Ectodomain Shedding by ADAM17 Increases the Release of Soluble CD40 from Human Endothelial Cells under Pro-Inflammatory Conditions
Abstract
1. Introduction
2. Material and Methods
2.1. Cell Culture and Treatments
2.2. DNA Extraction and Genotyping
2.3. Human Samples
2.4. Quantitative PCR
2.5. Western Blot, ELISA, and FACS
2.6. Statistical Analysis
3. Results
3.1. ADAM17-Mediated Ectodomain Shedding of CD40 and VCAM
3.2. Differential Inflammatory Activation of the ADAM17 Regulators iRhom1 and 2
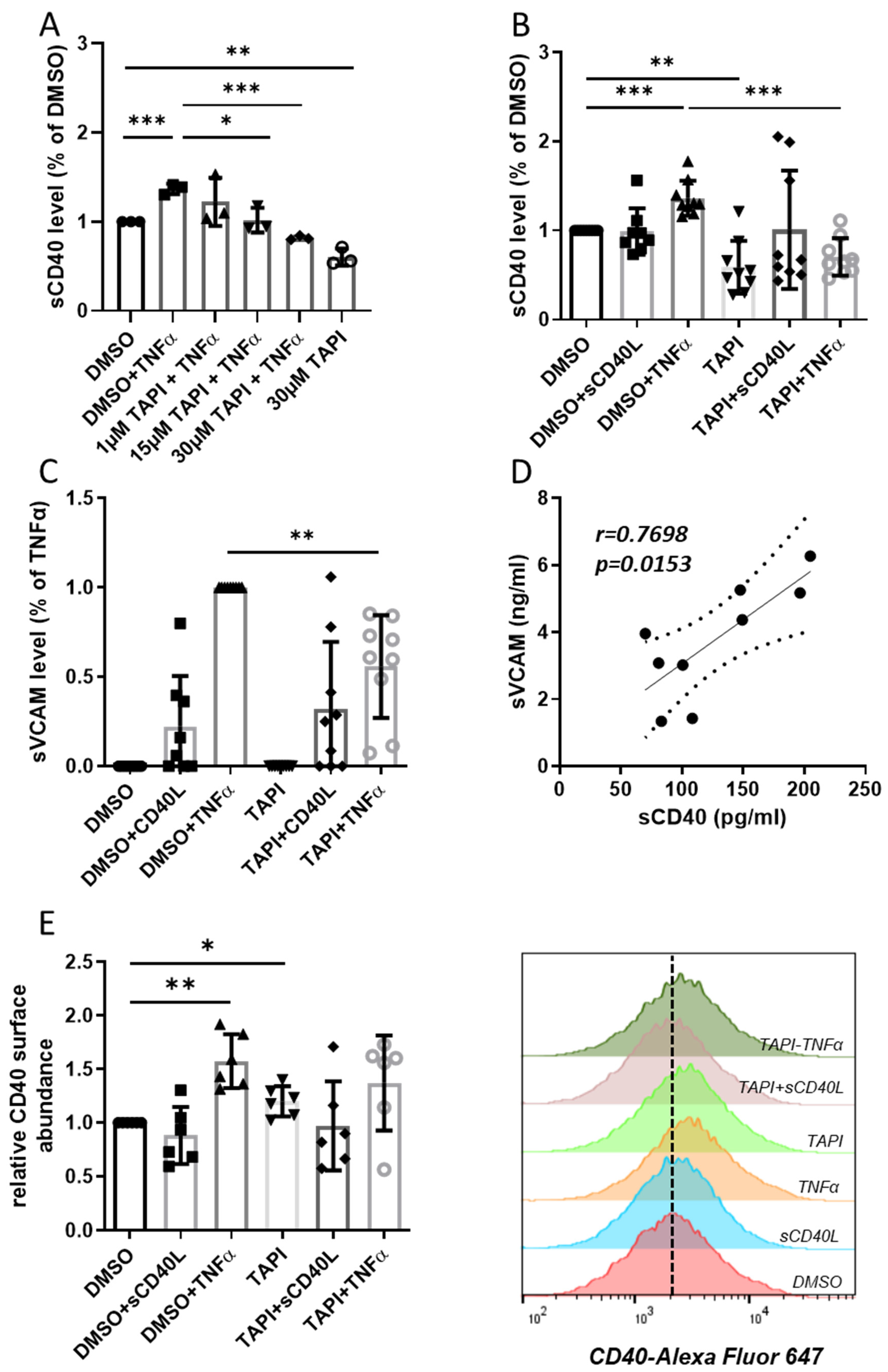
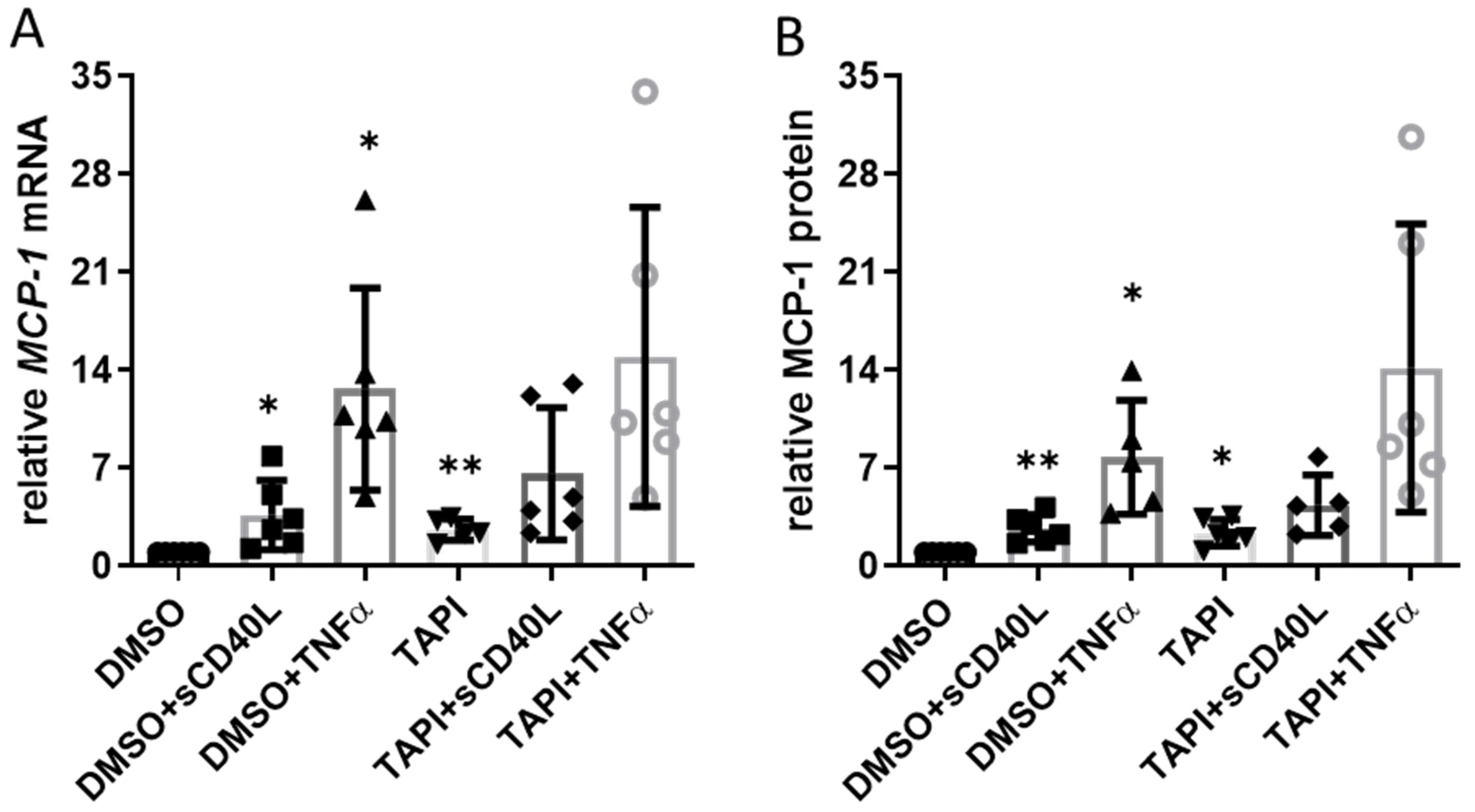
3.3. Impact of TAPI-0-Mediated ADAM17 Inhibition on CD40 Signaling
3.4. Correlation of sCD40 Plasma Levels with Other Biomarkers for CHD
4. Discussion
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strohm, L.; Ubbens, H.; Münzel, T.; Daiber, A.; Daub, S. Role of CD40(L)-TRAF signaling in inflammation and resolution—A double-edged sword. Front. Pharmacol. 2022, 13, 995061. [Google Scholar] [CrossRef] [PubMed]
- Libby, P.; Ridker, P.M.; Maseri, A. Inflammation and atherosclerosis. Circulation 2002, 105, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Elgueta, R.; Benson, M.J.; De Vries, V.C.; Wasiuk, A.; Guo, Y.; Noelle, R.J. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 2009, 229, 152–172. [Google Scholar] [CrossRef]
- Hassan, G.S.; Yacoub, D.; Alaaeddine, N.; Nadiri, A.; Merhi, Y.; Mourad, W. CD154: The atherosclerotic risk factor in rheumatoid arthritis? Arthritis Res. Ther. 2013, 15, 1–9. [Google Scholar] [CrossRef]
- Tomer, Y.; Concepcion, E.; Greenberg, D.A.; Kurylowicz, A.; Kula, D.; Ploski, R.; Skorka, A.; Jurecka-Lubieniecka, B.; Zebracka, J.; Steinhof-Radwanska, K.; et al. A C/T Single-Nucleotide Polymorphism in the Region of the CD40 Gene is Associated with Graves’ Disease. Thyroid 2002, 12, 1129–1135. [Google Scholar] [CrossRef]
- Sultan, C.S.; Weitnauer, M.; Turinsky, M.; Kessler, T.; Brune, M.; A Gleissner, C.; Leuschner, F.; Wagner, A.H.; Hecker, M. Functional association of a CD40 gene single-nucleotide polymorphism with the pathogenesis of coronary heart disease. Cardiovasc. Res. 2020, 116, 1214–1225. [Google Scholar] [CrossRef] [PubMed]
- Calligaris, M.; Cuffaro, D.; Bonelli, S.; Spanò, D.P.; Rossello, A.; Nuti, E.; Scilabra, S.D. Strategies to Target ADAM17 in Disease: From Its Discovery to the iRhom Revolution. Molecules 2021, 26, 944. [Google Scholar] [CrossRef]
- Zunke, F.; Rose-John, S. The shedding protease ADAM17: Physiology and pathophysiology. Biochim. Biophys. Acta 2017, 1864, 2059–2070. [Google Scholar] [CrossRef]
- Düsterhöft, S.; Babendreyer, A.; Giese, A.A.; Flasshove, C.; Ludwig, A. Status update on iRhom and ADAM17: It’s still complicated. Biochim. Biophys. Acta Mol. Cell Res. 2019, 1866, 1567–1583. [Google Scholar] [CrossRef]
- Wagner, A.H.; Güldenzoph, B.; Lienenlüke, B.; Hecker, M. CD154/CD40-mediated expression of CD154 in endothelial cells: Consequences for endothelial cell-monocyte interaction. Arter. Thromb. Vasc. Biol. 2004, 24, 715–720. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Contin, C.; Pitard, V.; Itai, T.; Nagata, S.; Moreau, J.-F.; Déchanet-Merville, J. Membrane-anchored CD40 Is Processed by the Tumor Necrosis Factor-α-converting Enzyme: Implications for CD40 signaling. J. Biol. Chem. 2003, 278, 32801–32809. [Google Scholar] [CrossRef]
- Chen, J.; Chen, L.; Wang, G.; Tang, H. Cholesterol-Dependent and -Independent CD40 Internalization and Signaling Activation in Cardiovascular Endothelial Cells. Arter. Thromb. Vasc. Biol. 2007, 27, 2005–2013. [Google Scholar] [CrossRef] [PubMed]
- Garton, K.J.; Gough, P.J.; Philalay, J.; Wille, P.T.; Blobel, C.P.; Whitehead, R.H.; Dempsey, P.J.; Raines, E.W. Stimulated Shedding of Vascular Cell Adhesion Molecule 1 (VCAM-1) Is Mediated by Tumor Necrosis Factor-α-converting Enzyme (ADAM 17). J. Biol. Chem. 2003, 278, 37459–37464. [Google Scholar] [CrossRef] [PubMed]
- Tone, M.; Tone, Y.; Fairchild, P.J.; Wykes, M.; Waldmann, H. Regulation of CD40 function by its isoforms generated through alternative splicing. Proc. Natl. Acad. Sci. USA 2001, 98, 1751–1756. [Google Scholar] [CrossRef]
- Eshel, D.; Toporik, A.; Efrati, T.; Nakav, S.; Chen, A.; Douvdevani, A. Characterization of natural human antagonistic soluble CD40 isoforms produced through alternative splicing. Mol. Immunol. 2008, 46, 250–257. [Google Scholar] [CrossRef]
- Nicolaou, A.; Zhao, Z.; Northoff, B.H.; Sass, K.; Herbst, A.; Kohlmaier, A.; Chalaris, A.; Wolfrum, C.; Weber, C.; Steffens, S.; et al. Adam17 Deficiency Promotes Atherosclerosis by Enhanced TNFR2 Signaling in Mice. Arter. Thromb. Vasc. Biol. 2017, 37, 247–257. [Google Scholar] [CrossRef]
- Tsakadze, N.L.; Sithu, S.D.; Sen, U.; English, W.R.; Murphy, G.; D’Souza, S.E. Tumor Necrosis Factor-α-converting Enzyme (TACE/ADAM-17) Mediates the Ectodomain Cleavage of Intercellular Adhesion Molecule-1 (ICAM-1). J. Biol. Chem. 2006, 281, 3157–3164. [Google Scholar] [CrossRef]
- Koenen, R.R.; Pruessmeyer, J.; Soehnlein, O.; Fraemohs, L.; Zernecke, A.; Schwarz, N.; Reiss, K.; Sarabi, A.; Lindbom, L.; Hackeng, T.M.; et al. Regulated release and functional modulation of junctional adhesion molecule A by disintegrin metalloproteinases. Blood 2009, 113, 4799–4809. [Google Scholar] [CrossRef] [PubMed]
- Henn, V.; Slupsky, J.R.; Gräfe, M.; Anagnostopoulos, I.; Förster, R.; Müller-Berghaus, G.; Kroczek, R.A. CD40 ligand on activated platelets triggers an inflammatory reaction of endothelial cells. Nature 1998, 391, 591–594. [Google Scholar] [CrossRef] [PubMed]
- Babendreyer, A.; Rojas-González, D.M.; Giese, A.A.; Fellendorf, S.; Düsterhöft, S.; Mela, P.; Ludwig, A. Differential Induction of the ADAM17 Regulators iRhom1 and 2 in Endothelial Cells. Front. Cardiovasc. Med. 2020, 7, 610344. [Google Scholar] [CrossRef] [PubMed]
- Lutgens, E. rs1883832: A CD40 single-nucleotide polymorphism for predicting coronary heart disease in humans. Cardiovasc. Res. 2020, 116, 1095–1096. [Google Scholar] [CrossRef] [PubMed]
- Heeschen, C.; Dimmeler, S.; Hamm, C.W.; Brand, M.J.v.D.; Boersma, E.; Zeiher, A.M.; Simoons, M.L. Soluble CD40 Ligand in Acute Coronary Syndromes. N. Engl. J. Med. 2003, 348, 1104–1111. [Google Scholar] [CrossRef] [PubMed]
- Gergei, I.; Kälsch, T.; Scharnagl, H.; Kleber, M.E.; Zirlik, A.; März, W.; Krämer, B.K.; Kälsch, A.-I. Association of soluble CD40L with short-term and long-term cardiovascular and all-cause mortality: The Ludwigshafen Risk and Cardiovascular Health (LURIC) study. Atherosclerosis 2019, 291, 127–131. [Google Scholar] [CrossRef]
- Ferrucci, L.; Fabbri, E. Inflammageing: Chronic inflammation in ageing, cardiovascular disease, and frailty. Nat. Rev. Cardiol. 2018, 15, 505–522. [Google Scholar] [CrossRef]
- Ridker, P.M. High-sensitivity C-reactive protein: Potential adjunct for global risk assessment in the primary prevention of cardiovascular disease. Circulation 2001, 103, 1813–1818. [Google Scholar] [CrossRef]
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global Burden of Cardiovascular Diseases and Risk Factors, 1990–2019: Update From the GBD 2019 Study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef]
- Dou, H.; Feher, A.; Davila, A.C.; Romero, M.J.; Patel, V.S.; Kamath, V.M.; Gooz, M.B.; Rudic, R.D.; Lucas, R.; Fulton, D.J.; et al. Role of Adipose Tissue Endothelial ADAM17 in Age-Related Coronary Microvascular Dysfunction. Arter. Thromb. Vasc. Biol. 2017, 37, 1180–1193. [Google Scholar] [CrossRef]
- Shami, A.; Edsfeldt, A.; Bengtsson, E.; Nilsson, J.; Shore, A.C.; Natali, A.; Khan, F.; Lutgens, E.; Gonçalves, I. Soluble CD40 Levels in Plasma Are Associated with Cardiovascular Disease and in Carotid Plaques with a Vulnerable Phenotype. J. Stroke 2021, 23, 367–376. [Google Scholar] [CrossRef]
- Leonetti, S.; Tricò, D.; Nesti, L.; Baldi, S.; Kozakova, M.; Goncalves, I.; Nilsson, J.; Shore, A.; Khan, F.; Natali, A. Soluble CD40 receptor is a biomarker of the burden of carotid artery atherosclerosis in subjects at high cardiovascular risk. Atherosclerosis 2022, 343, 1–9. [Google Scholar] [CrossRef]
- Lampinen, M.; Vessby, J.; Fredricsson, A.; Wanders, A.; Rorsman, F.; Carlson, M. High Serum sCD40 and a Distinct Colonic T Cell Profile in Ulcerative Colitis Associated With Primary Sclerosing Cholangitis. J. Crohn’s Colitis 2019, 13, 341–350. [Google Scholar] [CrossRef] [PubMed]
- Komura, K.; Fujimoto, M.; Matsushita, T.; Yanaba, K.; Kodera, M.; Kawasuji, A.; Hasegawa, M.; Takehara, K.; Sato, S. Increased serum soluble CD40 levels in patients with systemic sclerosis. J. Rheumatol. 2007, 34, 353–358. [Google Scholar] [PubMed]
- Bae, S.-C.; Lee, Y.H. Association between CD40 polymorphisms and systemic lupus erythematosus and correlation between soluble CD40 and CD40 ligand levels in the disease: A meta-analysis. Lupus 2019, 28, 1452–1459. [Google Scholar] [CrossRef]
- Esposito, P.; Gabanti, E.; Bianzina, S.; Rampino, T.; Dal Canton, A. CD40/SCD40 imbalance in hemodialysis patients. Clin. Biochem. 2011, 44, 268–269. [Google Scholar] [CrossRef]
- Ait-ghezala, G.; Abdullah, L.; Volmar, C.H.; Paris, D.; Luis, C.A.; Quadros, A.; Mouzon, B.; Mullan, M.A.; Keegan, A.P.; Parrish, J.; et al. Diagnostic utility of APOE, soluble CD40, CD40L, and Abeta1-40 levels in plasma in Alzheimer’s disease. Cytokine 2008, 44, 283–287. [Google Scholar] [CrossRef] [PubMed]
- Peter, K.; Nawroth, P.; Conradt, C.; Nordt, T.; Weiss, T.; Boehme, M.; Wunsch, A.; Allenberg, J.; Kübler, W.; Bode, C. Circulating Vascular Cell Adhesion Molecule-1 Correlates With the Extent of Human Atherosclerosis in Contrast to Circulating Intercellular Adhesion Molecule-1, E-Selectin, P-Selectin, and Thrombomodulin. Arter. Thromb. Vasc. Biol. 1997, 17, 505–512. [Google Scholar] [CrossRef]
- Rizza, S.; Copetti, M.; Cardellini, M.; Menghini, R.; Pecchioli, C.; Luzi, A.; Di Cola, G.; Porzio, O.; Ippoliti, A.; Romeo, F.; et al. A score including ADAM17 substrates correlates to recurring cardiovascular event in subjects with atherosclerosis. Atherosclerosis 2015, 239, 459–464. [Google Scholar] [CrossRef]


| Groups | CHD (n = 92) |
|---|---|
| Age (years) | 69.8 ± 9.7 |
| Sex (M/F) | 69/23 |
| Smoker (%) | 48.9 |
| Hypertension (%) | 93.5 |
| Diabetes (%) | 47.9 |
| Hypercholesterolemia (%) | 97.8 |
| Body mass index (kg/m2) | 97.8 ± 4.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klersy, A.; Meyer, S.; Leuschner, F.; Kessler, T.; Hecker, M.; Wagner, A.H. Ectodomain Shedding by ADAM17 Increases the Release of Soluble CD40 from Human Endothelial Cells under Pro-Inflammatory Conditions. Cells 2023, 12, 1926. https://doi.org/10.3390/cells12151926
Klersy A, Meyer S, Leuschner F, Kessler T, Hecker M, Wagner AH. Ectodomain Shedding by ADAM17 Increases the Release of Soluble CD40 from Human Endothelial Cells under Pro-Inflammatory Conditions. Cells. 2023; 12(15):1926. https://doi.org/10.3390/cells12151926
Chicago/Turabian StyleKlersy, Anton, Sören Meyer, Florian Leuschner, Thorsten Kessler, Markus Hecker, and Andreas H. Wagner. 2023. "Ectodomain Shedding by ADAM17 Increases the Release of Soluble CD40 from Human Endothelial Cells under Pro-Inflammatory Conditions" Cells 12, no. 15: 1926. https://doi.org/10.3390/cells12151926
APA StyleKlersy, A., Meyer, S., Leuschner, F., Kessler, T., Hecker, M., & Wagner, A. H. (2023). Ectodomain Shedding by ADAM17 Increases the Release of Soluble CD40 from Human Endothelial Cells under Pro-Inflammatory Conditions. Cells, 12(15), 1926. https://doi.org/10.3390/cells12151926








