Targeting Extracellular RNA Mitigates Hepatic Lipotoxicity and Liver Injury in NASH
Abstract
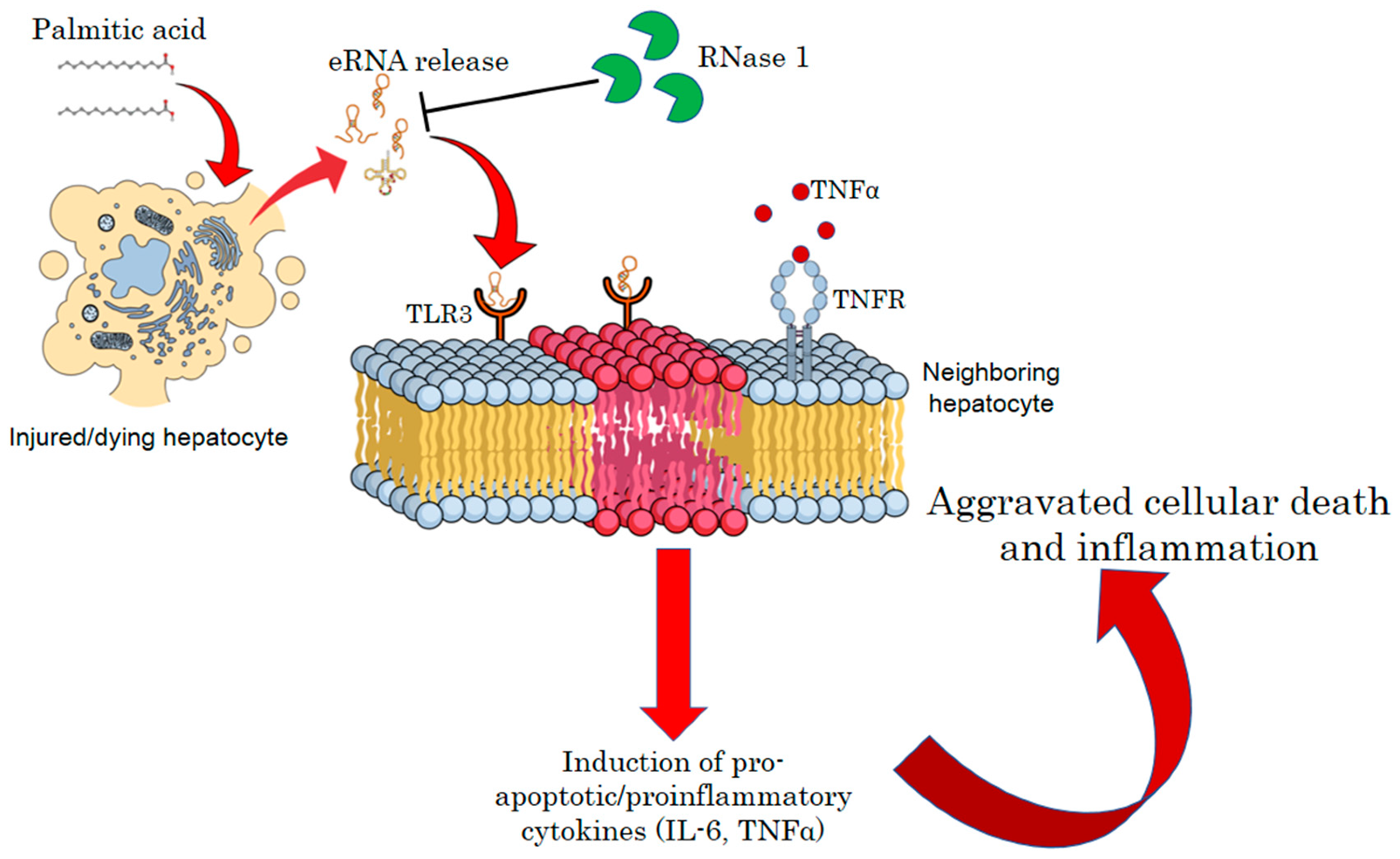
:1. Introduction
2. Materials and Methods
2.1. Cell Culture and Treatment
2.2. MTT Assay
2.3. Analysis of Total eRNA Levels
2.4. Oil Red O Staining
2.5. Animal Experiments
2.6. RNA Isolation and qRT-PCR
2.7. Western Blotting
2.8. H&E Staining
2.9. Serum ALT Test
2.10. Liver TG Analysis
2.11. Confocal Microscopy
2.12. Mitochondrial ROS Detection
2.13. Mitochondrial Membrane Potential (MMP)
2.14. Statistics
3. Results
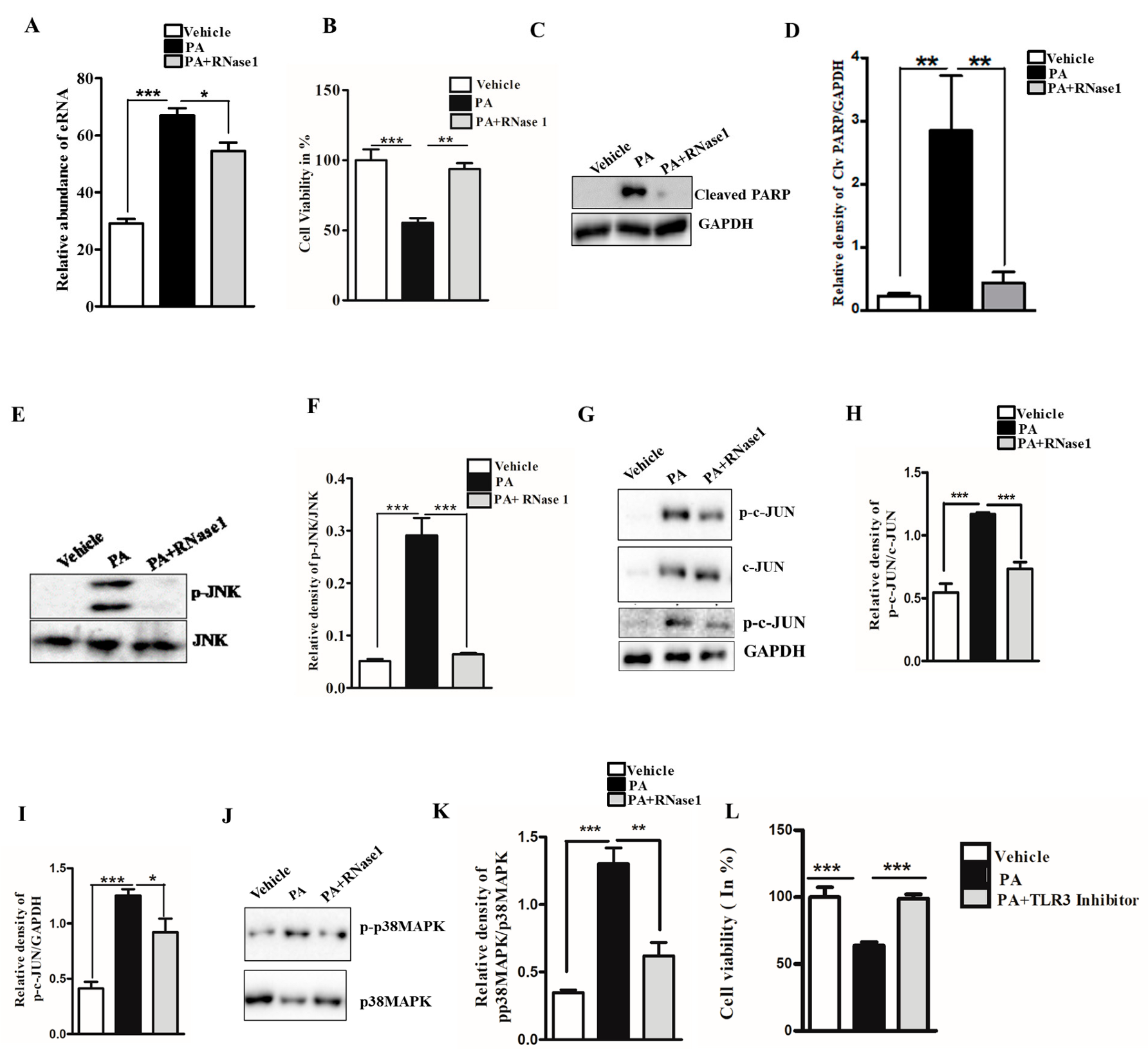
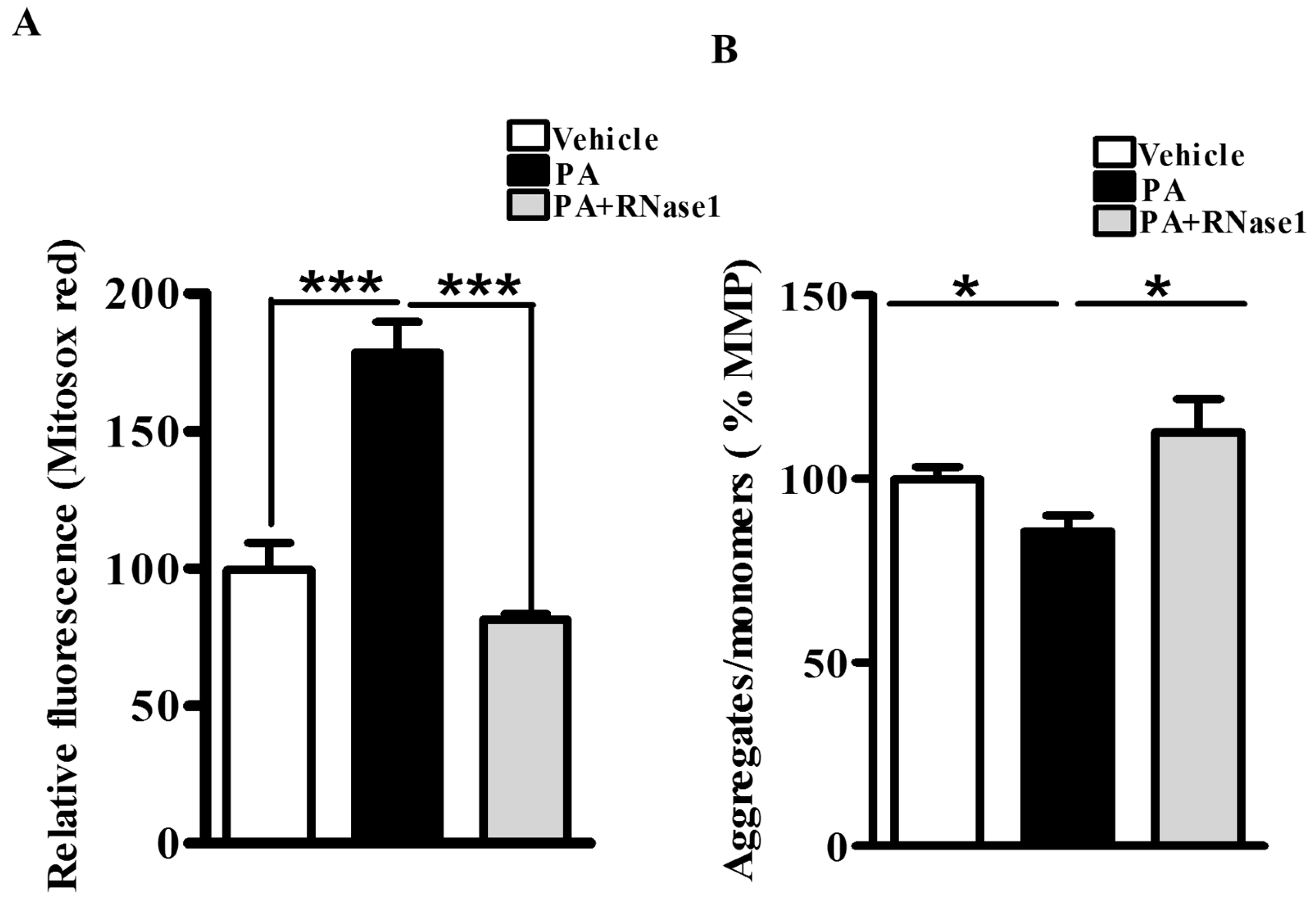
3.1. RNase 1 Attenuates PA-Induced Cellular Injury in HepG2 Cells
3.2. RNase 1 Attenuates PA-Induced Pro-Inflammatory Signaling in HepG2 Cells
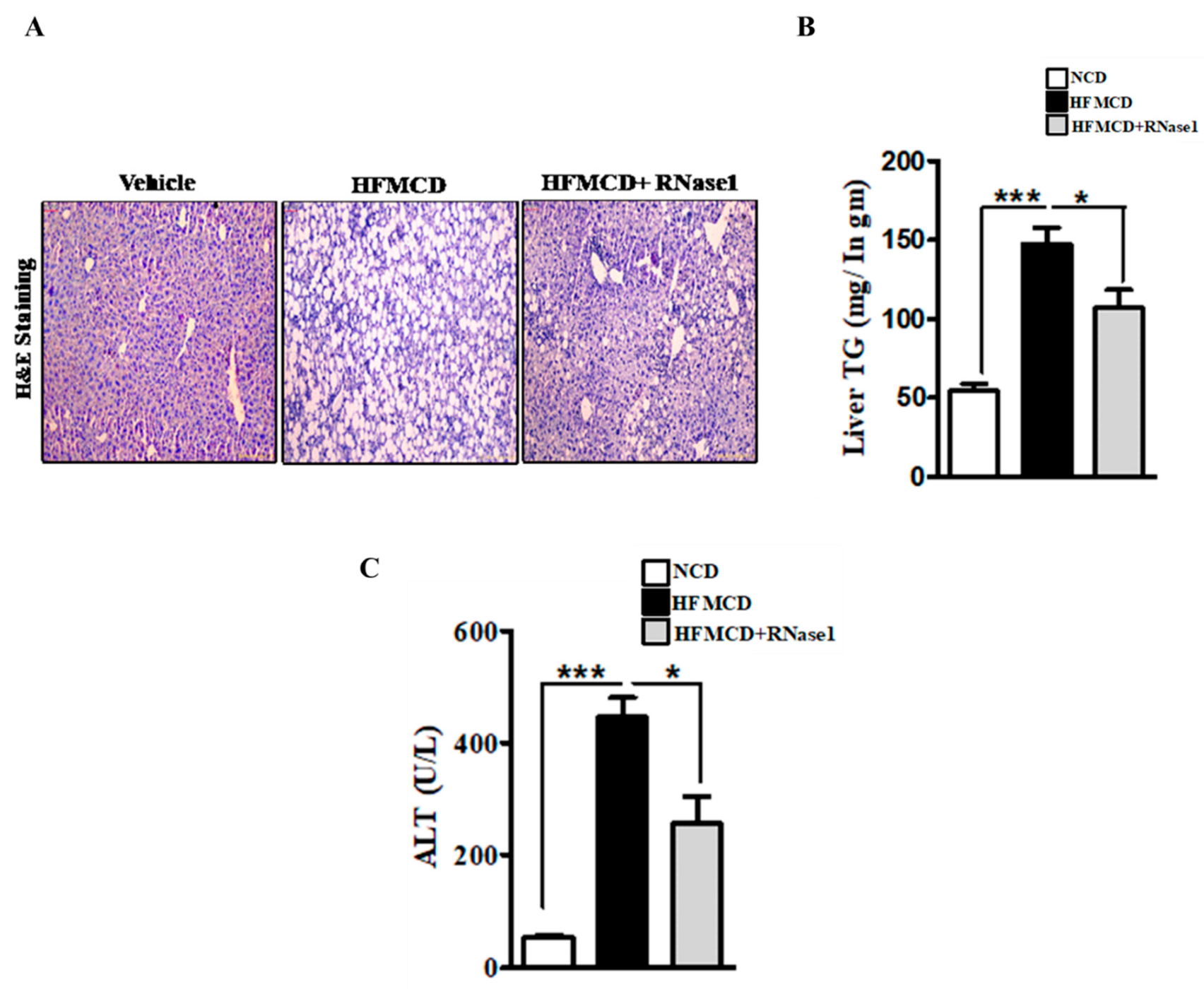
3.3. RNase 1 Administration Mitigates NASH-Induced Liver Injury in Mouse
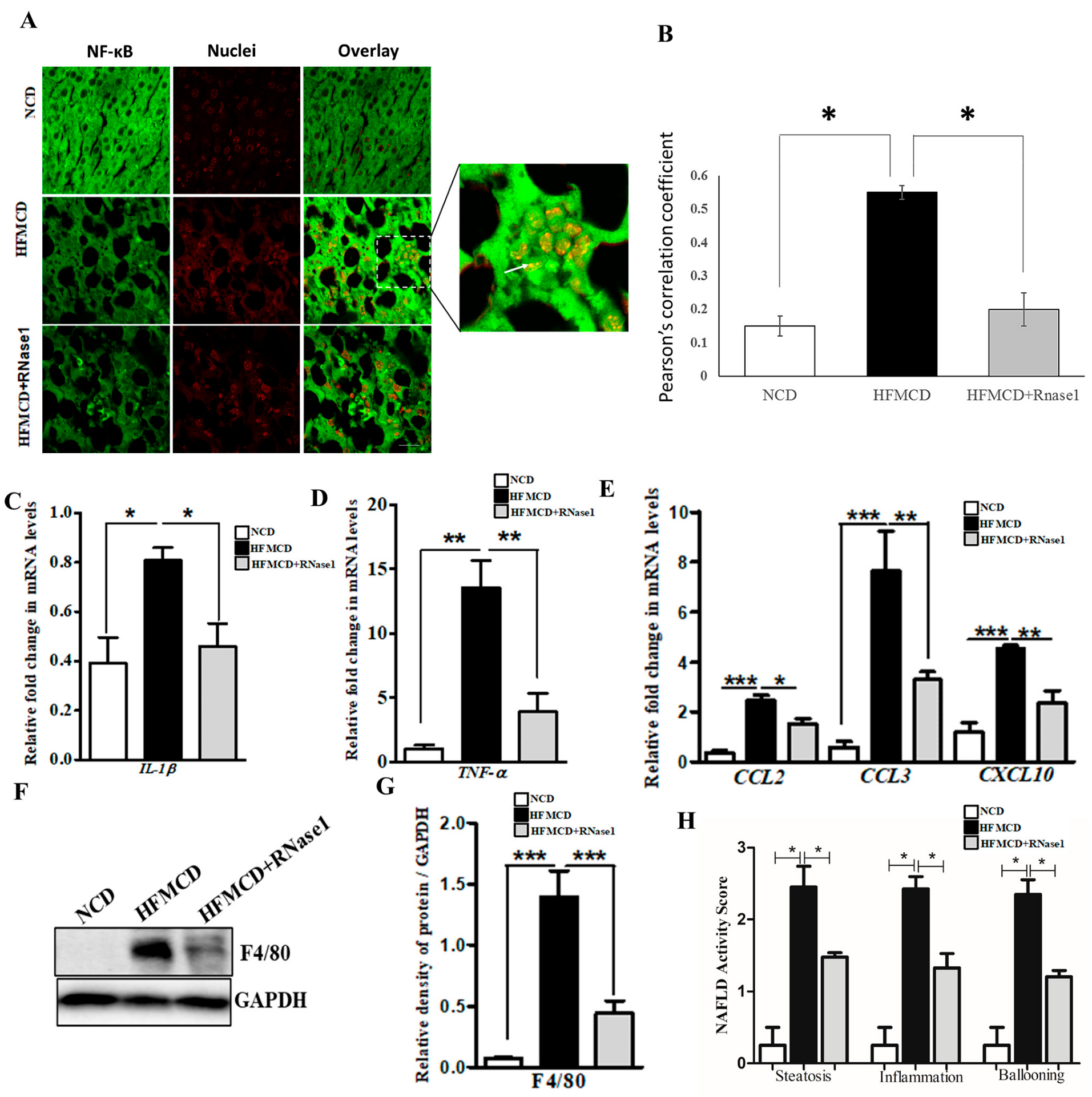
3.4. RNase 1 Administration Reduces NASH-Induced Liver Inflammation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- Younossi, Z.; Tacke, F.; Arrese, M.; Sharma, B.C.; Mostafa, I.; Bugianesi, E.; Wong, V.W.-S.; Yilmaz, Y.; George, J.; Fan, J.; et al. Global Perspectives on Nonalcoholic Fatty Liver Disease and Nonalcoholic Steatohepatitis. Hepatology 2019, 69, 2672–2682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheka, A.C.; Adeyi, O.; Thompson, J.; Hameed, B.; Crawford, P.A.; Ikramuddin, S. Nonalcoholic Steatohepatitis: A Review. JAMA 2020, 323, 1175–1183. [Google Scholar] [CrossRef] [PubMed]
- Raza, S.; Rajak, S.; Upadhyay, A.; Tewari, A.; Sinha, R.A. Current treatment paradigms and emerging therapies for NAFLD/NASH. Front. Biosci. 2021, 26, 206–237. [Google Scholar] [CrossRef] [PubMed]
- Engin, A.B. What Is Lipotoxicity? Adv. Exp. Med. Biol. 2017, 960, 197–220. [Google Scholar]
- Fromenty, B.; Roden, M. Mitochondrial alterations in fatty liver diseases. J. Hepatol. 2023, 78, 415–429. [Google Scholar] [CrossRef]
- Svegliati-Baroni, G.; Pierantonelli, I.; Torquato, P.; Marinelli, R.; Ferreri, C.; Chatgilialoglu, C.; Bartolini, D.; Galli, F. Lipidomic biomarkers and mechanisms of lipotoxicity in non-alcoholic fatty liver disease. Free. Radic. Biol. Med. 2019, 144, 293–309. [Google Scholar] [CrossRef]
- Mota, M.; Banini, B.A.; Cazanave, S.C.; Sanyal, A.J. Molecular mechanisms of lipotoxicity and glucotoxicity in nonalcoholic fatty liver disease. Metabolism 2016, 65, 1049–1061. [Google Scholar] [CrossRef] [Green Version]
- An, P.; Wei, L.-L.; Zhao, S.; Sverdlov, D.Y.; Vaid, K.A.; Miyamoto, M.; Kuramitsu, K.; Lai, M.; Popov, Y.V. Hepatocyte mitochondria-derived danger signals directly activate hepatic stellate cells and drive progression of liver fibrosis. Nat. Commun. 2020, 11, 2362. [Google Scholar] [CrossRef]
- Handa, P.; Vemulakonda, A.; Kowdley, K.V.; Uribe, M.; Méndez-Sánchez, N. Mitochondrial DNA from hepatocytes as a ligand for TLR9: Drivers of nonalcoholic steatohepatitis? World J. Gastroenterol. 2016, 22, 6965–6971. [Google Scholar] [CrossRef]
- Luedde, T.; Kaplowitz, N.; Schwabe, R.F. Cell Death and Cell Death Responses in Liver Disease: Mechanisms and Clinical Relevance. Gastroenterology 2014, 147, 765–783.e4. [Google Scholar] [CrossRef] [Green Version]
- Mihm, S. Danger-Associated Molecular Patterns (DAMPs): Molecular Triggers for Sterile Inflammation in the Liver. Int. J. Mol. Sci. 2018, 19, 3104. [Google Scholar] [CrossRef] [Green Version]
- Shaker, M.E. The contribution of sterile inflammation to the fatty liver disease and the potential therapies. Biomed. Pharmacother. 2022, 148, 112789. [Google Scholar] [CrossRef]
- Wallace, S.J.; Tacke, F.; Schwabe, R.F.; Henderson, N.C. Understanding the cellular interactome of non-alcoholic fatty liver disease. JHEP Rep. 2022, 4, 100524. [Google Scholar] [CrossRef]
- Preissner, K.T.; Fischer, S.; Deindl, E. Extracellular RNA as a Versatile DAMP and Alarm Signal That Influences Leukocyte Recruitment in Inflammation and Infection. Front. Cell Dev. Biol. 2020, 8, 619221. [Google Scholar] [CrossRef] [PubMed]
- Biswas, I.; Singh, B.; Sharma, M.; Agrawala, P.K.; Khan, G.A. Extracellular RNA facilitates hypoxia-induced leukocyte adhesion and infiltration in the lung through TLR3-IFN-γ-STAT1 signaling pathway. Eur. J. Immunol. 2015, 45, 3158–3173. [Google Scholar] [CrossRef] [PubMed]
- Cabrera-Fuentes, H.A.; Lopez, M.L.; McCurdy, S.; Fischer, S.; Meiler, S.; Baumer, Y.; Galuska, S.P.; Preissner, K.T.; Boisvert, W.A. Regulation of monocyte/macrophage polarisation by extracellular RNA. Thromb. Haemost. 2015, 113, 473–481. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.; Nasyrov, E.; Brosien, M.; Preissner, K.T.; Marti, H.H.; Kunze, R. Self-extracellular RNA promotes pro-inflammatory response of astrocytes to exogenous and endogenous danger signals. J. Neuroinflammation 2021, 18, 252. [Google Scholar] [CrossRef]
- Grote, K.; Nicolai, M.; Schubert, U.; Schieffer, B.; Troidl, C.; Preissner, K.T.; Bauer, S.; Fischer, S. Extracellular Ribosomal RNA Acts Synergistically with Toll-like Receptor 2 Agonists to Promote Inflammation. Cells 2022, 11, 1440. [Google Scholar] [CrossRef]
- Gruner, H.N.; McManus, M.T. Examining the evidence for extracellular RNA function in mammals. Nat. Rev. Genet. 2021, 22, 448–458. [Google Scholar] [CrossRef]
- Kluever, A.K.; Deindl, E. Extracellular RNA, a Potential Drug Target for Alleviating Atherosclerosis, Ischemia/Reperfusion Injury and Organ Transplantation. Curr. Pharm. Biotechnol. 2018, 19, 1189–1195. [Google Scholar] [CrossRef]
- Nation, G.K.; Saffold, C.E.; Pua, H.H. Secret messengers: Extracellular RNA communication in the immune system. Immunol. Rev. 2021, 304, 62–76. [Google Scholar] [CrossRef] [PubMed]
- Preissner, K.T.; Fischer, S. Functions and cellular signaling by ribosomal extracellular RNA (rexRNA): Facts and hypotheses on a non-typical DAMP. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2023, 1870, 119408. [Google Scholar] [CrossRef] [PubMed]
- Stieger, P.; Daniel, J.; Thölen, C.; Dutzmann, J.; Knöpp, K.; Gündüz, D.; Aslam, M.; Kampschulte, M.; Langheinrich, A.; Fischer, S.; et al. Targeting of Extracellular RNA Reduces Edema Formation and Infarct Size and Improves Survival After Myocardial Infarction in Mice. J. Am. Hear. Assoc. 2017, 6, e004541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zechendorf, E.; O’Riordan, C.; Stiehler, L.; Wischmeyer, N.; Chiazza, F.; Collotta, D.; Denecke, B.; Ernst, S.; Müller-Newen, G.; Coldewey, S.M.; et al. Ribonuclease 1 attenuates septic cardiomyopathy and cardiac apoptosis in a murine model of polymicrobial sepsis. J. Clin. Investig. 2020, 5, e131571. [Google Scholar] [CrossRef] [Green Version]
- Zhang, X.-Y.; Chen, C.; Bai, Y.-P.; Ma, G.; Zhang, Y.-B.; Liu, B. RNase attenuates acute lung injury induced by ischemia–reperfusion in mice. Int. Immunopharmacol. 2016, 40, 288–293. [Google Scholar] [CrossRef]
- Brunt, E.M.; Janney, C.G.; Di Bisceglie, A.M.; Neuschwander-Tetri, B.A.; Bacon, B.R. Nonalcoholic steatohepatitis: A proposal for grading and staging the histological lesions. Am. J. Gastroenterol. 1999, 94, 2467–2474. [Google Scholar] [CrossRef]
- Ji, J.; Zhang, L.; Wang, P.; Mu, Y.-M.; Zhu, X.-Y.; Wu, Y.-Y.; Yu, H.; Zhang, B.; Chen, S.-M.; Sun, X.-Z. Saturated free fatty acid, palmitic acid, induces apoptosis in fetal hepatocytes in culture. Exp. Toxicol. Pathol. 2005, 56, 369–376. [Google Scholar] [CrossRef]
- Gao, D.; Nong, S.; Huang, X.; Lu, Y.; Zhao, H.; Lin, Y.; Man, Y.; Wang, S.; Yang, J.; Li, J. The Effects of Palmitate on Hepatic Insulin Resistance Are Mediated by NADPH Oxidase 3-derived Reactive Oxygen Species through JNK and p38MAPK Pathways. J. Biol. Chem. 2010, 285, 29965–29973. [Google Scholar] [CrossRef] [Green Version]
- Sinha, R.A. Autophagy: A Cellular Guardian against Hepatic Lipotoxicity. Genes 2023, 14, 553. [Google Scholar] [CrossRef]
- Cheng, K.; Wang, X.; Yin, H. Small-molecule inhibitors of the TLR3/dsRNA complex. J. Am. Chem. Soc. 2011, 133, 3764–3767. [Google Scholar] [CrossRef] [Green Version]
- Ezquerro, S.; Mocha, F.; Frühbeck, G.; Guzmán-Ruiz, R.; Valentí, V.; Mugueta, C.; Becerril, S.; Catalán, V.; Gómez-Ambrosi, J.; Silva, C. Ghrelin Reduces TNF-alpha-Induced Human Hepatocyte Apoptosis, Autophagy, and Pyroptosis: Role in Obesity-Associated NAFLD. J. Clin. Endocrinol. Metab. 2019, 104, 21–37. [Google Scholar]
- Joshi-Barve, S.; Barve, S.S.; Amancherla, K.; Gobejishvili, L.; Hill, D.; Cave, M.; Hote, P.; McClain, C.J. Palmitic acid induces production of proinflammatory cytokine interleukin-8 from hepatocytes. Hepatology 2007, 46, 823–830. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, M.; Hada, N.; Sakamaki, Y.; Uno, A.; Shiga, T.; Tanaka, C.; Ito, T.; Katsume, A.; Sudoh, M. An improved mouse model that rapidly develops fibrosis in non-alcoholic steatohepatitis. Int. J. Exp. Pathol. 2013, 94, 93–103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajak, S.; Gupta, P.; Anjum, B.; Raza, S.; Tewari, A.; Ghosh, S.; Tripathi, M.; Singh, B.K.; Sinha, R.A. Role of AKR1B10 and AKR1B8 in the pathogenesis of non-alcoholic steatohepatitis (NASH) in mouse. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2022, 1868, 166319. [Google Scholar] [CrossRef] [PubMed]
- Rajak, S.; Tewari, A.; Raza, S.; Gupta, P.; Chakravarti, B.; Anjum, B.; Tripathi, M.; Singh, B.K.; Yen, P.M.; Goel, A.; et al. Pharmacological inhibition of CFTR attenuates nonalcoholic steatohepatitis (NASH) progression in mice. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2023, 1869, 166662. [Google Scholar] [CrossRef]
- Widjaja, A.A.; Singh, B.K.; Adami, E.; Viswanathan, S.; Dong, J.; D’agostino, G.A.; Ng, B.; Lim, W.W.; Tan, J.; Paleja, B.S.; et al. Inhibiting Interleukin 11 Signaling Reduces Hepatocyte Death and Liver Fibrosis, Inflammation, and Steatosis in Mouse Models of Nonalcoholic Steatohepatitis. Gastroenterology 2019, 157, 777–792.e14. [Google Scholar] [CrossRef] [Green Version]
- Vuppalanchi, R.; Noureddin, M.; Alkhouri, N.; Sanyal, A.J. Therapeutic pipeline in nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 373–392. [Google Scholar] [CrossRef]
- Pierantonelli, I.; Svegliati-Baroni, G. Nonalcoholic Fatty Liver Disease: Basic Pathogenetic Mechanisms in the Progression From NAFLD to NASH. Transplantation 2019, 103, e1–e13. [Google Scholar] [CrossRef]
- Rada, P.; González-Rodríguez, Á.; García-Monzón, C.; Valverde, Á.M. Understanding lipotoxicity in NAFLD pathogenesis: Is CD36 a key driver? Cell Death Dis. 2020, 11, 802. [Google Scholar] [CrossRef]
- Arguello, G.; Balboa, E.; Arrese, M.; Zanlungo, S. Recent insights on the role of cholesterol in non-alcoholic fatty liver disease. Biochim. Biophys. Acta (BBA)-Mol. Basis Dis. 2015, 1852, 1765–1778. [Google Scholar] [CrossRef] [Green Version]
- Seki, E.; Schwabe, R.F. Hepatic inflammation and fibrosis: Functional links and key pathways. Hepatology 2015, 61, 1066–1079. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.; Jeon, O.H.; Jeon, Y.-J. Extracellular RNA: Emerging roles in cancer cell communication and biomarkers. Cancer Lett. 2020, 495, 33–40. [Google Scholar] [CrossRef]
- Hooten, N.N. Extracellular vesicles and extracellular RNA in aging and age-related disease. Transl. Med. Aging 2020, 4, 96–98. [Google Scholar] [CrossRef]
- Hunter, R.W.; Dhaun, N. Extracellular RNA in kidney disease: Moving slowly but surely from bench to bedside. Clin. Sci. 2020, 134, 2893–2895. [Google Scholar] [CrossRef]
- Giraldez, M.D.; Spengler, R.M.; Etheridge, A.; Goicochea, A.J.; Tuck, M.; Choi, S.W.; Galas, D.J.; Tewari, M. Phospho-RNA-seq: A modified small RNA-seq method that reveals circulating mRNA and lncRNA fragments as potential biomarkers in human plasma. EMBO J. 2019, 38, e101695. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Li, J.; Tillman, B.; Morgan, T.R.; French, B.A.; French, S.W. TLR3/4 signaling is mediated via the NFkappaB-CXCR4/7 pathway in human alcoholic hepatitis and non-alcoholic steatohepatitis which formed Mallory-Denk bodies. Exp. Mol. Pathol. 2014, 97, 234–240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jordaan, S.; Akinrinmade, O.A.; Nachreiner, T.; Cremer, C.; Naran, K.; Chetty, S.; Barth, S. Updates in the Development of ImmunoRNases for the Selective Killing of Tumor Cells. Biomedicines 2018, 6, 28. [Google Scholar] [CrossRef] [PubMed] [Green Version]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tewari, A.; Rajak, S.; Raza, S.; Gupta, P.; Chakravarti, B.; Srivastava, J.; Chaturvedi, C.P.; Sinha, R.A. Targeting Extracellular RNA Mitigates Hepatic Lipotoxicity and Liver Injury in NASH. Cells 2023, 12, 1845. https://doi.org/10.3390/cells12141845
Tewari A, Rajak S, Raza S, Gupta P, Chakravarti B, Srivastava J, Chaturvedi CP, Sinha RA. Targeting Extracellular RNA Mitigates Hepatic Lipotoxicity and Liver Injury in NASH. Cells. 2023; 12(14):1845. https://doi.org/10.3390/cells12141845
Chicago/Turabian StyleTewari, Archana, Sangam Rajak, Sana Raza, Pratima Gupta, Bandana Chakravarti, Jyotika Srivastava, Chandra P. Chaturvedi, and Rohit A. Sinha. 2023. "Targeting Extracellular RNA Mitigates Hepatic Lipotoxicity and Liver Injury in NASH" Cells 12, no. 14: 1845. https://doi.org/10.3390/cells12141845
APA StyleTewari, A., Rajak, S., Raza, S., Gupta, P., Chakravarti, B., Srivastava, J., Chaturvedi, C. P., & Sinha, R. A. (2023). Targeting Extracellular RNA Mitigates Hepatic Lipotoxicity and Liver Injury in NASH. Cells, 12(14), 1845. https://doi.org/10.3390/cells12141845






