sanchez-Madrid, F. Functional insights on the polarized redistribution of leukocyte integrins and their ligands during leukocyte migration and immune interactions. Immunol. Rev. 2007, 218, 147–164. [Google Scholar] [CrossRef] [PubMed]
- Cavallaro, U.; Dejana, E. Adhesion molecule signalling: Not always a sticky business. Nat. Rev. Mol. Cell Biol. 2011, 12, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Dejana, E.; Orsenigo, F. Endothelial adherens junctions at a glance. J. Cell Sci. 2013, 126 Pt 12, 2545–2549. [Google Scholar] [CrossRef] [PubMed]
- McEver, R.P. Selectins: Initiators of leucocyte adhesion and signalling at the vascular wall. Cardiovasc. Res. 2015, 107, 331–339. [Google Scholar] [CrossRef] [PubMed]
- Leick, M.; Azcutia, V.; Newton, G.; Luscinskas, F.W. Leukocyte recruitment in inflammation: Basic concepts and new mechanistic insights based on new models and microscopic imaging technologies. Cell Tissue Res. 2014, 355, 647–656. [Google Scholar] [CrossRef] [PubMed]
- Fukuda, M.; Hiraoka, N.; Yeh, J.C. C-type lectins and sialyl Lewis X oligosaccharides. Versatile roles in cell-cell interaction. J. Cell Biol. 1999, 147, 467–470. [Google Scholar] [CrossRef] [PubMed]
- Ivetic, A.; Hoskins Green, H.L.; Hart, S.J. L-selectin: A Major Regulator of Leukocyte Adhesion, Migration and Signaling. Front. Immunol. 2019, 10, 1068. [Google Scholar] [CrossRef]
- Johnston, B.; Butcher, E.C. Chemokines in rapid leukocyte adhesion triggering and migration. Semin. Immunol. 2002, 14, 83–92. [Google Scholar] [CrossRef]
- Kuwano, Y.; Spelten, O.; Zhang, H.; Ley, K.; Zarbock, A. Rolling on E- or P-selectin induces the extended but not high-affinity conformation of LFA-1 in neutrophils. Blood 2010, 116, 617–624. [Google Scholar] [CrossRef]
- Humphries, J.D.; Byron, A.; Humphries, M.J. Integrin ligands at a glance. J. Cell Sci. 2006, 119 Pt 19, 3901–3903. [Google Scholar] [CrossRef]
- Dustin, M.L. Integrins and Their Role in Immune Cell Adhesion. Cell 2019, 177, 499–501. [Google Scholar] [CrossRef]
- Heit, B.; Colarusso, P.; Kubes, P. Fundamentally different roles for LFA-1, Mac-1 and alpha4-integrin in neutrophil chemotaxis. J. Cell Sci. 2005, 118, 5205–5220. [Google Scholar] [CrossRef] [PubMed]
- Sumagin, R.; Prizant, H.; Lomakina, E.; Waugh, R.E. Sarelius IH: LFA-1 and Mac-1 define characteristically different intralumenal crawling and emigration patterns for monocytes and neutrophils in situ. J. Immunol. 2010, 185, 7057–7066. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Madrid, F.; Nagy, J.A.; Robbins, E.; Simon, P.; Springer, T.A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: The lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J. Exp. Med. 1983, 158, 1785–1803. [Google Scholar] [CrossRef] [PubMed]
- Van der Vieren, M.; Le Trong, H.; Wood, C.L.; Moore, P.F.; St John, T.; Staunton, D.E.; Gallatin, W.M. A novel leukointegrin, alpha d beta 2, binds preferentially to ICAM-3. Immunity 1995, 3, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, Y.; Vieira-de-Abreu, A.; Harris, E.S.; Shah, A.M.; Weyrich, A.S.; Castro-Faria-Neto, H.C.; Zimmerman, G.A. Integrin alphaDbeta2 (CD11d/CD18) is expressed by human circulating and tissue myeloid leukocytes and mediates inflammatory signaling. PLoS ONE 2014, 9, e112770. [Google Scholar] [CrossRef] [PubMed]
- Lindquist, R.L.; Shakhar, G.; Dudziak, D.; Wardemann, H.; Eisenreich, T.; Dustin, M.L.; Nussenzweig, M.C. Visualizing dendritic cell networks in vivo. Nat. Immunol. 2004, 5, 1243–1250. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.; Hogg, N. Regulation of leukocyte integrin function: Affinity vs. avidity. J. Cell Biochem. 1996, 61, 554–561. [Google Scholar] [CrossRef]
- Sen, M.; Springer, T.A. Leukocyte integrin alphaLbeta2 headpiece structures: The alphaI domain, the pocket for the internal ligand, and concerted movements of its loops. Proc. Natl. Acad. Sci. USA 2016, 113, 2940–2945. [Google Scholar] [CrossRef]
- Shimaoka, M.; Xiao, T.; Liu, J.H.; Yang, Y.; Dong, Y.; Jun, C.D.; McCormack, A.; Zhang, R.; Joachimiak, A.; Takagi, J.; et al. Structures of the alpha L I domain and its complex with ICAM-1 reveal a shape-shifting pathway for integrin regulation. Cell 2003, 112, 99–111. [Google Scholar] [CrossRef] [PubMed]
- Sen, M.; Koksal, A.C.; Yuki, K.; Wang, J.; Springer, T.A. Ligand- and cation-induced structural alterations of the leukocyte integrin LFA-1. J. Biol. Chem. 2018, 293, 6565–6577. [Google Scholar] [CrossRef] [PubMed]
- Cabanas, C.; Hogg, N. Ligand intercellular adhesion molecule 1 has a necessary role in activation of integrin lymphocyte function-associated molecule 1. Proc. Natl. Acad. Sci. USA 1993, 90, 5838–5842. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.H.; Carman, C.V.; Springer, T.A. Structural basis of integrin regulation and signaling. Annu. Rev. Immunol. 2007, 25, 619–647. [Google Scholar] [CrossRef] [PubMed]
- Schurpf, T.; Springer, T.A. Regulation of integrin affinity on cell surfaces. EMBO J. 2011, 30, 4712–4727. [Google Scholar] [CrossRef] [PubMed]
- Swaminathan, V.; Kalappurakkal, J.M.; Mehta, S.B.; Nordenfelt, P.; Moore, T.I.; Koga, N.; Baker, D.A.; Oldenbourg, R.; Tani, T.; Mayor, S.; et al. Actin retrograde flow actively aligns and orients ligand-engaged integrins in focal adhesions. Proc. Natl. Acad. Sci. USA 2017, 114, 10648–10653. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Springer, T.A. Structural specializations of immunoglobulin superfamily members for adhesion to integrins and viruses. Immunol. Rev. 1998, 163, 197–215. [Google Scholar] [CrossRef]
- Hayflick, J.S.; Kilgannon, P.; Gallatin, W.M. The intercellular adhesion molecule (ICAM) family of proteins. New members and novel functions. Immunol. Res. 1998, 17, 313–327. [Google Scholar] [CrossRef]
- Gahmberg, C.G. Leukocyte adhesion: CD11/CD18 integrins and intercellular adhesion molecules. Curr. Opin. Cell Biol. 1997, 9, 643–650. [Google Scholar] [CrossRef]
- Williams, A.F.; Barclay, A.N. The immunoglobulin superfamily--domains for cell surface recognition. Annu. Rev. Immunol. 1988, 6, 381–405. [Google Scholar] [CrossRef] [PubMed]
- Aricescu, A.R.; Jones, E.Y. Immunoglobulin superfamily cell adhesion molecules: Zippers and signals. Curr. Opin. Cell Biol. 2007, 19, 543–550. [Google Scholar] [CrossRef]
- Appleby, S.L.; Cockshell, M.P.; Pippal, J.B.; Thompson, E.J.; Barrett, J.M.; Tooley, K.; Sen, S.; Sun, W.Y.; Grose, R.; Nicholson, I.; et al. Characterization of a distinct population of circulating human non-adherent endothelial forming cells and their recruitment via intercellular adhesion molecule-3. PLoS ONE 2012, 7, e46996. [Google Scholar] [CrossRef]
- Patey, N.; Vazeux, R.; Canioni, D.; Potter, T.; Gallatin, W.M.; Brousse, N. Intercellular adhesion molecule-3 on endothelial cells. Expression in tumors but not in inflammatory responses. Am. J. Pathol. 1996, 148, 465–472. [Google Scholar] [PubMed]
- Zennadi, R.; Whalen, E.J.; Soderblom, E.J.; Alexander, S.C.; Thompson, J.W.; Dubois, L.G.; Moseley, M.A.; Telen, M.J. Erythrocyte plasma membrane-bound ERK1/2 activation promotes ICAM-4-mediated sickle red cell adhesion to endothelium. Blood 2012, 119, 1217–1227. [Google Scholar] [CrossRef]
- Ihanus, E.; Uotila, L.M.; Toivanen, A.; Varis, M.; Gahmberg, C.G. Red-cell ICAM-4 is a ligand for the monocyte/macrophage integrin CD11c/CD18: Characterization of the binding sites on ICAM-4. Blood 2007, 109, 802–810. [Google Scholar] [CrossRef] [PubMed]
- Gahmberg, C.G.; Ning, L.; Paetau, S. ICAM-5: A neuronal dendritic adhesion molecule involved in immune and neuronal functions. Adv. Neurobiol. 2014, 8, 117–132. [Google Scholar] [PubMed]
- Gerard, A.; Cope, A.P.; Kemper, C.; Alon, R.; Kochl, R. LFA-1 in T cell priming, differentiation, and effector functions. Trends Immunol. 2021, 42, 706–722. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.W. 3. Adhesion molecules and receptors. J. Allergy Clin. Immunol. 2008, 121 (Suppl. S2), S375–S379, quiz S414. [Google Scholar] [CrossRef] [PubMed]
- Luster, A.D.; Alon, R.; von Andrian, U.H. Immune cell migration in inflammation: Present and future therapeutic targets. Nat. Immunol. 2005, 6, 1182–1190. [Google Scholar] [CrossRef] [PubMed]
- Sugino, H. ICAM-3, a ligand for DC-SIGN, was duplicated from ICAM-1 in mammalian evolution, but was lost in the rodent genome. FEBS Lett. 2005, 579, 2901–2906. [Google Scholar] [CrossRef]
- Staunton, D.E.; Dustin, M.L.; Springer, T.A. Functional cloning of ICAM-2, a cell adhesion ligand for LFA-1 homologous to ICAM-1. Nature 1989, 339, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Vazeux, R.; Hoffman, P.A.; Tomita, J.K.; Dickinson, E.S.; Jasman, R.L.; St John, T.; Gallatin, W.M. Cloning and characterization of a new intercellular adhesion molecule ICAM-R. Nature 1992, 360, 485–488. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.W.; Chen, J.; Chacko, B.K.; Traylor, J.G., Jr.; Orr, A.W.; Patel, R.P. Role of endothelial N-glycan mannose residues in monocyte recruitment during atherogenesis. Arterioscler. Thromb. Vasc. Biol. 2012, 32, e51–e59. [Google Scholar] [CrossRef] [PubMed]
- Diamond, M.S.; Staunton, D.E.; Marlin, S.D.; Springer, T.A. Binding of the integrin Mac-1 (CD11b/CD18) to the third immunoglobulin-like domain of ICAM-1 (CD54) and its regulation by glycosylation. Cell 1991, 65, 961–971. [Google Scholar] [CrossRef]
- Otto, V.I.; Schurpf, T.; Folkers, G.; Cummings, R.D. Sialylated complex-type N-glycans enhance the signaling activity of soluble intercellular adhesion molecule-1 in mouse astrocytes. J. Biol. Chem. 2004, 279, 35201–35209. [Google Scholar] [CrossRef]
- Staunton, D.E.; Marlin, S.D.; Stratowa, C.; Dustin, M.L.; Springer, T.A. Primary structure of ICAM-1 demonstrates interaction between members of the immunoglobulin and integrin supergene families. Cell 1988, 52, 925–933. [Google Scholar] [CrossRef]
- Bloom, J.W.; Madanat, M.S.; Ray, M.K. Cell line and site specific comparative analysis of the N-linked oligosaccharides on human ICAM-1des454-532 by electrospray ionization mass spectrometry. Biochemistry 1996, 35, 1856–1864. [Google Scholar] [CrossRef]
- Scott, D.W.; Dunn, T.S.; Ballestas, M.E.; Litovsky, S.H.; Patel, R.P. Identification of a high-mannose ICAM-1 glycoform: Effects of ICAM-1 hypoglycosylation on monocyte adhesion and outside in signaling. Am. J. Physiol. Cell Physiol. 2013, 305, C228–C237. [Google Scholar] [CrossRef]
- Regal-McDonald, K.; Somarathna, M.; Lee, T.; Litovsky, S.H.; Barnes, J.; Peretik, J.M.; Traylor, J.G., Jr.; Orr, A.W.; Patel, R.P. Assessment of ICAM-1 N-glycoforms in mouse and human models of endothelial dysfunction. PLoS ONE 2020, 15, e0230358. [Google Scholar] [CrossRef]
- Funatsu, O.; Sato, T.; Kotovuori, P.; Gahmberg, C.G.; Ikekita, M.; Furukawa, K. Structural study of N-linked oligosaccharides of human intercellular adhesion molecule-3 (CD50). Eur. J. Biochem. 2001, 268, 1020–1029. [Google Scholar] [CrossRef]
- de Fougerolles, A.R.; Klickstein, L.B.; Springer, T.A. Cloning and expression of intercellular adhesion molecule 3 reveals strong homology to other immunoglobulin family counter-receptors for lymphocyte function-associated antigen 1. J. Exp. Med. 1993, 177, 1187–1192. [Google Scholar] [CrossRef]
- Yang, Y.; Jun, C.D.; Liu, J.H.; Zhang, R.; Joachimiak, A.; Springer, T.A.; Wang, J.H. Structural basis for dimerization of ICAM-1 on the cell surface. Mol. Cell 2004, 14, 269–276. [Google Scholar] [CrossRef]
- Jimenez, D.; Roda-Navarro, P.; Springer, T.A.; Casasnovas, J.M. Contribution of N-linked glycans to the conformation and function of intercellular adhesion molecules (ICAMs). J. Biol. Chem. 2005, 280, 5854–5861. [Google Scholar] [CrossRef] [PubMed]
- Dalal, P.J.; Sumagin, R. Emerging Functions of ICAM-1 in Macrophage Efferocytosis and Wound Healing. J. Cell Immunol. 2020, 2, 250–253. [Google Scholar] [PubMed]
- Greve, J.M.; Davis, G.; Meyer, A.M.; Forte, C.P.; Yost, S.C.; Marlor, C.W.; Kamarck, M.E.; McClelland, A. The major human rhinovirus receptor is ICAM-1. Cell 1989, 56, 839–847. [Google Scholar] [CrossRef]
- Staunton, D.E.; Merluzzi, V.J.; Rothlein, R.; Barton, R.; Marlin, S.D.; Springer, T.A. A cell adhesion molecule, ICAM-1, is the major surface receptor for rhinoviruses. Cell 1989, 56, 849–853. [Google Scholar] [CrossRef]
- Xiao, C.; Bator, C.M.; Bowman, V.D.; Rieder, E.; He, Y.; Hebert, B.; Bella, J.; Baker, T.S.; Wimmer, E.; Kuhn, R.J.; et al. Interaction of coxsackievirus A21 with its cellular receptor, ICAM-1. J. Virol. 2001, 75, 2444–2451. [Google Scholar] [CrossRef]
- Ockenhouse, C.F.; Betageri, R.; Springer, T.A.; Staunton, D.E. Plasmodium falciparum-infected erythrocytes bind ICAM-1 at a site distinct from LFA-1, Mac-1, and human rhinovirus. Cell 1992, 68, 63–69. [Google Scholar] [CrossRef]
- Berendt, A.R.; McDowall, A.; Craig, A.G.; Bates, P.A.; Sternberg, M.J.; Marsh, K.; Newbold, C.I.; Hogg, N. The binding site on ICAM-1 for Plasmodium falciparum-infected erythrocytes overlaps, but is distinct from, the LFA-1-binding site. Cell 1992, 68, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Barragan, A.; Brossier, F.; Sibley, L.D. Transepithelial migration of Toxoplasma gondii involves an interaction of intercellular adhesion molecule 1 (ICAM-1) with the parasite adhesin MIC2. Cell Microbiol. 2005, 7, 561–568. [Google Scholar] [CrossRef]
- Jun, C.D.; Shimaoka, M.; Carman, C.V.; Takagi, J.; Springer, T.A. Dimerization and the effectiveness of ICAM-1 in mediating LFA-1-dependent adhesion. Proc. Natl. Acad. Sci. USA 2001, 98, 6830–6835. [Google Scholar] [CrossRef]
- Fan, Z.; Kiosses, W.B.; Sun, H.; Orecchioni, M.; Ghosheh, Y.; Zajonc, D.M.; Arnaout, M.A.; Gutierrez, E.; Groisman, A.; Ginsberg, M.H.; et al. High-Affinity Bent beta(2)-Integrin Molecules in Arresting Neutrophils Face Each Other through Binding to ICAMs in cis. Cell Rep. 2019, 26, 119–130. [Google Scholar] [CrossRef]
- Fan, Z.; McArdle, S.; Marki, A.; Mikulski, Z.; Gutierrez, E.; Engelhardt, B.; Deutsch, U.; Ginsberg, M.; Groisman, A.; Ley, K. Neutrophil recruitment limited by high-affinity bent beta2 integrin binding ligand in cis. Nat. Commun. 2016, 7, 12658. [Google Scholar] [CrossRef]
- Ramos, T.N.; Bullard, D.C.; Barnum, S.R. ICAM-1: Isoforms and phenotypes. J. Immunol. 2014, 192, 4469–4474. [Google Scholar] [CrossRef] [PubMed]
- Bullard, D.C.; Hu, X.; Crawford, D.; McDonald, K.; Ramos, T.N.; Barnum, S.R. Expression of a single ICAM-1 isoform on T cells is sufficient for development of experimental autoimmune encephalomyelitis. Eur. J. Immunol. 2014, 44, 1194–1199. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Barnum, S.R.; Wohler, J.E.; Schoeb, T.R.; Bullard, D.C. Differential ICAM-1 isoform expression regulates the development and progression of experimental autoimmune encephalomyelitis. Mol. Immunol. 2010, 47, 1692–1700. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; Vogel, S.M.; Gao, X.; Javaid, K.; Hu, G.; Danilov, S.M.; Malik, A.B.; Minshall, R.D. Src phosphorylation of endothelial cell surface intercellular adhesion molecule-1 mediates neutrophil adhesion and contributes to the mechanism of lung inflammation. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 1342–1350. [Google Scholar] [CrossRef] [PubMed]
- Lim, E.J.; Kang, J.H.; Kim, Y.J.; Kim, S.; Lee, S.J. ICAM-1 promotes cancer progression by regulating SRC activity as an adapter protein in colorectal cancer. Cell Death Dis. 2022, 13, 417. [Google Scholar] [CrossRef] [PubMed]
- Sithu, S.D.; English, W.R.; Olson, P.; Krubasik, D.; Baker, A.H.; Murphy, G.; D’Souza, S.E. Membrane-type 1-matrix metalloproteinase regulates intracellular adhesion molecule-1 (ICAM-1)-mediated monocyte transmigration. J. Biol. Chem. 2007, 282, 25010–25019. [Google Scholar] [CrossRef] [PubMed]
- Champagne, B.; Tremblay, P.; Cantin, A.; St Pierre, Y. Proteolytic cleavage of ICAM-1 by human neutrophil elastase. J. Immunol. 1998, 161, 6398–6405. [Google Scholar] [CrossRef] [PubMed]
- Wakatsuki, T.; Kimura, K.; Kimura, F.; Shinomiya, N.; Ohtsubo, M.; Ishizawa, M.; Yamamoto, M. A distinct mRNA encoding a soluble form of ICAM-1 molecule expressed in human tissues. Cell Adhes. Commun. 1995, 3, 283–292. [Google Scholar] [CrossRef] [PubMed]
- Rothlein, R.; Mainolfi, E.A.; Czajkowski, M.; Marlin, S.D. A form of circulating ICAM-1 in human serum. J. Immunol. 1991, 147, 3788–3793. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Hussain, S.; Kolhe, K.; Kumar, G.; Tripathi, D.M.; Tomar, A.; Kale, P.; Narayanan, A.; Bihari, C.; Bajpai, M.; et al. Elevated plasma ICAM1 levels predict 28-day mortality in cirrhotic patients with COVID-19 or bacterial sepsis. JHEP Rep. 2021, 3, 100303. [Google Scholar] [CrossRef] [PubMed]
- Sprenger, A.; Schardt, C.; Rotsch, M.; Zehrer, M.; Wolf, M.; Havemann, K.; Heymanns, J. Soluble intercellular adhesion molecule-1 in patients with lung cancer and benign lung diseases. J. Cancer Res. Clin. Oncol. 1997, 123, 632–638. [Google Scholar] [CrossRef] [PubMed]
- Sari, R.A.; Taysi, S.; Erdem, F.; Yilmaz, O.; Keles, S.; Kiziltunc, A.; Odabas, A.R.; Cetinkaya, R. Correlation of serum levels of soluble intercellular adhesion molecule-1 with disease activity in systemic lupus erythematosus. Rheumatol. Int. 2002, 21, 149–152. [Google Scholar] [CrossRef] [PubMed]
- Schopf, R.E.; Naumann, S.; Rehder, M.; Morsches, B. Soluble intercellular adhesion molecule-1 levels in patients with psoriasis. Br. J. Dermatol. 1993, 128, 34–37. [Google Scholar] [CrossRef]
- Cush, J.J.; Rothlein, R.; Lindsley, H.B.; Mainolfi, E.A.; Lipsky, P.E. Increased levels of circulating intercellular adhesion molecule 1 in the sera of patients with rheumatoid arthritis. Arthritis Rheum. 1993, 36, 1098–1102. [Google Scholar] [CrossRef]
- Tohma, S.; Ramberg, J.E.; Lipsky, P.E. Expression and distribution of CD11a/CD18 and CD54 during human T cell-B cell interactions. J. Leukoc. Biol. 1992, 52, 97–103. [Google Scholar] [CrossRef]
- Woodfin, A.; Beyrau, M.; Voisin, M.B.; Ma, B.; Whiteford, J.R.; Hordijk, P.L.; Hogg, N.; Nourshargh, S. ICAM-1-expressing neutrophils exhibit enhanced effector functions in murine models of endotoxemia. Blood 2016, 127, 898–907. [Google Scholar] [CrossRef]
- Kim, I.; Moon, S.O.; Kim, S.H.; Kim, H.J.; Koh, Y.S.; Koh, G.Y. Vascular endothelial growth factor expression of intercellular adhesion molecule 1 (ICAM-1), vascular cell adhesion molecule 1 (VCAM-1), and E-selectin through nuclear factor-kappa B activation in endothelial cells. J. Biol. Chem. 2001, 276, 7614–7620. [Google Scholar] [CrossRef]
- Ledebur, H.C.; Parks, T.P. Transcriptional regulation of the intercellular adhesion molecule-1 gene by inflammatory cytokines in human endothelial cells. Essential roles of a variant NF-kappa B site and p65 homodimers. J. Biol. Chem. 1995, 270, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, K.A.; Rahman, A.; Lakshminarayanan, V.; Janakidevi, K.; Malik, A.B. H2O2 and tumor necrosis factor-alpha activate intercellular adhesion molecule 1 (ICAM-1) gene transcription through distinct cis-regulatory elements within the ICAM-1 promoter. J. Biol. Chem. 1995, 270, 18966–18974. [Google Scholar] [CrossRef] [PubMed]
- Roebuck, K.A.; Finnegan, A. Regulation of intercellular adhesion molecule-1 (CD54) gene expression. J. Leukoc. Biol. 1999, 66, 876–888. [Google Scholar] [CrossRef] [PubMed]
- Haydinger, C.D.; Ashander, L.M.; Tan, A.C.R.; Smith, J.R. Intercellular Adhesion Molecule 1: More than a Leukocyte Adhesion Molecule. Biology 2023, 12, 743. [Google Scholar] [CrossRef] [PubMed]
- Radisavljevic, Z.; Avraham, H.; Avraham, S. Vascular endothelial growth factor up-regulates ICAM-1 expression via the phosphatidylinositol 3 OH-kinase/AKT/Nitric oxide pathway and modulates migration of brain microvascular endothelial cells. J. Biol. Chem. 2000, 275, 20770–20774. [Google Scholar] [CrossRef] [PubMed]
- Kevil, C.G.; Orr, A.W.; Langston, W.; Mickett, K.; Murphy-Ullrich, J.; Patel, R.P.; Kucik, D.F.; Bullard, D.C. Intercellular adhesion molecule-1 (ICAM-1) regulates endothelial cell motility through a nitric oxide-dependent pathway. J. Biol. Chem. 2004, 279, 19230–19238. [Google Scholar] [CrossRef] [PubMed]
- Langston, W.; Chidlow, J.H., Jr.; Booth, B.A.; Barlow, S.C.; Lefer, D.J.; Patel, R.P.; Kevil, C.G. Regulation of endothelial glutathione by ICAM-1 governs VEGF-A-mediated eNOS activity and angiogenesis. Free Radic. Biol. Med. 2007, 42, 720–729. [Google Scholar] [CrossRef]
- Liu, G.; Place, A.T.; Chen, Z.; Brovkovych, V.M.; Vogel, S.M.; Muller, W.A.; Skidgel, R.A.; Malik, A.B.; Minshall, R.D. ICAM-1-activated Src and eNOS signaling increase endothelial cell surface PECAM-1 adhesivity and neutrophil transmigration. Blood 2012, 120, 1942–1952. [Google Scholar] [CrossRef]
- Martinelli, R.; Gegg, M.; Longbottom, R.; Adamson, P.; Turowski, P.; Greenwood, J. ICAM-1-mediated endothelial nitric oxide synthase activation via calcium and AMP-activated protein kinase is required for transendothelial lymphocyte migration. Mol. Biol. Cell 2009, 20, 995–1005. [Google Scholar] [CrossRef]
- Galore-Haskel, G.; Baruch, E.N.; Berg, A.L.; Barshack, I.; Zilinsky, I.; Avivi, C.; Besser, M.J.; Schachter, J.; Markel, G. Histopathological expression analysis of intercellular adhesion molecule 1 (ICAM-1) along development and progression of human melanoma. Oncotarget 2017, 8, 99580–99586. [Google Scholar] [CrossRef]
- Guo, P.; Huang, J.; Wang, L.; Jia, D.; Yang, J.; Dillon, D.A.; Zurakowski, D.; Mao, H.; Moses, M.A.; Auguste, D.T. ICAM-1 as a molecular target for triple negative breast cancer. Proc. Natl. Acad. Sci. USA 2014, 111, 14710–14715. [Google Scholar] [CrossRef]
- Kotteas, E.A.; Boulas, P.; Gkiozos, I.; Tsagkouli, S.; Tsoukalas, G.; Syrigos, K.N. The intercellular cell adhesion molecule-1 (icam-1) in lung cancer: Implications for disease progression and prognosis. Anticancer. Res. 2014, 34, 4665–4672. [Google Scholar]
- Bui, T.M.; Wiesolek, H.L.; Sumagin, R. ICAM-1: A master regulator of cellular responses in inflammation, injury resolution, and tumorigenesis. J. Leukoc. Biol. 2020, 108, 787–799. [Google Scholar] [CrossRef]
- Wang, H.; Xu, X.; Wang, Y.; Xue, X.; Guo, W.; Guo, S.; Qiu, S.; Cui, J.; Qiao, Y. NMT1 sustains ICAM-1 to modulate adhesion and migration of tumor cells. Cell Signal 2023, 109, 110739. [Google Scholar] [CrossRef]
- Morelli, A.E.; Larregina, A.T.; Shufesky, W.J.; Sullivan, M.L.; Stolz, D.B.; Papworth, G.D.; Zahorchak, A.F.; Logar, A.J.; Wang, Z.; Watkins, S.C.; et al. Endocytosis, intracellular sorting, and processing of exosomes by dendritic cells. Blood 2004, 104, 3257–3266. [Google Scholar] [CrossRef]
- Lee, H.M.; Choi, E.J.; Kim, J.H.; Kim, T.D.; Kim, Y.K.; Kang, C.; Gho, Y.S. A membranous form of ICAM-1 on exosomes efficiently blocks leukocyte adhesion to activated endothelial cells. Biochem. Biophys. Res. Commun. 2010, 397, 251–256. [Google Scholar] [CrossRef]
- Zhang, W.; Zhong, W.; Wang, B.; Yang, J.; Yu, Z.; Qin, Z.; Shi, A.; Xu, W.; Zheng, C.; Schuchter, L.M.; et al. ICAM-1-mediated adhesion is a prerequisite for exosome-induced T cell suppression. Dev. Cell 2022, 57, 329–343 e327. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.T.; Mason, J.C.; Birdsey, G.M.; Amsellem, V.; Gerwin, N.; Haskard, D.O.; Ridley, A.J.; Randi, A.M. Endothelial intercellular adhesion molecule (ICAM)-2 regulates angiogenesis. Blood 2005, 106, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Thompson, P.W.; Randi, A.M.; Ridley, A.J. Intercellular adhesion molecule (ICAM)-1, but not ICAM-2, activates RhoA and stimulates c-fos and rhoA transcription in endothelial cells. J. Immunol. 2002, 169, 1007–1013. [Google Scholar] [CrossRef] [PubMed]
- Halai, K.; Whiteford, J.; Ma, B.; Nourshargh, S.; Woodfin, A. ICAM-2 facilitates luminal interactions between neutrophils and endothelial cells in vivo. J. Cell Sci. 2014, 127 Pt 3, 620–629. [Google Scholar] [PubMed]
- Geijtenbeek, T.B.; Krooshoop, D.J.; Bleijs, D.A.; van Vliet, S.J.; van Duijnhoven, G.C.; Grabovsky, V.; Alon, R.; Figdor, C.G.; van Kooyk, Y. DC-SIGN-ICAM-2 interaction mediates dendritic cell trafficking. Nat. Immunol. 2000, 1, 353–357. [Google Scholar] [CrossRef]
- Somersalo, K.; Carpen, O.; Saksela, E.; Gahmberg, C.G.; Nortamo, P.; Timonen, T. Activation of natural killer cell migration by leukocyte integrin-binding peptide from intracellular adhesion molecule-2 (ICAM-2). J. Biol. Chem. 1995, 270, 8629–8636. [Google Scholar] [CrossRef]
- Li, R.; Nortamo, P.; Kantor, C.; Kovanen, P.; Timonen, T.; Gahmberg, C.G. A leukocyte integrin binding peptide from intercellular adhesion molecule-2 stimulates T cell adhesion and natural killer cell activity. J. Biol. Chem. 1993, 268, 21474–21477. [Google Scholar] [CrossRef]
- Fawcett, J.; Holness, C.L.; Needham, L.A.; Turley, H.; Gatter, K.C.; Mason, D.Y.; Simmons, D.L. Molecular cloning of ICAM-3, a third ligand for LFA-1, constitutively expressed on resting leukocytes. Nature 1992, 360, 481–484. [Google Scholar] [CrossRef] [PubMed]
- Doussis-Anagnostopoulou, I.; Kaklamanis, L.; Cordell, J.; Jones, M.; Turley, H.; Pulford, K.; Simmons, D.; Mason, D.; Gatter, K. ICAM-3 expression on endothelium in lymphoid malignancy. Am. J. Pathol. 1993, 143, 1040–1043. [Google Scholar]
- Estecha, A.; Aguilera-Montilla, N.; Sanchez-Mateos, P.; Puig-Kroger, A. RUNX3 regulates intercellular adhesion molecule 3 (ICAM-3) expression during macrophage differentiation and monocyte extravasation. PLoS ONE 2012, 7, e33313. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, M.A.; Pulido, R.; Munoz, C.; Alvarez, V.; Humbria, A.; Campanero, M.R.; Sanchez-Madrid, F. Regulation of ICAM-3 (CD50) membrane expression on human neutrophils through a proteolytic shedding mechanism. Eur. J. Immunol. 1994, 24, 2586–2594. [Google Scholar] [CrossRef] [PubMed]
- de Fougerolles, A.R.; Diamond, M.S.; Springer, T.A. Heterogenous glycosylation of ICAM-3 and lack of interaction with Mac-1 and p150,95. Eur. J. Immunol. 1995, 25, 1008–1012. [Google Scholar] [CrossRef]
- Geijtenbeek, T.B.; Torensma, R.; van Vliet, S.J.; van Duijnhoven, G.C.; Adema, G.J.; van Kooyk, Y.; Figdor, C.G. Identification of DC-SIGN, a novel dendritic cell-specific ICAM-3 receptor that supports primary immune responses. Cell 2000, 100, 575–585. [Google Scholar] [CrossRef]
- Binnerts, M.E.; van Kooyk, Y.; Simmons, D.L.; Figdor, C.G. Distinct binding of T lymphocytes to ICAM-1, -2 or -3 upon activation of LFA-1. Eur. J. Immunol. 1994, 24, 2155–2160. [Google Scholar] [CrossRef]
- Woska, J.R., Jr.; Morelock, M.M.; Jeanfavre, D.D.; Caviness, G.O.; Bormann, B.J.; Rothlein, R. Molecular comparison of soluble intercellular adhesion molecule (sICAM)-1 and sICAM-3 binding to lymphocyte function-associated antigen-1. J. Biol. Chem. 1998, 273, 4725–4733. [Google Scholar] [CrossRef] [PubMed]
- de Fougerolles, A.R.; Qin, X.; Springer, T.A. Characterization of the function of intercellular adhesion molecule (ICAM)-3 and comparison with ICAM-1 and ICAM-2 in immune responses. J. Exp. Med. 1994, 179, 619–629. [Google Scholar] [CrossRef] [PubMed]
- Starling, G.C.; McLellan, A.D.; Egner, W.; Sorg, R.V.; Fawcett, J.; Simmons, D.L.; Hart, D.N. Intercellular adhesion molecule-3 is the predominant co-stimulatory ligand for leukocyte function antigen-1 on human blood dendritic cells. Eur. J. Immunol. 1995, 25, 2528–2532. [Google Scholar] [CrossRef] [PubMed]
- de Fougerolles, A.R.; Springer, T.A. Intercellular adhesion molecule 3, a third adhesion counter-receptor for lymphocyte function-associated molecule 1 on resting lymphocytes. J. Exp. Med. 1992, 175, 185–190. [Google Scholar] [CrossRef] [PubMed]
- Campanero, M.R.; del Pozo, M.A.; Arroyo, A.G.; Sanchez-Mateos, P.; Hernandez-Caselles, T.; Craig, A.; Pulido, R.; Sanchez-Madrid, F. ICAM-3 interacts with LFA-1 and regulates the LFA-1/ICAM-1 cell adhesion pathway. J. Cell Biol. 1993, 123, 1007–1016. [Google Scholar] [CrossRef]
- Montoya, M.C.; Sancho, D.; Bonello, G.; Collette, Y.; Langlet, C.; He, H.T.; Aparicio, P.; Alcover, A.; Olive, D.; Sanchez-Madrid, F. Role of ICAM-3 in the initial interaction of T lymphocytes and APCs. Nat. Immunol. 2002, 3, 159–168. [Google Scholar] [CrossRef]
- Carpen, O.; Pallai, P.; Staunton, D.E.; Springer, T.A. Association of intercellular adhesion molecule-1 (ICAM-1) with actin-containing cytoskeleton and alpha-actinin. J. Cell Biol. 1992, 118, 1223–1234. [Google Scholar] [CrossRef]
- Heiska, L.; Kantor, C.; Parr, T.; Critchley, D.R.; Vilja, P.; Gahmberg, C.G.; Carpen, O. Binding of the cytoplasmic domain of intercellular adhesion molecule-2 (ICAM-2) to alpha-actinin. J. Biol. Chem. 1996, 271, 26214–26219. [Google Scholar] [CrossRef]
- Garcia-Ortiz, A.; Serrador, J.M. ERM Proteins at the Crossroad of Leukocyte Polarization, Migration and Intercellular Adhesion. Int. J. Mol. Sci. 2020, 21, 1502. [Google Scholar] [CrossRef]
- Sanchez-Madrid, F.; Serrador, J.M. Bringing up the rear: Defining the roles of the uropod. Nat. Rev. Mol. Cell Biol. 2009, 10, 353–359. [Google Scholar] [CrossRef]
- Heiska, L.; Alfthan, K.; Gronholm, M.; Vilja, P.; Vaheri, A.; Carpen, O. Association of ezrin with intercellular adhesion molecule-1 and -2 (ICAM-1 and ICAM-2). Regulation by phosphatidylinositol 4, 5-bisphosphate. J. Biol. Chem. 1998, 273, 21893–21900. [Google Scholar] [CrossRef]
- Serrador, J.M.; Vicente-Manzanares, M.; Calvo, J.; Barreiro, O.; Montoya, M.C.; Schwartz-Albiez, R.; Furthmayr, H.; Lozano, F.; Sanchez-Madrid, F. A novel serine-rich motif in the intercellular adhesion molecule 3 is critical for its ezrin/radixin/moesin-directed subcellular targeting. J. Biol. Chem. 2002, 277, 10400–10409. [Google Scholar] [CrossRef]
- Hao, J.J.; Liu, Y.; Kruhlak, M.; Debell, K.E.; Rellahan, B.L.; Shaw, S. Phospholipase C-mediated hydrolysis of PIP2 releases ERM proteins from lymphocyte membrane. J. Cell Biol. 2009, 184, 451–462. [Google Scholar] [CrossRef]
- del Pozo, M.A.; Sanchez-Mateos, P.; Nieto, M.; Sanchez-Madrid, F. Chemokines regulate cellular polarization and adhesion receptor redistribution during lymphocyte interaction with endothelium and extracellular matrix. Involvement of cAMP signaling pathway. J. Cell Biol. 1995, 131, 495–508. [Google Scholar] [CrossRef] [PubMed]
- Serrador, J.M.; Alonso-Lebrero, J.L.; del Pozo, M.A.; Furthmayr, H.; Schwartz-Albiez, R.; Calvo, J.; Lozano, F.; Sanchez-Madrid, F. Moesin interacts with the cytoplasmic region of intercellular adhesion molecule-3 and is redistributed to the uropod of T lymphocytes during cell polarization. J. Cell Biol. 1997, 138, 1409–1423. [Google Scholar] [CrossRef] [PubMed]
- Alonso-Lebrero, J.L.; Serrador, J.M.; Dominguez-Jimenez, C.; Barreiro, O.; Luque, A.; del Pozo, M.A.; Snapp, K.; Kansas, G.; Schwartz-Albiez, R.; Furthmayr, H.; et al. Polarization and interaction of adhesion molecules P-selectin glycoprotein ligand 1 and intercellular adhesion molecule 3 with moesin and ezrin in myeloid cells. Blood 2000, 95, 2413–2419. [Google Scholar] [CrossRef] [PubMed]
- Helander, T.S.; Carpen, O.; Turunen, O.; Kovanen, P.E.; Vaheri, A.; Timonen, T. ICAM-2 redistributed by ezrin as a target for killer cells. Nature 1996, 382, 265–268. [Google Scholar] [CrossRef] [PubMed]
- del Pozo, M.A.; Cabanas, C.; Montoya, M.C.; Ager, A.; Sanchez-Mateos, P.; Sanchez-Madrid, F. ICAMs redistributed by chemokines to cellular uropods as a mechanism for recruitment of T lymphocytes. J. Cell Biol. 1997, 137, 493–508. [Google Scholar] [CrossRef] [PubMed]
- Hyun, Y.M.; Sumagin, R.; Sarangi, P.P.; Lomakina, E.; Overstreet, M.G.; Baker, C.M.; Fowell, D.J.; Waugh, R.E.; Sarelius, I.H.; Kim, M. Uropod elongation is a common final step in leukocyte extravasation through inflamed vessels. J. Exp. Med. 2012, 209, 1349–1362. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S.; Hirao, M.; Doi, Y.; Takahashi, N.; Kondo, T.; Tsukita, S. Ezrin/radixin/moesin (ERM) proteins bind to a positively charged amino acid cluster in the juxta-membrane cytoplasmic domain of CD44, CD43, and ICAM-2. J. Cell Biol. 1998, 140, 885–895. [Google Scholar] [CrossRef] [PubMed]
- Hayflick, J.S.; Stine, J.; Fox, R.; Hoekstra, D.; Gallatin, W.M. Functional mapping of the cytoplasmic region of intercellular adhesion molecule-3 reveals important roles for serine residues. J. Biol. Chem. 1997, 272, 22207–22214. [Google Scholar] [CrossRef] [PubMed]
- Yonemura, S.; Tsukita, S.; Tsukita, S. Direct involvement of ezrin/radixin/moesin (ERM)-binding membrane proteins in the organization of microvilli in collaboration with activated ERM proteins. J. Cell Biol. 1999, 145, 1497–1509. [Google Scholar] [CrossRef] [PubMed]
- Ivetic, A.; Florey, O.; Deka, J.; Haskard, D.O.; Ager, A.; Ridley, A.J. Mutagenesis of the ezrin-radixin-moesin binding domain of L-selectin tail affects shedding, microvillar positioning, and leukocyte tethering. J. Biol. Chem. 2004, 279, 33263–33272. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Di Bartolo, V.; Tubul, L.; Shimoni, E.; Kartvelishvily, E.; Dadosh, T.; Feigelson, S.W.; Alon, R.; Alcover, A.; Haran, G. ERM-Dependent Assembly of T Cell Receptor Signaling and Co-stimulatory Molecules on Microvilli prior to Activation. Cell Rep. 2020, 30, 3434–3447 e3436. [Google Scholar] [CrossRef] [PubMed]
- Barreiro, O.; Yanez-Mo, M.; Serrador, J.M.; Montoya, M.C.; Vicente-Manzanares, M.; Tejedor, R.; Furthmayr, H.; Sanchez-Madrid, F. Dynamic interaction of VCAM-1 and ICAM-1 with moesin and ezrin in a novel endothelial docking structure for adherent leukocytes. J. Cell Biol. 2002, 157, 1233–1245. [Google Scholar] [CrossRef] [PubMed]
- Carman, C.V.; Jun, C.D.; Salas, A.; Springer, T.A. Endothelial cells proactively form microvilli-like membrane projections upon intercellular adhesion molecule 1 engagement of leukocyte LFA-1. J. Immunol. 2003, 171, 6135–6144. [Google Scholar] [CrossRef]
- Millan, J.; Hewlett, L.; Glyn, M.; Toomre, D.; Clark, P.; Ridley, A.J. Lymphocyte transcellular migration occurs through recruitment of endothelial ICAM-1 to caveola- and F-actin-rich domains. Nat. Cell Biol. 2006, 8, 113–123. [Google Scholar] [CrossRef]
- Comrie, W.A.; Li, S.; Boyle, S.; Burkhardt, J.K. The dendritic cell cytoskeleton promotes T cell adhesion and activation by constraining ICAM-1 mobility. J. Cell Biol. 2015, 208, 457–473. [Google Scholar] [CrossRef]
- Ma, V.P.; Hu, Y.; Kellner, A.V.; Brockman, J.M.; Velusamy, A.; Blanchard, A.T.; Evavold, B.D.; Alon, R.; Salaita, K. The magnitude of LFA-1/ICAM-1 forces fine-tune TCR-triggered T cell activation. Sci. Adv. 2022, 8, eabg4485. [Google Scholar] [CrossRef]
- Kohlmeier, J.E.; Benedict, S.H. Alternate costimulatory molecules in T cell activation: Differential mechanisms for directing the immune response. Histol. Histopathol. 2003, 18, 1195–1204. [Google Scholar]
- Damle, N.K.; Klussman, K.; Linsley, P.S.; Aruffo, A.; Ledbetter, J.A. Differential regulatory effects of intercellular adhesion molecule-1 on costimulation by the CD28 counter-receptor B7. J. Immunol. 1992, 149, 2541–2548. [Google Scholar] [CrossRef] [PubMed]
- Damle, N.K.; Klussman, K.; Aruffo, A. Intercellular adhesion molecule-2, a second counter-receptor for CD11a/CD18 (leukocyte function-associated antigen-1), provides a costimulatory signal for T-cell receptor-initiated activation of human T cells. J. Immunol. 1992, 148, 665–671. [Google Scholar] [CrossRef]
- Salomon, B.; Bluestone, J.A. LFA-1 interaction with ICAM-1 and ICAM-2 regulates Th2 cytokine production. J. Immunol. 1998, 161, 5138–5142. [Google Scholar] [CrossRef] [PubMed]
- Verma, N.K.; Fazil, M.H.; Ong, S.T.; Chalasani, M.L.; Low, J.H.; Kottaiswamy, A.; Praseetha, P.; Kizhakeyil, A.; Kumar, S.; Panda, A.K.; et al. Correction: LFA-1/ICAM-1 Ligation in Human T Cells Promotes Th1 Polarization through a GSK3beta Signaling-Dependent Notch Pathway. J. Immunol. 2016, 197, 2039–2040. [Google Scholar] [CrossRef] [PubMed]
- Kolev, M.; West, E.E.; Kunz, N.; Chauss, D.; Moseman, E.A.; Rahman, J.; Freiwald, T.; Balmer, M.L.; Lotscher, J.; Dimeloe, S.; et al. Diapedesis-Induced Integrin Signaling via LFA-1 Facilitates Tissue Immunity by Inducing Intrinsic Complement C3 Expression in Immune Cells. Immunity 2020, 52, 513–527 e518. [Google Scholar] [CrossRef] [PubMed]
- Freeley, S.; Cardone, J.; Gunther, S.C.; West, E.E.; Reinheckel, T.; Watts, C.; Kemper, C.; Kolev, M.V. Asparaginyl Endopeptidase (Legumain) Supports Human Th1 Induction via Cathepsin L-Mediated Intracellular C3 Activation. Front. Immunol. 2018, 9, 2449. [Google Scholar] [CrossRef] [PubMed]
- Kolev, M.; Dimeloe, S.; Le Friec, G.; Navarini, A.; Arbore, G.; Povoleri, G.A.; Fischer, M.; Belle, R.; Loeliger, J.; Develioglu, L.; et al. Complement Regulates Nutrient Influx and Metabolic Reprogramming during Th1 Cell Responses. Immunity 2015, 42, 1033–1047. [Google Scholar] [CrossRef]
- Sharma, A.; Lawry, S.M.; Klein, B.S.; Wang, X.; Sherer, N.M.; Zumwalde, N.A.; Gumperz, J.E. LFA-1 Ligation by High-Density ICAM-1 Is Sufficient To Activate IFN-gamma Release by Innate T Lymphocytes. J. Immunol. 2018, 201, 2452–2461. [Google Scholar] [CrossRef]
- Tohma, S.; Hirohata, S.; Lipsky, P.E. The role of CD11a/CD18-CD54 interactions in human T cell-dependent B cell activation. J. Immunol. 1991, 146, 492–499. [Google Scholar] [CrossRef]
- Carrasco, Y.R.; Fleire, S.J.; Cameron, T.; Dustin, M.L.; Batista, F.D. LFA-1/ICAM-1 interaction lowers the threshold of B cell activation by facilitating B cell adhesion and synapse formation. Immunity 2004, 20, 589–599. [Google Scholar] [CrossRef]
- Balkow, S.; Heinz, S.; Schmidbauer, P.; Kolanus, W.; Holzmann, B.; Grabbe, S.; Laschinger, M. LFA-1 activity state on dendritic cells regulates contact duration with T cells and promotes T-cell priming. Blood 2010, 116, 1885–1894. [Google Scholar] [CrossRef] [PubMed]
- Kandula, S.; Abraham, C. LFA-1 on CD4+ T cells is required for optimal antigen-dependent activation in vivo. J. Immunol. 2004, 173, 4443–4451. [Google Scholar] [CrossRef] [PubMed]
- Scholer, A.; Hugues, S.; Boissonnas, A.; Fetler, L.; Amigorena, S. Intercellular adhesion molecule-1-dependent stable interactions between T cells and dendritic cells determine CD8+ T cell memory. Immunity 2008, 28, 258–270. [Google Scholar] [CrossRef]
- Segura, E.; Nicco, C.; Lombard, B.; Veron, P.; Raposo, G.; Batteux, F.; Amigorena, S.; Thery, C. ICAM-1 on exosomes from mature dendritic cells is critical for efficient naive T-cell priming. Blood 2005, 106, 216–223. [Google Scholar] [CrossRef] [PubMed]
- Feigelson, S.W.; Solomon, A.; Biram, A.; Hatzav, M.; Lichtenstein, M.; Regev, O.; Kozlovski, S.; Varol, D.; Curato, C.; Leshkowitz, D.; et al. ICAMs Are Not Obligatory for Functional Immune Synapses between Naive CD4 T Cells and Lymph Node DCs. Cell Rep. 2018, 22, 849–859. [Google Scholar] [CrossRef]
- Sapoznikov, A.; Kozlovski, S.; Levi, N.; Feigelson, S.W.; Regev, O.; Davidzohn, N.; Ben-Dor, S.; Haffner-Krausz, R.; Feldmesser, E.; Wigoda, N.; et al. Dendritic cell ICAM-1 strengthens synapses with CD8 T cells but is not required for their early differentiation. Cell Rep. 2023, 42, 112864. [Google Scholar] [CrossRef]
- Haghayegh Jahromi, N.; Marchetti, L.; Moalli, F.; Duc, D.; Basso, C.; Tardent, H.; Kaba, E.; Deutsch, U.; Pot, C.; Sallusto, F.; et al. Intercellular Adhesion Molecule-1 (ICAM-1) and ICAM-2 Differentially Contribute to Peripheral Activation and CNS Entry of Autoaggressive Th1 and Th17 Cells in Experimental Autoimmune Encephalomyelitis. Front. Immunol. 2019, 10, 3056. [Google Scholar] [CrossRef]
- Tran, D.Q.; Glass, D.D.; Uzel, G.; Darnell, D.A.; Spalding, C.; Holland, S.M.; Shevach, E.M. Analysis of adhesion molecules, target cells, and role of IL-2 in human FOXP3+ regulatory T cell suppressor function. J. Immunol. 2009, 182, 2929–2938. [Google Scholar] [CrossRef]
- Onishi, Y.; Fehervari, Z.; Yamaguchi, T.; Sakaguchi, S. Foxp3+ natural regulatory T cells preferentially form aggregates on dendritic cells in vitro and actively inhibit their maturation. Proc. Natl. Acad. Sci. USA 2008, 105, 10113–10118. [Google Scholar] [CrossRef]
- Deane, J.A.; Abeynaike, L.D.; Norman, M.U.; Wee, J.L.; Kitching, A.R.; Kubes, P.; Hickey, M.J. Endogenous regulatory T cells adhere in inflamed dermal vessels via ICAM-1: Association with regulation of effector leukocyte adhesion. J. Immunol. 2012, 188, 2179–2188. [Google Scholar] [CrossRef]
- Holland, J.; Owens, T. Signaling through intercellular adhesion molecule 1 (ICAM-1) in a B cell lymphoma line. The activation of Lyn tyrosine kinase and the mitogen-activated protein kinase pathway. J. Biol. Chem. 1997, 272, 9108–9112. [Google Scholar] [CrossRef]
- Chirathaworn, C.; Kohlmeier, J.E.; Tibbetts, S.A.; Rumsey, L.M.; Chan, M.A.; Benedict, S.H. Stimulation through intercellular adhesion molecule-1 provides a second signal for T cell activation. J. Immunol. 2002, 168, 5530–5537. [Google Scholar] [CrossRef]
- Kohlmeier, J.E.; Chan, M.A.; Benedict, S.H. Costimulation of naive human CD4 T cells through intercellular adhesion molecule-1 promotes differentiation to a memory phenotype that is not strictly the result of multiple rounds of cell division. Immunology 2006, 118, 549–558. [Google Scholar] [CrossRef]
- Hernandez-Caselles, T.; Rubio, G.; Campanero, M.R.; del Pozo, M.A.; Muro, M.; Sanchez-Madrid, F.; Aparicio, P. ICAM-3, the third LFA-1 counterreceptor, is a co-stimulatory molecule for both resting and activated T lymphocytes. Eur. J. Immunol. 1993, 23, 2799–2806. [Google Scholar] [CrossRef]
- Berney, S.M.; Schaan, T.; Alexander, J.S.; Peterman, G.; Hoffman, P.A.; Wolf, R.E.; van der Heyde, H.; Atkinson, T.P. ICAM-3 (CD50) cross-linking augments signaling in CD3-activated peripheral human T lymphocytes. J. Leukoc. Biol. 1999, 65, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Kohlmeier, J.E.; Rumsey, L.M.; Chan, M.A.; Benedict, S.H. The outcome of T-cell costimulation through intercellular adhesion molecule-1 differs from costimulation through leucocyte function-associated antigen-1. Immunology 2003, 108, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Bhatta, A.; Chan, M.A.; Benedict, S.H. Transcription factor activation and protein phosphorylation patterns are distinct for CD28 and ICAM-1 co-stimulatory molecules. Immunobiology 2021, 226, 152067. [Google Scholar] [CrossRef] [PubMed]
- Wiesolek, H.L.; Bui, T.M.; Lee, J.J.; Dalal, P.; Finkielsztein, A.; Batra, A.; Thorp, E.B.; Sumagin, R. Intercellular Adhesion Molecule 1 Functions as an Efferocytosis Receptor in Inflammatory Macrophages. Am. J. Pathol. 2020, 190, 874–885. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, O.D.; Devitt, A.; Bell, E.D.; Simmons, D.L.; Gregory, C.D. Macrophage recognition of ICAM-3 on apoptotic leukocytes. J. Immunol. 1999, 162, 6800–6810. [Google Scholar] [CrossRef] [PubMed]
- Torr, E.E.; Gardner, D.H.; Thomas, L.; Goodall, D.M.; Bielemeier, A.; Willetts, R.; Griffiths, H.R.; Marshall, L.J.; Devitt, A. Apoptotic cell-derived ICAM-3 promotes both macrophage chemoattraction to and tethering of apoptotic cells. Cell Death Differ. 2012, 19, 671–679. [Google Scholar] [CrossRef] [PubMed]
- Perez, O.D.; Kinoshita, S.; Hitoshi, Y.; Payan, D.G.; Kitamura, T.; Nolan, G.P.; Lorens, J.B. Activation of the PKB/AKT pathway by ICAM-2. Immunity 2002, 16, 51–65. [Google Scholar] [CrossRef] [PubMed]
- Ahn, K.C.; Choi, J.Y.; Kim, J.S.; Hwang, S.G.; Kim, W.J.; Park, J.K.; Um, H.D. ICAM-3 endows anticancer drug resistance against microtubule-damaging agents via activation of the ICAM-3-AKT/ERK-CREB-2 pathway and blockage of apoptosis. Biochem. Biophys. Res. Commun. 2013, 441, 507–513. [Google Scholar] [CrossRef] [PubMed]
- Shen, W.; Zhang, X.; Du, R.; Fan, Y.; Luo, D.; Bao, Y.; Yang, W.; Luo, N.; Luo, Y.; Zhao, S. ICAM3 mediates tumor metastasis via a LFA-1-ICAM3-ERM dependent manner. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 2566–2578. [Google Scholar] [CrossRef] [PubMed]
- de la Fuente, H.; Mittelbrunn, M.; Sanchez-Martin, L.; Vicente-Manzanares, M.; Lamana, A.; Pardi, R.; Cabanas, C.; Sanchez-Madrid, F. Synaptic clusters of MHC class II molecules induced on DCs by adhesion molecule-mediated initial T-cell scanning. Mol. Biol. Cell 2005, 16, 3314–3322. [Google Scholar] [CrossRef]
- Juan, M.; Vinas, O.; Pino-Otin, M.R.; Places, L.; Martinez-Caceres, E.; Barcelo, J.J.; Miralles, A.; Vilella, R.; de la Fuente, M.A.; Vives, J.; et al. CD50 (intercellular adhesion molecule 3) stimulation induces calcium mobilization and tyrosine phosphorylation through p59fyn and p56lck in Jurkat T cell line. J. Exp. Med. 1994, 179, 1747–1756. [Google Scholar] [CrossRef]
- Feldhaus, M.J.; Kessel, J.M.; Zimmerman, G.A.; McIntyre, T.M. Engagement of ICAM-3 activates polymorphonuclear leukocytes: Aggregation without degranulation or beta 2 integrin recruitment. J. Immunol. 1998, 161, 6280–6287. [Google Scholar] [CrossRef]
- Skubitz, K.M.; Ahmed, K.; Campbell, K.D.; Skubitz, A.P. CD50 (ICAM-3) is phosphorylated on tyrosine and is associated with tyrosine kinase activity in human neutrophils. J. Immunol. 1995, 154, 2888–2895. [Google Scholar] [CrossRef]
- Arroyo, A.G.; Campanero, M.R.; Sanchez-Mateos, P.; Zapata, J.M.; Ursa, M.A.; del Pozo, M.A.; Sanchez-Madrid, F. Induction of tyrosine phosphorylation during ICAM-3 and LFA-1-mediated intercellular adhesion, and its regulation by the CD45 tyrosine phosphatase. J. Cell Biol. 1994, 126, 1277–1286. [Google Scholar] [CrossRef]
- Gottrand, G.; Courau, T.; Thomas-Vaslin, V.; Prevel, N.; Vazquez, T.; Ruocco, M.G.; Lambrecht, B.; Bellier, B.; Colombo, B.M.; Klatzmann, D. Regulatory T-cell development and function are impaired in mice lacking membrane expression of full length intercellular adhesion molecule-1. Immunology 2015, 146, 657–670. [Google Scholar] [CrossRef]
- Thummler, K.; Leipe, J.; Ramming, A.; Schulze-Koops, H.; Skapenko, A. Immune regulation by peripheral suppressor T cells induced upon homotypic T cell/T cell interactions. J. Leukoc. Biol. 2010, 88, 1041–1050. [Google Scholar] [CrossRef]
- Zumwalde, N.A.; Domae, E.; Mescher, M.F.; Shimizu, Y. ICAM-1-dependent homotypic aggregates regulate CD8 T cell effector function and differentiation during T cell activation. J. Immunol. 2013, 191, 3681–3693. [Google Scholar] [CrossRef] [PubMed]
- Sabatos, C.A.; Doh, J.; Chakravarti, S.; Friedman, R.S.; Pandurangi, P.G.; Tooley, A.J.; Krummel, M.F. A synaptic basis for paracrine interleukin-2 signaling during homotypic T cell interaction. Immunity 2008, 29, 238–248. [Google Scholar] [CrossRef] [PubMed]
- Okada, T.; Miller, M.J.; Parker, I.; Krummel, M.F.; Neighbors, M.; Hartley, S.B.; O’Garra, A.; Cahalan, M.D.; Cyster, J.G. Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 2005, 3, e150. [Google Scholar] [CrossRef] [PubMed]
- Zaretsky, I.; Atrakchi, O.; Mazor, R.D.; Stoler-Barak, L.; Biram, A.; Feigelson, S.W.; Gitlin, A.D.; Engelhardt, B.; Shulman, Z. ICAMs support B cell interactions with T follicular helper cells and promote clonal selection. J. Exp. Med. 2017, 214, 3435–3448. [Google Scholar] [CrossRef] [PubMed]
- Kishimoto, T.K.; Hollander, N.; Roberts, T.M.; Anderson, D.C.; Springer, T.A. Heterogeneous mutations in the beta subunit common to the LFA-1, Mac-1, and p150,95 glycoproteins cause leukocyte adhesion deficiency. Cell 1987, 50, 193–202. [Google Scholar] [CrossRef]
- Hanna, S.; Etzioni, A. Leukocyte adhesion deficiencies. Ann. N. Y. Acad. Sci. 2012, 1250, 50–55. [Google Scholar] [CrossRef]
- Pflugfelder, S.C.; Stern, M.; Zhang, S.; Shojaei, A. LFA-1/ICAM-1 Interaction as a Therapeutic Target in Dry Eye Disease. J. Ocul. Pharmacol. Ther. 2017, 33, 5–12. [Google Scholar] [CrossRef]
- Zhong, M.; Gadek, T.R.; Bui, M.; Shen, W.; Burnier, J.; Barr, K.J.; Hanan, E.J.; Oslob, J.D.; Yu, C.H.; Zhu, J.; et al. Discovery and Development of Potent LFA-1/ICAM-1 Antagonist SAR 1118 as an Ophthalmic Solution for Treating Dry Eye. ACS Med. Chem. Lett. 2012, 3, 203–206. [Google Scholar] [CrossRef]
- Holland, E.J.; Whitley, W.O.; Sall, K.; Lane, S.S.; Raychaudhuri, A.; Zhang, S.Y.; Shojaei, A. Lifitegrast clinical efficacy for treatment of signs and symptoms of dry eye disease across three randomized controlled trials. Curr. Med. Res. Opin. 2016, 32, 1759–1765. [Google Scholar] [CrossRef]
- Connolly, E.S., Jr.; Winfree, C.J.; Springer, T.A.; Naka, Y.; Liao, H.; Yan, S.D.; Stern, D.M.; Solomon, R.A.; Gutierrez-Ramos, J.C.; Pinsky, D.J. Cerebral protection in homozygous null ICAM-1 mice after middle cerebral artery occlusion. Role of neutrophil adhesion in the pathogenesis of stroke. J. Clin. Investig. 1996, 97, 209–216. [Google Scholar] [CrossRef]
- Kelly, K.J.; Williams, W.W., Jr.; Colvin, R.B.; Meehan, S.M.; Springer, T.A.; Gutierrez-Ramos, J.C.; Bonventre, J.V. Intercellular adhesion molecule-1-deficient mice are protected against ischemic renal injury. J. Clin. Investig. 1996, 97, 1056–1063. [Google Scholar] [CrossRef]
- Habas, K.; Shang, L. Alterations in intercellular adhesion molecule 1 (ICAM-1) and vascular cell adhesion molecule 1 (VCAM-1) in human endothelial cells. Tissue Cell 2018, 54, 139–143. [Google Scholar] [CrossRef]
- Vainer, B.; Nielsen, O.H. Changed colonic profile of P-selectin, platelet-endothelial cell adhesion molecule-1 (PECAM-1), intercellular adhesion molecule-1 (ICAM-1), ICAM-2, and ICAM-3 in inflammatory bowel disease. Clin. Exp. Immunol. 2000, 121, 242–247. [Google Scholar] [CrossRef]
- Bendjelloul, F.; Maly, P.; Mandys, V.; Jirkovska, M.; Prokesova, L.; Tuckova, L.; Tlaskalova-Hogenova, H. Intercellular adhesion molecule-1 (ICAM-1) deficiency protects mice against severe forms of experimentally induced colitis. Clin. Exp. Immunol. 2000, 119, 57–63. [Google Scholar] [CrossRef]
- Beck-Schimmer, B.; Madjdpour, C.; Kneller, S.; Ziegler, U.; Pasch, T.; Wuthrich, R.P.; Ward, P.A.; Schimmer, R.C. Role of alveolar epithelial ICAM-1 in lipopolysaccharide-induced lung inflammation. Eur. Respir. J. 2002, 19, 1142–1150. [Google Scholar] [CrossRef]
- Tsoutsou, P.G.; Gourgoulianis, K.I.; Petinaki, E.; Mpaka, M.; Efremidou, S.; Maniatis, A.; Molyvdas, P.A. ICAM-1, ICAM-2 and ICAM-3 in the sera of patients with idiopathic pulmonary fibrosis. Inflammation 2004, 28, 359–364. [Google Scholar] [CrossRef]
- Kraus, J.; Oschmann, P.; Engelhardt, B.; Bauer, R.; Schiel, C.; Kern, A.; Stolz, E.; Traupe, A.; Dorndorf, W. Soluble and cell surface ICAM-3 in blood and cerebrospinal fluid of patients with multiple sclerosis: Influence of methylprednisolone treatment and relevance as markers for disease activity. Acta Neurol. Scand. 2000, 101, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Lerma, I.; Estrach, M.T. A distinct profile of serum levels of soluble intercellular adhesion molecule-1 and intercellular adhesion molecule-3 in mycosis fungoides and Sezary syndrome. J. Am. Acad. Dermatol. 2009, 61, 263–270. [Google Scholar] [CrossRef] [PubMed]
- Richmond, J.M.; Harris, J.E. Immunology and skin in health and disease. Cold Spring Harb. Perspect. Med. 2014, 4, a015339. [Google Scholar] [CrossRef] [PubMed]
- Griffiths, C.E.; Boffa, M.J.; Gallatin, W.M.; Martin, S. Elevated levels of circulating intercellular adhesion molecule-3 (cICAM-3) in Psoriasis. Acta Derm. Venereol. 1996, 76, 2–5. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Zhou, N.; Liu, Y.; Zhang, W.; Li, X.; Wang, Y.; Zheng, R.; Zhang, Y. Mesenchymal stem cell exosome-derived miR-223 alleviates acute graft-versus-host disease via reducing the migration of donor T cells. Stem Cell Res. Ther. 2021, 12, 153. [Google Scholar] [CrossRef]
- Liu, Y.F.; Zhang, S.Y.; Chen, Y.Y.; Shi, K.; Zou, B.; Liu, J.; Yang, Q.; Jiang, H.; Wei, L.; Li, C.Z.; et al. ICAM-1 Deficiency in the Bone Marrow Niche Impairs Quiescence and Repopulation of Hematopoietic Stem Cells. Stem Cell Rep. 2018, 11, 258–273. [Google Scholar] [CrossRef] [PubMed]
- Park, J.K.; Park, S.H.; So, K.; Bae, I.H.; Yoo, Y.D.; Um, H.D. ICAM-3 enhances the migratory and invasive potential of human non-small cell lung cancer cells by inducing MMP-2 and MMP-9 via Akt and CREB. Int. J. Oncol. 2010, 36, 181–192. [Google Scholar] [CrossRef] [PubMed]
- de Chaisemartin, L.; Goc, J.; Damotte, D.; Validire, P.; Magdeleinat, P.; Alifano, M.; Cremer, I.; Fridman, W.H.; Sautes-Fridman, C.; Dieu-Nosjean, M.C. Characterization of chemokines and adhesion molecules associated with T cell presence in tertiary lymphoid structures in human lung cancer. Cancer Res. 2011, 71, 6391–6399. [Google Scholar] [CrossRef] [PubMed]
- Muro, S.; Garnacho, C.; Champion, J.A.; Leferovich, J.; Gajewski, C.; Schuchman, E.H.; Mitragotri, S.; Muzykantov, V.R. Control of endothelial targeting and intracellular delivery of therapeutic enzymes by modulating the size and shape of ICAM-1-targeted carriers. Mol. Ther. 2008, 16, 1450–1458. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Liu, W.; Rui, Z.; Zheng, W.; Tan, J.; Li, N.; Yu, Y. Immunotherapy with a biologically active ICAM-1 mAb and an siRNA targeting TSHR in a BALB/c mouse model of Graves’ disease. Endokrynol. Pol. 2021, 72, 592–600. [Google Scholar] [CrossRef] [PubMed]
- Zheng, S.; Huang, K.; Xia, W.; Shi, J.; Liu, Q.; Zhang, X.; Li, G.; Chen, J.; Wang, T.; Chen, X.; et al. Mesenchymal Stromal Cells Rapidly Suppress TCR Signaling-Mediated Cytokine Transcription in Activated T Cells through the ICAM-1/CD43 Interaction. Front. Immunol. 2021, 12, 609544. [Google Scholar] [CrossRef] [PubMed]
- Min, I.M.; Shevlin, E.; Vedvyas, Y.; Zaman, M.; Wyrwas, B.; Scognamiglio, T.; Moore, M.D.; Wang, W.; Park, S.; Park, S.; et al. CAR T Therapy Targeting ICAM-1 Eliminates Advanced Human Thyroid Tumors. Clin. Cancer Res. 2017, 23, 7569–7583. [Google Scholar] [CrossRef]
- Li, R.; Xie, J.; Kantor, C.; Koistinen, V.; Altieri, D.C.; Nortamo, P.; Gahmberg, C.G. A peptide derived from the intercellular adhesion molecule-2 regulates the avidity of the leukocyte integrins CD11b/CD18 and CD11c/CD18. J. Cell Biol. 1995, 129, 1143–1153. [Google Scholar] [CrossRef]
- Bleijs, D.A.; Binnerts, M.E.; van Vliet, S.J.; Figdor, C.G.; van Kooyk, Y. Low-affinity LFA-1/ICAM-3 interactions augment LFA-1/ICAM-1-mediated T cell adhesion and signaling by redistribution of LFA-1. J. Cell Sci. 2000, 113 Pt 3, 391–400. [Google Scholar] [CrossRef]
- Cid, M.C.; Esparza, J.; Juan, M.; Miralles, A.; Ordi, J.; Vilella, R.; Urbano-Marquez, A.; Gaya, A.; Vives, J.; Yague, J. Signaling through CD50 (ICAM-3) stimulates T lymphocyte binding to human umbilical vein endothelial cells and extracellular matrix proteins via an increase in beta 1 and beta 2 integrin function. Eur. J. Immunol. 1994, 24, 1377–1382. [Google Scholar] [CrossRef]
- Verma, N.K.; Kelleher, D. Not Just an Adhesion Molecule: LFA-1 Contact Tunes the T Lymphocyte Program. J. Immunol. 2017, 199, 1213–1221. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, J.C.; Jablonski-Westrich, D.; Haubold, U.; Gutierrez-Ramos, J.C.; Springer, T.; Hamann, A. Overlapping and selective roles of endothelial intercellular adhesion molecule-1 (ICAM-1) and ICAM-2 in lymphocyte trafficking. J. Immunol. 2003, 171, 2588–2593. [Google Scholar] [CrossRef] [PubMed]
- Perez, O.D.; Mitchell, D.; Jager, G.C.; Nolan, G.P. LFA-1 signaling through p44/42 is coupled to perforin degranulation in CD56+CD8+ natural killer cells. Blood 2004, 104, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Perez, O.D.; Mitchell, D.; Nolan, G.P. Differential role of ICAM ligands in determination of human memory T cell differentiation. BMC Immunol. 2007, 8, 2. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guerra-Espinosa, C.; Jiménez-Fernández, M.; Sánchez-Madrid, F.; Serrador, J.M. ICAMs in Immunity, Intercellular Adhesion and Communication. Cells 2024, 13, 339. https://doi.org/10.3390/cells13040339
Guerra-Espinosa C, Jiménez-Fernández M, Sánchez-Madrid F, Serrador JM. ICAMs in Immunity, Intercellular Adhesion and Communication. Cells. 2024; 13(4):339. https://doi.org/10.3390/cells13040339
Chicago/Turabian StyleGuerra-Espinosa, Claudia, María Jiménez-Fernández, Francisco Sánchez-Madrid, and Juan M. Serrador. 2024. "ICAMs in Immunity, Intercellular Adhesion and Communication" Cells 13, no. 4: 339. https://doi.org/10.3390/cells13040339
APA StyleGuerra-Espinosa, C., Jiménez-Fernández, M., Sánchez-Madrid, F., & Serrador, J. M. (2024). ICAMs in Immunity, Intercellular Adhesion and Communication. Cells, 13(4), 339. https://doi.org/10.3390/cells13040339






