Characteristics of Serum Exosomes after Burn Injury and Dermal Fibroblast Regulation by Exosomes In Vitro
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients and Blood Samples
2.2. EXO Isolation
2.3. Transmission Electron Microscope (TEM)
2.4. Western Blot
2.5. Dynamic Light Scattering (DLS)
2.6. Multiplex Cytokine Assay
2.7. EXO Uptake Assay
2.8. Fibroblast Regulation by EXOs In Vitro
2.9. Statistical Analysis
3. Results
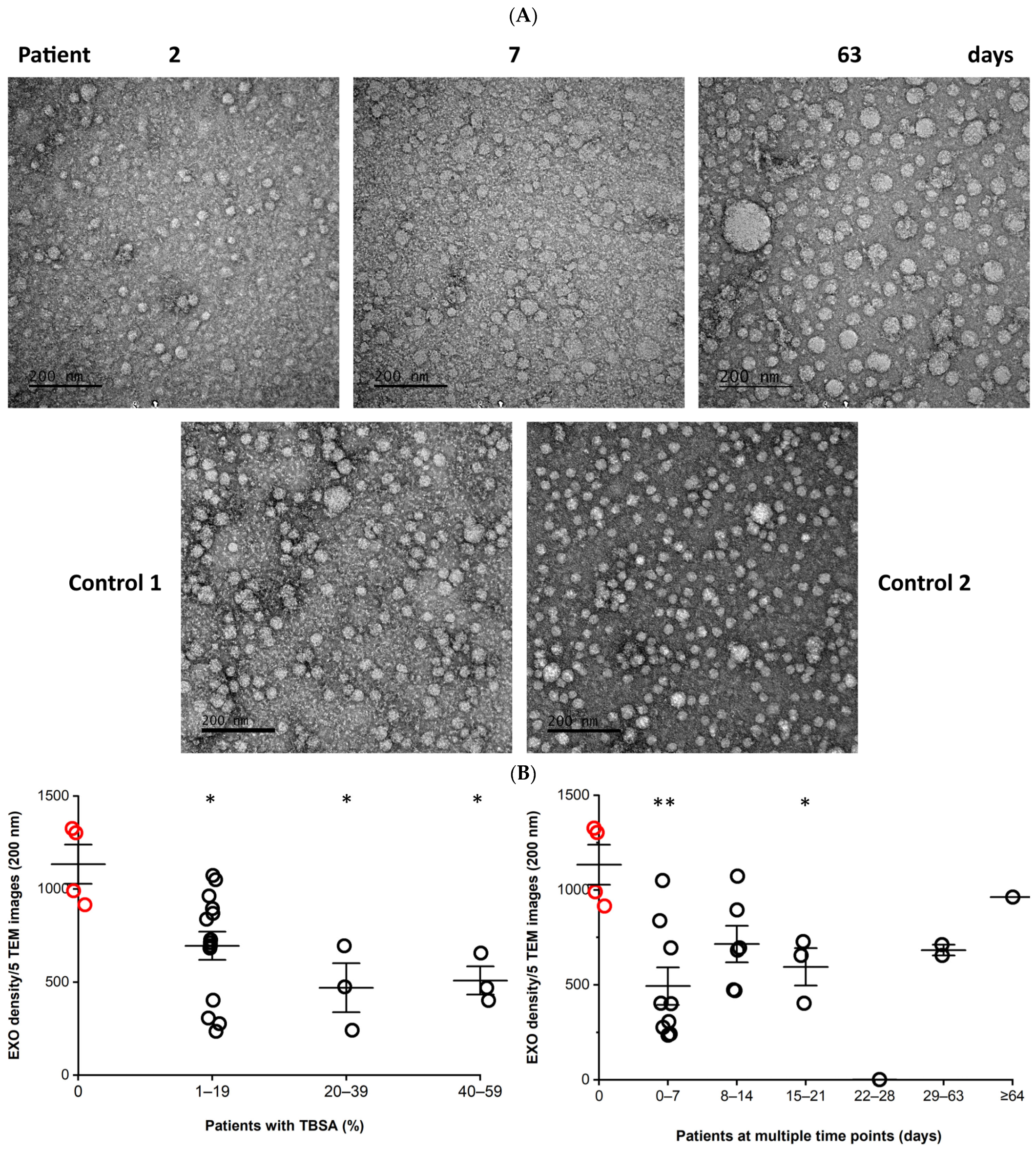
3.1. Morphology and Density of Serum EXOs
3.2. Marker Expression of EXOs
3.3. Concentration of EXOs
3.4. EXO Size Distribution
3.5. Cytokine and Cytokine Correlation with Patient TBSA
3.6. EXO Uptake by Dermal Fibroblasts
3.7. Fibroblast Regulation by EXOs
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singer, A.J.; Clark, R.A. Cutaneous wound healing. N. Engl. J. Med. 1999, 341, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Almadani, Y.H.; Vorstenbosch, J.; Davison, P.G.; Murphy, A.M. Wound Healing: A Comprehensive Review. Semin. Plast. Surg. 2021, 35, 141–144. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.C.; Zhao, W.Y.; Cao, Y.; Liu, Y.Q.; Sun, Q.; Shi, P.; Cai, J.Q.; Shen, X.Z.; Tan, W.Q. The Roles of Inflammation in Keloid and Hypertrophic Scars. Front. Immunol. 2020, 11, 603187. [Google Scholar] [CrossRef] [PubMed]
- Kalluri, R.; LeBleu, V.S. The biology, function, and biomedical applications of exosomes. Science 2020, 367, eaau6977. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Wu, N. Mechanism and application of exosomes in the wound healing process in diabetes mellitus. Diabetes Res. Clin. Pract. 2022, 187, 109882. [Google Scholar] [CrossRef]
- Prasai, A.; Jay, J.W.; Jupiter, D.; Wolf, S.E.; El Ayadi, A. Role of Exosomes in Dermal Wound Healing: A Systematic Review. J. Investig. Dermatol. 2022, 142 Pt A, 662–678.e8. [Google Scholar] [CrossRef]
- Yang, G.; Waheed, S.; Wang, C.; Shekh, M.; Li, Z.; Wu, J. Exosomes and Their Bioengineering Strategies in the Cutaneous Wound Healing and Related Complications: Current Knowledge and Future Perspectives. Int. J. Biol. Sci. 2023, 19, 1430–1454. [Google Scholar] [CrossRef]
- Scott, P.; Dodd, C.; Ghahary, A.; Shen, Y.; Tredget, E. Fibroblasts from post-burn hypertrophic scar tissue synthesize less decorin than normal dermal fibroblasts. Clin. Sci. 1998, 94, 541–547. [Google Scholar] [CrossRef] [Green Version]
- Harding, C.; Stahl, P. Transferrin recycling in reticulocytes: pH and iron are important determinants of ligand binding and processing. Biochem. Biophys. Res. Commun. 1983, 113, 650–658. [Google Scholar] [CrossRef]
- Pan, B.T.; Johnstone, R.M. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: Selective externalization of the receptor. Cell 1983, 33, 967–978. [Google Scholar] [CrossRef]
- Johnstone, R.M.; Adam, M.; Hammond, J.R.; Orr, L.; Turbide, C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes). J. Biol. Chem. 1987, 262, 9412–9420. [Google Scholar] [CrossRef]
- Harding, C.V.; Heuser, J.E.; Stahl, P.D. Exosomes: Looking back three decades and into the future. J. Cell Biol. 2013, 200, 367–371. [Google Scholar] [CrossRef] [Green Version]
- He, C.; Zheng, S.; Luo, Y.; Wang, B. Exosome Theranostics: Biology and Translational Medicine. Theranostics 2018, 8, 237–255. [Google Scholar] [CrossRef]
- Basu, J.; Ludlow, J.W. Exosomes for repair, regeneration and rejuvenation. Expert Opin. Biol. Ther. 2016, 16, 489–506. [Google Scholar] [CrossRef]
- Hough, K.P.; Chanda, D.; Duncan, S.R.; Thannickal, V.J.; Deshane, J.S. Exosomes in immunoregulation of chronic lung diseases. Allergy 2017, 72, 534–544. [Google Scholar] [CrossRef] [Green Version]
- Zhang, S.; Chuah, S.J.; Lai, R.C.; Hui, J.H.P.; Lim, S.K.; Toh, W.S. MSC exosomes mediate cartilage repair by enhancing proliferation, attenuating apoptosis and modulating immune reactivity. Biomaterials 2018, 156, 16–27. [Google Scholar] [CrossRef]
- Vidal, M. Exosomes: Revisiting their role as “garbage bags”. Traffic 2019, 20, 815–828. [Google Scholar] [CrossRef]
- Li, Y.; Wen, Q.; Chen, H.; Wu, X.; Liu, B.; Li, H.; Su, L.; Tong, H. Exosomes Derived from Heat Stroke Cases Carry miRNAs Associated with Inflammation and Coagulation Cascade. Front. Immunol. 2021, 12, 624753. [Google Scholar] [CrossRef]
- Liu, H.; Shen, M.; Zhao, D.; Ru, D.; Duan, Y.; Ding, C.; Li, H. The Effect of Triptolide-Loaded Exosomes on the Proliferation and Apoptosis of Human Ovarian Cancer SKOV3 Cells. BioMed Res. Int. 2019, 2019, 2595801. [Google Scholar] [CrossRef]
- Perocheau, D.; Touramanidou, L.; Gurung, S.; Gissen, P.; Baruteau, J. Clinical applications for exosomes: Are we there yet? Br. J. Pharmacol. 2021, 178, 2375–2392. [Google Scholar] [CrossRef]
- Hu, Y.; Rao, S.S.; Wang, Z.X.; Cao, J.; Tan, Y.J.; Luo, J.; Li, H.M.; Zhang, W.S.; Chen, C.Y.; Xie, H. Exosomes from human umbilical cord blood accelerate cutaneous wound healing through miR-21-3p-mediated promotion of angiogenesis and fibroblast function. Theranostics 2018, 8, 169–184. [Google Scholar] [CrossRef] [PubMed]
- Vu, N.B.; Nguyen, H.T.; Palumbo, R.; Pellicano, R.; Fagoonee, S.; Pham, P.V. Stem cell-derived exosomes for wound healing: Current status and promising directions. Minerva Medica 2021, 112, 384–400. [Google Scholar] [CrossRef] [PubMed]
- An, Y.; Lin, S.; Tan, X.; Zhu, S.; Nie, F.; Zhen, Y.; Gu, L.; Zhang, C.; Wang, B.; Wei, W.; et al. Exosomes from adipose-derived stem cells and application to skin wound healing. Cell Prolif. 2021, 54, e12993. [Google Scholar] [CrossRef] [PubMed]
- Ha, D.H.; Kim, H.K.; Lee, J.; Kwon, H.H.; Park, G.H.; Yang, S.H.; Jung, J.Y.; Choi, H.; Lee, J.H.; Sung, S.; et al. Mesenchymal Stem/Stromal Cell-Derived Exosomes for Immunomodulatory Therapeutics and Skin Regeneration. Cells 2020, 9, 1157. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, M.; Xie, D.; Wang, L.; Ye, C.; Zhu, Q.; Liu, F.; Yang, L. Melatonin-stimulated MSC-derived exosomes improve diabetic wound healing through regulating macrophage M1 and M2 polarization by targeting the PTEN/AKT pathway. Stem Cell Res. Ther. 2020, 11, 259. [Google Scholar] [CrossRef]
- Wang, J.; Wu, H.; Peng, Y.; Zhao, Y.; Qin, Y.; Zhang, Y.; Xiao, Z. Hypoxia adipose stem cell-derived exosomes promote high-quality healing of diabetic wound involves activation of PI3K/Akt pathways. J. Nanobio. 2021, 19, 202. [Google Scholar] [CrossRef]
- Abdul Kareem, N.; Aijaz, A.; Jeschke, M.G. Stem Cell Therapy for Burns: Story so Far. Biologics 2021, 15, 379–397. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, J.; Shi, J.; Liu, K.; Wang, X.; Jia, Y.; Ting, H.; Shen, K.; Wang, Y.; Liu, J.; et al. Exosomes derived from human adipose mesenchymal stem cells attenuate hypertrophic scar fibrosis by miR-192-5p/IL-17RA/Smad axis. Stem Cell Res. Ther. 2021, 12, 221. [Google Scholar] [CrossRef]
- Li, C.; Wei, S.; Xu, Q.; Sun, Y.; Ning, X.; Wang, Z. Application of ADSCs and their Exosomes in Scar Prevention. Stem Cell Rev. Rep. 2022, 18, 952–967. [Google Scholar] [CrossRef]
- Simons, M.; Raposo, G. Exosomes—vesicular carriers for intercellular communication. Curr. Opin. Cell Biol. 2009, 21, 575–581. [Google Scholar] [CrossRef]
- Zhu, Z.; Ding, J.; Tredget, E.E. The molecular basis of hypertrophic scars. Burn. Trauma 2016, 4, 2. [Google Scholar] [CrossRef] [Green Version]
- Mony, M.P.; Harmon, K.A.; Hess, R.; Dorafshar, A.H.; Shafikhani, S.H. An Updated Review of Hypertrophic Scarring. Cells 2023, 12, 678. [Google Scholar] [CrossRef] [PubMed]
- Tredget, E.E.; Ding, J. Wound healing: From embryos to adults and back again. Lancet 2009, 373, 1226–1228. [Google Scholar] [CrossRef]
- Wang, J.; Jiao, H.; Stewart, T.L.; Shankowsky, H.A.; Scott, P.G.; Tredget, E.E. Improvement in postburn hypertrophic scar after treatment with IFN-alpha2b is associated with decreased fibrocytes. J. Interferon Cytokine Res. 2007, 27, 921–930. [Google Scholar] [CrossRef] [PubMed]
- Albuquerque Pereira, M.F.; Morais de Avila, L.G.; Dos Santos Cruz, B.C.; Campos Silva, B.; Licursi de Oliveira, L.; Vilela Goncalves, R.; de Oliveira Barros Ribon, A.; de Oliveira Mendesd, T.A.; do Carmo Gouveia Peluzio, M. The role of IL-10 in regulating inflammation and gut microbiome in mice consuming milk kefir and orally challenged with S. Typhimurium. Food Funct. 2023, 14, 3804–3814. [Google Scholar] [CrossRef] [PubMed]
- Yin, H.; Yu, S.; Xie, Y.; Dai, X.; Dong, M.; Sheng, C.; Hu, J. Cancer-associated fibroblasts-derived exosomes upregulate microRNA-135b-5p to promote colorectal cancer cell growth and angiogenesis by inhibiting thioredoxin-interacting protein. Cell. Signal. 2021, 84, 110029. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ding, J.; Pan, Y.; Raj, S.; Schaffrick, L.; Wong, J.; Nguyen, A.; Manchikanti, S.; Unsworth, L.; Kwan, P.; Tredget, E. Characteristics of Serum Exosomes after Burn Injury and Dermal Fibroblast Regulation by Exosomes In Vitro. Cells 2023, 12, 1738. https://doi.org/10.3390/cells12131738
Ding J, Pan Y, Raj S, Schaffrick L, Wong J, Nguyen A, Manchikanti S, Unsworth L, Kwan P, Tredget E. Characteristics of Serum Exosomes after Burn Injury and Dermal Fibroblast Regulation by Exosomes In Vitro. Cells. 2023; 12(13):1738. https://doi.org/10.3390/cells12131738
Chicago/Turabian StyleDing, Jie, Yingying Pan, Shammy Raj, Lindy Schaffrick, Jolene Wong, Antoinette Nguyen, Sharada Manchikanti, Larry Unsworth, Peter Kwan, and Edward Tredget. 2023. "Characteristics of Serum Exosomes after Burn Injury and Dermal Fibroblast Regulation by Exosomes In Vitro" Cells 12, no. 13: 1738. https://doi.org/10.3390/cells12131738
APA StyleDing, J., Pan, Y., Raj, S., Schaffrick, L., Wong, J., Nguyen, A., Manchikanti, S., Unsworth, L., Kwan, P., & Tredget, E. (2023). Characteristics of Serum Exosomes after Burn Injury and Dermal Fibroblast Regulation by Exosomes In Vitro. Cells, 12(13), 1738. https://doi.org/10.3390/cells12131738





