Chronic Inflammation, Oxidative Stress and Metabolic Plasticity: Three Players Driving the Pro-Tumorigenic Microenvironment in Malignant Mesothelioma
Abstract
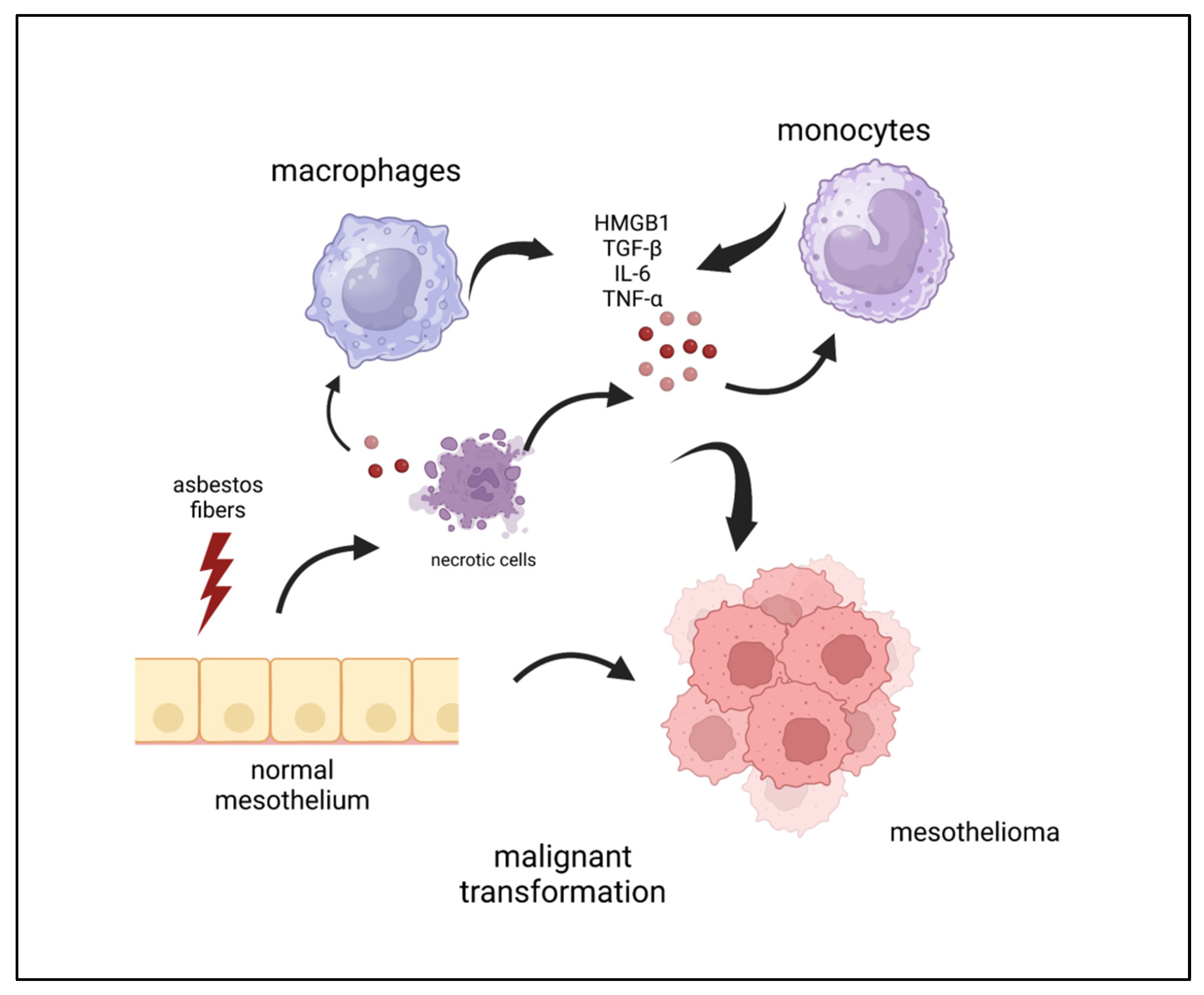
1. Introduction
2. Inflammation and Mesothelioma Pathogenesis
3. Mesothelioma Microenvironment: Tumor–Host Crosstalk Driving Chronic Inflammation
3.1. Secretome/Soluble Factors
3.1.1. Transforming Growth Factor-β (TGF-β)
3.1.2. IL-6 in Mesothelioma
3.1.3. Tumor Necrosis Factor-α (TNF-α) and Asbestos Exposure
3.1.4. DAMP and Mesothelioma
3.1.5. HMGB1: What It Is and How It Can Play a Role in MPM
3.1.6. Peripheral Blood Markers
3.2. Oxidative Stress and ROS
Iron and ROS
3.3. Metabolic Crosstalk and Reprogramming
3.3.1. Hypoxic Conditions and Nutrients Competition
3.3.2. Adenosine Pathway
3.3.3. BAP1 and Metabolic Reprogramming
4. Conclusions and Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yap, T.A.; Aerts, J.G.; Popat, S.; Fennell, D.A. Novel Insights into Mesothelioma Biology and Implications for Therapy. Nat. Rev. Cancer 2017, 17, 475–488. [Google Scholar] [PubMed]
- Sun, H.H.; Vaynblat, A.; Pass, H.I. Diagnosis and Prognosis-Review of Biomarkers for Mesothelioma. Ann. Transl. Med. 2017, 5, 244. [Google Scholar] [CrossRef]
- Kindler, H.L.; Ismaila, N.; Armato, S.G.; Bueno, R.; Hesdorffer, M.; Jahan, T.; Jones, C.M.; Miettinen, M.; Pass, H.; Rimner, A.; et al. Treatment of Malignant Pleural Mesothelioma: American Society of Clinical Oncology Clinical Practice Guideline. J. Clin. Oncol. 2018, 36, 1343–1373. [Google Scholar] [PubMed]
- Vogelzang, N.J.; Rusthoven, J.J.; Symanowski, J.; Denham, C.; Kaukel, E.; Ruffie, P.; Gatzemeier, U.; Boyer, M.; Emri, S.; Manegold, C.; et al. Phase III Study of Pemetrexed in Combination with Cisplatin versus Cisplatin Alone in Patients with Malignant Pleural Mesothelioma. J. Clin. Oncol. 2003, 21, 2636–2644. [Google Scholar] [CrossRef] [PubMed]
- Robert, C. A Decade of Immune-Checkpoint Inhibitors in Cancer Therapy. Nat. Commun. 2020, 11, 3801. [Google Scholar] [CrossRef] [PubMed]
- Scherpereel, A.; Wallyn, F.; Albelda, S.M.; Munck, C. Novel Therapies for Malignant Pleural Mesothelioma. Lancet Oncol. 2018, 19, e161–e172. [Google Scholar] [PubMed]
- Tsao, A.S.; Pass, H.I.; Rimner, A.; Mansfield, A.S. New Era for Malignant Pleural Mesothelioma: Updates on Therapeutic Options. J. Clin. Oncol. 2022, 40, 681–692. [Google Scholar] [PubMed]
- Nicolini, F.; Bocchini, M.; Bronte, G.; Delmonte, A.; Guidoboni, M.; Crinò, L.; Mazza, M. Malignant Pleural Mesothelioma: State-of-the-Art on Current Therapies and Promises for the Future. Front. Oncol. 2020, 9, 1519. [Google Scholar]
- Baas, P.; Scherpereel, A.; Nowak, A.K.; Fujimoto, N.; Peters, S.; Tsao, A.S.; Mansfield, A.S.; Popat, S.; Jahan, T.; Antonia, S.; et al. First-Line Nivolumab plus Ipilimumab in Unresectable Malignant Pleural Mesothelioma (CheckMate 743): A Multicentre, Randomised, Open-Label, Phase 3 Trial. Lancet 2021, 397, 375–386. [Google Scholar] [CrossRef] [PubMed]
- Zauderer, M.G.; Kass, S.L.; Woo, K.; Sima, C.S.; Ginsberg, M.S.; Krug, L.M. Vinorelbine and Gemcitabine as Second- or Third-Line Therapy for Malignant Pleural Mesothelioma. Lung Cancer 2014, 84, 271–274. [Google Scholar] [CrossRef]
- Popat, S.; Curioni-Fontecedro, A.; Dafni, U.; Shah, R.; O’Brien, M.; Pope, A.; Fisher, P.; Spicer, J.; Roy, A.; Gilligan, D.; et al. A Multicentre Randomised Phase III Trial Comparing Pembrolizumab versus Single-Agent Chemotherapy for Advanced Pre-Treated Malignant Pleural Mesothelioma: The European Thoracic Oncology Platform (ETOP 9–15) PROMISE-Meso Trial. Ann. Oncol. 2020, 31, 1734–1745. [Google Scholar] [CrossRef] [PubMed]
- Fennell, D.; Ottensmeier, C.; Califano, R.; Hanna, G.; Ewings, S.; Hill, K.; Wilding, S.; Danson, S.; Nye, M.; Steele, N.; et al. Nivolumab Versus Placebo in Relapsed Malignant Mesothelioma: The Confirm Phase 3 Trial. J. Thorac. Oncol. 2021, 16, S62. [Google Scholar]
- Rivalland, G.; Kao, S.C.-H.; Pavlakis, N.; Hughes, B.G.M.; Thapa, B.; Pal, A.; Walkiewicz, M.; Zimet, A.S.; White, S.; O’Byrne, K.; et al. Outcomes of Anti-PD-1 Therapy in Mesothelioma and Correlation with PD-L1 Expression. J. Clin. Oncol. 2017, 35, 8514. [Google Scholar] [CrossRef]
- Huang, S.X.L.; Jaurand, M.C.; Kamp, D.W.; Whysner, J.; Hei, T.K. Role of Mutagenicity in Asbestos Fiber-Induced Carcinogenicity and Other Diseases. J. Toxicol. Environ. Health B Crit. Rev. 2011, 14, 179–245. [Google Scholar] [PubMed]
- Felley-Bosco, E.; Macfarlane, M. Asbestos: Modern Insights for Toxicology in the Era of Engineered Nanomaterials. Chem. Res. Toxicol. 2018, 31, 994–1008. [Google Scholar] [PubMed]
- Brcic, L.; Kern, I. Clinical Significance of Histologic Subtyping of Malignant Pleural Mesothelioma. Transl. Lung Cancer Res. 2020, 9, 924–933. [Google Scholar] [CrossRef] [PubMed]
- Betti, M.; Casalone, E.; Ferrante, D.; Aspesi, A.; Morleo, G.; Biasi, A.; Sculco, M.; Mancuso, G.; Guarrera, S.; Righi, L.; et al. Germline Mutations in DNA Repair Genes Predispose Asbestos-Exposed Patients to Malignant Pleural Mesothelioma. Cancer Lett. 2017, 405, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Nagai, H.; Ishihara, T.; Lee, W.H.; Ohara, H.; Okazaki, Y.; Okawa, K.; Toyokuni, S. Asbestos Surface Provides a Niche for Oxidative Modification. Cancer Sci. 2011, 102, 2118–2125. [Google Scholar] [CrossRef]
- Gaudino, G.; Xue, J.; Yang, H. How Asbestos and Other Fibers Cause Mesothelioma. Transl. Lung Cancer Res. 2020, 9, S39–S46. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Zhang, Y. HMGB1 in Inflammation and Cancer. J. Hematol. Oncol. 2020, 13, 1–4. [Google Scholar] [CrossRef]
- Zolondick, A.A.; Gaudino, G.; Xue, J.; Pass, H.I.; Carbone, M.; Yang, H. Asbestos-Induced Chronic Inflammation in Malignant Pleural Mesothelioma and Related Therapeutic Approaches—A Narrative Review. Precis. Cancer Med. 2021, 4, 27. [Google Scholar] [PubMed]
- Chu, G.J.; van Zandwijk, N.; Rasko, J.E.J. The Immune Microenvironment in Mesothelioma: Mechanisms of Resistance to Immunotherapy. Front. Oncol. 2019, 9, 1366. [Google Scholar]
- Greten, F.R.; Grivennikov, S.I. Inflammation and Cancer: Triggers, Mechanisms, and Consequences. Immunity 2019, 51, 27–41. [Google Scholar] [PubMed]
- Chéné, A.L.; D’Almeida, S.; Blondy, T.; Tabiasco, J.; Deshayes, S.; Fonteneau, J.F.; Cellerin, L.; Delneste, Y.; Grégoire, M.; Blanquart, C. Pleural Effusions from Patients with Mesothelioma Induce Recruitment of Monocytes and Their Differentiation into M2 Macrophages. J. Thorac. Oncol. 2016, 11, 1765–1773. [Google Scholar] [CrossRef] [PubMed]
- Sarode, P.; Schaefer, M.B.; Grimminger, F.; Seeger, W.; Savai, R. Macrophage and Tumor Cell Cross-Talk Is Fundamental for Lung Tumor Progression: We Need to Talk. Front. Oncol. 2020, 10, 324. [Google Scholar] [PubMed]
- Cersosimo, F.; Barbarino, M.; Lonardi, S.; Vermi, W.; Giordano, A.; Bellan, C.; Giurisato, E. Mesothelioma Malignancy and the Microenvironment: Molecular Mechanisms. Cancers 2021, 13, 5664. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Guo, N.; Zhou, Y.; Chen, J.; Wei, Q.; Han, M. The Role of Tumor-Associated Macrophages (TAMs) in Tumor Progression and Relevant Advance in Targeted Therapy. Acta Pharm. Sin. B 2020, 10, 2156–2170. [Google Scholar]
- Xu, F.; Wei, Y.; Tang, Z.; Liu, B.; Dong, J. Tumor-Associated Macrophages in Lung Cancer: Friend or Foe? Mol. Med. Rep. 2020, 22, 4107–4115. [Google Scholar]
- Burt, B.M.; Rodig, S.J.; Tilleman, T.R.; Elbardissi, A.W.; Bueno, R.; Sugarbaker, D.J. Circulating and Tumor-Infiltrating Myeloid Cells Predict Survival in Human Pleural Mesothelioma. Cancer 2011, 117, 5234–5244. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2018, 19, 92. [Google Scholar]
- Désage, A.L.; Karpathiou, G.; Peoc’h, M.; Froudarakis, M.E. The Immune Microenvironment of Malignant Pleural Mesothelioma: A Literature Review. Cancers 2021, 13, 3205. [Google Scholar] [PubMed]
- Chen, Y.; Song, Y.; Du, W.; Gong, L.; Chang, H.; Zou, Z. Tumor-Associated Macrophages: An Accomplice in Solid Tumor Progression. J. Biomed. Sci. 2019, 26, 78. [Google Scholar]
- Andón, F.T.; Digifico, E.; Maeda, A.; Erreni, M.; Mantovani, A.; Alonso, M.J.; Allavena, P. Targeting Tumor Associated Macrophages: The New Challenge for Nanomedicine. Semin. Immunol. 2017, 34, 103–113. [Google Scholar] [PubMed]
- Colak, S.; ten Dijke, P. Targeting TGF-β Signaling in Cancer. Trends Cancer 2017, 3, 56–71. [Google Scholar] [CrossRef]
- Yin, M.; Tissari, M.; Tamminen, J.; Ylivinkka, I.; Rönty, M.; von Nandelstadh, P.; Lehti, K.; Hyytiäinen, M.; Myllärniemi, M.; Koli, K. Gremlin-1 Is a Key Regulator of the Invasive Cell Phenotype in Mesothelioma. Oncotarget 2017, 8, 98280–98297. [Google Scholar] [CrossRef]
- Hoda, M.A.; Dong, Y.; Rozsas, A.; Klikovits, T.; Laszlo, V.; Ghanim, B.; Stockhammer, P.; Ozsvar, J.; Jakopovic, M.; Samarzija, M.; et al. Circulating Activin A Is a Novel Prognostic Biomarker in Malignant Pleural Mesothelioma—A Multi-Institutional Study. Eur. J. Cancer 2016, 63, 64–73. [Google Scholar] [CrossRef]
- Fujii, M.; Nakanishi, H.; Toyoda, T.; Tanaka, I.; Kondo, Y.; Osada, H.; Sekido, Y. Convergent Signaling in the Regulation of Connective Tissue Growth Factor in Malignant Mesothelioma: TGFβ Signaling and Defects in the Hippo Signaling Cascade. Cell Cycle 2012, 11, 3373–3379. [Google Scholar] [PubMed]
- Gerwin, B.I.; Lechner, J.F.; Reddel, R.R.; Roberts, A.B.; Robbins, K.C.; Gabrielson, E.W.; Harris, C.C. Comparison of Production of Transforming Growth Factor-Beta and Platelet-Derived Growth Factor by Normal Human Mesothelial Cells and Mesothelioma Cell Lines. Cancer Res. 1987, 47, 6180–6184. [Google Scholar] [PubMed]
- Suzuki, E.; Kim, S.; Cheung, H.K.; Corbley, M.J.; Zhang, X.; Sun, L.; Shan, F.; Singh, J.; Lee, W.C.; Albelda, S.M.; et al. A Novel Small-Molecule Inhibitor of Transforming Growth Factor β Type I Receptor Kinase (SM16) Inhibits Murine Mesothelioma Tumor Growth in Vivo and Prevents Tumor Recurrence after Surgical Resection. Cancer Res. 2007, 67, 2351–2359. [Google Scholar] [CrossRef] [PubMed]
- Hmeljak, J.; Sanchez-Vega, F.; Hoadley, K.A.; Shih, J.; Stewart, C.; Heiman, D.; Tarpey, P.; Danilova, L.; Drill, E.; Gibb, E.A.; et al. Integrative Molecular Characterization of Malignant Pleural Mesothelioma. Cancer Discov. 2018, 8, 1548–1565. [Google Scholar] [CrossRef] [PubMed]
- Stevenson, J.P.; Kindler, H.L.; Papasavvas, E.; Sun, J.; Jacobs-Small, M.; Hull, J.; Schwed, D.; Ranganathan, A.; Newick, K.; Heitjan, D.F.; et al. Immunological Effects of the TGFβ-Blocking Antibody GC1008 in Malignant Pleural Mesothelioma Patients. Oncoimmunology 2013, 2, e26218. [Google Scholar] [CrossRef]
- Stockhammer, P.; Ploenes, T.; Theegarten, D.; Schuler, M.; Maier, S.; Aigner, C.; Hegedus, B. Detection of TGF-β in Pleural Effusions for Diagnosis and Prognostic Stratification of Malignant Pleural Mesothelioma. Lung Cancer 2020, 139, 124–132. [Google Scholar] [CrossRef]
- Naka, T.; Nishimoto, N.; Kishimoto, T. The Paradigm of IL-6: From Basic Science to Medicine. Arthritis Res. 2002, 4, S233–S242. [Google Scholar] [PubMed]
- Tanaka, H.; Sun, T.; Kinashi, H.; Kamiya, K.; Yamaguchi, M.; Nobata, H.; Sakata, F.; Kim, H.; Mizuno, M.; Kunoki, S.; et al. Interleukin-6 Blockade Reduces Salt-Induced Cardiac Inflammation and Fibrosis in Subtotal Nephrectomized Mice. Am. J. Physiol. Renal Physiol. 2022, 323, F654–F665. [Google Scholar] [CrossRef] [PubMed]
- Bielefeldt-Ohmann, H.; Marzo, A.L.; Himbeck, R.P.; Jarnicki, A.G.; Robinson, B.W.S.; Fitzpatrick, D.R. Interleukin-6 Involvement in Mesothelioma Pathobiology: Inhibition by Interferon α Immunotherapy. Cancer Immunol. Immunother. 1995, 40, 241–250. [Google Scholar] [CrossRef]
- Schmitter, D.; Lauber, B.; Fagg, B.; Stahel, R.A. Hematopoietic Growth Factors Secreted by Seven Human Pleural Mesothelioma Cell Lines: Interleukin-6 Production as a Common Feature. Int. J. Cancer 1992, 51, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Monti, G.; Jaurand, M.C.; Monnet, I.; Chretien, P.; Saint-Etienne, L.; Zeng, L.; Portier, A.; Devillier, P.; Galanaud, P.; Bignon, J. Intrapleural Production of Interleukin 6 during Mesothelioma and Its Modulation by Gamma-Interferon Treatment. Cancer Res. 1994, 54, 4419–4423. [Google Scholar]
- Adachi, Y.; Aoki, C.; Yoshio-Hoshino, N.; Takayama, K.; Curiel, D.T.; Nishimoto, N. Interleukin-6 Induces Both Cell Growth and VEGF Production in Malignant Mesotheliomas. Int. J. Cancer 2006, 119, 1303–1311. [Google Scholar] [CrossRef]
- Songür, N.; Kuru, B.; Kalkan, F.; Özdilekcan, Ç.; Çakmak, H.; Hizel, N. Serum Interleukin-6 Levels Correlate with Malnutrition and Survival in Patients with Advanced Non-Small Cell Lung Cancer. Tumori 2004, 90, 196–200. [Google Scholar] [CrossRef]
- McLaren, B.R.; Robinson, B.W.S.; Lake, R.A. New Chemotherapeutics in Malignant Mesothelioma: Effects on Cell Growth and IL-6 Production. Cancer Chemother. Pharmacol. 2000, 45, 502–508. [Google Scholar] [CrossRef]
- Philip, M.; Rowley, D.A.; Schreiber, H. Inflammation as a Tumor Promoter in Cancer Induction. Semin. Cancer Biol. 2004, 14, 433–439. [Google Scholar] [CrossRef] [PubMed]
- Acencio, M.M.P.; Soares, B.; Marchi, E.; Silva, C.S.R.; Teixeira, L.R.; Broaddus, V.C. Inflammatory Cytokines Contribute to Asbestos-Induced Injury of Mesothelial Cells. Lung 2015, 193, 831–837. [Google Scholar] [CrossRef] [PubMed]
- Yang, H.; Rivera, Z.; Jube, S.; Nasu, M.; Bertino, P.; Goparaju, C.; Franzoso, G.; Lotze, M.T.; Krausz, T.; Pass, H.I.; et al. Programmed Necrosis Induced by Asbestos in Human Mesothelial Cells Causes High-Mobility Group Box 1 Protein Release and Resultant Inflammation. Proc. Natl. Acad. Sci. USA 2010, 107, 12611–12616. [Google Scholar] [CrossRef]
- Yang, H.; Bocchetta, M.; Kroczynska, B.; Elmishad, A.G.; Chen, Y.; Liu, Z.; Bubici, C.; Mossman, B.T.; Pass, H.I.; Testa, J.R.; et al. TNF-α Inhibits Asbestos-Induced Cytotoxicity via a NF-ΚB-Dependent Pathway, a Possible Mechanism for Asbestos-Induced Oncogenesis. Proc. Natl. Acad. Sci. USA 2006, 103, 10397–10402. [Google Scholar] [CrossRef] [PubMed]
- Bonsignore, G.; Martinotti, S.; Grosso, F.; Ranzato, E. New Developments in Mesothelioma Markers. In Mesothelioma: Risk Factors, Treatment and Prognosis; Martin, A.K., Ed.; Nova Science Publishers: Hauppauge, NY, USA, 2021. [Google Scholar]
- Gong, T.; Liu, L.; Jiang, W.; Zhou, R. DAMP-Sensing Receptors in Sterile Inflammation and Inflammatory Diseases. Nat. Rev. Immunol. 2020, 20, 95–112. [Google Scholar]
- Goodwin, G.H.; Sanders, C.; Johns, E.W. A New Group of Chromatin-Associated Proteins with a High Content of Acidic and Basic Amino Acids. Eur. J. Biochem. 1973, 38, 14–19. [Google Scholar] [CrossRef]
- Ranzato, E.; Martinotti, S.; Patrone, M. Emerging Roles for HMGB1 Protein in Immunity, Inflammation, and Cancer. Immunotargets Ther. 2015, 4, 101–109. [Google Scholar] [CrossRef]
- Huebener, P.; Gwak, G.Y.; Pradere, J.P.; Quinzii, C.M.; Friedman, R.; Lin, C.S.; Trent, C.M.; Mederacke, I.; Zhao, E.; Dapito, D.H.; et al. High-Mobility Group Box 1 Is Dispensable for Autophagy, Mitochondrial Quality Control, and Organ Function in Vivo. Cell Metab. 2014, 19, 539–547. [Google Scholar] [CrossRef]
- Ellerman, J.E.; Brown, C.K.; De Vera, M.; Zeh, H.J.; Billiar, T.; Rubartelli, A.; Lotze, M.T. Masquerader: High Mobility Group Box-1 and Cancer. Clin. Cancer Res. 2007, 13, 2836–2848. [Google Scholar]
- Rrapaj, E.; Trisolini, E.; Bertero, L.; Salvo, M.; Indellicato, R.; Andorno, S.; Garcia-Manteiga, J.M.; Rena, O.; Boldorini, R.L. Expression Analysis of HMGB1 in Histological Samples of Malignant Pleural Mesothelioma. Histopathology 2018, 72, 1039–1050. [Google Scholar] [CrossRef]
- Ying, S.; Jiang, Z.; He, X.; Yu, M.; Chen, R.; Chen, J.; Ru, G.; Chen, Y.; Chen, W.; Zhu, L.; et al. Serum HMGB1 as a Potential Biomarker for Patients with Asbestos-Related Diseases. Dis. Markers 2017, 2017, 1–9. [Google Scholar] [CrossRef]
- Cheng, Y.Y.; Rath, E.M.; Linton, A.; Yuen, M.L.; Takahashi, K.; Lee, K. The Current Understanding of Asbestos-Induced Epigenetic Changes Associated with Lung Cancer. Lung Cancer Targets Ther. 2020, 11, 1–11. [Google Scholar]
- Yamagishi, T.; Fujimoto, N.; Nishi, H.; Miyamoto, Y.; Hara, N.; Asano, M.; Fuchimoto, Y.; Wada, S.; Kitamura, K.; Ozaki, S.; et al. Prognostic Significance of the Lymphocyte-to-Monocyte Ratio in Patients with Malignant Pleural Mesothelioma. Lung Cancer 2015, 90, 111–117. [Google Scholar] [CrossRef]
- Tagawa, T.; Anraku, M.; Morodomi, Y.; Takenaka, T.; Okamoto, T.; Takenoyama, M.; Ichinose, Y.; Maehara, Y.; John Cho, B.C.; Feld, R.; et al. Clinical Role of a New Prognostic Score Using Platelet-Tolymphocyte Ratio in Patients with Malignant Pleural Mesothelioma Undergoing Extrapleural Pneumonectomy. J. Thorac. Dis. 2015, 7, 1898–1906. [Google Scholar] [CrossRef] [PubMed]
- Urso, L.; Cavallari, I.; Sharova, E.; Ciccarese, F.; Pasello, G.; Ciminale, V. Metabolic Rewiring and Redox Alterations in Malignant Pleural Mesothelioma. Br. J. Cancer 2020, 122, 52–61. [Google Scholar] [CrossRef] [PubMed]
- Hayes, J.D.; Dinkova-Kostova, A.T.; Tew, K.D. Oxidative Stress in Cancer. Cancer Cell 2020, 38, 167–197. [Google Scholar] [CrossRef]
- Weinberg, F.; Ramnath, N.; Nagrath, D. Reactive Oxygen Species in the Tumor Microenvironment: An Overview. Cancers 2019, 11, 1191. [Google Scholar] [PubMed]
- Circu, M.L.; Aw, T.Y. Reactive Oxygen Species, Cellular Redox Systems, and Apoptosis. Free Radic. Biol. Med. 2010, 48, 749–762. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of Cancer: The next Generation. Cell 2011, 144, 646–674. [Google Scholar] [PubMed]
- Murphy, M.P. How Mitochondria Produce Reactive Oxygen Species. Biochem. J. 2009, 417, 1–13. [Google Scholar] [PubMed]
- D’Autréaux, B.; Toledano, M.B. ROS as Signalling Molecules: Mechanisms That Generate Specificity in ROS Homeostasis. Nat. Rev. Mol. Cell Biol. 2007, 8, 813–824. [Google Scholar] [PubMed]
- Nakamura, H.; Takada, K. Reactive Oxygen Species in Cancer: Current Findings and Future Directions. Cancer Sci. 2021, 112, 3945. [Google Scholar] [CrossRef] [PubMed]
- Perillo, B.; Di Donato, M.; Pezone, A.; Di Zazzo, E.; Giovannelli, P.; Galasso, G.; Castoria, G.; Migliaccio, A. ROS in Cancer Therapy: The Bright Side of the Moon. Exp. Mol. Med. 2020, 52, 192–203. [Google Scholar] [CrossRef] [PubMed]
- Kennedy, L.; Sandhu, J.K.; Harper, M.E.; Cuperlovic-culf, M. Role of Glutathione in Cancer: From Mechanisms to Therapies. Biomolecules 2020, 10, 1429. [Google Scholar] [CrossRef]
- Rhee, S.G.; Yang, K.S.; Kang, S.W.; Woo, H.A.; Chang, T.S. Controlled Elimination of Intracellular H2O2: Regulation of Peroxiredoxin, Catalase, and Glutathione Peroxidase via Post-Translational Modification. Antioxid. Redox Signal. 2005, 7, 619–626. [Google Scholar]
- Kirtonia, A.; Sethi, G.; Garg, M. The Multifaceted Role of Reactive Oxygen Species in Tumorigenesis. Cell. Mol. Life Sci. 2020, 77, 4459–4483. [Google Scholar]
- Benedetti, S.; Nuvoli, B.; Catalani, S.; Galati, R. Reactive Oxygen Species a Double-Edged Sword for Mesothelioma. Oncotarget 2015, 6, 16848–16865. [Google Scholar] [CrossRef]
- Donaldson, K.; Murphy, F.A.; Duffin, R.; Poland, C.A. Asbestos, Carbon Nanotubes and the Pleural Mesothelium: A Review of the Hypothesis Regarding the Role of Long Fibre Retention in the Parietal Pleura, Inflammation and Mesothelioma. Part. Fibre Toxicol. 2010, 7, 5. [Google Scholar] [PubMed]
- Schiavello, M.; Gazzano, E.; Bergandi, L.; Silvagno, F.; Libener, R.; Riganti, C.; Aldieri, E. Identification of Redox-Sensitive Transcription Factors as Markers of Malignant Pleural Mesothelioma. Cancers 2021, 13, 1138. [Google Scholar] [CrossRef]
- Derynck, R.; Akhurst, R.J.; Balmain, A. TGF-β Signaling in Tumor Suppression and Cancer Progression. Nat. Genet. 2001, 29, 117–129. [Google Scholar] [PubMed]
- Suzuki, E.; Kapoor, V.; Cheung, H.-K.; Ling, L.E.; Delong, P.A.; Kaiser, L.R.; Albelda, S.M. Soluble Type II Transforming Growth Factor-Receptor Inhibits Established Murine Malignant Mesothelioma Tumor Growth by Augmenting Host Antitumor Immunity. Clin. Cancer Res. 2004, 10, 5907–5918. [Google Scholar] [PubMed]
- Fujii, M.; Toyoda, T.; Nakanishi, H.; Yatabe, Y.; Sato, A.; Matsudaira, Y.; Ito, H.; Murakami, H.; Kondo, Y.; Kondo, E.; et al. TGF-β Synergizes with Defects in the Hippo Pathway to Stimulate Human Malignant Mesothelioma Growth. J. Exp. Med. 2012, 209, 479–494. [Google Scholar] [CrossRef]
- Krstić, J.; Trivanović, D.; Mojsilović, S.; Santibanez, J.F. Transforming Growth Factor-Beta and Oxidative Stress Interplay: Implications in Tumorigenesis and Cancer Progression. Oxid. Med. Cell. Longev. 2015, 2015, 1–15. [Google Scholar] [CrossRef]
- Jobling, M.F.; Mott, J.D.; Finnegan, M.T.; Jurukovski, V.; Erickson, A.C.; Walian, P.J.; Taylor, S.E.; Ledbetter, S.; Lawrence, C.M.; Rifkin, D.B.; et al. Isoform-Specific Activation of Latent Transforming Growth Factor β (LTGF-β) by Reactive Oxygen Species. Radiat. Res. 2006, 166, 839–848. [Google Scholar] [CrossRef] [PubMed]
- Pociask, D.A.; Sime, P.J.; Brody, A.R. Asbestos-Derived Reactive Oxygen Species Activate TGF-Β1. Lab. Investig. 2004, 84, 1013–1023. [Google Scholar] [CrossRef]
- Tamminen, J.A.; Myllärniemi, M.; Hyytiäinen, M.; Keski-Oja, J.; Koli, K. Asbestos Exposure Induces Alveolar Epithelial Cell Plasticity through MAPK/Erk Signaling. J. Cell. Biochem. 2012, 113, 2234–2247. [Google Scholar] [CrossRef] [PubMed]
- Tsubakihara, Y.; Moustakas, A. Epithelial-Mesenchymal Transition and Metastasis under the Control of Transforming Growth Factor β. Int. J. Mol. Sci. 2018, 19, 3672. [Google Scholar] [CrossRef]
- Ramundo, V.; Zanirato, G.; Aldieri, E. The Epithelial-to-Mesenchymal Transition (Emt) in the Development and Metastasis of Malignant Pleural Mesothelioma. Int. J. Mol. Sci. 2021, 22, 12216. [Google Scholar] [CrossRef] [PubMed]
- Turini, S.; Bergandi, L.; Gazzano, E.; Prato, M.; Aldieri, E. Epithelial to Mesenchymal Transition in Human Mesothelial Cells Exposed to Asbestos Fibers: Role of TGF-β as Mediator of Malignant Mesothelioma Development or Metastasis via EMT Event. Int. J. Mol. Sci. 2019, 20, 150. [Google Scholar] [CrossRef]
- Byun, H.O.; Jung, H.J.; Seo, Y.H.; Lee, Y.K.; Hwang, S.C.; Seong Hwang, E.; Yoon, G. GSK3 Inactivation Is Involved in Mitochondrial Complex IV Defect in Transforming Growth Factor (TGF) Β1-Induced Senescence. Exp. Cell Res. 2012, 318, 1808–1819. [Google Scholar] [CrossRef] [PubMed]
- Gorrini, C.; Harris, I.S.; Mak, T.W. Modulation of Oxidative Stress as an Anticancer Strategy. Nat. Rev. Drug Discov. 2013, 12, 931–947. [Google Scholar] [CrossRef]
- Torti, S.V.; Manz, D.H.; Paul, B.T.; Blanchette-Farra, N.; Torti, F.M. Iron and Cancer. Annu. Rev. Nutr. 2018, 38, 97–125. [Google Scholar] [PubMed]
- Guo, Q.; Li, L.; Hou, S.; Yuan, Z.; Li, C.; Zhang, W.; Zheng, L.; Li, X. The Role of Iron in Cancer Progression. Front. Oncol. 2021, 11. [Google Scholar] [CrossRef]
- Fiorito, V.; Chiabrando, D.; Petrillo, S.; Bertino, F.; Tolosano, E. The Multifaceted Role of Heme in Cancer. Front. Oncol. 2020, 9, 1540. [Google Scholar] [CrossRef]
- Eales, K.L.; Hollinshead, K.E.R.; Tennant, D.A. Hypoxia and Metabolic Adaptation of Cancer Cells. Oncogenesis 2016, 5, e190. [Google Scholar] [CrossRef] [PubMed]
- Lei, G.; Zhuang, L.; Gan, B. Targeting Ferroptosis as a Vulnerability in Cancer. Nat. Rev. Cancer 2022, 22, 381–396. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Cao, X.; Wang, D.; Zhu, H. Iron Metabolism: State of the Art in Hypoxic Cancer Cell Biology. Arch. Biochem. Biophys. 2022, 723, 109199. [Google Scholar]
- Toyokuni, S. Role of Iron in Carcinogenesis: Cancer as a Ferrotoxic Disease. Cancer Sci. 2009, 100, 9–16. [Google Scholar] [PubMed]
- Aung, W.; Hasegawa, S.; Furukawa, T.; Saga, T. Potential Role of Ferritin Heavy Chain in Oxidative Stress and Apoptosis in Human Mesothelial and Mesothelioma Cells: Implications for Asbestos-Induced Oncogenesis. Carcinogenesis 2007, 28, 2047–2052. [Google Scholar] [CrossRef] [PubMed]
- Kambara, T.; Amatya, V.J.; Kushitani, K.; Fujii, Y.; Endo, I.; Takeshima, Y. Downregulation of FTL Decreases Proliferation of Malignant Mesothelioma Cells by Inducing G1 Cell Cycle Arrest. Oncol. Lett. 2022, 23, 1–9. [Google Scholar] [CrossRef]
- Toyokuni, S. Iron Addiction with Ferroptosis-Resistance in Asbestos-Induced Mesothelial Carcinogenesis: Toward the Era of Mesothelioma Prevention. Free Radic. Biol. Med. 2019, 133, 206–215. [Google Scholar] [PubMed]
- Hanahan, D. Hallmarks of Cancer: New Dimensions. Cancer Discov. 2022, 12, 31–46. [Google Scholar]
- Cairns, R.A.; Harris, I.S.; Mak, T.W. Regulation of Cancer Cell Metabolism. Nat. Rev. Cancer 2011, 11, 85–95. [Google Scholar] [CrossRef] [PubMed]
- Maynard, R.S.; Hellmich, C.; Bowles, K.M.; Rushworth, S.A. Acute Myeloid Leukaemia Drives Metabolic Changes in the Bone Marrow Niche. Front. Oncol. 2022, 12, 924567. [Google Scholar]
- Martínez-Reyes, I.; Chandel, N.S. Cancer Metabolism: Looking Forward. Nat. Rev. Cancer 2021, 21, 669–680. [Google Scholar] [PubMed]
- Elia, I.; Haigis, M.C. Metabolites and the Tumour Microenvironment: From Cellular Mechanisms to Systemic Metabolism. Nat. Metab. 2021, 3, 21–32. [Google Scholar] [PubMed]
- de la Cruz-López, K.G.; Castro-Muñoz, L.J.; Reyes-Hernández, D.O.; García-Carrancá, A.; Manzo-Merino, J. Lactate in the Regulation of Tumor Microenvironment and Therapeutic Approaches. Front. Oncol. 2019, 9, 1143. [Google Scholar] [PubMed]
- San-Millán, I.; Brooks, G.A. Reexamining Cancer Metabolism: Lactate Production for Carcinogenesis Could Be the Purpose and Explanation of the Warburg Effect. Carcinogenesis 2017, 38, 119–133. [Google Scholar]
- Gatenby, R.A.; Gillies, R.J. Why Do Cancers Have High Aerobic Glycolysis? Nat. Rev. Cancer 2004, 4, 891–899. [Google Scholar] [PubMed]
- O’Neill, L.A.J.; Grahame Hardie, D. Metabolism of Inflammation Limited by AMPK and Pseudo-Starvation. Nature 2013, 493, 346–355. [Google Scholar]
- Biswas, S.K.; Mantovani, A. Orchestration of Metabolism by Macrophages. Cell Metab. 2012, 15, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.C.; Hwang, S.H.; Kim, N.Y.; Lee, H.S.; Ji, S.; Yang, Y.; Kim, Y. Hypoxia Promotes Acquisition of Aggressive Phenotypes in Human Malignant Mesothelioma. BMC Cancer 2018, 18, 1–11. [Google Scholar] [CrossRef]
- Kaira, K.; Oriuchi, N.; Takahashi, T.; Nakagawa, K.; Ohde, Y.; Okumura, T.; Murakami, H.; Shukuya, T.; Kenmotsu, H.; Naito, T.; et al. L-Type Amino Acid Transporter 1 (LAT1) Expression in Malignant Pleural Mesothelioma. Anticancer Res. 2011, 31, 4075–4082. [Google Scholar]
- Klabatsa, A.; Sheaff, M.T.; Steele, J.P.C.; Evans, M.T.; Rudd, R.M.; Fennell, D.A. Expression and Prognostic Significance of Hypoxia-Inducible Factor 1α (HIF-1α) in Malignant Pleural Mesothelioma (MPM). Lung Cancer 2006, 51, 53–59. [Google Scholar] [CrossRef]
- Francis, R.J.; Segard, T.; Morandeau, L.; Lee, Y.C.G.; Millward, M.J.; Segal, A.; Nowak, A.K. Characterization of Hypoxia in Malignant Pleural Mesothelioma with FMISO PET-CT. Lung Cancer 2015, 90, 55–60. [Google Scholar] [CrossRef] [PubMed]
- Minato, H.; Kurose, N.; Fukushima, M.; Nojima, T.; Usuda, K.; Sagawa, M.; Sakuma, T.; Ooi, A.; Matsumoto, I.; Oda, M.; et al. Comparative Immunohistochemical Analysis of IMP3, GLUT1, EMA, CD146, and Desmin for Distinguishing Malignant Mesothelioma from Reactive Mesothelial Cells. Am. J. Clin. Pathol. 2014, 141, 85–93. [Google Scholar] [CrossRef] [PubMed]
- Hommell-Fontaine, J.; Isaac, S.; Passot, G.; Decullier, E.; Traverse-Glehen, A.; Cotte, E.; You, B.; Mohamed, F.; Gilly, F.N.; Glehen, O.; et al. Malignant Peritoneal Mesothelioma Treated by Cytoreductive Surgery and Hyperthermic Intraperitoneal Chemotherapy: Is GLUT1 Expression a Major Prognostic Factor? A Preliminary Study. Ann. Surg. Oncol. 2013, 20, 3892–3898. [Google Scholar] [CrossRef] [PubMed]
- Corzo, C.A.; Condamine, T.; Lu, L.; Cotter, M.J.; Youn, J.I.; Cheng, P.; Cho, H.-I.; Celis, E.; Quiceno, D.G.; Padhya, T.; et al. HIF-1α Regulates Function and Differentiation of Myeloid-Derived Suppressor Cells in the Tumor Microenvironment. J. Exp. Med. 2010, 207, 2439–2453. [Google Scholar] [CrossRef]
- Doedens, A.L.; Stockmann, C.; Rubinstein, M.P.; Liao, D.; Zhang, N.; DeNardo, D.G.; Coussens, L.M.; Karin, M.; Goldrath, A.W.; Johnson, R.S. Macrophage Expression of Hypoxia-Inducible Factor-1α Suppresses T-Cell Function and Promotes Tumor Progression. Cancer Res. 2010, 70, 7465–7475. [Google Scholar] [CrossRef]
- Sugiura, A.; Rathmell, J.C. Metabolic Barriers to T Cell Function in Tumors. J. Immunol. 2018, 200, 400–407. [Google Scholar] [CrossRef] [PubMed]
- Napoli, F.; Listì, A.; Zambelli, V.; Witel, G.; Bironzo, P.; Papotti, M.; Volante, M.; Scagliotti, G.; Righi, L. Pathological Characterization of Tumor Immune Microenvironment (Time) in Malignant Pleural Mesothelioma. Cancers 2021, 13, 2564. [Google Scholar] [CrossRef] [PubMed]
- Salaroglio, I.C.; Campia, I.; Kopecka, J.; Gazzano, E.; Sara, O.; Ghigo, D.; Riganti, C. Zoledronic Acid Overcomes Chemoresistance and Immunosuppression of Malignant Mesothelioma. Oncotarget 2015, 6, 1128–1142. [Google Scholar] [CrossRef]
- Tanrıverdi, Z.; Meteroglu, F.; Yüce, H.; Şenyiğit, A.; Işcan, M.; Unüvar, S. The Usefulness of Biomarkers in Diagnosis of Asbestos-Induced Malignant Pleural Mesothelioma. Hum. Exp. Toxicol. 2021, 40, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Ohta, A. A Metabolic Immune Checkpoint: Adenosine in Tumor Microenvironment. Front. Immunol. 2016, 7, 109. [Google Scholar] [PubMed]
- Blay, J.; White, T.D.; Hoskin, D.W. The Extracellular Fluid of Solid Carcinomas Contains Immunosuppressive Concentrations of Adenosine. Cancer Res. 1997, 57, 2602–2605. [Google Scholar]
- Synnestvedt, K.; Furuta, G.T.; Comerford, K.M.; Louis, N.; Karhausen, J.; Eltzschig, H.K.; Hansen, K.R.; Thompson, L.F.; Colgan, S.P. Ecto-5′-Nucleotidase (CD73) Regulation by Hypoxia-Inducible Factor-1 Mediates Permeability Changes in Intestinal Epithelia. J. Clin. Investig. 2002, 110, 993–1002. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Allard, B.; Beavis, P.A.; Darcy, P.K.; Stagg, J. Immunosuppressive Activities of Adenosine in Cancer. Curr. Opin. Pharmacol. 2016, 29, 7–16. [Google Scholar] [CrossRef]
- Reyna-Jeldes, M.; Díaz-Muñoz, M.; Madariaga, J.A.; Coddou, C.; Vázquez-Cuevas, F.G. Autocrine and Paracrine Purinergic Signaling in the Most Lethal Types of Cancer. Purinergic Signal. 2021, 17, 345–370. [Google Scholar]
- Shi, L.; Wu, Z.; Miao, J.; Du, S.; Ai, S.; Xu, E.; Feng, M.; Song, J.; Guan, W. Adenosine Interaction with Adenosine Receptor A2a Promotes Gastric Cancer Metastasis by Enhancing PI3K-AKT-MTOR Signaling. Mol. Biol. Cell 2019, 30, 2527–2534. [Google Scholar] [CrossRef]
- Wang, J.; Wang, Y.; Chu, Y.; Li, Z.; Yu, X.; Huang, Z.; Xu, J.; Zheng, L. Tumor-Derived Adenosine Promotes Macrophage Proliferation in Human Hepatocellular Carcinoma. J. Hepatol. 2021, 74, 627–637. [Google Scholar] [CrossRef]
- Al-Taei, S.; Salimu, J.; Spary, L.K.; Clayton, A.; Lester, J.F.; Tabi, Z. Prostaglandin E2-Mediated Adenosinergic Effects on CD14+ Cells: Self-Amplifying Immunosuppression in Cancer. Oncoimmunology 2017, 6, e1268308. [Google Scholar] [CrossRef] [PubMed]
- Clayton, A.; Al-Taei, S.; Webber, J.; Mason, M.D.; Tabi, Z. Cancer Exosomes Express CD39 and CD73, Which Suppress T Cells through Adenosine Production. J. Immunol. 2011, 187, 676–683. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Kuribayashi, K.; Ishigaki, H.; Tada, A.; Negi, Y.; Minami, T.; Takahashi, R.; Doi, H.; Kitajima, K.; Yokoi, T.; et al. Adenosine Deaminase in Pleural Effusion and Its Relationship with Clinical Parameters in Patients with Malignant Pleural Mesothelioma. Cancer Investig. 2020, 38, 356–364. [Google Scholar] [CrossRef]
- Testa, J.R.; Cheung, M.; Pei, J.; Below, J.E.; Tan, Y.; Sementino, E.; Cox, N.J.; Dogan, A.U.; Pass, H.I.; Trusa, S.; et al. Germline BAP1 Mutations Predispose to Malignant Mesothelioma. Nat. Genet. 2011, 43, 1022–1025. [Google Scholar] [CrossRef] [PubMed]
- Carbone, M.; Pass, H.I.; Ak, G.; Alexander, H.R.; Baas, P.; Baumann, F.; Blakely, A.M.; Bueno, R.; Bzura, A.; Cardillo, G.; et al. Medical and Surgical Care of Patients with Mesothelioma and Their Relatives Carrying Germline BAP1 Mutations. J. Thorac. Oncol. 2022, 17, 873–889. [Google Scholar]
- Xu, J.; Kadariya, Y.; Cheung, M.; Pei, J.; Talarchek, J.; Sementino, E.; Tan, Y.; Menges, C.W.; Cai, K.Q.; Litwin, S.; et al. Germline Mutation of Bap1 Accelerates Development of Asbestos-Induced Malignant Mesothelioma. Cancer Res. 2014, 74, 4388–4397. [Google Scholar] [CrossRef]
- Betti, M.; Aspesi, A.; Ferrante, D.; Sculco, M.; Righi, L.; Mirabelli, D.; Napoli, F.; Rondón-Lagos, M.; Casalone, E.; Vignolo Lutati, F.; et al. Sensitivity to Asbestos Is Increased in Patients with Mesothelioma and Pathogenic Germline Variants in BAP1 or Other DNA Repair Genes. Genes Chromosomes Cancer 2018, 57, 573–583. [Google Scholar] [CrossRef]
- Carbone, M.; Harbour, J.W.; Brugarolas, J.; Bononi, A.; Pagano, I.; Dey, A.; Krausz, T.; Pass, H.I.; Yang, H.; Gaudino, G. Biological Mechanisms and Clinical Signifi Cance of BAP1 Mutations in Human Cancer. Cancer Discov. 2020, 10, 1103–1120. [Google Scholar] [CrossRef]
- Bononi, A.; Yang, H.; Giorgi, C.; Patergnani, S.; Pellegrini, L.; Su, M.; Xie, G.; Signorato, V.; Pastorino, S.; Morris, P.; et al. Germline BAP1 Mutations Induce a Warburg Effect. Cell Death Differ. 2017, 24, 1694–1704. [Google Scholar] [CrossRef]
- De Berardinis, R.J.; Chandel, N.S. Fundamentals of Cancer Metabolism. Sci. Adv. 2016, 2, e1600200. [Google Scholar] [CrossRef]
- Romero-Garcia, S.; Moreno-Altamirano, M.M.B.; Prado-Garcia, H.; Sánchez-García, F.J. Lactate Contribution to the Tumor Microenvironment: Mechanisms, Effects on Immune Cells and Therapeutic Relevance. Front. Immunol. 2016, 7, 52. [Google Scholar] [PubMed]
- Végran, F.; Boidot, R.; Michiels, C.; Sonveaux, P.; Feron, O. Lactate Influx through the Endothelial Cell Monocarboxylate Transporter MCT1 Supports an NF-KB/IL-8 Pathway That Drives Tumor Angiogenesis. Cancer Res. 2011, 71, 2550–2560. [Google Scholar] [CrossRef]
- Colegio, O.R.; Chu, N.Q.; Szabo, A.L.; Chu, T.; Rhebergen, A.M.; Jairam, V.; Cyrus, N.; Brokowski, C.E.; Eisenbarth, S.C.; Phillips, G.M.; et al. Functional Polarization of Tumour-Associated Macrophages by Tumour-Derived Lactic Acid. Nature 2014, 513, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Baughman, J.M.; Rose, C.M.; Kolumam, G.; Webster, J.D.; Wilkerson, E.M.; Merrill, A.E.; Rhoads, T.W.; Noubade, R.; Katavolos, P.; Lesch, J.; et al. NeuCode Proteomics Reveals Bap1 Regulation of Metabolism. Cell Rep. 2016, 16, 583–595. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Ma, J.; Li, W.; Mo, R.; Zhang, P.; Gao, K.; Jin, X.; Xiao, J.; Wang, C.; Fan, J. Stabilization of MCRS1 by BAP1 Prevents Chromosome Instability in Renal Cell Carcinoma. Cancer Lett. 2015, 369, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Ruan, H.B.; Han, X.; Li, M.D.; Singh, J.P.; Qian, K.; Azarhoush, S.; Zhao, L.; Bennett, A.M.; Samuel, V.T.; Wu, J.; et al. O-GlcNAc Transferase/Host Cell Factor C1 Complex Regulates Gluconeogenesis by Modulating PGC-1α Stability. Cell Metab. 2012, 16, 226–237. [Google Scholar] [CrossRef]
- Gray, S.G.; Mutti, L. Immunotherapy for Mesothelioma: A Critical Review of Current Clinical Trials and Future Perspectives. Transl. Lung Cancer Res. 2020, 9, S100–S119. [Google Scholar]
- Obacz, J.; Yung, H.; Shamseddin, M.; Linnane, E.; Liu, X.; Azad, A.A.; Rassl, D.M.; Fairen-Jimenez, D.; Rintoul, R.C.; Nikolić, M.Z.; et al. Biological Basis for Novel Mesothelioma Therapies. Br. J. Cancer 2021, 125, 1039–1055. [Google Scholar]
- Tedesco, J.; Jaradeh, M.; Vigneswaran, W.T. Malignant Pleural Mesothelioma: Current Understanding of the Immune Microenvironment and Treatments of a Rare Disease. Cancers 2022, 14, 4415. [Google Scholar]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fiorilla, I.; Martinotti, S.; Todesco, A.M.; Bonsignore, G.; Cavaletto, M.; Patrone, M.; Ranzato, E.; Audrito, V. Chronic Inflammation, Oxidative Stress and Metabolic Plasticity: Three Players Driving the Pro-Tumorigenic Microenvironment in Malignant Mesothelioma. Cells 2023, 12, 2048. https://doi.org/10.3390/cells12162048
Fiorilla I, Martinotti S, Todesco AM, Bonsignore G, Cavaletto M, Patrone M, Ranzato E, Audrito V. Chronic Inflammation, Oxidative Stress and Metabolic Plasticity: Three Players Driving the Pro-Tumorigenic Microenvironment in Malignant Mesothelioma. Cells. 2023; 12(16):2048. https://doi.org/10.3390/cells12162048
Chicago/Turabian StyleFiorilla, Irene, Simona Martinotti, Alberto Maria Todesco, Gregorio Bonsignore, Maria Cavaletto, Mauro Patrone, Elia Ranzato, and Valentina Audrito. 2023. "Chronic Inflammation, Oxidative Stress and Metabolic Plasticity: Three Players Driving the Pro-Tumorigenic Microenvironment in Malignant Mesothelioma" Cells 12, no. 16: 2048. https://doi.org/10.3390/cells12162048
APA StyleFiorilla, I., Martinotti, S., Todesco, A. M., Bonsignore, G., Cavaletto, M., Patrone, M., Ranzato, E., & Audrito, V. (2023). Chronic Inflammation, Oxidative Stress and Metabolic Plasticity: Three Players Driving the Pro-Tumorigenic Microenvironment in Malignant Mesothelioma. Cells, 12(16), 2048. https://doi.org/10.3390/cells12162048









