Cissus quadrangularis (Hadjod) Inhibits RANKL-Induced Osteoclastogenesis and Augments Bone Health in an Estrogen-Deficient Preclinical Model of Osteoporosis Via Modulating the Host Osteoimmune System
Abstract
1. Introduction
2. Materials and Methods
2.1. Animals and Treatments
2.2. Collection of Plant Material
2.3. Preparation of CQ Extract
2.4. Reagents and Antibodies
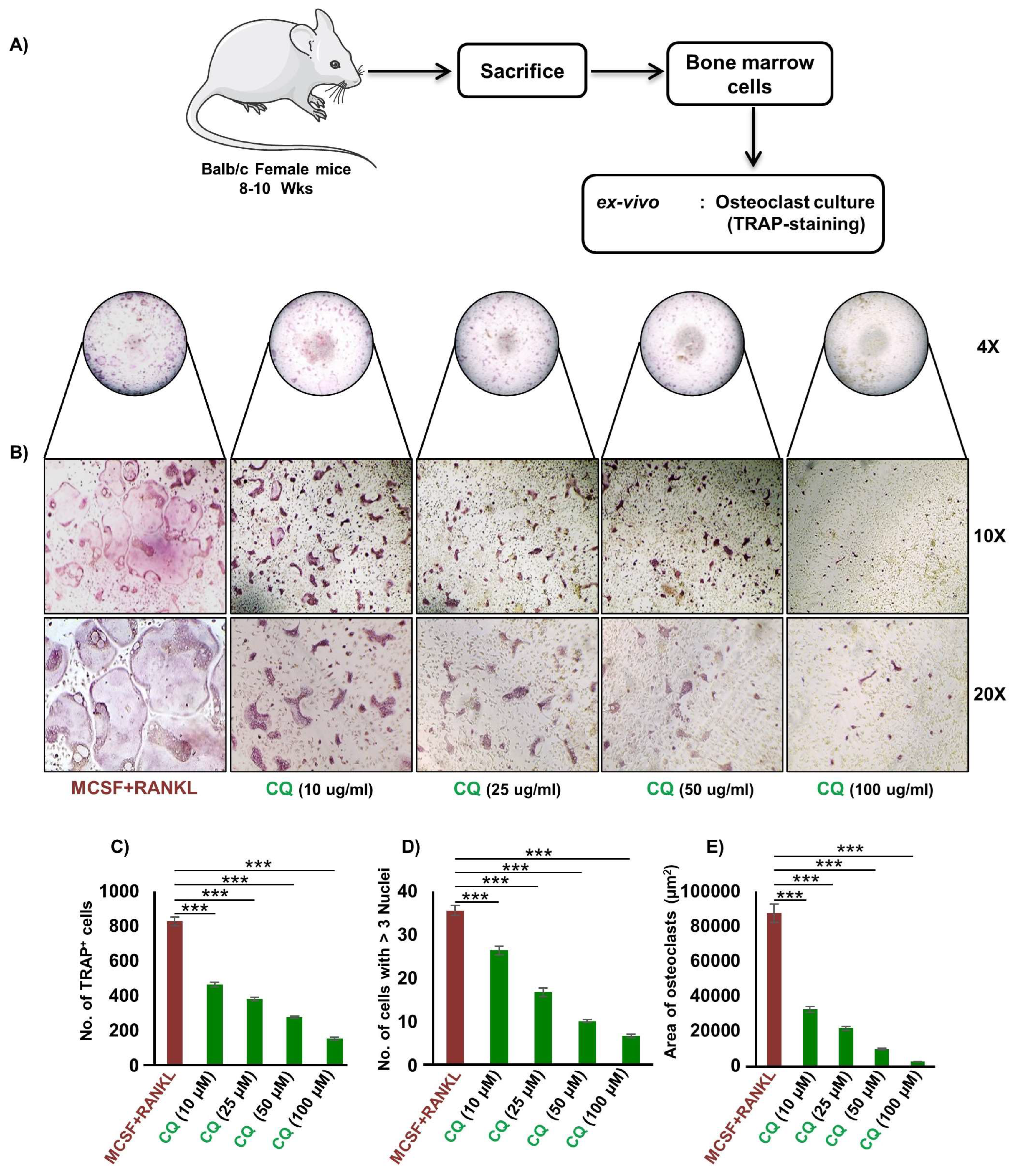
2.5. Osteoclasts Differentiation and TRAP Staining
2.6. F-Actin Ring Polymerization Assay
2.7. Cell Viability or Metabolic Activity Assay
2.8. Scanning Electron Microscopy (SEM)
2.9. Micro-Computed Tomography (µ-CT)
2.10. Flow Cytometry
2.11. Enzyme-Linked Immunosorbent Assay (ELISA)
2.12. Histologic Analysis
2.13. Statistical Analysis of Data
3. Results
3.1. CQ Inhibits RANKL-Induced Osteoclastogenesis
3.2. CQ Attenuates the Functional Activity of Osteoclasts
3.3. CQ Augments Bone Health under Postmenopausal Osteoporotic Conditions
3.4. CQ Enhances Bone Micro-Architecture and Histomorphometric Indices
3.5. CQ Improves Bone Mineral Density (BMD)
3.6. CQ Augments Bone Health Via Modulating Immunoporotic Cells
3.7. CQ Skews the Cytokine Balance under Estrogen-Deficient Conditions
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Sözen, T.; Özışık, L.; Başaran, N.Ç. An Overview and Management of Osteoporosis. Eur. J. Rheumatol. 2017, 4, 46. [Google Scholar] [CrossRef] [PubMed]
- Boyle, W.J.; Simonet, W.S.; Lacey, D.L. Osteoclast Differentiation and Activation. Nature 2003, 423, 337–342. [Google Scholar] [CrossRef] [PubMed]
- Redlich, K.; Smolen, J.S. Inflammatory Bone Loss: Pathogenesis and Therapeutic Intervention. Nat. Rev. Drug Discov. 2012, 11, 234–250. [Google Scholar] [CrossRef] [PubMed]
- McClung, M.; Harris, S.T.; Miller, P.D.; Bauer, D.C.; Davison, K.S.; Dian, L.; Hanley, D.A.; Kendler, D.L.; Yuen, C.K.; Lewiecki, E.M. Bisphosphonate Therapy for Osteoporosis: Benefits, Risks, and Drug Holiday. Am. J. Med. 2013, 126, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Tanvetyanon, T.; Stiff, P. Management of the Adverse Effects Associated with Intravenous Bisphosphonates. Ann. Oncol. 2006, 17, 897–907. [Google Scholar] [CrossRef] [PubMed]
- Bowring, C.; Francis, R. National Osteoporosis Society’s Position Statement on Hormone Replacement Therapy in the Prevention and Treatment of Osteoporosis. Menopause Int. 2011, 17, 63–65. [Google Scholar] [CrossRef]
- Keshishi, D.; Makunts, T.; Abagyan, R. Common Osteoporosis Drug Associated with Increased Rates of Depression and Anxiety. Sci. Rep. 2021, 11, 23956. [Google Scholar] [CrossRef]
- Riancho, J.A.; Zarrabeitia, M.T.; Gonzalezmacias, J. Interleukin-4 Modulates Osteoclast Differentiation and Inhibits the Formation of Resorption Pits in Mouse Osteoclast Cultures. Biochem. Biophys. Res. Commun. 1993, 196, 678–685. [Google Scholar] [CrossRef]
- Miossec, P.; Chomarat, P.; Dechanet, J.; Moreau, J.-F.; Roux, J.-P.; Delmas, P.; Banchereau, J. Interleukin-4 Inhibits Bone Resorption through an Effect on Osteoclasts and Proinflammatory Cytokines in an Ex Vivo Model of Bone Resorption in Rheumatoid Arthritis. Arthritis Rheum. 1994, 37, 1715–1722. [Google Scholar] [CrossRef]
- McInnes, I.B.; Schett, G. Cytokines in the Pathogenesis of Rheumatoid Arthritis. Nat. Rev. Immunol. 2007, 7, 429–442. [Google Scholar] [CrossRef]
- Pacifici, R. T Cells: Critical Bone Regulators in Health and Disease. Bone 2010, 47, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Ghoreschi, K.; Laurence, A.; Yang, X.-P.; Hirahara, K.; O’Shea, J.J. T Helper 17 Cell Heterogeneity and Pathogenicity in Autoimmune Disease. Trends Immunol. 2011, 32, 395–401. [Google Scholar] [CrossRef] [PubMed]
- Raphael, I.; Nalawade, S.; Eagar, T.N.; Forsthuber, T.G. T Cell Subsets and Their Signature Cytokines in Autoimmune and Inflammatory Diseases. Cytokine 2015, 74, 5–17. [Google Scholar] [CrossRef] [PubMed]
- Dar, H.Y.; Pal, S.; Shukla, P.; Mishra, P.K.; Tomar, G.B.; Chattopadhyay, N.; Srivastava, R.K. Bacillus Clausii Inhibits Bone Loss by Skewing Treg-Th17 Cell Equilibrium in Postmenopausal Osteoporotic Mice Model. Nutrition 2018, 54, 118–128. [Google Scholar] [CrossRef]
- Dar, H.Y.; Shukla, P.; Mishra, P.K.; Anupam, R.; Mondal, R.K.; Tomar, G.B.; Sharma, V.; Srivastava, R.K. Lactobacillus Acidophilus Inhibits Bone Loss and Increases Bone Heterogeneity in Osteoporotic Mice via Modulating Treg-Th17 Cell Balance. Bone Rep. 2018, 8, 46–56. [Google Scholar] [CrossRef]
- Azam, Z.; Sapra, L.; Bhardwaj, A.; Yadav, S.; Mishra, P.K.; Shukla, P.; Sharma, V.; Srivastava, R.K. Crocin Attenuates Osteoclastogenesis and Enhances Bone Health by Skewing the Immunoporotic “Treg-Th17” Cell Axis in Post-Menopausal Osteoporotic Mice Model. Phytomed. Plus 2022, 2, 100302. [Google Scholar] [CrossRef]
- Dar, H.Y.; Azam, Z.; Anupam, R.; Mondal, R.K.; Srivastava, R.K. Osteoimmunology: The Nexus between Bone and Immune System. Front. Biosci.-Landmark 2018, 23, 464–492. [Google Scholar]
- Dar, H.Y.; Singh, A.; Shukla, P.; Anupam, R.; Mondal, R.K.; Mishra, P.K.; Srivastava, R.K. High Dietary Salt Intake Correlates with Modulated Th17-Treg Cell Balance Resulting in Enhanced Bone Loss and Impaired Bone-Microarchitecture in Male Mice. Sci. Rep. 2018, 8, 2503. [Google Scholar] [CrossRef]
- Srivastava, R.K.; Dar, H.Y.; Mishra, P.K. Immunoporosis: Immunology of Osteoporosis—Role of T Cells. Front. Immunol. 2018, 9, 657. [Google Scholar] [CrossRef]
- Fischer, L.; Herkner, C.; Kitte, R.; Dohnke, S.; Riewaldt, J.; Kretschmer, K.; Garbe, A.I. Foxp3+ Regulatory T Cells in Bone and Hematopoietic Homeostasis. Front. Endocrinol. 2019, 10, 578. [Google Scholar] [CrossRef]
- Sapra, L.; Bhardwaj, A.; Mishra, P.K.; Garg, B.; Verma, B.; Mishra, G.C.; Srivastava, R.K. Regulatory B Cells (Bregs) Inhibit Osteoclastogenesis and Play a Potential Role in Ameliorating Ovariectomy-Induced Bone Loss. Front. Immunol. 2021, 12, 691081. [Google Scholar] [CrossRef] [PubMed]
- Sapra, L.; Shokeen, N.; Gupta, K.; Saini, C.; Bhardwaj, A.; Mathew, M.; Mishra, P.K.; Chattopadhyay, N.; Verma, B.; Srivastava, R.K. Bifidobacterium Longum Attenuates Ovariectomy-Induced Bone Loss Via Modulating the Immunoporotic Breg-Treg-Th17 Cell Axis. Front. Immunol. 2022, 13, 875788. [Google Scholar] [CrossRef] [PubMed]
- Sofowora, A.; Ogunbodede, E.; Onayade, A. The Role and Place of Medicinal Plants in the Strategies for Disease Prevention. Afr. J. Tradit. Complement. Altern. Med. 2013, 10, 210–229. [Google Scholar] [CrossRef] [PubMed]
- Udupa, K.; Chaturvedi, G.; Tripathi, S. Advances in Research in Indian Medicine; Banaras Hindu University: Varanasi, India, 1970. [Google Scholar]
- Ganguly, A.; Ganguly, D.; Banerjee, S. Topical Phytotherapeutic Treatment: Management of Normalization of Elevated Levels of Biochemical Parameter During Osteoarthritic Disorders: A Prospective Study. J. Orthop. Rheumatol. 2018, 5, 14. [Google Scholar]
- Sivarajan, V.; Balachandran, I. Ayurvedic Drugs and Their Plant Sources; Oxford and IBH Publishing: New Delhi, India, 1994. [Google Scholar]
- Udupa, K.N.; Prasad, G.C. Further Studies on the Effect of Cissus Quadrangularis in Accelerating Fracture Healing. Indian J. Med. Res. 1964, 52, 26–35. [Google Scholar]
- Shirwaikar, A.; Khan, S.; Malini, S. Antiosteoporotic Effect of Ethanol Extract of Cissus Quadrangularis Linn. On Ovariectomized Rat. J. Ethnopharmacol. 2003, 89, 245–250. [Google Scholar] [CrossRef]
- Stohs, S.J.; Ray, S.D. A Review and Evaluation of the Efficacy and Safety of Cissus Quadrangularis Extracts. Phytother. Res. 2013, 27, 1107–1114. [Google Scholar] [CrossRef]
- Udupa, K.N.; Prasad, G.; Sen, S.P. The Effect of Phytogenic Anabolic Steroid in the Acceleration of Fracture Repair. Life Sci. 1965, 4, 317–327. [Google Scholar] [CrossRef]
- Prasad, G.C.; Udupa, K.N. Pathways and Site of Action of a Phytogenic Steroid from Cissus Quadrangularis. J. Res. Indian Med. 1972, 4, 132. [Google Scholar]
- Padmalochana, K.; Rajan, M.S.D.; Lalitha, R.; Sivasankari, H. Evaluation of the Antioxidant and Hepatoprotective Activity of Cryptolepis Buchanani. J. Appl. Pharm. Sci. 1930, 3, 099–104. [Google Scholar] [CrossRef]
- Muthusami, S.; Ramachandran, I.; Krishnamoorthy, S.; Govindan, R.; Narasimhan, S. Cissus Quadrangularis Augments Igf System Components in Human Osteoblast Like Saos-2 Cells. Growth Horm. IGF Res. 2011, 21, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Sapra, L.; Dar, H.Y.; Bhardwaj, A.; Pandey, A.; Kumari, S.; Azam, Z.; Upmanyu, V.; Anwar, A.; Shukla, P.; Mishra, P.K.; et al. Lactobacillus Rhamnosus Attenuates Bone Loss and Maintains Bone Health by Skewing Treg-Th17 Cell Balance in Ovx Mice. Sci. Rep. 2021, 11, 1807. [Google Scholar] [CrossRef] [PubMed]
- Dempster, D.W.; Compston, J.E.; Drezner, M.K.; Glorieux, F.H.; Kanis, J.A.; Malluche, H.; Meunier, P.J.; Ott, S.M.; Recker, R.R.; Parfitt, A.M. Standardized Nomenclature, Symbols, and Units for Bone Histomorphometry: A 2012 Update of the Report of the Asbmr Histomorphometry Nomenclature Committee. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2013, 28, 2. [Google Scholar] [CrossRef] [PubMed]
- Li, J.Y.; Chassaing, B.; Tyagi, A.M.; Vaccaro, C.; Luo, T.; Adams, J.; Darby, T.M.; Weitzmann, M.N.; Mulle, J.G.; Gewirtz, A.T.; et al. Sex Steroid Deficiency–Associated Bone Loss Is Microbiota Dependent and Prevented by Probiotics. J. Clin. Investig. 2016, 126, 2049–2063. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Tomar, G.B.; Barhanpurkar, A.P.; Gupta, N.; Pote, S.T.; Mishra, G.C.; Wani, M.R. Il-3 Attenuates Collagen-Induced Arthritis by Modulating the Development of Foxp3+ Regulatory T Cells. J. Immunol. 2011, 186, 2262–2272. [Google Scholar] [CrossRef]
- Azam, Z.; Pandey, V.; Gupta, N.; Sapra, L.; Dar, H.Y.; Shokeen, N.; Soni, V.; Srivastava, R.K. Phytoconstituents as Novel Osteo-Protective Agents: Implications in Bone Health. Front. Biosci.-Landmark 2020, 25, 1259–1296. [Google Scholar]
- Mahmoudi, Z.; Soleimani, M.; Khamisipour, G.; Azizsoltani, A. Effects of Foeniculum Vulgare Ethanol Extract on Osteogenesis in Human Mecenchymal Stem Cells. Avicenna J. Phytomed. 2013, 3, 135. [Google Scholar]
- Kanis, J.A.; McCloskey, E.V.; Johansson, H.; Oden, A.; Melton, L.J., III; Khaltaev, N. A Reference Standard for the Description of Osteoporosis. Bone 2008, 42, 467–475. [Google Scholar] [CrossRef]
- Park, J.A.; Ha, S.K.; Kang, T.H.; Oh, M.S.; Cho, M.H.; Lee, S.Y.; Park, J.-H.; Kim, S.Y. Protective Effect of Apigenin on Ovariectomy-Induced Bone Loss in Rats. Life Sci. 2008, 82, 1217–1223. [Google Scholar] [CrossRef]
- Kalalinia, F.; Ghasim, H.; Farzad, S.A.; Pishavar, E.; Ramezani, M.; Hashemi, M. Comparison of the Effect of Crocin and Crocetin, Two Major Compounds Extracted from Saffron, on Osteogenic Differentiation of Mesenchymal Stem Cells. Life Sci. 2018, 208, 262–267. [Google Scholar] [CrossRef]
- Mishra, G.; Srivastava, S.; Nagori, B. Pharmacological and Therapeutic Activity of Cissus Quadrangularis: An Overview. Int. J. Pharmtech Res. 2010, 2, 1298–1310. [Google Scholar]










| Bone Parameters | Sham | Ovx | Ovx + CQ |
|---|---|---|---|
| LV-5 | |||
| BV/TV (%) | 68.8 ± 0.1 | 56.4 ± 2.5 | 83.35 ± 1.35 ** |
| Tb. Th (mm) | 0.05 ± 0 | 0.045 ± 0.005 | 0.055 ± 0.005 * |
| Tb. Sp (mm−3) | 0.038 ± 0.002 | 0.058 ± 0.002 | 0.037 ± 0.0015 * |
| Conn. D (mm−3) | 198.38 ± 40.22 | 93.87 ± 27.52 | 223.153 ± 17.29 * |
| Tb. N (mm−1) | 9.8 ± 0.1 | 13.45 ± 0.45 | 16.95 ± 1.75 * |
| Tb. Pf (%) | 15.05 ± 2.65 | 21.25 ± 0.25 | 7.05 ± 0.65 * |
| Femur Trabecular | |||
| BV/TV (%) | 85.3 ± 2.6 | 63.35 ± 1.25 | 89.15 ± 0.75 ** |
| Tb. Th (mm) | 0.05 ± 0 | 0.041 ± 0.0015 | 0.07 ± 0 *** |
| Tb. Sp (mm−3) | 0.2 ± 0 | 0.4 ± 0 | 0.75 ± 0.0015 ** |
| Conn. D (mm−3) | 185.51 ± 14.85 | 83.39 ± 7.75 | 179.04 ± 9.13 *** |
| Tb. N (mm−1) | 7.36 ± 4.78 | 3.56 ± 2.21 | 11.4 ± 7.20 * |
| Tb. Pf (%) | 14.4 ± 0.62 | 19.03 ± 1.26 | 12.53 ± 1.65 ** |
| Tibia Trabecular | |||
| BV/TV (%) | 81.65 ± 2.75 | 62.15 ± 2.05 | 86.35 ± 2.85 * |
| Tb. Th (mm) | 0.07 ± 0 | 0.046 ± 0.005 | 0.00071 ± 0 *** |
| Tb. Sp (mm−3) | 0.037 ± 0.0035 | 0.046 ± 0.0015 | 0.025 ± 0.005 ** |
| Conn. D (mm−3) | 50.33 ± | 27.89 ± | 86.30 ± 53.60 * |
| Tb. N (mm−1) | 9.13 ± 1.73 | 6.7 ± 0.22 | 13.5 ± 1.20 ** |
| Tb. Pf (%) | 13.63 ± 2.35 | 20 ± 1.79 | 12.86 ± 0.96 * |
| Femur Cortical | |||
| Tt. Ar (mm2) | 1.35 ± 0.01 | 0.8 ± 0.1 | 2.03 ± 0.06 ** |
| T. Pm (mm) | 4.38 ± 0.025 | 3.22 ± 0.045 | 5.4 ±0.08 *** |
| Ct. Th (mm) | 0.16 ± 0.005 | 0.13 ± 0.005 | 0.17 ± 0.005 * |
| Ct. Ar (mm2) | 0.59 ± 0.57 | 0.54 ± 0.55 | 0.7 ± 0.08 * |
| B. Pm (mcm) | 7.60 ± 0.03 | 6.57 ± 0.56 | 9.39 ± 0.77 |
| J (mm4) | 0.21 ± 0.02 | 0.13 ± 0.04 | 0.26 ± 0.08 |
| Tibia Cortical | |||
| Tt. Ar (mm2) | 0.975 ± 0.015 | 0.65 ± 0.05 | 1.39 ± 0.05 ** |
| T. Pm (mm) | 4.51 ± 0 | 4.68 ± 0.02 | 5.57 ± 0.115 ** |
| Ct. Th (mm) | 0.14 ± 0.01 | 0.11 ± 0.01 | 0.16 ± 0.01 * |
| Ct. Ar (mm2) | 0.53 ± 0.05 | 0.46 ± 0.02 | 0.66 ± 0.02 ** |
| B. Pm (mcm) | 5.27 ± 3.22 | 4.72 ± 2.90 | 6.77 ± 4.15 * |
| J (mm4) | 0.14 ± 0.02 | 0.13 ± 0.01 | 0.31 ± 0.03 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Azam, Z.; Sapra, L.; Baghel, K.; Sinha, N.; Gupta, R.K.; Soni, V.; Saini, C.; Mishra, P.K.; Srivastava, R.K. Cissus quadrangularis (Hadjod) Inhibits RANKL-Induced Osteoclastogenesis and Augments Bone Health in an Estrogen-Deficient Preclinical Model of Osteoporosis Via Modulating the Host Osteoimmune System. Cells 2023, 12, 216. https://doi.org/10.3390/cells12020216
Azam Z, Sapra L, Baghel K, Sinha N, Gupta RK, Soni V, Saini C, Mishra PK, Srivastava RK. Cissus quadrangularis (Hadjod) Inhibits RANKL-Induced Osteoclastogenesis and Augments Bone Health in an Estrogen-Deficient Preclinical Model of Osteoporosis Via Modulating the Host Osteoimmune System. Cells. 2023; 12(2):216. https://doi.org/10.3390/cells12020216
Chicago/Turabian StyleAzam, Zaffar, Leena Sapra, Kalpana Baghel, Niharika Sinha, Rajesh K. Gupta, Vandana Soni, Chaman Saini, Pradyumna K. Mishra, and Rupesh K. Srivastava. 2023. "Cissus quadrangularis (Hadjod) Inhibits RANKL-Induced Osteoclastogenesis and Augments Bone Health in an Estrogen-Deficient Preclinical Model of Osteoporosis Via Modulating the Host Osteoimmune System" Cells 12, no. 2: 216. https://doi.org/10.3390/cells12020216
APA StyleAzam, Z., Sapra, L., Baghel, K., Sinha, N., Gupta, R. K., Soni, V., Saini, C., Mishra, P. K., & Srivastava, R. K. (2023). Cissus quadrangularis (Hadjod) Inhibits RANKL-Induced Osteoclastogenesis and Augments Bone Health in an Estrogen-Deficient Preclinical Model of Osteoporosis Via Modulating the Host Osteoimmune System. Cells, 12(2), 216. https://doi.org/10.3390/cells12020216








