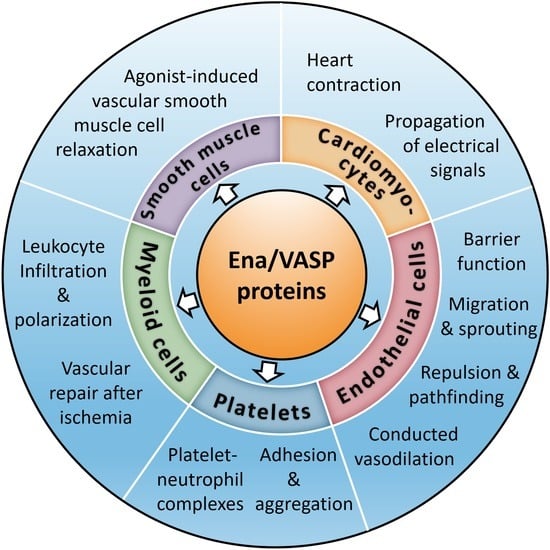
Cardiovascular Functions of Ena/VASP Proteins: Past, Present and Beyond
Abstract
1. Introduction
2. Ena/VASP Proteins
3. Domain Organization and Protein–Protein Interactions of Ena/VASP Proteins
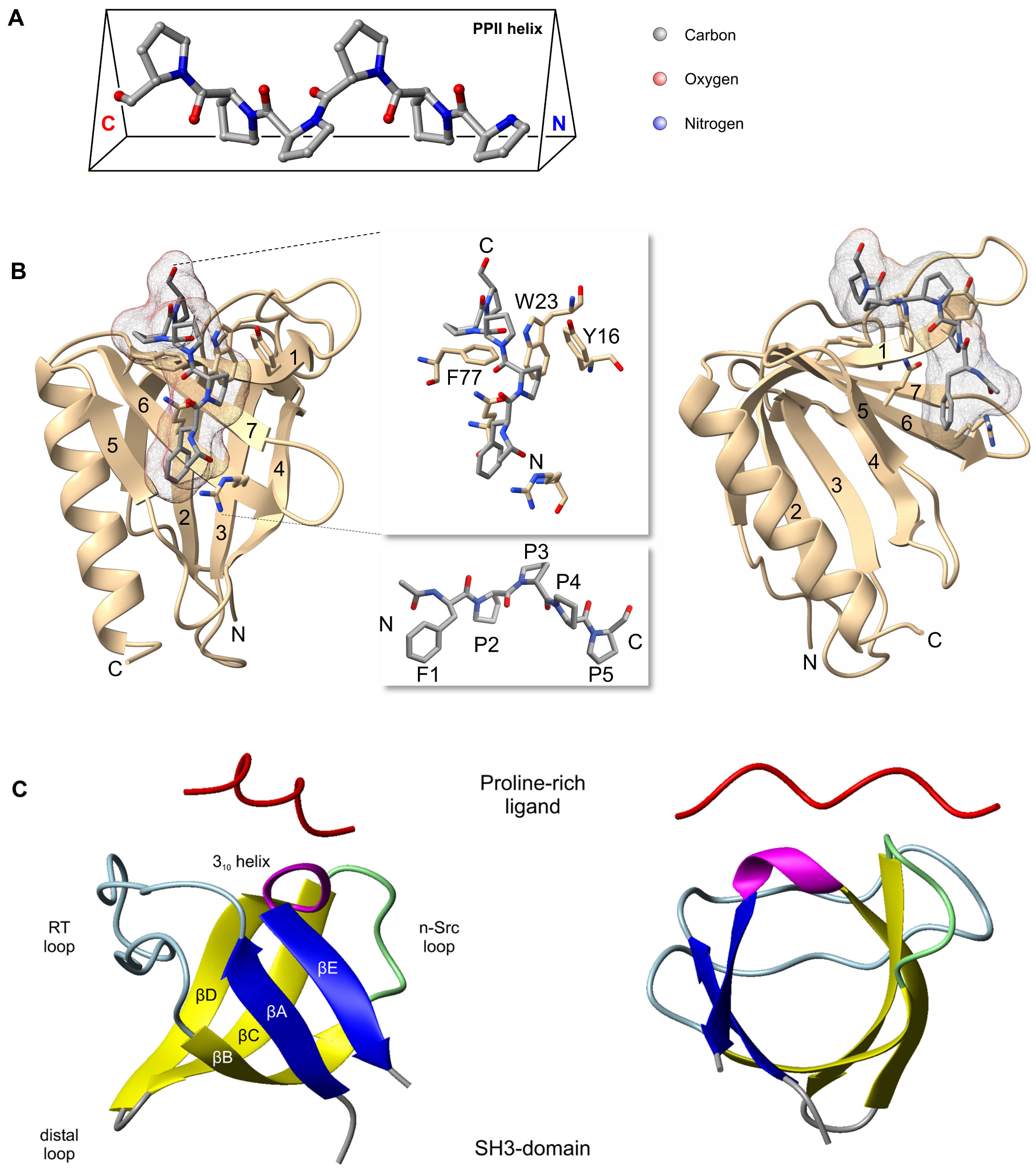
3.1. EVH1 Domain
3.2. LERER Repeats
3.3. Proline-Rich Region
3.4. EVH2 Domain
4. Regulation of Ena/VASP Proteins by Phosphorylation
4.1. Ena/VASP Serine/Threonine Phosphorylation
4.2. Ena/VASP Tyrosine Phosphorylation and Its Implication in Ephrin/EphB Signaling
5. Ena/VASP-Deficient Mouse Models
6. Role of VASP in Platelets
7. Role of Ena/VASP Proteins in Endothelial Barrier Function
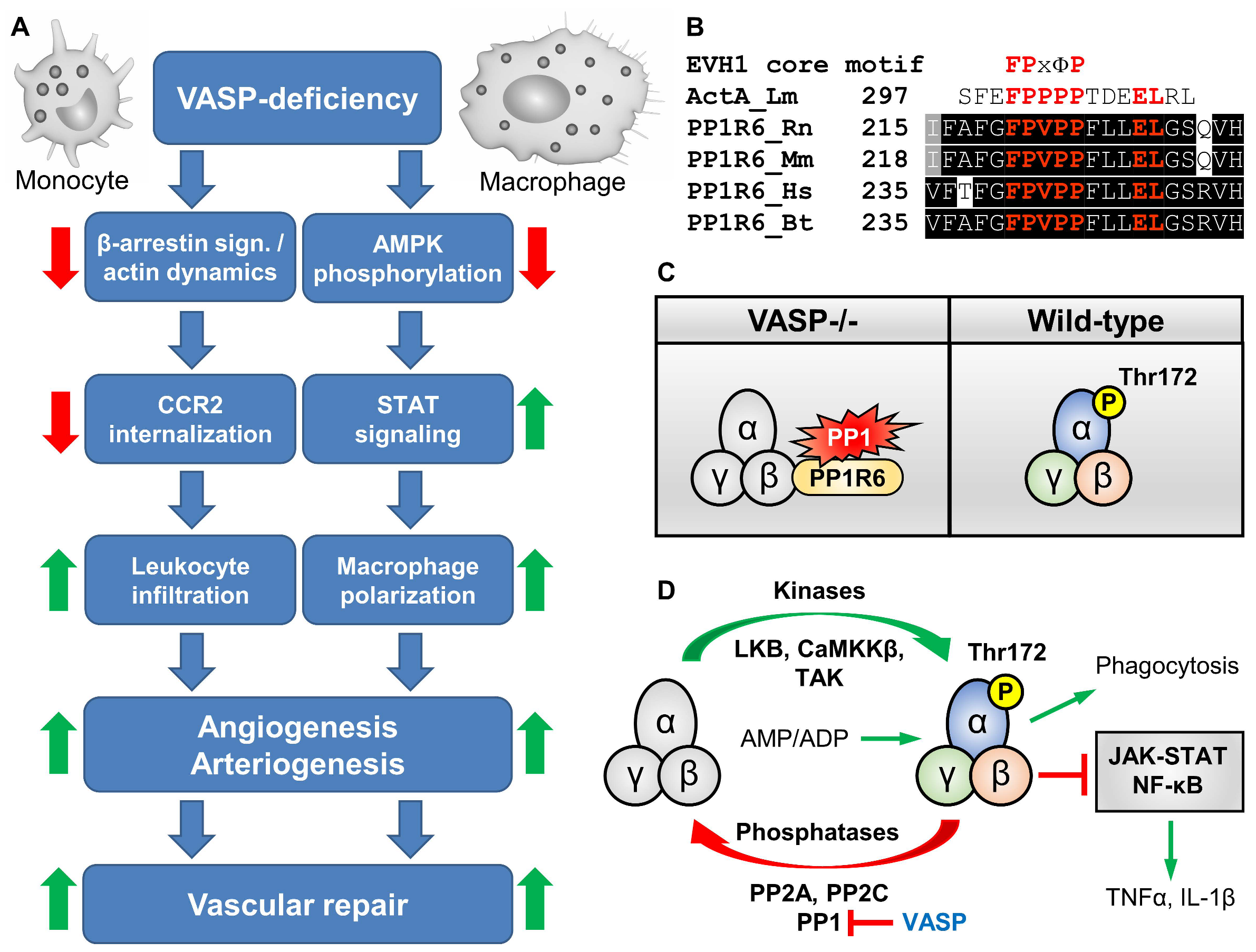
8. Role of VASP in Leukocyte Infiltration, Polarization, and Vascular Repair after Ischemia
9. Role of Ena/VASP Proteins in the Mammalian Heart
10. Role of VASP in Supporting the Conducted Vasodilation along the Vessel Wall
11. Role of Mena and VASP in Smooth Muscle Cell Relaxation
12. Interaction of VASP and AKAP12 in VEGF-Induced Endothelial Cell Migration and Sprouting
13. EVL Regulates VEGF Receptor 2 Internalization and Signaling in Developmental Angiogenesis
14. Role of Ena/VASP Proteins in Endocytosis and Receptor Trafficking
15. Redundant and Non-Redundant Functions of Ena/VASP Proteins
16. Ena/VASP Proteins as Therapeutic Targets
17. Conclusions and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kaksonen, M.; Toret, C.P.; Drubin, D.G. Harnessing actin dynamics for clathrin-mediated endocytosis. Nat. Rev. Mol. Cell Biol. 2006, 7, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Perrin, B.J.; Ervasti, J.M. The actin gene family: Function follows isoform. Cytoskeleton 2010, 67, 630–634. [Google Scholar] [CrossRef] [PubMed]
- Pollard, T.D. Actin and Actin-Binding Proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a018226. [Google Scholar] [CrossRef] [PubMed]
- Winder, S.J.; Ayscough, K.R. Actin-binding proteins. J. Cell Sci. 2005, 118, 651–654. [Google Scholar] [CrossRef]
- Wu, L.G.; Chan, C.Y. Multiple Roles of Actin in Exo- and Endocytosis. Front. Synaptic Neurosci. 2022, 14, 841704. [Google Scholar] [CrossRef]
- Rottner, K.; Faix, J.; Bogdan, S.; Linder, S.; Kerkhoff, E. Actin assembly mechanisms at a glance. J. Cell Sci. 2017, 130, 3427–3435. [Google Scholar] [CrossRef]
- Sechi, A.S.; Wehland, J. ENA/VASP proteins: Multifunctional regulators of actin cytoskeleton dynamics. Front. Biosci. 2004, 9, 1294–1310. [Google Scholar] [CrossRef]
- Waldmann, R.; Nieberding, M.; Walter, U. Vasodilator-stimulated protein phosphorylation in platelets is mediated by cAMP- and cGMP-dependent protein kinases. Eur. J. Biochem. 1987, 167, 441–448. [Google Scholar] [CrossRef]
- Halbrugge, M.; Walter, U. Purification of a vasodilator-regulated phosphoprotein from human platelets. Eur. J. Biochem. 1989, 185, 41–50. [Google Scholar] [CrossRef]
- Gertler, F.B.; Niebuhr, K.; Reinhard, M.; Wehland, J.; Soriano, P. Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell 1996, 87, 227–239. [Google Scholar] [CrossRef]
- Lambrechts, A.; Kwiatkowski, A.V.; Lanier, L.M.; Bear, J.E.; Vandekerckhove, J.; Ampe, C.; Gertler, F.B. cAMP-dependent protein kinase phosphorylation of EVL, a Mena/VASP relative, regulates its interaction with actin and SH3 domains. J. Biol. Chem. 2000, 275, 36143–36151. [Google Scholar] [CrossRef]
- Benz, P.M.; Merkel, C.J.; Offner, K.; Abesser, M.; Ullrich, M.; Fischer, T.; Bayer, B.; Wagner, H.; Gambaryan, S.; Ursitti, J.A.; et al. Mena/VASP and alphaII-Spectrin complexes regulate cytoplasmic actin networks in cardiomyocytes and protect from conduction abnormalities and dilated cardiomyopathy. Cell Commun. Signal. 2013, 11, 56. [Google Scholar] [CrossRef]
- Di Modugno, F.; Iapicca, P.; Boudreau, A.; Mottolese, M.; Terrenato, I.; Perracchio, L.; Carstens, R.P.; Santoni, A.; Bissell, M.J.; Nistico, P. Splicing program of human MENA produces a previously undescribed isoform associated with invasive, mesenchymal-like breast tumors. Proc. Natl. Acad. Sci. USA 2012, 109, 19280–19285. [Google Scholar] [CrossRef]
- Goswami, S.; Philippar, U.; Sun, D.; Patsialou, A.; Avraham, J.; Wang, W.; Di Modugno, F.; Nistico, P.; Gertler, F.B.; Condeelis, J.S. Identification of invasion specific splice variants of the cytoskeletal protein Mena present in mammary tumor cells during invasion in vivo. Clin. Exp. Metastasis 2009, 26, 153–159. [Google Scholar] [CrossRef]
- Philippar, U.; Roussos, E.T.; Oser, M.; Yamaguchi, H.; Kim, H.D.; Giampieri, S.; Wang, Y.; Goswami, S.; Wyckoff, J.B.; Lauffenburger, D.A.; et al. A Mena invasion isoform potentiates EGF-induced carcinoma cell invasion and metastasis. Dev. Cell 2008, 15, 813–828. [Google Scholar] [CrossRef]
- Veniere, S.; Waterschoot, D.; Vandekerckhove, J.; Lambrechts, A.; Ampe, C. Identification and expression analysis of splice variants of mouse enabled homologue during development and in adult tissues. BMC Mol. Biol. 2010, 11, 45. [Google Scholar] [CrossRef]
- Urbanelli, L.; Massini, C.; Emiliani, C.; Orlacchio, A.; Bernardi, G. Characterization of human Enah gene. Biochim. Biophys. Acta 2006, 1759, 99–107. [Google Scholar] [CrossRef]
- Benz, P.M.; Blume, C.; Seifert, S.; Wilhelm, S.; Waschke, J.; Schuh, K.; Gertler, F.; Munzel, T.; Renne, T. Differential VASP phosphorylation controls remodeling of the actin cytoskeleton. J. Cell Sci. 2009, 122, 3954–3965. [Google Scholar] [CrossRef]
- Faix, J.; Rottner, K. Ena/VASP proteins in cell edge protrusion, migration and adhesion. J. Cell Sci. 2022, 135, jcs259226. [Google Scholar] [CrossRef]
- De Smet, F.; Segura, I.; De Bock, K.; Hohensinner, P.J.; Carmeliet, P. Mechanisms of vessel branching: Filopodia on endothelial tip cells lead the way. Arterioscler. Thromb. Vasc. Biol. 2009, 29, 639–649. [Google Scholar] [CrossRef]
- Benz, P.M.; Laban, H.; Zink, J.; Gunther, L.; Walter, U.; Gambaryan, S.; Dib, K. Vasodilator-Stimulated Phosphoprotein (VASP)-dependent and -independent pathways regulate thrombin-induced activation of Rap1b in platelets. Cell Commun. Signal. 2016, 14, 21. [Google Scholar] [CrossRef] [PubMed]
- Benz, P.M.; Blume, C.; Moebius, J.; Oschatz, C.; Schuh, K.; Sickmann, A.; Walter, U.; Feller, S.M.; Renne, T. Cytoskeleton assembly at endothelial cell-cell contacts is regulated by alphaII-spectrin-VASP complexes. J. Cell Biol. 2008, 180, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Poley, P.; Benz, P.M.; de Wit, C. The vasodilator-stimulated phosphoprotein (VASP) supports the conduction of vasodilator signals and NO-induced arteriolar dilations in murine arterioles in vivo. bioRxiv 2023. [Google Scholar] [CrossRef]
- Vasioukhin, V.; Fuchs, E. Actin dynamics and cell-cell adhesion in epithelia. Curr. Opin. Cell Biol. 2001, 13, 76–84. [Google Scholar] [CrossRef] [PubMed]
- Laban, H.; Weigert, A.; Zink, J.; Elgheznawy, A.; Schurmann, C.; Gunther, L.; Abdel Malik, R.; Bothur, S.; Wingert, S.; Bremer, R.; et al. VASP regulates leukocyte infiltration, polarization, and vascular repair after ischemia. J. Cell Biol. 2018, 217, 1503–1519. [Google Scholar] [CrossRef]
- Vehlow, A.; Soong, D.; Vizcay-Barrena, G.; Bodo, C.; Law, A.L.; Perera, U.; Krause, M. Endophilin, Lamellipodin, and Mena cooperate to regulate F-actin-dependent EGF-receptor endocytosis. EMBO J. 2013, 32, 2722–2734. [Google Scholar] [CrossRef]
- Damiano-Guercio, J.; Kurzawa, L.; Mueller, J.; Dimchev, G.; Schaks, M.; Nemethova, M.; Pokrant, T.; Bruhmann, S.; Linkner, J.; Blanchoin, L.; et al. Loss of Ena/VASP interferes with lamellipodium architecture, motility and integrin-dependent adhesion. Elife 2020, 9, e55351. [Google Scholar] [CrossRef]
- Breitsprecher, D.; Kiesewetter, A.K.; Linkner, J.; Vinzenz, M.; Stradal, T.E.; Small, J.V.; Curth, U.; Dickinson, R.B.; Faix, J. Molecular mechanism of Ena/VASP-mediated actin-filament elongation. EMBO J. 2011, 30, 456–467. [Google Scholar] [CrossRef]
- Hansen, S.D.; Mullins, R.D. VASP is a processive actin polymerase that requires monomeric actin for barbed end association. J. Cell Biol. 2010, 191, 571–584. [Google Scholar] [CrossRef]
- Lebrand, C.; Dent, E.W.; Strasser, G.A.; Lanier, L.M.; Krause, M.; Svitkina, T.M.; Borisy, G.G.; Gertler, F.B. Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 2004, 42, 37–49. [Google Scholar] [CrossRef]
- Barzik, M.; Kotova, T.I.; Higgs, H.N.; Hazelwood, L.; Hanein, D.; Gertler, F.B.; Schafer, D.A. Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J. Biol. Chem. 2005, 280, 28653–28662. [Google Scholar] [CrossRef]
- Skoble, J.; Auerbuch, V.; Goley, E.D.; Welch, M.D.; Portnoy, D.A. Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility. J. Cell Biol. 2001, 155, 89–100. [Google Scholar] [CrossRef]
- Winkelman, J.D.; Bilancia, C.G.; Peifer, M.; Kovar, D.R. Ena/VASP Enabled is a highly processive actin polymerase tailored to self-assemble parallel-bundled F-actin networks with Fascin. Proc. Natl. Acad. Sci. USA 2014, 111, 4121–4126. [Google Scholar] [CrossRef]
- Vasioukhin, V.; Bauer, C.; Yin, M.; Fuchs, E. Directed actin polymerization is the driving force for epithelial cell-cell adhesion. Cell 2000, 100, 209–219. [Google Scholar] [CrossRef]
- Scott, J.A.; Shewan, A.M.; den Elzen, N.R.; Loureiro, J.J.; Gertler, F.B.; Yap, A.S. Ena/VASP proteins can regulate distinct modes of actin organization at cadherin-adhesive contacts. Mol. Biol. Cell 2006, 17, 1085–1095. [Google Scholar] [CrossRef]
- Hoelzle, M.K.; Svitkina, T. The cytoskeletal mechanisms of cell-cell junction formation in endothelial cells. Mol. Biol. Cell 2012, 23, 310–323. [Google Scholar] [CrossRef]
- Kraft, P.; Benz, P.M.; Austinat, M.; Brede, M.E.; Schuh, K.; Walter, U.; Stoll, G.; Kleinschnitz, C. Deficiency of vasodilator-stimulated phosphoprotein (VASP) increases blood-brain-barrier damage and edema formation after ischemic stroke in mice. PLoS ONE 2010, 5, e15106. [Google Scholar] [CrossRef]
- Furman, C.; Sieminski, A.L.; Kwiatkowski, A.V.; Rubinson, D.A.; Vasile, E.; Bronson, R.T.; Fassler, R.; Gertler, F.B. Ena/VASP is required for endothelial barrier function in vivo. J. Cell Biol. 2007, 179, 761–775. [Google Scholar] [CrossRef]
- Kay, B.K.; Williamson, M.P.; Sudol, M. The importance of being proline: The interaction of proline-rich motifs in signaling proteins with their cognate domains. FASEB J. 2000, 14, 231–241. [Google Scholar] [CrossRef]
- Peterson, F.C.; Volkman, B.F. Diversity of polyproline recognition by EVH1 domains. Front. Biosci. 2009, 14, 833–846. [Google Scholar] [CrossRef]
- Ball, L.J.; Kuhne, R.; Schneider-Mergener, J.; Oschkinat, H. Recognition of proline-rich motifs by protein-protein-interaction domains. Angew. Chem. Int. Ed. 2005, 44, 2852–2869. [Google Scholar] [CrossRef] [PubMed]
- Li, S.S. Specificity and versatility of SH3 and other proline-recognition domains: Structural basis and implications for cellular signal transduction. Biochem. J. 2005, 390, 641–653. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.J.; Jarchau, T.; Oschkinat, H.; Walter, U. EVH1 domains: Structure, function and interactions. FEBS Lett. 2002, 513, 45–52. [Google Scholar] [CrossRef] [PubMed]
- Ball, L.J.; Kuhne, R.; Hoffmann, B.; Hafner, A.; Schmieder, P.; Volkmer-Engert, R.; Hof, M.; Wahl, M.; Schneider-Mergener, J.; Walter, U.; et al. Dual epitope recognition by the VASP EVH1 domain modulates polyproline ligand specificity and binding affinity. EMBO J. 2000, 19, 4903–4914. [Google Scholar] [CrossRef]
- Prehoda, K.E.; Lee, D.J.; Lim, W.A. Structure of the enabled/VASP homology 1 domain-peptide complex: A key component in the spatial control of actin assembly. Cell 1999, 97, 471–480. [Google Scholar] [CrossRef]
- Renfranz, P.J.; Beckerle, M.C. Doing (F/L)PPPPs: EVH1 domains and their proline-rich partners in cell polarity and migration. Curr. Opin. Cell Biol. 2002, 14, 88–103. [Google Scholar] [CrossRef]
- Niebuhr, K.; Ebel, F.; Frank, R.; Reinhard, M.; Domann, E.; Carl, U.D.; Walter, U.; Gertler, F.B.; Wehland, J.; Chakraborty, T. A novel proline-rich motif present in ActA of Listeria monocytogenes and cytoskeletal proteins is the ligand for the EVH1 domain, a protein module present in the Ena/VASP family. EMBO J. 1997, 16, 5433–5444. [Google Scholar] [CrossRef]
- Chakraborty, T.; Ebel, F.; Domann, E.; Niebuhr, K.; Gerstel, B.; Pistor, S.; Temm-Grove, C.J.; Jockusch, B.M.; Reinhard, M.; Walter, U.; et al. A focal adhesion factor directly linking intracellularly motile Listeria monocytogenes and Listeria ivanovii to the actin-based cytoskeleton of mammalian cells. EMBO J. 1995, 14, 1314–1321. [Google Scholar] [CrossRef]
- Cossart, P.; Bierne, H. The use of host cell machinery in the pathogenesis of Listeria monocytogenes. Curr. Opin. Immunol. 2001, 13, 96–103. [Google Scholar] [CrossRef]
- Hoffman, L.M.; Jensen, C.C.; Kloeker, S.; Wang, C.L.; Yoshigi, M.; Beckerle, M.C. Genetic ablation of zyxin causes Mena/VASP mislocalization, increased motility, and deficits in actin remodeling. J. Cell Biol. 2006, 172, 771–782. [Google Scholar] [CrossRef]
- Petit, M.M.; Fradelizi, J.; Golsteyn, R.M.; Ayoubi, T.A.; Menichi, B.; Louvard, D.; Van de Ven, W.J.; Friederich, E. LPP, an actin cytoskeleton protein related to zyxin, harbors a nuclear export signal and transcriptional activation capacity. Mol. Biol. Cell 2000, 11, 117–129. [Google Scholar] [CrossRef]
- Brindle, N.P.; Holt, M.R.; Davies, J.E.; Price, C.J.; Critchley, D.R. The focal-adhesion vasodilator-stimulated phosphoprotein (VASP) binds to the proline-rich domain in vinculin. Biochem. J. 1996, 318 Pt 3, 753–757. [Google Scholar] [CrossRef]
- Drees, B.; Friederich, E.; Fradelizi, J.; Louvard, D.; Beckerle, M.C.; Golsteyn, R.M. Characterization of the interaction between zyxin and members of the Ena/vasodilator-stimulated phosphoprotein family of proteins. J. Biol. Chem. 2000, 275, 22503–22511. [Google Scholar] [CrossRef]
- Krause, M.; Leslie, J.D.; Stewart, M.; Lafuente, E.M.; Valderrama, F.; Jagannathan, R.; Strasser, G.A.; Rubinson, D.A.; Liu, H.; Way, M.; et al. Lamellipodin, an Ena/VASP ligand, is implicated in the regulation of lamellipodial dynamics. Dev. Cell 2004, 7, 571–583. [Google Scholar] [CrossRef]
- Lafuente, E.M.; van Puijenbroek, A.A.; Krause, M.; Carman, C.V.; Freeman, G.J.; Berezovskaya, A.; Constantine, E.; Springer, T.A.; Gertler, F.B.; Boussiotis, V.A. RIAM, an Ena/VASP and Profilin ligand, interacts with Rap1-GTP and mediates Rap1-induced adhesion. Dev. Cell 2004, 7, 585–595. [Google Scholar] [CrossRef]
- Moeller, M.J.; Soofi, A.; Braun, G.S.; Li, X.; Watzl, C.; Kriz, W.; Holzman, L.B. Protocadherin FAT1 binds Ena/VASP proteins and is necessary for actin dynamics and cell polarization. EMBO J. 2004, 23, 3769–3779. [Google Scholar] [CrossRef]
- Castellano, F.; Le Clainche, C.; Patin, D.; Carlier, M.F.; Chavrier, P. A WASp-VASP complex regulates actin polymerization at the plasma membrane. EMBO J. 2001, 20, 5603–5614. [Google Scholar] [CrossRef]
- Parast, M.M.; Otey, C.A. Characterization of palladin, a novel protein localized to stress fibers and cell adhesions. J. Cell Biol. 2000, 150, 643–656. [Google Scholar] [CrossRef]
- Bashaw, G.J.; Kidd, T.; Murray, D.; Pawson, T.; Goodman, C.S. Repulsive axon guidance: Abelson and Enabled play opposing roles downstream of the roundabout receptor. Cell 2000, 101, 703–715. [Google Scholar] [CrossRef]
- Klostermann, A.; Lutz, B.; Gertler, F.; Behl, C. The orthologous human and murine semaphorin 6A-1 proteins (SEMA6A-1/Sema6A-1) bind to the enabled/vasodilator-stimulated phosphoprotein-like protein (EVL) via a novel carboxyl-terminal zyxin-like domain. J. Biol. Chem. 2000, 275, 39647–39653. [Google Scholar] [CrossRef]
- van der Ven, P.F.; Ehler, E.; Vakeel, P.; Eulitz, S.; Schenk, J.A.; Milting, H.; Micheel, B.; Furst, D.O. Unusual splicing events result in distinct Xin isoforms that associate differentially with filamin c and Mena/VASP. Exp. Cell Res. 2006, 312, 2154–2167. [Google Scholar] [CrossRef] [PubMed]
- Krause, M.; Sechi, A.S.; Konradt, M.; Monner, D.; Gertler, F.B.; Wehland, J. Fyn-binding protein (Fyb)/SLP-76-associated protein (SLAP), Ena/vasodilator-stimulated phosphoprotein (VASP) proteins and the Arp2/3 complex link T cell receptor (TCR) signaling to the actin cytoskeleton. J. Cell Biol. 2000, 149, 181–194. [Google Scholar] [CrossRef] [PubMed]
- Laban, H.; Froemel, T.; Fleming, I.; Benz, P.M. Impaired AMPK activity contributes to the inflammatory phenotype and the reduced phagocytosis capacity of VASP-deficient macrophages. bioRxiv 2023. [Google Scholar] [CrossRef]
- Chen, X.J.; Squarr, A.J.; Stephan, R.; Chen, B.; Higgins, T.E.; Barry, D.J.; Martin, M.C.; Rosen, M.K.; Bogdan, S.; Way, M. Ena/VASP proteins cooperate with the WAVE complex to regulate the actin cytoskeleton. Dev. Cell 2014, 30, 569–584. [Google Scholar] [CrossRef] [PubMed]
- Boeda, B.; Briggs, D.C.; Higgins, T.; Garvalov, B.K.; Fadden, A.J.; McDonald, N.Q.; Way, M. Tes, a specific Mena interacting partner, breaks the rules for EVH1 binding. Mol. Cell 2007, 28, 1071–1082. [Google Scholar] [CrossRef]
- Applewhite, D.A.; Barzik, M.; Kojima, S.; Svitkina, T.M.; Gertler, F.B.; Borisy, G.G. Ena/VASP proteins have an anti-capping independent function in filopodia formation. Mol. Biol. Cell 2007, 18, 2579–2591. [Google Scholar] [CrossRef]
- Bear, J.E.; Loureiro, J.J.; Libova, I.; Fassler, R.; Wehland, J.; Gertler, F.B. Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 2000, 101, 717–728. [Google Scholar] [CrossRef]
- Drees, F.; Gertler, F.B. Ena/VASP: Proteins at the tip of the nervous system. Curr. Opin. Neurobiol. 2008, 18, 53–59. [Google Scholar] [CrossRef]
- Barone, M.; Muller, M.; Chiha, S.; Ren, J.; Albat, D.; Soicke, A.; Dohmen, S.; Klein, M.; Bruns, J.; van Dinther, M.; et al. Designed nanomolar small-molecule inhibitors of Ena/VASP EVH1 interaction impair invasion and extravasation of breast cancer cells. Proc. Natl. Acad. Sci. USA 2020, 117, 29684–29690. [Google Scholar] [CrossRef]
- Gupton, S.L.; Riquelme, D.; Hughes-Alford, S.K.; Tadros, J.; Rudina, S.S.; Hynes, R.O.; Lauffenburger, D.; Gertler, F.B. Mena binds alpha5 integrin directly and modulates alpha5beta1 function. J. Cell Biol. 2012, 198, 657–676. [Google Scholar] [CrossRef]
- Krause, M.; Dent, E.W.; Bear, J.E.; Loureiro, J.J.; Gertler, F.B. Ena/VASP proteins: Regulators of the actin cytoskeleton and cell migration. Annu. Rev. Cell Dev. Biol. 2003, 19, 541–564. [Google Scholar] [CrossRef]
- Reinhard, M.; Giehl, K.; Abel, K.; Haffner, C.; Jarchau, T.; Hoppe, V.; Jockusch, B.M.; Walter, U. The proline-rich focal adhesion and microfilament protein VASP is a ligand for profilins. EMBO J. 1995, 14, 1583–1589. [Google Scholar] [CrossRef]
- Kang, F.; Laine, R.O.; Bubb, M.R.; Southwick, F.S.; Purich, D.L. Profilin interacts with the Gly-Pro-Pro-Pro-Pro-Pro sequences of vasodilator-stimulated phosphoprotein (VASP): Implications for actin-based Listeria motility. Biochemistry 1997, 36, 8384–8392. [Google Scholar] [CrossRef]
- Ferron, F.; Rebowski, G.; Lee, S.H.; Dominguez, R. Structural basis for the recruitment of profilin-actin complexes during filament elongation by Ena/VASP. EMBO J. 2007, 26, 4597–4606. [Google Scholar] [CrossRef]
- Breitsprecher, D.; Kiesewetter, A.K.; Linkner, J.; Urbanke, C.; Resch, G.P.; Small, J.V.; Faix, J. Clustering of VASP actively drives processive, WH2 domain-mediated actin filament elongation. EMBO J. 2008, 27, 2943–2954. [Google Scholar] [CrossRef]
- Pasic, L.; Kotova, T.; Schafer, D.A. Ena/VASP proteins capture actin filament barbed ends. J. Biol. Chem. 2008, 283, 9814–9819. [Google Scholar] [CrossRef]
- Bruhmann, S.; Ushakov, D.S.; Winterhoff, M.; Dickinson, R.B.; Curth, U.; Faix, J. Distinct VASP tetramers synergize in the processive elongation of individual actin filaments from clustered arrays. Proc. Natl. Acad. Sci. USA 2017, 114, E5815–E5824. [Google Scholar] [CrossRef]
- Skruber, K.; Warp, P.V.; Shklyarov, R.; Thomas, J.D.; Swanson, M.S.; Henty-Ridilla, J.L.; Read, T.A.; Vitriol, E.A. Arp2/3 and Mena/VASP Require Profilin 1 for Actin Network Assembly at the Leading Edge. Curr. Biol. 2020, 30, 2651–2664.e2655. [Google Scholar] [CrossRef]
- Ermekova, K.S.; Zambrano, N.; Linn, H.; Minopoli, G.; Gertler, F.; Russo, T.; Sudol, M. The WW domain of neural protein FE65 interacts with proline-rich motifs in Mena, the mammalian homolog of Drosophila enabled. J. Biol. Chem. 1997, 272, 32869–32877. [Google Scholar] [CrossRef]
- Meiyappan, M.; Birrane, G.; Ladias, J.A.A. Structural basis for polyproline recognition by the FE65 WW domain. J. Mol. Biol. 2007, 372, 970–980. [Google Scholar] [CrossRef][Green Version]
- Musacchio, A. How SH3 domains recognize proline. Adv. Protein Chem. 2002, 61, 211–268. [Google Scholar] [PubMed]
- Musacchio, A.; Noble, M.; Pauptit, R.; Wierenga, R.; Saraste, M. Crystal structure of a Src-homology 3 (SH3) domain. Nature 1992, 359, 851–855. [Google Scholar] [CrossRef] [PubMed]
- Ahern-Djamali, S.M.; Bachmann, C.; Hua, P.; Reddy, S.K.; Kastenmeier, A.S.; Walter, U.; Hoffmann, F.M. Identification of profilin and src homology 3 domains as binding partners for Drosophila enabled. Proc. Natl. Acad. Sci. USA 1999, 96, 4977–4982. [Google Scholar] [CrossRef] [PubMed]
- Howe, A.K.; Hogan, B.P.; Juliano, R.L. Regulation of vasodilator-stimulated phosphoprotein phosphorylation and interaction with Abl by protein kinase A and cell adhesion. J. Biol. Chem. 2002, 277, 38121–38126. [Google Scholar] [CrossRef] [PubMed]
- Keicher, C.; Gambaryan, S.; Schulze, E.; Marcus, K.; Meyer, H.E.; Butt, E. Phosphorylation of mouse LASP-1 on threonine 156 by cAMP- and cGMP-dependent protein kinase. Biochem. Biophys. Res. Commun. 2004, 324, 308–316. [Google Scholar] [CrossRef]
- Rotter, B.; Bournier, O.; Nicolas, G.; Dhermy, D.; Lecomte, M.C. AlphaII-spectrin interacts with Tes and EVL, two actin-binding proteins located at cell contacts. Biochem. J. 2005, 388, 631–638. [Google Scholar] [CrossRef]
- Krugmann, S.; Jordens, I.; Gevaert, K.; Driessens, M.; Vandekerckhove, J.; Hall, A. Cdc42 induces filopodia by promoting the formation of an IRSp53:Mena complex. Curr. Biol. 2001, 11, 1645–1655. [Google Scholar] [CrossRef]
- Tsai, F.C.; Henderson, J.M.; Jarin, Z.; Kremneva, E.; Senju, Y.; Pernier, J.; Mikhajlov, O.; Manzi, J.; Kogan, K.; Le Clainche, C.; et al. Activated I-BAR IRSp53 clustering controls the formation of VASP-actin-based membrane protrusions. Sci. Adv. 2022, 8, eabp8677. [Google Scholar] [CrossRef]
- Salazar, M.A.; Kwiatkowski, A.V.; Pellegrini, L.; Cestra, G.; Butler, M.H.; Rossman, K.L.; Serna, D.M.; Sondek, J.; Gertler, F.B.; De Camilli, P. Tuba, a novel protein containing bin/amphiphysin/Rvs and Dbl homology domains, links dynamin to regulation of the actin cytoskeleton. J. Biol. Chem. 2003, 278, 49031–49043. [Google Scholar] [CrossRef]
- Tani, K.; Sato, S.; Sukezane, T.; Kojima, H.; Hirose, H.; Hanafusa, H.; Shishido, T. Abl interactor 1 promotes tyrosine 296 phosphorylation of mammalian enabled (Mena) by c-Abl kinase. J. Biol. Chem. 2003, 278, 21685–21692. [Google Scholar] [CrossRef]
- Maruoka, M.; Sato, M.; Yuan, Y.; Ichiba, M.; Fujii, R.; Ogawa, T.; Ishida-Kitagawa, N.; Takeya, T.; Watanabe, N. Abl-1-bridged tyrosine phosphorylation of VASP by Abelson kinase impairs association of VASP to focal adhesions and regulates leukaemic cell adhesion. Biochem. J. 2012, 441, 889–899. [Google Scholar] [CrossRef]
- Mayer, B.J. SH3 domains: Complexity in moderation. J. Cell Sci. 2001, 114, 1253–1263. [Google Scholar] [CrossRef]
- Harbeck, B.; Huttelmaier, S.; Schluter, K.; Jockusch, B.M.; Illenberger, S. Phosphorylation of the vasodilator-stimulated phosphoprotein regulates its interaction with actin. J. Biol. Chem. 2000, 275, 30817–30825. [Google Scholar] [CrossRef]
- Bachmann, C.; Fischer, L.; Walter, U.; Reinhard, M. The EVH2 domain of the vasodilator-stimulated phosphoprotein mediates tetramerization, F-actin binding, and actin bundle formation. J. Biol. Chem. 1999, 274, 23549–23557. [Google Scholar] [CrossRef]
- Huttelmaier, S.; Harbeck, B.; Steffens, O.; Messerschmidt, T.; Illenberger, S.; Jockusch, B.M. Characterization of the actin binding properties of the vasodilator-stimulated phosphoprotein VASP. FEBS Lett. 1999, 451, 68–74. [Google Scholar] [CrossRef]
- Walders-Harbeck, B.; Khaitlina, S.Y.; Hinssen, H.; Jockusch, B.M.; Illenberger, S. The vasodilator-stimulated phosphoprotein promotes actin polymerisation through direct binding to monomeric actin. FEBS Lett. 2002, 529, 275–280. [Google Scholar] [CrossRef]
- Dominguez, R. The WH2 Domain and Actin Nucleation: Necessary but Insufficient. Trends Biochem Sci 2016, 41, 478–490. [Google Scholar] [CrossRef]
- Van Troys, M.; Dewitte, D.; Goethals, M.; Carlier, M.F.; Vandekerckhove, J.; Ampe, C. The actin binding site of thymosin beta 4 mapped by mutational analysis. EMBO J. 1996, 15, 201–210. [Google Scholar] [CrossRef]
- Reinhard, M.; Halbrugge, M.; Scheer, U.; Wiegand, C.; Jockusch, B.M.; Walter, U. The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J. 1992, 11, 2063–2070. [Google Scholar] [CrossRef]
- Laurent, V.; Loisel, T.P.; Harbeck, B.; Wehman, A.; Grobe, L.; Jockusch, B.M.; Wehland, J.; Gertler, F.B.; Carlier, M.F. Role of proteins of the Ena/VASP family in actin-based motility of Listeria monocytogenes. J. Cell Biol. 1999, 144, 1245–1258. [Google Scholar] [CrossRef]
- Trichet, L.; Sykes, C.; Plastino, J. Relaxing the actin cytoskeleton for adhesion and movement with Ena/VASP. J. Cell Biol. 2008, 181, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Doppler, H.; Storz, P. Regulation of VASP by phosphorylation: Consequences for cell migration. Cell Adhes. Migr. 2013, 7, 492–496. [Google Scholar] [CrossRef] [PubMed]
- Blume, C.; Benz, P.M.; Walter, U.; Ha, J.; Kemp, B.E.; Renne, T. AMP-activated protein kinase impairs endothelial actin cytoskeleton assembly by phosphorylating vasodilator-stimulated phosphoprotein. J. Biol. Chem. 2007, 282, 4601–4612. [Google Scholar] [CrossRef] [PubMed]
- Munzel, T.; Feil, R.; Mulsch, A.; Lohmann, S.M.; Hofmann, F.; Walter, U. Physiology and pathophysiology of vascular signaling controlled by guanosine 3′,5′-cyclic monophosphate-dependent protein kinase [corrected]. Circulation 2003, 108, 2172–2183. [Google Scholar] [CrossRef]
- Bonello, L.; Camoin-Jau, L.; Arques, S.; Boyer, C.; Panagides, D.; Wittenberg, O.; Simeoni, M.C.; Barragan, P.; Dignat-George, F.; Paganelli, F. Adjusted clopidogrel loading doses according to vasodilator-stimulated phosphoprotein phosphorylation index decrease rate of major adverse cardiovascular events in patients with clopidogrel resistance: A multicenter randomized prospective study. J. Am. Coll. Cardiol. 2008, 51, 1404–1411. [Google Scholar] [CrossRef]
- Benz, P.M.; Fleming, I. Can erythrocytes release biologically active NO? Cell Commun. Signal. 2016, 14, 22. [Google Scholar] [CrossRef]
- Rukoyatkina, N.; Butt, E.; Subramanian, H.; Nikolaev, V.O.; Mindukshev, I.; Walter, U.; Gambaryan, S.; Benz, P.M. Protein kinase A activation by the anti-cancer drugs ABT-737 and thymoquinone is caspase-3-dependent and correlates with platelet inhibition and apoptosis. Cell Death Dis. 2017, 8, e2898. [Google Scholar] [CrossRef]
- Walter, U.; Gambaryan, S. cGMP and cGMP-dependent protein kinase in platelets and blood cells. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2009; pp. 533–548. [Google Scholar] [CrossRef]
- Butt, E.; Abel, K.; Krieger, M.; Palm, D.; Hoppe, V.; Hoppe, J.; Walter, U. cAMP- and cGMP-dependent protein kinase phosphorylation sites of the focal adhesion vasodilator-stimulated phosphoprotein (VASP) in vitro and in intact human platelets. J. Biol. Chem. 1994, 269, 14509–14517. [Google Scholar] [CrossRef]
- Zhuang, S.; Nguyen, G.T.; Chen, Y.; Gudi, T.; Eigenthaler, M.; Jarchau, T.; Walter, U.; Boss, G.R.; Pilz, R.B. Vasodilator-stimulated phosphoprotein activation of serum-response element-dependent transcription occurs downstream of RhoA and is inhibited by cGMP-dependent protein kinase phosphorylation. J. Biol. Chem. 2004, 279, 10397–10407. [Google Scholar] [CrossRef]
- Doppler, H.R.; Bastea, L.I.; Lewis-Tuffin, L.J.; Anastasiadis, P.Z.; Storz, P. Protein kinase D1-mediated phosphorylations regulate vasodilator-stimulated phosphoprotein (VASP) localization and cell migration. J. Biol. Chem. 2013, 288, 24382–24393. [Google Scholar] [CrossRef]
- Doppler, H.; Bastea, L.; Borges, S.; Geiger, X.; Storz, P. The phosphorylation status of VASP at serine 322 can be predictive for aggressiveness of invasive ductal carcinoma. Oncotarget 2015, 6, 29740–29752. [Google Scholar] [CrossRef]
- Wentworth, J.K.; Pula, G.; Poole, A.W. Vasodilator-stimulated phosphoprotein (VASP) is phosphorylated on Ser157 by protein kinase C-dependent and -independent mechanisms in thrombin-stimulated human platelets. Biochem. J. 2006, 393, 555–564. [Google Scholar] [CrossRef]
- Chitaley, K.; Chen, L.; Galler, A.; Walter, U.; Daum, G.; Clowes, A.W. Vasodilator-stimulated phosphoprotein is a substrate for protein kinase C. FEBS Lett. 2004, 556, 211–215. [Google Scholar] [CrossRef]
- Thomson, D.M.; Ascione, M.P.; Grange, J.; Nelson, C.; Hansen, M.D. Phosphorylation of VASP by AMPK alters actin binding and occurs at a novel site. Biochem. Biophys. Res. Commun. 2011, 414, 215–219. [Google Scholar] [CrossRef]
- Lara, R.; Mauri, F.A.; Taylor, H.; Derua, R.; Shia, A.; Gray, C.; Nicols, A.; Shiner, R.J.; Schofield, E.; Bates, P.A.; et al. An siRNA screen identifies RSK1 as a key modulator of lung cancer metastasis. Oncogene 2011, 30, 3513–3521. [Google Scholar] [CrossRef]
- Janssens, K.; De Kimpe, L.; Balsamo, M.; Vandoninck, S.; Vandenheede, J.R.; Gertler, F.; Van Lint, J. Characterization of EVL-I as a protein kinase D substrate. Cell. Signal. 2009, 21, 282–292. [Google Scholar] [CrossRef]
- Ha, C.H.; Jin, Z.G. Protein kinase D1, a new molecular player in VEGF signaling and angiogenesis. Mol. Cells 2009, 28, 1–5. [Google Scholar] [CrossRef]
- Wong, C.; Jin, Z.G. Protein kinase C-dependent protein kinase D activation modulates ERK signal pathway and endothelial cell proliferation by vascular endothelial growth factor. J. Biol. Chem. 2005, 280, 33262–33269. [Google Scholar] [CrossRef]
- Zink, J.; Frye, M.; Fromel, T.; Carlantoni, C.; John, D.; Schreier, D.; Weigert, A.; Laban, H.; Salinas, G.; Stingl, H.; et al. EVL regulates VEGF receptor-2 internalization and signaling in developmental angiogenesis. EMBO Rep. 2021, 22, e48961. [Google Scholar] [CrossRef]
- Rikova, K.; Guo, A.; Zeng, Q.; Possemato, A.; Yu, J.; Haack, H.; Nardone, J.; Lee, K.; Reeves, C.; Li, Y.; et al. Global survey of phosphotyrosine signaling identifies oncogenic kinases in lung cancer. Cell 2007, 131, 1190–1203. [Google Scholar] [CrossRef]
- Jorgensen, C.; Sherman, A.; Chen, G.I.; Pasculescu, A.; Poliakov, A.; Hsiung, M.; Larsen, B.; Wilkinson, D.G.; Linding, R.; Pawson, T. Cell-specific information processing in segregating populations of Eph receptor ephrin-expressing cells. Science 2009, 326, 1502–1509. [Google Scholar] [CrossRef] [PubMed]
- Helou, Y.A.; Nguyen, V.; Beik, S.P.; Salomon, A.R. ERK positive feedback regulates a widespread network of tyrosine phosphorylation sites across canonical T cell signaling and actin cytoskeletal proteins in Jurkat T cells. PLoS ONE 2013, 8, e69641. [Google Scholar] [CrossRef] [PubMed]
- Colicelli, J. ABL tyrosine kinases: Evolution of function, regulation, and specificity. Sci. Signal. 2010, 3, re6. [Google Scholar] [CrossRef] [PubMed]
- Kania, A.; Klein, R. Mechanisms of ephrin-Eph signalling in development, physiology and disease. Nat. Rev. Mol. Cell Biol. 2016, 17, 240–256. [Google Scholar] [CrossRef] [PubMed]
- Evans, I.R.; Renne, T.; Gertler, F.B.; Nobes, C.D. Ena/VASP proteins mediate repulsion from ephrin ligands. J. Cell Sci. 2007, 120, 289–298. [Google Scholar] [CrossRef]
- Zink, J.; Froemel, T.; Wittig, I.; Fleming, I.; Benz, P.M. Ena/VASP proteins mediate endothelial cell repulsion from ephrin ligands. bioRxiv 2023. [Google Scholar] [CrossRef]
- Coppolino, M.G.; Krause, M.; Hagendorff, P.; Monner, D.A.; Trimble, W.; Grinstein, S.; Wehland, J.; Sechi, A.S. Evidence for a molecular complex consisting of Fyb/SLAP, SLP-76, Nck, VASP and WASP that links the actin cytoskeleton to Fcgamma receptor signalling during phagocytosis. J. Cell Sci. 2001, 114, 4307–4318. [Google Scholar] [CrossRef]
- Kwiatkowski, A.V.; Rubinson, D.A.; Dent, E.W.; Edward van Veen, J.; Leslie, J.D.; Zhang, J.; Mebane, L.M.; Philippar, U.; Pinheiro, E.M.; Burds, A.A.; et al. Ena/VASP Is Required for neuritogenesis in the developing cortex. Neuron 2007, 56, 441–455. [Google Scholar] [CrossRef]
- Aszodi, A.; Pfeifer, A.; Ahmad, M.; Glauner, M.; Zhou, X.H.; Ny, L.; Andersson, K.E.; Kehrel, B.; Offermanns, S.; Fassler, R. The vasodilator-stimulated phosphoprotein (VASP) is involved in cGMP- and cAMP-mediated inhibition of agonist-induced platelet aggregation, but is dispensable for smooth muscle function. EMBO J. 1999, 18, 37–48. [Google Scholar] [CrossRef]
- Hauser, W.; Knobeloch, K.P.; Eigenthaler, M.; Gambaryan, S.; Krenn, V.; Geiger, J.; Glazova, M.; Rohde, E.; Horak, I.; Walter, U.; et al. Megakaryocyte hyperplasia and enhanced agonist-induced platelet activation in vasodilator-stimulated phosphoprotein knockout mice. Proc. Natl. Acad. Sci. USA 1999, 96, 8120–8125. [Google Scholar] [CrossRef]
- Lanier, L.M.; Gates, M.A.; Witke, W.; Menzies, A.S.; Wehman, A.M.; Macklis, J.D.; Kwiatkowski, D.; Soriano, P.; Gertler, F.B. Mena is required for neurulation and commissure formation. Neuron 1999, 22, 313–325. [Google Scholar] [CrossRef]
- Massberg, S.; Gruner, S.; Konrad, I.; Garcia Arguinzonis, M.I.; Eigenthaler, M.; Hemler, K.; Kersting, J.; Schulz, C.; Muller, I.; Besta, F.; et al. Enhanced in vivo platelet adhesion in vasodilator-stimulated phosphoprotein (VASP)-deficient mice. Blood 2004, 103, 136–142. [Google Scholar] [CrossRef]
- Menzies, A.S.; Aszodi, A.; Williams, S.E.; Pfeifer, A.; Wehman, A.M.; Goh, K.L.; Mason, C.A.; Fassler, R.; Gertler, F.B. Mena and vasodilator-stimulated phosphoprotein are required for multiple actin-dependent processes that shape the vertebrate nervous system. J. Neurosci. 2004, 24, 8029–8038. [Google Scholar] [CrossRef]
- Ulrich, L.; Gliem, C.; Groneberg, D.; Froemel, T.; Schuh, K.; Fleming, I.; Benz, P.M. Mena and VASP are required for vascular smooth muscle relaxation. bioRxiv 2023. [Google Scholar] [CrossRef]
- Gambaryan, S.; Hauser, W.; Kobsar, A.; Glazova, M.; Walter, U. Distribution, cellular localization, and postnatal development of VASP and Mena expression in mouse tissues. Histochem. Cell Biol. 2001, 116, 535–543. [Google Scholar] [CrossRef]
- Ruggeri, Z.M. Platelets in atherothrombosis. Nat. Med. 2002, 8, 1227–1234. [Google Scholar] [CrossRef]
- Bertoni, A.; Tadokoro, S.; Eto, K.; Pampori, N.; Parise, L.V.; White, G.C.; Shattil, S.J. Relationships between Rap1b, affinity modulation of integrin alpha IIbbeta 3, and the actin cytoskeleton. J. Biol. Chem. 2002, 277, 25715–25721. [Google Scholar] [CrossRef]
- Chrzanowska-Wodnicka, M.; Smyth, S.S.; Schoenwaelder, S.M.; Fischer, T.H.; White, G.C., 2nd. Rap1b is required for normal platelet function and hemostasis in mice. J. Clin. Invest. 2005, 115, 680–687. [Google Scholar] [CrossRef]
- Gutierrez-Herrero, S.; Fernandez-Infante, C.; Hernandez-Cano, L.; Ortiz-Rivero, S.; Guijas, C.; Martin-Granado, V.; Gonzalez-Porras, J.R.; Balsinde, J.; Porras, A.; Guerrero, C. C3G contributes to platelet activation and aggregation by regulating major signaling pathways. Signal Transduct. Target. Ther. 2020, 5, 29. [Google Scholar] [CrossRef]
- Oda, A.; Ochs, H.D.; Lasky, L.A.; Spencer, S.; Ozaki, K.; Fujihara, M.; Handa, M.; Ikebuchi, K.; Ikeda, H. CrkL is an adapter for Wiskott-Aldrich syndrome protein and Syk. Blood 2001, 97, 2633–2639. [Google Scholar] [CrossRef]
- Kohler, D.; Bibli, S.I.; Klammer, L.P.; Roth, J.M.; Lehmann, R.; Fleming, I.; Granja, T.F.; Straub, A.; Benz, P.M.; Rosenberger, P. Phosphorylation of vasodilator-stimulated phosphoprotein contributes to myocardial ischemic preconditioning. Basic Res. Cardiol. 2018, 113, 11. [Google Scholar] [CrossRef] [PubMed]
- Kohler, D.; Straub, A.; Weissmuller, T.; Faigle, M.; Bender, S.; Lehmann, R.; Wendel, H.P.; Kurz, J.; Walter, U.; Zacharowski, K.; et al. Phosphorylation of vasodilator-stimulated phosphoprotein prevents platelet-neutrophil complex formation and dampens myocardial ischemia-reperfusion injury. Circulation 2011, 123, 2579–2590. [Google Scholar] [CrossRef] [PubMed]
- Claesson-Welsh, L.; Dejana, E.; McDonald, D.M. Permeability of the Endothelial Barrier: Identifying and Reconciling Controversies. Trends Mol. Med. 2021, 27, 314–331. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Ponce, A.; Citalan-Madrid, A.F.; Velazquez-Avila, M.; Vargas-Robles, H.; Schnoor, M. The role of actin-binding proteins in the control of endothelial barrier integrity. Thromb. Haemost. 2015, 113, 20–36. [Google Scholar] [CrossRef] [PubMed]
- Rosenberger, P.; Khoury, J.; Kong, T.; Weissmuller, T.; Robinson, A.M.; Colgan, S.P. Identification of vasodilator-stimulated phosphoprotein (VASP) as an HIF-regulated tissue permeability factor during hypoxia. FASEB J. 2007, 21, 2613–2621. [Google Scholar] [CrossRef][Green Version]
- Comerford, K.M.; Lawrence, D.W.; Synnestvedt, K.; Levi, B.P.; Colgan, S.P. Role of vasodilator-stimulated phosphoprotein in PKA-induced changes in endothelial junctional permeability. FASEB J. 2002, 16, 583–585. [Google Scholar] [CrossRef]
- Rentsendorj, O.; Mirzapoiazova, T.; Adyshev, D.; Servinsky, L.E.; Renne, T.; Verin, A.D.; Pearse, D.B. Role of vasodilator-stimulated phosphoprotein in cGMP-mediated protection of human pulmonary artery endothelial barrier function. Am. J. Physiol. Lung Cell Mol. Physiol. 2008, 294, L686–L697. [Google Scholar] [CrossRef]
- Profirovic, J.; Han, J.; Andreeva, A.V.; Neamu, R.F.; Pavlovic, S.; Vogel, S.M.; Walter, U.; Voyno-Yasenetskaya, T.A. Vasodilator-stimulated phosphoprotein deficiency potentiates PAR-1-induced increase in endothelial permeability in mouse lungs. J. Cell Physiol. 2011, 226, 1255–1264. [Google Scholar] [CrossRef]
- Mascarenhas, J.B.; Song, J.H.; Gaber, A.A.; Jacobson, J.R.; Cress, A.E.; Camp, S.M.; Dudek, S.M.; Garcia, J.G.N. An Actin-, Cortactin- and Ena-VASP-Linked Complex Contributes to Endothelial Cell Focal Adhesion and Vascular Barrier Regulation. Cell Physiol. Biochem. 2022, 56, 329–339. [Google Scholar] [CrossRef]
- Mascarenhas, J.B.; Gaber, A.A.; Larrinaga, T.M.; Mayfield, R.; Novak, S.; Camp, S.M.; Gregorio, C.; Jacobson, J.R.; Cress, A.E.; Dudek, S.M.; et al. EVL is a novel focal adhesion protein involved in the regulation of cytoskeletal dynamics and vascular permeability. Pulm. Circ. 2021, 11, 20458940211049002. [Google Scholar] [CrossRef]
- Zamorano, P.; Marin, N.; Cordova, F.; Aguilar, A.; Meininger, C.; Boric, M.P.; Golenhofen, N.; Contreras, J.E.; Sarmiento, J.; Duran, W.N.; et al. S-nitrosylation of VASP at cysteine 64 mediates the inflammation-stimulated increase in microvascular permeability. Am. J. Physiol. Heart Circ. Physiol. 2017, 313, H66–H71. [Google Scholar] [CrossRef]
- Tang, M.; Tian, Y.; Li, D.; Lv, J.; Li, Q.; Kuang, C.; Hu, P.; Wang, Y.; Wang, J.; Su, K.; et al. TNF-alpha mediated increase of HIF-1alpha inhibits VASP expression, which reduces alveolar-capillary barrier function during acute lung injury (ALI). PLoS ONE 2014, 9, e102967. [Google Scholar] [CrossRef]
- Schlegel, N.; Burger, S.; Golenhofen, N.; Walter, U.; Drenckhahn, D.; Waschke, J. The role of VASP in regulation of cAMP- and Rac 1-mediated endothelial barrier stabilization. Am. J. Physiol. Cell Physiol. 2008, 294, C178–C188. [Google Scholar] [CrossRef]
- Schlegel, N.; Waschke, J. VASP is involved in cAMP-mediated Rac 1 activation in microvascular endothelial cells. Am. J. Physiol. Cell Physiol. 2009, 296, C453–C462. [Google Scholar] [CrossRef]
- Schlegel, N.; Waschke, J. Impaired cAMP and Rac 1 signaling contribute to TNF-alpha-induced endothelial barrier breakdown in microvascular endothelium. Microcirculation 2009, 16, 521–533. [Google Scholar] [CrossRef]
- Schlegel, N.; Waschke, J. Impaired integrin-mediated adhesion contributes to reduced barrier properties in VASP-deficient microvascular endothelium. J. Cell Physiol. 2009, 220, 357–366. [Google Scholar] [CrossRef]
- Waschke, J.; Curry, F.E.; Adamson, R.H.; Drenckhahn, D. Regulation of actin dynamics is critical for endothelial barrier functions. Am. J Physiol. Heart Circ. Physiol. 2005, 288, H1296–H1305. [Google Scholar] [CrossRef]
- Henes, J.; Schmit, M.A.; Morote-Garcia, J.C.; Mirakaj, V.; Kohler, D.; Glover, L.; Eldh, T.; Walter, U.; Karhausen, J.; Colgan, S.P.; et al. Inflammation-associated repression of vasodilator-stimulated phosphoprotein (VASP) reduces alveolar-capillary barrier function during acute lung injury. FASEB J. 2009, 23, 4244–4255. [Google Scholar] [CrossRef]
- Schmit, M.A.; Mirakaj, V.; Stangassinger, M.; Konig, K.; Kohler, D.; Rosenberger, P. Vasodilator phosphostimulated protein (VASP) protects endothelial barrier function during hypoxia. Inflammation 2012, 35, 566–573. [Google Scholar] [CrossRef][Green Version]
- Davis, B.; Tang, J.; Zhang, L.; Mu, D.; Jiang, X.; Biran, V.; Vexler, Z.; Ferriero, D.M. Role of vasodilator stimulated phosphoprotein in VEGF induced blood-brain barrier permeability in endothelial cell monolayers. Int. J. Dev. Neurosci. 2010, 28, 423–428. [Google Scholar] [CrossRef]
- Benz, P.M.; Feller, S.M.; Sickmann, A.; Walter, U.; Renne, T. Prostaglandin-induced VASP phosphorylation controls alpha II-spectrin breakdown in apoptotic cells. Int. Immunopharmacol. 2008, 8, 319–324. [Google Scholar] [CrossRef] [PubMed]
- Schlegel, N.; Waschke, J. Vasodilator-stimulated phosphoprotein: Crucial for activation of Rac1 in endothelial barrier maintenance. Cardiovasc. Res. 2010, 87, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Limbourg, A.; Korff, T.; Napp, L.C.; Schaper, W.; Drexler, H.; Limbourg, F.P. Evaluation of postnatal arteriogenesis and angiogenesis in a mouse model of hind-limb ischemia. Nat. Protoc. 2009, 4, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Cochain, C.; Channon, K.M.; Silvestre, J.S. Angiogenesis in the infarcted myocardium. Antioxid. Redox Signal. 2013, 18, 1100–1113. [Google Scholar] [CrossRef]
- Markkanen, J.E.; Rissanen, T.T.; Kivela, A.; Yla-Herttuala, S. Growth factor-induced therapeutic angiogenesis and arteriogenesis in the heart--gene therapy. Cardiovasc. Res. 2005, 65, 656–664. [Google Scholar] [CrossRef]
- Shireman, P.K. The chemokine system in arteriogenesis and hind limb ischemia. J. Vasc. Surg. 2007, 45, A48–A56. [Google Scholar] [CrossRef]
- Cannata, A.; Ali, H.; Sinagra, G.; Giacca, M. Gene Therapy for the Heart Lessons Learned and Future Perspectives. Circ. Res. 2020, 126, 1394–1414. [Google Scholar] [CrossRef]
- Heil, M.; Ziegelhoeffer, T.; Wagner, S.; Fernandez, B.; Helisch, A.; Martin, S.; Tribulova, S.; Kuziel, W.A.; Bachmann, G.; Schaper, W. Collateral artery growth (arteriogenesis) after experimental arterial occlusion is impaired in mice lacking CC-chemokine receptor-2. Circ. Res. 2004, 94, 671–677. [Google Scholar] [CrossRef]
- Griffith, J.W.; Sokol, C.L.; Luster, A.D. Chemokines and chemokine receptors: Positioning cells for host defense and immunity. Annu. Rev. Immunol. 2014, 32, 659–702. [Google Scholar] [CrossRef]
- Sag, D.; Carling, D.; Stout, R.D.; Suttles, J. Adenosine 5′-monophosphate-activated protein kinase promotes macrophage polarization to an anti-inflammatory functional phenotype. J. Immunol. 2008, 181, 8633–8641. [Google Scholar] [CrossRef]
- O’Neill, L.A.; Hardie, D.G. Metabolism of inflammation limited by AMPK and pseudo-starvation. Nature 2013, 493, 346–355. [Google Scholar] [CrossRef]
- Meares, G.P.; Qin, H.; Liu, Y.; Holdbrooks, A.T.; Benveniste, E.N. AMP-activated protein kinase restricts IFN-gamma signaling. J. Immunol. 2013, 190, 372–380. [Google Scholar] [CrossRef]
- Mounier, R.; Theret, M.; Arnold, L.; Cuvellier, S.; Bultot, L.; Goransson, O.; Sanz, N.; Ferry, A.; Sakamoto, K.; Foretz, M.; et al. AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 2013, 18, 251–264. [Google Scholar] [CrossRef]
- Tateya, S.; Rizzo-De Leon, N.; Handa, P.; Cheng, A.M.; Morgan-Stevenson, V.; Ogimoto, K.; Kanter, J.E.; Bornfeldt, K.E.; Daum, G.; Clowes, A.W.; et al. VASP increases hepatic fatty acid oxidation by activating AMPK in mice. Diabetes 2013, 62, 1913–1922. [Google Scholar] [CrossRef]
- Bae, H.B.; Zmijewski, J.W.; Deshane, J.S.; Tadie, J.M.; Chaplin, D.D.; Takashima, S.; Abraham, E. AMP-activated protein kinase enhances the phagocytic ability of macrophages and neutrophils. FASEB J. 2011, 25, 4358–4368. [Google Scholar] [CrossRef]
- He, Z.; Mao, F.; Lin, Y.; Li, J.; Zhang, X.; Zhang, Y.; Xiang, Z.; Noor, Z.; Yu, Z. Molecular characteristics of AMPK and its role in regulating the phagocytosis of oyster hemocytes. Fish Shellfish. Immunol. 2019, 93, 416–427. [Google Scholar] [CrossRef]
- Liu, J.; Shen, D.; Wei, C.; Wu, W.; Luo, Z.; Hu, L.; Xiao, Z.; Hu, T.; Sun, Q.; Wang, X.; et al. Inhibition of the LRRC8A channel promotes microglia/macrophage phagocytosis and improves outcomes after intracerebral hemorrhagic stroke. Iscience 2022, 25, 105527. [Google Scholar] [CrossRef]
- Korber, S.; Faix, J. VASP boosts protrusive activity of macroendocytic cups and drives phagosome rocketing after internalization. Eur. J. Cell Biol. 2022, 101, 151200. [Google Scholar] [CrossRef]
- Jeon, S.M. Regulation and function of AMPK in physiology and diseases. Exp. Mol. Med. 2016, 48, e245. [Google Scholar] [CrossRef]
- Garcia-Haro, L.; Garcia-Gimeno, M.A.; Neumann, D.; Beullens, M.; Bollen, M.; Sanz, P. The PP1-R6 protein phosphatase holoenzyme is involved in the glucose-induced dephosphorylation and inactivation of AMP-activated protein kinase, a key regulator of insulin secretion, in MIN6 beta cells. FASEB J. 2010, 24, 5080–5091. [Google Scholar] [CrossRef]
- Abel, K.; Mieskes, G.; Walter, U. Dephosphorylation of the focal adhesion protein VASP in vitro and in intact human platelets. FEBS Lett. 1995, 370, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Visweshwaran, S.P.; Nayab, H.; Hoffmann, L.; Gil, M.; Liu, F.; Kuhne, R.; Maritzen, T. Ena/VASP proteins at the crossroads of actin nucleation pathways in dendritic cell migration. Front. Cell Dev. Biol. 2022, 10, 1008898. [Google Scholar] [CrossRef] [PubMed]
- Estin, M.L.; Thompson, S.B.; Traxinger, B.; Fisher, M.H.; Friedman, R.S.; Jacobelli, J. Ena/VASP proteins regulate activated T-cell trafficking by promoting diapedesis during transendothelial migration. Proc. Natl. Acad. Sci. USA 2017, 114, E2901–E2910. [Google Scholar] [CrossRef] [PubMed]
- Waldman, M.M.; Rahkola, J.T.; Sigler, A.L.; Chung, J.W.; Willett, B.A.S.; Kedl, R.M.; Friedman, R.S.; Jacobelli, J. Ena/VASP Protein-Mediated Actin Polymerization Contributes to Naive CD8(+) T Cell Activation and Expansion by Promoting T Cell-APC Interactions In Vivo. Front. Immunol. 2022, 13, 856977. [Google Scholar] [CrossRef]
- Wilton, K.M.; Billadeau, D.D. VASP Regulates NK Cell Lytic Granule Convergence. J. Immunol. 2018, 201, 2899–2909. [Google Scholar] [CrossRef]
- Kee, A.J.; Gunning, P.W.; Hardeman, E.C. Diverse roles of the actin cytoskeleton in striated muscle. J. Muscle Res. Cell Motil. 2009, 30, 187–197. [Google Scholar] [CrossRef]
- Bennett, P.M.; Maggs, A.M.; Baines, A.J.; Pinder, J.C. The transitional junction: A new functional subcellular domain at the intercalated disc. Mol. Biol. Cell 2006, 17, 2091–2100. [Google Scholar] [CrossRef]
- Larsson, H.; Lindberg, U. The effect of divalent cations on the interaction between calf spleen profilin and different actins. Biochim. Biophys. Acta 1988, 953, 95–105. [Google Scholar] [CrossRef]
- Al-Hasani, J.; Sens-Albert, C.; Ghosh, S.; Trogisch, F.A.; Nahar, T.; Friede, P.A.P.; Reil, J.C.; Hecker, M. Zyxin protects from hypertension-induced cardiac dysfunction. Cell Mol. Life Sci. 2022, 79, 93. [Google Scholar] [CrossRef]
- Xu, W.; Baribault, H.; Adamson, E.D. Vinculin knockout results in heart and brain defects during embryonic development. Development 1998, 125, 327–337. [Google Scholar] [CrossRef]
- Stankewich, M.C.; Cianci, C.D.; Stabach, P.R.; Ji, L.; Nath, A.; Morrow, J.S. Cell organization, growth, and neural and cardiac development require αII-spectrin. J. Cell Sci. 2011, 124, 3956–3966. [Google Scholar] [CrossRef]
- Sartoretto, J.L.; Jin, B.Y.; Bauer, M.; Gertler, F.B.; Liao, R.; Michel, T. Regulation of VASP phosphorylation in cardiac myocytes: Differential regulation by cyclic nucleotides and modulation of protein expression in diabetic and hypertrophic heart. Am. J. Physiol. Heart Circ. Physiol. 2009, 297, H1697–H1710. [Google Scholar] [CrossRef]
- Aguilar, F.; Belmonte, S.L.; Ram, R.; Noujaim, S.F.; Dunaevsky, O.; Protack, T.L.; Jalife, J.; Todd Massey, H.; Gertler, F.B.; Blaxall, B.C. Mammalian enabled (Mena) is a critical regulator of cardiac function. Am. J. Physiol. Heart Circ. Physiol. 2011, 300, H1841–H1852. [Google Scholar] [CrossRef]
- Eigenthaler, M.; Engelhardt, S.; Schinke, B.; Kobsar, A.; Schmitteckert, E.; Gambaryan, S.; Engelhardt, C.M.; Krenn, V.; Eliava, M.; Jarchau, T.; et al. Disruption of cardiac Ena-VASP protein localization in intercalated disks causes dilated cardiomyopathy. Am. J. Physiol. Heart Circ. Physiol. 2003, 285, H2471–H2481. [Google Scholar] [CrossRef]
- Shu, X.; Ruddiman, C.A.; Keller, T.C.S.t.; Keller, A.S.; Yang, Y.; Good, M.E.; Best, A.K.; Columbus, L.; Isakson, B.E. Heterocellular Contact Can Dictate Arterial Function. Circ. Res. 2019, 124, 1473–1481. [Google Scholar] [CrossRef]
- Schmidt, K.; de Wit, C. Endothelium-Derived Hyperpolarizing Factor and Myoendothelial Coupling: The in vivo Perspective. Front. Physiol. 2020, 11, 602930. [Google Scholar] [CrossRef]
- Straub, A.C.; Zeigler, A.C.; Isakson, B.E. The myoendothelial junction: Connections that deliver the message. Physiology 2014, 29, 242–249. [Google Scholar] [CrossRef]
- Schmidt, V.J.; Wolfle, S.E.; Boettcher, M.; de Wit, C. Gap junctions synchronize vascular tone within the microcirculation. Pharmacol. Rep. 2008, 60, 68–74. [Google Scholar]
- Bagher, P.; Segal, S.S. Regulation of blood flow in the microcirculation: Role of conducted vasodilation. Acta Physiol. 2011, 202, 271–284. [Google Scholar] [CrossRef]
- Milkau, M.; Kohler, R.; de Wit, C. Crucial importance of the endothelial K+ channel SK3 and connexin40 in arteriolar dilations during skeletal muscle contraction. FASEB J. 2010, 24, 3572–3579. [Google Scholar] [CrossRef]
- de Wit, C.; Roos, F.; Bolz, S.S.; Kirchhoff, S.; Kruger, O.; Willecke, K.; Pohl, U. Impaired conduction of vasodilation along arterioles in connexin40-deficient mice. Circ. Res. 2000, 86, 649–655. [Google Scholar] [CrossRef] [PubMed]
- Jackson, W.F. Boosting the signal: Endothelial inward rectifier K(+) channels. Microcirculation 2017, 24, e12319. [Google Scholar] [CrossRef] [PubMed]
- Jobs, A.; Schmidt, K.; Schmidt, V.J.; Lubkemeier, I.; van Veen, T.A.; Kurtz, A.; Willecke, K.; de Wit, C. Defective Cx40 maintains Cx37 expression but intact Cx40 is crucial for conducted dilations irrespective of hypertension. Hypertension 2012, 60, 1422–1429. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.R.; Graceffa, P.; Ferron, F.; Gallant, C.; Boczkowska, M.; Dominguez, R.; Morgan, K.G. Actin polymerization in differentiated vascular smooth muscle cells requires vasodilator-stimulated phosphoprotein. Am. J. Physiol. Cell Physiol. 2010, 298, C559–C571. [Google Scholar] [CrossRef]
- Yamin, R.; Morgan, K.G. Deciphering actin cytoskeletal function in the contractile vascular smooth muscle cell. J. Physiol. 2012, 590, 4145–4154. [Google Scholar] [CrossRef]
- Tang, D.D. Critical role of actin-associated proteins in smooth muscle contraction, cell proliferation, airway hyperresponsiveness and airway remodeling. Respir. Res. 2015, 16, 134. [Google Scholar] [CrossRef]
- Gunst, S.J.; Zhang, W. Actin cytoskeletal dynamics in smooth muscle: A new paradigm for the regulation of smooth muscle contraction. Am. J. Physiol. Cell Physiol. 2008, 295, C576–C587. [Google Scholar] [CrossRef]
- Markert, T.; Krenn, V.; Leebmann, J.; Walter, U. High expression of the focal adhesion- and microfilament-associated protein VASP in vascular smooth muscle and endothelial cells of the intact human vessel wall. Basic Res. Cardiol. 1996, 91, 337–343. [Google Scholar] [CrossRef]
- Wu, Y.; Gunst, S.J. Vasodilator-stimulated phosphoprotein (VASP) regulates actin polymerization and contraction in airway smooth muscle by a vinculin-dependent mechanism. J. Biol. Chem. 2015, 290, 11403–11416. [Google Scholar] [CrossRef]
- Defawe, O.D.; Kim, S.; Chen, L.; Huang, D.; Kenagy, R.D.; Renne, T.; Walter, U.; Daum, G.; Clowes, A.W. VASP phosphorylation at serine239 regulates the effects of NO on smooth muscle cell invasion and contraction of collagen. J. Cell Physiol. 2010, 222, 230–237. [Google Scholar] [CrossRef]
- Calejo, A.I.; Tasken, K. Targeting protein-protein interactions in complexes organized by A kinase anchoring proteins. Front. Pharmacol. 2015, 6, 192. [Google Scholar] [CrossRef]
- Benz, P.M.; Ding, Y.; Stingl, H.; Loot, A.E.; Zink, J.; Wittig, I.; Popp, R.; Fleming, I. AKAP12 deficiency impairs VEGF-induced endothelial cell migration and sprouting. Acta Physiol. 2019, 228, e13325. [Google Scholar] [CrossRef]
- Zhang, D.; Ouyang, J.; Wang, N.; Zhang, Y.; Bie, J. Promotion of PDGF-induced endothelial cell migration by phosphorylated VASP depends on PKA anchoring via AKAP. Mol. Cell Biochem. 2010, 335, 1–11. [Google Scholar] [CrossRef]
- Choi, Y.K.; Kim, J.H.; Kim, W.J.; Lee, H.Y.; Park, J.A.; Lee, S.W.; Yoon, D.K.; Kim, H.H.; Chung, H.; Yu, Y.S.; et al. AKAP12 regulates human blood-retinal barrier formation by downregulation of hypoxia-inducible factor-1alpha. J. Neurosci. 2007, 27, 4472–4481. [Google Scholar] [CrossRef]
- Radeva, M.Y.; Kugelmann, D.; Spindler, V.; Waschke, J. PKA compartmentalization via AKAP220 and AKAP12 contributes to endothelial barrier regulation. PLoS ONE 2014, 9, e106733. [Google Scholar] [CrossRef]
- Carmeliet, P.; De Smet, F.; Loges, S.; Mazzone, M. Branching morphogenesis and antiangiogenesis candidates: Tip cells lead the way. Nat. Rev. Clin. Oncol. 2009, 6, 315–326. [Google Scholar] [CrossRef]
- Simons, M.; Gordon, E.; Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell Biol. 2016, 17, 611–625. [Google Scholar] [CrossRef]
- Sakurai, Y.; Ohgimoto, K.; Kataoka, Y.; Yoshida, N.; Shibuya, M. Essential role of Flk-1 (VEGF receptor 2) tyrosine residue 1173 in vasculogenesis in mice. Proc. Natl. Acad. Sci. USA 2005, 102, 1076–1081. [Google Scholar] [CrossRef]
- Zink, J.; Froemel, T.; Boon, R.A.; Fleming, I.; Benz, P.M. Impaired H19 lncRNA expression contributes to the compromised developmental angiogenesis in EVL-deficient mice. bioRxiv 2023. [Google Scholar] [CrossRef]
- Fischer, R.S.; Lam, P.Y.; Huttenlocher, A.; Waterman, C.M. Filopodia and focal adhesions: An integrated system driving branching morphogenesis in neuronal pathfinding and angiogenesis. Dev. Biol. 2018, 451, 86–95. [Google Scholar] [CrossRef]
- Adams, R.H.; Eichmann, A. Axon guidance molecules in vascular patterning. Cold Spring Harb. Perspect. Biol. 2010, 2, a001875. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Levine, Y.C.; Golan, D.E.; Michel, T.; Lin, A.J. Atrial natriuretic peptide-initiated cGMP pathways regulate vasodilator-stimulated phosphoprotein phosphorylation and angiogenesis in vascular endothelium. J. Biol. Chem. 2008, 283, 4439–4447. [Google Scholar] [CrossRef] [PubMed]
- Hofmann, P.; Sommer, J.; Theodorou, K.; Kirchhof, L.; Fischer, A.; Li, Y.; Perisic, L.; Hedin, U.; Maegdefessel, L.; Dimmeler, S.; et al. Long non-coding RNA H19 regulates endothelial cell aging via inhibition of STAT3 signalling. Cardiovasc. Res. 2019, 115, 230–242. [Google Scholar] [CrossRef] [PubMed]
- Jia, P.; Cai, H.; Liu, X.; Chen, J.; Ma, J.; Wang, P.; Liu, Y.; Zheng, J.; Xue, Y. Long non-coding RNA H19 regulates glioma angiogenesis and the biological behavior of glioma-associated endothelial cells by inhibiting microRNA-29a. Cancer Lett. 2016, 381, 359–369. [Google Scholar] [CrossRef]
- Li, J.; Jiang, H.; Peng, P.; Zhang, Q.; Bai, W.; Yang, Y.; Huo, S.; Zhou, G.; Shao, J. Biliverdin modulates the long non-coding RNA H19/microRNA-181b-5p/endothelial cell specific molecule 1 axis to alleviate cerebral ischemia reperfusion injury. Biomed. Pharmacother. 2022, 153, 113455. [Google Scholar] [CrossRef]
- Rocha, S.F.; Schiller, M.; Jing, D.; Li, H.; Butz, S.; Vestweber, D.; Biljes, D.; Drexler, H.C.; Nieminen-Kelha, M.; Vajkoczy, P.; et al. Esm1 modulates endothelial tip cell behavior and vascular permeability by enhancing VEGF bioavailability. Circ. Res. 2014, 115, 581–590. [Google Scholar] [CrossRef]
- Liu, Z.Z.; Tian, Y.F.; Wu, H.; Ouyang, S.Y.; Kuang, W.L. LncRNA H19 promotes glioma angiogenesis through miR-138/HIF-1alpha/VEGF axis. Neoplasma 2020, 67, 111–118. [Google Scholar] [CrossRef]
- Toyofuku, T.; Zhang, H.; Kumanogoh, A.; Takegahara, N.; Yabuki, M.; Harada, K.; Hori, M.; Kikutani, H. Guidance of myocardial patterning in cardiac development by Sema6D reverse signalling. Nat. Cell Biol. 2004, 6, 1204–1211. [Google Scholar] [CrossRef]
- Wills, Z.; Bateman, J.; Korey, C.A.; Comer, A.; Van Vactor, D. The tyrosine kinase Abl and its substrate enabled collaborate with the receptor phosphatase Dlar to control motor axon guidance. Neuron 1999, 22, 301–312. [Google Scholar] [CrossRef]
- Tu, K.; Li, J.; Verma, V.K.; Liu, C.; Billadeau, D.D.; Lamprecht, G.; Xiang, X.; Guo, L.; Dhanasekaran, R.; Roberts, L.R.; et al. Vasodilator-stimulated phosphoprotein promotes activation of hepatic stellate cells by regulating Rab11-dependent plasma membrane targeting of transforming growth factor beta receptors. Hepatology 2015, 61, 361–374. [Google Scholar] [CrossRef]
- Bennett, L.D.; Fox, J.M.; Signoret, N. Mechanisms regulating chemokine receptor activity. Immunology 2011, 134, 246–256. [Google Scholar] [CrossRef]
- Lettau, M.; Pieper, J.; Janssen, O. Nck adapter proteins: Functional versatility in T cells. Cell Commun. Signal. 2009, 7, 1. [Google Scholar] [CrossRef]
- Dubrac, A.; Genet, G.; Ola, R.; Zhang, F.; Pibouin-Fragner, L.; Han, J.; Zhang, J.; Thomas, J.L.; Chedotal, A.; Schwartz, M.A.; et al. Targeting NCK-Mediated Endothelial Cell Front-Rear Polarity Inhibits Neovascularization. Circulation 2016, 133, 409–421. [Google Scholar] [CrossRef]
- Basagiannis, D.; Zografou, S.; Murphy, C.; Fotsis, T.; Morbidelli, L.; Ziche, M.; Bleck, C.; Mercer, J.; Christoforidis, S. VEGF induces signalling and angiogenesis by directing VEGFR2 internalisation through macropinocytosis. J. Cell Sci. 2016, 129, 4091–4104. [Google Scholar] [CrossRef]
- Miklavc, P.; Frick, M. Actin and Myosin in Non-Neuronal Exocytosis. Cells 2020, 9, 1455. [Google Scholar] [CrossRef]
- Ahern-Djamali, S.M.; Comer, A.R.; Bachmann, C.; Kastenmeier, A.S.; Reddy, S.K.; Beckerle, M.C.; Walter, U.; Hoffmann, F.M. Mutations in Drosophila enabled and rescue by human vasodilator-stimulated phosphoprotein (VASP) indicate important functional roles for Ena/VASP homology domain 1 (EVH1) and EVH2 domains. Mol. Biol. Cell 1998, 9, 2157–2171. [Google Scholar] [CrossRef]
- Young, L.E.; Latario, C.J.; Higgs, H.N. Roles for Ena/VASP proteins in FMNL3-mediated filopodial assembly. J. Cell Sci. 2018, 131, jcs220814. [Google Scholar] [CrossRef]
- Puleo, J.I.; Parker, S.S.; Roman, M.R.; Watson, A.W.; Eliato, K.R.; Peng, L.; Saboda, K.; Roe, D.J.; Ros, R.; Gertler, F.B.; et al. Mechanosensing during directed cell migration requires dynamic actin polymerization at focal adhesions. J. Cell Biol. 2019, 218, 4215–4235. [Google Scholar] [CrossRef]
- Pula, G.; Krause, M. Role of Ena/VASP proteins in homeostasis and disease. In Handbook of Experimental Pharmacology; Springer: Berlin/Heidelberg, Germany, 2008; pp. 39–65. [Google Scholar] [CrossRef]
- Khodadi, E. Platelet Function in Cardiovascular Disease: Activation of Molecules and Activation by Molecules. Cardiovasc. Toxicol. 2020, 20, 1–10. [Google Scholar] [CrossRef]
- Angiolillo, D.J.; Bernardo, E.; Sabate, M.; Jimenez-Quevedo, P.; Costa, M.A.; Palazuelos, J.; Hernandez-Antolin, R.; Moreno, R.; Escaned, J.; Alfonso, F.; et al. Impact of platelet reactivity on cardiovascular outcomes in patients with type 2 diabetes mellitus and coronary artery disease. J. Am. Coll. Cardiol. 2007, 50, 1541–1547. [Google Scholar] [CrossRef]
- Zaccolo, M.; Movsesian, M.A. cAMP and cGMP signaling cross-talk: Role of phosphodiesterases and implications for cardiac pathophysiology. Circ. Res. 2007, 100, 1569–1578. [Google Scholar] [CrossRef] [PubMed]
- Petraina, A.; Nogales, C.; Krahn, T.; Mucke, H.; Luscher, T.F.; Fischmeister, R.; Kass, D.A.; Burnett, J.C.; Hobbs, A.J.; Schmidt, H. Cyclic GMP modulating drugs in cardiovascular diseases: Mechanism-based network pharmacology. Cardiovasc. Res. 2022, 118, 2085–2102. [Google Scholar] [CrossRef] [PubMed]
- Schwarz, U.R.; Geiger, J.; Walter, U.; Eigenthaler, M. Flow cytometry analysis of intracellular VASP phosphorylation for the assessment of activating and inhibitory signal transduction pathways in human platelets--definition and detection of ticlopidine/clopidogrel effects. Thromb. Haemost. 1999, 82, 1145–1152. [Google Scholar] [CrossRef] [PubMed]
- Aleil, B.; Ravanat, C.; Cazenave, J.P.; Rochoux, G.; Heitz, A.; Gachet, C. Flow cytometric analysis of intraplatelet VASP phosphorylation for the detection of clopidogrel resistance in patients with ischemic cardiovascular diseases. J. Thromb. Haemost. 2005, 3, 85–92. [Google Scholar] [CrossRef]
- Yamaguchi, H.; Condeelis, J. Regulation of the actin cytoskeleton in cancer cell migration and invasion. Biochim. Biophys. Acta 2007, 1773, 642–652. [Google Scholar] [CrossRef]
- Olson, M.F.; Sahai, E. The actin cytoskeleton in cancer cell motility. Clin. Exp. Metastasis 2009, 26, 273–287. [Google Scholar] [CrossRef]
- Izdebska, M.; Zielinska, W.; Halas-Wisniewska, M.; Grzanka, A. Involvement of Actin and Actin-Binding Proteins in Carcinogenesis. Cells 2020, 9, 2245. [Google Scholar] [CrossRef]
- Hu, L.D.; Zou, H.F.; Zhan, S.X.; Cao, K.M. EVL (Ena/VASP-like) expression is up-regulated in human breast cancer and its relative expression level is correlated with clinical stages. Oncol. Rep. 2008, 19, 1015–1020. [Google Scholar] [CrossRef]
- Tian, Y.; Xu, L.; He, Y.; Xu, X.; Li, K.; Ma, Y.; Gao, Y.; Wei, D.; Wei, L. Knockdown of RAC1 and VASP gene expression inhibits breast cancer cell migration. Oncol. Lett. 2018, 16, 2151–2160. [Google Scholar] [CrossRef]
- Dertsiz, L.; Ozbilim, G.; Kayisli, Y.; Gokhan, G.A.; Demircan, A.; Kayisli, U.A. Differential expression of VASP in normal lung tissue and lung adenocarcinomas. Thorax 2005, 60, 576–581. [Google Scholar] [CrossRef]
- Roussos, E.T.; Wang, Y.; Wyckoff, J.B.; Sellers, R.S.; Wang, W.; Li, J.; Pollard, J.W.; Gertler, F.B.; Condeelis, J.S. Mena deficiency delays tumor progression and decreases metastasis in polyoma middle-T transgenic mouse mammary tumors. Breast Cancer Res. 2010, 12, R101. [Google Scholar] [CrossRef]
- Na, S.; Cui, H.; Guo, Z.; Liang, X.; Sakran, K.A.; Guo, X.; Li, X.; Xie, L.; Zhu, Y.; Qi, H.; et al. Overexpression of Mena is associated with tumor progression and poor prognosis in oral squamous cell carcinoma via EMT. Front. Oncol. 2022, 12, 1052375. [Google Scholar] [CrossRef]
- Xiang, X.; Wang, Y.; Zhang, H.; Piao, J.; Muthusamy, S.; Wang, L.; Deng, Y.; Zhang, W.; Kuang, R.; Billadeau, D.D.; et al. Vasodilator-stimulated phosphoprotein promotes liver metastasis of gastrointestinal cancer by activating a beta1-integrin-FAK-YAP1/TAZ signaling pathway. NPJ Precis. Oncol. 2018, 2, 2. [Google Scholar] [CrossRef]
- Kim, Y.M.; Renne, C.; Seifert, S.; Schuh, K.; Renne, T. Impaired melanoma growth in VASP deficient mice. FEBS Lett. 2011, 585, 2533–2536. [Google Scholar] [CrossRef]








Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Benz, P.M.; Frömel, T.; Laban, H.; Zink, J.; Ulrich, L.; Groneberg, D.; Boon, R.A.; Poley, P.; Renne, T.; de Wit, C.; et al. Cardiovascular Functions of Ena/VASP Proteins: Past, Present and Beyond. Cells 2023, 12, 1740. https://doi.org/10.3390/cells12131740
Benz PM, Frömel T, Laban H, Zink J, Ulrich L, Groneberg D, Boon RA, Poley P, Renne T, de Wit C, et al. Cardiovascular Functions of Ena/VASP Proteins: Past, Present and Beyond. Cells. 2023; 12(13):1740. https://doi.org/10.3390/cells12131740
Chicago/Turabian StyleBenz, Peter M., Timo Frömel, Hebatullah Laban, Joana Zink, Lea Ulrich, Dieter Groneberg, Reinier A. Boon, Philip Poley, Thomas Renne, Cor de Wit, and et al. 2023. "Cardiovascular Functions of Ena/VASP Proteins: Past, Present and Beyond" Cells 12, no. 13: 1740. https://doi.org/10.3390/cells12131740
APA StyleBenz, P. M., Frömel, T., Laban, H., Zink, J., Ulrich, L., Groneberg, D., Boon, R. A., Poley, P., Renne, T., de Wit, C., & Fleming, I. (2023). Cardiovascular Functions of Ena/VASP Proteins: Past, Present and Beyond. Cells, 12(13), 1740. https://doi.org/10.3390/cells12131740