Pathogenic Th2 Cytokine Profile Skewing by IFN-γ-Responding Vitiligo Fibroblasts via CCL2/CCL8
Abstract
:1. Introduction
2. Materials and Methods
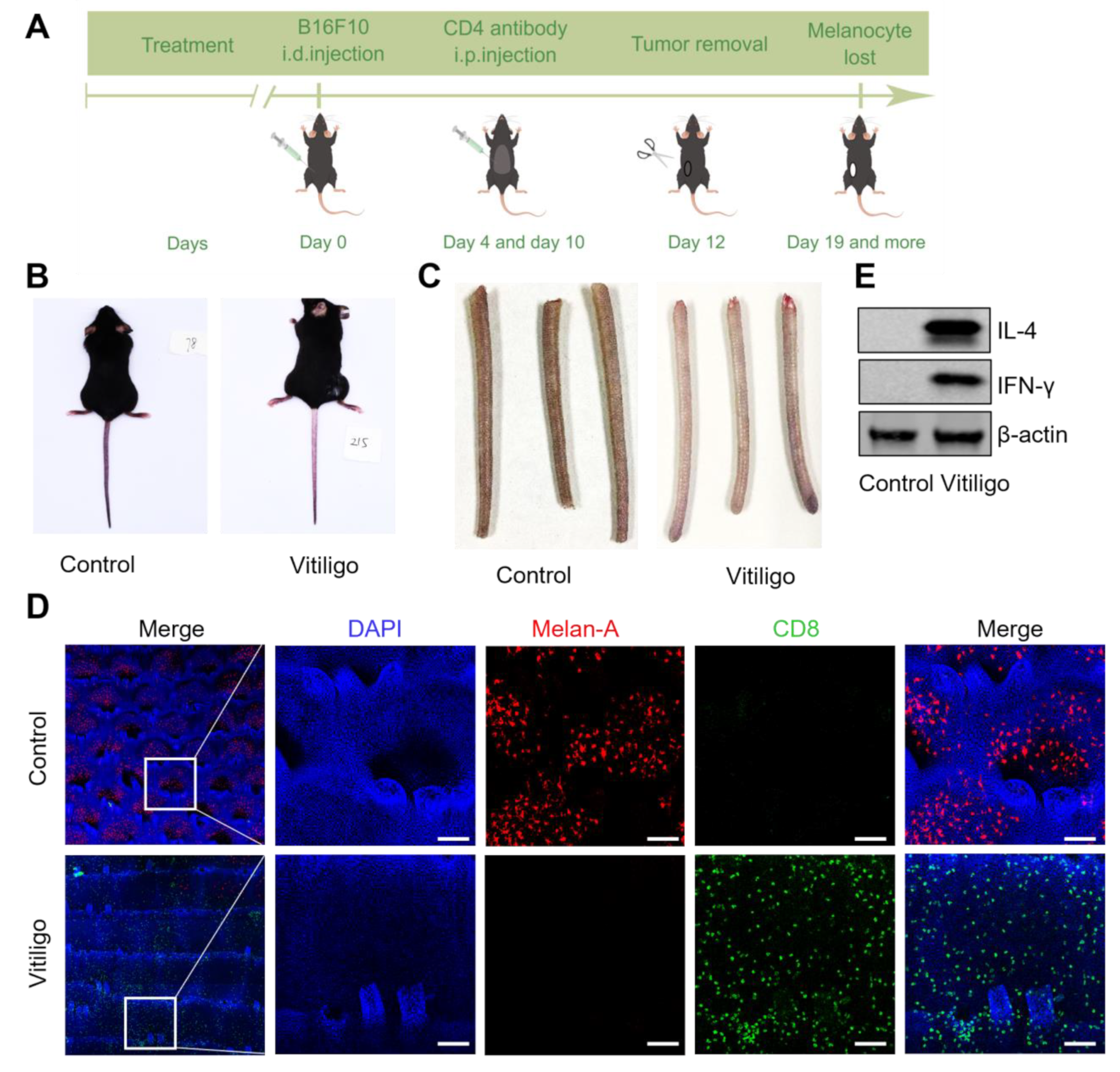
2.1. Mouse Vitiligo Model
2.2. Immunofluorescence
2.3. ROS Staining
2.4. RNA Extraction and Library Construction
2.5. Bioinformatics Analysis of RNA-Sequencing
2.6. mRNA Extraction Analysis by Quantitative PCR
- Ccl2 Forward, GAATCACCAGCAGCAAGTGTC;
- Ccl2 Reverse, CGGAGTTTGGGTTTGCTTGTC;
- Ccl8 Forward, TGGAGAGCTACACAAGAATCACC;
- Ccl8 Reverse, TGGTCCAGATGCTTCATGGAA;
- Jak2 Forward, CGAATGGTGTTTCTGATGTACC;
- Jak2 Reverse, GTCTCCTACTTCTCTTCGTACG;
- Stat1 Forward, CAGCTTGACTCAAAATTCCTGGA;
- Stat1 Reverse, TGAAGATTACGCTTGCTTTTCCT;
- β-actin Forward, CCTTCCTGGGCATGGAGTC;
- β-actin Reverse, TGATCTTCATTGTGCTGGGTG.
2.7. ELISA
2.8. Western Blotting
2.9. Immunohistochemistry
2.10. T Cell Differentiation
2.11. Flow Cytometry and Intracellular Cytokine Staining
2.12. Statistical Analysis
3. Results
3.1. The CD8-Mediated Mouse Vitiligo Model Features Human Vitiligo Hallmarks
3.2. Altered Fibroblasts Set an Inflammatory Tone at the Stromal Site
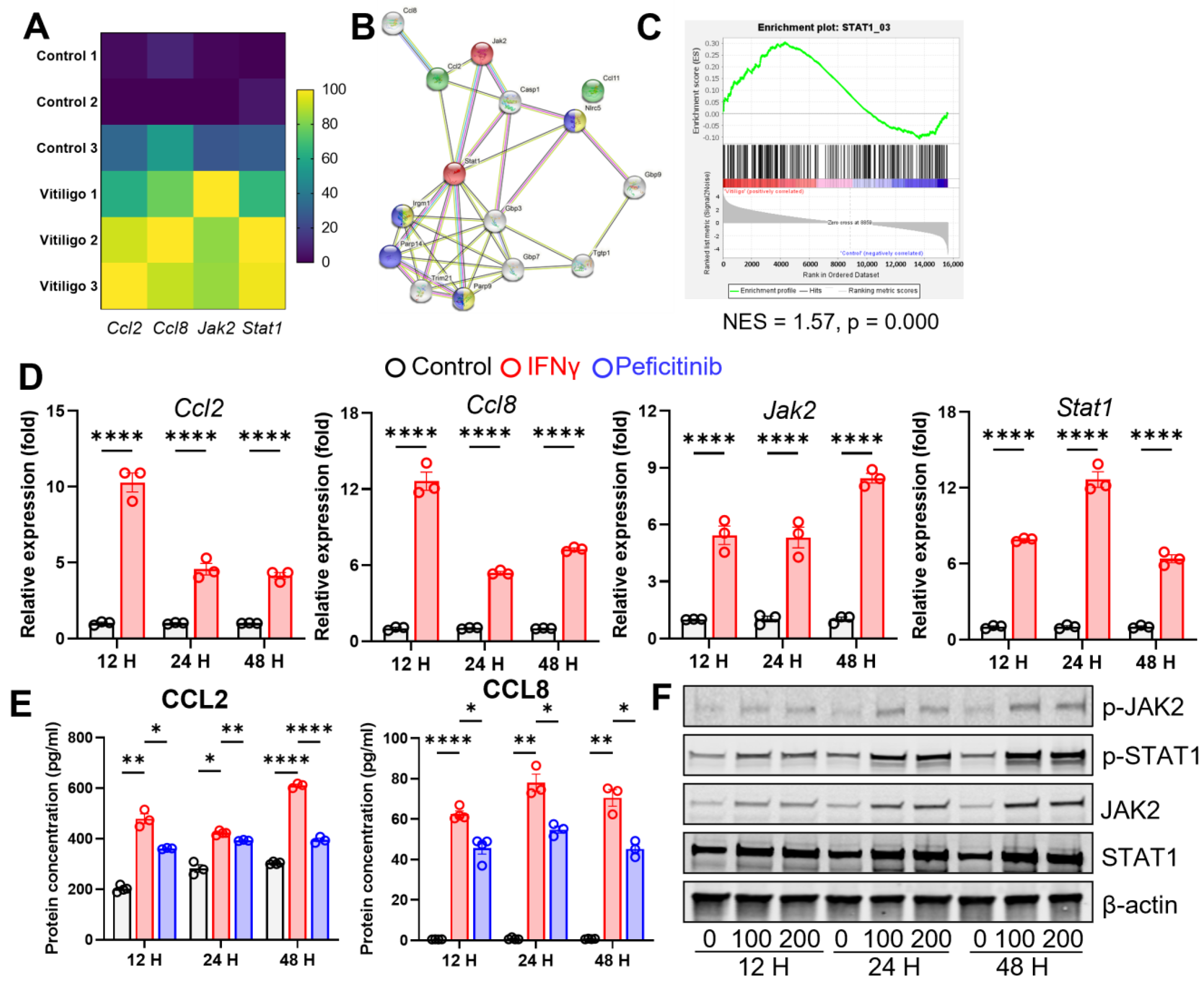
3.3. Vitiligo Fibroblasts Show a T Cell-Related Transcription Profile
3.4. IFN-γ Stimulates CCL2/CCL8 Expression in Fibroblasts through JAK2/STAT1 Signaling
3.5. CCL2 and CCL8 Skew the T Cell Population towards a Pathogenic Type 2 Profile
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Picardo, M.; Dell’anna, M.L.; Ezzedine, K.; Hamzavi, I.; Harris, J.E.; Parsad, D.; Taieb, A. Vitiligo. Nat. Rev. Dis. Primers 2015, 1, 15011. [Google Scholar] [CrossRef] [PubMed]
- Migayron, L.; Boniface, K.; Seneschal, J. Vitiligo, From Physiopathology to Emerging Treatments: A Review. Dermatol. Ther. (Heidelb) 2020, 10, 1185–1198. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Chen, D.; Hu, Y.; Jiang, K.; Huang, H.; Du, Y.; Wu, W.; Wang, J.; Sui, J.; Wang, W.; et al. Anatomically distinct fibroblast subsets determine skin autoimmune patterns. Nature 2022, 601, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Gegotek, A.; Domingues, P.; Wronski, A.; Skrzydlewska, E. Changes in Proteome of Fibroblasts Isolated from Psoriatic Skin Lesions. Int. J. Mol. Sci. 2020, 21, 5363. [Google Scholar] [CrossRef]
- Berroth, A.; Kuhnl, J.; Kurschat, N.; Schwarz, A.; Stab, F.; Schwarz, T.; Wenck, H.; Folster-Holst, R.; Neufang, G. Role of fibroblasts in the pathogenesis of atopic dermatitis. J. Allergy Clin. Immunol. 2013, 131, 1547–1554. [Google Scholar] [CrossRef]
- Cario-Andre, M.; Pain, C.; Gauthier, Y.; Casoli, V.; Taieb, A. In vivo and in vitro evidence of dermal fibroblasts influence on human epidermal pigmentation. Pigment Cell Res. 2006, 19, 434–442. [Google Scholar] [CrossRef]
- Schiaffino, M.V. Signaling pathways in melanosome biogenesis and pathology. Int. J. Biochem. Cell Biol. 2010, 42, 1094–1104. [Google Scholar] [CrossRef] [Green Version]
- Bastonini, E.; Bellei, B.; Filoni, A.; Kovacs, D.; Iacovelli, P.; Picardo, M. Involvement of non-melanocytic skin cells in vitiligo. Exp. Dermatol. 2019, 28, 667–673. [Google Scholar] [CrossRef] [Green Version]
- Correa-Gallegos, D.; Jiang, D.; Rinkevich, Y. Fibroblasts as confederates of the immune system. Immunol. Rev. 2021, 302, 147–162. [Google Scholar] [CrossRef]
- She, S.; Ren, L.; Chen, P.; Wang, M.; Chen, D.; Wang, Y.; Chen, H. Functional Roles of Chemokine Receptor CCR2 and Its Ligands in Liver Disease. Front. Immunol. 2022, 13, 812431. [Google Scholar] [CrossRef]
- Sierra-Filardi, E.; Nieto, C.; Dominguez-Soto, A.; Barroso, R.; Sanchez-Mateos, P.; Puig-Kroger, A.; Lopez-Bravo, M.; Joven, J.; Ardavin, C.; Rodriguez-Fernandez, J.L.; et al. CCL2 shapes macrophage polarization by GM-CSF and M-CSF: Identification of CCL2/CCR2-dependent gene expression profile. J. Immunol. 2014, 192, 3858–3867. [Google Scholar] [CrossRef] [Green Version]
- Vitiello, P.F.; Shainheit, M.G.; Allison, E.M.; Adler, E.P.; Kurt, R.A. Impact of tumor-derived CCL2 on T cell effector function. Immunol. Lett. 2004, 91, 239–245. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Takahashi, N.; Ushiki, M.; Monya, M.; Aihara, F.; Kuboki, E.; Moriyama, S.; Iida, M.; Kitamura, H.; Qiu, C.H.; et al. Intestinal CD169(+) macrophages initiate mucosal inflammation by secreting CCL8 that recruits inflammatory monocytes. Nat. Commun. 2015, 6, 7802. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Debes, G.F.; Diehl, M.C. CCL8 and skin T cells--an allergic attraction. Nat. Immunol. 2011, 12, 111–112. [Google Scholar] [CrossRef] [PubMed]
- Antonelli, A.; Ferrari, S.M.; Fallahi, P. The role of the Th1 chemokine CXCL10 in vitiligo. Ann. Transl. Med. 2015, 3, S16. [Google Scholar] [CrossRef]
- Martins, C.; Migayron, L.; Drullion, C.; Jacquemin, C.; Lucchese, F.; Rambert, J.; Merhi, R.; Michon, P.; Taieb, A.; Rezvani, H.R.; et al. Vitiligo Skin T Cells Are Prone to Produce Type 1 and Type 2 Cytokines to Induce Melanocyte Dysfunction and Epidermal Inflammatory Response Through Jak Signaling. J. Investig. Dermatol. 2022, 142, 1194–1205. [Google Scholar] [CrossRef]
- Qi, F.; Liu, F.; Gao, L. Janus Kinase Inhibitors in the Treatment of Vitiligo: A Review. Front. Immunol. 2021, 12, 790125. [Google Scholar] [CrossRef]
- Hu, X.; Li, J.; Fu, M.; Zhao, X.; Wang, W. The JAK/STAT signaling pathway: From bench to clinic. Signal. Transduct. Target Ther. 2021, 6, 402. [Google Scholar] [CrossRef]
- Chen, D.; Xu, Z.; Cui, J.; Chen, T. A mouse model of vitiligo based on endogenous auto-reactive CD8 + T cell targeting skin melanocyte. Cell Regen. 2022, 11, 31. [Google Scholar] [CrossRef]
- Zhang, P.; Cote, A.L.; de Vries, V.C.; Usherwood, E.J.; Turk, M.J. Induction of postsurgical tumor immunity and T-cell memory by a poorly immunogenic tumor. Cancer Res. 2007, 67, 6468–6476. [Google Scholar] [CrossRef]
- Chen, J.; Li, S.; Li, C. Mechanisms of melanocyte death in vitiligo. Med. Res. Rev. 2021, 41, 1138–1166. [Google Scholar] [CrossRef]
- Riding, R.L.; Richmond, J.M.; Harris, J.E. Mouse Model for Human Vitiligo. Curr. Protoc. Immunol. 2019, 124, e63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van den Boorn, J.G.; Konijnenberg, D.; Dellemijn, T.A.; van der Veen, J.P.; Bos, J.D.; Melief, C.J.; Vyth-Dreese, F.A.; Luiten, R.M. Autoimmune destruction of skin melanocytes by perilesional T cells from vitiligo patients. J. Investig. Dermatol. 2009, 129, 2220–2232. [Google Scholar] [CrossRef] [Green Version]
- Kovacs, D.; Bastonini, E.; Ottaviani, M.; Cota, C.; Migliano, E.; Dell’Anna, M.L.; Picardo, M. Vitiligo Skin: Exploring the Dermal Compartment. J. Investig. Dermatol. 2018, 138, 394–404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, J.; Tredget, E.E. The Role of Chemokines in Fibrotic Wound Healing. Adv. Wound Care (New Rochelle) 2015, 4, 673–686. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zdanowska, N.; Kasprowicz-Furmanczyk, M.; Placek, W.; Owczarczyk-Saczonek, A. The Role of Chemokines in Psoriasis—An Overview. Medicina (Kaunas) 2021, 57, 754. [Google Scholar] [CrossRef]
- Fallahi, P.; Foddis, R.; Elia, G.; Ragusa, F.; Patrizio, A.; Benvenga, S.; Cristaudo, A.; Antonelli, A.; Ferrari, S.M. CXCL8 and CXCL11 chemokine secretion in dermal fibroblasts is differentially modulated by vanadium pentoxide. Mol. Med. Rep. 2018, 18, 1798–1803. [Google Scholar] [CrossRef] [Green Version]
- Gu, L.; Tseng, S.; Horner, R.M.; Tam, C.; Loda, M.; Rollins, B.J. Control of TH2 polarization by the chemokine monocyte chemoattractant protein-1. Nature 2000, 404, 407–411. [Google Scholar] [CrossRef]
- Zhang, J.; Patel, L.; Pienta, K.J. Targeting chemokine (C-C motif) ligand 2 (CCL2) as an example of translation of cancer molecular biology to the clinic. Prog. Mol. Biol. Transl. Sci. 2010, 95, 31–53. [Google Scholar] [CrossRef] [Green Version]
- Islam, S.A.; Chang, D.S.; Colvin, R.A.; Byrne, M.H.; McCully, M.L.; Moser, B.; Lira, S.A.; Charo, I.F.; Luster, A.D. Mouse CCL8, a CCR8 agonist, promotes atopic dermatitis by recruiting IL-5+ T(H)2 cells. Nat. Immunol. 2011, 12, 167–177. [Google Scholar] [CrossRef]
- Frisoli, M.L.; Essien, K.; Harris, J.E. Vitiligo: Mechanisms of Pathogenesis and Treatment. Annu. Rev. Immunol. 2020, 38, 621–648. [Google Scholar] [CrossRef] [Green Version]
- Lowa, A.; Graff, P.; Kaessmeyer, S.; Hedtrich, S. Fibroblasts from atopic dermatitis patients trigger inflammatory processes and hyperproliferation in human skin equivalents. J. Eur. Acad. Dermatol. Venereol. 2020, 34, e262–e265. [Google Scholar] [CrossRef]
- Bastonini, E.; Kovacs, D.; Picardo, M. Skin Pigmentation and Pigmentary Disorders: Focus on Epidermal/Dermal Cross-Talk. Ann. Dermatol. 2016, 28, 279–289. [Google Scholar] [CrossRef] [Green Version]
- Choi, W.; Wolber, R.; Gerwat, W.; Mann, T.; Batzer, J.; Smuda, C.; Liu, H.; Kolbe, L.; Hearing, V.J. The fibroblast-derived paracrine factor neuregulin-1 has a novel role in regulating the constitutive color and melanocyte function in human skin. J. Cell Sci. 2010, 123, 3102–3111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yamaguchi, Y.; Morita, A.; Maeda, A.; Hearing, V.J. Regulation of Skin Pigmentation and Thickness by Dickkopf 1 (DKK1). J. Investig. Derm. Symp. P 2009, 14, 73–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rashighi, M.; Agarwal, P.; Richmond, J.M.; Harris, T.H.; Dresser, K.; Su, M.W.; Zhou, Y.; Deng, A.; Hunter, C.A.; Luster, A.D.; et al. CXCL10 is critical for the progression and maintenance of depigmentation in a mouse model of vitiligo. Sci. Transl. Med. 2014, 6, 223ra223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riding, R.L.; Harris, J.E. The Role of Memory CD8(+) T Cells in Vitiligo. J. Immunol. 2019, 203, 11–19. [Google Scholar] [CrossRef]
- Esche, C.; Stellato, C.; Beck, L.A. Chemokines: Key players in innate and adaptive immunity. J. Investig. Dermatol. 2005, 125, 615–628. [Google Scholar] [CrossRef] [Green Version]
- Yla-Herttuala, S.; Lipton, B.A.; Rosenfeld, M.E.; Sarkioja, T.; Yoshimura, T.; Leonard, E.J.; Witztum, J.L.; Steinberg, D. Expression of monocyte chemoattractant protein 1 in macrophage-rich areas of human and rabbit atherosclerotic lesions. Proc. Natl. Acad. Sci. USA 1991, 88, 5252–5256. [Google Scholar] [CrossRef] [Green Version]
- Yu, X.; Barnhill, R.L.; Graves, D.T. Expression of monocyte chemoattractant protein-1 in delayed type hypersensitivity reactions in the skin. Lab. Investig. 1994, 71, 226–235. [Google Scholar]
- Yang, P.; Chen, W.; Xu, H.; Yang, J.; Jiang, J.; Jiang, Y.; Xu, G. Correlation of CCL8 expression with immune cell infiltration of skin cutaneous melanoma: Potential as a prognostic indicator and therapeutic pathway. Cancer Cell Int. 2021, 21, 635. [Google Scholar] [CrossRef] [PubMed]
- Asano, K.; Kikuchi, K.; Tanaka, M. CD169 macrophages regulate immune responses toward particulate materials in the circulating fluid. J. Biochem. 2018, 164, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Chensue, S.W.; Warmington, K.S.; Ruth, J.H.; Sanghi, P.S.; Lincoln, P.; Kunkel, S.L. Role of monocyte chemoattractant protein-1 (MCP-1) in Th1 (mycobacterial) and Th2 (schistosomal) antigen-induced granuloma formation: Relationship to local inflammation, Th cell expression, and IL-12 production. J. Immunol. 1996, 157, 4602–4608. [Google Scholar] [CrossRef] [PubMed]
- Palomino, D.C.; Marti, L.C. Chemokines and immunity. Einstein (Sao Paulo) 2015, 13, 469–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Relke, N.; Gooderham, M. The Use of Janus Kinase Inhibitors in Vitiligo: A Review of the Literature. J. Cutan. Med. Surg. 2019, 23, 298–306. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, R.; Zhou, M.; Lin, F.; Xu, W.; Xu, A. Pathogenic Th2 Cytokine Profile Skewing by IFN-γ-Responding Vitiligo Fibroblasts via CCL2/CCL8. Cells 2023, 12, 217. https://doi.org/10.3390/cells12020217
Jin R, Zhou M, Lin F, Xu W, Xu A. Pathogenic Th2 Cytokine Profile Skewing by IFN-γ-Responding Vitiligo Fibroblasts via CCL2/CCL8. Cells. 2023; 12(2):217. https://doi.org/10.3390/cells12020217
Chicago/Turabian StyleJin, Rong, Miaoni Zhou, Fuquan Lin, Wen Xu, and Aie Xu. 2023. "Pathogenic Th2 Cytokine Profile Skewing by IFN-γ-Responding Vitiligo Fibroblasts via CCL2/CCL8" Cells 12, no. 2: 217. https://doi.org/10.3390/cells12020217




