Sox9 Inhibits Cochlear Hair Cell Fate by Upregulating Hey1 and HeyL Antagonists of Atoh1
Abstract
1. Introduction
2. Materials and Methods
2.1. Mouse Strains and Tamoxifen Treatment
2.2. Plasmid Constructions
2.3. Cell Culture and Transfection
2.4. Western Blot
2.5. RT-qPCR
2.6. Luciferase Assay
2.7. Organotypic Cochlear Cultures
2.7.1. Tissue Isolation and Culture
2.7.2. Electroporation
2.8. Immunohistochemistry
2.9. Quantification of HC Fate in Cultured Explants
2.10. Supernumerary HC Counts
2.11. In Situ Hybridization
2.12. Statistical Analysis
3. Results
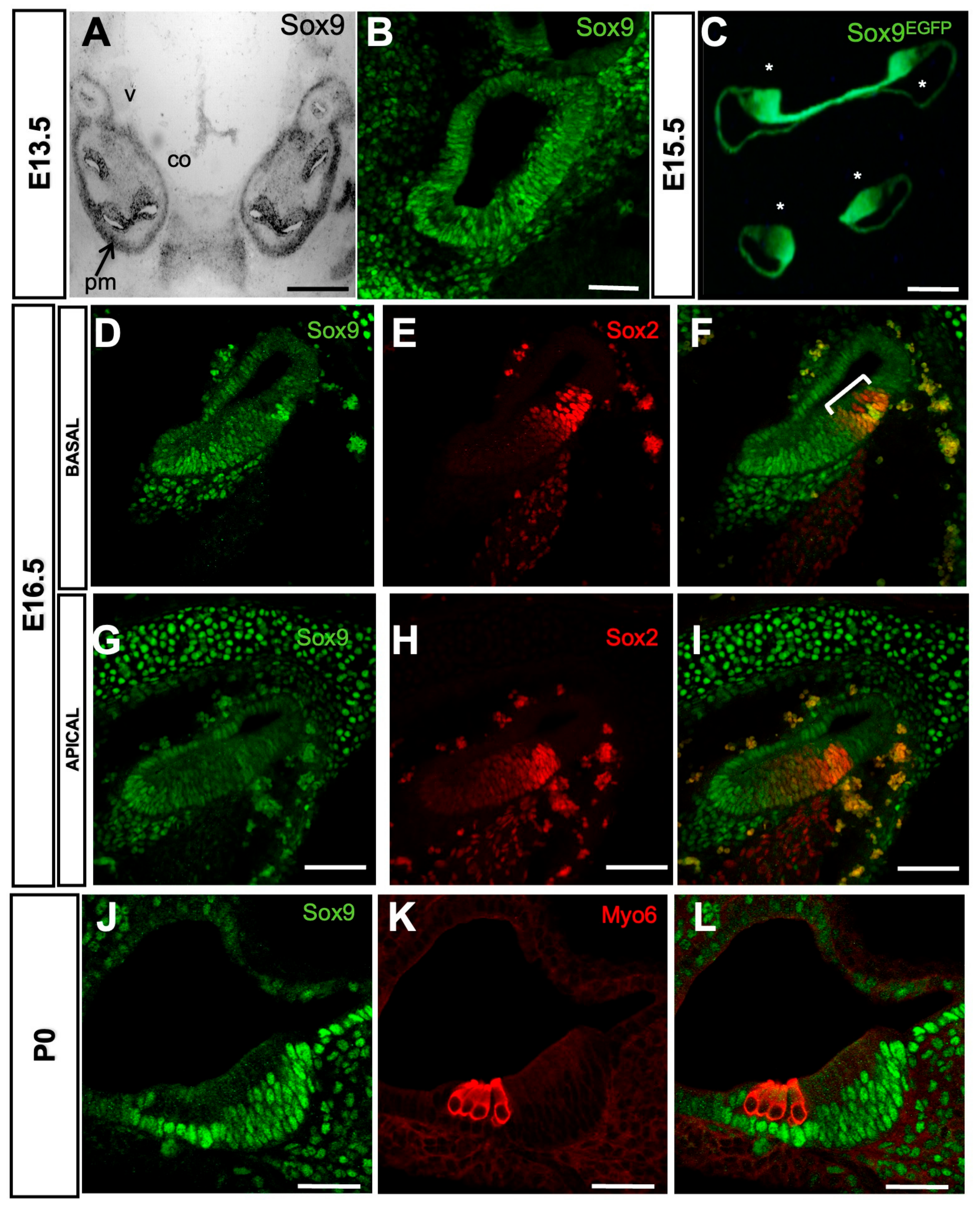
3.1. Analysis of Sox9 Expression Pattern in the Developing Mouse Cochlea
3.2. Sox9 Inhibits Cochlear Hair Cell Fate Ex Vivo
3.3. Sox9 Inhibits Atoh1 Activity
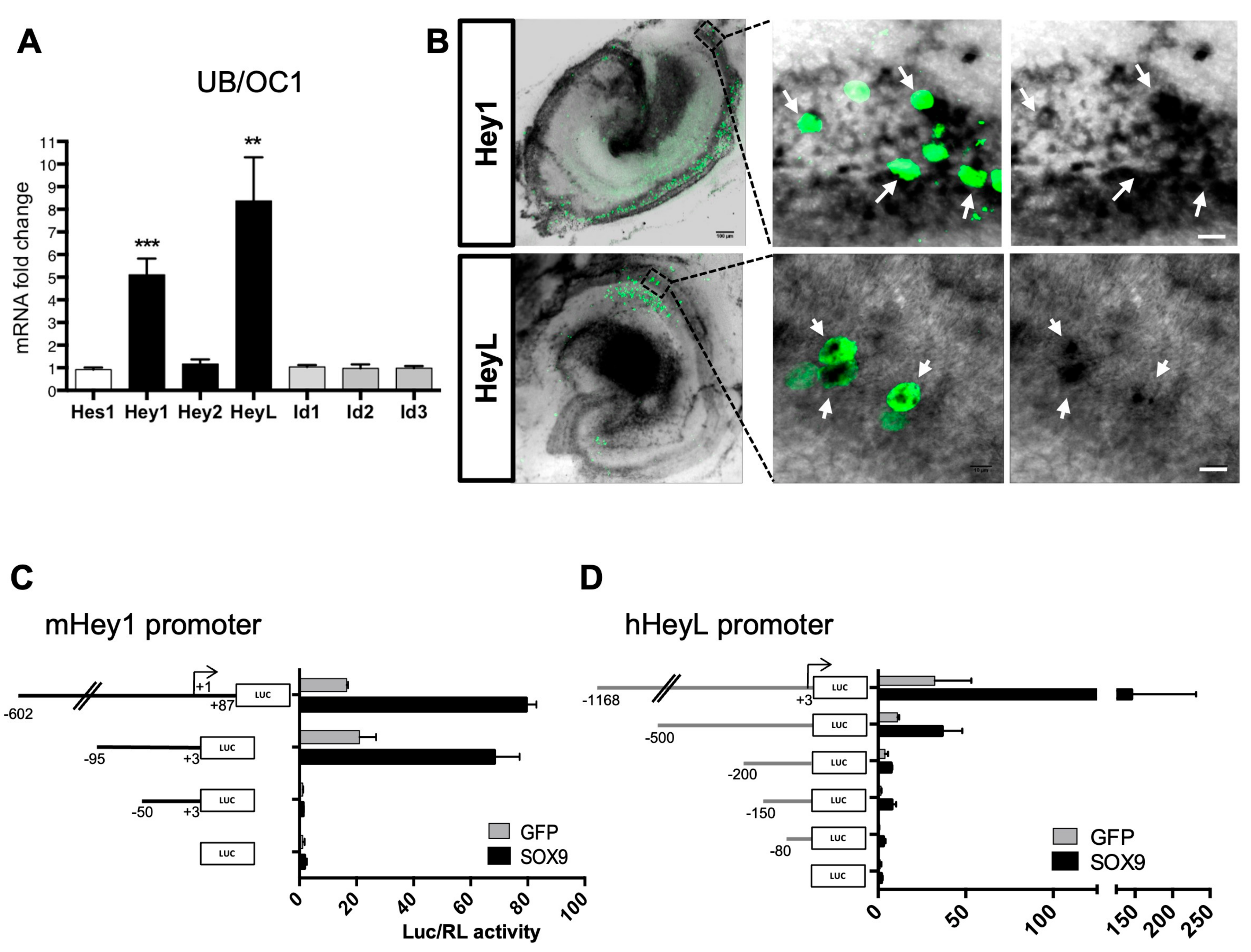
3.4. Sox9 Activates Hey1 and HeyL Gene Expression
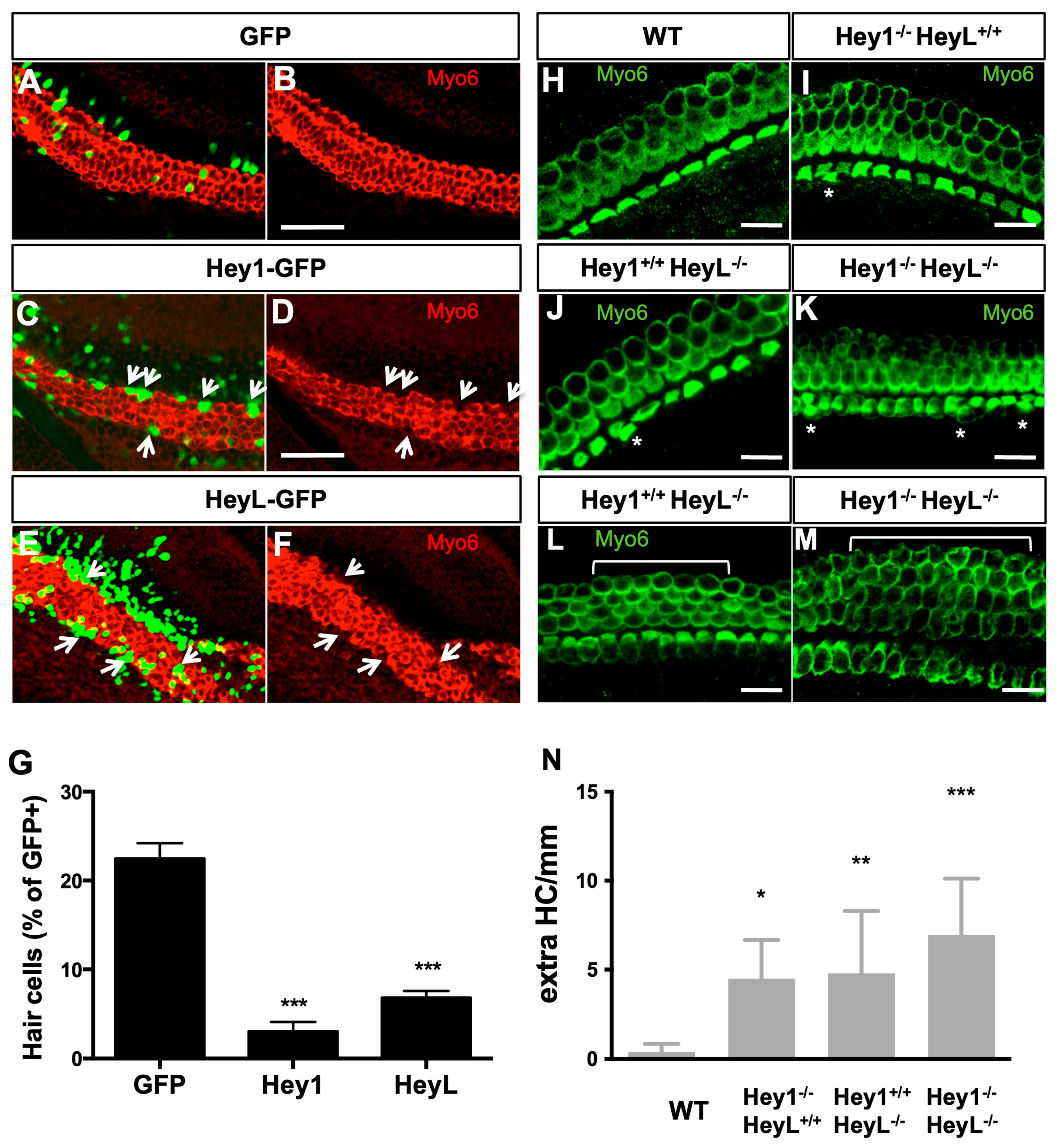
3.5. Overexpression of Hey1 and HeyL Prevents Hair Cell Differentiation
3.6. Sox9 Inhibition of Atoh1 Partially Relies on Hey1 and HeyL Upregulation
3.7. Sox9 Loss Leads to Supernumerary HCs but Not upon Sox2 Hypomorphism
4. Discussion
4.1. Sox9 Cell Fate Determiner Is an Inhibitor of Atoh1 in the Cochlea
4.2. Functional Redundancy for Sox Factors in the Differentiating Organ of Corti?
4.3. Hey1 and HeyL Are Downstream Effectors of Sox9
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kelley, M.W. Regulation of cell fate in the sensory epithelia of the inner ear. Nat. Rev. Neurosci. 2006, 7, 837–849. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.; Segil, N. p27(Kip1) links cell proliferation to morphogenesis in the developing organ of Corti. Development 1999, 126, 1581–1590. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Zhang, S.; Chai, R.; Li, H. Hair Cell Regeneration. In Hearing Loss: Mechanisms, Prevention and Cure; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; Volume 1130, pp. 1–16. [Google Scholar] [CrossRef]
- Atkinson, P.J.; Huarcaya Najarro, E.; Sayyid, Z.N.; Cheng, A.G. Sensory hair cell development and regeneration: Similarities and differences. Development 2015, 142, 1561–1571. [Google Scholar] [CrossRef] [PubMed]
- Bermingham, N.A.; Hassan, B.A.; Price, S.D.; Vollrath, M.A.; Ben-Arie, N.; Eatock, R.A.; Bellen, H.J.; Lysakowski, A.; Zoghbi, H.Y. Math1: An essential gene for the generation of inner ear hair cells. Science 1999, 284, 1837–1841. [Google Scholar] [CrossRef]
- Chen, P.; Johnson, J.E.; Zoghbi, H.Y.; Segil, N. The role of Math1 in inner ear development: Uncoupling the establishment of the sensory primordium from hair cell fate determination. Development 2002, 129, 2495–2505. [Google Scholar] [CrossRef]
- Zheng, J.L.; Gao, W.Q. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat. Neurosci. 2000, 3, 580–586. [Google Scholar] [CrossRef]
- Lanford, P.J.; Shailam, R.; Norton, C.R.; Gridley, T.; Kelley, M.W. Expression of Math1 and HES5 in the cochleae of wildtype and Jag2 mutant mice. J. Assoc. Res. Otolaryngol. 2000, 1, 161–171. [Google Scholar] [CrossRef]
- Morrison, A.; Hodgetts, C.; Gossler, A.; Hrabe de Angelis, M.; Lewis, J. Expression of Delta1 and Serrate1 (Jagged1) in the mouse inner ear. Mech. Dev. 1999, 84, 169–172. [Google Scholar] [CrossRef]
- Lanford, P.J.; Lan, Y.; Jiang, R.; Lindsell, C.; Weinmaster, G.; Gridley, T.; Kelley, M.W. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat. Genet. 1999, 21, 289–292. [Google Scholar] [CrossRef]
- Brooker, R.; Hozumi, K.; Lewis, J. Notch ligands with contrasting functions: Jagged1 and Delta1 in the mouse inner ear. Development 2006, 133, 1277–1286. [Google Scholar] [CrossRef]
- Kiernan, A.E.; Cordes, R.; Kopan, R.; Gossler, A.; Gridley, T. The Notch ligands DLL1 and JAG2 act synergistically to regulate hair cell development in the mammalian inner ear. Development 2005, 132, 4353–4362. [Google Scholar] [CrossRef] [PubMed]
- Artavanis-Tsakonas, S.; Rand, M.D.; Lake, R.J. Notch signaling: Cell fate control and signal integration in development. Science 1999, 284, 770–776. [Google Scholar] [CrossRef] [PubMed]
- Akazawa, C.; Ishibashi, M.; Shimizu, C.; Nakanishi, S.; Kageyama, R. A mammalian helix-loop-helix factor structurally related to the product of Drosophila proneural gene atonal is a positive transcriptional regulator expressed in the developing nervous system. J. Biol. Chem. 1995, 270, 8730–8738. [Google Scholar] [CrossRef] [PubMed]
- Zine, A.; Aubert, A.; Qiu, J.; Therianos, S.; Guillemot, F.; Kageyama, R.; de Ribaupierre, F. Hes1 and Hes5 activities are required for the normal development of the hair cells in the mammalian inner ear. J. Neurosci. 2001, 21, 4712–4720. [Google Scholar] [CrossRef]
- Dy, P.; Wang, W.; Bhattaram, P.; Wang, Q.; Wang, L.; Ballock, R.T.; Lefebvre, V. Sox9 directs hypertrophic maturation and blocks osteoblast differentiation of growth plate chondrocytes. Dev. Cell 2012, 22, 597–609. [Google Scholar] [CrossRef] [PubMed]
- Bagheri-Fam, S.; Barrionuevo, F.; Dohrmann, U.; Gunther, T.; Schule, R.; Kemler, R.; Mallo, M.; Kanzler, B.; Scherer, G. Long-range upstream and downstream enhancers control distinct subsets of the complex spatiotemporal Sox9 expression pattern. Dev. Biol. 2006, 291, 382–397. [Google Scholar] [CrossRef]
- Mak, A.C.; Szeto, I.Y.; Fritzsch, B.; Cheah, K.S. Differential and overlapping expression pattern of SOX2 and SOX9 in inner ear development. Gene Expr. Patterns—GEP 2009, 9, 444–453. [Google Scholar] [CrossRef]
- Seymour, P.A.; Freude, K.K.; Tran, M.N.; Mayes, E.E.; Jensen, J.; Kist, R.; Scherer, G.; Sander, M. SOX9 is required for maintenance of the pancreatic progenitor cell pool. Proc. Natl. Acad. Sci. USA 2007, 104, 1865–1870. [Google Scholar] [CrossRef]
- Stolt, C.C.; Lommes, P.; Sock, E.; Chaboissier, M.C.; Schedl, A.; Wegner, M. The Sox9 transcription factor determines glial fate choice in the developing spinal cord. Genes. Dev. 2003, 17, 1677–1689. [Google Scholar] [CrossRef]
- Pritchett, J.; Athwal, V.; Roberts, N.; Hanley, N.A.; Hanley, K.P. Understanding the role of SOX9 in acquired diseases: Lessons from development. Trends Mol. Med. 2011, 17, 166–174. [Google Scholar] [CrossRef]
- Mansour, S.; Offiah, A.C.; McDowall, S.; Sim, P.; Tolmie, J.; Hall, C. The phenotype of survivors of campomelic dysplasia. J. Med. Genet. 2002, 39, 597–602. [Google Scholar] [CrossRef] [PubMed]
- Tokita, N.; Chandra-Sekhar, H.K.; Daly, J.F.; Becker, M.H.; Aleksic, S. The Campomelic syndrome. Temporal bone histopathologic features and otolaryngologic manifestations. Arch. Otolaryngol. 1979, 105, 449–454. [Google Scholar] [CrossRef]
- Savarirayan, R.; Robertson, S.P.; Bankier, A.; Rogers, J.G. Variable expression of campomelic dysplasia in a father and his 46, XY daughter. Pediatr. Pathol. Mol. Med. 2003, 22, 37–46. [Google Scholar] [CrossRef] [PubMed]
- Saint-Germain, N.; Lee, Y.H.; Zhang, Y.; Sargent, T.D.; Saint-Jeannet, J.P. Specification of the otic placode depends on Sox9 function in Xenopus. Development 2004, 131, 1755–1763. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.M.; Labonne, C. SoxE factors function equivalently during neural crest and inner ear development and their activity is regulated by SUMOylation. Dev. Cell 2005, 9, 593–603. [Google Scholar] [CrossRef]
- Yan, Y.L.; Willoughby, J.; Liu, D.; Crump, J.G.; Wilson, C.; Miller, C.T.; Singer, A.; Kimmel, C.; Westerfield, M.; Postlethwait, J.H. A pair of Sox: Distinct and overlapping functions of zebrafish sox9 co-orthologs in craniofacial and pectoral fin development. Development 2005, 132, 1069–1083. [Google Scholar] [CrossRef]
- Barrionuevo, F.; Naumann, A.; Bagheri-Fam, S.; Speth, V.; Taketo, M.M.; Scherer, G.; Neubuser, A. Sox9 is required for invagination of the otic placode in mice. Dev. Biol. 2008, 317, 213–224. [Google Scholar] [CrossRef]
- Jo, A.; Denduluri, S.; Zhang, B.; Wang, Z.; Yin, L.; Yan, Z.; Kang, R.; Shi, L.L.; Mok, J.; Lee, M.J.; et al. The versatile functions of Sox9 in development, stem cells, and human diseases. Genes. Dis. 2014, 1, 149–161. [Google Scholar] [CrossRef]
- Fukada, S.; Yamaguchi, M.; Kokubo, H.; Ogawa, R.; Uezumi, A.; Yoneda, T.; Matev, M.M.; Motohashi, N.; Ito, T.; Zolkiewska, A.; et al. Hesr1 and Hesr3 are essential to generate undifferentiated quiescent satellite cells and to maintain satellite cell numbers. Development 2011, 138, 4609–4619. [Google Scholar] [CrossRef]
- Formeister, E.J.; Sionas, A.L.; Lorance, D.K.; Barkley, C.L.; Lee, G.H.; Magness, S.T. Distinct SOX9 levels differentially mark stem/progenitor populations and enteroendocrine cells of the small intestine epithelium. Am. J. Physiology. Gastrointest. Liver Physiol. 2009, 296, G1108–G1118. [Google Scholar] [CrossRef]
- Arnold, K.; Sarkar, A.; Yram, M.A.; Polo, J.M.; Bronson, R.; Sengupta, S.; Seandel, M.; Geijsen, N.; Hochedlinger, K. Sox2(+) adult stem and progenitor cells are important for tissue regeneration and survival of mice. Cell Stem Cell 2011, 9, 317–329. [Google Scholar] [CrossRef]
- Kist, R.; Schrewe, H.; Balling, R.; Scherer, G. Conditional inactivation of Sox9: A mouse model for campomelic dysplasia. Genesis 2002, 32, 121–123. [Google Scholar] [CrossRef] [PubMed]
- Srinivas, S.; Watanabe, T.; Lin, C.S.; William, C.M.; Tanabe, Y.; Jessell, T.M.; Costantini, F. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev. Biol. 2001, 1, 4. [Google Scholar] [CrossRef] [PubMed]
- Creppe, C.; Malinouskaya, L.; Volvert, M.L.; Gillard, M.; Close, P.; Malaise, O.; Laguesse, S.; Cornez, I.; Rahmouni, S.; Ormenese, S.; et al. Elongator controls the migration and differentiation of cortical neurons through acetylation of alpha-tubulin. Cell 2009, 136, 551–564. [Google Scholar] [CrossRef] [PubMed]
- Flora, A.; Garcia, J.J.; Thaller, C.; Zoghbi, H.Y. The E-protein Tcf4 interacts with Math1 to regulate differentiation of a specific subset of neuronal progenitors. Proc. Natl. Acad. Sci. USA 2007, 104, 15382–15387. [Google Scholar] [CrossRef]
- Wilhelm, D.; Hiramatsu, R.; Mizusaki, H.; Widjaja, L.; Combes, A.N.; Kanai, Y.; Koopman, P. SOX9 regulates prostaglandin D synthase gene transcription in vivo to ensure testis development. J. Biol. Chem. 2007, 282, 10553–10560. [Google Scholar] [CrossRef]
- Lefebvre, V.; Zhou, G.; Mukhopadhyay, K.; Smith, C.N.; Zhang, Z.; Eberspaecher, H.; Zhou, X.; Sinha, S.; Maity, S.N.; de Crombrugghe, B. An 18-base-pair sequence in the mouse proalpha1(II) collagen gene is sufficient for expression in cartilage and binds nuclear proteins that are selectively expressed in chondrocytes. Mol. Cell. Biol. 1996, 16, 4512–4523. [Google Scholar] [CrossRef] [PubMed]
- Maier, M.M.; Gessler, M. Comparative analysis of the human and mouse Hey1 promoter: Hey genes are new Notch target genes. Biochem. Biophys. Res. Commun. 2000, 275, 652–660. [Google Scholar] [CrossRef]
- Kanai, Y.; Koopman, P. Structural and functional characterization of the mouse Sox9 promoter: Implications for campomelic dysplasia. Hum. Mol. Genet. 1999, 8, 691–696. [Google Scholar] [CrossRef][Green Version]
- Rivolta, M.N.; Grix, N.; Lawlor, P.; Ashmore, J.F.; Jagger, D.J.; Holley, M.C. Auditory hair cell precursors immortalized from the mammalian inner ear. Proc. Biol. Sci. 1998, 265, 1595–1603. [Google Scholar] [CrossRef]
- Breuskin, I.; Bodson, M.; Thelen, N.; Thiry, M.; Borgs, L.; Nguyen, L.; Stolt, C.; Wegner, M.; Lefebvre, P.P.; Malgrange, B. Glial but not neuronal development in the cochleo-vestibular ganglion requires Sox10. J. Neurochem. 2010, 114, 1827–1839. [Google Scholar] [CrossRef] [PubMed]
- Matei, V.; Pauley, S.; Kaing, S.; Rowitch, D.; Beisel, K.W.; Morris, K.; Feng, F.; Jones, K.; Lee, J.; Fritzsch, B. Smaller inner ear sensory epithelia in Neurog 1 null mice are related to earlier hair cell cycle exit. Dev. Dyn. 2005, 234, 633–650. [Google Scholar] [CrossRef] [PubMed]
- Woods, C.; Montcouquiol, M.; Kelley, M.W. Math1 regulates development of the sensory epithelium in the mammalian cochlea. Nat. Neurosci. 2004, 7, 1310–1318. [Google Scholar] [CrossRef]
- Yang, H.; Xie, X.; Deng, M.; Chen, X.; Gan, L. Generation and characterization of Atoh1-Cre knock-in mouse line. Genesis 2010, 48, 407–413. [Google Scholar] [CrossRef] [PubMed]
- Lefebvre, V.; Huang, W.; Harley, V.R.; Goodfellow, P.N.; de Crombrugghe, B. SOX9 is a potent activator of the chondrocyte-specific enhancer of the pro alpha1(II) collagen gene. Mol. Cell Biol. 1997, 17, 2336–2346. [Google Scholar] [CrossRef]
- Kageyama, R.; Nakanishi, S. Helix-loop-helix factors in growth and differentiation of the vertebrate nervous system. Curr. Opin. Genet. Dev. 1997, 7, 659–665. [Google Scholar] [CrossRef]
- Jones, J.M.; Montcouquiol, M.; Dabdoub, A.; Woods, C.; Kelley, M.W. Inhibitors of differentiation and DNA binding (Ids) regulate Math1 and hair cell formation during the development of the organ of Corti. J. Neurosci. Off. J. Soc. Neurosci. 2006, 26, 550–558. [Google Scholar] [CrossRef]
- Tateya, T.; Imayoshi, I.; Tateya, I.; Ito, J.; Kageyama, R. Cooperative functions of Hes/Hey genes in auditory hair cell and supporting cell development. Dev. Biol. 2011, 352, 329–340. [Google Scholar] [CrossRef]
- Doetzlhofer, A.; Basch, M.L.; Ohyama, T.; Gessler, M.; Groves, A.K.; Segil, N. Hey2 regulation by FGF provides a Notch-independent mechanism for maintaining pillar cell fate in the organ of Corti. Dev. Cell 2009, 16, 58–69. [Google Scholar] [CrossRef]
- Atkinson, P.J.; Dong, Y.; Gu, S.; Liu, W.; Najarro, E.H.; Udagawa, T.; Cheng, A.G. Sox2 haploinsufficiency primes regeneration and Wnt responsiveness in the mouse cochlea. J. Clin. Invest. 2018, 128, 1641–1656. [Google Scholar] [CrossRef]
- Yang, J.; Bouvron, S.; Lv, P.; Chi, F.; Yamoah, E.N. Functional features of trans-differentiated hair cells mediated by Atoh1 reveals a primordial mechanism. J. Neurosci. 2012, 32, 3712–3725. [Google Scholar] [CrossRef] [PubMed]
- Dabdoub, A.; Puligilla, C.; Jones, J.M.; Fritzsch, B.; Cheah, K.S.; Pevny, L.H.; Kelley, M.W. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc. Natl. Acad. Sci. USA 2008, 105, 18396–18401. [Google Scholar] [CrossRef]
- Cox, B.C.; Chai, R.; Lenoir, A.; Liu, Z.; Zhang, L.; Nguyen, D.H.; Chalasani, K.; Steigelman, K.A.; Fang, J.; Rubel, E.W.; et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development 2014, 141, 816–829. [Google Scholar] [CrossRef]
- Helms, A.W.; Abney, A.L.; Ben-Arie, N.; Zoghbi, H.Y.; Johnson, J.E. Autoregulation and multiple enhancers control Math1 expression in the developing nervous system. Development 2000, 127, 1185–1196. [Google Scholar] [CrossRef] [PubMed]
- Akiyama, H.; Chaboissier, M.C.; Behringer, R.R.; Rowitch, D.H.; Schedl, A.; Epstein, J.A.; de Crombrugghe, B. Essential role of Sox9 in the pathway that controls formation of cardiac valves and septa. Proc. Natl. Acad. Sci. USA 2004, 101, 6502–6507. [Google Scholar] [CrossRef] [PubMed]
- Cheung, M.; Chaboissier, M.C.; Mynett, A.; Hirst, E.; Schedl, A.; Briscoe, J. The transcriptional control of trunk neural crest induction, survival, and delamination. Dev. Cell 2005, 8, 179–192. [Google Scholar] [CrossRef] [PubMed]
- Bermingham-McDonogh, O.; Oesterle, E.C.; Stone, J.S.; Hume, C.R.; Huynh, H.M.; Hayashi, T. Expression of Prox1 during mouse cochlear development. J. Comp. Neurol. 2006, 496, 172–186. [Google Scholar] [CrossRef]
- Locher, H.; Frijns, J.H.; van Iperen, L.; de Groot, J.C.; Huisman, M.A.; Chuva de Sousa Lopes, S.M. Neurosensory development and cell fate determination in the human cochlea. Neural Dev. 2013, 8, 20. [Google Scholar] [CrossRef]
- Ahmed, M.; Wong, E.Y.; Sun, J.; Xu, J.; Wang, F.; Xu, P.X. Eya1-Six1 interaction is sufficient to induce hair cell fate in the cochlea by activating Atoh1 expression in cooperation with Sox2. Dev. Cell 2012, 22, 377–390. [Google Scholar] [CrossRef]
- Neves, J.; Uchikawa, M.; Bigas, A.; Giraldez, F. The prosensory function of Sox2 in the chicken inner ear relies on the direct regulation of Atoh1. PLoS ONE 2012, 7, e30871. [Google Scholar] [CrossRef]
- Li, S.; Mark, S.; Radde-Gallwitz, K.; Schlisner, R.; Chin, M.T.; Chen, P. Hey2 functions in parallel with Hes1 and Hes5 for mammalian auditory sensory organ development. BMC Dev. Biol. 2008, 8, 20. [Google Scholar] [CrossRef] [PubMed]
- Fischer, A.; Gessler, M. Delta-Notch--and then? Protein interactions and proposed modes of repression by Hes and Hey bHLH factors. Nucleic Acids Res. 2007, 35, 4583–4596. [Google Scholar] [CrossRef] [PubMed]
- Szeto, I.Y.Y.; Chu, D.K.H.; Chen, P.; Chu, K.C.; Au, T.Y.K.; Leung, K.K.H.; Huang, Y.H.; Wynn, S.L.; Mak, A.C.Y.; Chan, Y.S.; et al. SOX9 and SOX10 control fluid homeostasis in the inner ear for hearing through independent and cooperative mechanisms. Proc. Natl. Acad. Sci. USA 2022, 119, e2122121119. [Google Scholar] [CrossRef] [PubMed]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veithen, M.; Huyghe, A.; Van Den Ackerveken, P.; Fukada, S.-i.; Kokubo, H.; Breuskin, I.; Nguyen, L.; Delacroix, L.; Malgrange, B. Sox9 Inhibits Cochlear Hair Cell Fate by Upregulating Hey1 and HeyL Antagonists of Atoh1. Cells 2023, 12, 2148. https://doi.org/10.3390/cells12172148
Veithen M, Huyghe A, Van Den Ackerveken P, Fukada S-i, Kokubo H, Breuskin I, Nguyen L, Delacroix L, Malgrange B. Sox9 Inhibits Cochlear Hair Cell Fate by Upregulating Hey1 and HeyL Antagonists of Atoh1. Cells. 2023; 12(17):2148. https://doi.org/10.3390/cells12172148
Chicago/Turabian StyleVeithen, Mona, Aurélia Huyghe, Priscilla Van Den Ackerveken, So-ichiro Fukada, Hiroki Kokubo, Ingrid Breuskin, Laurent Nguyen, Laurence Delacroix, and Brigitte Malgrange. 2023. "Sox9 Inhibits Cochlear Hair Cell Fate by Upregulating Hey1 and HeyL Antagonists of Atoh1" Cells 12, no. 17: 2148. https://doi.org/10.3390/cells12172148
APA StyleVeithen, M., Huyghe, A., Van Den Ackerveken, P., Fukada, S.-i., Kokubo, H., Breuskin, I., Nguyen, L., Delacroix, L., & Malgrange, B. (2023). Sox9 Inhibits Cochlear Hair Cell Fate by Upregulating Hey1 and HeyL Antagonists of Atoh1. Cells, 12(17), 2148. https://doi.org/10.3390/cells12172148






