Immunotherapy around the Clock: Impact of Infusion Timing on Stage IV Melanoma Outcomes
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Outcomes
2.4. Treatment Groups and Allocation
2.5. Statistical Analysis
3. Results
3.1. Patient Characteristics and Disease Burden by Treatment Group
3.2. Immunotherapy Toxicities
3.3. Progression-Free and Overall Survival
3.4. Subgroup Analysis
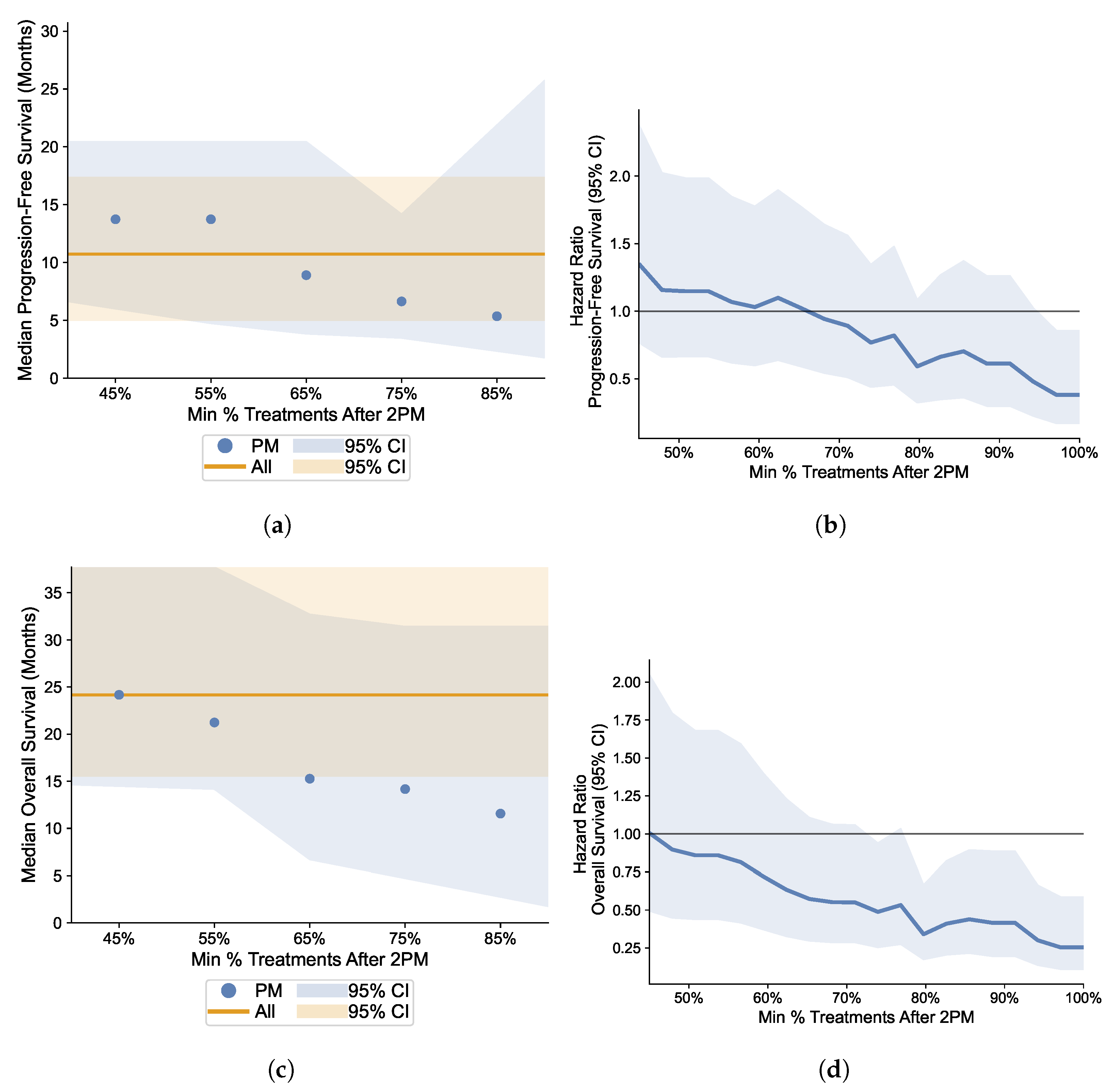
3.5. Robustness Analysis: Varying the Proportion Cutoff of Afternoon Infusions
4. Discussion
4.1. Concurrent Research on Chronoimmunotherapy
4.2. Potential Underlying Mechanisms
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CI | Confidence interval |
| CNS | Central nervous system |
| CTC | Circulating tumor cells |
| CTCAE | Common Terminology Criteria for Adverse Events |
| CTLA-4 | Anti-cytotoxic lymphocyte antigen-4 |
| DC | Dendritic cell |
| ECOG | Estearn Cooperative Oncology Group |
| HR | Hazard ratio |
| ICI | Immune checkpoint inhibitor |
| Ig | Immunoglobulin |
| irAE(s) | Immune-related adverse effect(s) |
| LDH | Lactate dehydrogenase |
| MHC | major histocompatibility complex |
| NK | Natural killer |
| NSCLC | Non-small cell lung cancer |
| OS | Overall survival |
| PD-1 | Programmed death protein-1 |
| PD-L1 | Programmed death ligand-1 |
| PFS | Progression-free survival |
| PS | Performance status |
| RECIST | Response Evaluation Criteria in Solid tumors |
| TAM | tumor associated macrophage |
| TCR | T cell receptor |
| ULN | Upper limit of normal |
References
- Forsea, A.M. Melanoma Epidemiology and Early Detection in Europe: Diversity and Disparities. Dermatol. Pract. Concept. 2020, 10, e2020033. [Google Scholar] [CrossRef]
- Rebordão Pires, M.; Caetano, A.C.; Amorim Costa, A.C.; Monteiro, J.C.; Santos, R.A.; Félix Soares, R.; Piedade Domingues, I.C.; Costa Jesus, E.D.; Sousa, G.M. 114P Systemic inflammatory index as a prognostic biomarker in metastatic melanoma patients under immune checkpoint inhibitors. Immuno-Oncol. Technol. 2022, 16, 100218. [Google Scholar] [CrossRef]
- Keilholz, U.; Ascierto, P.A.; Dummer, R.; Robert, C.; Lorigan, P.; van Akkooi, A.; Arance, A.; Blank, C.U.; Chiarion Sileni, V.; Donia, M.; et al. ESMO consensus conference recommendations on the management of metastatic melanoma: Under the auspices of the ESMO Guidelines Committee. Ann. Oncol. 2020, 33, 1435–1448. [Google Scholar] [CrossRef] [PubMed]
- Diamantopoulos, P.; Gogas, H. Melanoma immunotherapy dominates the field. Ann. Transl. Med. 2016, 4, 269. [Google Scholar] [CrossRef] [PubMed]
- Passarelli, A.; Mannavola, F.; Stucci, L.S.; Tucci, M.; Silvestris, F. Immune system and melanoma biology: A balance between immunosurveillance and immune escape. Oncotarget 2017, 8, 106132–106142. [Google Scholar] [CrossRef]
- Postow, M.; Harding, J.; Wolchok, J. Targeting Immune Checkpoints: Releasing the Restraints on Anti-Tumor Immunity for Patients With Melanoma. Cancer J. 2012, 18, 153–159. [Google Scholar] [CrossRef]
- Carlino, M.; Larkin, J.; Long, G.V. Immune checkpoint inhibitors in melanoma. Lancet 2021, 398, 1002–1014. [Google Scholar] [CrossRef] [PubMed]
- Hanaizi, Z.; van Zwieten-Boot, B.; Calvo, G.; Lopez, A.S.; van Dartel, M.; Camarero, J.; Abadie, E.; Pignatti, F. The European Medicines Agency review of ipilimumab (Yervoy) for the treatment of advanced (unresectable or metastatic) melanoma in adults who have received prior therapy: Summary of the scientific assessment of the Committee for Medicinal Products for Human Use. Eur. J. Cancer 2012, 48, 237–242. [Google Scholar] [CrossRef]
- Postow, M.A.; Chesney, J.; Pavlick, A.C.; Robert, C.; Grossmann, K.; McDermott, D.; Linette, G.P.; Meyer, N.; Giguere, J.K.; Agarwala, S.S.; et al. Nivolumab and ipilimumab versus ipilimumab in untreated melanoma. N. Engl. J. Med. 2015, 372, 2006–2017. [Google Scholar] [CrossRef]
- Robert, C.; Schachter, J.; Long, G.V.; Arance, A.; Grob, J.J.; Mortier, L.; Daud, A.; Carlino, M.S.; McNeil, C.; Lotem, M.; et al. Pembrolizumab versus Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2015, 372, 2521–2532. [Google Scholar] [CrossRef]
- EMA. New Treatment Option Recommended for Patients with Advanced Melanoma. European Medicines Agency Press Release 2015. Available online: https://www.ema.europa.eu/en/documents/press-release/new-treatment-option-recommended-patients-advanced-melanoma_en.pdf (accessed on 22 May 2015).
- EMA. New Treatment for Advanced Melanoma. European Medicines Agency Press Release 2015. Available online: https://www.ema.europa.eu/en/documents/press-release/new-treatment-advanced-melanoma_en.pdf (accessed on 24 April 2015).
- Wolchok, J.D.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Outcomes With Nivolumab Plus Ipilimumab or Nivolumab Alone Versus Ipilimumab in Patients With Advanced Melanoma. J. Clin. Oncol. 2022, 40, 127–137. [Google Scholar] [CrossRef]
- Larkin, J.; Chiarion-Sileni, V.; Gonzalez, R.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Five-Year Survival with Combined Nivolumab and Ipilimumab in Advanced Melanoma. N. Engl. J. Med. 2019, 381, 1535–1546. [Google Scholar] [CrossRef] [PubMed]
- Anderson, N.M.; Simon, M.C. The tumor microenvironment. Curr. Biol. 2020, 30, R921–R925. [Google Scholar] [CrossRef] [PubMed]
- Jin, M.Z.; Jin, W.L. The updated landscape of tumor microenvironment and drug repurposing. Signal Transduct. Target. Ther. 2020, 5, 166. [Google Scholar] [CrossRef] [PubMed]
- Dimitrov, S.; Benedict, C.; Heutling, D.; Westermann, J.; Born, J.; Lange, T. Cortisol and Epinephrine Control Opposing Circadian Rhythms in T Cell Subsets. Blood 2009, 113, 5134–5143. [Google Scholar] [CrossRef]
- Haus, E.; Smolensky, M.H. Biologic Rhythms in the Immune System. Chronobiol. Int. 1999, 16, 581–622. [Google Scholar] [CrossRef]
- Scheiermann, C.; Kunisaki, Y.; Frenette, P.S. Circadian control of the immune system. Nat. Rev. Immunol. 2013, 13, 190–198. [Google Scholar] [CrossRef]
- Wang, C.; Lutes, L.K.; Barnoud, C.; Scheiermann, C. The circadian immune system. Sci. Immunol. 2022, 7, eabm2465. [Google Scholar] [CrossRef]
- Wang, C.; Barnoud, C.; Cenerenti, M.; Sun, M.; Caffa, I.; Kizil, B.; Bill, R.; Liu, Y.; Pick, R.; Garnier, L.; et al. Dendritic Cells Direct Circadian Anti-Tumor Immune Response. Nature 2023, 614, 136–143. [Google Scholar] [CrossRef]
- Pick, R.; He, W.; Chen, C.S.; Scheiermann, C. Time-of-Day-Dependent Trafficking and Function of Leukocyte Subsets. Trends Immunol. 2019, 40, 524–537. [Google Scholar] [CrossRef]
- Kim, M.J.; Ha, S.-J. Differential Role of PD-1 Expressed by Various Immune and Tumor Cells in the Tumor Immune Microenvironment: Expression, Function, Therapeutic Efficacy, and Resistance to Cancer Immunotherapy. Front. Cell Dev. Biol. 2021, 9, 767466. [Google Scholar] [CrossRef]
- Gordon, S.R.; Maute, R.L.; Dulken, B.W.; Hutter, G.; George, B.M.; McCracken, M.N.; Gupta, R.; Tsai, J.M.; Sinha, R.; Corey, D.; et al. PD-1 Expression by Tumour-Associated Macrophages Inhibits Phagocytosis and Tumour Immunity. Nature 2017, 545, 495–499. [Google Scholar] [CrossRef]
- Tsuruta, A.; Shiiba, Y.; Matsunaga, N.; Fujimoto, M.; Yoshida, Y.; Koyanagi, S.; Ohdo, S. Diurnal Expression of PD-1 on Tumor-Associated Macrophages Underlies the Dosing Time-Dependent Antitumor Effects of the PD-1/PD-L1 Inhibitor BMS-1 in B16/BL6 Melanoma-Bearing Mice. Mol. Cancer Res. 2022, 20, 972–982. [Google Scholar] [CrossRef]
- Qian, D.C.; Kleber, T.; Brammer, B.; Xu, K.M.; Switchenko, J.M.; Janopaul-Naylor, J.R.; Zhong, J.; Yushak, M.L.; Harvey, R.D.; Paulos, C.M.; et al. Effect of Immunotherapy Time-of-Day Infusion on Overall Survival Among Patients with Advanced Melanoma in the USA (MEMOIR): A Propensity Score-Matched Analysis of a Single-Centre, Longitudinal Study. Lancet Oncol. 2021, 22, 1777–1786. [Google Scholar] [CrossRef]
- Karaboué, A.; Collon, T.; Pavese, I.; Bodiguel, V.; Cucherousset, J.; Zakine, E.; Innominato, P.F.; Bouchahda, M.; Adam, R.; Lévi, F. Time-Dependent Efficacy of Checkpoint Inhibitor Nivolumab: Results from a Pilot Study in Patients with Metastatic Non-Small-Cell Lung Cancer. Cancers 2022, 14, 896. [Google Scholar] [CrossRef] [PubMed]
- Dizman, N.; Govindarajan, A.; Zengin, Z.B.; Meza, L.A.; Tripathi, N.; Sayegh, N.; Castro, D.V.; Chan, E.H.; Lee, K.O.; Prajapati, S.R.; et al. Association Between Time-of-Day of the Immune Checkpoint Inhibitor (ICI) Infusion and Disease Outcomes Among Patients with Metastatic Renal Cell Carcinoma (mRCC). J. Clin. Oncol. 2023, 41, 678. [Google Scholar] [CrossRef]
- Fernandez-Mañas, L.; Gonzalez Aguado, L.; Aversa, C.; Ferrer-Mileo, L.; Garcia de Herreros, M.; Jiménez, N.; Febrer, A.; Vernet, R.; García-Esteve, S.; Mellado, B.; et al. Does the Time-of-Day Administration of Immune Checkpoint Inhibitors Affect Efficacy in Patients with Metastatic Renal Cell Carcinoma? A Single-Center Study. J. Clin. Oncol. 2023, 41, 681. [Google Scholar] [CrossRef]
- Janse van Rensburg, H.J.; Liu, Z.; Abdul Razak, A.R.; Spreafico, A.; Bedard, P.L.; Hansen, A.R.; Lheureux, S.; Butler, M.O.; Siu, L.L. The Effect of Circadian Rhythm on Clinical Outcome in Patients Receiving Pembrolizumab in the INSPIRE Pan-Cancer Trial. J. Clin. Oncol. 2022, 40, 2589. [Google Scholar] [CrossRef]
- Cortellini, A.; Barrichello, A.P.C.; Alessi, J.V.; Ricciuti, B.; Vaz, V.R.; Newsom-Davis, T.; Evans, J.S.; Lamberti, G.; Pecci, F.; Viola, P.; et al. A multicentre study of pembrolizumab time-of-day infusion patterns and clinical outcomes in non-small-cell lung cancer: Too soon to promote morning infusions. Ann. Oncol. 2022, 33, 1202–1204. [Google Scholar] [CrossRef]
- Freites-Martinez, A.; Santana, N.; Arias-Santiago, S.; Viera, A. Using the Common Terminology Criteria for Adverse Events (CTCAE – Version 5.0) to Evaluate the Severity of Adverse Events of Anticancer Therapies. Actas Dermo-Sifiliográficas 2021, 112, 90–92. [Google Scholar] [CrossRef]
- Eisenhauer, E.; Therasse, P.; Bogaerts, J.; Schwartz, L.; Sargent, D.; Ford, R.; Dancey, J.; Arbuck, S.; Gwyther, S.; Mooney, M.; et al. New Response Evaluation Criteria in Solid Tumours: Revised RECIST Guideline (version 1.1). Eur. J. Cancer 2009, 45, 228–247. [Google Scholar] [CrossRef] [PubMed]
- Lévi, F. From Circadian Rhythms to Cancer Chronotherapeutics. Chronobiol. Int. 2002, 19, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yuan, G.; Xie, H.; Wei, T.; Zhu, D.; Cui, J.; Liu, X.; Shen, R.; Zhu, Y.; Yang, X. Circadian Clock Associates with Tumor Microenvironment in Thoracic Cancers. Aging 2019, 11, 11814–11828. [Google Scholar] [CrossRef] [PubMed]
- Mirian, M.; Hariri, A.; Yadollahi, M.; Kohandel, M. Circadian and Immunity Cycle Talk in Cancer Destination: From Biological Aspects to In Silico Analysis. Cancers 2022, 14, 1578. [Google Scholar] [CrossRef]
- Cortés-Hernández, L.E.; Eslami-S, Z.; Dujon, A.M.; Giraudeau, M.; Ujvari, B.; Thomas, F.; Alix-Panabières, C. Do Malignant Cells Sleep at Night? Genome Biol. 2020, 21, 276. [Google Scholar] [CrossRef]
- Diamantopoulou, Z.; Castro-Giner, F.; Schwab, F.D.; Foerster, C.; Saini, M.; Budinjas, S.; Strittmatter, K.; Krol, I.; Seifert, B.; Heinzelmann-Schwarz, V.; et al. The Metastatic Spread of Breast Cancer Accelerates During Sleep. Nature 2022, 607, 156–162. [Google Scholar] [CrossRef]
- Gorbacheva, V.Y.; Kondratov, R.V.; Zhang, R.; Cherukuri, S.; Gudkov, A.V.; Takahashi, J.S.; Antoch, M.P. Circadian Sensitivity to the Chemotherapeutic Agent Cyclophosphamide Depends on the Functional Status of the CLOCK/BMAL1 Transactivation Complex. Proc. Natl. Acad. Sci. USA 2005, 102, 3407–3412. [Google Scholar] [CrossRef]
- Centanni, M.; Moes, D.; Trocóniz, I.; Ciccolini, J.; Hasselt, J.V. Clinical Pharmacokinetics and Pharmacodynamics of Immune Checkpoint Inhibitors. Clin. Pharmacokinet. 2019, 58, 835–857. [Google Scholar] [CrossRef]
- Gunn, P.J.; Middleton, B.; Davies, S.K.; Revell, V.L.; Skene, D.J. Sex differences in the circadian profiles of melatonin and cortisol in plasma and urine matrices under constant routine conditions. Chronobiol. Int. 2016, 33, 39–50. [Google Scholar] [CrossRef]
- Larsson, C.A.; Gullberg, B.; Rastam, L.; Lindblad, U. Salivary cortisol differs with age and sex and shows inverse associations with WHR in Swedish women: A cross-sectional study. BMC Endocr. Disord. 2009, 9, 16. [Google Scholar] [CrossRef]
- Bree, L.d.; Mourits, V.; Koeken, V.; Moorlag, S.; Janssen, R.; Folkman, L.; Barreca, D.; Krausgruber, T.; Fife-Gernedl, V.; Novakovic, B.; et al. Circadian Rhythm Influences Induction of Trained Immunity by BCG Vaccination. J. Clin. Investig. 2020, 130, 5603–5617. [Google Scholar] [CrossRef] [PubMed]
- Phillips, A.C.; Gallagher, S.; Carroll, D.; Drayson, M. Preliminary Evidence That Morning Vaccination Is Associated with an Enhanced Antibody Response in Men. Psychophysiology 2008, 45, 663–666. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Suo, Y.; Fu, Y.; Zhang, F.; Ding, N.; Pang, K.; Xie, C.; Weng, X.; Tian, M.; He, H.; et al. In Vivo Flow Cytometry Reveals a Circadian Rhythm of Circulating Tumor Cells. Light Sci. Appl. 2021, 10, 110. [Google Scholar] [CrossRef] [PubMed]
- Hodi, F.S.; Chiarion Sileni, V.; Lewis, K.D.; Grob, J.J.; Rutkowski, P.; Lao, C.D.; Cowey, C.L.; Schadendorf, D.; Wagstaff, J.; Dummer, R.; et al. Long-Term Survival in Advanced Melanoma for Patients Treated with Nivolumab Plus Ipilimumab in CheckMate 067. J. Clin. Oncol. 2022, 40, 9522. [Google Scholar] [CrossRef]

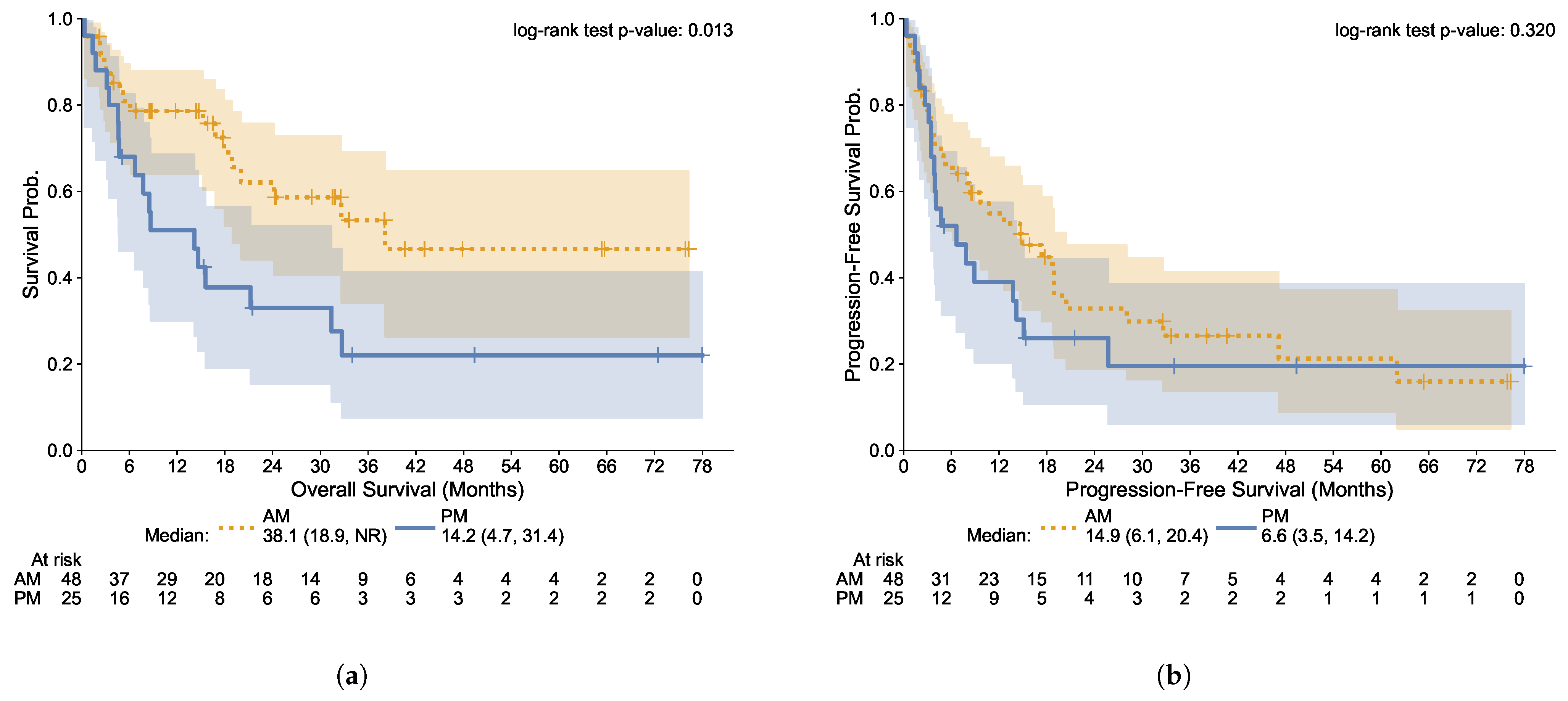
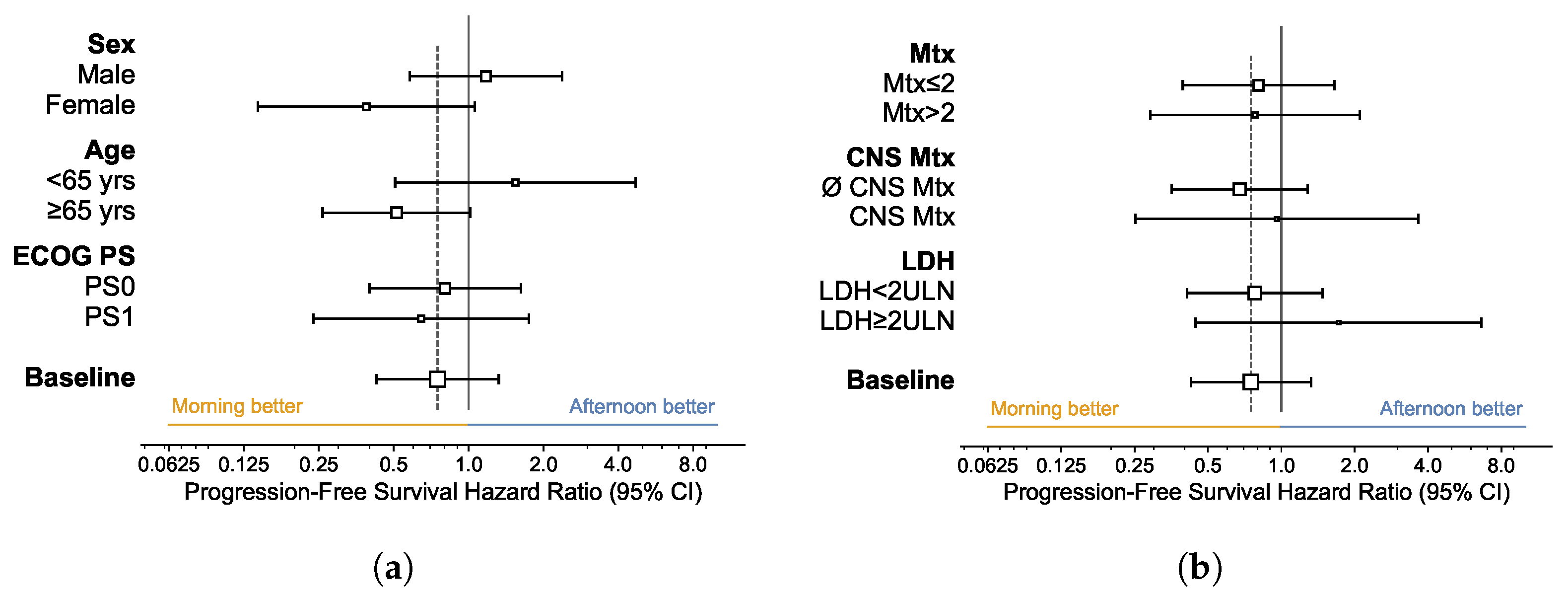
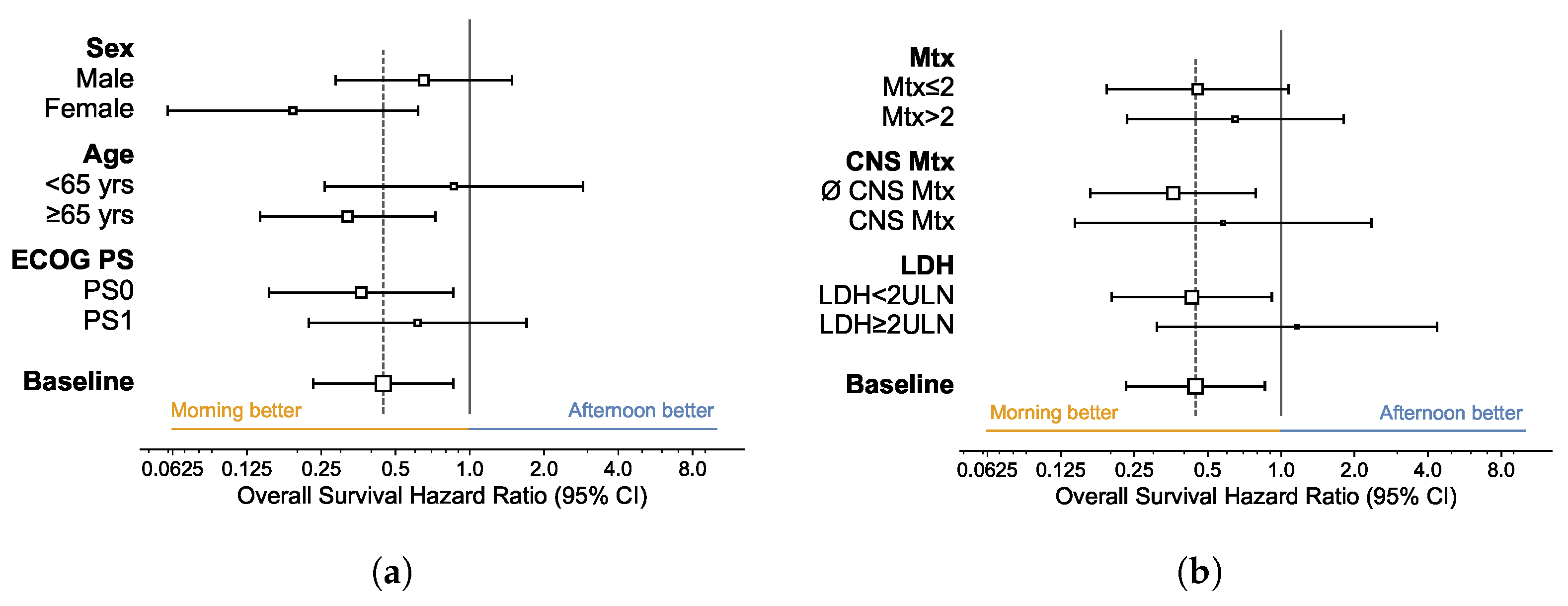
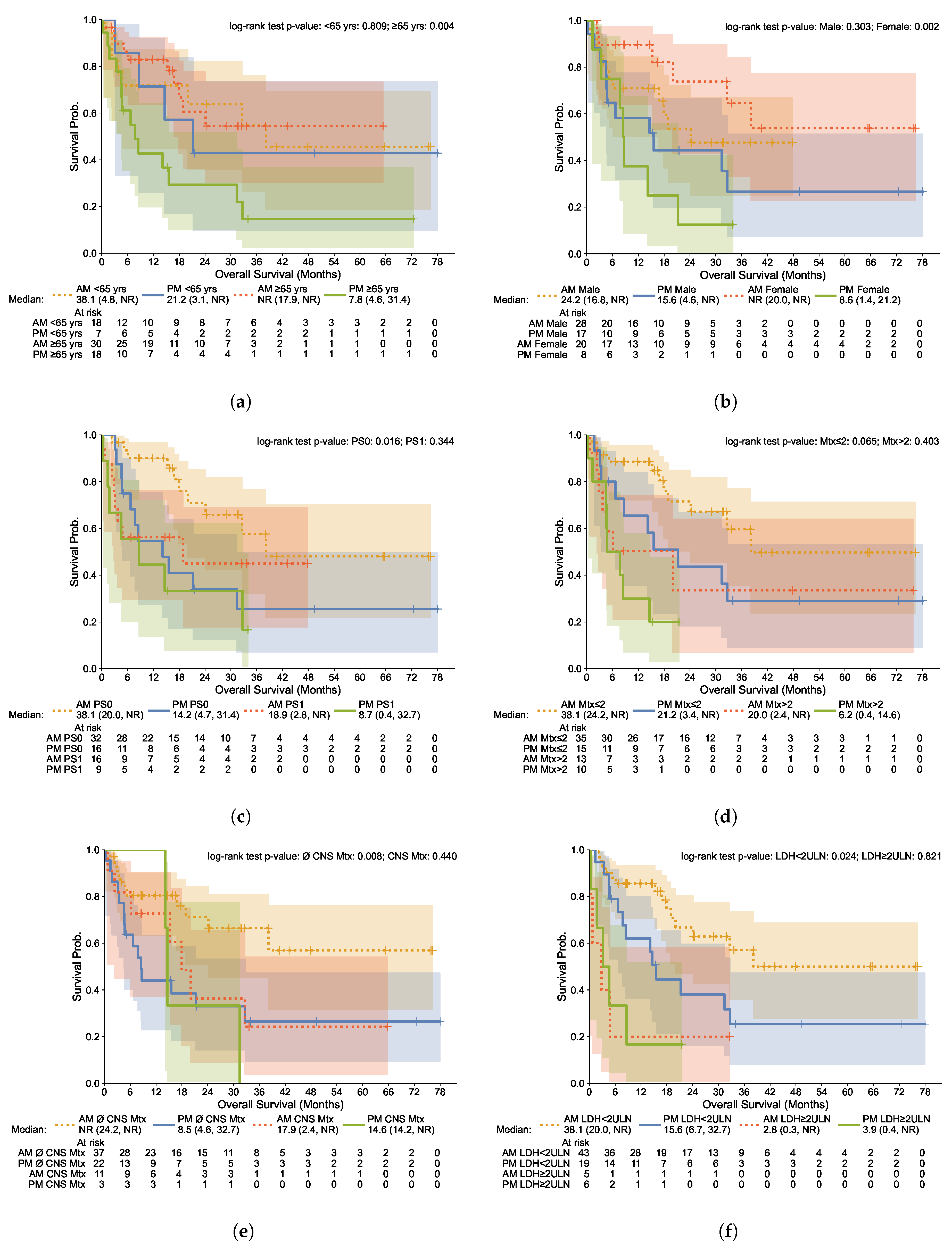

| All Patients | Morning Group | Afternoon Group | Statistic | |
|---|---|---|---|---|
| () | () | () | (p-Value) | |
| Age (years) | 0.30 (0.581) | |||
| Median (range) | 70 (29–91) | 69 (35–91) | 75 (29–86) | |
| <65 | 25 (34.2%) | 18 (37.5%) | 7 (28.0%) | |
| ≥65 | 48 (65.8%) | 30 (62.5%) | 18 (72.0%) | |
| Sex | 0.31 (0.581) | |||
| Female | 28 (38.4%) | 20 (41.7%) | 8 (32.0%) | |
| Male | 45 (61.6%) | 28 (58.3%) | 17 (68.0%) | |
| ECOG PS | 0.00 (≥0.999) | |||
| 0 | 48 (65.8%) | 32 (66.7%) | 16 (64.0%) | |
| 1 | 25 (34.2%) | 16 (33.3%) | 9 (36.0%) | |
| Melanoma subtype | 1.84 (0.398) | |||
| Cutaneous | 62 (84.9%) | 39 (81.2%) | 23 (92.0%) | |
| Mucosal | 2 (2.7%) | 2 (4.2%) | 0 (0.0%) | |
| Ocular | 9 (12.3%) | 7 (14.6%) | 2 (8.0%) |
| All Patients | Morning Group | Afternoon Group | Statistic | |
|---|---|---|---|---|
| () | () | () | (p-Value) | |
| Metastatic Sites (N) | 1.71 (0.634) | |||
| 1 | 23 (31.5%) | 17 (35.4%) | 6 (24.0%) | |
| 2 | 27 (37.0%) | 18 (37.5%) | 9 (36.0%) | |
| 3 | 17 (23.3%) | 10 (20.8%) | 7 (28.0%) | |
| ≥4 | 6 (8.2%) | 3 (6.2%) | 3 (12.0%) | |
| CNS Metastases | 0.66 (0.417) | |||
| Yes | 14 (19.2%) | 11 (22.9%) | 3 (12.0%) | |
| No | 59 (80.8%) | 37 (77.1%) | 22 (88.0%) | |
| LDH (U/L) | ||||
| Median (range) | 225 (117–4529) | 223 (117–4529) | 301 (135–1922) | |
| ≥250 U/L | 31 (42.5%) | 17 (35.4%) | 14 (56.0%) | 2.07 (0.150) |
| ≥2 ULN | 11 (15.1%) | 5 (10.4%) | 6 (24.0%) | 1.43 (0.232) |
| All Patients | Morning Group | Afternoon Group | Statistic | |
|---|---|---|---|---|
| () | () | () | (p-Value) | |
| Overall Toxicity | 6.94 (0.225) | |||
| No Toxicity | 22 (30.1%) | 15 (31.2%) | 7 (28.0%) | |
| G1 | 12 (16.4%) | 7 (14.6%) | 5 (20.0%) | |
| G2 | 27 (37.0%) | 16 (33.3%) | 11 (44.0%) | |
| G3 | 7 (9.6%) | 7 (14.6%) | 0 (0.0%) | |
| G4 | 2 (2.7%) | 2 (4.2%) | 0 (0.0%) | |
| N/I | 3 (4.1%) | 1 (2.1%) | 2 (8.0%) | |
| Fatigue | 1.66 (0.435) | |||
| G1/G2 | 27 (37.0%) | 19 (39.6%) | 8 (32.0%) | |
| G3/G4 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Cutaneous | 1.98 (0.577) | |||
| G1/G2 | 23 (31.5%) | 15 (31.2%) | 8 (32.0%) | |
| G3/G4 | 1 (1.4%) | 1 (2.1%) | 0 (0.0%) | |
| Endocrine | 3.65 (0.302) | |||
| G1/G2 | 14 (19.2%) | 11 (22.9%) | 3 (12.0%) | |
| G3/G4 | 2 (2.7%) | 2 (4.2%) | 0 (0.0%) | |
| Hepatitis | 3.21 (0.360) | |||
| G1/G2 | 6 (8.2%) | 3 (6.2%) | 3 (12.0%) | |
| G3/G4 | 2 (2.7%) | 2 (4.2%) | 0 (0.0%) | |
| Pneumonitis | 2.96 (0.397) | |||
| G1/G2 | 2 (2.7%) | 2 (4.2%) | 0 (0.0%) | |
| G3/G4 | 1 (1.4%) | 1 (2.1%) | 0 (0.0%) | |
| Renal Insufficiency | 1.55 (0.461) | |||
| G1/G2 | 8 (11.0%) | 5 (10.4%) | 3 (12.0%) | |
| G3/G4 | 0 (0%) | 0 (0%) | 0 (0%) | |
| Uveitis | 2.45 (0.485) | |||
| G1/G2 | 1 (1.4%) | 1 (2.1%) | 0 (0.0%) | |
| G3/G4 | 1 (1.4%) | 1 (2.1%) | 0 (0.0%) | |
| Encephalitis | 2.45 (0.485) | |||
| G1/G2 | 1 (1.4%) | 1 (2.1%) | 0 (0.0%) | |
| G3/G4 | 1 (1.4%) | 1 (2.1%) | 0 (0.0%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gonçalves, L.; Gonçalves, D.; Esteban-Casanelles, T.; Barroso, T.; Soares de Pinho, I.; Lopes-Brás, R.; Esperança-Martins, M.; Patel, V.; Torres, S.; Teixeira de Sousa, R.; et al. Immunotherapy around the Clock: Impact of Infusion Timing on Stage IV Melanoma Outcomes. Cells 2023, 12, 2068. https://doi.org/10.3390/cells12162068
Gonçalves L, Gonçalves D, Esteban-Casanelles T, Barroso T, Soares de Pinho I, Lopes-Brás R, Esperança-Martins M, Patel V, Torres S, Teixeira de Sousa R, et al. Immunotherapy around the Clock: Impact of Infusion Timing on Stage IV Melanoma Outcomes. Cells. 2023; 12(16):2068. https://doi.org/10.3390/cells12162068
Chicago/Turabian StyleGonçalves, Lisa, Duarte Gonçalves, Teresa Esteban-Casanelles, Tiago Barroso, Inês Soares de Pinho, Raquel Lopes-Brás, Miguel Esperança-Martins, Vanessa Patel, Sofia Torres, Rita Teixeira de Sousa, and et al. 2023. "Immunotherapy around the Clock: Impact of Infusion Timing on Stage IV Melanoma Outcomes" Cells 12, no. 16: 2068. https://doi.org/10.3390/cells12162068
APA StyleGonçalves, L., Gonçalves, D., Esteban-Casanelles, T., Barroso, T., Soares de Pinho, I., Lopes-Brás, R., Esperança-Martins, M., Patel, V., Torres, S., Teixeira de Sousa, R., Mansinho, A., & Costa, L. (2023). Immunotherapy around the Clock: Impact of Infusion Timing on Stage IV Melanoma Outcomes. Cells, 12(16), 2068. https://doi.org/10.3390/cells12162068








