Notch Blockade Specifically in Bone Marrow-Derived FSP-1-Positive Cells Ameliorates Renal Fibrosis
Abstract
:1. Introduction
2. Methods
2.1. Animals
2.2. DNMAML1 Transgenic Mice
2.3. Generation of Reagents
2.4. Renal Interstitial Fibrosis Model
2.5. Histology and Immunohistochemistry
2.6. Isolation of BM–Derived FSP-1+ and BM Transplantation
2.7. Isolation of Renal Cells from Control and Obstructive Kidneys
2.8. BrdU Experiments
2.9. Cell Culture
2.10. Measurements of mRNA Expression
2.11. Western Blot Analysis
2.12. Statistical Analysis
3. Result
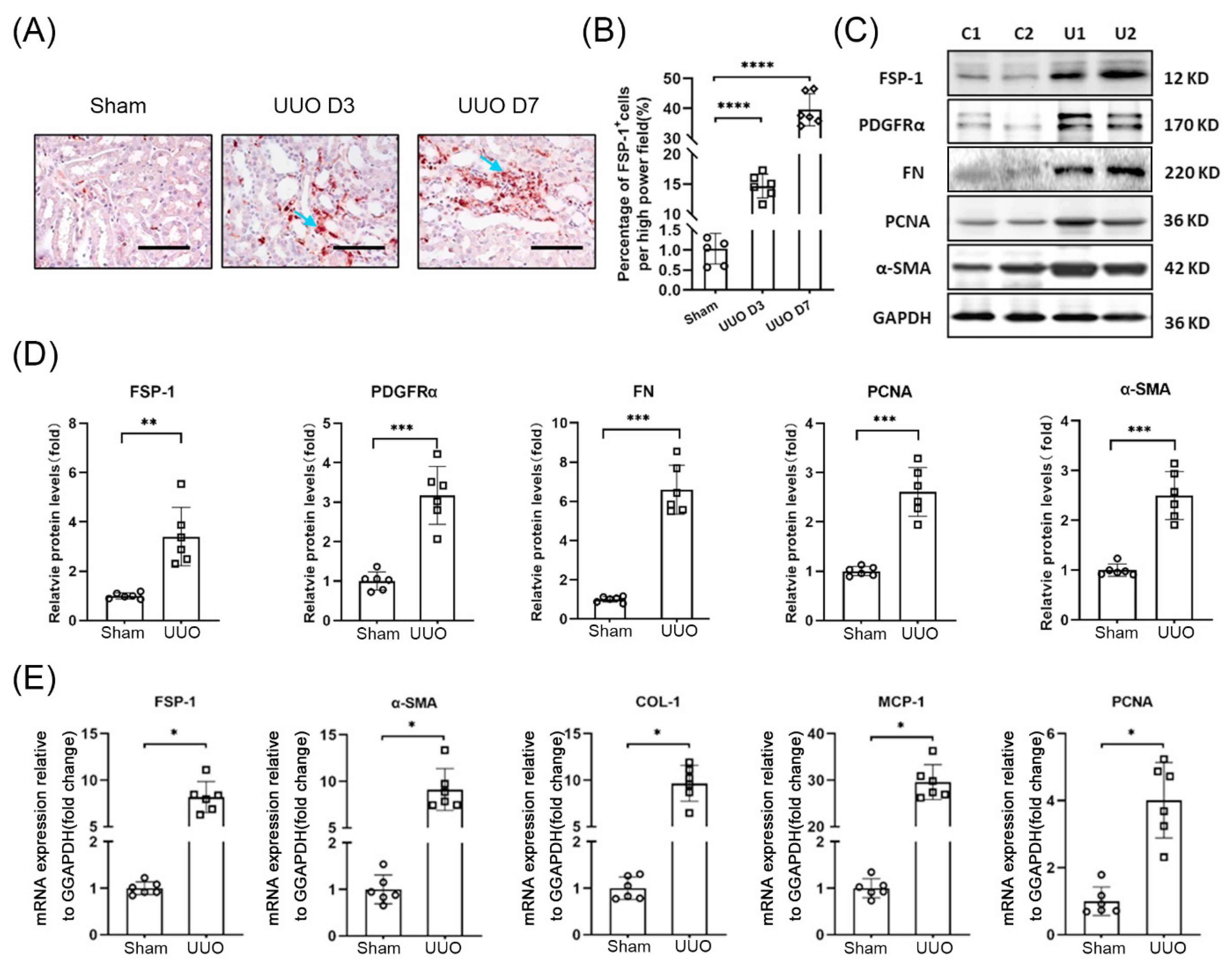
3.1. FSP-1 Positive Cells Accumulate in Obstructed Kidneys
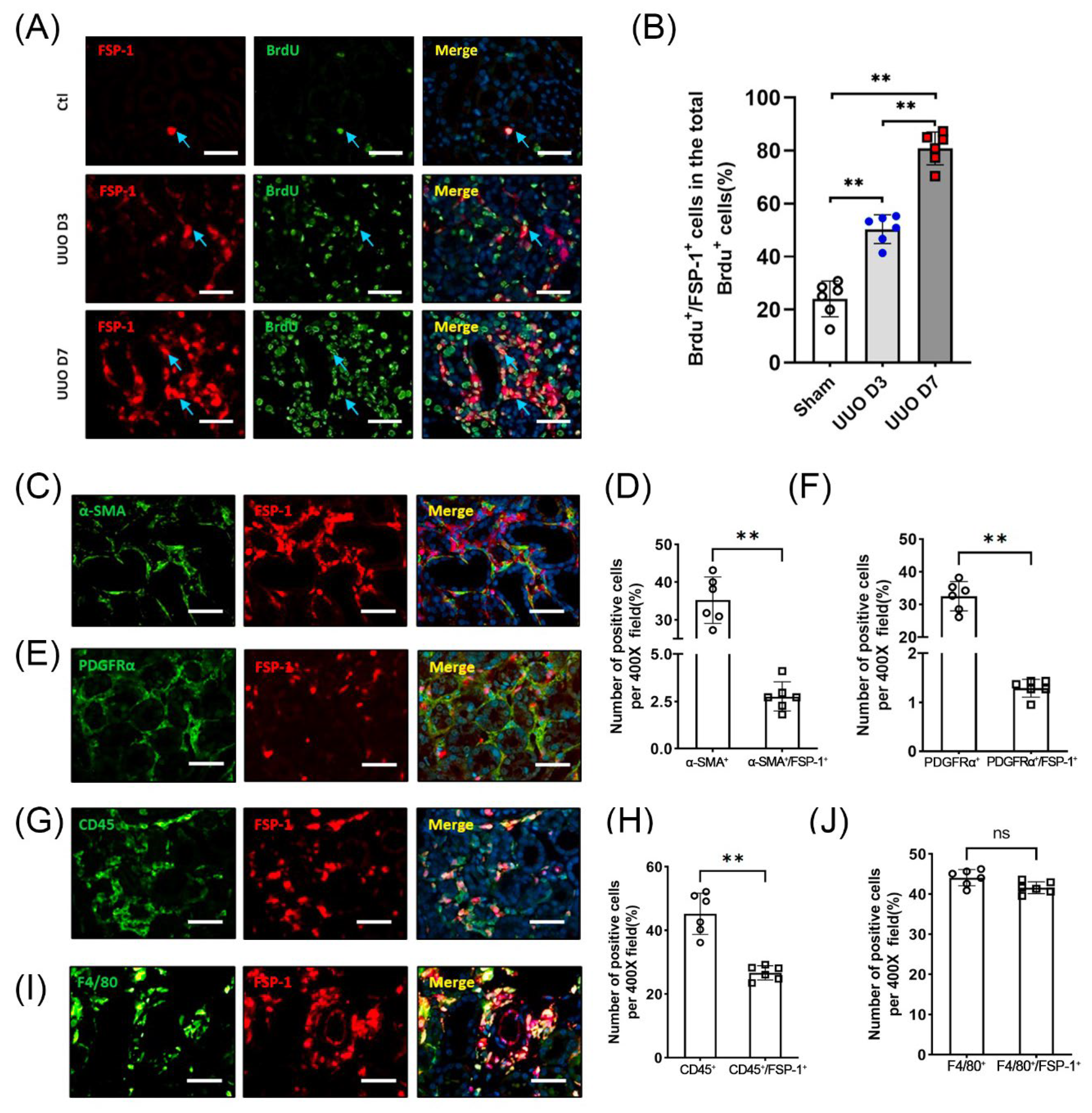
3.2. FSP-1 Is Not a Specific Marker for Myofibroblasts in Fibrogenic Kidneys
3.3. The Majority of FSP-1+ Cells in the Obstructed Kidneys Are Identified as BM-Derived Hematopoietic Cells
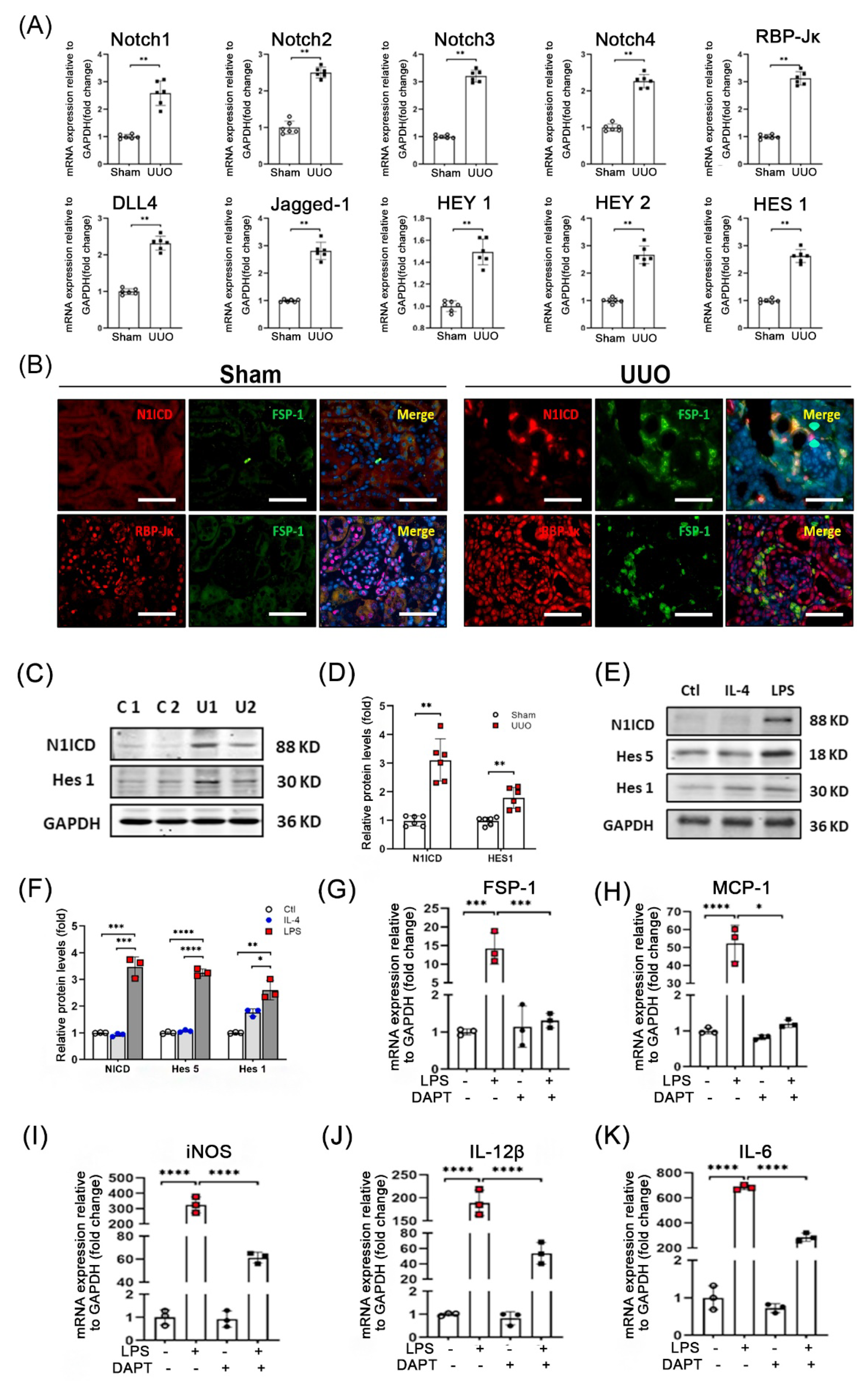
3.4. Notch Signaling Is Activated during UUO
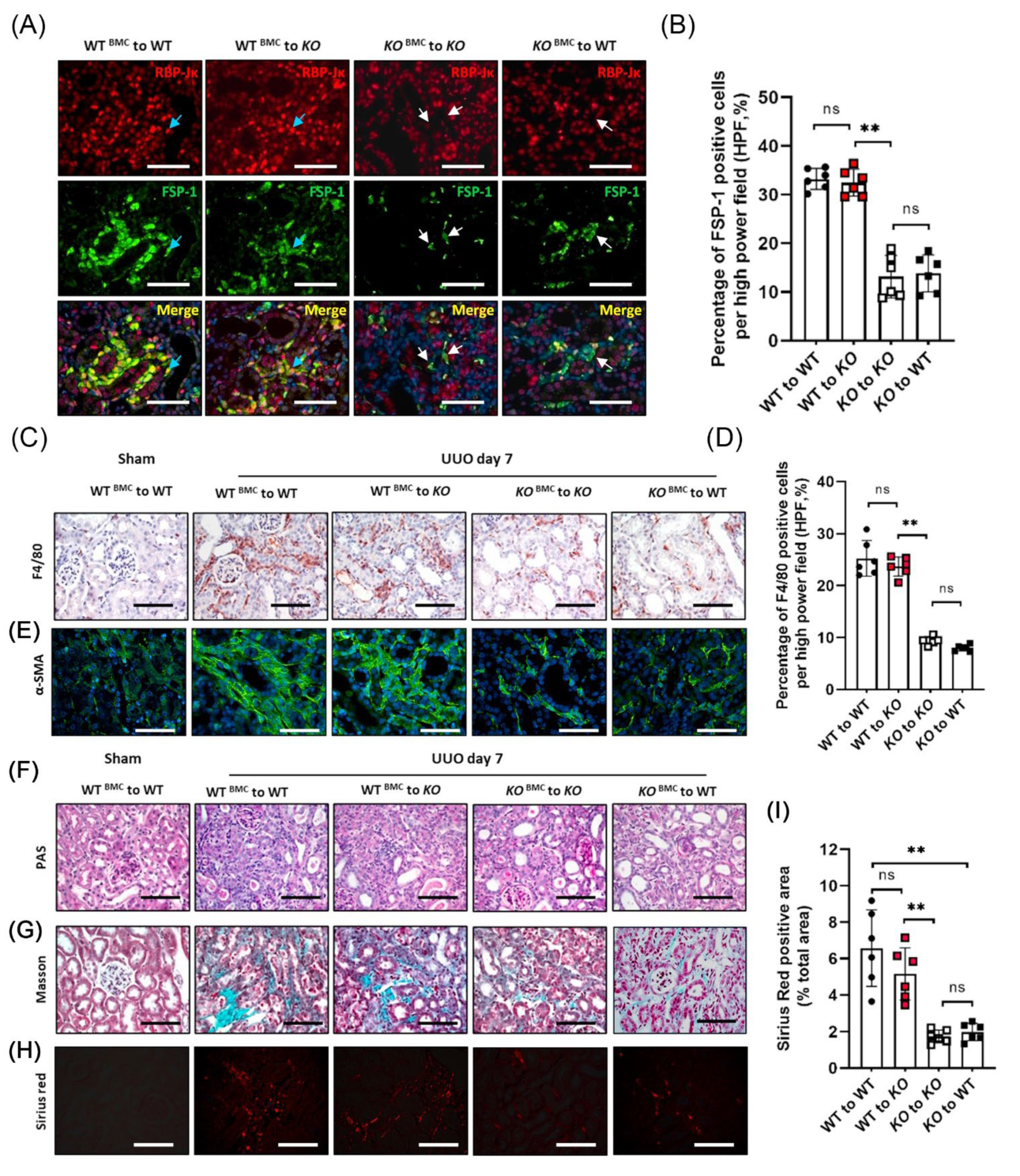
3.5. Genetic Ablation of RBP-Jκ in FSP-1+ Cells Suppresses Tubulointerstitial Fibrosis Caused by UUO
3.6. BM-Derived FSP-1+ Cells Are Functionally Important for Renal Fibrosis
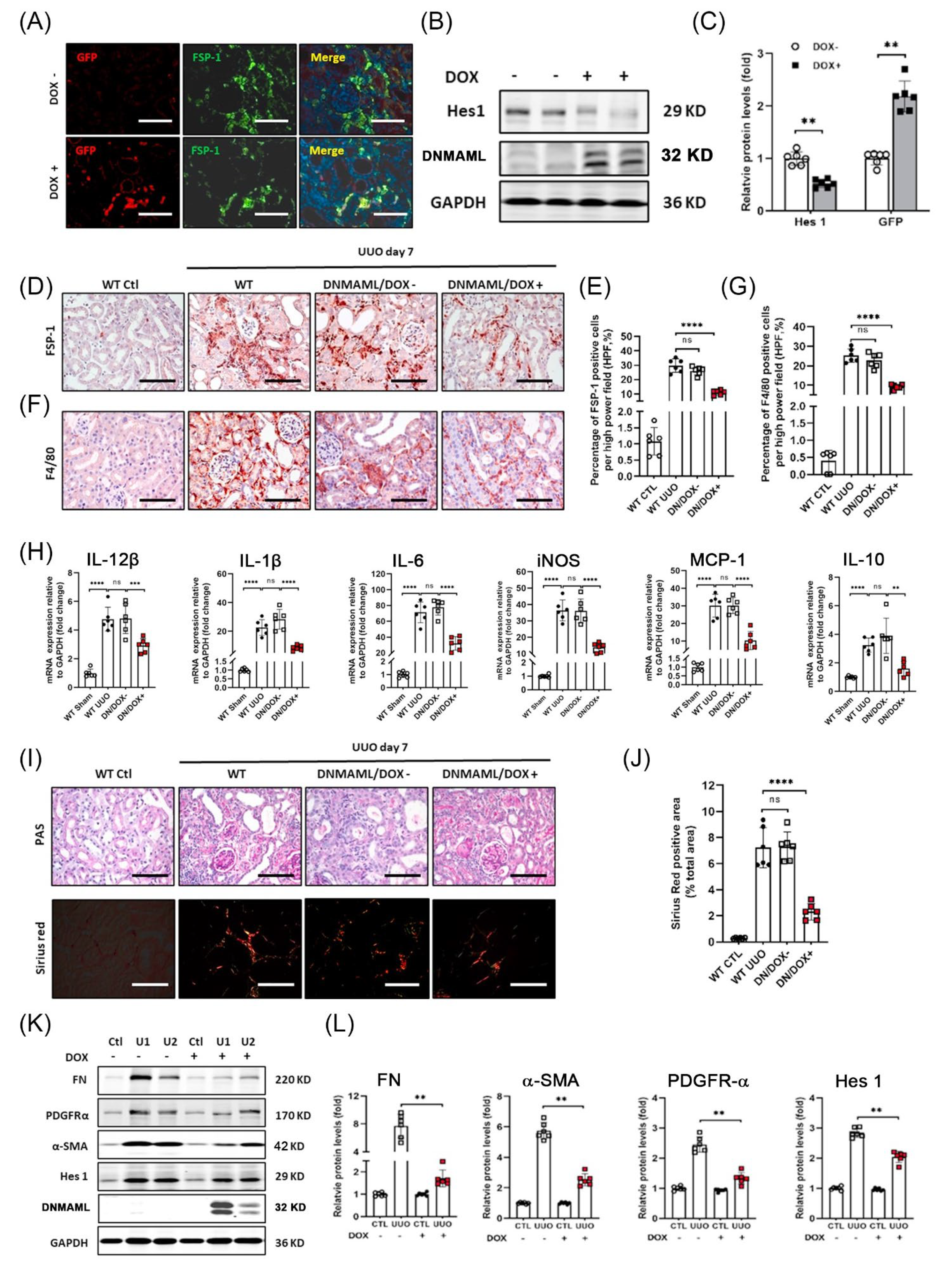
3.7. Overexpression of DNMAML1 in FSP-1+ Cells Inhibits UUO-Induced Renal Inflammation and Fibrosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sean Eardley, K.; Cockwell, P. Macrophages and progressive tubulointerstitial disease. Kidney Int. 2005, 68, 437–455. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rodriguez-Iturbe, B.; Pons, H.; Herrera-Acosta, J.; Johnson, R.J. Role of immunocompetent cells in nonimmune renal diseases. Kidney Int. 2001, 59, 1626–1640. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boye, K.; Maelandsmo, G.M. S100A4 and metastasis: A small actor playing many roles. Am. J. Pathol. 2010, 176, 528–535. [Google Scholar] [CrossRef] [PubMed]
- Lawson, W.E.; Polosukhin, V.V.; Zoia, O.; Stathopoulos, G.T.; Han, W.; Plieth, D.; Loyd, J.E.; Neilson, E.G.; Blackwell, T.S. Characterization of fibroblast-specific protein 1 in pulmonary fibrosis. Am. J. Respir. Crit. Care Med. 2005, 171, 899–907. [Google Scholar] [CrossRef] [PubMed]
- Strutz, F.; Okada, H.; Lo, C.W.; Danoff, T.; Carone, R.L.; Tomaszewski, J.E.; Neilson, E.G. Identification and characterization of a fibroblast marker: FSP1. J. Cell Biol. 1995, 130, 393–405. [Google Scholar] [CrossRef] [Green Version]
- Kong, P.; Christia, P.; Saxena, A.; Su, Y.; Frangogiannis, N.G. Lack of specificity of fibroblast-specific protein 1 in cardiac remodeling and fibrosis. Am. J. Physiol. Heart Circ. Physiol. 2013, 305, H1363–H1372. [Google Scholar] [CrossRef]
- Osterreicher, C.H.; Penz-Osterreicher, M.; Grivennikov, S.I.; Guma, M.; Koltsova, E.K.; Datz, C.; Sasik, R.; Hardiman, G.; Karin, M.; Brenner, D.A. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc. Natl. Acad. Sci. USA 2011, 108, 308–313. [Google Scholar] [CrossRef] [Green Version]
- Okada, H.; Danoff, T.M.; Kalluri, R.; Neilson, E.G. Early role of Fsp1 in epithelial-mesenchymal transformation. Am. J. Physiol. 1997, 273, F563–F574. [Google Scholar] [CrossRef]
- Picard, N.; Baum, O.; Vogetseder, A.; Kaissling, B.; Le Hir, M. Origin of renal myofibroblasts in the model of unilateral ureter obstruction in the rat. Histochem. Cell Biol. 2008, 130, 141–155. [Google Scholar] [CrossRef] [Green Version]
- Le Hir, M.; Hegyi, I.; Cueni-Loffing, D.; Loffing, J.; Kaissling, B. Characterization of renal interstitial fibroblast-specific protein 1/S100A4-positive cells in healthy and inflamed rodent kidneys. Histochem. Cell Biol. 2005, 123, 335–346. [Google Scholar] [CrossRef]
- Cheng, H.T.; Miner, J.H.; Lin, M.; Tansey, M.G.; Roth, K.; Kopan, R. Gamma-secretase activity is dispensable for mesenchyme-to-epithelium transition but required for podocyte and proximal tubule formation in developing mouse kidney. Development 2003, 130, 5031–5042. [Google Scholar] [CrossRef] [Green Version]
- Hsu, Y.C.; Chang, P.J.; Tung, C.W.; Shih, Y.H.; Ni, W.C.; Li, Y.C.; Uto, T.; Shoyama, Y.; Ho, C.; Lin, C.L. De-Glycyrrhizinated Licorice Extract Attenuates High Glucose-Stimulated Renal Tubular Epithelial-Mesenchymal Transition via Suppressing the Notch2 Signaling Pathway. Cells 2020, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Niranjan, T.; Bielesz, B.; Gruenwald, A.; Ponda, M.P.; Kopp, J.B.; Thomas, D.B.; Susztak, K. The Notch pathway in podocytes plays a role in the development of glomerular disease. Nat. Med. 2008, 14, 290–298. [Google Scholar] [CrossRef]
- Djudjaj, S.; Chatziantoniou, C.; Raffetseder, U.; Guerrot, D.; Dussaule, J.C.; Boor, P.; Kerroch, M.; Hanssen, L.; Brandt, S.; Dittrich, A.; et al. Notch-3 receptor activation drives inflammation and fibrosis following tubulointerstitial kidney injury. J. Pathol. 2012, 228, 286–299. [Google Scholar] [CrossRef]
- Xiao, Z.; Zhang, J.; Peng, X.; Dong, Y.; Jia, L.; Li, H.; Du, J. The Notch gamma-secretase inhibitor ameliorates kidney fibrosis via inhibition of TGF-beta/Smad2/3 signaling pathway activation. Int. J. Biochem. Cell Biol. 2014, 55, 65–71. [Google Scholar] [CrossRef]
- Waters, A.M.; Wu, M.Y.; Huang, Y.W.; Liu, G.Y.; Holmyard, D.; Onay, T.; Jones, N.; Egan, S.E.; Robinson, L.A.; Piscione, T.D. Notch promotes dynamin-dependent endocytosis of nephrin. J. Am. Soc. Nephrol. 2012, 23, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Boonyatecha, N.; Sangphech, N.; Wongchana, W.; Kueanjinda, P.; Palaga, T. Involvement of Notch signaling pathway in regulating IL-12 expression via c-Rel in activated macrophages. Mol. Immunol. 2012, 51, 255–262. [Google Scholar] [CrossRef] [Green Version]
- Liang, A.; Wang, Y.; Han, G.; Truong, L.; Cheng, J. Chronic kidney disease accelerates endothelial barrier dysfunction in a mouse model of an arteriovenous fistula. Am. J. Physiol.-Ren. Physiol. 2013, 304, F1413–F1420. [Google Scholar] [CrossRef] [Green Version]
- Cheng, J.; Wang, Y.; Liang, A.; Jia, L.; Du, J. FSP-1 silencing in bone marrow cells suppresses neointima formation in vein graft. Circ. Res. 2012, 110, 230–240. [Google Scholar] [CrossRef] [Green Version]
- Liang, M.; Wang, Y.; Liang, A.; Dong, J.F.; Du, J.; Cheng, J. Impaired integrin beta3 delays endothelial cell regeneration and contributes to arteriovenous graft failure in mice. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 607–615. [Google Scholar] [CrossRef]
- Bielesz, B.; Sirin, Y.; Si, H.; Niranjan, T.; Gruenwald, A.; Ahn, S.; Kato, H.; Pullman, J.; Gessler, M.; Haase, V.H.; et al. Epithelial Notch signaling regulates interstitial fibrosis development in the kidneys of mice and humans. J. Clin. Investig. 2010, 120, 4040–4054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Li, S.; Abedin, M.J.; Wang, L.; Schneider, E.; Najafian, B.; Rosenberg, M. Effect of Notch activation on the regenerative response to acute renal failure. Am. J. Physiol.-Ren. Physiol. 2010, 298, F209–F215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Conway, B.; Hughes, J. Cellular orchestrators of renal fibrosis. QJM 2012, 105, 611–615. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chaves, L.D.; Mathew, L.; Shakaib, M.; Chang, A.; Quigg, R.J.; Puri, T.S. Contrasting effects of systemic monocyte/macrophage and CD4+ T cell depletion in a reversible ureteral obstruction mouse model of chronic kidney disease. Clin. Dev. Immunol. 2013, 2013, 836989. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ricardo, S.D.; van Goor, H.; Eddy, A.A. Macrophage diversity in renal injury and repair. J. Clin. Investig. 2008, 118, 3522–3530. [Google Scholar] [CrossRef] [Green Version]
- Lepenies, J.; Eardley, K.S.; Kienitz, T.; Hewison, M.; Ihl, T.; Stewart, P.M.; Cockwell, P.; Quinkler, M. Renal TLR4 mRNA expression correlates with inflammatory marker MCP-1 and profibrotic molecule TGF-beta(1) in patients with chronic kidney disease. Nephron Clin. Pract. 2011, 119, c97–c104. [Google Scholar] [CrossRef]
- Kulkarni, O.; Pawar, R.D.; Purschke, W.; Eulberg, D.; Selve, N.; Buchner, K.; Ninichuk, V.; Segerer, S.; Vielhauer, V.; Klussmann, S.; et al. Spiegelmer inhibition of CCL2/MCP-1 ameliorates lupus nephritis in MRL-(Fas)lpr mice. J. Am. Soc. Nephrol. 2007, 18, 2350–2358. [Google Scholar] [CrossRef] [Green Version]
- Klessens, C.Q.F.; Zandbergen, M.; Wolterbeek, R.; Bruijn, J.A.; Rabelink, T.J.; Bajema, I.M.; IJpelaar, D.H. Macrophages in diabetic nephropathy in patients with type 2 diabetes. Nephrol. Dial. Transplant. 2017, 32, 1322–1329. [Google Scholar] [CrossRef] [Green Version]
- Ellson, C.D.; Dunmore, R.; Hogaboam, C.M.; Sleeman, M.A.; Murray, L.A. Danger-associated molecular patterns and danger signals in idiopathic pulmonary fibrosis. Am. J. Respir. Cell Mol. Biol. 2014, 51, 163–168. [Google Scholar] [CrossRef]
- Zhang, J.; Jiao, Y.; Hou, S.; Tian, T.; Yuan, Q.; Hao, H.; Wu, Z.; Bao, X. S100A4 contributes to colitis development by increasing the adherence of Citrobacter rodentium in intestinal epithelial cells. Sci. Rep. 2017, 7, 12099. [Google Scholar] [CrossRef]
- Zhang, W.; Ohno, S.; Steer, B.; Klee, S.; Staab-Weijnitz, C.A.; Wagner, D.; Lehmann, M.; Stoeger, T.; Konigshoff, M.; Adler, H. S100a4 Is Secreted by Alternatively Activated Alveolar Macrophages and Promotes Activation of Lung Fibroblasts in Pulmonary Fibrosis. Front. Immunol. 2018, 9, 1216. [Google Scholar] [CrossRef] [Green Version]
- Du, X.; Shimizu, A.; Masuda, Y.; Kuwahara, N.; Arai, T.; Kataoka, M.; Uchiyama, M.; Kaneko, T.; Akimoto, T.; Iino, Y.; et al. Involvement of matrix metalloproteinase-2 in the development of renal interstitial fibrosis in mouse obstructive nephropathy. Lab. Investig. 2012, 92, 1149–1160. [Google Scholar] [CrossRef] [Green Version]
- Loeffler, I.; Liebisch, M.; Allert, S.; Kunisch, E.; Kinne, R.W.; Wolf, G. FSP1-specific SMAD2 knockout in renal tubular, endothelial, and interstitial cells reduces fibrosis and epithelial-to-mesenchymal transition in murine STZ-induced diabetic nephropathy. Cell Tissue Res. 2018, 372, 115–133. [Google Scholar] [CrossRef]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Tang, P.M.; Nikolic-Paterson, D.J.; Lan, H.Y. Macrophages: Versatile players in renal inflammation and fibrosis. Nat. Rev. Nephrol. 2019, 15, 144–158. [Google Scholar] [CrossRef]
- Xu, H.; Zhu, J.; Smith, S.; Foldi, J.; Zhao, B.; Chung, A.Y.; Outtz, H.; Kitajewski, J.; Shi, C.; Weber, S.; et al. Notch-RBP-J signaling regulates the transcription factor IRF8 to promote inflammatory macrophage polarization. Nat. Immunol. 2012, 13, 642–650. [Google Scholar] [CrossRef] [Green Version]
- Monsalve, E.; Ruiz-Garcia, A.; Baladron, V.; Ruiz-Hidalgo, M.J.; Sanchez-Solana, B.; Rivero, S.; Garcia-Ramirez, J.J.; Rubio, A.; Laborda, J.; Diaz-Guerra, M.J. Notch1 upregulates LPS-induced macrophage activation by increasing NF-kappaB activity. Eur. J. Immunol. 2009, 39, 2556–2570. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, Y.; Ma, P.; An, D.; Zhao, J.; Liang, S.; Ye, Y.; Lu, Y.; Zhang, P.; Liu, X.; et al. Myeloid-specific targeting of Notch ameliorates murine renal fibrosis via reduced infiltration and activation of bone marrow-derived macrophage. Protein Cell 2019, 10, 196–210. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wu, Y.; Liang, M.; Huang, F.; Cheng, O.H.; Xiao, X.; Lee, T.H.; Truong, L.; Cheng, J. Notch Blockade Specifically in Bone Marrow-Derived FSP-1-Positive Cells Ameliorates Renal Fibrosis. Cells 2023, 12, 214. https://doi.org/10.3390/cells12020214
Wu Y, Liang M, Huang F, Cheng OH, Xiao X, Lee TH, Truong L, Cheng J. Notch Blockade Specifically in Bone Marrow-Derived FSP-1-Positive Cells Ameliorates Renal Fibrosis. Cells. 2023; 12(2):214. https://doi.org/10.3390/cells12020214
Chicago/Turabian StyleWu, Yongdong, Ming Liang, Fengzhang Huang, Owen H. Cheng, Xiaoguang Xiao, Tae Hoon Lee, Luan Truong, and Jizhong Cheng. 2023. "Notch Blockade Specifically in Bone Marrow-Derived FSP-1-Positive Cells Ameliorates Renal Fibrosis" Cells 12, no. 2: 214. https://doi.org/10.3390/cells12020214




