Human Macrophages Polarized by Interaction with Apoptotic Cells Produce Fibrosis-Associated Mediators and Enhance Pro-Fibrotic Activity of Dermal Fibroblasts In Vitro
Abstract
:1. Introduction
2. Materials and Methods
3. Results
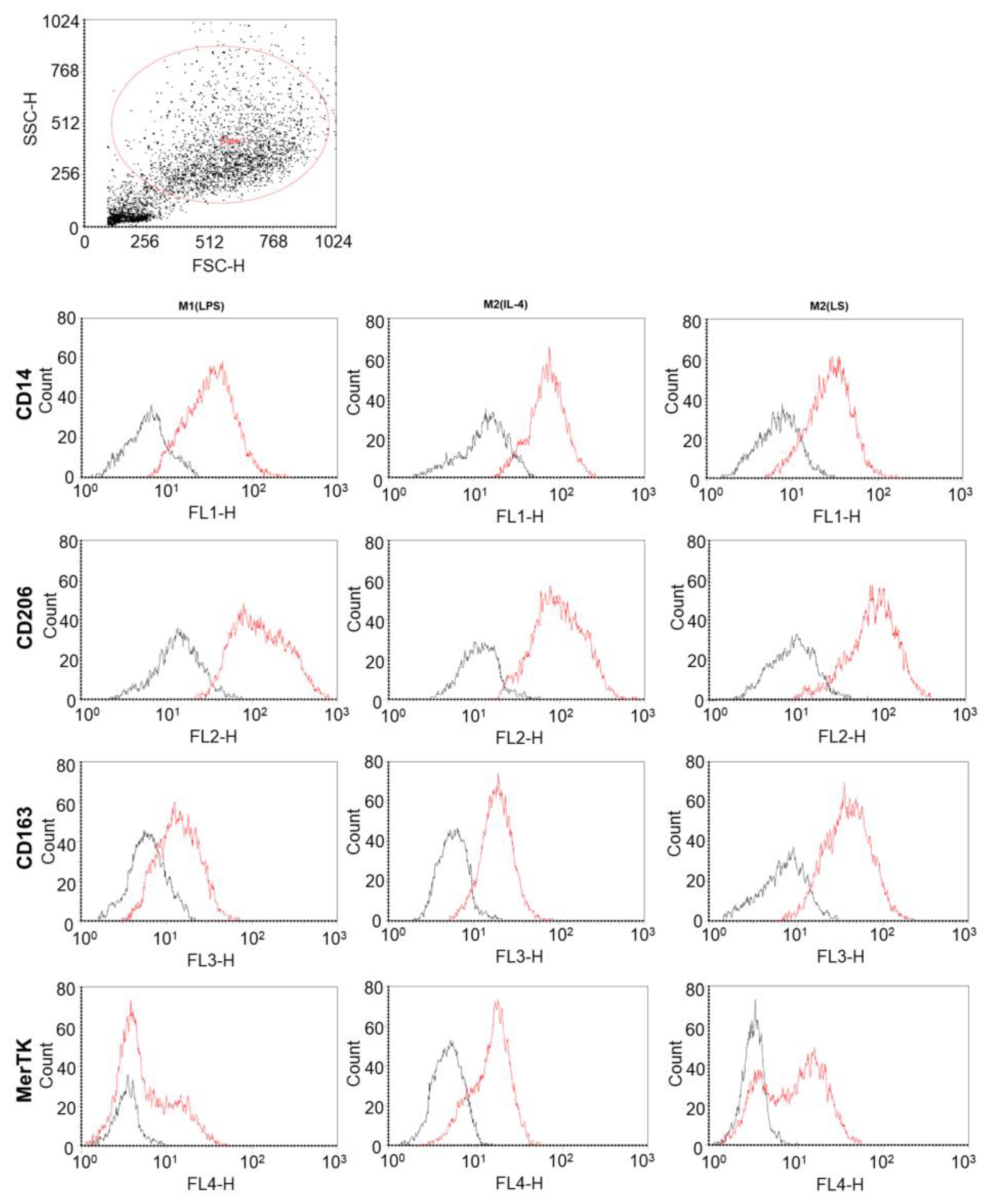
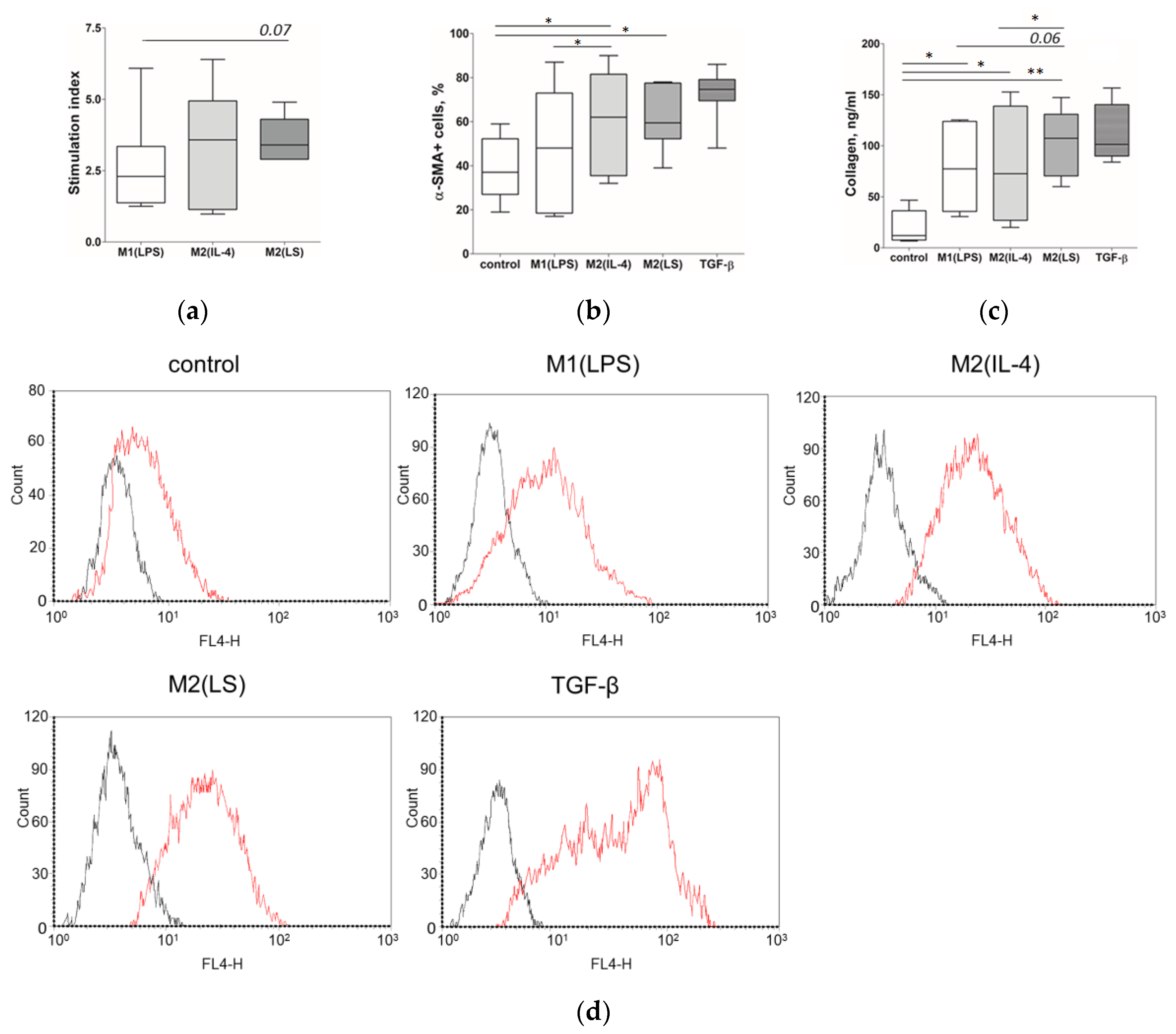
3.1. Characteristic of Polarized Macrophages
3.2. Characteristic of Polarized Macrophages
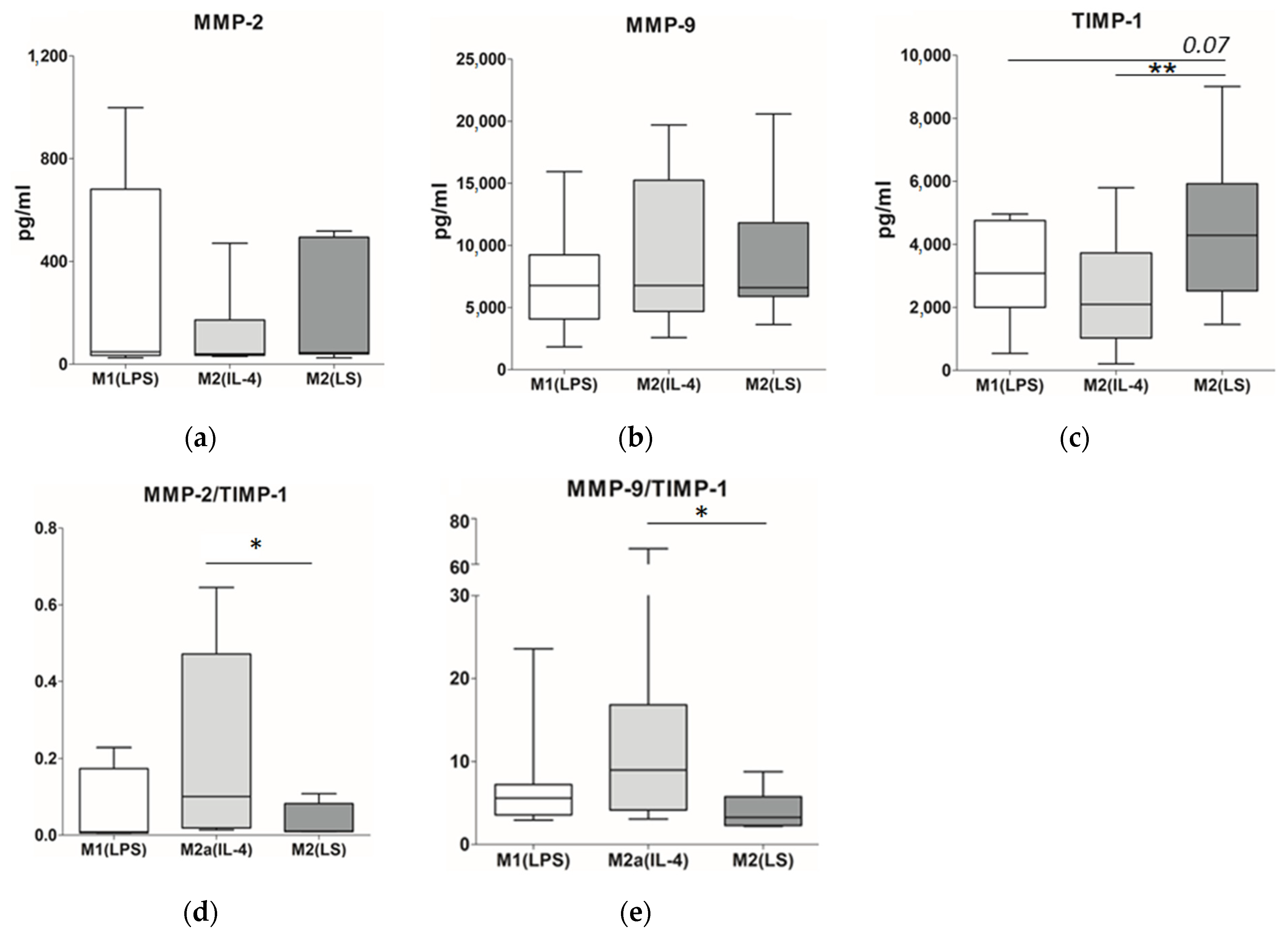
3.3. MMP and TIMP Production
3.4. Production of TGF-β, VEGF and Angiogenin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Wynn, T.A.; Barron, L. Macrophages: Master regulators of inflammation and fibrosis. Semin. Liver Dis. 2010, 30, 245–257. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mescher, A.L. Macrophages and fibroblasts during inflammation and tissue repair in models of organ regeneration. Regeneration 2017, 4, 39–53. [Google Scholar] [CrossRef] [PubMed]
- Krafts, K.P. Tissue repair: The hidden drama. Organogenesis 2010, 6, 225–233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Darby, I.A.; Zakuan, N.; Billet, F.; Desmoulière, A. The myofibroblast, a key cell in normal and pathological tissue repair. Cell. Mol. Life Sci. 2016, 73, 1145–1157. [Google Scholar] [CrossRef]
- Wynn, T.A.; Vannella, K.M. Macrophages in tissue repair, regeneration, and fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Adhyatmika, A.; Putri, K.S.; Beljaars, L.; Melgert, B.N. The Elusive Antifibrotic Macrophage. Front. Med. 2015, 2, 81. [Google Scholar] [CrossRef] [Green Version]
- Pakshir, P.; Hinz, B. The big five in fibrosis: Macrophages, myofibroblasts, matrix, mechanics, and miscommunication. Matrix Biol. 2018, 68–69, 81–93. [Google Scholar] [CrossRef]
- Webster, N.L.; Crowe, S.M. Matrix metalloproteinases, their production by monocytes and macrophages and their potential role in HIV-related diseases. J. Leukoc. Biol. 2006, 80, 1052–1066. [Google Scholar] [CrossRef]
- Madsen, D.H.; Leonard, D.; Masedunskas, A.; Moyer, A.; Jürgensen, H.J.; Peters, D.E.; Amornphimoltham, P.; Selvaraj, A.; Yamada, S.S.; Brenner, D.A.; et al. M2-like macrophages are responsible for collagen degradation through a mannose receptor-mediated pathway. J. Cell Biol. 2013, 202, 951–966. [Google Scholar] [CrossRef]
- Sprangers, S.; Behrendt, N.; Engelholm, L.; Cao, Y.; Everts, V. Phagocytosis of Collagen Fibrils by Fibroblasts In Vivo Is Independent of the uPARAP/Endo180 Receptor. J. Cell. Biochem. 2017, 118, 1590–1595. [Google Scholar] [CrossRef]
- Locati, M.; Curtale, G.; Mantovani, A. Diversity, Mechanisms, and Significance of Macrophage Plasticity. Annu. Rev. Pathol. 2020, 15, 123–147. [Google Scholar] [CrossRef] [Green Version]
- Orekhov, A.N.; Orekhova, V.A.; Nikiforov, N.G.; Myasoedova, V.A.; Grechko, A.V.; Romanenko, E.B.; Zhang, D.; Chistiakov, D.A. Monocyte differentiation and macrophage polarization. Vessel Plus 2019, 3, 10. [Google Scholar] [CrossRef]
- Martinez, F.O.; Sica, A.; Mantovani, A.; Locati, M. Macrophage activation and polarization. Front. Biosci. 2008, 13, 453–461. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Yao, Y.; Xu, X.H.; Jin, L. Macrophage Polarization in Physiological and Pathological Pregnancy. Front. Immunol. 2019, 10, 792. [Google Scholar] [CrossRef]
- Rai, N.K.; Tripathi, K.; Sharma, D.; Shukla, V.K. Apoptosis: A basic physiologic process in wound healing. Int. J. Low Extrem. Wounds 2005, 4, 138–144. [Google Scholar] [CrossRef]
- Wu, Y.S.; Chen, S.N. Apoptotic cell: Linkage of inflammation and wound healing. Front. Pharmacol. 2014, 5, 1. [Google Scholar] [CrossRef] [Green Version]
- Johnson, A.; DiPietro, L.A. Apoptosis and angiogenesis: An evolving mechanism for fibrosis. FASEB J. 2013, 27, 3893–3901. [Google Scholar] [CrossRef] [Green Version]
- Sindrilaru, A.; Peters, T.; Schymeinsky, J.; Oreshkova, T.; Wang, H.L.; Gompf, A.; Mannella, F.; Wlaschek, M.; Sunderkötter, C.; Rudolph, K.L.; et al. Wound healing defect of Vav3(-/-) mice due to impaired beta(2)-integrin-dependent macrophage phagocytosis of apoptotic neutrophils. Blood 2009, 113, 5266–5276. [Google Scholar] [CrossRef] [Green Version]
- Chernykh, E.R.; Shevela, E.Y.; Sakhno, L.V.; Tikhonova, M.A.; Petrovsky, Y.L.; Ostanin, A.A. The generation and properties of human M2-like macrophages: Potential candidates for CNS repair? Cell. Ther. Transpl. 2010, 2, 1–8. [Google Scholar]
- Yankovskaya, A.A.; Shevela, E.Y.; Sakhno, L.V.; Tikhonova, M.A.; Dome, A.S.; Ostanin, A.A.; Chernykh, E.R. Allostimulatory activity as a criterion of the functional phenotype of human macrophages. Hum. Immunol. 2019, 80, 890–896. [Google Scholar] [CrossRef] [PubMed]
- Sakhno, L.V.; Shevela, E.Y.; Tikhonova, M.A.; Ostanin, A.A.; Chernykh, E.R. The Phenotypic and Functional Features of Human M2 Macrophages Generated Under Low Serum Conditions. Scand. J. Immunol. 2016, 83, 151–159. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fadok, V.A.; Bratton, D.L.; Konowal, A.; Freed, P.W.; Westcott, J.Y.; Henson, P.M. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-beta, PGE2, and PAF. J. Clin. Investig. 1998, 101, 890–898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferracini, M.; Rios, F.J.; Pecenin, M.; Jancar, S. Clearance of apoptotic cells by macrophages induces regulatory phenotype and involves stimulation of CD36 and platelet-activating factor receptor. Mediat. Inflamm. 2013, 2013, 950273. [Google Scholar] [CrossRef] [Green Version]
- Tassiulas, I.; Park-Min, K.H.; Hu, Y.; Kellerman, L.; Mevorach, D.; Ivashkiv, L.B. Apoptotic cells inhibit LPS-induced cytokine and chemokine production and IFN responses in macrophages. Hum. Immunol. 2007, 68, 156–164. [Google Scholar] [CrossRef] [Green Version]
- Elliott, M.R.; Koster, K.M.; Murphy, P.S. Efferocytosis signaling in the regulation of macrophage inflammatory responses. J. Immunol. 2017, 198, 1387–1394. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.-H.; Chun, T. Anti-inflammatory role of TAM family of receptor tyrosine kinases via modulating macrophage function. Mol. Cells 2019, 42, 1–7. [Google Scholar]
- Haskó, G.; Pacher, P. Regulation of macrophage function by adenosine. Arterioscler. Thromb. Vasc. Biol. 2012, 32, 865–869. [Google Scholar] [CrossRef] [Green Version]
- Han, J.; Bae, J.; Choi, C.-Y.; Choi, S.-P.; Kang, H.-S.; Jo, E.-K.; Park, J.; Lee, Y.S.; Moon, H.S.; Park, C.G.; et al. Autophagy induced by AXL receptor tyrosine kinase alleviates acute liver injury via inhibition of NLRP3 inflammasome activation in mice. Autophagy 2016, 12, 2326–2343. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Chen, Z.; Chen, K.; Ma, D.; Chen, L.; DiPietro, L.A. Phagocytosis of apoptotic endothelial cells reprograms macrophages in skin wounds. J. Immunol. Regen. Med. 2021, 12, 100038. [Google Scholar] [CrossRef]
- Kurosaka, K.; Watanabe, N.; Kobayashi, Y. Production of proinflammatory cytokines by phorbol myristate acetate-treated THP-1 Cells and monocyte-derived macrophages after phagocytosis of apoptotic CTLL-2 cells. J. Immunol. 1998, 161, 6245–6249. [Google Scholar] [CrossRef]
- Kawagishi, C.; Kurosaka, K.; Watanabe, N.; Kobayashi, Y. Cytokine production by macrophages in association with phagocytosis of etoposide-treated P388 cells in vitro and in vivo. Biochim. Biophys. Acta 2001, 1541, 221–230. [Google Scholar] [CrossRef] [Green Version]
- Song, E.; Ouyang, N.; Hörbelt, M.; Antus, B.; Wang, M.; Exton, M.S. Influence of alternatively and classically activated macrophages on fibrogenic activities of human fibroblasts. Cell. Immunol. 2000, 204, 19–28. [Google Scholar] [CrossRef]
- Ploeger, D.T.; Hosper, N.A.; Schipper, M.; Koerts, J.A.; de Rond, S.; Bank, R.A. Cell plasticity in wound healing: Paracrine factors of M1/ M2 polarized macrophages influence the phenotypical state of dermal fibroblasts. Cell Commun. Signal. 2013, 11, 29. [Google Scholar] [CrossRef] [Green Version]
- Glim, J.E.; Niessen, F.B.; Everts, V.; van Egmond, M.; Beelen, R.H. Platelet derived growth factor-CC secreted by M2 macrophages induces alpha-smooth muscle actin expression by dermal and gingival fibroblasts. Immunobiology 2013, 218, 924–929. [Google Scholar] [CrossRef]
- Nacu, N.; Luzina, I.G.; Highsmith, K.; Lockatell, V.; Pochetuhen, K.; Cooper, Z.A.; Gillmeister, M.P.; Todd, N.W.; Atamas, S.P. Macrophages produce TGF-beta-induced (beta-ig-h3) following ingestion of apoptotic cells and regulate MMP14 levels and collagen turnover in fibroblasts. J. Immunol. 2008, 180, 5036–5044. [Google Scholar] [CrossRef]
- Kim, Y.B.; Yoon, Y.S.; Choi, Y.H.; Park, E.M.; Kang, J.L. Interaction of macrophages with apoptotic cells inhibits transdifferentiation and invasion of lung fibroblasts. Oncotarget 2017, 8, 112297–112312. [Google Scholar] [CrossRef] [Green Version]
- Krzyszczyk, P.; Schloss, R.; Palmer, A.; Berthiaume, F. The Role of Macrophages in Acute and Chronic Wound Healing and Interventions to Promote Pro-wound Healing Phenotypes. Front. Physiol. 2018, 9, 419. [Google Scholar] [CrossRef]
- Borthwick, L.A.; Wynn, T.A.; Fisher, A.J. Cytokine mediated tissue fibrosis. Biochim. Biophys. Acta 2013, 1832, 1049–1060. [Google Scholar] [CrossRef] [Green Version]
- Newby, A.C. Metalloproteinase production from macrophages—A perfect storm leading to atherosclerotic plaque rupture and myocardial infarction. Exp. Physiol. 2016, 101, 1327–1337. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, W.C.; Sala-Newby, G.B.; Susana, A.; Johnson, J.L.; Newby, A.C. Classical macrophage activation up-regulates several matrix metalloproteinases through mitogen activated protein kinases and nuclear factor-κB. PLoS ONE 2012, 7, e42507. [Google Scholar] [CrossRef] [Green Version]
- Hanania, R.; Sun, H.S.; Xu, K.; Pustylnik, S.; Jeganathan, S.; Harrison, R.E. Classically activated macrophages use stable microtubules for matrix metalloproteinase-9 (MMP-9) secretion. J. Biol. Chem. 2012, 287, 8468–8483. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spiller, K.L.; Anfang, R.R.; Spiller, K.J.; Ng, J.; Nakazawa, K.R.; Daulton, J.W.; Vunjak-Novakovic, G. The role of macrophage phenotype in vascularization of tissue engineering scaffolds. Biomaterials 2014, 35, 4477–4488. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Y.; Liu, S.; Zhang, S.; Cai, G.; Jiang, H.; Su, H.; Li, X.; Hong, Q.; Zhang, X.; Chen, X. Tissue inhibitor of metalloproteinase-1 promotes NIH3T3 fibroblast proliferation by activating p-Akt and cell cycle progression. Mol. Cells 2011, 31, 225–230. [Google Scholar] [CrossRef] [Green Version]
- Sa, Y.; Li, C.; Li, H.; Guo, H. TIMP-1 Induces α-Smooth Muscle Actin in Fibroblasts to Promote Urethral Scar Formation. Cell. Physiol. Biochem. 2015, 35, 2233–2243. [Google Scholar] [CrossRef]
- Lao, G.; Ren, M.; Wang, X.; Zhang, J.; Huang, Y.; Liu, D.; Luo, H.; Yang, C.; Yan, L. Human tissue inhibitor of metalloproteinases-1 improved wound healing in diabetes through its anti-apoptotic effect. Exp. Dermatol. 2019, 28, 528–535. [Google Scholar] [CrossRef]
- Freire-de-Lima, C.G.; Xiao, Y.Q.; Gardai, S.J.; Bratton, D.L.; Schiemann, W.P.; Henson, P.M. Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J. Biol. Chem. 2006, 281, 38376–38384. [Google Scholar] [CrossRef] [Green Version]
- Meng, X.; Nikolic-Paterson, D.; Lan, H. TGF-β: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Budi, E.H.; Schaub, J.R.; Decaris, M.; Turner, S.; Derynck, R. TGF-β as a driver of fibrosis: Physiological roles and therapeutic opportunities. J. Pathol. 2021, 254, 358–373. [Google Scholar] [CrossRef]
- Chung, C.C.; Lin, Y.K.; Chen, Y.C.; Kao, Y.H.; Lee, T.I.; Chen, Y.J. Vascular endothelial growth factor enhances profibrotic activities through modulation of calcium homeostasis in human atrial fibroblasts. Lab. Investig. 2020, 100, 285–296. [Google Scholar] [CrossRef]
- Larsson-Callerfelt, A.; Sjöland, A.A.; Hallgren, O.; Bagher, M.; Thiman, L.; Löfdahl, C.; Bjermer, L.H.; Westergren-Thorsson, G. VEGF induces ECM synthesis and fibroblast activity in human lung fibroblasts. Eur. Respir. J. 2017, 50, PA1045. [Google Scholar]
- Lai, Y.S.; Wahyuningtyas, R.; Aui, S.P.; Chang, K.T. Autocrine VEGF signalling on M2 macrophages regulates PD-L1 expression for immunomodulation of T cells. J. Cell. Mol. Med. 2019, 23, 1257–1267. [Google Scholar]
- Golpon, H.A.; Fadok, V.A.; Taraseviciene-Stewart, L.; Scerbavicius, R.; Sauer, C.; Welte, T.; Henson, P.M.; Voelkel, N.F. Life after corpse engulfment: Phagocytosis of apoptotic cells leads to VEGF secretion and cell growth. FASEB J. 2004, 18, 1716–1718. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maksimova, A.; Shevela, E.; Sakhno, L.; Tikhonova, M.; Ostanin, A.; Chernykh, E. Human Macrophages Polarized by Interaction with Apoptotic Cells Produce Fibrosis-Associated Mediators and Enhance Pro-Fibrotic Activity of Dermal Fibroblasts In Vitro. Cells 2023, 12, 1928. https://doi.org/10.3390/cells12151928
Maksimova A, Shevela E, Sakhno L, Tikhonova M, Ostanin A, Chernykh E. Human Macrophages Polarized by Interaction with Apoptotic Cells Produce Fibrosis-Associated Mediators and Enhance Pro-Fibrotic Activity of Dermal Fibroblasts In Vitro. Cells. 2023; 12(15):1928. https://doi.org/10.3390/cells12151928
Chicago/Turabian StyleMaksimova, Aleksandra, Ekaterina Shevela, Lyudmila Sakhno, Marina Tikhonova, Aleksandr Ostanin, and Elena Chernykh. 2023. "Human Macrophages Polarized by Interaction with Apoptotic Cells Produce Fibrosis-Associated Mediators and Enhance Pro-Fibrotic Activity of Dermal Fibroblasts In Vitro" Cells 12, no. 15: 1928. https://doi.org/10.3390/cells12151928
APA StyleMaksimova, A., Shevela, E., Sakhno, L., Tikhonova, M., Ostanin, A., & Chernykh, E. (2023). Human Macrophages Polarized by Interaction with Apoptotic Cells Produce Fibrosis-Associated Mediators and Enhance Pro-Fibrotic Activity of Dermal Fibroblasts In Vitro. Cells, 12(15), 1928. https://doi.org/10.3390/cells12151928







