Alzheimer’s Disease-like Pathological Features in the Dorsal Hippocampus of Wild-Type Rats Subjected to Methionine-Diet-Evoked Mild Hyperhomocysteinaemia
Abstract
:1. Introduction
2. Materials and Methods
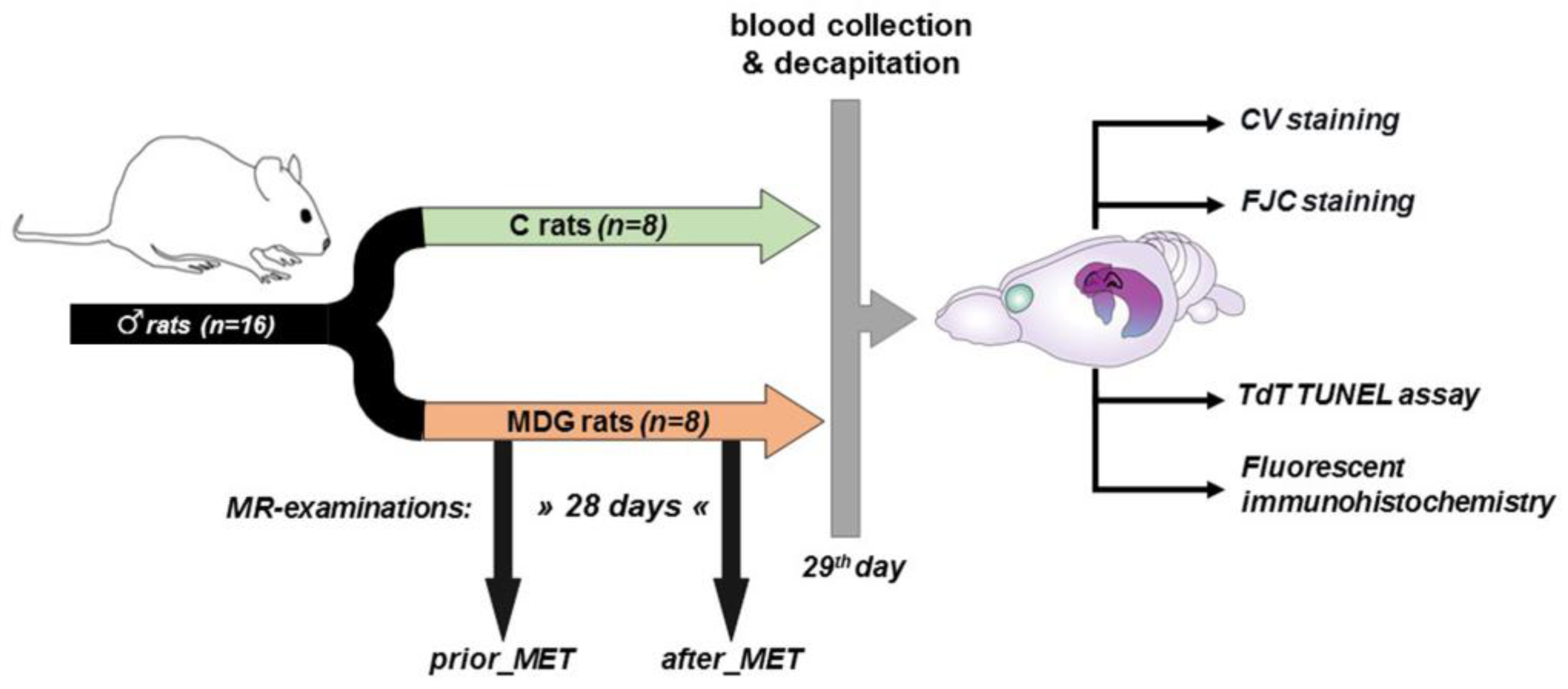
2.1. Experimental Procedures with Mild hHcy Induction
2.2. MR Examination in Living Subjects
2.3. Cresyl Violet (CV) Staining
2.4. Fluoro-Jade C (FJC) Staining
2.5. Terminal Deoxynucleotidyl Transferase (TdT) dUTP Nick-End Labelling (TUNEL) Assay
2.6. Fluorescent Immunohistochemistry
2.7. Quantitative Image Analysis
2.8. Statistical Analysis
3. Results
3.1. MR Spectroscopic Analysis
3.2. Histomorphological Analysis
3.2.1. CV, FJC, and TUNEL Analyses
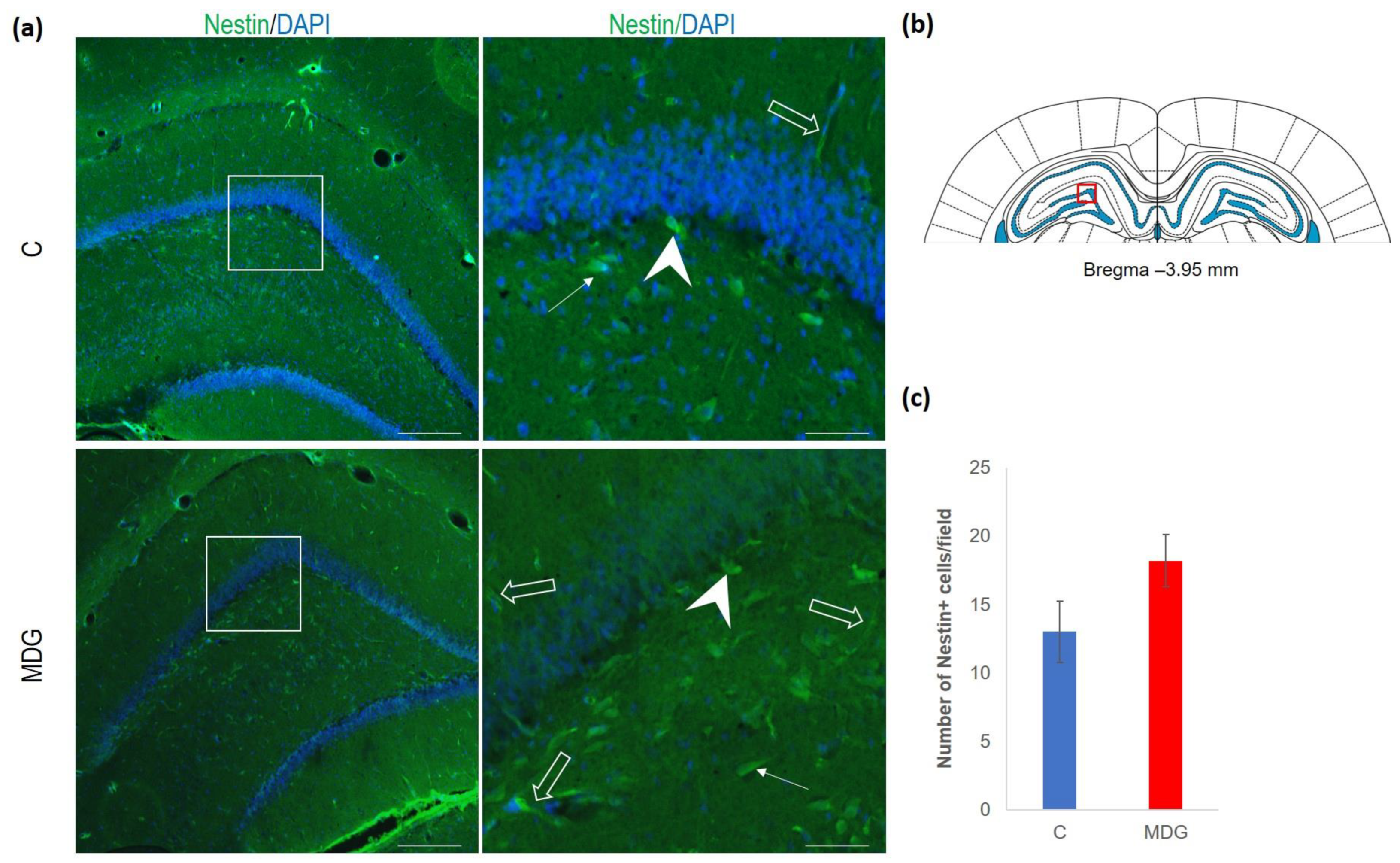
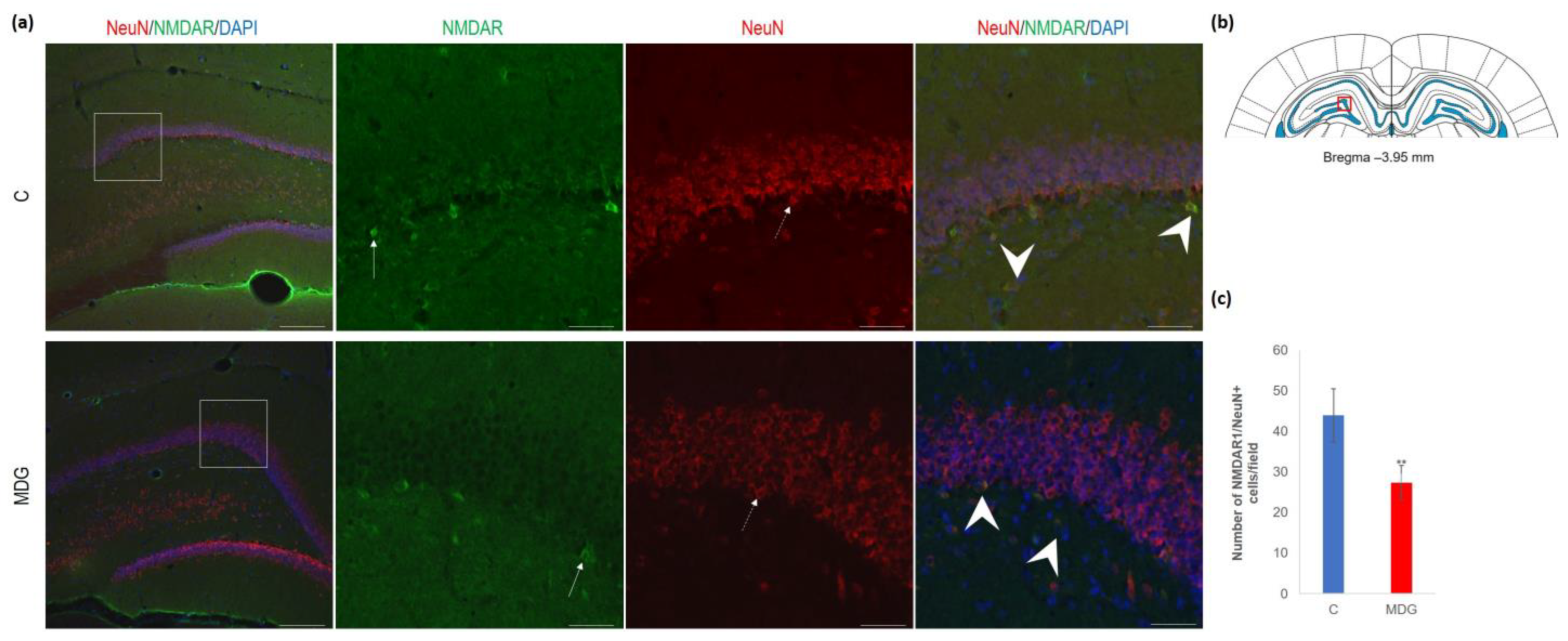
3.2.2. Immunofluorescent Analysis
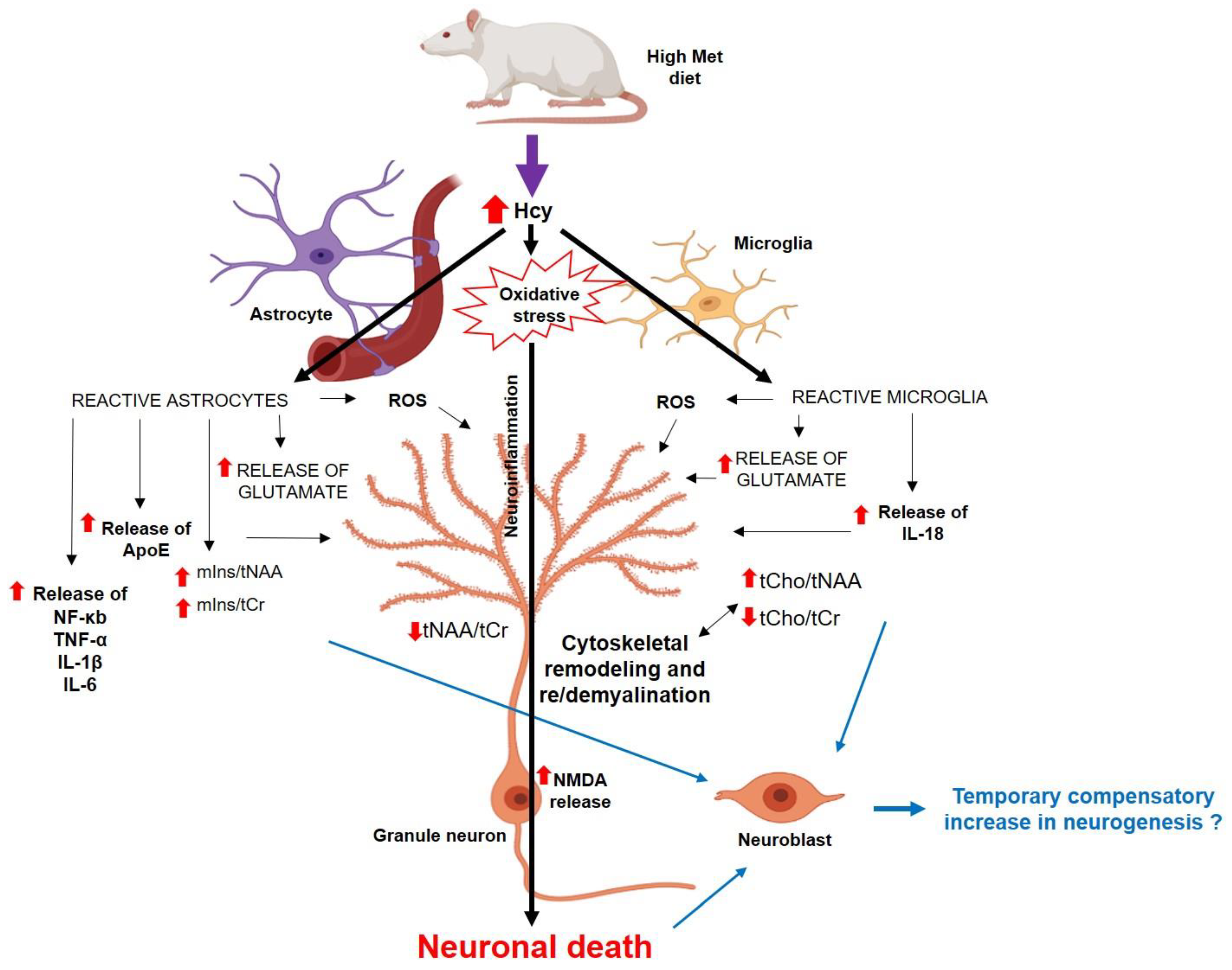
4. Discussion
4.1. In Vivo Metabolic Changes in the Dorsal Hippocampus in Met-Diet-Induced hHcy Conditions
4.2. Histomorphological Alterations in the DG in Met-Diet-Induced Mild hHcy Conditions
4.3. Neurogenesis in Met-Diet-Induced hHcy Conditions
4.4. Alterations in NMDAR and ApoE in the DG in Met-Diet-Induced Mild hHcy Conditions
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 1HMRS | Proton MRS |
| ApoE | Apolipoprotein E |
| CV | Cresyl violet |
| DCX | Doublecortin |
| DG | Dentate gyrus |
| FJC | Fluoro-Jade C |
| Hcy | Homocysteine |
| hHcy | Hyperhomocysteinaemia |
| MDG | Met diet group |
| Met | Methionine |
| MR | Magnetic resonance |
| MRS | MR spectroscopy |
| NeuN | Neural nuclei |
| NMDAR1 | N-methyl-D-aspartate receptor 1 |
| TUNEL | Terminal deoxynucleotidyl transferase dUTP nick end labelling |
References
- Xu, W.; Tan, L.; Wang, H.-F.; Jiang, T.; Tan, M.-S.; Tan, L.; Zhao, Q.-F.; Li, J.-Q.; Wang, J.; Yu, J.-T. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.D.; Refsum, H.; Bottiglieri, T.; Fenech, M.; Hooshmand, B.; McCaddon, A.; Miller, J.W.; Rosenberg, I.H.; Obeid, R. Homocysteine and Dementia: An International Consensus Statement. J. Alzheimer’s Dis. 2018, 62, 561–570. [Google Scholar] [CrossRef] [PubMed]
- Lehotsky, J.; Kovalska, M.; Tomascova, A.; Kalenska, D.; Baranovicova, E.; Kaplan, P. Ischemic brain injury in hyperhomocysteinemic conditions and the development of Alzheimer’s disease. In Brain Ischemia: Alzheimer’s Disease Mechanisms, 1st ed.; Pluta, R., Ed.; Nova Science Pub. Inc.: New York, NY, USA, 2019; pp. 115–156. [Google Scholar]
- Lehotsky, J.; Kovalska, M.; Baranovicova, E.; Hnilicova, P.; Kalenska, D.; Kaplan, P. Ischemic Brain Injury in Hyperhomocysteinemia. Cereb. Ischemia 2021, 16, 648. [Google Scholar] [CrossRef]
- Thelen, M.; Brown-Borg, H.M. Does Diet Have a Role in the Treatment of Alzheimer’s Disease? Front. Aging Neurosci. 2020, 12, 617071. [Google Scholar] [CrossRef] [PubMed]
- Pi, T.; Wei, S.; Jiang, Y.; Shi, J.-S. High Methionine Diet-Induced Alzheimer’s Disease like Symptoms Are Accompanied by 5-Methylcytosine Elevated Levels in the Brain. Behav. Neurol. 2021, 2021, 6683318. [Google Scholar] [CrossRef] [PubMed]
- Xing, S.-H.; Zhu, C.-X.; Zhang, R.; An, L. Huperzine A in the Treatment of Alzheimer’s Disease and Vascular Dementia: A Meta-Analysis. Evid. Based Complement. Altern. Med. 2014, 2014, 363985. [Google Scholar] [CrossRef] [PubMed]
- Boissonnas, C.C.; Jouannet, P.; Jammes, H. Epigenetic disorders and male subfertility. Fertil. Steril. 2013, 99, 624–631. [Google Scholar] [CrossRef]
- Futschek, I.E.; Schernhammer, E.; Haslacher, H.; Stögmann, E.; Lehrner, J. Homocysteine—A predictor for five year-mortality in patients with subjective cognitive decline, mild cognitive impairment and Alzheimer’s dementia. Exp. Gerontol. 2023, 172, 112045. [Google Scholar] [CrossRef] [PubMed]
- Han, L.; Wu, G.; Feng, C.; Yang, Y.; Li, B.; Ge, Y.; Jiang, Y.; Shi, Y.; Le, G. Dietary methionine restriction improves the impairment of cardiac function in middle-aged obese mice. Food Funct. 2020, 11, 1764–1778. [Google Scholar] [CrossRef]
- Esse, R.; Barroso, M.; De Almeida, I.T.; Castro, R. The Contribution of Homocysteine Metabolism Disruption to Endothelial Dysfunction: State-of-the-Art. Int. J. Mol. Sci. 2019, 20, 867. [Google Scholar] [CrossRef]
- Tripathi, M.; Singh, B.K.; Zhou, J.; Tikno, K.; Widjaja, A.; Sandireddy, R.; Arul, K.; Ghani, S.A.B.A.; Bee, G.G.B.; Wong, K.A.; et al. Vitamin B12 and folate decrease inflammation and fibrosis in NASH by preventing syntaxin 17 homocysteinylation. J. Hepatol. 2022, 77, 1246–1255. [Google Scholar] [CrossRef]
- Nuru, M.; Muradashvili, N.; Kalani, A.; Lominadze, D.; Tyagi, N. High methionine, low folate and low vitamin B6/B12 (HM-LF-LV) diet causes neurodegeneration and subsequent short-term memory loss. Metab. Brain Dis. 2018, 33, 1923–1934. [Google Scholar] [CrossRef]
- Weekman, E.M.; Sudduth, T.L.; Price, B.R.; Woolums, A.E.; Hawthorne, D.; Seaks, C.E.; Wilcock, D.M. Time course of neuropathological events in hyperhomocysteinemic amyloid depositing mice reveals early neuroinflammatory changes that precede amyloid changes and cerebrovascular events. J. Neuroinflamm. 2019, 16, 284. [Google Scholar] [CrossRef]
- Sudduth, T.L.; Weekman, E.M.; Price, B.R.; Gooch, J.L.; Woolums, A.; Norris, C.M.; Wilcock, D.M. Time-course of glial changes in the hyperhomocysteinemia model of vascular cognitive impairment and dementia (VCID). Neuroscience 2017, 341, 42–51. [Google Scholar] [CrossRef] [PubMed]
- Rizk, T.; Turtzo, L.C.; Cota, M.; Van Der Merwe, A.J.; Latour, L.; Whiting, M.D.; Chan, L. Traumatic microbleeds persist for up to five years following traumatic brain injury despite resolution of other acute findings on MRI. Brain Inj. 2020, 34, 775–783. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tian, Y.; Wei, H.-J.; Dong, J.-F.; Zhang, J.-N. Methionine diet-induced hyperhomocysteinemia accelerates cerebral aneurysm formation in rats. Neurosci. Lett. 2011, 494, 139–144. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, H.; Ji, Z.-H.; Yu, X.-Y. Hyperhomocysteinemia Induces Rat Memory Impairment Via Injuring Hippocampal CA3 Neurons and Downregulating cAMP Response Element-Binding Protein (CREB) Phosphorylation. Neurochem. Res. 2021, 47, 762–767. [Google Scholar] [CrossRef] [PubMed]
- Alachkar, A.; Agrawal, S.; Baboldashtian, M.; Nuseir, K.; Salazar, J.; Agrawal, A. L-methionine enhances neuroinflammation and impairs neurogenesis: Implication for Alzheimer’s disease. J. Neuroimmunol. 2022, 366, 577843. [Google Scholar] [CrossRef] [PubMed]
- Pluta, R.; Ułamek-Kozioł, M.; Kocki, J.; Bogucki, J.; Januszewski, S.; Bogucka-Kocka, A.; Czuczwar, S.J. Expression of the Tau Protein and Amyloid Protein Precursor Processing Genes in the CA3 Area of the Hippocampus in the Ischemic Model of Alzheimer’s Disease in the Rat. Mol. Neurobiol. 2019, 57, 1281–1290. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, Y.; Müller, M.K.; von Engelhardt, J.; Sprengel, R.; Seeburg, P.H.; Monyer, H. Age-Dependent Degeneration of Mature Dentate Gyrus Granule Cells Following NMDA Receptor Ablation. Front. Mol. Neurosci. 2016, 8, 87. [Google Scholar] [CrossRef]
- Kovalska, M.; Hnilicova, P.; Kalenska, D.; Tothova, B.; Adamkov, M.; Lehotsky, J. Effect of Methionine Diet on Metabolic and Histopathological Changes of Rat Hippocampus. Int. J. Mol. Sci. 2019, 20, 6234. [Google Scholar] [CrossRef] [PubMed]
- Kovalska, M.; Baranovicova, E.; Kalenska, D.; Tomascova, A.; Adamkov, M.; Kovalska, L.; Lehotsky, J. Methionine Diet Evoked Hyperhomocysteinemia Causes Hippocampal Alterations, Metabolomics Plasma Changes and Behavioral Pattern in Wild Type Rats. Int. J. Mol. Sci. 2021, 22, 4961. [Google Scholar] [CrossRef] [PubMed]
- Kovalska, M.; Hnilicova, P.; Kalenska, D.; Tomascova, A.; Adamkov, M.; Lehotsky, J. Effect of Methionine Diet on Time-Related Metabolic and Histopathological Changes of Rat Hippocampus in the Model of Global Brain Ischemia. Biomolecules 2020, 10, 1128. [Google Scholar] [CrossRef]
- Kawles, A.; Minogue, G.; Zouridakis, A.; Keszycki, R.; Gill, N.; Nassif, C.; Coventry, C.; Zhang, H.; Rogalski, E.; Flanagan, M.E.; et al. Differential vulnerability of the dentate gyrus to tauopathies in dementias. Acta Neuropathol. Commun. 2023, 11, 1. [Google Scholar] [CrossRef]
- Richetin, K.; Steullet, P.; Pachoud, M.; Perbet, R.; Parietti, E.; Maheswaran, M.; Eddarkaoui, S.; Bégard, S.; Pythoud, C.; Rey, M.; et al. Tau accumulation in astrocytes of the dentate gyrus induces neuronal dysfunction and memory deficits in Alzheimer’s disease. Nat. Neurosci. 2020, 23, 1567–1579. [Google Scholar] [CrossRef]
- Armstrong, R.A. Pathology of the dentate gyrus in neurodegenerative disease. In Dentate Gyrus: Structure, Role in Disease, and Potential Health Implications, 1st ed.; Lowes, Z., Ed.; Nova Science: New York, NY, USA, 2015; pp. 47–68. [Google Scholar]
- Faghihi, F.; Moustafa, A.A. Impaired neurogenesis of the dentate gyrus is associated with pattern separation deficits: A computational study. J. Integr. Neurosci. 2016, 15, 277–293. [Google Scholar] [CrossRef]
- Tchantchou, F.; Hsia, R.-C.; Puche, A.; Fiskum, G. Hippocampal vulnerability to hyperhomocysteinemia worsens pathological outcomes of mild traumatic brain injury in rats. J. Central Nerv. Syst. Dis. 2023, 15, 11795735231160025. [Google Scholar] [CrossRef]
- Paris, J.J.; Chen, X.; Anderson, J.; Qrareya, A.N.; Mahdi, F.; Du, F.; McLaughlin, J.P.; Kaufman, M.J. In vivo proton magnetic resonance spectroscopy detection of metabolite abnormalities in aged Tat-transgenic mouse brain. Geroscience 2021, 43, 1851–1862. [Google Scholar] [CrossRef] [PubMed]
- Siani, F.; Greco, R.; Levandis, G.; Ghezzi, C.; Daviddi, F.; Demartini, C.; Vegeto, E.; Fuzzati-Armentero, M.-T.; Blandini, F. Influence of Estrogen Modulation on Glia Activation in a Murine Model of Parkinson’s Disease. Front. Neurosci. 2017, 11, 306. [Google Scholar] [CrossRef]
- Robison, L.S.; Gannon, O.J.; Salinero, A.E.; Zuloaga, K.L. Contributions of sex to cerebrovascular function and pathology. Brain Res. 2018, 1710, 43–60. [Google Scholar] [CrossRef]
- Zhu, D.; Zhang, M.; He, B.; Wan, Y.; Wang, L.; Gao, F. The role of sex and ovarian hormones in hippocampal damage and cognitive deficits induced by chronic exposure to hypobaric hypoxia. Front. Neurosci. 2022, 16, 953417. [Google Scholar] [CrossRef] [PubMed]
- Chaudry, O.; Ndukwe, K.; Xie, L.; Figueiredo-Pereira, M.E.; Serrano, P.; Rockwell, P. Females exhibit higher GluA2 levels and outperform males in active place avoidance despite increased amyloid plaques in TgF344-Alzheimer’s rats. Sci Rep. 2022, 12, 19129. [Google Scholar] [CrossRef] [PubMed]
- Labinjoh, C.; Newby, D.E.; Wilkinson, I.B.; Megson, I.L.; Maccallum, H.; Melville, V.; Boon, N.A.; Webb, D.J. Effects of acute methionine loading and vitamin C on endogenous fibrinolysis, endothelium-dependent vasomotion and platelet aggregation. Clin. Sci. 2001, 100, 127–135. [Google Scholar] [CrossRef]
- Nestel, P. Arterial stiffness is rapidly induced by raising the plasma homocysteine concentration with methionine. Atherosclerosis 2003, 171, 83–86. [Google Scholar] [CrossRef]
- Paxinos, G.; Watson, C. The Rat Brain in Stereotaxic Coordinates, 6th ed.; Academic Press: London, UK, 2006. [Google Scholar]
- Dusart, P.; Fagerberg, L.; Perisic, L.; Civelek, M.; Struck, E.; Hedin, U.; Uhlén, M.; Trégouët, D.-A.; Renné, T.; Odeberg, J.; et al. A systems-approach reveals human nestin is an endothelial-enriched, angiogenesis-independent intermediate filament protein. Sci. Rep. 2018, 8, 14668. [Google Scholar] [CrossRef]
- Kovalska, M.; Tothova, B.; Kalenska, D.; Tomascova, A.; Kovalska, L.; Adamkov, M.; Lehotsky, J. Association of induced hyperhomocysteinemia with neurodegeneration in rat entorhinal cortex-hippocampal system after global brain ischemia: A progression of Alzheimer’s disease-like pathological features. Act. Nerv. Super. Rediviva 2019, 61, 31–38. [Google Scholar]
- Brandt, R.; Bakota, L. Microtubule dynamics and the neurodegenerative triad of Alzheimer’s disease: The hidden connection. J. Neurochem. 2017, 143, 409–417. [Google Scholar] [CrossRef]
- Hooshmand, B.; Refsum, H.; Smith, A.D.; Kalpouzos, G.; Mangialasche, F.; von Arnim, C.A.F.; Kåreholt, I.; Kivipelto, M.; Fratiglioni, L. Association of Methionine to Homocysteine Status with Brain Magnetic Resonance Imaging Measures and Risk of Dementia. JAMA Psychiatry 2019, 76, 1198–1205. [Google Scholar] [CrossRef]
- Sam, R.C.; Burns, P.J.; Hobbs, S.D.; Marshall, T.; Wilmink, A.B.; Silverman, S.H.; Bradbury, A.W. The prevalence of hyperhomocysteinemia, methylene tetrahydrofolate reductase C677T mutation, and vitamin B12 and folate deficiency in patients with chronic venous insufficiency. J. Vasc. Surg. 2003, 38, 904–908. [Google Scholar] [CrossRef]
- Pardon, M.-C.; Lopez, M.Y.; Yuchun, D.; Marjańska, M.; Prior, M.; Brignell, C.; Parhizkar, S.; Agostini, A.; Bai, L.; Auer, D.P.; et al. Magnetic Resonance Spectroscopy discriminates the response to microglial stimulation of wild type and Alzheimer’s disease models. Sci. Rep. 2016, 6, 19880. [Google Scholar] [CrossRef]
- Ford, J.; Dogan, N.; Young, L.; Yang, F. Quantitative Radiomics: Impact of Pulse Sequence Parameter Selection on MRI-Based Textural Features of the Brain. Contrast Media Mol. Imaging 2018, 2018, 1729071. [Google Scholar] [CrossRef]
- Le Stunff, H.; Véret, J.; Kassis, N.; Denom, J.; Meneyrol, K.; Paul, J.-L.; Cruciani-Guglielmacci, C.; Magnan, C.; Janel, N. Deciphering the Link Between Hyperhomocysteinemia and Ceramide Metabolism in Alzheimer-Type Neurodegeneration. Front. Neurol. 2019, 10, 807. [Google Scholar] [CrossRef] [PubMed]
- Chang, L.; Munsaka, S.M.; Kraft-Terry, S.; Ernst, T. Magnetic Resonance Spectroscopy to Assess NeuroInflammation and Neuropathic Pain. J. Neuroimmune Pharmacol. 2013, 8, 576–593. [Google Scholar] [CrossRef] [PubMed]
- Harris, J.L.; Choi, I.-Y.; Brooks, W.M. Probing astrocyte metabolism in vivo: Proton magnetic resonance spectroscopy in the injured and aging brain. Front. Aging Neurosci. 2015, 7, 202. [Google Scholar] [CrossRef] [PubMed]
- Filibian, M.; Frasca, A.; Maggioni, D.; Micotti, E.; Vezzani, A.; Ravizza, T. In vivo imaging of glia activation using1H-magnetic resonance spectroscopy to detect putative biomarkers of tissue epileptogenicity. Epilepsia 2012, 53, 1907–1916. [Google Scholar] [CrossRef]
- Jakubowski, H. Homocysteine Modification in Protein Structure/Function and Human Disease. Physiol. Rev. 2019, 99, 555–604. [Google Scholar] [CrossRef] [PubMed]
- Hanzel, C.E.; Pichet-Binette, A.; Pimentel, L.S.; Iulita, M.F.; Allard, S.; Ducatenzeiler, A.; Carmo, S.D.; Cuello, A.C. Neuronal driven pre-plaque inflammation in a transgenic rat model of Alzheimer’s disease. Neurobiol. Aging 2014, 35, 2249–2262. [Google Scholar] [CrossRef]
- Seifert, B.; Eckenstaler, R.; Rönicke, R.; Leschik, J.; Lutz, B.; Reymann, K.; Lessmann, V.; Brigadski, T. Amyloid-Beta Induced Changes in Vesicular Transport of BDNF in Hippocampal Neurons. Neural Plast. 2016, 2016, 4145708. [Google Scholar] [CrossRef]
- Jaworski, J.; Kapitein, L.C.; Gouveia, S.M.; Dortland, B.R.; Wulf, P.S.; Grigoriev, I.; Camera, P.; Spangler, S.A.; Di Stefano, P.; Demmers, J.; et al. Dynamic Microtubules Regulate Dendritic Spine Morphology and Synaptic Plasticity. Neuron 2009, 61, 85–100. [Google Scholar] [CrossRef]
- Fanara, P.; Wong, P.-Y.A.; Husted, K.H.; Liu, S.; Liu, V.M.; Kohlstaedt, L.A.; Riiff, T.; Protasio, J.C.; Boban, D.; Killion, S.; et al. Cerebrospinal fluid–based kinetic biomarkers of axonal transport in monitoring neurodegeneration. J. Clin. Investig. 2012, 122, 3159–3169. [Google Scholar] [CrossRef]
- Baron, R.; Nemirovsky, A.; Harpaz, I.; Cohen, H.; Owens, T.; Monsonego, A. IFN-γ enhances neurogenesis in wild-type mice and in a mouse model of Alzheimer’s disease. FASEB J. 2008, 22, 2843–2852. [Google Scholar] [CrossRef] [PubMed]
- Owens, G.D.; Jadavji, N.M.; Smith, P.D. Neurogenesis Unchanged by MTHFR Deficiency in Three-Week-Old Mice. J. Young Investig. 2016, 31, 39–43. [Google Scholar] [CrossRef]
- Sakurai, M.; Suzuki, H.; Tomita, N.; Sunden, Y.; Shimada, A.; Miyata, H.; Morita, T. Enhanced neurogenesis and possible synaptic reorganization in the piriform cortex of adult rat following kainic acid-induced status epilepticus. Neuropathology 2017, 38, 135–143. [Google Scholar] [CrossRef] [PubMed]
- Korwek, K.M.; Trotter, J.H.; LaDu, M.J.; Sullivan, P.M.; Weeber, E.J. ApoE isoform-dependent changes in hippocampal synaptic function. Mol. Neurodegener. 2009, 4, 21. [Google Scholar] [CrossRef]
- Dayon, L.; Guiraud, S.P.; Corthésy, J.; Da Silva, L.; Migliavacca, E.; Tautvydaitė, D.; Oikonomidi, A.; Moullet, B.; Henry, H.; Métairon, S.; et al. One-carbon metabolism, cognitive impairment and CSF measures of Alzheimer pathology: Homocysteine and beyond. Alzheimer’s Res. Ther. 2017, 9, 43. [Google Scholar] [CrossRef] [PubMed]
- Trusca, V.G.; Mihai, A.D.; Fuior, E.V.; Fenyo, I.M.; Gafencu, A.V. High levels of homocysteine downregulate apolipoprotein E expressionvianuclear factor kappa B. World J. Biol. Chem. 2016, 7, 178–187. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Initiative, A.D.N.; Yamada, K.; Liddelow, S.A.; Smith, S.T.; Zhao, L.; Luo, W.; Tsai, R.M.; Spina, S.; Grinberg, L.T.; et al. ApoE4 markedly exacerbates tau-mediated neurodegeneration in a mouse model of tauopathy. Nature 2017, 549, 523–527. [Google Scholar] [CrossRef]
- Lin, Y.-T.; Seo, J.; Gao, F.; Feldman, H.M.; Wen, H.-L.; Penney, J.; Cam, H.P.; Gjoneska, E.; Raja, W.K.; Cheng, J.; et al. APOE4 Causes Widespread Molecular and Cellular Alterations Associated with Alzheimer’s Disease Phenotypes in Human iPSC-Derived Brain Cell Types. Neuron 2018, 98, 1141–1154. [Google Scholar] [CrossRef]
- Poddar, R.; Paul, S. Homocysteine-NMDA receptor-mediated activation of extracellular signal-regulated kinase leads to neuronal cell death. J. Neurochem. 2009, 110, 1095–1106. [Google Scholar] [CrossRef]
- Moretti, R.; Caruso, P. The Controversial Role of Homocysteine in Neurology: From Labs to Clinical Practice. Int. J. Mol. Sci. 2019, 20, 231. [Google Scholar] [CrossRef]
- Shibasaki, K.; Hosoi, N.; Kaneko, R.; Tominaga, M.; Yamada, K. Glycine release from astrocytes via functional reversal of GlyT1. J. Neurochem. 2016, 140, 395–403. [Google Scholar] [CrossRef] [PubMed]
- Rosi, S.; Ramirez-Amaya, V.; Hauss-Wegrzyniak, B.; Wenk, G.L. Chronic brain inflammation leads to a decline in hippocampal NMDA-R1 receptors. J. Neuroinflamm. 2004, 1, 12. [Google Scholar] [CrossRef] [PubMed]
- Sugimoto, A.; Takeda, A.; Kogure, K.; Onodera, H. NMDA receptor (NMDAR1) expression in the rat hippocampus after forebrain ischemia. Neurosci. Lett. 1994, 170, 39–42. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Liang, X.; Zhang, Q.; Luo, S.; Liu, H.; Wang, X.; Sai, N.; Zhang, X. Homocysteine can aggravate depressive like behaviors in a middle cerebral artery occlusion/reperfusion rat model: A possible role for NMDARs-mediated synaptic alterations. Nutr. Neurosci. 2022, 26, 483–495. [Google Scholar] [CrossRef]
- Ramakrishna, S.; Jhaveri, V.; Konings, S.C.; Nawalpuri, B.; Chakraborty, S.; Holst, B.; Schmid, B.; Gouras, G.K.; Freude, K.K.; Muddashetty, R.S. APOE4 Affects Basal and NMDAR-Mediated Protein Synthesis in Neurons by Perturbing Calcium Homeostasis. J. Neurosci. 2021, 41, 8686–8709. [Google Scholar] [CrossRef]
- Petráš, M.; Drgová, A.; Kovalská, M.; Tatarková, Z.; Tóthová, B.; Križanová, O.; Lehotský, J. Effect of Hyperhomocysteinemia on Redox Balance and Redox Defence Enzymes in Ischemia–Reperfusion Injury and/or After Ischemic Preconditioning in Rats. Cell. Mol. Neurobiol. 2017, 37, 1417–1431. [Google Scholar] [CrossRef] [PubMed]
- Ostrakhovitch, E.; Tabibzadeh, S. Homocysteine and age-associated disorders. Ageing Res. Rev. 2018, 49, 144–164. [Google Scholar] [CrossRef]
- Ravaglia, G.; Forti, P.; Maioli, F.; Scali, R.; Saccheitti, L.; Talerico, T.; Mantovani, V.; Bianchin, M. HOMOCYSTEINE AND COGNITIVE PERFORMANCE IN HEALTHY ELDERLY SUBJECTS. Arch. Gerontol. Geriatr. 2004, 38, 349–357. [Google Scholar] [CrossRef]
- Irizarry, M.C.; Gurol, M.E.; Raju, S.; Diaz-Arrastia, R.; Locascio, J.J.; Tennis, M.; Hyman, B.T.; Growdon, J.H.; Greenberg, S.M.; Bottiglieri, T. Association of homocysteine with plasma amyloid β protein in aging and neurodegenerative disease. Neurology 2005, 65, 1402–1408. [Google Scholar] [CrossRef]
- Miles, L.M.; Allen, E.; Mills, K.; Clarke, R.; Uauy, R.; Dangour, A.D. Vitamin B-12 status and neurologic function in older people: A cross-sectional analysis of baseline trial data from the Older People and Enhanced Neurological Function (OPEN) study. Am. J. Clin. Nutr. 2016, 104, 790–796. [Google Scholar] [CrossRef]
- Tannorella, P.; Stoccoro, A.; Tognoni, G.; Petrozzi, L.; Salluzzo, M.G.; Ragalmuto, A.; Siciliano, G.; Haslberger, A.; Bosco, P.; Bonuccelli, U.; et al. Methylation analysis of multiple genes in blood DNA of Alzheimer’s disease and healthy individuals. Neurosci. Lett. 2015, 600, 143–147. [Google Scholar] [CrossRef] [PubMed]
- Coppo, L.; Scheggi, S.; DeMontis, G.; Priora, R.; Frosali, S.; Margaritis, A.; Summa, D.; Di Giuseppe, D.; Ulivelli, M.; DiSimplicio, P.C. Does Risk of Hyperhomocysteinemia Depend on Thiol-Disulfide Exchange Reactions of Albumin and Homocysteine? Antioxid. Redox Signal. 2023, 38, 920–958. [Google Scholar] [CrossRef] [PubMed]







| Dorsal Hippocampus | ||||
|---|---|---|---|---|
| Animal Group | prior_Met /n = 8/ (Mean ± SD) | after_MET /n = 8/ (Mean ± SD) | prior_MET vs. after_MET p-Value | |
| MRS Ratio | ||||
| tNAA/tCr | 0.958 ± 0.144 | 0.888 ± 0.074 | 0.107 | |
| mIns/tNAA | 0.442 ± 0.176 | 0.493 ± 0.140 | 0.584 | |
| mIns/tCr | 0.408 ± 0.129 | 0.438 ± 0.135 | 0.710 | |
| tCho/tNAA | 0.216 ± 0.053 | 0.223 ± 0.056 | 0.735 | |
| tCho/tCr | 0.203 ± 0.037 | 0.195 ± 0.040 | 0.632 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kovalska, M.; Hnilicova, P.; Kalenska, D.; Adamkov, M.; Kovalska, L.; Lehotsky, J. Alzheimer’s Disease-like Pathological Features in the Dorsal Hippocampus of Wild-Type Rats Subjected to Methionine-Diet-Evoked Mild Hyperhomocysteinaemia. Cells 2023, 12, 2087. https://doi.org/10.3390/cells12162087
Kovalska M, Hnilicova P, Kalenska D, Adamkov M, Kovalska L, Lehotsky J. Alzheimer’s Disease-like Pathological Features in the Dorsal Hippocampus of Wild-Type Rats Subjected to Methionine-Diet-Evoked Mild Hyperhomocysteinaemia. Cells. 2023; 12(16):2087. https://doi.org/10.3390/cells12162087
Chicago/Turabian StyleKovalska, Maria, Petra Hnilicova, Dagmar Kalenska, Marian Adamkov, Libusa Kovalska, and Jan Lehotsky. 2023. "Alzheimer’s Disease-like Pathological Features in the Dorsal Hippocampus of Wild-Type Rats Subjected to Methionine-Diet-Evoked Mild Hyperhomocysteinaemia" Cells 12, no. 16: 2087. https://doi.org/10.3390/cells12162087
APA StyleKovalska, M., Hnilicova, P., Kalenska, D., Adamkov, M., Kovalska, L., & Lehotsky, J. (2023). Alzheimer’s Disease-like Pathological Features in the Dorsal Hippocampus of Wild-Type Rats Subjected to Methionine-Diet-Evoked Mild Hyperhomocysteinaemia. Cells, 12(16), 2087. https://doi.org/10.3390/cells12162087






