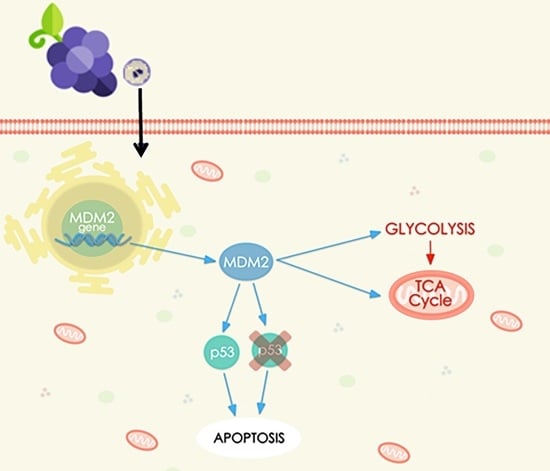
Aglianico Grape Seed Semi-Polar Extract Exerts Anticancer Effects by Modulating MDM2 Expression and Metabolic Pathways
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. AGS Extract Preparation
2.3. Cell Proliferation Assay
2.4. Apoptosis Detection by Flow Cytometry Analysis
2.5. Transcriptomic Analysis
2.6. IPA Analysis
2.7. Quantitative PCR
2.8. Western Blot
2.9. Metabolomics Analysis
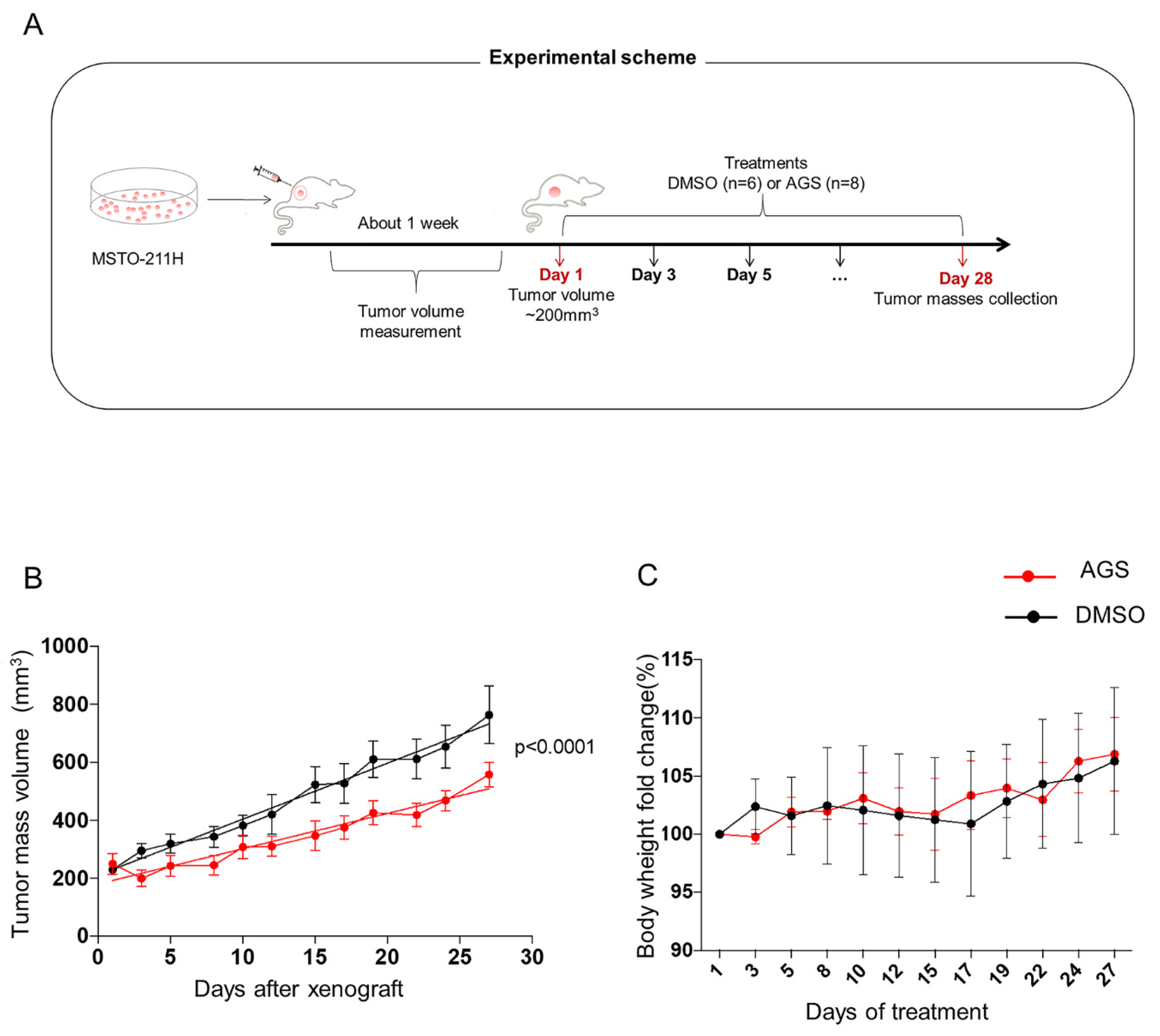
2.10. In Vivo Xenograft Model
2.11. Histology and Immunohistochemistry
2.12. Statistical Analysis
3. Results
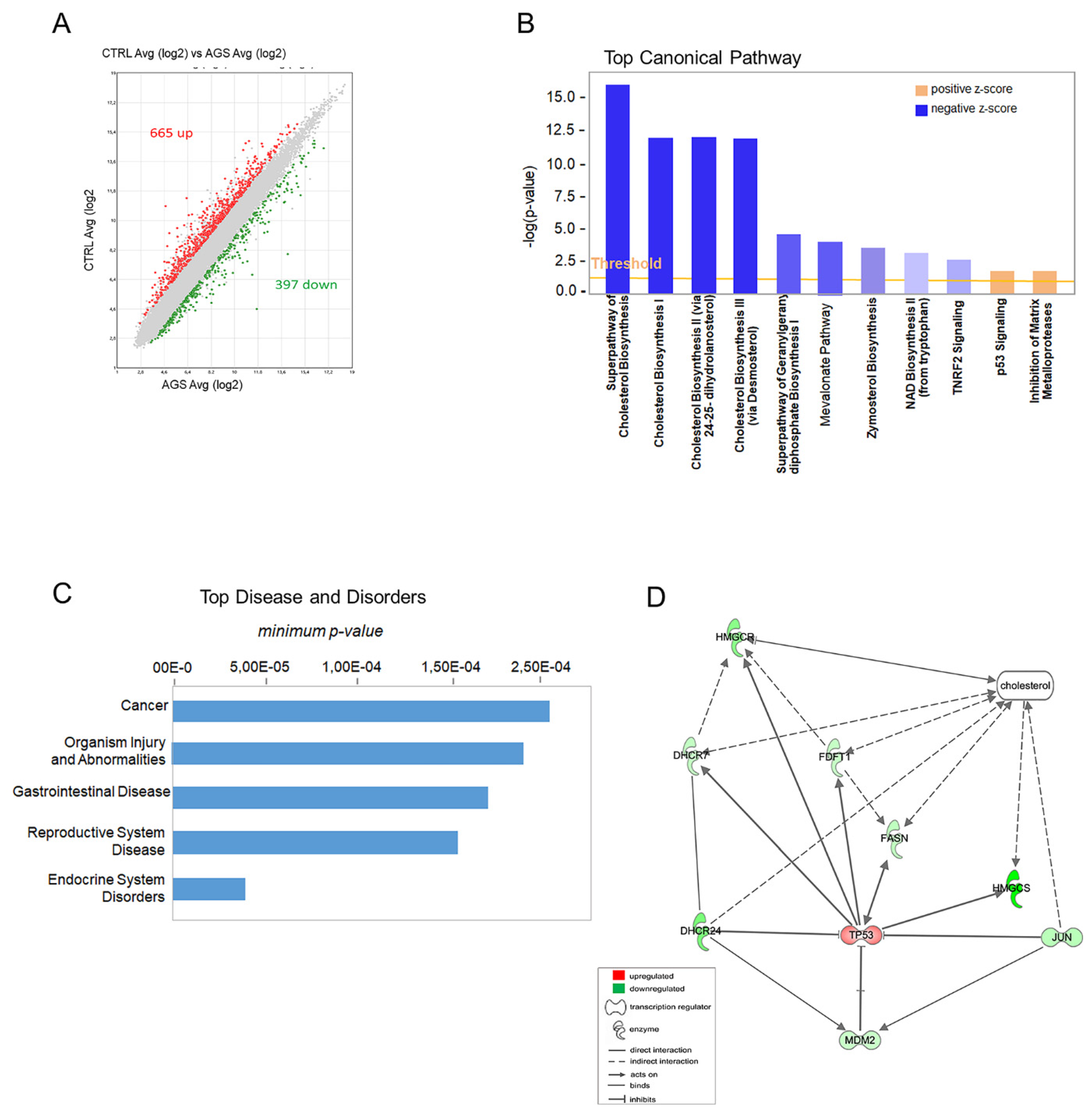
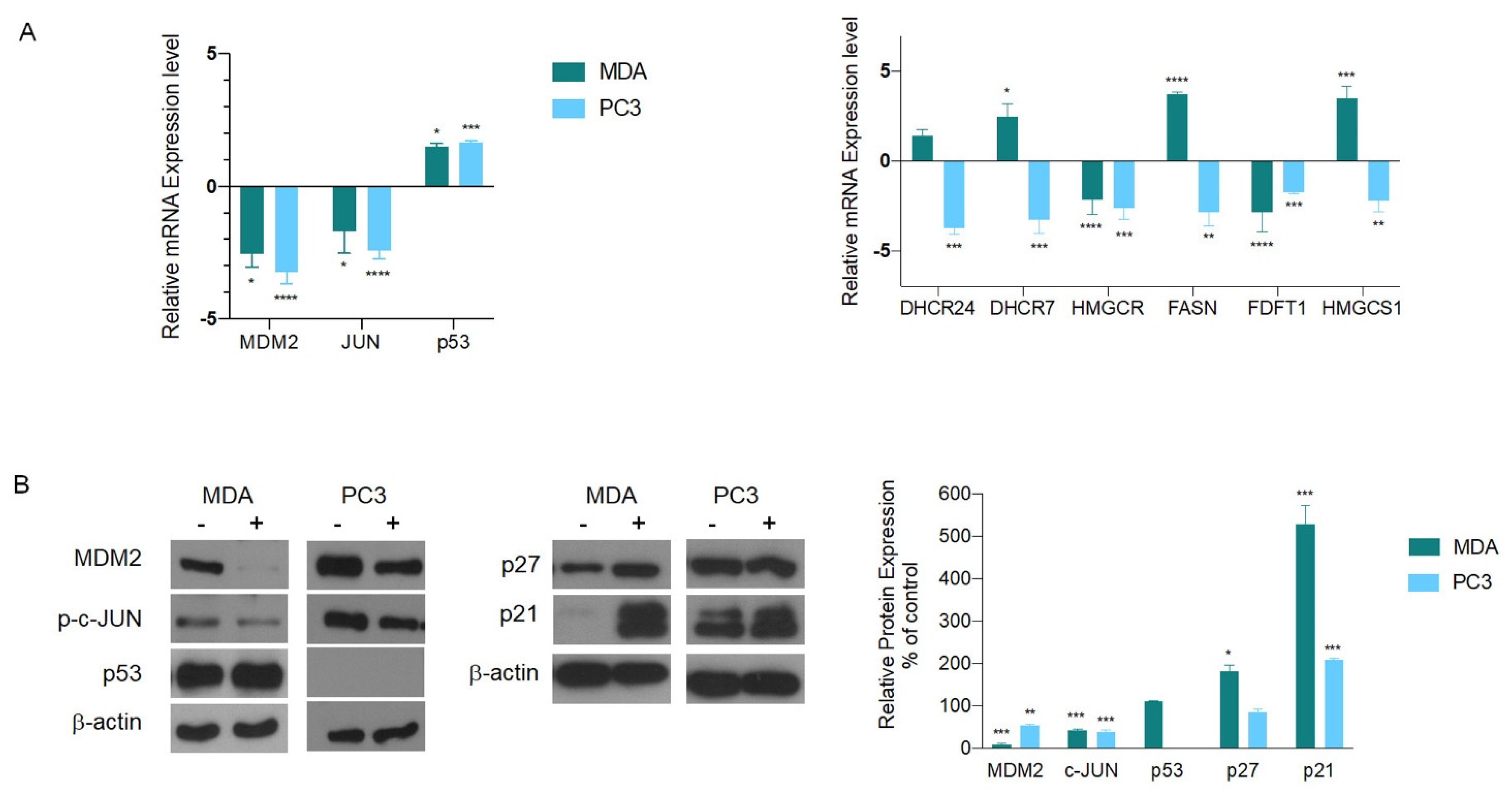
3.1. Transcriptomics Profiling
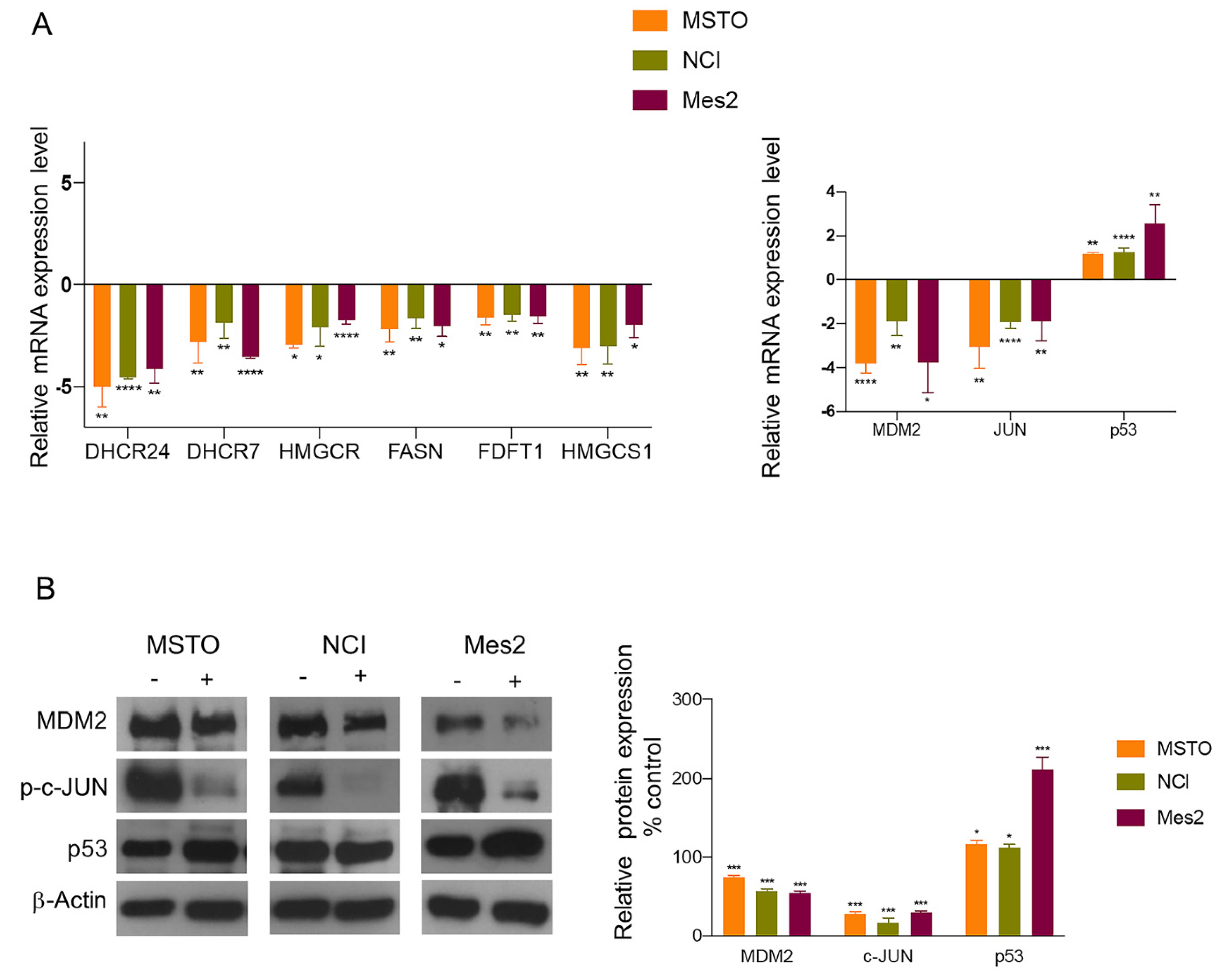
3.2. Biological Validation of Gene and Protein Expression
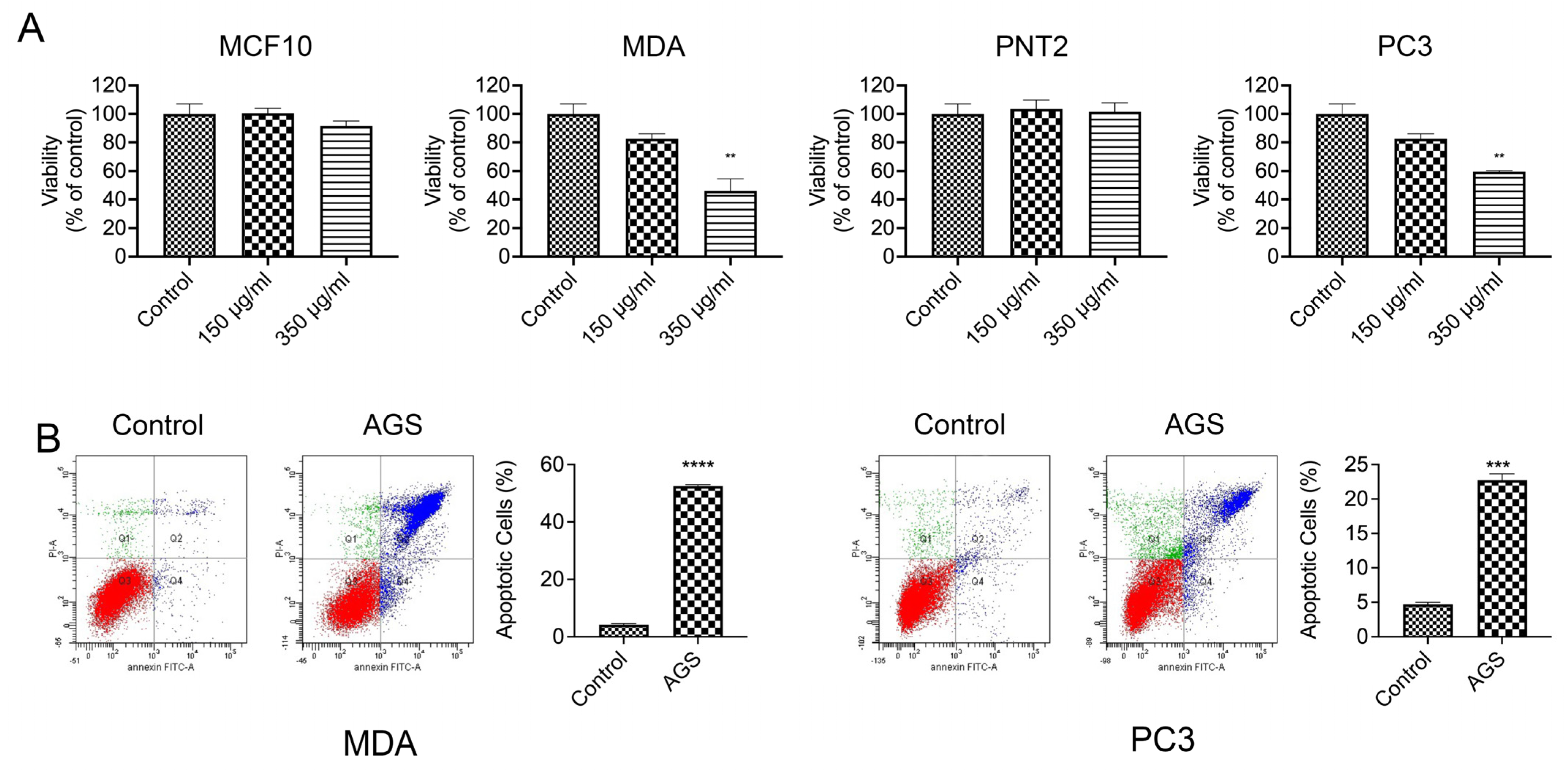
3.3. AGS Induces Apoptosis in p53 Defective Cancer Cell Lines
3.4. Metabolomic Analysis
3.5. In Vivo Analysis of AGS Treatment
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Shi, J.; Yu, J.; Pohorly, J.E.; Kakuda, Y. Polyphenolics in grape seeds-biochemistry and functionality. J. Med. Food 2003, 6, 291–299. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Abu-Izneid, T.; Iahfisham-Ul-Haq; Patel, S.; Pan, X.; Naz, S.; Silva, A.; Saeed, F.; Suleria, H. Proanthocyanidins: A comprehensive review. Biomed. Pharmacother. 2019, 116, 108999. [Google Scholar] [CrossRef] [PubMed]
- He, F.; Pan, Q.H.; Shi, Y.; Duan, C.Q. Biosynthesis and genetic regulation of proanthocyanidins in plants. Molecules 2008, 13, 2674–2703. [Google Scholar] [CrossRef] [Green Version]
- Xu, Z.; Du, P.; Meiser, P.; Jacob, C. Proanthocyanidins: Oligomeric structures with unique biochemical properties and great therapeutic promise. Nat. Prod. Commun. 2012, 7, 381–388. [Google Scholar] [CrossRef] [Green Version]
- Shimura, T.; Sharma, P.; Sharma, G.G.; Banwait, J.K.; Goel, A. Enhanced anti-cancer activity of andrographis with oligomeric proanthocyanidins through activation of metabolic and ferroptosis pathways in colorectal cancer. Sci. Rep. 2021, 11, 7548. [Google Scholar] [CrossRef] [PubMed]
- Dinicola, S.; Pasqualato, A.; Cucina, A.; Coluccia, P.; Ferranti, F.; Canipari, R.; Catizone, A.; Proietti, S.; D’Anselmi, F.; Ricci, G.; et al. Grape seed extract suppresses MDA-MB231 breast cancer cell migration and invasion. Eur. J. Nutr. 2014, 53, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Na, W.; Ma, B.; Shi, S.; Chen, Y.; Zhang, H.; Zhan, Y.; An, H. Procyanidin B1, a novel and specific inhibitor of Kv10.1 channel, suppresses the evolution of hepatoma. Biochem. Pharm. 2020, 178, 114089. [Google Scholar] [CrossRef] [PubMed]
- Brás, N.F.; Gonçalves, R.; Fernandes, P.A.; Mateus, N.; Ramos, M.J.; de Freitas, V. Understanding the binding of procyanidins to pancreatic elastase by experimental and computational methods. Biochemistry 2010, 49, 5097–5108. [Google Scholar] [CrossRef]
- Di Meo, F.; Aversano, R.; Diretto, G.; Demurtas, O.; Villano, C.; Cozzolino, S.; Filosa, S.; Carputo, D.; Crispi, S. Anti-cancer activity of grape seed semi-polar extracts in human mesothelioma cell lines. J. Funct. Foods 2019, 61, 103515. [Google Scholar] [CrossRef]
- Baldi, A.; Piccolo, M.; Boccellino, M.; Donizetti, A.; Cardillo, I.; La Porta, R.; Quagliuolo, L.; Spugnini, E.; Cordero, F.; Citro, G.; et al. Apoptosis Induced by Piroxicam plus Cisplatin Combined Treatment Is Triggered by p21 in Mesothelioma. PLoS ONE 2011, 6, e23569. [Google Scholar] [CrossRef]
- Crispi, S.; Calogero, R.A.; Santini, M.; Mellone, P.; Vincenzi, B.; Citro, G.; Vicidomini, G.; Fasano, S.; Meccariello, R.; Cobellis, G.; et al. Global gene expression profiling of human pleural mesotheliomas: Identification of matrix metalloproteinase 14 (MMP-14) as potential tumour target. PLoS ONE 2009, 4, e7016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahrazem, O.; Argandona, J.; Fiore, A.; Aguado, C.; Lujan, R.; Rubio-Moraga, A.; Marro, M.; Araujo-Andrade, C.; Loza-Alvarez, P.; Diretto, G.; et al. Transcriptome analysis in tissue sectors with contrasting crocins accumulation provides novel insights into apocarotenoid biosynthesis and regulation during chromoplast biogenesis. Sci. Rep. 2018, 8, 2843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grosso, V.; Farina, A.; Giorgi, D.; Nardi, L.; Diretto, G.; Lucretti, S. A high-throughput flow cytometry system for early screening of in vitro made polyploids in Dendrobium hybrids. Plant Cell Tissue Organ Cult. 2018, 132, 57–70. [Google Scholar] [CrossRef]
- Spugnini, E.; Menicagli, F.; Giaconella, R.; Zanni, F.; Camponi, C.; De Luca, A.; Santoro, A.; Baldi, A. Filling the gap between histology and cytology: Description of an innovative technology (Cytomatrix) to increase the diagnostic effectiveness of fine needle aspirates data. J. Clin. Pathol. 2021, 74, 269–270. [Google Scholar] [CrossRef] [PubMed]
- Diretto, G.; Ahrazem, O.; Rubio-Moraga, A.; Fiore, A.; Sevi, F.; Argandoa, A.; Gmez-Gmez, L. UGT709G1: A novel uridine diphosphate glycosyltransferase involved in the biosynthesis of picrocrocin, the precursor of safranal in saffron (Crocus sativus). New Phytol. 2019, 224, 725–740. [Google Scholar] [CrossRef]
- Huang, B.; Song, B.L.; Xu, C. Cholesterol metabolism in cancer: Mechanisms and therapeutic opportunities. Nat. Metab. 2020, 2, 132–141. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.; Miloslavskaya, I.; Demontis, S.; Maestro, R.; Galaktionov, K. Regulation of cellular response to oncogenic and oxidative stress by Seladin-1. Nature 2004, 432, 640–645. [Google Scholar] [CrossRef] [Green Version]
- Ganguli, G.; Wasylyk, B. p53-independent functions of MDM2. Mol. Cancer Res. 2003, 1, 1027–1035. [Google Scholar]
- Li, Q.; Lozano, G. Molecular Pathways: Targeting Mdm2 and Mdm4 in Cancer Therapy. Clin. Cancer Res. 2013, 19, 34–41. [Google Scholar] [CrossRef] [Green Version]
- Battista, M.C.; Guimond, M.O.; Roberge, C.; Doueik, A.A.; Fazli, L.; Gleave, M.; Sabbagh, R.; Gallo-Payet, N. Inhibition of DHCR24/seladin-1 impairs cellular homeostasis in prostate cancer. Prostate 2010, 70, 921–933. [Google Scholar] [CrossRef]
- Dai, M.; Zhu, X.; Liu, F.; Xu, Q.; Ge, Q.; Jiang, S.; Yang, X.; Li, J.; Wang, Y.; Wu, Q.; et al. Cholesterol Synthetase DHCR24 Induced by Insulin Aggravates Cancer Invasion and Progesterone Resistance in Endometrial Carcinoma. Sci. Rep. 2017, 7, 41404. [Google Scholar] [CrossRef] [PubMed]
- Qiu, T.; Cao, J.; Chen, W.; Wang, J.; Wang, Y.; Zhao, L.; Liu, M.; He, L.; Wu, G.; Li, H.; et al. 24-Dehydrocholesterol reductase promotes the growth of breast cancer stem-like cells through the Hedgehog pathway. Cancer Sci. 2020, 111, 3653–3664. [Google Scholar] [CrossRef] [PubMed]
- Zerenturk, E.J.; Kristiana, I.; Gill, S.; Brown, A.J. The endogenous regulator 24(S),25-epoxycholesterol inhibits cholesterol synthesis at DHCR24 (Seladin-1). Biochim. Biophys. Acta 2012, 1821, 1269–1277. [Google Scholar] [CrossRef] [PubMed]
- Chavez, K.J.; Garimella, S.V.; Lipkowitz, S. Triple negative breast cancer cell lines: One tool in the search for better treatment of triple negative breast cancer. Breast Dis. 2010, 32, 35–48. [Google Scholar] [CrossRef] [Green Version]
- Bartek, J.; Iggo, R.; Gannon, J.; Lane, D.P. Genetic and immunochemical analysis of mutant p53 in human breast cancer cell lines. Oncogene 1990, 5, 893–899. [Google Scholar] [PubMed]
- Isaacs, W.B.; Carter, B.S.; Ewing, C.M. Wild-type p53 suppresses growth of human prostate cancer cells containing mutant p53 alleles. Cancer Res. 1991, 51, 4716–4720. [Google Scholar] [PubMed]
- Kim, S.; Han, J.; Kim, N.; Lee, S.; Cho, D.; Choi, M.; Kim, J.; Kim, J.; Choe, J.; Nam, S.; et al. Effect of berberine on p53 expression by TPA in breast cancer cells. Oncol. Rep. 2012, 27, 210–215. [Google Scholar] [CrossRef]
- Carroll, A.G.; Voeller, H.J.; Sugars, L.; Gelmann, E.P. p53 oncogene mutations in three human prostate cancer cell lines. Prostate 1993, 23, 123–134. [Google Scholar] [CrossRef]
- Qi, D.L.; Cobrinik, D. MDM2 but not MDM4 promotes retinoblastoma cell proliferation through p53-independent regulation of MYCN translation. Oncogene 2017, 36, 1760–1769. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Li, M.; Wang, H.; Agrawal, S.; Zhang, R. Antisense therapy targeting MDM2 oncogene in prostate cancer: Effects on proliferation, apoptosis, multiple gene expression, and chemotherapy. Proc. Natl. Acad. Sci. USA 2003, 100, 11636–11641. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Wang, H.; Li, M.; Agrawal, S.; Chen, X.; Zhang, R. MDM2 is a negative regulator of p21WAF1/CIP1, independent of p53. J. Biol. Chem. 2004, 279, 16000–16006. [Google Scholar] [CrossRef]
- Miranda, P.; Buckley, D.; Raghu, D.; Pang, J.; Takano, E.; Vijayakumaran, R.; Teunisse, A.; Posner, A.; Procter, T.; Herold, M.; et al. MDM4 is a rational target for treating breast cancers with mutant p53. J. Pathol. 2017, 241, 661–670. [Google Scholar] [CrossRef]
- Huang, K.; Tang, Y.; He, L.; Dai, Y. MicroRNA-340 inhibits prostate cancer cell proliferation and metastasis by targeting the MDM2-p53 pathway. Oncol. Rep. 2016, 35, 887–895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kong, Y.; Lu, Z.L.; Wang, J.J.; Zhou, R.; Guo, J.; Liu, J.; Sun, H.L.; Wang, H.; Song, W.; Yang, J.; et al. Platycodin D, a metabolite of Platycodin grandiflorum, inhibits highly metastatic MDA-MB-231 breast cancer growth in vitro and in vivo by targeting the MDM2 oncogene. Oncol. Rep. 2016, 36, 1447–1456. [Google Scholar] [CrossRef] [Green Version]
- Lieu, E.; Nguyen, T.; Rhyne, S.; Kim, J. Amino acids in cancer. Exp. Mol. Med. 2020, 52, 15–30. [Google Scholar] [CrossRef]
- Watabe, T.; Kanai, M.; Isobe, M.; Ozawa, N. Cholesterol alpha-epoxides and beta-epoxides as obligatory intermediates in the hepatic-microsomal metabolism of cholesterol to cholestanetriol. Biochim. Et Biophys. Acta 1980, 619, 414–419. [Google Scholar] [CrossRef]
- Derbal, Y. The Adaptive Complexity of Cancer. Biomed. Res. Int. 2018, 2018, 5837235. [Google Scholar] [CrossRef] [Green Version]
- Kotecha, R.; Takami, A.; Espinoza, J.L. Dietary phytochemicals and cancer chemoprevention: A review of the clinical evidence. Oncotarget 2016, 7, 52517–52529. [Google Scholar] [CrossRef] [Green Version]
- Cragg, G.M.; Newman, D.J. Plants as a source of anti-cancer agents. J. Ethnopharmacol. 2005, 100, 72–79. [Google Scholar] [CrossRef] [Green Version]
- Ammoun, S.; Schmid, M.C.; Zhou, L.; Hilton, D.A.; Barczyk, M.; Hanemann, C.O. The p53/mouse double minute 2 homolog complex deregulation in merlin-deficient tumours. Mol. Oncol. 2015, 9, 236–248. [Google Scholar] [CrossRef]
- Momand, J.; Jung, D.; Wilczynski, S.; Niland, J. The MDM2 gene amplification database. Nucleic Acids Res. 1998, 26, 3453–3459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, J. The Cell-Cycle Arrest and Apoptotic Functions of p53 in Tumor Initiation and Progression. Cold Spring Harb. Perspect. Med. 2016, 6, a026104. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaulian, E.; Karin, M. AP-1 as a regulator of cell life and death. Nat. Cell Biol. 2002, 4, E131–E136. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Lu, C.; Shen, Q.; Munoz-Medellin, D.; Kim, H.; Brown, P. AP-1 blockade in breast cancer cells causes cell cycle arrest by suppressing G1 cyclin expression and reducing cyclin-dependent kinase activity. Oncogene 2004, 23, 8238–8246. [Google Scholar] [CrossRef] [Green Version]
- Blau, L.; Knirsh, R.; Ben-Dror, I.; Oren, S.; Kuphal, S.; Hau, P.; Proescholdt, M.; Bosserhoff, A.; Vardimon, L. Aberrant expression of c-Jun in glioblastoma by internal ribosome entry site (IRES)-mediated translational activation. Proc. Natl. Acad. Sci. USA 2012, 109, E2875–E2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Momand, J.; Zambetti, G.P.; Olson, D.C.; George, D.; Levine, A.J. The mdm-2 oncogene product forms a complex with the p53 protein and inhibits p53-mediated transactivation. Cell 1992, 69, 1237–1245. [Google Scholar] [CrossRef]
- Bohlman, S.; Manfredi, J.J. p53-independent effects of Mdm2. Subcell Biochem. 2014, 85, 235–246. [Google Scholar]
- Zhu, J.; Thompson, C.B. Metabolic regulation of cell growth and proliferation. Nat. Rev. Mol. Cell Biol. 2019, 20, 436–450. [Google Scholar] [CrossRef]
- Mondul, A.M.; Moore, S.C.; Weinstein, S.J.; Evans, A.M.; Karoly, E.D.; Männistö, S.; Sampson, J.N.; Albanes, D. Serum Metabolomic Response to Long-Term Supplementation with. J. Nutr. Metab. 2016, 2016, 6158436. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, Y.; Yoshizawa, K.; Hamazaki, K.; Emoto, Y.; Yuri, T.; Yuki, M.; Shikata, N.; Kawashima, H.; Tsubura, A. Mead acid inhibits the growth of KPL-1 human breast cancer cells in vitro and in vivo. Oncol. Rep. 2014, 32, 1385–1394. [Google Scholar] [CrossRef] [Green Version]
- Lope, V.; Guerrero-Zotano, Á.; Casas, A.; Baena-Cañada, J.M.; Bermejo, B.; Pérez-Gómez, B.; Criado-Navarro, I.; Antolín, S.; Sánchez-Rovira, P.; Ramos-Vázquez, M.; et al. Serum Phospholipids Fatty Acids and Breast Cancer Risk by Pathological Subtype. Nutrients 2020, 12, 3132. [Google Scholar] [CrossRef]
- Liu, H.; Toman, R.E.; Goparaju, S.K.; Maceyka, M.; Nava, V.E.; Sankala, H.; Payne, S.G.; Bektas, M.; Ishii, I.; Chun, J.; et al. Sphingosine kinase type 2 is a putative BH3-only protein that induces apoptosis. J. Biol. Chem. 2003, 278, 40330–40336. [Google Scholar] [CrossRef]
- Riscal, R.; Schrepfer, E.; Arena, G.; Cissé, M.Y.; Bellvert, F.; Heuillet, M.; Rambow, F.; Bonneil, E.; Sabourdy, F.; Vincent, C.; et al. Chromatin-Bound MDM2 Regulates Serine Metabolism and Redox Homeostasis Independently of p53. Mol. Cell 2016, 62, 890–902. [Google Scholar] [CrossRef] [Green Version]
- Pacilli, A.; Calienni, M.; Margarucci, S.; D’Apolito, M.; Petillo, O.; Rocchi, L.; Pasquinelli, G.; Nicolai, R.; Koverech, A.; Calvani, M.; et al. Carnitine-acyltransferase system inhibition, cancer cell death, and prevention of myc-induced lymphomagenesis. J. Natl. Cancer Inst. 2013, 105, 489–498. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.I. Folate and carcinogenesis: Evidence, mechanisms, and implications. J. Nutr. Biochem. 1999, 10, 66–88. [Google Scholar] [CrossRef]
- Anantharaju, P.G.; Reddy, B.D.; Padukudru, M.A.; Kumari Chitturi, C.M.; Vimalambike, M.G.; Madhunapantula, S.V. Naturally occurring benzoic acid derivatives retard cancer cell growth by inhibiting histone deacetylases (HDAC). Cancer Biol. 2017, 18, 492–504. [Google Scholar] [CrossRef] [Green Version]
- Kurmi, K.; Haigis, M.C. Nitrogen Metabolism in Cancer and Immunity. Trends Cell Biol. 2020, 30, 408–424. [Google Scholar] [CrossRef]
- Li, J.; Qu, P.; Zhou, X.; Ji, Y.; Yuan, S.; Liu, S.; Zhang, Q. Pimozide inhibits the growth of breast cancer cells by alleviating the Warburg effect through the P53 signaling pathway. Biomed. Pharmacother. 2022, 150, 113063. [Google Scholar] [CrossRef]
- Liu, M.; Liu, W.; Qin, Y.; Xu, X.; Yu, X.; Zhuo, Q.; Ji, S. Regulation of metabolic reprogramming by tumor suppressor genes in pancreatic cancer. Exp. Hematol. Oncol. 2020, 9, 23. [Google Scholar] [CrossRef]



| Gene | Forward | Reverse |
|---|---|---|
| p53 | 5′-TCTGTCCCTTCCCAGAAAACC-3′ | 5′-CAAGAAGCCCAGACGGAAAC-3′ |
| JUN | 5′-TTTTGCAAGCCTTTCCTGCG-3′ | 5′-TCTTCTCTTGCGTGGCTCTC-3′ |
| MDM2 | 5′-AAGGTGGGAGTGATCAAAAGGA-3′ | 5′-TAGAAACCAAATGTGAAGATGAAGGT-3′ |
| DHCR24 | 5′-ACATCTGCACTGCTTACGAG-3′ | 5′- AAACCCAGCGTCCCACAG-3′ |
| DHCR7 | 5′- CGCAGGACTTTAGCCGGT-3′ | 5′-TGGCTTTGGGAATGTTGGGT-3′ |
| FDFT1 | 5′-GGAAGGTGATGCCCAAGATG-3′ | 5′-ACTGGTCTGATTGAGATACTTGTAGCA-3′ |
| HMGCR | 5′-CCTTTCCAGAGCAAGCACATTA-3′ | 5′-TTTCCCTTACTTCATCCTGTGAGTT-3′ |
| HMGCS1 | 5′-TGCTGTCTTCAATGCTGTTAACTG-3′ | 5′-ACCAGGGCATACCGTCCAT-3′ |
| GAPDH | 5′-CAAGGCTGTGGGCAAGGT-3′ | 5′-GGAAGGCCATGCCAGTGA-3′ |
| MDM2 | Effect on Disease | Disease or Function |
|---|---|---|
| decreased | affects | Aberration of Chromosomes, Abnormal cell cycle |
| decreased | affects | Abnormal cell cycle |
| decreased | increases | Apoptosis |
| decreased | increases | Apoptosis of lymphocytes |
| decreased | decreases | Cell viability of lumphoma cell lines |
| decreased | affects | Abnormal morphologyof granule neuron progenitors cells |
| decreased | increases | Arrest in G1 phase of lung cancer cell lines |
| increased | decreases | Apoptosis of hematopoietic cell lines |
| increased | affects | Morphology of bone cancer and sarcoma cell lines |
| increased | decreases | Stabilization of chromosomes |
| increased | decreases | Arrest in G1 phase of bone cancer cell lines |
| increased | increases | Invasion of tumor cell lines |
| increased | increases | Cancers and tumors |
| increased | increases | Arrest in cell cycle progression |
| increased | increases | Proliferation of cells |
| increased | decreases | Apoptosis |
| increased | increases | Development of mammary tumor |
| increased | increases | Lung metastasis by tumor |
| increased | decreases | Arrest in proliferation of cells |
| MSTO | PC3 | MDA |
|---|---|---|
| 14-Demethyllanosterol ↓ | 1,3-bisphosphoglyceric acid ↑ | 11,12,19-/11,12,20-trihydroxy-5,8,14-eicosatrienoic acid ↑ |
| 20-alpha,22beta-Dihydroxycholesterol ↓ | 11,12,19-/11,12,20-trihydroxy-5,8,14-eicosatrienoic acid ↑ | 13-keto-9Z,11E-octadecadienoic acid ↑ |
| 3-alpha,7alpha-Dihydroxy-5beta-cholestane ↓ | 13-keto-9Z,11E-octadecadienoic acid ↑ | 14,15-dihydroxy-5,-8,11-eicosatrienoic acid ↑ |
| 3-alpha,7alpha,26-Trihydroxy-5 beta-cholestan ↑ | 14-Demethyllanosterol ↓ | 14,15,19-/14,15,20-trihydroxy-5,8,11-eicosatrienoic acid ↑ |
| 4alpha-Methylzymosterol-4-carboxylate ↓ | 14,15-dihydroxy-5,-8,11-eicosatrienoic acid ↑ | 2,3-phosphoglyceric acid ↑ |
| a-ketoglutaric acid ↑ | 14,15,19-/14,15,20-trihydroxy-5,8,11-eicosatrienoic acid ↑ | 3-alpha,7alpha,12alpha,26-Tetrahydroxy-5beta ↑ |
| alanine, 3-cyano ↓ | 19-oxoarachidonic acid ↑ | 3-alpha,7alpha,26-Trihydroxy-5 beta-cholestan ↑ |
| Aminobenzoate ↑ | 2-piperidinecarboxylic acid ↑ | 4alpha-Methylzymosterol-4-carboxylate↓ |
| butanoic acid, 4-amino ↓ | 2,3-phosphoglyceric acid ↑ | 6-O-phosphono-D-gluconic acid ↑ |
| Calcitetrol ↓ | 20-alpha,22beta-Dihydroxycholesterol↓ | 6-phosphonoglucono-D-lactone ↑ |
| Calcitriol ↑ | 3-alpha,7alpha-Dihydroxy-5beta-cholestane ↓ | a-ketoglutaric acid ↓ |
| cholesterol alpha-epoxide (5, 6 alpha-epoxy-5-alpha-cholestan-3 beta-ol) ↑ | 4-alpha-Methylzymosterol ↑ | Alanine ↓ |
| cis-9,10-epoxyoctadecanoic acid (cis-EODA) ↑ | 4alpha-Methylzymosterol-4-carboxylate ↓ | Aminobenzoate ↑ |
| citric acid ↑ | 6-O-phosphono-D-gluconic acid ↑ | AMP ↓ |
| erythrose-4-phosphate ↓ | 6-phosphonoglucono-D-lactone ↑ | aspartic acid ↓ |
| folic acid ↑ | Adenine ↑ | benzoic acid, 4-hydroxy ↑ |
| glyceraldehyde-3-Phosphate (GAP) ↓ | Alanine ↑ | butanoic acid, 4-amino ↓ |
| glyceric acid ↑ | Aminobenzoate ↑ | Calcitetrol ↓ |
| isocitric acid ↑ | AMP ↓ | cholestanetriol (5 alpha-cholestane-3 beta, 5, 6 beta-triol) ↑ |
| lactic acid ↓ | benzoic acid, 4-hydroxy ↑ | citric acid ↑ |
| succinic acid ↓ | Calcitetrol ↓ | Cytosine ↑ |
| Thymidine ↓ | cholestanetriol (5 alpha-cholestane-3 beta, 5, 6 beta-triol) ↑ | dihydroxyacetone phosphate ↑ |
| Valine ↓ | cholesterol alpha-epoxide (5, 6 alpha-epoxy-5-alpha-cholestan-3 beta-ol) ↑ | Dimethylarginine ↑ |
| cis-9,10-epoxyoctadecanoic acid (cis-EODA) ↑ | erythrose-4-phosphate ↑ | |
| Cytidine ↑ | folic acid ↑ | |
| folic acid ↑ | fructose-1,6-bisphosphate ↑ | |
| glucose-6P/fructose-6P ↑ | fumaric acid ↓ | |
| glucose/fructose ↑ | Glucosamine ↑ | |
| glutaric acid, 2-hydroxy ↑ | glucose-6P/fructose-6P ↑ | |
| glyceraldehyde-3-Phosphate (GAP) ↓ | glucose/fructose ↑ | |
| glyceric acid ↑ | glutamic acid ↓ | |
| Isoleucine ↑ | glyceraldehyde-3-Phosphate (GAP) ↓ | |
| lactic acid | glyceric acid ↓ | |
| Leucine ↑ | isocitric acid ↑ | |
| Lysine ↑ | Isoleucine ↓ | |
| malic acid ↓ | lactic acid ↓ | |
| Methionine ↑ | malic acid ↑ | |
| PEP ↑ | NADP ↓ | |
| phosphoric acid ↑ | PEP ↑ | |
| Pregnenolone ↓ | phosphoric acid ↑ | |
| pyroglutamic acid ↑ | Pregnenolone ↓ | |
| pyruvic acid ↓ | sedoheptulose-7-phosphate ↑ | |
| succinic acid ↓ | serine, o-acetyl ↓ | |
| Thymidine ↓ | Trimethyllysine ↑ | |
| Trimethyllysine ↑ | Valine ↓ | |
| Tyrosine ↑ |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cuciniello, R.; Di Meo, F.; Sulli, M.; Demurtas, O.C.; Tanori, M.; Mancuso, M.; Villano, C.; Aversano, R.; Carputo, D.; Baldi, A.; et al. Aglianico Grape Seed Semi-Polar Extract Exerts Anticancer Effects by Modulating MDM2 Expression and Metabolic Pathways. Cells 2023, 12, 210. https://doi.org/10.3390/cells12020210
Cuciniello R, Di Meo F, Sulli M, Demurtas OC, Tanori M, Mancuso M, Villano C, Aversano R, Carputo D, Baldi A, et al. Aglianico Grape Seed Semi-Polar Extract Exerts Anticancer Effects by Modulating MDM2 Expression and Metabolic Pathways. Cells. 2023; 12(2):210. https://doi.org/10.3390/cells12020210
Chicago/Turabian StyleCuciniello, Rossana, Francesco Di Meo, Maria Sulli, Olivia Costantina Demurtas, Mirella Tanori, Mariateresa Mancuso, Clizia Villano, Riccardo Aversano, Domenico Carputo, Alfonso Baldi, and et al. 2023. "Aglianico Grape Seed Semi-Polar Extract Exerts Anticancer Effects by Modulating MDM2 Expression and Metabolic Pathways" Cells 12, no. 2: 210. https://doi.org/10.3390/cells12020210