Anti-Tumor Effect of Turandot Proteins Induced via the JAK/STAT Pathway in the mxc Hematopoietic Tumor Mutant in Drosophila
Abstract
:1. Introduction
2. Materials and Methods
2.1. Drosophila Stocks and Husbandry
2.2. LG Preparation
2.3. Quantitative Real-Time PCR (qRT-PCR) Analysis
2.4. Immunostaining of the LGs
2.5. Preparation and Immunostaining of Hemocytes
2.6. Apoptosis Assay
2.7. Statistical Analysis
3. Results
3.1. The Hemolymph of the Mutant Larvae Contained Some Hemocytes with Ectopic Expression of Upd3
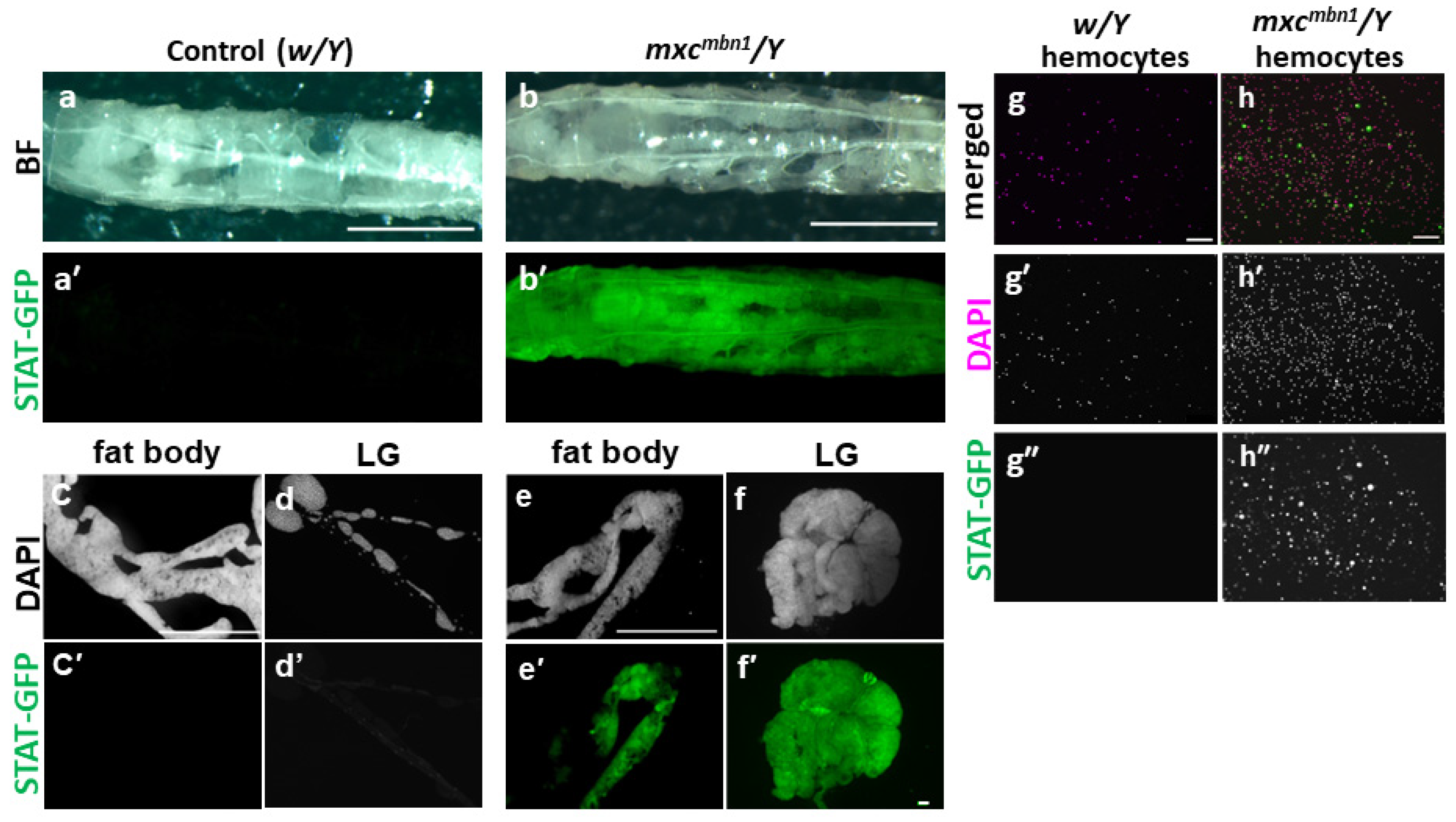
3.2. Hyperactivation of the JAK/STAT Pathway in mxcmbn1 Mutant Larvae
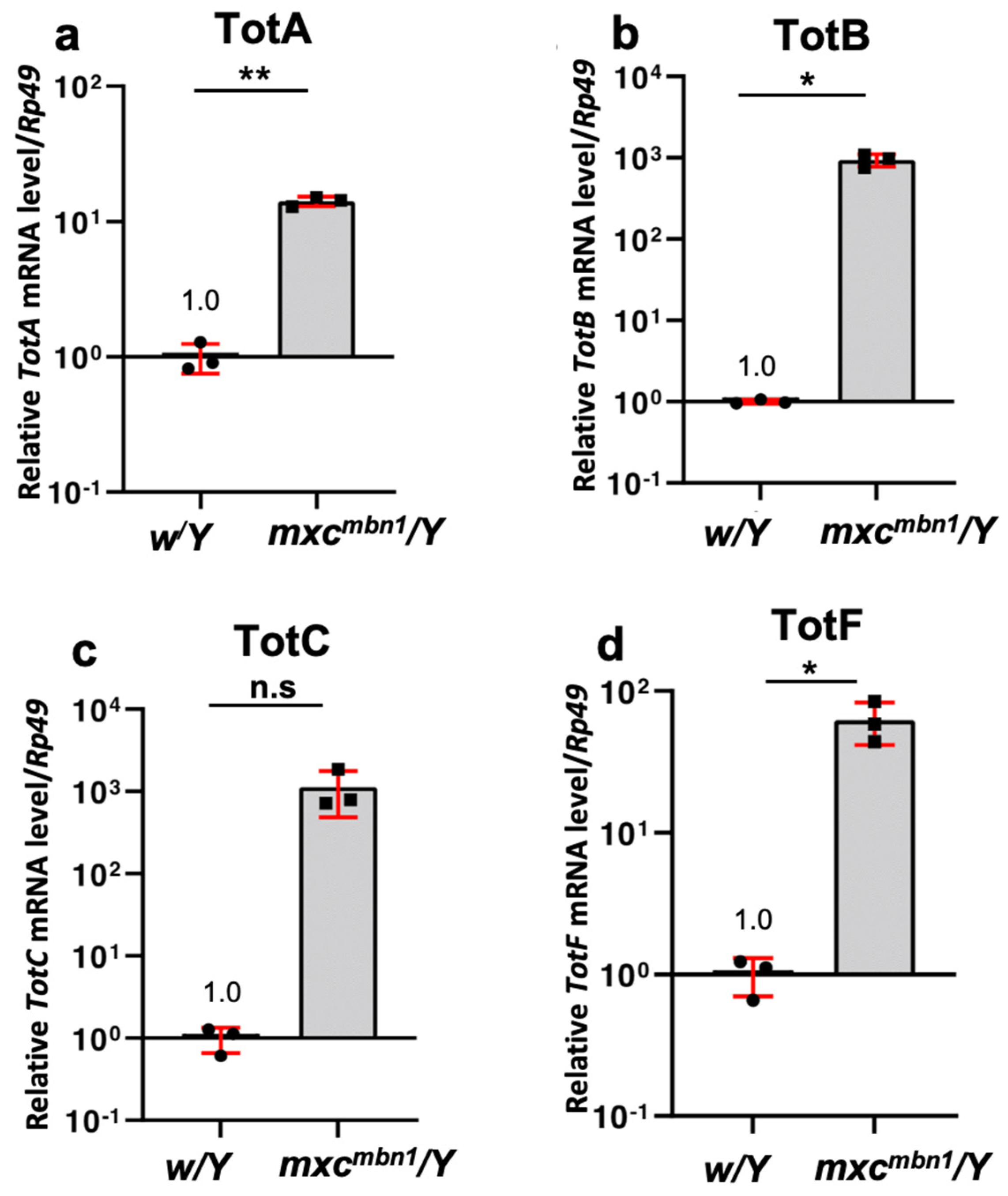
3.3. Remarkably Increased mRNA Levels of Turandot (Tot) Genes in mxcmbn1 Mutant Larvae
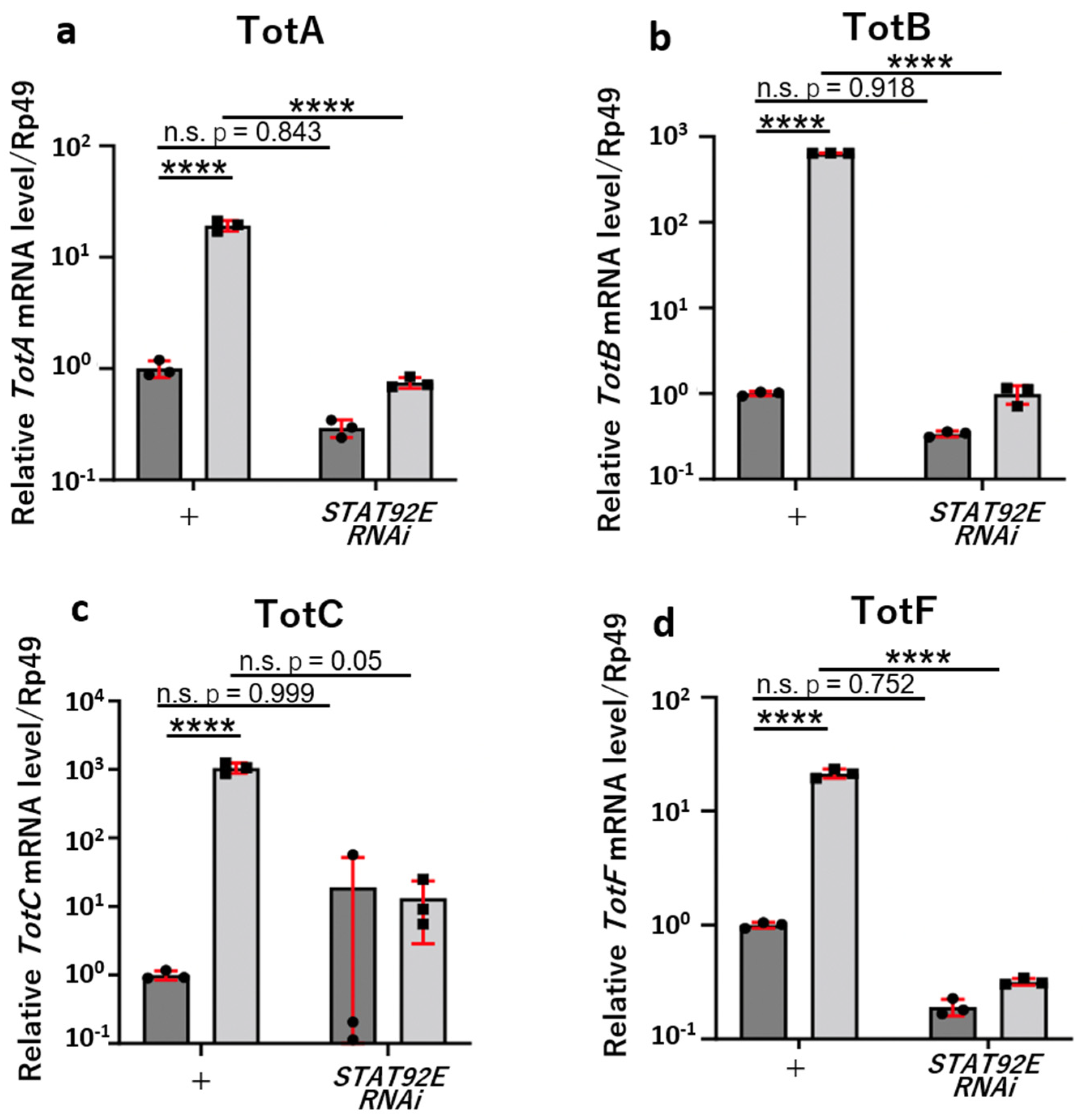
3.4. JAK/STAT-Dependent Induction of Gene Expression of Four Tot Genes in the Fat Body of mxcmbn1 Larvae
3.5. Silencing TotA, TotB, or TotF Gene by Expressing dsRNAs against the Relevant mRNAs in the Fat Body Enhanced LG Hyperplasia in mxcmbn1 Larvae
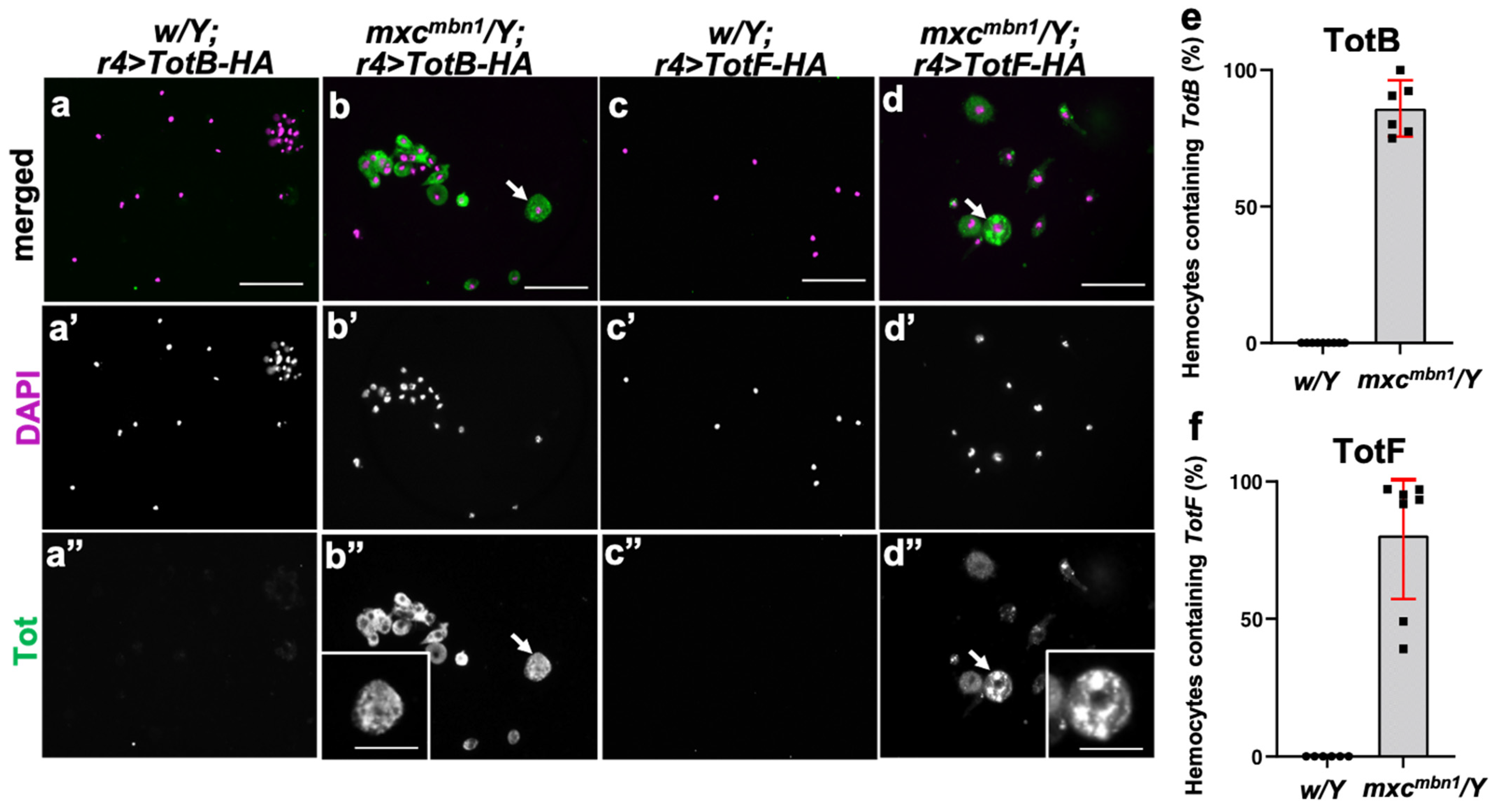
3.6. Intake of the Tot B and F Proteins Produced in the Fat Body into the Circulating Hemocytes in mxcmbn1 Larvae
3.7. More Hemocytes Containing TotF Proteins Were Associated with the LG Tumors in mxcmbn1 Larvae
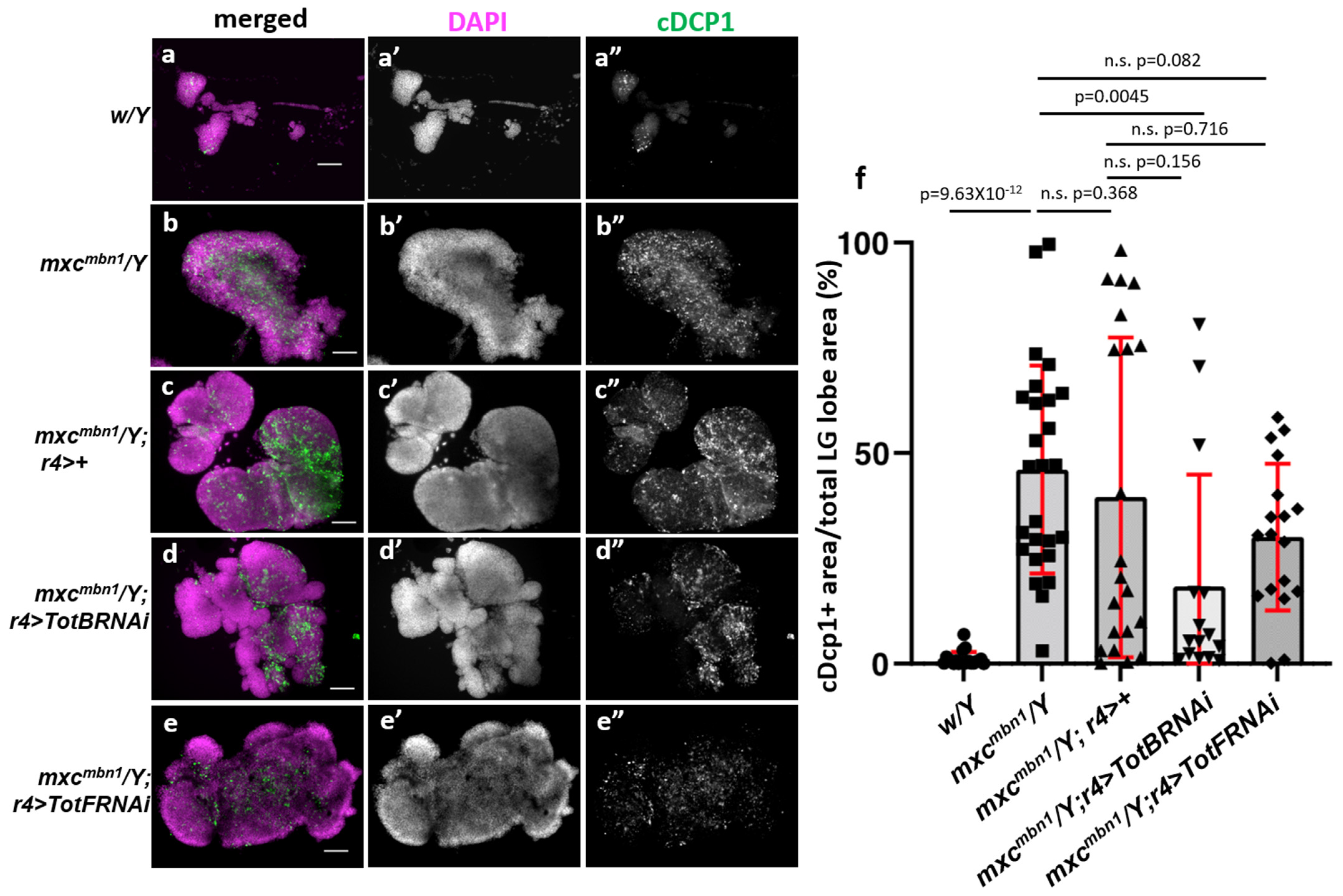
3.8. Fat-Body-Specific Depletion of Tot Genes Enhanced Apoptosis in mxcmbn1 LG Tumor
3.9. RNAi-Based Silencing of Tot Genes Also Increased Mitotic Cells in the LGs of mxcmbn1 Larvae
4. Discussion
4.1. Activation of the JAK/STAT Pathway by Ectopic Expression of Upd3 from Macrophage-like Cells and Subsequent Induction of Tot Proteins in the Fat Body
4.2. Tot Proteins Possess Antitumor Property That Induced Apoptosis in the Hematopoietic Tissue Tumors in a Drosophila Model of Hematopoietic Tumor
4.3. Another Antitumor Effect of the Tot Proteins due to the Suppression of LG Tumor Cell Proliferation
4.4. Possible Origin of Bimodal Distribution of Apoptosis and Mitotic Cells in LGs of mxcmbn1 and the Mutant Larvae Harboring Fat-Body-Specific Tot Depletion
4.5. Contribution of the Innate Immune System to Suppression of Tumor Growth in Drosophila
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hoffmann, J.A. The immune response of Drosophila. Nature 2003, 426, 33–38. [Google Scholar] [CrossRef]
- Buchon, N.; Silverman, N.; Cherry, S. Immunity in Drosophila melanogaster--from microbial recognition to whole-organism physiology. Nat. Rev. Immunol. 2014, 14, 796–810. [Google Scholar] [CrossRef] [PubMed]
- Hoffmann, J.A.; Reichhart, J.-M. Drosophila innate immunity: An evolutionary perspective. Nat. Immunol. 2002, 3, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Tzou, P.; De Gregorio, E.; Lemaitre, B. How Drosophila combats microbial infection: A model to study innate immunity and host-pathogen interactions. Curr. Opin. Microbiol. 2002, 5, 102–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- Leulier, F.; Rodriguez, A.; Khush, R.S.; Abrams, J.M.; Lemaitre, B. Drosophila caspase Dredd is required for resistance to gram-negative bacterial infections. EMBO Rep. 2000, 1, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Araki, M.; Kurihara, M.; Kinoshita, S.; Awane, R.; Sato, T.; Ohkawa, Y.; Inoue, Y.H. Anti-tumor effects of antimicrobial peptides, components of the innate immune system, against hematopoietic tumours in Drosophila mxc mutants. Dis. Models Mech. 2019, 12, dmm037721. [Google Scholar] [CrossRef] [Green Version]
- Parvy, J.-P.; Yu, Y.; Dostalova, A.; Kondo, S.; Kurjan, A.; Bulet, P.; Lemaître, B.; Vidal, M.; Cordero, J.B. The antimicrobial peptide defensin cooperates with the tumor necrosis factor to drive tumor cell death in Drosophila. ELife 2019, 8, e45061. [Google Scholar] [CrossRef]
- Dostert, C.; Jouanguy, E.; Irving, P.; Troxler, L.; Galiana-Arnoux, D.; Hetru, C.; Hoffmann, J.A.; Imler, J.L. The Jak-STAT signaling pathway is required but not sufficient for the antiviral response of Drosophila. Nat. Immunol. 2005, 6, 946–953. [Google Scholar] [CrossRef]
- Arbouzova, N.I.; Zeidler, M.P. JAK/STAT signalling in Drosophila: Insights into conserved regulatory and cellular functions. Development 2006, 133, 2605–2616. [Google Scholar] [CrossRef] [Green Version]
- Kemp, C.; Mueller, S.; Goto, A.; Barbier, V.; Paro, S.; Bonnay, F.; Dostert, C.; Troxler, L.; Hetru, C.; Meignin, C.; et al. Broad RNA mediated antiviral immunity and virus-specific inducible responses in Drosophila. J. Immunol. 2013, 190, 650–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houtz, P.; Bonfini, A.; Liu, X.; Revah, J.; Guillou, A.; Poidevin, M.; Hens, K.; Huang, H.Y.; Deplancke, B.; Tsai, Y.C.; et al. Hippo, TGF-β, and Src-MAPK pathways regulate transcription of the upd3 cytokine in Drosophila enterocytes upon bacterial infection. PLoS Genet. 2017, 13, e1007091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pastor-Pareja, J.C.; Wu, M.; Xu, T. The innate immune response of blood cells to tumors and tissue damage in Drosophila. Dis. Models Mech. 2008, 1, 144–154. [Google Scholar] [CrossRef] [Green Version]
- Agaisse, H.; Petersen, U.-M.; Boutros, M.; Mathey-Prevot, B.; Perrimon, N. Signaling role of hemocytes in Drosophila JAK/STAT-dependent response to septic injury. Dev. Cell 2003, 5, 441–450. [Google Scholar] [CrossRef] [Green Version]
- Agaisse, H.; Perrimon, N. The roles of JAK/STAT signaling in Drosophila immune responses. Immunol. Rev. 2004, 198, 72–82. [Google Scholar] [CrossRef]
- Ekengren, S.; Tryselius, Y.; Dushay, M.S.; Liu, G.; Steiner, H.; Hultmark, D. A humoral stress response in Drosophila. Curr. Biol. 2001, 11, 714–718. [Google Scholar] [CrossRef] [Green Version]
- Myllymäki, H.; Valanne, S.; Rämet, M. Drosophila Imd signaling pathways. J. Immunol. 2014, 192, 3455–3462. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parisi, F.; Stefanatos, R.K.; Strathdee, K.; Yu, Y.; Vidal, M. Transformed epithelia trigger non-tissue-autonomous tumor suppressor response by adipocytes via activation of Toll and Eiger/TNF signaling. Cell Rep. 2014, 6, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Shrestha, R.; Gateff, E. Ultrastructure and cytochemistry of cell types in larval hematopoietic organs and hemolymph of Drosophila melanogaster. Dev. Growth Diff. 1982, 24, 65–82. [Google Scholar] [CrossRef]
- Remillieux-Leschelle, N.; Santamaria, P.; Randsholt, N.B. Regulation of larval hematopoiesis in Drosophila melanogaster: A role for the multi-sex comb gene. Genetics 2002, 162, 1259–1274. [Google Scholar] [CrossRef]
- Kurihara, M.; Komatsu, K.; Awane, R.; Inoue, Y.H. Loss of Histone Locus Bodies in mature hemocytes of larval lymph glands results in tissue hyperplasia in mxc mutants of Drosophila. Int. J. Mol. Sci. 2020, 21, 1586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kurihara, M.; Takarada, K.; Inoue, Y.H. Enhancement of leukemia-like phenotypes in Drosophila mxc mutant larvae due to activation of the RAS-MAP kinase cascade, possibly via downregulation of DE-cadherin. Genes Cell. 2020, 5, 757–769. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, S.; Takarada, K.; Kinoshita, Y.; Inoue, Y.H. Drosophila hemocytes recognize lymph gland tumors in mxc mutants and activate the innate immune pathway in a ROS-dependent manner. Biol. Open 2022, 11, bio059523. [Google Scholar] [CrossRef]
- Oka, S.; Hirai, J.; Yasukawa, T.; Nakahara, Y.; Inoue, Y.H. Correlation between reactive oxygen species accumulation and depletion of superoxide dismutases with age-dependent impairment in the nervous system and muscles of adult Drosophila. Biogerontology 2015, 16, 485–501. [Google Scholar] [CrossRef]
- Lee, G.; Park, J.H. Hemolymph sugar homeostasis and starvation-induced hyperactivity affected by genetic manipulations of the adipokinetic hormone-encoding gene in Drosophila melanogaster. Genetics 2004, 167, 311–323. [Google Scholar] [CrossRef] [Green Version]
- Jung, S.-H.; Evans, C.J.; Uemura, C.; Banerjee, U. The Drosophila lymph gland is used as a developmental model of hematopoiesis. Development 2005, 132, 2521–2533. [Google Scholar] [CrossRef] [Green Version]
- Ekas, L.A.; Baeg, G.-H.; Flaherty, M.S.; Ayala-Camargo, A.; Bach, E.A. JAK/STAT signaling promotes regional specification by negatively regulating wingless expression in Drosophila. Development 2006, 133, 4721–4729. [Google Scholar] [CrossRef] [Green Version]
- Neely, G.G.; Hess, A.; Costigan, M.; Keene, A.C.; Goulas, S.; Langeslag, M.; Griffin, R.S.; Belfer, I.; Dai, F.; Smith, S.B.; et al. A genome-wide Drosophila screen for heat nociception identifies α2δ3 as an evolutionarily conserved pain gene. Cell 2010, 143, 628–638. [Google Scholar] [CrossRef] [Green Version]
- Aw, S.S.; Lim, I.K.H.; Tang, M.X.M.; Cohen, S.M. A glio-protective role of mir-263a by tuning sensitivity to glutamate. Cell Rep. 2017, 19, 1783–1793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dietzl, G.; Chen, D.; Schnorrer, F.; Su, K.-C.; Barinova, Y.; Fellner, M.; Gasser, B.; Kinsey, K.; Oppel, S.; Scheiblauer, S.; et al. A genome-wide transgenic RNAi library for conditional gene inactivation in Drosophila. Nature 2007, 448, 151–156. [Google Scholar] [CrossRef]
- Kurucz, E.; Márkus, R.; Zsámboki, J.; Folkl-Medzihradszky, K.; Darula, Z.; Vilmos, P.; Udvardy, A.; Krausz, I.; Lukacsovich, T.; Gateff, E.; et al. Nimrod, a putative phagocytosis receptor with EGF repeats in Drosophila plasmatocytes. Curr. Biol. 2007, 17, 649–654. [Google Scholar] [CrossRef] [Green Version]
- Honti, V.; Kurucz, E.; Csordás, G.; Laurinyecz, B.; Márkus, R.; Andó, I. In vivo detection of lamellocytes in Drosophila melanogaster. Immunol. Lett. 2009, 126, 83–84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gilbert, M.M.; Beam, C.K.; Robinson, B.S.; Moberg, K.H. Genetic interactions between the Drosophila tumor suppressor gene and the stat92E transcription factor. PLoS ONE 2009, 4, e7083. [Google Scholar] [CrossRef] [Green Version]
- Gong, S.; Zhang, Y.; Tian, A.; Deng, W.M. Tumor models in various Drosophila tissues. WIREs Mech. Dis. 2021, 13, e1525. [Google Scholar] [CrossRef]
- Felcher, C.M.; Bogni, E.S.; Kordon, E.C. IL-6 cytokine family: A putative target for breast cancer prevention and treatment. Int. J. Mol. Sci. 2022, 23, 1809. [Google Scholar] [CrossRef]
- Szulc-Kielbik, I.; Kielbik, M.; Nowak, M.; Klink, M. Role of IL-6 in ovarian cancer cell invasiveness and chemoresistance A systematic review of its potential role as a biomarker in ovarian cancer patients. Biochim. Biophys. Acta Rev. Cancer 2021, 1876, 188639. [Google Scholar] [CrossRef] [PubMed]
- Harrison, D.A.; McCoon, P.E.; Binari, R.; Gilman, M.; Perrimon, N. Drosophila Ununpaired encodes a secreted protein that activates the JAK signaling pathway. Genes Dev. 1998, 12, 3252–3263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hombría, J.C.-G.; Brown, S.; Häder, S.; Zeidler, M.P. Characterization of Upd2, a Drosophila JAK/STAT pathway ligand. Dev. Biol. 2005, 288, 420–433. [Google Scholar] [CrossRef] [Green Version]
- Amoyel, M.; Anderson, A.M.; Bach, E.A. JAK/STAT pathway dysregulation in tumors: A Drosophila perspective. Semin. Cell Dev. Biol. 2014, 28, 96–103. [Google Scholar] [CrossRef] [Green Version]
- Santabárbara-Ruiz, P.; López-Santillán, M.; Martínez-Rodríguez, I.; Binagui-Casas, A.; Pérez, L.; Milán, M.; Corominas, M.; Serras, F. ROS-induced JNK and p38 signaling is required for unpaired cytokine activation during Drosophila regeneration. PLoS Genet. 2015, 11, e1005595. [Google Scholar] [CrossRef] [Green Version]
- La Fortezza, M.; Schenk, M.; Cosolo, A.; Kolybaba, A.; Grass, I.; Classen, A.K. JAK/STAT signalling mediates cell survival in response to tissue stress. Development 2016, 143, 2907–2919. [Google Scholar] [CrossRef] [Green Version]
- Diwanji, N.; Bergmann, A. Basement membrane damage by ROS- and JNK-mediated Mmp2 activation drives macrophage recruitment to overgrown tissue. Nat. Commun. 2020, 11, 3631. [Google Scholar] [CrossRef]
- Srivastava, A.; Pastor-Pareja, J.C.; Igaki, T.; Pagliarini, R.; Xu, T. Basement membrane remodeling is essential for Drosophila disc eversion and tumor invasion. Proc. Natl. Acad. Sci. USA 2007, 104, 2721–2726. [Google Scholar] [CrossRef] [PubMed]
- Hultmark, D.; Ekengren, S. Cytokines in Drosophila stress response. Dev. Cell 2003, 5, 360–361. [Google Scholar] [CrossRef] [Green Version]
- Cho, J.; Hwang, I.S.; Choi, H.; Hwang, J.H.; Hwang, J.S.; Lee, D.G. The novel biological action of antimicrobial peptides via apoptosis induction. J. Microbiol. Biotechnol. 2012, 22, 1457–1466. [Google Scholar] [CrossRef] [Green Version]
- Bilder, D.; Ong, K.; Hsi, T.C.; Adiga, K.; Kim, J. Tumour-host interactions through the lens of Drosophila. Nat. Rev. Cancer 2021, 21, 687–700. [Google Scholar] [CrossRef] [PubMed]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef] [PubMed]
- Lai, Y.; Gallo, R.L. AMPed up immunity: How antimicrobial peptides have multiple roles in immune defense. Trends Immunol. 2009, 30, 131–141. [Google Scholar] [CrossRef] [Green Version]
- Brun, S.; Vidal, S.; Spellman, P.; Takahashi, K.; Tricoire, H.; Lemaitre, B. The MAPKKK Mekk1 regulates the expression of Turandot stress genes in response to septic injury in Drosophila. Genes Cell. 2006, 11, 397–407. [Google Scholar] [CrossRef] [Green Version]
- Jin, L.; Shen, F.; Weinfeld, M.; Sergi, C. Insulin growth factor-binding protein 7 (IGFBP7)-related cancer, and IGFBP3 and IGFBP7 crosstalk. Front. Oncol. 2020, 10, 727. [Google Scholar] [CrossRef]
- Burger, A.M.; Zhang, X.; Li, H.; Ostrowski, J.L.; Beatty, B.; Venanzoni, M.; Papas, T.; Seth, A. Down-regulation of T1A12/mac25, a novel insulin-like growth factor binding protein related gene, is associated with disease progression in breast carcinomas. Oncogene 1998, 16, 2459–2467. [Google Scholar] [CrossRef] [Green Version]
- Sato, Y.; Chen, Z.; Miyazaki, K. Strong suppression of tumor growth by insulin-like growth factor-binding protein-related protein 1/tumor-derived cell adhesion factor/mac25. Cancer Sci. 2007, 98, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Figueroa-Clarevega, A.; Bilder, D. Malignant Drosophila tumors interrupt insulin signaling to induce cachexia-like wasting. Dev. Cell 2015, 33, 47–55. [Google Scholar] [CrossRef] [Green Version]
- Duneau, D.; Ferdy, J.B.; Revah, J.; Kondolf, H.; Ortiz, G.A.; Lazzaro, B.P.; Buchon, N. Stochastic variation in the initial phase of bacterial infection predicts the probability of survival in D. melanogaster. eLife 2017, 6, e28298. [Google Scholar] [CrossRef]
- Ellner, S.P.; Buchon, N.; Dörr, T.; Lazzaro, B.P. Host-pathogen immune feedbacks can explain widely divergent outcomes from similar infections. Proc. Biol. Sci. 2021, 288, 20210786. [Google Scholar] [CrossRef]
- Marusyk, A.; Janiszewska, M.; Polyak, K. Intratumor Heterogeneity: The rosetta stone of therapy resistance. Cancer Cell. 2020, 37, 471–484. [Google Scholar] [CrossRef]
- Yu, S.; Luo, F.; Xu, Y.; Zhang, Y.; Jin, L.H. Drosophila innate immunity involves multiple signaling pathways and coordinated communication between different tissues. Front. Immunol. 2022, 13, 905370. [Google Scholar] [CrossRef] [PubMed]
- Tanji, T.; Ip, Y.T. Regulators of the Toll and imd pathways in the Drosophila innate immune response. Trends Immunol. 2005, 26, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Valanne, S.; Wang, J.H.; Rämet, M. Drosophila Toll signaling pathway. J. Immunol. 2011, 186, 649–656. [Google Scholar] [CrossRef] [Green Version]
- Leulier, F.; Lemaitre, B. Toll-like receptors using an evolutionary approach. Nat. Rev. Genet. 2008, 9, 165–178. [Google Scholar] [CrossRef] [Green Version]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kinoshita, Y.; Shiratsuchi, N.; Araki, M.; Inoue, Y.H. Anti-Tumor Effect of Turandot Proteins Induced via the JAK/STAT Pathway in the mxc Hematopoietic Tumor Mutant in Drosophila. Cells 2023, 12, 2047. https://doi.org/10.3390/cells12162047
Kinoshita Y, Shiratsuchi N, Araki M, Inoue YH. Anti-Tumor Effect of Turandot Proteins Induced via the JAK/STAT Pathway in the mxc Hematopoietic Tumor Mutant in Drosophila. Cells. 2023; 12(16):2047. https://doi.org/10.3390/cells12162047
Chicago/Turabian StyleKinoshita, Yuriko, Naoka Shiratsuchi, Mayo Araki, and Yoshihiro H. Inoue. 2023. "Anti-Tumor Effect of Turandot Proteins Induced via the JAK/STAT Pathway in the mxc Hematopoietic Tumor Mutant in Drosophila" Cells 12, no. 16: 2047. https://doi.org/10.3390/cells12162047
APA StyleKinoshita, Y., Shiratsuchi, N., Araki, M., & Inoue, Y. H. (2023). Anti-Tumor Effect of Turandot Proteins Induced via the JAK/STAT Pathway in the mxc Hematopoietic Tumor Mutant in Drosophila. Cells, 12(16), 2047. https://doi.org/10.3390/cells12162047






