Can Galectin-3 Be a Novel Biomarker in Chronic Lymphocytic Leukemia?
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Material and Patients’ Characteristics
2.2. Flow Cytometry Assessment
2.2.1. PBMCs Isolation
2.2.2. Cell Surface and Intracellular Stainings
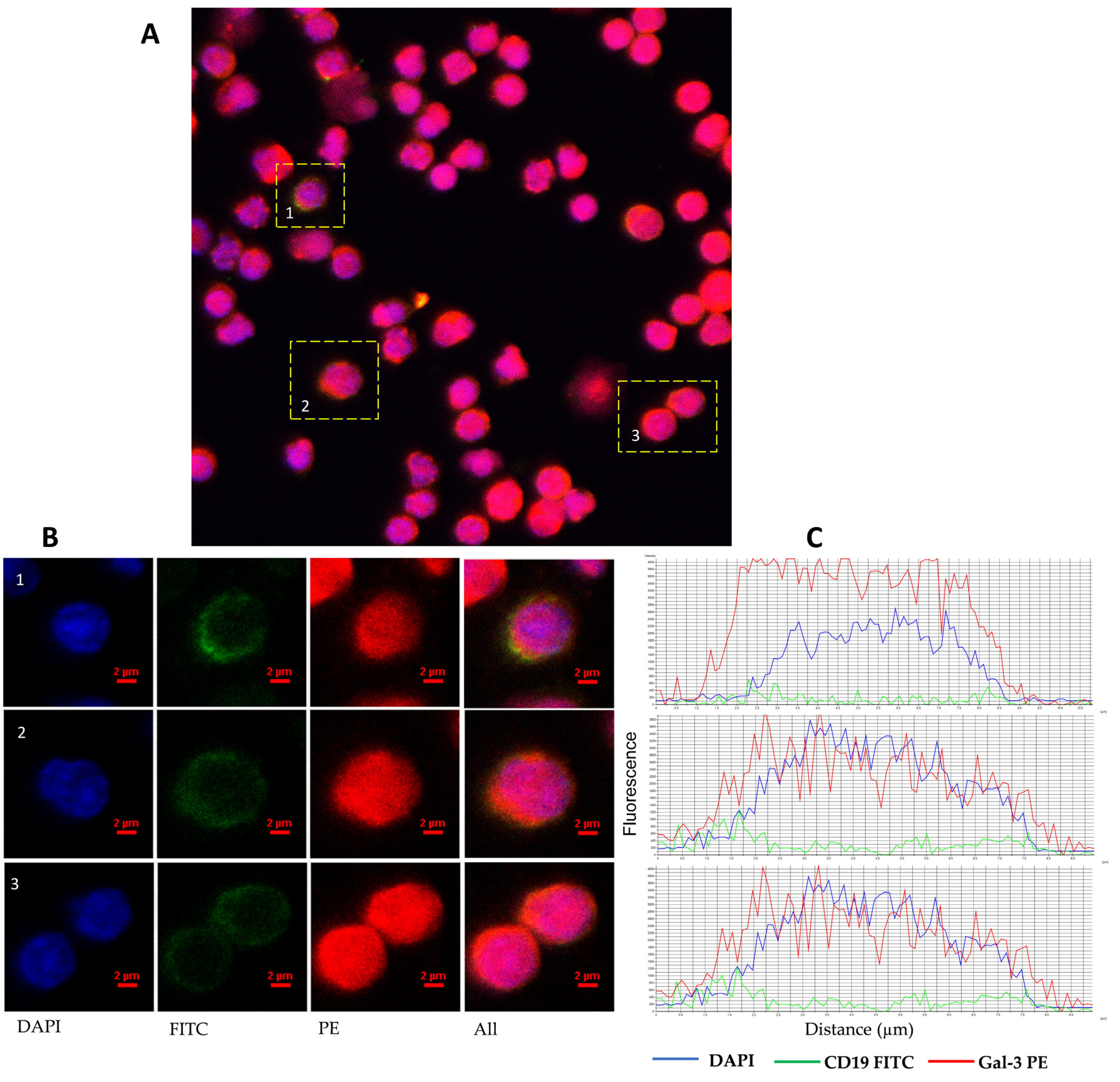
2.3. Confocal Microscopy
2.4. Quantitative Measurement of Gal-3 mRNA Expression
2.4.1. Magnetic Isolation of CD19+ Cells
2.4.2. RT-qPCR
2.5. Gal-3 Detection in Plasma
2.6. Fluorescent In Situ Hybridization (FISH)
2.7. Statistical Analysis
3. Results
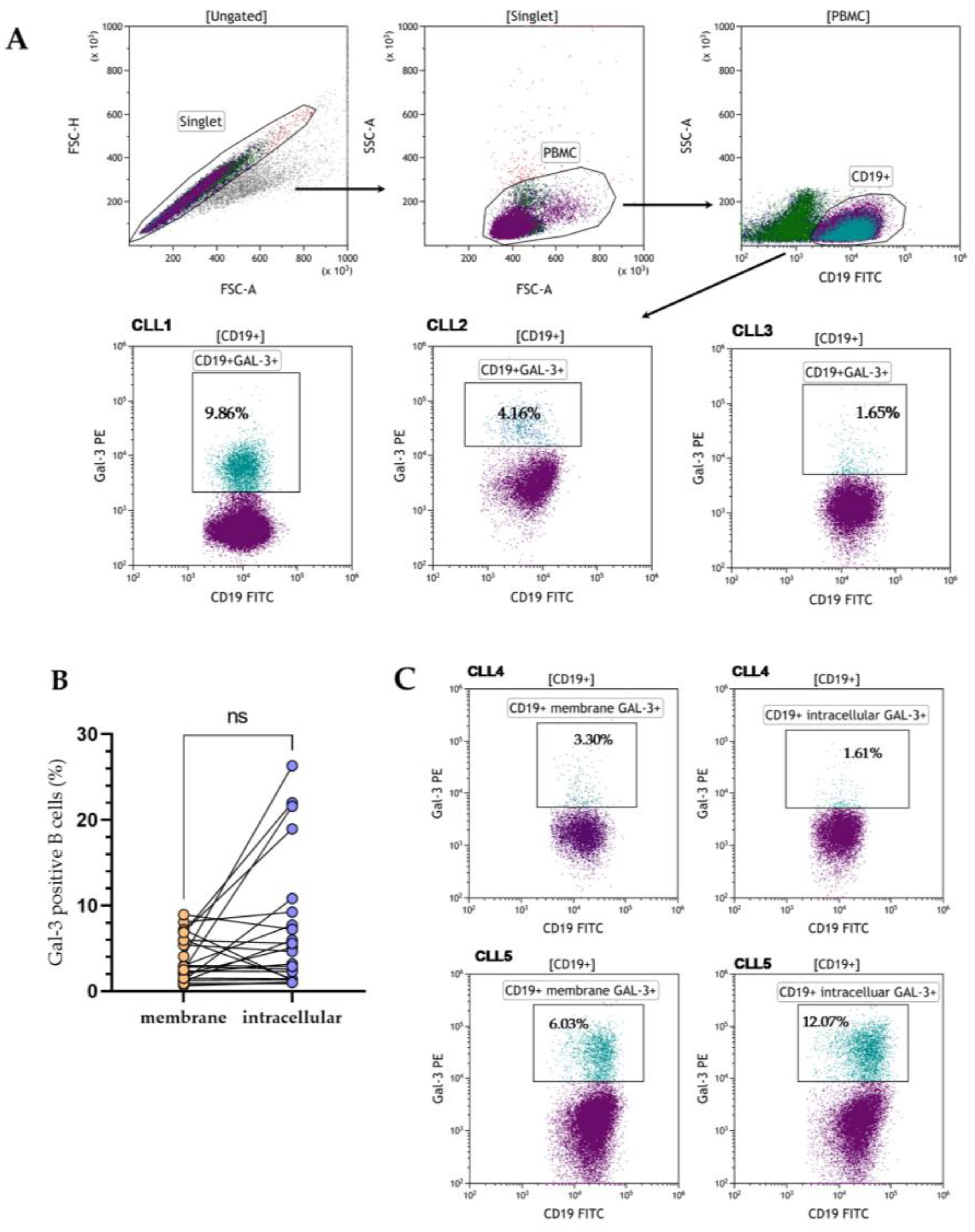
3.1. Heterogenous Gal-3 Expression in CLL Patients
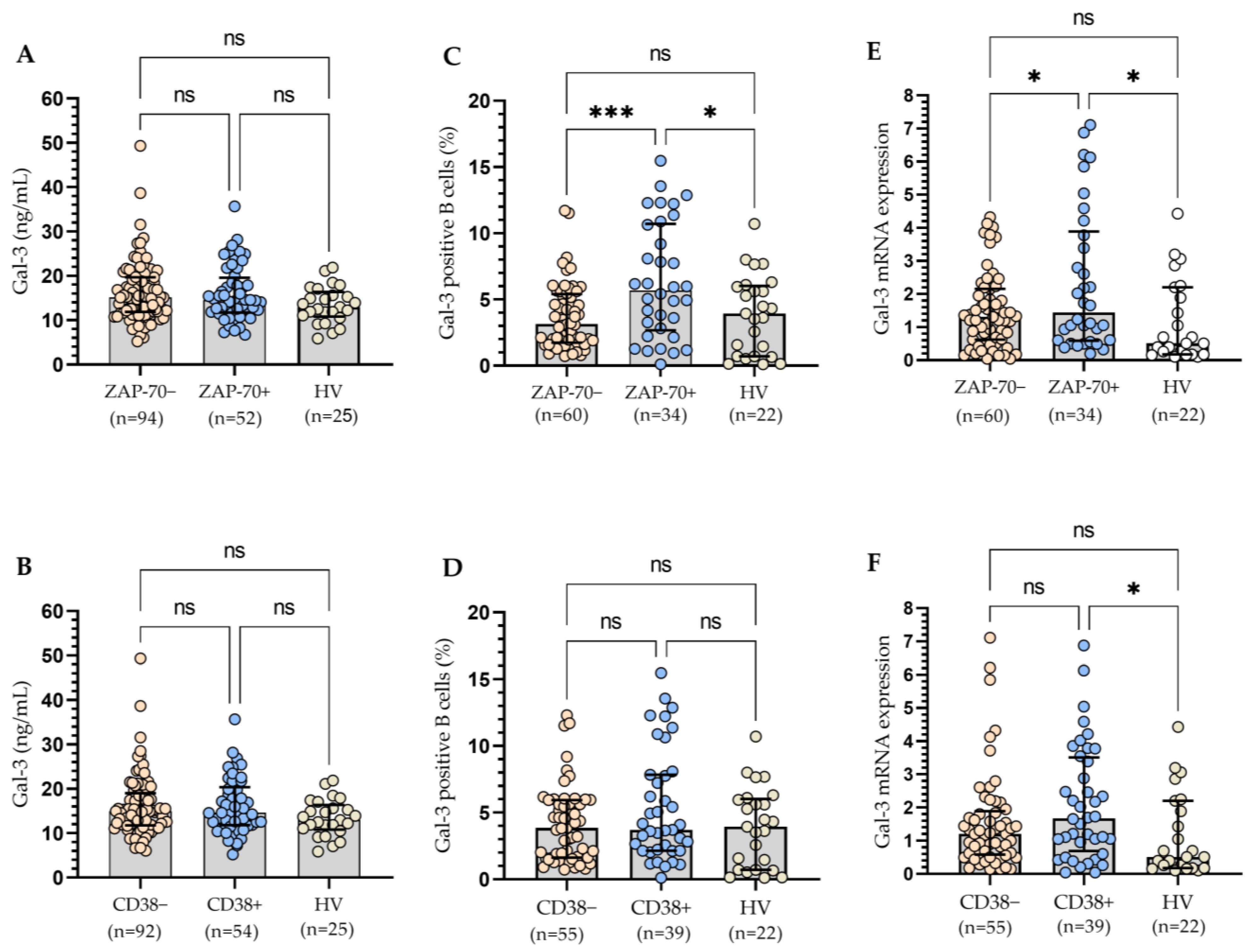
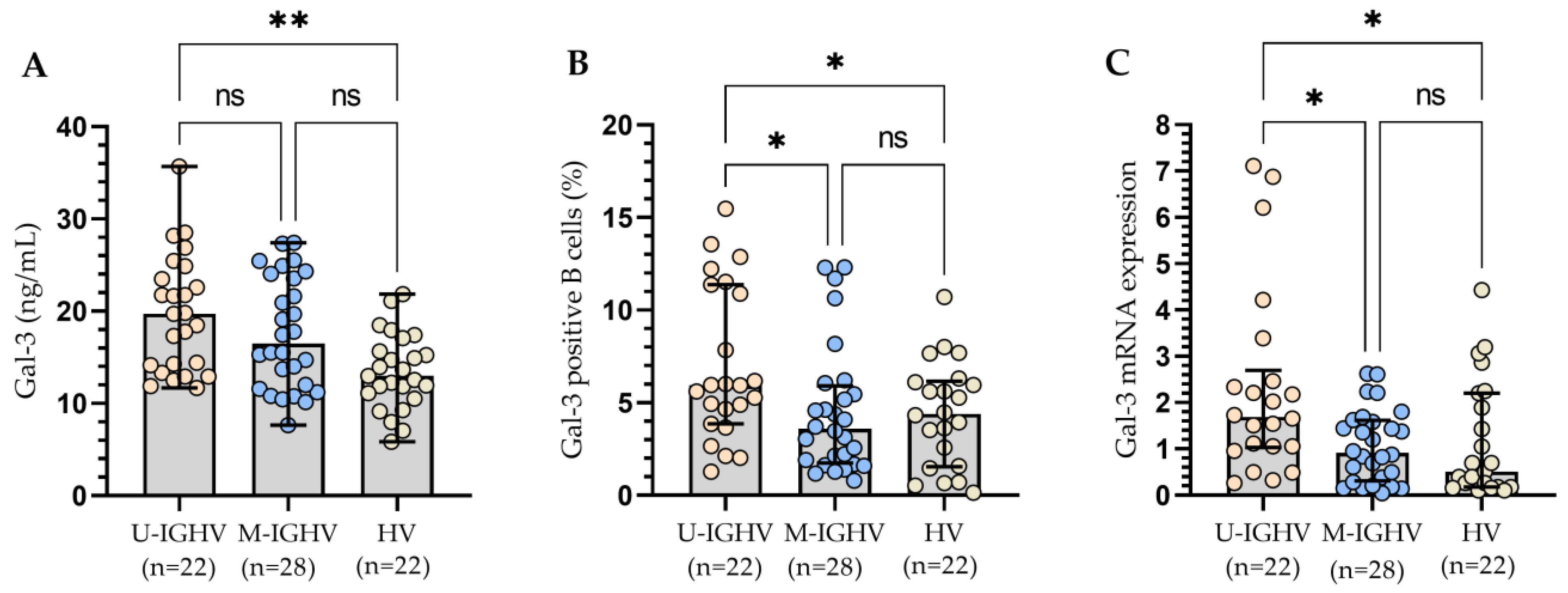
3.2. The Relationship between the Molecular Profile of Patients and Gal-3 Levels
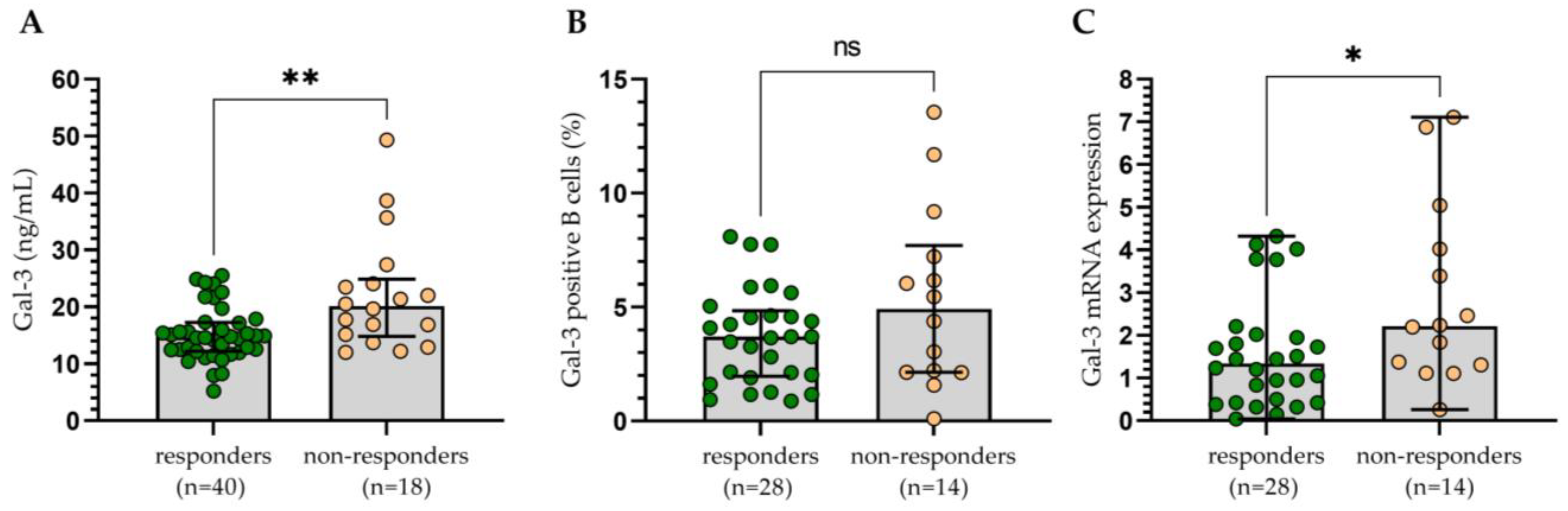
3.3. Assessment of the Influence of Gal-3 Expression on the Clinical Outcomes
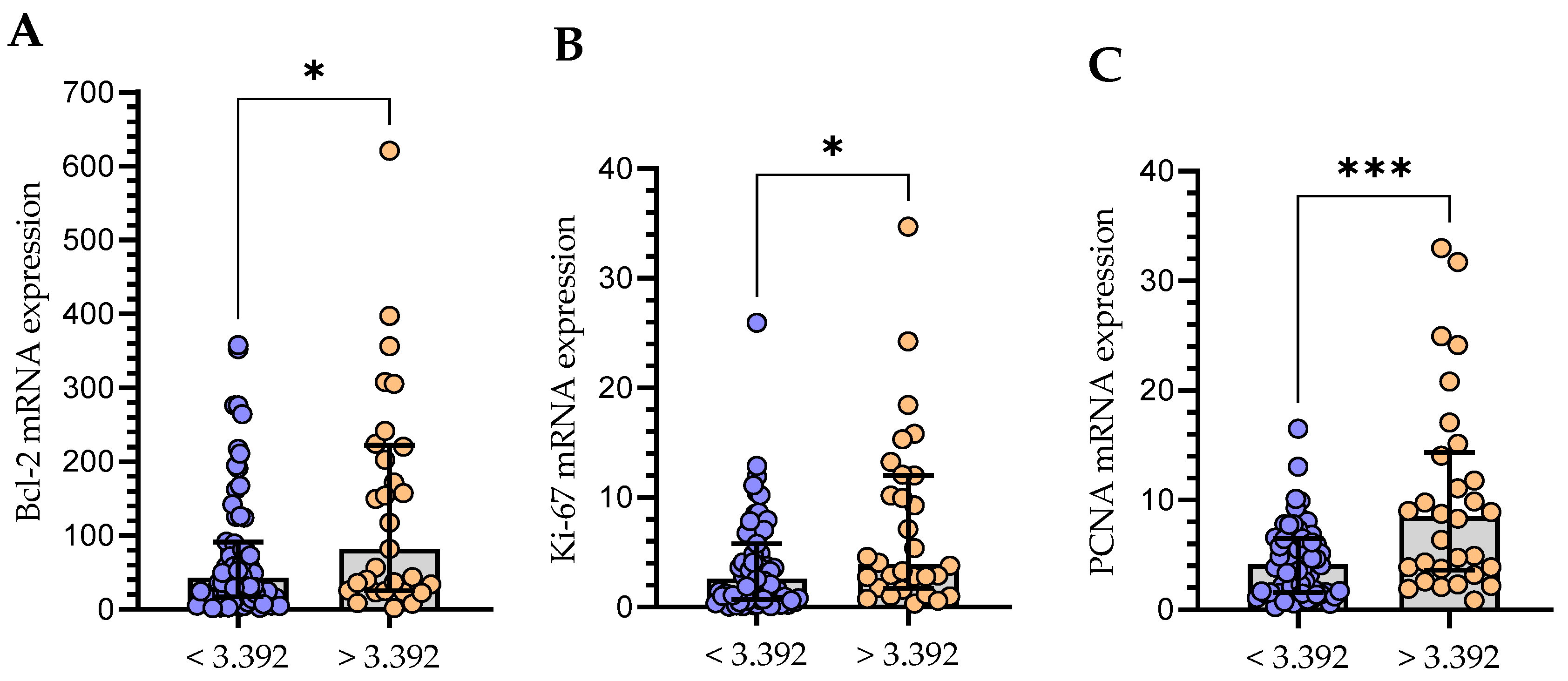
3.4. The Relationship between the Level of mRNA Expression of Gal-3 and Bcl-2 and Proliferation Markers (Ki-67 and PCNA)
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fiorcari, S.; Maffei, R.; Atene, C.G.; Potenza, L.; Luppi, M.; Marasca, R. Nurse-Like Cells and Chronic Lymphocytic Leukemia B Cells: A Mutualistic Crosstalk inside Tissue Microenvironments. Cells 2021, 10, 217. [Google Scholar] [CrossRef] [PubMed]
- Hermansen, J.U.; Yin, Y.; Urban, A.; Myklebust, C.V.; Karlsen, L.; Melvold, K.; Tveita, A.A.; Taskén, K.; Munthe, L.A.; Tjønnfjord, G.E.; et al. A Tumor Microenvironment Model of Chronic Lymphocytic Leukemia Enables Drug Sensitivity Testing to Guide Precision Medicine. Cell Death Discov. 2023, 9, 125. [Google Scholar] [CrossRef] [PubMed]
- Pena, C.; Mirandola, L.; Figueroa, J.A.; Hosiriluck, N.; Suvorava, N.; Trotter, K.; Reidy, A.; Rakhshanda, R.; Payne, D.; Jenkins, M.; et al. Galectins as Therapeutic Targets for Hematological Malignancies: A Hopeful Sweetness. Ann. Transl. Med. 2014, 2, 87. [Google Scholar] [CrossRef] [PubMed]
- Kapetanakis, N.-I.; Busson, P. Galectins as Pivotal Components in Oncogenesis and Immune Exclusion in Human Malignancies. Front. Immunol. 2023, 14, 1145268. [Google Scholar] [CrossRef] [PubMed]
- Kruk, L.; Braun, A.; Cosset, E.; Gudermann, T.; Mammadova-Bach, E. Galectin Functions in Cancer-Associated Inflammation and Thrombosis. Front. Cardiovasc. Med. 2023, 10, 1052959. [Google Scholar] [CrossRef] [PubMed]
- Radziejewska, I. Galectin-3 and Epithelial MUC1 Mucin—Interactions Supporting Cancer Development. Cancers 2023, 15, 2680. [Google Scholar] [CrossRef] [PubMed]
- Nangia-Makker, P.; Balan, V.; Raz, A. Regulation of Tumor Progression by Extracellular Galectin-3. Cancer Microenviron. 2008, 1, 43–51. [Google Scholar] [CrossRef]
- Pereira, J.X.; dos Santos, S.N.; Pereira, T.C.; Cabanel, M.; Chammas, R.; de Oliveira, F.L.; Bernardes, E.S.; El-Cheikh, M.C. Galectin-3 Regulates the Expression of Tumor Glycosaminoglycans and Increases the Metastatic Potential of Breast Cancer. J. Oncol. 2019, 2019, 9827147. [Google Scholar] [CrossRef]
- Ruvolo, P.P. Galectins as Regulators of Cell Survival in the Leukemia Niche. Adv. Biol. Regul. 2019, 71, 41–54. [Google Scholar] [CrossRef]
- Ruvolo, P.P. Galectin 3 as a Guardian of the Tumor Microenvironment. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2016, 1863, 427–437. [Google Scholar] [CrossRef]
- Song, L.; Tang, J.; Owusu, L.; Sun, M.-Z.; Wu, J.; Zhang, J. Galectin-3 in Cancer. Clin. Chim. Acta 2014, 431, 185–191. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, M.; Hu, Q.; Zheng, S.; Soh, A.; Zheng, Y.; Yuan, H. Galectin-3 as a Novel Biomarker for Disease Diagnosis and a Target for Therapy (Review). Int. J. Mol. Med. 2017, 41, 599–614. [Google Scholar] [CrossRef] [PubMed]
- Farhad, M.; Rolig, A.S.; Redmond, W.L. The Role of Galectin-3 in Modulating Tumor Growth and Immunosuppression within the Tumor Microenvironment. OncoImmunology 2018, 7, e1434467. [Google Scholar] [CrossRef] [PubMed]
- Hsu, D.K.; Chen, H.; Liu, F. Galectin-3 Regulates T-cell Functions. Immunol. Rev. 2009, 230, 114–127. [Google Scholar] [CrossRef]
- Kim, S.-J.; Chun, K.-H. Non-Classical Role of Galectin-3 in Cancer Progression: Translocation to Nucleus by Carbohydrate-Recognition Independent Manner. BMB Rep. 2020, 53, 173–180. [Google Scholar] [CrossRef]
- Dumic, J.; Dabelic, S.; Flögel, M. Galectin-3: An Open-Ended Story. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2006, 1760, 616–635. [Google Scholar] [CrossRef]
- Fortuna-Costa, A.; Gomes, A.M.; Kozlowski, E.O.; Stelling, M.P.; Pavão, M.S.G. Extracellular Galectin-3 in Tumor Progression and Metastasis. Front. Oncol. 2014, 4, 138. [Google Scholar] [CrossRef]
- Mansueto, G.; Di Vito, A.; Belluomo, C.; Murino, P.; Natella, V.; Camastra, C.; Presta, I.; Malara, N.; de Rosa, G.; Donato, G.; et al. A Case of Intravascular Large B Cell Lymphoma: New Clinical and Immunohistochemical Findings. Neuropathology 2016, 36, 496–503. [Google Scholar] [CrossRef]
- Kay, N.E.; Hampel, P.J.; Van Dyke, D.L.; Parikh, S.A. CLL Update 2022: A Continuing Evolution in Care. Blood Rev. 2022, 54, 100930. [Google Scholar] [CrossRef]
- Farzadfard, E.; Kalantari, T.; Tamaddon, G. Serum Expression of Seven MicroRNAs in Chronic Lymphocytic Leukemia Patients. J. Blood Med. 2020, 11, 97–102. [Google Scholar] [CrossRef]
- Hallek, M.; Cheson, B.D.; Catovsky, D.; Caligaris-Cappio, F.; Dighiero, G.; Döhner, H.; Hillmen, P.; Keating, M.; Montserrat, E.; Chiorazzi, N.; et al. iwCLL Guidelines for Diagnosis, Indications for Treatment, Response Assessment, and Supportive Management of CLL. Blood 2018, 131, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Puiggros, A.; Blanco, G.; Espinet, B. Genetic Abnormalities in Chronic Lymphocytic Leukemia: Where We Are and Where We Go. BioMed Res. Int. 2014, 2014, 435983. [Google Scholar] [CrossRef] [PubMed]
- Lima, T.; Perpétuo, L.; Henrique, R.; Fardilha, M.; Leite-Moreira, A.; Bastos, J.; Vitorino, R. Galectin-3 in Prostate Cancer and Heart Diseases: A Biomarker for These Two Frightening Pathologies? Mol. Biol. Rep. 2023, 50, 2763–2778. [Google Scholar] [CrossRef] [PubMed]
- Rai, K.R.; Sawitsky, A.; Cronkite, E.P.; Chanana, A.D.; Levy, R.N.; Pasternack, B.S. Clinical Staging of Chronic Lymphocytic Leukemia. Blood 1975, 46, 219–234. [Google Scholar] [CrossRef] [PubMed]
- Zarobkiewicz, M.; Kowalska, W.; Chocholska, S.; Tomczak, W.; Szymańska, A.; Morawska, I.; Wojciechowska, A.; Bojarska-Junak, A. High M-MDSC Percentage as a Negative Prognostic Factor in Chronic Lymphocytic Leukaemia. Cancers 2020, 12, 2614. [Google Scholar] [CrossRef] [PubMed]
- Chocholska, S.; Zarobkiewicz, M.; Szymańska, A.; Lehman, N.; Woś, J.; Bojarska-Junak, A. Prognostic Value of the miR-17~92 Cluster in Chronic Lymphocytic Leukemia. Int. J. Med. Sci. 2023, 24, 1705. [Google Scholar] [CrossRef]
- Guo, Y.; Shen, R.; Yu, L.; Zheng, X.; Cui, R.; Song, Y.; Wang, D. Roles of Galectin-3 in the Tumor Microenvironment and Tumor Metabolism (Review). Oncol. Rep. 2020, 44, 1799–1809. [Google Scholar] [CrossRef]
- Asgarian-Omran, H.; Forghani, P.; Hojjat-Farsangi, M.; Roohi, A.; Sharifian, R.A.; Razavi, S.M.; Jeddi-Tehrani, M.; Rabbani, H.; Shokri, F. Expression Profile of Galectin-1 and Galectin-3 Molecules in Different Subtypes of Chronic Lymphocytic Leukemia. Cancer Investig. 2010, 28, 717–725. [Google Scholar] [CrossRef]
- Dhirapong, A.; Lleo, A.; Leung, P.; Gershwin, M.E.; Liu, F.-T. The Immunological Potential of Galectin-1 and -3. Autoimmun. Rev. 2009, 8, 360–363. [Google Scholar] [CrossRef]
- Michalová, Z.; Čoma, M.; Kičová, M.; Gabzdilová, J.; Dedinská, K.; Guman, T.; Hájiková, M.; Veselinyová, D.; Giertlova, M.; Gál, P.; et al. Overexpression of Galectin-3 in Chronic Lymphocytic Leukemia Is Associated With 17p Deletion: A Short Report. Anticancer Res. 2019, 39, 2805–2810. [Google Scholar] [CrossRef]
- Fei, F.; Joo, E.J.; Tarighat, S.S.; Schiffer, I.; Paz, H.; Fabbri, M.; Abdel-Azim, H.; Groffen, J.; Heisterkamp, N. B-Cell Precursor Acute Lymphoblastic Leukemia and Stromal Cells Communicate through Galectin-3. Oncotarget 2015, 6, 11378–11394. [Google Scholar] [CrossRef] [PubMed]
- Wdowiak, K.; Gallego-Colon, E.; Francuz, T.; Czajka-Francuz, P.; Ruiz-Agamez, N.; Kubeczko, M.; Grochoła, I.; Wybraniec, M.; Chudek, J.; Wojnar, J. Increased Serum Levels of Galectin-9 in Patients with Chronic Lymphocytic Leukemia. Oncol. Lett. 2018, 17, 1019–1029. [Google Scholar] [CrossRef] [PubMed]
- D’Arena, G.; Tarnani, M.; Rumi, C.; Vaisitti, T.; Aydin, S.; De Filippi, R.; Perrone, F.; Pinto, A.; Chiusolo, P.; Deaglio, S.; et al. Prognostic Significance of Combined Analysis of ZAP-70 and CD38 in Chronic Lymphocytic Leukemia. Am. J. Hematol. 2007, 82, 787–791. [Google Scholar] [CrossRef]
- Amaya-Chanaga, C.I.; Rassenti, L.Z. Biomarkers in Chronic Lymphocytic Leukemia: Clinical Applications and Prognostic Markers. Best Pract. Res. Clin. Haematol. 2016, 29, 79–89. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.; Ahn, I.E. Prognostic Markers in the Era of Targeted Therapies. Acta Haematol. 2023, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Stiasny, A.; Freier, C.P.; Kuhn, C.; Schulze, S.; Mayr, D.; Alexiou, C.; Janko, C.; Wiest, I.; Dannecker, C.; Jeschke, U.; et al. The Involvement of E6, P53, P16, MDM2 and Gal-3 in the Clinical Outcome of Patients with Cervical Cancer. Oncol. Lett. 2017, 14, 4467–4476. [Google Scholar] [CrossRef]
- Chavez, J.C.; Kharfan-Dabaja, M.A.; Kim, J.; Yue, B.; Dalia, S.; Pinilla-Ibarz, J.; Anasetti, C.; Locke, F.L. Genomic Aberrations Deletion 11q and Deletion 17p Independently Predict for Worse Progression-Free and Overall Survival after Allogeneic Hematopoietic Cell Transplantation for Chronic Lymphocytic Leukemia. Leuk. Res. 2014, 38, 1165–1172. [Google Scholar] [CrossRef]
- Fukumori, T.; Kanayama, H.; Raz, A. The Role of Galectin-3 in Cancer Drug Resistance. Drug Resist. Updates 2007, 10, 101–108. [Google Scholar] [CrossRef]
- Thijssen, V.L.; Heusschen, R.; Caers, J.; Griffioen, A.W. Galectin Expression in Cancer Diagnosis and Prognosis: A Systematic Review. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2015, 1855, 235–247. [Google Scholar] [CrossRef]
- Perini, G.F.; Feres, C.C.P.; Teixeira, L.L.C.; Hamerschlak, N. BCL-2 Inhibition as Treatment for Chronic Lymphocytic Leukemia. Curr. Treat. Options Oncol. 2021, 22, 66. [Google Scholar] [CrossRef]
- Roberts, A.W. Therapeutic Development and Current Uses of BCL-2 Inhibition. Hematology 2020, 2020, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Tzifi, F.; Economopoulou, C.; Gourgiotis, D.; Ardavanis, A.; Papageorgiou, S.; Scorilas, A. The Role of BCL2 Family of Apoptosis Regulator Proteins in Acute and Chronic Leukemias. Adv. Hematol. 2012, 2012, 524308. [Google Scholar] [CrossRef] [PubMed]
- Herishanu, Y.; Pérez-Galán, P.; Liu, D.; Biancotto, A.; Pittaluga, S.; Vire, B.; Gibellini, F.; Njuguna, N.; Lee, E.; Stennett, L.; et al. The Lymph Node Microenvironment Promotes B-Cell Receptor Signaling, NF-κB Activation, and Tumor Proliferation in Chronic Lymphocytic Leukemia. Blood 2011, 117, 563–574. [Google Scholar] [CrossRef] [PubMed]
- Bruey, J.-M.; Kantarjian, H.; Ma, W.; Estrov, Z.; Yeh, C.; Donahue, A.; Sanders, H.; O’Brien, S.; Keating, M.; Albitar, M. Circulating Ki-67 Index in Plasma as a Biomarker and Prognostic Indicator in Chronic Lymphocytic Leukemia. Leuk. Res. 2010, 34, 1320–1324. [Google Scholar] [CrossRef] [PubMed]
- Kipps, T.J.; Stevenson, F.K.; Wu, C.J.; Croce, C.M.; Packham, G.; Wierda, W.G.; O’Brien, S.; Gribben, J.; Rai, K. Chronic Lymphocytic Leukaemia. Nat. Rev. Dis. Primers 2017, 3, 16096. [Google Scholar] [CrossRef] [PubMed]
- Giglio, A.D.; O’brien, S.; Ford, R.J.; Manning, J.; Saya, H.; Keating, M.; Johnston, D.; Chamone, D.F.; Deisseroth, A.B. Proliferating Cell Nuclear Antigen (PCNA) Expression in Chronic Lymphocytic Leukemia (CLL). Leuk. Lymphoma 1993, 10, 265–271. [Google Scholar] [CrossRef]
- Del Giglio, A.; O’Brien, S.; Ford, R.; Saya, H.; Manning, J.; Keating, M.; Johnston, D.; Khetan, R.; el-Naggar, A.; Deisseroth, A. Prognostic Value of Proliferating Cell Nuclear Antigen Expression in Chronic Lymphoid Leukemia. Blood 1992, 79, 2717–2720. [Google Scholar] [CrossRef]
- Wang, W.; Guo, H.; Geng, J.; Zheng, X.; Wei, H.; Sun, R.; Tian, Z. Tumor-Released Galectin-3, a Soluble Inhibitory Ligand of Human NKp30, Plays an Important Role in Tumor Escape from NK Cell Attack. J. Biol. Chem. 2014, 289, 33311–33319. [Google Scholar] [CrossRef]
- Krijgsman, D.; Roelands, J.; Andersen, M.N.; Wieringa, C.H.L.A.; Tollenaar, R.A.E.M.; Hendrickx, W.; Bedognetti, D.; Hokland, M.; Kuppen, P.J.K. Expression of NK Cell Receptor Ligands in Primary Colorectal Cancer Tissue in Relation to the Phenotype of Circulating NK- and NKT Cells, and Clinical Outcome. Mol. Immunol. 2020, 128, 205–218. [Google Scholar] [CrossRef]





| Characteristics | CLL Patients (n = 146) | HV (n = 26) |
|---|---|---|
| Gender | ||
| Female, n (%) | 71 (48.6) | 12 (46.2) |
| Male, n (%) | 75 (51.4) | 14 (56.8) |
| Age (years) | ||
| Median (IQR) | 66 (59–72) | 58 (53–62) |
| Min–max | 37–88 | 35–64 |
| Rai Stage [24] | ||
| 0, n (%) | 63 (43.2) | |
| I, n (%) | 31 (21.2) | |
| II, n (%) | 25 (17.1) | |
| III, n (%) | 16 (11.0) | |
| IV, n (%) | 11 (7.5) | |
| ZAP-70-positive, n (%) * | 52 (35.6) | |
| CD38-positive, n (%) * | 54 (37.0) | |
| IGHV mutational status | ||
| U-IGHV, n (%) | 22 (15.1) | |
| M-IGHV, n (%) | 28 (19.2) | |
| not available, n (%) | 96 (65.7) | |
| Chromosomal aberrations | ||
| 17p-, n (%) | 12 (8.2) | |
| 11q-, n (%) | 13 (8.9) | |
| +12, n (%) | 5 (3.4) | |
| sole 13q-, n (%) | 40 (27.4) | |
| Patients treated during observation period, n (%) # | 58 (39.7) | |
| Complete remission (CR), n (%) | 12/58 (20.7) | |
| Partial remission (PR), n (%) | 28/58 (48.3) | |
| Stable disease (SD), n (%) | 13/58 (22.4) | |
| Disease progression (PD), n (%) | 5/58 (8.6) | |
| First line therapy | ||
| fludarabine + cyclophosphamide + rituximab | 10/58 | |
| bendamustine + rituximab | 15/58 | |
| chlorambucil and obinutuzumab | 14/58 | |
| rituximab + cyclophosphamide + deksametazon | 4/58 | |
| venetoklax and obinutuzumab | 8/58 | |
| ibrutinib | 4/58 | |
| acalabrutinib | 3/58 | |
| WBC (G/L), median (IQR) | 24.63 (17.2–55.16) | |
| Lymphocyte count (G/L), median (IQR) | 18.32 (10.87–47.58) | |
| LDH level (IU/L), median (IQR) | 349 (285–412) | |
| β2M level (mg/dL), median (IQR) | 2.60 (2.08–3.40) |
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| Risk Factors | HR | 95% CI | p-Value | HR | 95% CI | p-Value |
| High ZAP-70 expression | 1.803 | 1.240–2.620 | 0.002 | 1.676 | 0.797–3.527 | 0.173 |
| High CD38 expression | 1.339 | 0.908–1.975 | 0.140 | na | ||
| High β2M level | 2.437 | 1.631–3.643 | <0.0001 | 2.319 | 1.103–4.875 | 0.026 |
| Positive 17p− and/or 11q− | 2.480 | 1.372–4.614 | <0.001 | 3.038 | 1.343–6.871 | 0.007 |
| High Gal-3 mRNA level | 2.123 | 1.172–3.846 | 0.013 | 2.492 | 0.706–8.793 | 0.155 |
| High percentage of Gal-3-positive B cells | 2.226 | 1.164–4.259 | 0.015 | 0.696 | 0.220–2.205 | 0.538 |
| High Gal-3 plasma level | 1.037 | 0.634–1.698 | 0.886 | na | ||
| Membrane Gal-3 (%) | Intracellular Gal-3 (%) | Gal-3 mRNA (2−ΔCq) | ||||
|---|---|---|---|---|---|---|
| Patient No. | At the Time of Diagnosis | After Treatment | At the Time of Diagnosis | After Treatment | At the Time of Diagnosis | After Treatment |
| 1. | 2.89 | 2.86 | 2.59 | 3.39 | 1.75 | 3.78 |
| 2. | 6.93 | 4.56 | 18.86 | 14.16 | 5.32 | 4.32 |
| 3. | 8.96 | 5.41 | 7.66 | 6.02 | 3.80 | 3.63 |
| 4. | 2.5 | 2.53 | 21.6 | 26.26 | 5.21 | 6.5 |
| 5. | 4.1 | 8.06 | 23.75 | 26.34 | 2.58 | 3.22 |
| 6. | 6.89 | 3.88 | 22.01 | 8.75 | 3.88 | 3.09 |
| 7. | 3.02 | 4.02 | 9.89 | 11.82 | 3.21 | 4.58 |
| 8. | 5.57 | 4.56 | 8.36 | 6.73 | 4.64 | 3.66 |
| Median | 4.835 * | 4.29 * | 14.38 # | 10.29 # | 3.84 ^ | 3.72 ^ |
| IQR | 2.92–6.92 | 3.12–5.19 | 7.83–21.91 | 6.19–23.24 | 2.74–5.07 | 3.32–4.51 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Woś, J.; Szymańska, A.; Lehman, N.; Chocholska, S.; Zarobkiewicz, M.; Pożarowski, P.; Bojarska-Junak, A. Can Galectin-3 Be a Novel Biomarker in Chronic Lymphocytic Leukemia? Cells 2024, 13, 30. https://doi.org/10.3390/cells13010030
Woś J, Szymańska A, Lehman N, Chocholska S, Zarobkiewicz M, Pożarowski P, Bojarska-Junak A. Can Galectin-3 Be a Novel Biomarker in Chronic Lymphocytic Leukemia? Cells. 2024; 13(1):30. https://doi.org/10.3390/cells13010030
Chicago/Turabian StyleWoś, Justyna, Agata Szymańska, Natalia Lehman, Sylwia Chocholska, Michał Zarobkiewicz, Piotr Pożarowski, and Agnieszka Bojarska-Junak. 2024. "Can Galectin-3 Be a Novel Biomarker in Chronic Lymphocytic Leukemia?" Cells 13, no. 1: 30. https://doi.org/10.3390/cells13010030