Early Administration of the Phytocannabinoid Cannabidivarin Prevents the Neurobehavioral Abnormalities Associated with the Fmr1-KO Mouse Model of Fragile X Syndrome
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals
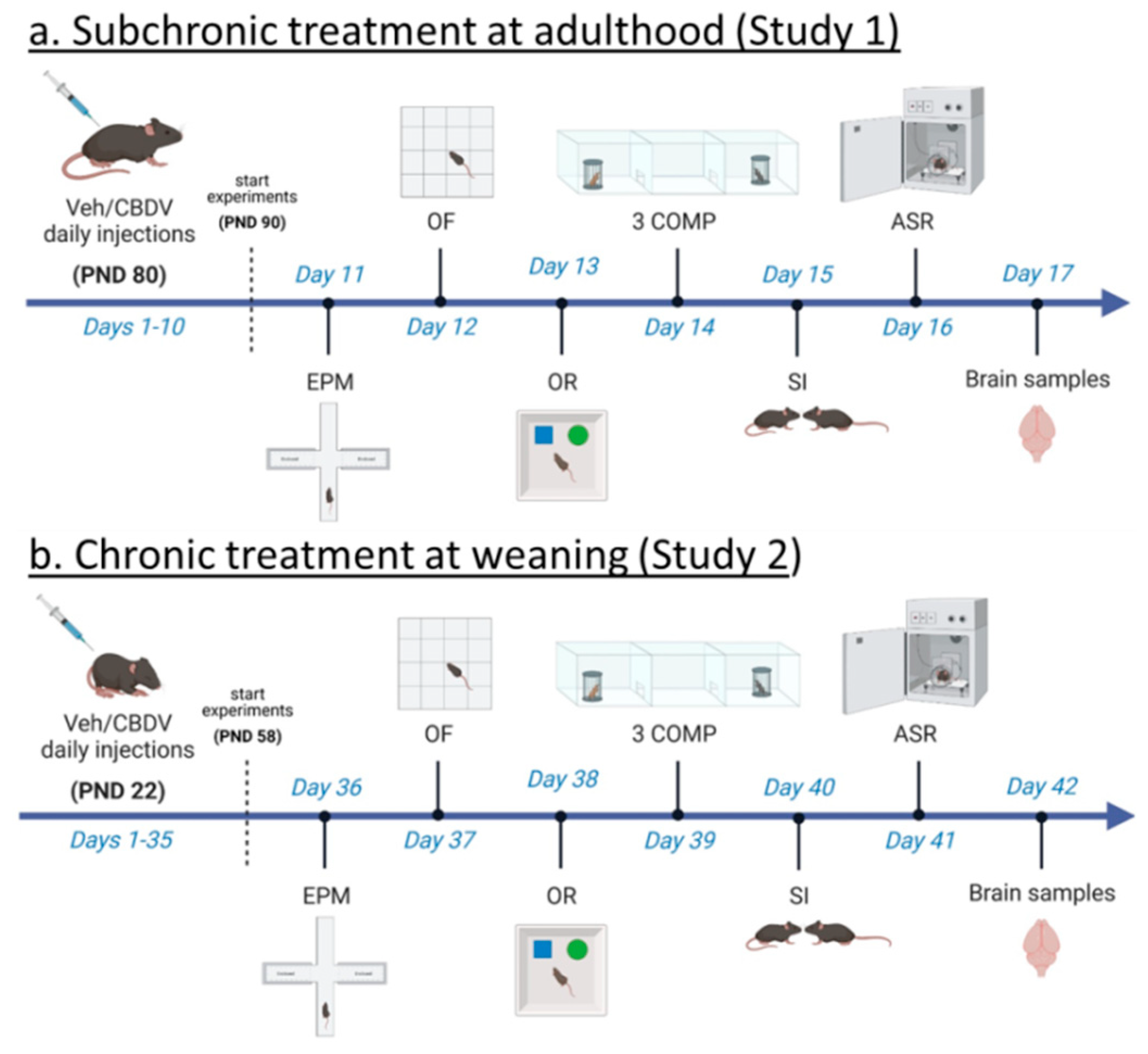
2.2. Experimental Procedures
2.2.1. CBDV Administration at Adulthood (Study 1)
2.2.2. CBDV Administration at Weaning (Study 2)
2.2.3. Behavioral Assessment (Studies 1 and 2)
2.2.4. Brain Assessment of Inflammatory and Plasticity Markers through RT-qPCR (Studies 1 and 2)
2.3. Statistical Analysis
3. Results
3.1. Study 1: Effects of Subchronic Administration of CBDV at Adulthood
3.1.1. Behavioral Profiling
- Elevated plus maze
- Object recognition test
- Three-compartment test for sociability and social novelty
- Direct social interaction with an adult female
- Acoustic startle response
3.1.2. Brain Analyses
- Hippocampus
- Prefrontal cortex
3.2. Study 2: Effects of Chronic Administration of CBDV at Weaning
3.2.1. Behavioral Profiling
- Elevated plus maze
- Object recognition test
- Three-compartment test for sociability and social novelty
- Direct social interaction with an adult female
- Acoustic startle response
3.2.2. Brain Analyses
- Hippocampus
- Prefrontal cortex
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parolaro, D.; Realini, N.; Vigano, D.; Guidali, C.; Rubino, T. The Endocannabinoid System and Psychiatric Disorders. Exp. Neurol. 2010, 224, 3–14. [Google Scholar] [CrossRef]
- Mackie, K. Distribution of Cannabinoid Receptors in the Central and Peripheral Nervous System. Handb. Exp. Pharmacol. 2005, 168, 299–325. [Google Scholar]
- Basavarajappa, B.S.; Nixon, R.A.; Arancio, O. Endocannabinoid System: Emerging Role from Neurodevelopment to Neurodegeneration. Mini Rev. Med. Chem. 2009, 9, 448–462. [Google Scholar] [CrossRef]
- Berghuis, P.; Rajnicek, A.M.; Morozov, Y.M.; Ross, R.A.; Mulder, J.; Urban, G.M.; Monory, K.; Marsicano, G.; Matteoli, M.; Canty, A.; et al. Hardwiring the Brain: Endocannabinoids Shape Neuronal Connectivity. Science 2007, 316, 1212–1216. [Google Scholar] [CrossRef]
- Monory, K.; Massa, F.; Egertova, M.; Eder, M.; Blaudzun, H.; Westenbroek, R.; Kelsch, W.; Jacob, W.; Marsch, R.; Ekker, M.; et al. The Endocannabinoid System Controls Key Epileptogenic Circuits in the Hippocampus. Neuron 2006, 51, 455–466. [Google Scholar] [CrossRef]
- Vigli, D.; Cosentino, L.; Raggi, C.; Laviola, G.; Woolley-Roberts, M.; De Filippis, B. Chronic Treatment with the Phytocannabinoid Cannabidivarin (Cbdv) Rescues Behavioural Alterations and Brain Atrophy in a Mouse Model of Rett Syndrome. Neuropharmacology 2018, 140, 121–129. [Google Scholar] [CrossRef]
- Zamberletti, E.; Gabaglio, M.; Piscitelli, F.; Brodie, J.S.; Woolley-Roberts, M.; Barbiero, I.; Tramarin, M.; Binelli, G.; Landsberger, N.; Kilstrup-Nielsen, C.; et al. Cannabidivarin Completely Rescues Cognitive Deficits and Delays Neurological and Motor Defects in Male Mecp2 Mutant Mice. J. Psychopharmacol. 2019, 33, 894–907. [Google Scholar] [CrossRef]
- Navarro-Romero, A.; Galera-Lopez, L.; Ortiz-Romero, P.; Llorente-Ovejero, A.; de Los Reyes-Ramirez, L.; de Tena, I.B.; Garcia-Elias, A.; Mas-Stachurska, A.; Reixachs-Sole, M.; Pastor, A.; et al. Cannabinoid Signaling Modulation through Jzl184 Restores Key Phenotypes of a Mouse Model for Williams-Beuren Syndrome. Elife 2022, 11, e72560. [Google Scholar] [CrossRef]
- Busquets-Garcia, A.; Gomis-Gonzalez, M.; Guegan, T.; Agustin-Pavon, C.; Pastor, A.; Mato, S.; Perez-Samartin, A.; Matute, C.; de la Torre, R.; Dierssen, M.; et al. Targeting the Endocannabinoid System in the Treatment of Fragile X Syndrome. Nat. Med. 2013, 19, 603–607. [Google Scholar] [CrossRef]
- Jung, K.M.; Sepers, M.; Henstridge, C.M.; Lassalle, O.; Neuhofer, D.; Martin, H.; Ginger, M.; Frick, A.; DiPatrizio, N.V.; Mackie, K.; et al. Uncoupling of the Endocannabinoid Signalling Complex in a Mouse Model of Fragile X Syndrome. Nat. Commun. 2012, 3, 1080. [Google Scholar] [CrossRef]
- Maccarrone, M.; Rossi, S.; Bari, M.; De Chiara, V.; Rapino, C.; Musella, A.; Bernardi, G.; Bagni, C.; Centonze, D. Abnormal Mglu 5 Receptor/Endocannabinoid Coupling in Mice Lacking Fmrp and Bc1 Rna. Neuropsychopharmacology 2010, 35, 1500–1509. [Google Scholar] [CrossRef]
- Zhang, L.; Alger, B.E. Enhanced Endocannabinoid Signaling Elevates Neuronal Excitability in Fragile X Syndrome. J. Neurosci. 2010, 30, 5724–5729. [Google Scholar] [CrossRef]
- Pieretti, M.; Zhang, F.P.; Fu, Y.H.; Warren, S.T.; Oostra, B.A.; Caskey, C.T.; Nelson, D.L. Absence of Expression of the Fmr-1 Gene in Fragile X Syndrome. Cell 1991, 66, 817–822. [Google Scholar] [CrossRef] [PubMed]
- Verkerk, A.J.; Pieretti, M.; Sutcliffe, J.S.; Fu, Y.H.; Kuhl, D.P.; Pizzuti, A.; Reiner, O.; Richards, S.; Victoria, M.F.; Zhang, F.P. Identification of a Gene (Fmr-1) Containing a Cgg Repeat Coincident with a Breakpoint Cluster Region Exhibiting Length Variation in Fragile X Syndrome. Cell 1991, 65, 905–914. [Google Scholar] [CrossRef]
- Bear, M.F.; Huber, K.M.; Warren, S.T. The Mglur Theory of Fragile X Mental Retardation. Trends Neurosci. 2004, 27, 370–377. [Google Scholar] [CrossRef]
- Michalon, A.; Bruns, A.; Risterucci, C.; Honer, M.; Ballard, T.M.; Ozmen, L.; Jaeschke, G.; Wettstein, J.G.; von Kienlin, M.; Kunnecke, B.; et al. Chronic Metabotropic Glutamate Receptor 5 Inhibition Corrects Local Alterations of Brain Activity and Improves Cognitive Performance in Fragile X Mice. Biol. Psychiatry 2014, 75, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Hoeffer, C.A.; Sanchez, E.; Hagerman, R.J.; Mu, Y.; Nguyen, D.V.; Wong, H.; Whelan, A.M.; Zukin, R.S.; Klann, E.; Tassone, F. Altered Mtor Signaling and Enhanced Cyfip2 Expression Levels in Subjects with Fragile X Syndrome. Genes Brain Behav. 2012, 11, 332–341. [Google Scholar] [CrossRef]
- Price, T.J.; Rashid, M.H.; Millecamps, M.; Sanoja, R.; Entrena, J.M.; Cervero, F. Decreased Nociceptive Sensitization in Mice Lacking the Fragile X Mental Retardation Protein: Role of Mglur1/5 and Mtor. J. Neurosci. 2007, 27, 13958–13967. [Google Scholar] [CrossRef] [PubMed]
- Sharma, A.; Hoeffer, C.A.; Takayasu, Y.; Miyawaki, T.; McBride, S.M.; Klann, E.; Zukin, R.S. Dysregulation of Mtor Signaling in Fragile X Syndrome. J. Neurosci. 2010, 30, 694–702. [Google Scholar] [CrossRef] [PubMed]
- Kano, M.; Ohno-Shosaku, T.; Hashimotodani, Y.; Uchigashima, M.; Watanabe, M. Endocannabinoid-Mediated Control of Synaptic Transmission. Physiol. Rev. 2009, 89, 309–380. [Google Scholar] [CrossRef]
- Varma, N.; Carlson, G.C.; Ledent, C.; Alger, B.E. Metabotropic Glutamate Receptors Drive the Endocannabinoid System in Hippocampus. J. Neurosci. 2001, 21, RC188. [Google Scholar] [CrossRef] [PubMed]
- Puighermanal, E.; Busquets-Garcia, A.; Maldonado, R.; Ozaita, A. Cellular and Intracellular Mechanisms Involved in the Cognitive Impairment of Cannabinoids. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2012, 367, 3254–3263. [Google Scholar] [CrossRef] [PubMed]
- Puighermanal, E.; Marsicano, G.; Busquets-Garcia, A.; Lutz, B.; Maldonado, R.; Ozaita, A. Cannabinoid Modulation of Hippocampal Long-Term Memory Is Mediated by Mtor Signaling. Nat. Neurosci. 2009, 12, 1152–1158. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Bellocchio, L.; Oddi, D.; D’Amato, F.R.; Marsicano, G.; Crusio, W.E. The Fmr1 Mouse Line as a Model for Autism: The Relevance of the Genetic Background and of the Endocannabinoid System. In Proceedings of the FENS Annual Meeting, Amsterdam, The Netherlands, 3–7 July 2010. [Google Scholar]
- Busquets-Garcia, A.; Maldonado, R.; Ozaita, A. New Insights into the Molecular Pathophysiology of Fragile X Syndrome and Therapeutic Perspectives from the Animal Model. Int. J. Biochem. Cell Biol. 2014, 53, 121–126. [Google Scholar] [CrossRef]
- Bisogno, T.; Howell, F.; Williams, G.; Minassi, A.; Cascio, M.G.; Ligresti, A.; Matias, I.; Schiano-Moriello, A.; Paul, P.; Williams, E.J.; et al. Cloning of the First Sn1-Dag Lipases Points to the Spatial and Temporal Regulation of Endocannabinoid Signaling in the Brain. J. Cell Biol. 2003, 163, 463–468. [Google Scholar] [CrossRef]
- Blessing, E.M.; Steenkamp, M.M.; Manzanares, J.; Marmar, C.R. Cannabidiol as a Potential Treatment for Anxiety Disorders. Neurotherapeutics 2015, 12, 825–836. [Google Scholar] [CrossRef]
- Burstein, S. Cannabidiol (Cbd) and Its Analogs: A Review of Their Effects on Inflammation. Bioorg. Med. Chem. 2015, 23, 1377–1385. [Google Scholar] [CrossRef]
- Campos, A.C.; Fogaca, M.V.; Sonego, A.B.; Guimaraes, F.S. Cannabidiol, Neuroprotection and Neuropsychiatric Disorders. Pharmacol. Res. 2016, 112, 119–127. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, B.; Ravi, J.; Ganju, R.K. Cannabinoids as Therapeutic Agents in Cancer: Current Status and Future Implications. Oncotarget 2014, 5, 5852–5872. [Google Scholar] [CrossRef]
- Fasinu, P.S.; Phillips, S.; ElSohly, M.A.; Walker, L.A. Current Status and Prospects for Cannabidiol Preparations as New Therapeutic Agents. Pharmacotherapy 2016, 36, 781–796. [Google Scholar] [CrossRef]
- Hill, A.J.; Williams, C.M.; Whalley, B.J.; Stephens, G.J. Phytocannabinoids as Novel Therapeutic Agents in Cns Disorders. Pharmacol. Ther. 2012, 133, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Hill, A.J.; Mercier, M.S.; Hill, T.D.; Glyn, S.E.; Jones, N.A.; Yamasaki, Y.; Futamura, T.; Duncan, M.; Stott, C.G.; Stephens, G.J.; et al. Cannabidivarin Is Anticonvulsant in Mouse and Rat. Br. J. Pharmacol. 2012, 167, 1629–1642. [Google Scholar] [CrossRef] [PubMed]
- Deiana, S.; Watanabe, A.; Yamasaki, Y.; Amada, N.; Arthur, M.; Fleming, S.; Woodcock, H.; Dorward, P.; Pigliacampo, B.; Close, S.; et al. Plasma and Brain Pharmacokinetic Profile of Cannabidiol (Cbd), Cannabidivarine (Cbdv), Delta(9)-Tetrahydrocannabivarin (Thcv) and Cannabigerol (Cbg) in Rats and Mice Following Oral and Intraperitoneal Administration and Cbd Action on Obsessive-Compulsive Behaviour. Psychopharmacology 2012, 219, 859–873. [Google Scholar] [PubMed]
- Zamberletti, E.; Gabaglio, M.; Woolley-Roberts, M.; Bingham, S.; Rubino, T.; Parolaro, D. Cannabidivarin Treatment Ameliorates Autism-Like Behaviors and Restores Hippocampal Endocannabinoid System and Glia Alterations Induced by Prenatal Valproic Acid Exposure in Rats. Front. Cell Neurosci. 2019, 13, 367. [Google Scholar] [CrossRef] [PubMed]
- Pietropaolo, S.; Subashi, E. Mouse Models of Fragile X Syndrome. In Behavioral Genetics of the Mouse; Pietropaolo, S., Sluyter, F., Crusio, W.E., Eds.; Cambridge University Press: Cambridge, UK, 2014; pp. 146–163. [Google Scholar]
- Spear, L.P. The Adolescent Brain and Age-Related Behavioral Manifestations. Neurosci. Biobehav. Rev. 2000, 24, 417–463. [Google Scholar] [CrossRef]
- Hebert, B.; Pietropaolo, S.; Meme, S.; Laudier, B.; Laugeray, A.; Doisne, N.; Quartier, A.; Lefeuvre, S.; Got, L.; Cahard, D.; et al. Rescue of Fragile X Syndrome Phenotypes in Fmr1 Ko Mice by a Bkca Channel Opener Molecule. Orphanet J. Rare Dis. 2014, 9, 124. [Google Scholar] [CrossRef]
- Oddi, D.; Subashi, E.; Middei, S.; Bellocchio, L.; Lemaire-Mayo, V.; Guzman, M.; Crusio, W.E.; D’Amato, F.R.; Pietropaolo, S. Early Social Enrichment Rescues Adult Behavioral and Brain Abnormalities in a Mouse Model of Fragile X Syndrome. Neuropsychopharmacology 2015, 40, 1113–1122. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Goubran, M.G.; Joffre, C.; Aubert, A.; Lemaire-Mayo, V.; Crusio, W.E.; Laye, S. Dietary Supplementation of Omega-3 Fatty Acids Rescues Fragile X Phenotypes in Fmr1-Ko Mice. Psychoneuroendocrinology 2014, 49, 119–129. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Guilleminot, A.; Martin, B.; D’Amato, F.R.; Crusio, W.E. Genetic-Background Modulation of Core and Variable Autistic-Like Symptoms in Fmr1 Knock-out Mice. PLoS ONE 2011, 6, e17073. [Google Scholar] [CrossRef]
- Zhang, Y.; Bonnan, A.; Bony, G.; Ferezou, I.; Pietropaolo, S.; Ginger, M.; Sans, N.; Rossier, J.; Oostra, B.; LeMasson, G.; et al. Dendritic Channelopathies Contribute to Neocortical and Sensory Hyperexcitability in Fmr1(-/Y) Mice. Nat. Neurosci. 2014, 17, 1701–1709. [Google Scholar] [CrossRef]
- Khandjian, E.W. Biology of the Fragile X Mental Retardation Protein, an Rna-Binding Protein. Biochem. Cell Biol. 1999, 77, 331–342. [Google Scholar] [CrossRef] [PubMed]
- Bakker, C.E.; de Diego Otero, Y.; Bontekoe, C.; Raghoe, P.; Luteijn, T.; Hoogeveen, A.T.; Oostra, B.A.; Willemsen, R. Immunocytochemical and Biochemical Characterization of Fmrp, Fxr1p, and Fxr2p in the Mouse. Exp. Cell Res. 2000, 258, 162–170. [Google Scholar] [CrossRef] [PubMed]
- Dutch-Belgian Fragile X Consortium. Fmr1 Knockout Mice: A Model to Study Fragile X Mental Retardation. Cell 1994, 78, 23–33. [Google Scholar]
- Moles, A.; D’Amato, R.F. Ultrasonic Vocalization by Female Mice in the Presence of a Conspecific Carrying Food Cues. Anim. Behav. 2000, 60, 689–694. [Google Scholar] [CrossRef]
- Gaudissard, J.; Ginger, M.; Premoli, M.; Memo, M.; Frick, A.; Pietropaolo, S. Behavioral Abnormalities in the Fmr1-Ko2 Mouse Model of Fragile X Syndrome: The Relevance of Early Life Phases. Autism Res. 2017, 10, 1584–1596. [Google Scholar] [CrossRef]
- Gauducheau, M.; Lemaire-Mayo, V.; D’Amato, F.R.; Oddi, D.; Crusio, W.E.; Pietropaolo, S. Age-Specific Autistic-Like Behaviors in Heterozygous Fmr1-Ko Female Mice. Autism Res. 2017, 10, 1067–1078. [Google Scholar] [CrossRef]
- Pietropaolo, S.; Crusio, W.E. Strain-Dependent Changes in Acoustic Startle Response and Its Plasticity across Adolescence in Mice. Behav. Genet. 2009, 39, 623–631. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The Miqe Guidelines: Minimum Information for Publication of Quantitative Real-Time Pcr Experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative Pcr and the 2−ΔΔCT Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Vandesquille, M.; Baudonnat, M.; Decorte, L.; Louis, C.; Lestage, P.; Beracochea, D. Working Memory Deficits and Related Disinhibition of the Camp/Pka/Creb Are Alleviated by Prefrontal Alpha4beta2 *-Nachrs Stimulation in Aged Mice. Neurobiol. Aging 2013, 34, 1599–1609. [Google Scholar] [CrossRef]
- Kat, R.; Arroyo-Araujo, M.; de Vries, R.B.M.; Koopmans, M.A.; de Boer, S.F.; Kas, M.J.H. Translational Validity and Methodological Underreporting in Animal Research: A Systematic Review and Meta-Analysis of the Fragile X Syndrome (Fmr1 Ko) Rodent Model. Neurosci. Biobehav. Rev. 2022, 139, 104722. [Google Scholar] [CrossRef]
- Bhattacharya, A.; Kaphzan, H.; Alvarez-Dieppa, A.C.; Murphy, J.P.; Pierre, P.; Klann, E. Genetic Removal of P70 S6 Kinase 1 Corrects Molecular, Synaptic, and Behavioral Phenotypes in Fragile X Syndrome Mice. Neuron 2012, 76, 325–337. [Google Scholar] [CrossRef] [PubMed]
- Dahlhaus, R.; El-Husseini, A. Altered Neuroligin Expression Is Involved in Social Deficits in a Mouse Model of the Fragile X Syndrome. Behav. Brain Res. 2010, 208, 96–105. [Google Scholar] [CrossRef] [PubMed]
- De Diego-Otero, Y.; Romero-Zerbo, Y.; el Bekay, R.; Decara, J.; Sanchez, L.; Fonseca, F.R.-D.; del Arco-Herrera, I. Alpha-Tocopherol Protects against Oxidative Stress in the Fragile X Knockout Mouse: An Experimental Therapeutic Approach for the Fmr1 Deficiency. Neuropsychopharmacology 2009, 34, 1011–1026. [Google Scholar] [CrossRef]
- Eadie, B.D.; Zhang, W.N.; Boehme, F.; Gil-Mohapel, J.; Kainer, L.; Simpson, J.M.; Christie, B.R. Fmr1 Knockout Mice Show Reduced Anxiety and Alterations in Neurogenesis That Are Specific to the Ventral Dentate Gyrus. Neurobiol. Dis. 2009, 36, 361–373. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M.L.; Rao, B.S.; Seo, J.S.; Choi, H.S.; Dolan, B.M.; Choi, S.Y.; Chattarji, S.; Tonegawa, S. Inhibition of P21-Activated Kinase Rescues Symptoms of Fragile X Syndrome in Mice. Proc. Natl. Acad. Sci. USA 2007, 104, 11489–11494. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.H.; Chuang, D.M.; Smith, C.B. Lithium Ameliorates Phenotypic Deficits in a Mouse Model of Fragile X Syndrome. Int. J. Neuropsychopharmacol. 2011, 14, 1–13. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Sluyter, F.; de Wit, S.; Oostra, B.A.; Crusio, W.E. Behavioral and Neuroanatomical Characterization of the Fmr1 Knockout Mouse. Hippocampus 2002, 12, 39–46. [Google Scholar] [CrossRef]
- Olmos-Serrano, J.L.; Corbin, J.G.; Burns, M.P. The Gaba(a) Receptor Agonist Thip Ameliorates Specific Behavioral Deficits in the Mouse Model of Fragile X Syndrome. Dev. Neurosci. 2011, 33, 395–403. [Google Scholar] [CrossRef]
- Peier, A.M.; McIlwain, K.L.; Kenneson, A.; Warren, S.T.; Paylor, R.; Nelson, D.L. (Over) Correction of Fmr1 Deficiency with Yac Transgenics: Behavioral and Physical Features. Hum. Mol. Genet. 2000, 9, 1145–1159. [Google Scholar] [CrossRef]
- Restivo, L.; Ferrari, F.; Passino, E.; Sgobio, C.; Bock, J.; Oostra, B.A.; Bagni, C.; Ammassari-Teule, M. Enriched Environment Promotes Behavioral and Morphological Recovery in a Mouse Model for the Fragile X Syndrome. Proc. Natl. Acad. Sci. USA 2005, 102, 11557–11562. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.M.; Alekseyenko, O.; Hamilton, S.M.; Thomas, A.M.; Serysheva, E.; Yuva-Paylor, L.A.; Paylor, R. Modifying Behavioral Phenotypes in Fmr1ko Mice: Genetic Background Differences Reveal Autistic-Like Responses. Autism Res. 2011, 4, 40–56. [Google Scholar] [CrossRef] [PubMed]
- Spencer, C.M.; Alekseyenko, O.; Serysheva, E.; Yuva-Paylor, L.A.; Paylor, R. Altered Anxiety-Related and Social Behaviors in the Fmr1 Knockout Mouse Model of Fragile X Syndrome. Genes Brain Behav. 2005, 4, 420–430. [Google Scholar] [CrossRef]
- Thomas, A.M.; Bui, N.; Graham, D.; Perkins, J.R.; Yuva-Paylor, L.A.; Paylor, R. Genetic Reduction of Group 1 Metabotropic Glutamate Receptors Alters Select Behaviors in a Mouse Model for Fragile X Syndrome. Behav. Brain Res. 2011, 223, 310–321. [Google Scholar] [CrossRef]
- Uutela, M.; Lindholm, J.; Louhivuori, V.; Wei, H.; Louhivuori, L.M.; Pertovaara, A.; Akerman, K.; Castren, E.; Castren, M.L. Reduction of Bdnf Expression in Fmr1 Knockout Mice Worsens Cognitive Deficits but Improves Hyperactivity and Sensorimotor Deficits. Genes Brain Behav. 2012, 11, 513–523. [Google Scholar] [CrossRef]
- Mineur, Y.S.; Huynh, L.X.; Crusio, W.E. Social Behavior Deficits in the Fmr1 Mutant Mouse. Behav. Brain Res. 2006, 168, 172–175. [Google Scholar] [CrossRef]
- Heitzer, A.M.; Roth, A.K.; Nawrocki, L.; Wrenn, C.C.; Valdovinos, M.G. Brief Report: Altered Social Behavior in Isolation-Reared Fmr1 Knockout Mice. J. Autism Dev. Disord 2012, 43, 1452–1458. [Google Scholar] [CrossRef]
- Mines, M.A.; Yuskaitis, C.J.; King, M.K.; Beurel, E.; Jope, R.S. Gsk3 Influences Social Preference and Anxiety-Related Behaviors During Social Interaction in a Mouse Model of Fragile X Syndrome and Autism. PLoS ONE 2010, 5, e9706. [Google Scholar] [CrossRef]
- Michalon, A.; Sidorov, M.; Ballard, T.M.; Ozmen, L.; Spooren, W.; Wettstein, J.G.; Jaeschke, G.; Bear, M.F.; Lindemann, L. Chronic Pharmacological Mglu5 Inhibition Corrects Fragile X in Adult Mice. Neuron 2012, 74, 49–56. [Google Scholar] [CrossRef]
- Ventura, R.; Pascucci, T.; Catania, M.V.; Musumeci, S.A.; Puglisi-Allegra, S. Object Recognition Impairment in Fmr1 Knockout Mice Is Reversed by Amphetamine: Involvement of Dopamine in the Medial Prefrontal Cortex. Behav. Pharmacol. 2004, 15, 433–442. [Google Scholar] [CrossRef]
- Nielsen, D.M.; Derber, W.J.; McClellan, D.A.; Crnic, L.S. Alterations in the Auditory Startle Response in Fmr1 Targeted Mutant Mouse Models of Fragile X Syndrome. Brain Res. 2002, 927, 8–17. [Google Scholar] [CrossRef]
- Veeraragavan, S.; Graham, D.; Bui, N.; Yuva-Paylor, L.A.; Wess, J.; Paylor, R. Genetic Reduction of Muscarinic M4 Receptor Modulates Analgesic Response and Acoustic Startle Response in a Mouse Model of Fragile X Syndrome (Fxs). Behav. Brain Res. 2011, 228, 1–8. [Google Scholar] [CrossRef]
- Heulens, I.; D’Hulst, C.; Van Dam, D.; De Deyn, P.P.; Kooy, R.F. Pharmacological Treatment of Fragile X Syndrome with Gabaergic Drugs in a Knockout Mouse Model. Behav. Brain Res. 2012, 229, 244–249. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P. Adolescent Brain Development and Animal Models. Ann. N. Y. Acad. Sci. 2004, 1021, 23–26. [Google Scholar] [CrossRef] [PubMed]
- Spear, L.P.; Brake, S.C. Periadolescence: Age-Dependent Behavior and Psychopharmacological Responsivity in Rats. Dev. Psychobiol. 1983, 16, 83–109. [Google Scholar] [CrossRef]
- Gantois, I.; Khoutorsky, A.; Popic, J.; Aguilar-Valles, A.; Freemantle, E.; Cao, R.; Sharma, V.; Pooters, T.; Nagpal, A.; Skalecka, A.; et al. Metformin Ameliorates Core Deficits in a Mouse Model of Fragile X Syndrome. Nat. Med. 2017, 23, 674–677. [Google Scholar] [CrossRef]
- Lemaire-Mayo, V.; Piquemal, M.; Crusio, W.E.; Louette, E.; Pietropaolo, S. Therapeutic Effects of Chlorzoxazone, a Bkca Channel Agonist, in a Mouse Model of Fragile X Syndrome. bioRxiv 2020. bioRxiv:2020.12.11.389569. [Google Scholar]
- Cheng, D.; Low, J.K.; Logge, W.; Garner, B.; Karl, T. Chronic Cannabidiol Treatment Improves Social and Object Recognition in Double Transgenic Appswe/Ps1e9 Mice. Psychopharmacology 2014, 231, 3009–3017. [Google Scholar] [CrossRef]
- Zupan, B.; Toth, M. Wild-Type Male Offspring of Fmr-1+/− Mothers Exhibit Characteristics of the Fragile X Phenotype. Neuropsychopharmacology 2008, 33, 2667–2675. [Google Scholar] [CrossRef]
- Lemaire-Mayo, V.; ESubashi; Henkous, N.; Beracochea, D.; Pietropaolo, S. Behavioral Effects of Chronic Stress in the Fmr1 Mouse Model for Fragile X Syndrome. Behav. Brain Res. 2017, 320, 128–135. [Google Scholar] [CrossRef]
- Markham, J.A.; Beckel-Mitchener, A.C.; Estrada, C.M.; Greenough, W.T. Corticosterone Response to Acute Stress in a Mouse Model of Fragile X Syndrome. Psychoneuroendocrinology 2006, 31, 781–785. [Google Scholar] [CrossRef]
- Petroni, V.; Subashi, E.; Premoli, M.; Memo, M.; Lemaire, V.; Pietropaolo, S. Long-Term Behavioral Effects of Prenatal Stress in the Fmr1-Knock-out Mouse Model for Fragile X Syndrome. Front. Cell Neurosci. 2022, 16, 917183. [Google Scholar] [CrossRef]
- Petroni, V.; Subashi, E.; Premoli, M.; Wohr, M.; Crusio, W.E.; Lemaire, V.; Pietropaolo, S. Autistic-Like Behavioral Effects of Prenatal Stress in Juvenile Fmr1 Mice: The Relevance of Sex Differences and Gene-Environment Interactions. Sci. Rep. 2022, 12, 7269. [Google Scholar] [CrossRef] [PubMed]
- Qin, M.; Xia, Z.; Huang, T.; Smith, C.B. Effects of Chronic Immobilization Stress on Anxiety-Like Behavior and Basolateral Amygdala Morphology in Fmr1 Knockout Mice. Neuroscience 2011, 194, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Daskalakis, N.P.; De Kloet, E.R.; Yehuda, R.; Malaspina, D.; Kranz, T.M. Early Life Stress Effects on Glucocorticoid-Bdnf Interplay in the Hippocampus. Front. Mol. Neurosci. 2015, 8, 68. [Google Scholar] [CrossRef]
- Nowacka, M.; Obuchowicz, E. Bdnf and Vegf in the Pathogenesis of Stress-Induced Affective Diseases: An Insight from Experimental Studies. Pharmacol. Rep. 2013, 65, 535–546. [Google Scholar] [CrossRef]
- Pardon, M.C. Role of Neurotrophic Factors in Behavioral Processes: Implications for the Treatment of Psychiatric and Neurodegenerative Disorders. Vitam. Horm. 2010, 82, 185–200. [Google Scholar]
- Schaaf, M.J.; De Kloet, E.R.; Vreugdenhil, E. Corticosterone Effects on Bdnf Expression in the Hippocampus. Implications for Memory Formation. Stress 2000, 3, 201–208. [Google Scholar] [CrossRef]
- Smith, M.A. Hippocampal Vulnerability to Stress and Aging: Possible Role of Neurotrophic Factors. Behav. Brain Res. 1996, 78, 25–36. [Google Scholar] [CrossRef]
- Suri, D.; Vaidya, V.A. Glucocorticoid Regulation of Brain-Derived Neurotrophic Factor: Relevance to Hippocampal Structural and Functional Plasticity. Neuroscience 2013, 239, 196–213. [Google Scholar] [CrossRef]
- Lee, J.; Duan, W.; Long, J.M.; Ingram, D.K.; Mattson, M.P. Dietary Restriction Increases the Number of Newly Generated Neural Cells, and Induces Bdnf Expression, in the Dentate Gyrus of Rats. J. Mol. Neurosci. 2000, 15, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Gronli, J.; Bramham, C.; Murison, R.; Kanhema, T.; Fiske, E.; Bjorvatn, B.; Ursin, R.; Portas, C.M. Chronic Mild Stress Inhibits Bdnf Protein Expression and Creb Activation in the Dentate Gyrus but Not in the Hippocampus Proper. Pharmacol. Biochem. Behav. 2006, 85, 842–849. [Google Scholar] [CrossRef] [PubMed]
- Smith, M.A.; Makino, S.; Kvetnansky, R.; Post, R.M. Stress and Glucocorticoids Affect the Expression of Brain-Derived Neurotrophic Factor and Neurotrophin-3 Mrnas in the Hippocampus. J. Neurosci. 1995, 15 Pt 1, 1768–1777. [Google Scholar] [CrossRef]
- Onishchenko, N.; Karpova, N.; Sabri, F.; Castren, E.; Ceccatelli, S. Long-Lasting Depression-Like Behavior and Epigenetic Changes of Bdnf Gene Expression Induced by Perinatal Exposure to Methylmercury. J. Neurochem. 2008, 106, 1378–1387. [Google Scholar] [CrossRef] [PubMed]
- Goshen, I.; Kreisel, T.; Ounallah-Saad, H.; Renbaum, P.; Zalzstein, Y.; Ben-Hur, T.; Levy-Lahad, E.; Yirmiya, R. A Dual Role for Interleukin-1 in Hippocampal-Dependent Memory Processes. Psychoneuroendocrinology 2007, 32, 1106–1115. [Google Scholar] [CrossRef] [PubMed]
- Dantzer, R.; O’Connor, J.C.; Freund, G.G.; Johnson, R.W.; Kelley, K.W. From Inflammation to Sickness and Depression: When the Immune System Subjugates the Brain. Nat. Rev. Neurosci. 2008, 9, 46–56. [Google Scholar] [CrossRef]
- Schaefer, T.L.; Davenport, M.H.; Grainger, L.M.; Robinson, C.K.; Earnheart, A.T.; Stegman, M.S.; Lang, A.L.; Ashworth, A.A.; Molinaro, G.; Huber, K.M.; et al. Acamprosate in a Mouse Model of Fragile X Syndrome: Modulation of Spontaneous Cortical Activity, Erk1/2 Activation, Locomotor Behavior, and Anxiety. J. Neurodev. Disord. 2017, 9, 6. [Google Scholar] [CrossRef]
- Rotschafer, S.E.; Trujillo, M.S.; Dansie, L.E.; Ethell, I.M.; Razak, K.A. Minocycline Treatment Reverses Ultrasonic Vocalization Production Deficit in a Mouse Model of Fragile X Syndrome. Brain Res. 2012, 1439, 7–14. [Google Scholar] [CrossRef]
- Toledo, M.A.; TWen, H.; Binder, D.K.; Ethell, I.M.; Razak, K.A. Reversal of Ultrasonic Vocalization Deficits in a Mouse Model of Fragile X Syndrome with Minocycline Treatment or Genetic Reduction of Mmp-9. Behav. Brain Res. 2019, 372, 112068. [Google Scholar] [CrossRef]
- Morales, P.; Hurst, D.P.; Reggio, P.H. Molecular Targets of the Phytocannabinoids: A Complex Picture. Prog. Chem. Org. Nat. Prod. 2017, 103, 103–131. [Google Scholar]
- Pietropaolo, S.; Provenzano, G. Editorial: Targeting Excitation-Inhibition Imbalance in Neurodevelopmental and Autism Spectrum Disorders. Front. Neurosci. 2022, 16, 968115. [Google Scholar] [CrossRef] [PubMed]






| Brain Region | Brain Marker | SUBCHRONIC TREATMENT AT ADULTHOOD | ||||||
|---|---|---|---|---|---|---|---|---|
| VEH | CBDV-20 | CBDV-100 | Significant Differences | |||||
| WT | KO | WT | KO | WT | KO | |||
| CA1 | BDNF | 1.00 ± 0.03 | 1.01 ± 0.02 | 0.99 ± 0.05 | 0.98 ± 0.03 | 0.93 ± 0.03 | 0.96 ± 0.04 | |
| IL-10 | 1.18 ± 0.23 | 1.11 ± 0.21 | 1.57 ± 0.44 | 1.16 ± 0.24 | 0.65 ± 0.12 | 0.82 ± 0.15 | ||
| IL-1β | 1.03 ± 0.10 | 0.95 ± 0.10 | 0.86 ± 0.07 | 1.09 ± 0.12 | 1.00 ± 0.07 | 1.14 ± 0.21 | ||
| IL-6 | 1.02 ± 0.08 | 1.05 ± 0.13 | 1.33 ± 0.22 | 1.10 ± 0.24 | 0.93 ± 0.13 | 0.95 ± 0.10 | ||
| CD11b | 1.00 ± 0.02 | 1.00 ± 0.02 | 1.12 ± 0.04 | 0.95 ± 0.04 | 1.05 ± 0.04 | 1.04 ± 0.07 | ||
| CD45 | 1.00 ± 0.03 | 0.95 ± 0.05 | 0.92 ± 0.05 | 0.88 ± 0.03 | 0.95 ± 0.06 | 1.00 ± 0.07 | ||
| TNF-α | 1.11 ± 0.18 | 0.91 ± 0.21 | 1.12 ± 0.15 | 0.84 ± 0.12 | 1.13 ± 0.20 | 1.04 ± 0.20 | ||
| CA3 | BDNF | 1.01 ± 0.04 | 1.10 ± 0.04 | 1.06 ± 0.06 | 1.04 ± 0.04 | 1.06 ± 0.06 | 1.03 ± 0.04 | |
| IL-10 | 1.13 ± 0.20 | 0.92 ± 0.23 | 1.07 ± 0.22 | 0.82 ± 0.16 | 1.58 ± 0.67 | 0.96 ± 0.14 | ||
| IL-1β | 1.14 ± 0.19 | 1.28 ± 0.25 | 0.94 ± 0.14 | 1.28 ± 0.25 | 2.37 ± 0.46 | 1.46 ± 0.26 | Treatment effect (CBDV-100 > CBDV-20 and VEH) only in WT | |
| IL-6 | 1.06 ± 0.12 | 0.59 ± 0.08 | 1.07 ± 0.18 | 1.19 ± 0.18 | 0.91 ± 0.22 | 0.90 ± 0.10 | ||
| CD11b | 1.01 ± 0.06 | 0.92 ± 0.05 | 1.05 ± 0.10 | 0.97 ± 0.04 | 0.89 ± 0.04 | 0.99 ± 0.06 | ||
| CD45 | 1.03 ± 0.09 | 0.92 ± 0.07 | 0.87 ± 0.06 | 1.07 ± 0.07 | 1.14 ± 0.08 | 1.26 ± 0.06 | Overall treatment effect (CBDV-100 > CBDV-20 and VEH) | |
| TNF-α | 1.06 ± 0.13 | 0.41 ± 0.04 | 1.03 ± 0.26 | 0.69 ± 0.16 | 0.86 ± 0.19 | 0.76 ± 0.09 | Overall genotype effect (KO < WT) | |
| DG | BDNF | 1.00 ± 0.03 | 1.24 ± 0.08 | 1.15 ± 0.06 | 1.11 ± 0.06 | 1.14 ± 0.04 | 1.18 ± 0.02 | Genotype effect (KO > WT) only in VEH |
| IL-10 | 1.10 ± 0.18 | 1.05 ± 0.19 | 1.03 ± 0.30 | 1.00 ± 0.19 | 0.82 ± 0.15 | 0.74 ± 0.14 | ||
| IL-1β | 1.03 ± 0.10 | 1.22 ± 0.22 | 1.53 ± 0.23 | 1.01 ± 0.06 | 1.45 ± 0.35 | 0.72 ± 0.06 | ||
| IL-6 | 1.17 ± 0.24 | 1.19 ± 0.12 | 1.30 ± 0.28 | 1.16 ± 0.13 | 1.14 ± 0.16 | 0.94 ± 0.08 | ||
| CD11b | 1.01 ± 0.06 | 1.04 ± 0.02 | 1.10 ± 0.05 | 1.02 ± 0.04 | 1.23 ± 0.10 | 1.06 ± 0.05 | ||
| CD45 | 1.02 ± 0.08 | 1.19 ± 0.09 | 1.14 ± 0.06 | 0.93 ± 0.09 | 1.23 ± 0.14 | 1.08 ± 0.07 | ||
| TNF-α | 1.06 ± 0.13 | 1.04 ± 0.19 | 0.92 ± 0.19 | 0.55 ± 0.08 | 0.72 ± 0.11 | 0.78 ± 0.21 | ||
| PFC | BDNF | 1.01 ± 0.04 | 0.95 ± 0.07 | 0.94 ± 0.10 | 0.91 ± 0.09 | 0.95 ± 0.06 | 0.92 ± 0.06 | |
| IL-10 | 0.93 ± 0.25 | 0.57 ± 0.04 | 1.44 ± 0.37 | 0.92 ± 0.18 | 0.64 ± 0.09 | 1.44 ± 0.39 | ||
| IL-1β | 1.05 ± 0.09 | 0.96 ± 0.13 | 1.20 ± 0.16 | 0.98 ± 0.09 | 0.86 ± 0.11 | 0.99 ± 0.08 | ||
| IL-6 | 0.83 ± 0.11 | 0.95 ± 0.20 | 1.33 ± 0.24 | 1.06 ± 0.11 | 0.90 ± 0.09 | 1.14 ± 0.14 | ||
| CD11b | 1.01 ± 0.06 | 0.98 ± 0.11 | 0.97 ± 0.06 | 0.91 ± 0.05 | 0.93 ± 0.07 | 1.03 ± 0.04 | ||
| CD45 | 0.55 ± 0.21 | 1.06 ± 0.11 | 0.95 ± 0.15 | 0.80 ± 0.03 | 1.05 ± 0.07 | 1.01 ± 0.08 | ||
| TNF-α | 0.56 ± 0.21 | 0.54 ± 0.13 | 0.68 ± 0.23 | 0.60 ± 0.12 | 0.59 ± 0.05 | 0.90 ± 0.16 | ||
| Brain Region | Brain Marker | CHRONIC TREATMENT AT WEANING | ||||||
|---|---|---|---|---|---|---|---|---|
| VEH | CBDV-20 | CBDV-100 | Significant Differences | |||||
| WT | KO | WT | KO | WT | KO | |||
| CA1 | BDNF | 1.00 ± 0.04 | 1.02 ± 0.03 | 1.13 ± 0.06 | 1.15 ± 0.06 | 0.90 ± 0.05 | 1.12 ± 0.09 | |
| IL-10 | 1.96 ± 0.77 | 1.28 ± 0.52 | 0.55 ± 0.25 | 2.54 ± 1.08 | 2.36 ± 1.22 | 0.78 ± 0.41 | ||
| IL-1β | 1.43 ± 0.40 | 1.11 ± 0.42 | 0.59 ± 0.19 | 1.73 ± 0.54 | 1.88 ± 0.75 | 0.63 ± 0.23 | ||
| IL-6 | 1.90 ± 0.65 | 1.17 ± 0.52 | 0.34 ± 0.12 | 2.06 ± 0.77 | 2.29 ± 1.21 | 0.76 ± 0.36 | ||
| CD11b | 1.01 ± 0.07 | 1.02 ± 0.05 | 1.00 ± 0.06 | 1.04 ± 0.07 | 0.84 ± 0.08 | 0.99 ± 0.08 | ||
| CD45 | 1.12 ± 0.22 | 1.09 ± 0.27 | 0.80 ± 0.16 | 1.28 ± 0.30 | 1.35 ± 0.38 | 0.79 ± 0.10 | ||
| TNF-α | 1.75 ± 0.69 | 1.17 ± 0.49 | 0.33 ± 0.11 | 1.93 ± 0.72 | 2.08 ± 1.07 | 1.23 ± 0.61 | ||
| CA3 | BDNF | 1.03 ± 0.11 | 0.96 ± 0.04 | 1.09 ± 0.06 | 1.09 ± 0.05 | 1.04 ± 0.07 | 0.94 ± 0.05 | |
| IL-10 | 0.87 ± 0.29 | 1.97 ± 0.76 | 4.57 ± 1.83 | 4.95 ± 2.04 | 6.54 ± 2.85 | 0.79 ± 0.19 | ||
| IL-1β | 1.02 ± 0.46 | 2.17 ± 0.76 | 2.74 ± 0.81 | 3.22 ± 1.30 | 3.53 ± 1.34 | 0.89 ± 0.11 | ||
| IL-6 | 2.59 ± 1.35 | 3.47 ± 1.17 | 4.14 ± 1.38 | 6.36 ± 2.27 | 4.90 ± 2.17 | 1.41 ± 0.38 | ||
| CD11b | 1.01 ± 0.05 | 0.99 ± 0.04 | 1.26 ± 0.03 | 1.24 ± 0.07 | 1.13 ± 0.04 | 1.11 ± 0.03 | Overall treatment effect (CBDV-100 and CBDV-20 > VEH) | |
| CD45 | 1.10 ± 0.21 | 1.14 ± 0.12 | 1.26 ± 0.15 | 1.51 ± 0.33 | 1.18 ± 0.22 | 0.80 ± 0.10 | ||
| TNF-α | 1.78 ± 0.80 | 1.74 ± 0.60 | 3.25 ± 1.12 | 3.12 ± 1.33 | 4.83 ± 2.26 | 0.59 ± 0.18 | ||
| DG | BDNF | 1.01 ± 0.07 | 1.14 ± 0.06 | 1.05 ± 0.05 | 1.11 ± 0.07 | 1.05 ± 0.04 | 1.04 ± 0.05 | |
| IL-10 | 0.77 ± 0.17 | 1.43 ± 0.21 | 2.42 ± 0.87 | 0.77 ± 0.15 | 1.19 ± 0.24 | 1.08 ± 0.19 | ||
| IL-1β | 1.03 ± 0.11 | 1.10 ± 0.14 | 1.31 ± 0.28 | 0.95 ± 0.10 | 1.25 ± 0.24 | 1.19 ± 0.25 | ||
| IL-6 | 1.11 ± 0.22 | 1.07 ± 0.19 | 1.71 ± 0.32 | 1.59 ± 0.30 | 1.97 ± 0.36 | 1.31 ± 0.23 | ||
| CD11b | 1.01 ± 0.07 | 0.97 ± 0.05 | 0.91 ± 0.06 | 0.95 ± 0.04 | 0.99 ± 0.04 | 1.13 ± 0.03 | Overall treatment effect (CBDV-100 > CBDV-20) | |
| CD45 | 1.07 ± 0.16 | 1.14 ± 0.12 | 1.06 ± 0.06 | 1.19 ± 0.09 | 1.56 ± 0.15 | 1.35 ± 0.13 | Overall treatment effect (CBDV100 > CBDV-20 and VEH) | |
| TNF-α | 0.82 ± 0.09 | 1.37 ± 0.13 | 1.20 ± 0.15 | 0.97 ± 0.21 | 1.56 ± 0.36 | 1.44 ± 0.47 | ||
| PFC | BDNF | 1.02 ± 0.08 | 1.00 ± 0.11 | 1.17 ± 0.11 | 1.26 ± 0.13 | 0.90 ± 0.07 | 0.92 ± 0.17 | Overall treatment effect (CBDV-100 < CBDV-20) |
| IL-10 | 1.36 ± 0.37 | 1.31 ± 0.35 | 1.45 ± 0.26 | 1.17 ± 0.46 | 1.34 ± 0.31 | 1.62 ± 0.75 | ||
| IL-1β | 1.05 ± 0.13 | 1.05 ± 0.21 | 1.07 ± 0.17 | 0.86 ± 0.12 | 1.09 ± 0.19 | 1.43 ± 0.29 | ||
| IL-6 | 1.47 ± 0.47 | 1.47 ± 0.38 | 0.71 ± 0.12 | 1.07 ± 0.25 | 1.05 ± 0.17 | 3.15 ± 1.23 | ||
| CD11b | 1.12 ± 0.05 | 0.90 ± 0.05 | 1.11 ± 0.06 | 1.06 ± 0.08 | 1.01 ± 0.06 | 1.05 ± 0.12 | ||
| CD45 | 1.23 ± 0.31 | 0.92 ± 0.14 | 1.22 ± 0.10 | 1.08 ± 0.11 | 1.44 ± 0.18 | 1.41 ± 0.25 | ||
| TNF-α | 1.39 ± 0.42 | 0.98 ± 0.22 | 0.70 ± 0.13 | 1.02 ± 0.36 | 1.38 ± 0.37 | 0.88 ± 0.09 | ||
| Behavioral Domain | Test | Variable Measured | ADULTS (Study 1) | JUVENILES (Study 2) | ||||
|---|---|---|---|---|---|---|---|---|
| Figures | KO Genotype Effect | CBDV Treatment Effect | Figures | KO Genotype Effect | CBDV Treatment Effect | |||
| Anxiety | EPM | % Time open arms | Figure S1 | none | none | Figure S2 | ↓ anxiety | none |
| OF | %Time center | Figure 2b | ↓ anxiety in CBDV-100 only | CBDV-100 induces a KO phenotype | Figure 5b | ↓ anxiety | none | |
| Locomotor activity | EPM | total arm entries | Figure S1 | none | none | Figure S3 | ↑ activity | none |
| OF | Total distance moved | Figure 2a | ↑ activity | none | Figure 5a | ↑ activity | none | |
| Learning & memory | OR | % OR index | Figure 2d | ↓ object memory | ↓ in WT mice, no rescue in KOs | Figure 5d | ↓ object memory | ↓ in WT mice, rescue in KOs |
| Social interest | 3-COMP | % sociability index (trial 2) | Figure 3d | none | none | Figure 6d | none | none |
| % social novelty index (trial 3) | Figure 3f | ↓ social novelty preference | ↓ in WT mice, no rescue in KOs | Figure 6f | ↓ social memory | ↓ in WT mice, rescue in KOs | ||
| SI | Affiliation time | Figure 4a | ↓ social interaction | ↓ in WT mice, no rescue in KOs | Figure 7a | ↓ social interaction | ↓ in WT mice, rescue in KOs | |
| Sensory responsiveness | ASR | startle body response | Figure 4b | ↑ acoustic startle | rescue in KOs only with CBDV-20 | Figure 7b | ↑ acoustic startle | rescue in KOs |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Premoli, M.; Fyke, W.; Bellocchio, L.; Lemaire, V.; Wolley-Roberts, M.; Bontempi, B.; Pietropaolo, S. Early Administration of the Phytocannabinoid Cannabidivarin Prevents the Neurobehavioral Abnormalities Associated with the Fmr1-KO Mouse Model of Fragile X Syndrome. Cells 2023, 12, 1927. https://doi.org/10.3390/cells12151927
Premoli M, Fyke W, Bellocchio L, Lemaire V, Wolley-Roberts M, Bontempi B, Pietropaolo S. Early Administration of the Phytocannabinoid Cannabidivarin Prevents the Neurobehavioral Abnormalities Associated with the Fmr1-KO Mouse Model of Fragile X Syndrome. Cells. 2023; 12(15):1927. https://doi.org/10.3390/cells12151927
Chicago/Turabian StylePremoli, Marika, William Fyke, Luigi Bellocchio, Valerie Lemaire, Marie Wolley-Roberts, Bruno Bontempi, and Susanna Pietropaolo. 2023. "Early Administration of the Phytocannabinoid Cannabidivarin Prevents the Neurobehavioral Abnormalities Associated with the Fmr1-KO Mouse Model of Fragile X Syndrome" Cells 12, no. 15: 1927. https://doi.org/10.3390/cells12151927
APA StylePremoli, M., Fyke, W., Bellocchio, L., Lemaire, V., Wolley-Roberts, M., Bontempi, B., & Pietropaolo, S. (2023). Early Administration of the Phytocannabinoid Cannabidivarin Prevents the Neurobehavioral Abnormalities Associated with the Fmr1-KO Mouse Model of Fragile X Syndrome. Cells, 12(15), 1927. https://doi.org/10.3390/cells12151927






