Early Regional Patterning in the Human Prefrontal Cortex Revealed by Laminar Dynamics of Deep Projection Neuron Markers
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Brain Tissue Processing
2.2. Histological and Immunofluorescence (IF) Stainings
2.3. Immunochemicals and Reagents
2.4. Imaging
2.5. Image Processing and Statistical Analysis
3. Results
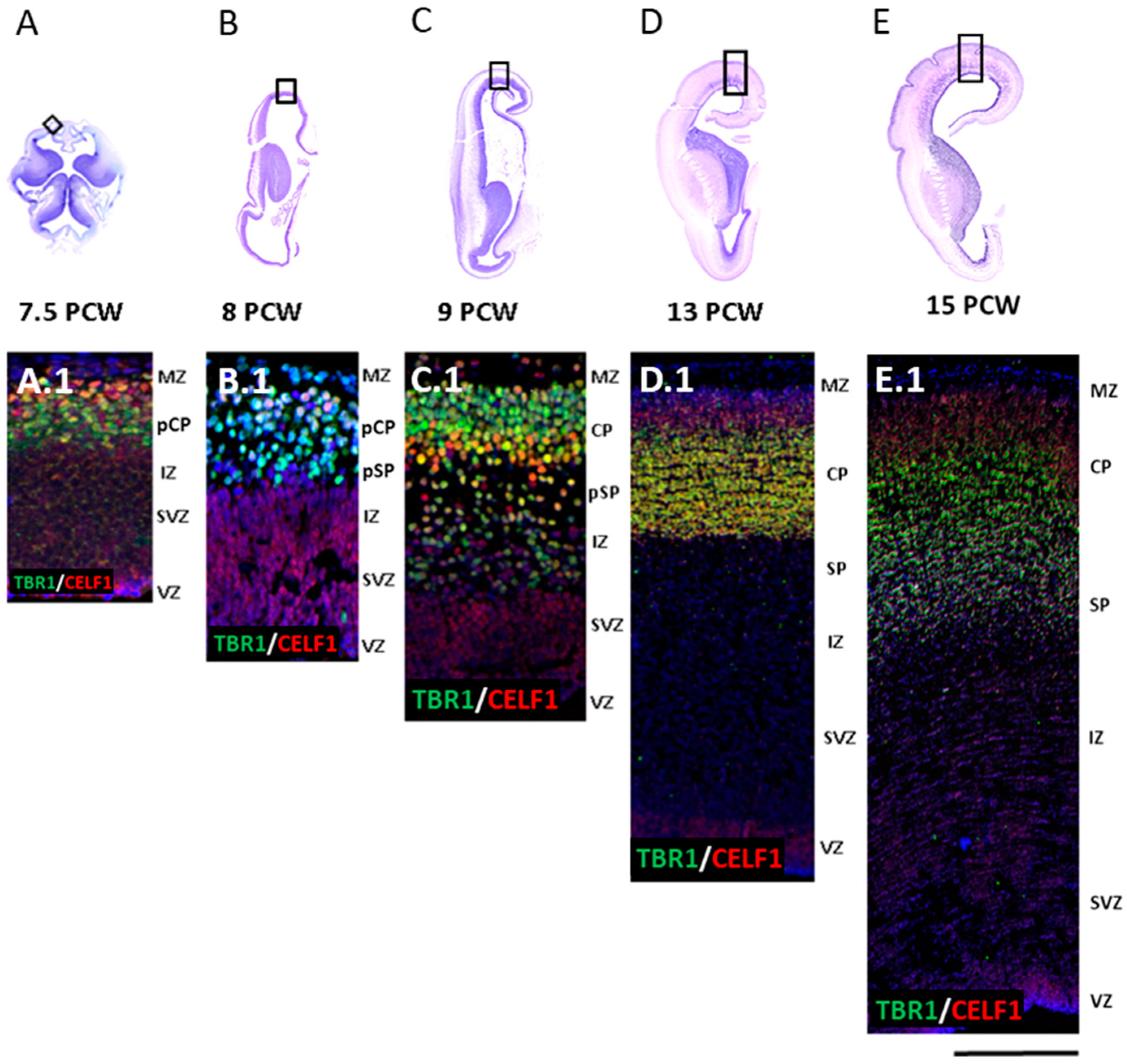
3.1. Late Preplate Phase (7.5 PCW)
3.2. Initial Formation of Pioneering CP Phase (8 PCW, Carnegie Stage 23)
3.3. First Condensation of the CP Phase (9 PCW)
3.4. Formation of the Subplate Phase (13 PCW)
3.5. Typical Fetal Lamination Phase (15 PCW)
4. Discussion
4.1. The DPN Role in the Early Basic Frontal Cortex Regionalization and Architecture Establishment
4.2. DPN Distribution during SP Formation-Expansion Phase and Establishment of the Regional Frontal Cortex Geography
4.3. Basic Neural Network Components of Highly Ordered Association Frontal Cortex Differentiate during Early Fetal Period
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- His, W. Die Entwicklung des Menschlichen Gehirns Wahrend der Ersten Monate, 1st ed.; Verlag von Hirzel: Leipzig, Germany, 1904. [Google Scholar]
- Von Economo, C.B.; Koskinas, G.N. Atlas of Cytoarchitectonics of the Adult Human Cerebral Cortex, 1st ed.; Verlag von Julius Springer: Wien, Austria, 1925. [Google Scholar]
- Rose, M. Uber das histogenetische Prinzip der Einteilung der Grosshirnrinde. J. Psychol. Neurol. 1926, 32, 97–160. [Google Scholar]
- Filimonoff, I.N. A Rational Subdivision of the Cerebral Cortex. Arch. Neurol. Psych. 1947, 58, 296–311. [Google Scholar] [CrossRef]
- Kostovic, I.; Rakic, P. Developmental History of the Transient Subplate Zone in the Visual and Somatosensory Cortex of the Macaque Monkey and Human Brain. J. Comp. Neurol. 1990, 297, 441–470. [Google Scholar] [CrossRef]
- Meyer, G.; Schaaps, J.P.; Moreau, L.; Goffinet, A.M. Embryonic and Early Fetal Development of the Human Neocortex. J. Neurosci. 2000, 20, 1858–1868. [Google Scholar] [CrossRef]
- Meyer, G. Human neocortical development: The importance of embryonic and early fetal events. Neuroscientist. 2001, 7, 303–314. [Google Scholar] [CrossRef]
- Bystron, I.; Blakemore, C.; Rakic, P. Development of the Human Cerebral Cortex: Boulder Committee Revisited. Nat. Rev. Neurosci. 2008, 9, 110–122. [Google Scholar] [CrossRef]
- Kostović, I.; Judaš, M. Embryonic and Fetal Development of the Human Cerebral Cortex. In Brain Mapping: An Encyclopedic Reference, 1st ed.; Toga, A.W., Ed.; Academic Press: London, UK, 2015; Volume 2, pp. 167–175. [Google Scholar]
- Kostović, I.; Išasegi, I.Ž.; Krsnik, Ž. Sublaminar Organization of the Human Subplate: Developmental Changes in the Distribution of Neurons, Glia, Growing Axons and Extracellular Matrix. J. Anat. 2019, 235, 481–506. [Google Scholar] [CrossRef]
- Clascá, F.; Angelucci, A.; Sur, M. Layer-Specific Programs of Development in Neocortical Projection Neurons. Proc. Natl. Acad. Sci. USA 1995, 92, 11145–11149. [Google Scholar] [CrossRef]
- Han, W.; Kwan, K.Y.; Shim, S.; Lam, M.M.S.; Shin, Y.; Xu, M.; Zhu, Y.; Li, M.; Šestan, N. TBR1 Directly Represses Fezf2 to Control the Laminar Origin and Development of the Corticospinal Tract. Proc. Natl. Acad. Sci. USA 2011, 108, 3041–3046. [Google Scholar] [CrossRef]
- Kwan, K.Y.; Šestan, N.; Anton, E.S. Transcriptional Co-Regulation of Neuronal Migration and Laminar Identity in the Neocortex. Development 2012, 139, 1535–1546. [Google Scholar] [CrossRef]
- Hevner, R.F.; Shi, L.; Justice, N.; Hsueh, Y.P.; Sheng, M.; Smiga, S.; Bulfone, A.; Goffinet, A.M.; Campagnoni, A.T.; Rubenstein, J.L.R. Tbr1 Regulates Differentiation of the Preplate and Layer 6. Neuron 2001, 29, 353–366. [Google Scholar] [CrossRef]
- Arlotta, P.; Molyneaux, B.J.; Chen, J.; Inoue, J.; Kominami, R.; MacKlis, J.D. Neuronal Subtype-Specific Genes That Control Corticospinal Motor Neuron Development In Vivo. Neuron 2005, 45, 207–221. [Google Scholar] [CrossRef] [PubMed]
- Molyneaux, B.J.; Arlotta, P.; Menezes, J.R.L.; Macklis, J.D. Neuronal Subtype Specification in the Cerebral Cortex. Nat. Rev. Neurosci. 2007, 8, 427–437. [Google Scholar] [CrossRef]
- Greig, L.C.; Woodworth, M.B.; Galazo, M.J.; Padmanabhan, H.; Macklis, J.D. Molecular Logic of Neocortical Projection Neuron Specification, Development and Diversity. Nat. Rev. Neurosci. 2013, 14, 755–769. [Google Scholar] [CrossRef] [PubMed]
- Ohtaka-Maruyama, C.; Okamoto, M.; Endo, K.; Oshima, M.; Kaneko, N.; Yura, K.; Okado, H.; Miyata, T.; Maeda, N. Synaptic Transmission from Subplate Neurons Controls Radial Migration of Neocortical Neurons. Science 2018, 360, 313–317. [Google Scholar] [CrossRef]
- Marin-Padilla, M. Structural Organization of the Human Cerebral Cortex Prior to the Appearance of the Cortical Plate. Anat. Embryol. 1983, 168, 21–40. [Google Scholar] [CrossRef]
- Mrzljak, L.; Uylings, H.B.M.; Kostović, I.; Van Eden, C.G. Prenatal development of neurons in the human prefrontal cortex. I: A qualitative Golgi study. J. Comp. Neurol. 1988, 271, 355–386. [Google Scholar] [CrossRef] [PubMed]
- Kwan, K.Y.; Lam, M.M.S.; Krsnik, Ž.; Kawasawa, Y.I.; Lefebvre, V.; Šestan, N. SOX5 Postmitotically Regulates Migration, Postmigratory Differentiation, and Projections of Subplate and Deep-Layer Neocortical Neurons. Proc. Natl. Acad. Sci. USA 2008, 10, 16021–16026. [Google Scholar] [CrossRef] [PubMed]
- Dubois, J.; Kostovic, I.; Judas, M. Development of Structural and Functional Connectivity. In Brain Mapping: An Encyclopedic Reference, 1st ed.; Toga, A.W., Ed.; Elsevier: Amsterdam, The Netherlands; Academic Press: London, UK, 2015; Volume 3, pp. 423–437. [Google Scholar]
- Rakic, P. Specification of cerebral cortical areas. Science 1988, 241, 170–176. [Google Scholar] [CrossRef] [PubMed]
- Popovitchenko, T.; Park, Y.; Page, N.F.; Luo, X.; Krsnik, Z.; Liu, Y.; Salamon, I.; Stephenson, J.D.; Kraushar, M.L.; Volk, N.L.; et al. Translational Derepression of Elavl4 Isoforms at Their Alternative 5′ UTRs Determines Neuronal Development. Nat. Commun. 2020, 11, 1674. [Google Scholar] [CrossRef]
- Salamon, I.; Rasin, M.R. Evolution of the Neocortex Through RNA-Binding Proteins and Post-Transcriptional Regulation. Front. Neurosci. 2022, 15, 1840. [Google Scholar] [CrossRef] [PubMed]
- Kostovic, I. Zentralnervensystem. In Humanembryologie: Lehrbuch Und Atlas Der Vorgwburtlichen Entwicklung Des Menschen, 1st ed.; Hinrichsen, K.V., Beier, H.M., Breucker, H., Christ, B., Duncker, H.R., Dvořák, M., Gaudecker, B., Dorsche, H.H., Holstein, A.F., et al., Eds.; Springer: Berlin/Heidelberg, Germany, 1990; pp. 381–448. [Google Scholar]
- Kast, R.J.; Levitt, P. Precision in the Development of Neocortical Architecture: From Progenitors to Cortical Networks. Prog. Neurobiol. 2019, 175, 77–95. [Google Scholar] [CrossRef] [PubMed]
- Kostović, I.; Judaš, M. Correlation between the sequential ingrowth of afferents and transient patterns of cortical lamination in preterm infants. Anat Rec. 2002, 267, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Silbereis, J.C.; Pochareddy, S.; Zhu, Y.; Li, M.; Sestan, N. The Cellular and Molecular Landscapes of the Developing Human Central Nervous System. Neuron 2016, 89, 248–268. [Google Scholar] [CrossRef]
- Nowakowski, T.J.; Bhaduri, A.; Pollen, A.A.; Alvarado, B.; Mostajo-Radji, M.A.; Di Lullo, E.; Haeussler, M.; Sandoval-Espinosa, C.; Liu, S.J.; Velmeshev, D.; et al. Spatiotemporal Gene Expression Trajectories Reveal Developmental Hierarchies of the Human Cortex. Science 2017, 358, 1318–1323. [Google Scholar] [CrossRef] [PubMed]
- Sestan, N.; State, M.W. Lost in Translation: Traversing the Complex Path from Genomics to Therapeutics in Autism Spectrum Disorder. Neuron 2018, 100, 406–423. [Google Scholar] [CrossRef]
- Cadwell, C.R.; Bhaduri, A.; Mostajo-Radji, M.A.; Keefe, M.G.; Nowakowski, T.J. Development and Arealization of the Cerebral Cortex. Neuron 2019, 103, 980–1004. [Google Scholar] [CrossRef]
- Miller, J.A.; Ding, S.L.; Sunkin, S.M.; Smith, K.A.; Ng, L.; Szafer, A.; Ebbert, A.; Riley, Z.L.; Royall, J.J.; Aiona, K.; et al. Transcriptional Landscape of the Prenatal Human Brain. Nature 2014, 508, 199–206. [Google Scholar] [CrossRef]
- Polioudakis, D.; de la Torre-Ubieta, L.; Langerman, J.; Elkins, A.G.; Shi, X.; Stein, J.L.; Vuong, C.K.; Nichterwitz, S.; Gevorgian, M.; Opland, C.K.; et al. A Single Cell Transcriptomic Atlas of Human Neocortical developmentduring Mid-Gestation. Neuron 2019, 103, 785. [Google Scholar] [CrossRef]
- Willsey, A.J.; Sanders, S.J.; Li, M.; Dong, S.; Tebbenkamp, A.T.; Muhle, R.A.; Reilly, S.K.; Lin, L.; Fertuzinhos, S.; Miller, J.A.; et al. Coexpression Networks Implicate Human Midfetal Deep Cortical Projection Neurons in the Pathogenesis of Autism. Cell 2013, 155, 997–1007. [Google Scholar] [CrossRef]
- Fazel Darbandi, S.; Robinson Schwartz, S.E.; Qi, Q.; Catta-Preta, R.; Pai, E.L.L.; Mandell, J.D.; Everitt, A.; Rubin, A.; Krasnoff, R.A.; Katzman, S.; et al. Neonatal Tbr1 Dosage Controls Cortical Layer 6 Connectivity. Neuron 2018, 100, 831–845. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Z.; Luhmann, H.J.; Kanold, P.O. Transient Cortical Circuits Match Spontaneous and Sensory-Driven Activity during Development. Science 2020, 370, 6514. [Google Scholar] [CrossRef] [PubMed]
- Nauta, W.J. Neural associations of the frontal cortex. Acta Neurobiol. Exp. 1972, 32, 125–140. [Google Scholar]
- Goldman, P.S.; Nauta, W.J.H. An Intricately Patterned Prefronto-Caudate Projection in the Rhesus Monkey. J. Comp. Neurol. 1977, 72, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Stephan, H. Mikroskopische Anatomie. In Allocortex, 1st ed.; Stephan, H., Ed.; Springer: Berlin/Heidelberg, Germany, 1975; Volume 4/9, pp. 213–838. [Google Scholar]
- Kahle, W. Die Entwicklung Der Menschlichen Großhirnhemisphäre, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1969. [Google Scholar]
- Molliver, M.E.; Kostović, I.; Van Der Loos, H. The Development of Synapses in Cerebral Cortex of the Human Fetus. Brain Res. 1973, 50, 403–407. [Google Scholar] [CrossRef]
- Kostovic, I.; Goldman-Rakic, P.S. Transient Cholinesterase Staining in the Mediodorsal Nucleus of the Thalamus and Its Connections in the Developing Human and Monkey Brain. J. Comp. Neurol. 1983, 219, 431–447. [Google Scholar] [CrossRef]
- Goldman-Rakic, P.S. Development of cortical circuitry and cognitive function. Child Dev. 1987, 58, 601–622. [Google Scholar] [CrossRef]
- Fuster, J.M. The Prefrontal Cortex, 4th ed.; Academic Press: London, UK, 2008. [Google Scholar]
- Kolk, S.M.; Rakic, P. Development of Prefrontal Cortex. Neuropsychopharmacology 2021, 47, 41–57. [Google Scholar] [CrossRef]
- Eyre, J.A.; Clowry, G.J. Development of the human spinal cord. Brain 2002, 125, 2134–2136. [Google Scholar] [CrossRef]
- Duque, A.; Krsnik, Z.; Kostović, I.; Rakic, P. Secondary Expansion of the Transient Subplate Zone in the Developing Cerebrum of Human and Nonhuman Primates. Proc. Natl. Acad. Sci. USA 2016, 113, 9892–9897. [Google Scholar] [CrossRef]
- Englund, C.; Fink, A.; Lau, C.; Pham, D.; Daza, R.A.M.; Bulfone, A.; Kowalczyk, T.; Hevner, R.F. Pax6, Tbr2, and Tbr1 Are Expressed Sequentially by Radial Glia, Intermediate Progenitor Cells, and Postmitotic Neurons in Developing Neocortex. J. Neurosci. 2005, 25, 247–251. [Google Scholar] [CrossRef] [PubMed]
- Ip, B.K.; Bayatti, N.; Howard, N.J.; Lindsay, S.; Clowry, G.J. The Corticofugal Neuron-Associated Genes ROBO1, SRGAP1, and CTIP2 Exhibit an Anterior to Posterior Gradient of Expression in Early Fetal Human Neocortex Development. Cereb. Cortex 2011, 21, 1395–1407. [Google Scholar] [CrossRef] [PubMed]
- Bulfone, A.; Smiga, S.M.; Shimamura, K.; Peterson, A.; Puelles, L.; Rubenstein, J.L.R. T-Brain-1: A Homolog of Brachyury Whose Expression Defines Molecularly Distinct Domains within the Cerebral Cortex. Neuron 1995, 15, 63–78. [Google Scholar] [CrossRef] [PubMed]
- Kolk, S.M.; Whitman, M.C.; Yun, M.E.; Shete, P.; Donoghue, M.J. A Unique Subpopulation of Tbr1-Expressing Deep Layer Neurons in the Developing Cerebral Cortex. Mol. Cell. Neurosci. 2006, 32, 200–214. [Google Scholar] [CrossRef] [PubMed]
- Aboitiz, F.; Montiel, J.; García, R.R. Ancestry of the Mammalian Preplate and Its Derivatives: Evolutionary Relicts or Embryonic Adaptations? Rev. Neurosci. 2005, 16, 359–376. [Google Scholar] [CrossRef]
- O’Rahilly, R.; Müller, F. Significant Features in the Early Prenatal Development of the Human. Brain. Ann. Anat. 2008, 190, 105–118. [Google Scholar] [CrossRef]
- Papadopulos, F.; Spinelli, M.; Valente, S.; Foroni, L.; Orrico, C.; Alviano, F.; Pasquinelli, G. Common Tasks in Microscopic and Ultrastructural Image Analysis Using ImageJ. Ultrastruct. Pathol. 2007, 31, 401–407. [Google Scholar] [CrossRef]
- Grishagin, I.V. Automatic Cell Counting with ImageJ. Anal. Biochem. 2015, 473, 63–65. [Google Scholar] [CrossRef]
- Marin-Padilla, M. Prenatal and Early Postnatal Ontogenesis of the Human Motor Cortex: A Golgi Study. I. The Sequential Development of the Cortical Layers. Brain Res. 1970, 23, 167–183. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.D.M.; Chou, S.J.; Sahara, S. Area Patterning of the Mammalian Cortex. Neuron 2007, 56, 252–269. [Google Scholar] [CrossRef]
- Chou, S.J.; Babot, Z.; Leingärtner, A.; Studer, M.; Nakagawa, Y.; O’Leary, D.D.M. Geniculocortical Input Drives Genetic Distinctions between Primary and Higher-Order Visual Areas. Science 2013, 340, 1239–1242. [Google Scholar] [CrossRef] [PubMed]
- Simi, A.; Studer, M. Developmental Genetic Programs and Activity-Dependent Mechanisms Instruct Neocortical Area Mapping. Curr. Opin. Neurobiol. 2018, 53, 96–102. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, D.D.M. Do Cortical Areas Emerge from a Protocortex? Trends Neurosci. 1989, 12, 400–406. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P. Radial Unit Hypothesis of Neocortical Expansion. Novartis Found. Symp. 2000, 228, 30–45. [Google Scholar] [PubMed]
- Fukuchi-Shimogori, T.; Grove, E.A. Neocortex Patterning by the Secreted Signaling Molecute FGF8. Science 2001, 294, 1071–1074. [Google Scholar] [CrossRef] [PubMed]
- Rakic, P.; Ayoub, A.E.; Breunig, J.J.; Dominguez, M.H. Decision by Division: Making Cortical Maps. Trends Neurosci. 2009, 32, 291–301. [Google Scholar] [CrossRef]
- Kraushar, M.L.; Viljetic, B.; Wijeratne, H.R.S.; Thompson, K.; Jiao, X.; Pike, J.W.; Medvedeva, V.; Groszer, M.; Kiledjian, M.; Hart, R.P.; et al. Thalamic WNT3 Secretion Spatiotemporally Regulates the Neocortical Ribosome Signature and MRNA Translation to Specify Neocortical Cell Subtypes. J. Neurosci. 2015, 35, 10911–10926. [Google Scholar] [CrossRef]
- Luhmann, H.J.; Khazipov, R. Neuronal Activity Patterns in the Developing Barrel Cortex. Neuroscience 2018, 368, 256–267. [Google Scholar] [CrossRef] [PubMed]
- Bystron, I.; Rakic, P.; Molnár, Z.; Blakemore, C. The First Neurons of the Human Cerebral Cortex. Nat. Neurosci. 2006, 9, 880–886. [Google Scholar] [CrossRef] [PubMed]
- Bayatti, N.; Sarma, S.; Shaw, C.; Eyre, J.A.; Vouyiouklis, D.A.; Lindsay, S.; Clowry, G.J. Progressive Loss of PAX6, TBR2, NEUROD and TBR1 MRNA Gradients Correlates with Translocation of EMX2 to the Cortical Plate during Human Cortical Development. Eur. J. Neurosci. 2008, 28, 1449–1456. [Google Scholar] [CrossRef]
- Kostović-Knežević, L.; Kostović, I.; Krmpotić-Nemanić, J.; Kelović, Z.; Vuković, B. The cortical plate of the human neocortex during the early fetal period (at 31–65 mm CRL). Verh. Anat. Ges. 1978, 72, 721–723. [Google Scholar]
- Molnár, Z.; Rockland, K.S. Cortical columns. In Neural Circuit and Cognitive Development, 2nd ed.; Rubenstein, J., Rakic, P., Chen, B., Kwan, K.Y., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 103–126. [Google Scholar]
- Marín-Padilla, M. Ontogenesis of the pyramidal cell of the mammalian neocortex and developmental cytoarchitectonics: A unifying theory. J. Comp. Neurol. 1992, 321, 223–240. [Google Scholar] [CrossRef] [PubMed]
- Brodmann, K. Vergleichende Lokalisationslehre der Grosshirnrinde in Ihren Prinzipien Dargestellt auf Grund des Zellenbaues, 1st ed.; Barth: Leipzig, Germany, 1909. [Google Scholar]
- Bayer, S.A.; Altman, J. The Human Brain During the Early First Trimester, 1st ed.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Kostović, I.; Sedmak, G.; Judaš, M. Neural Histology and Neurogenesis of the Human Fetal and Infant Brain. Neuroimage 2019, 188, 743–773. [Google Scholar] [CrossRef] [PubMed]
- Žunić Išasegi, I.; Radoš, M.; Krsnik, Ž.; Radoš, M.; Benjak, V.; Kostović, I. Interactive Histogenesis of Axonal Strata and Proliferative Zones in the Human Fetal Cerebral Wall. Brain Struct. Funct. 2018, 223, 3919–3943. [Google Scholar] [CrossRef] [PubMed]
- Haydar, T.F.; Wang, F.; Schwartz, M.L.; Rakic, P. Differential Modulation of Proliferation in the Neocortical Ventricular and Subventricular Zones. J. Neurosci. 2000, 20, 5764–5774. [Google Scholar] [CrossRef] [PubMed]
- Haubensak, W.; Attardo, A.; Denk, W.; Huttner, W.B. Neurons Arise in the Basal Neuroepithelium of the Early Mammalian Telencephalon: A Major Site of Neurogenesis. Proc. Natl. Acad. Sci. USA 2004, 101, 3196–3201. [Google Scholar] [CrossRef]
- Vasung, L.; Lepage, C.; Radoš, M.; Pletikos, M.; Goldman, J.S.; Richiardi, J.; Raguž, M.; Fischi-Gómez, E.; Karama, S.; Huppi, P.S.; et al. Quantitative and Qualitative Analysis of Transient Fetal Compartments during Prenatal Human Brain Development. Front. Neuroanat. 2016, 10, 11. [Google Scholar] [CrossRef]
- Osheroff, H.; Hatten, M.E. Gene Expression Profiling of Preplate Neurons Destined for the Subplate: Genes Involved in Transcription, Axon Extension, Neurotransmitter Regulation, Steroid Hormone Signaling, and Neuronal Survival. Cereb. Cortex 2009, 19 (Suppl. S1), 126–134. [Google Scholar] [CrossRef]
- Poliakov, G.I. Structural organization of the human cerebral cortex during ontogenetic development. In Cytoarchitectonics of the Cerebral Cortex in Man, 1st ed.; Sarkisov, A.S., Filimonof, I.N., Preobrazenskaya, N.S., Eds.; Medgiz: Moscow, Russia, 1949; pp. 33–92. [Google Scholar]
- Hevner, R.F. Layer-Specific Markers as Probes for Neuron Type Identity in Human Neocortex and Malformations of Cortical Development. J. Neuropathol. Exp. Neurol. 2007, 66, 101–109. [Google Scholar] [CrossRef]
- Bedogni, F.; Hodge, R.D.; Elsen, G.E.; Nelson, B.R.; Daza, R.A.M.; Beyer, R.P.; Bammler, T.K.; Rubenstein, J.L.R.; Hevner, R.F. Tbr1 Regulates Regional and Laminar Identity of Postmitotic Neurons in Developing Neocortex. Proc. Natl. Acad. Sci. USA 2010, 107, 13129–13134. [Google Scholar] [CrossRef]
- Kostović, I. Structural and histochemical reorganization of the human prefrontal cortex during perinatal and postnatal life. Prog. Brain Res. 1991, 85, 223–240. [Google Scholar]
- Kelava, I.; Reillo, I.; Murayama, A.Y.; Kalinka, A.T.; Stenzel, D.; Tomancak, P.; Matsuzaki, F.; Lebrand, S.E.; Schwamborn, J.C.; Okano, H.; et al. Abundant occurrence of basal radial glia in the subventricular zone of embryonic neocortex of a lissencephalic primate, the common marmoset callithrix jacchus. Cereb. Cortex 2012, 22, 469–481. [Google Scholar] [CrossRef] [PubMed]
- Kalebic, N.; Huttner, W.B. Basal Progenitor Morphology and Neocortex Evolution. Trends Neurosci. 2020, 43, 843–853. [Google Scholar] [CrossRef]
- Smart, I.H.M.; Dehay, C.; Giroud, P.; Berland, M.; Kennedy, H. Unique Morphological Features of the Proliferative Zones and Postmitotic Compartments of the Neural Epithelium Giving Rise to Striate and Extrastriate Cortex in the Monkey. Cereb. Cortex 2002, 12, 37–53. [Google Scholar] [CrossRef]
- Molnár, Z.; Clowry, G. Cerebral Cortical Development in Rodents and Primates. Prog. Brain Res. 2012, 195, 45–70. [Google Scholar]
- Kriegstein, A.; Noctor, S.; Martínez-Cerdeño, V. Patterns of neural stem and progenitor cell division may underlie evolutionary cortical expansion. Nat. Rev. Neurosci. 2006, 7, 883–890. [Google Scholar] [CrossRef]
- Rakic, P. Evolution of the neocortex: A perspective from developmental biology. Nat. Rev. Neurosci. 2009, 10, 724–735. [Google Scholar] [CrossRef]
- Zecevic, N.; Chen, Y.; Filipovic, R. Contributions of cortical subventricular zone to the development of the human cerebral cortex. J. Comp. Neurol. 2005, 491, 109–122. [Google Scholar] [CrossRef]
- DeAzevedo, L.C.; Hedin-Pereira, C.; Lent, R. Callosal neurons in the cingulate cortical plate and subplate of human fetuses. J. Comp. Neurol. 1997, 386, 60–70. [Google Scholar] [CrossRef]
- Sidman, R.L.; Rakic, P. Neuronal Migration, with Special Reference to Developing Human Brain: A Review. Brain Res. 1973, 62, 1–35. [Google Scholar] [CrossRef]
- Kostović, I.; Sedmak, G.; Vukšić, M.; Judaš, M. The Relevance of Human Fetal Subplate Zone for Developmental Neuropathology of Neuronal Migration Disorders and Cortical Dysplasia. CNS Neurosci. Ther. 2015, 21, 74. [Google Scholar] [CrossRef]
- Ohtaka-Maruyama, C. Subplate Neurons as an Organizer of Mammalian Neocortical Development. Front. Neuroanat. 2020, 14, 8. [Google Scholar] [CrossRef]
- Ozair, M.Z.; Kirst, C.; van den Berg, B.L.; Ruzo, A.; Rito, T.; Brivanlou, A.H. HPSC Modeling Reveals That Fate Selection of Cortical Deep Projection Neurons Occurs in the Subplate. Cell Stem Cell 2018, 23, 60–73. [Google Scholar] [CrossRef]
- Rakic, P. Neurons in Rhesus Monkey Visual Cortex: Systematic Relation between Time of Origin and Eventual Disposition. Science 1974, 183, 425–427. [Google Scholar] [CrossRef]
- Meyer, G.; González-Gómez, M. The Subpial Granular Layer and Transient Versus Persisting Cajal-Retzius Neurons of the Fetal Human Cortex. Cereb. Cortex 2018, 28, 2043–2058. [Google Scholar] [CrossRef]
- Bakken, T.E.; Miller, J.A.; Ding, S.L.; Sunkin, S.M.; Smith, K.A.; Ng, L.; Szafer, A.; Dalley, R.A.; Royall, J.J.; Lemon, T.; et al. A Comprehensive Transcriptional Map of Primate Brain Development. Nature 2016, 535, 367–375. [Google Scholar] [CrossRef] [PubMed]
- Berg, J.; Sorensen, S.A.; Ting, J.T.; Miller, J.A.; Chartrand, T.; Buchin, A.; Bakken, T.E.; Budzillo, A.; Dee, N.; Ding, S.L.; et al. Human Neocortical Expansion Involves Glutamatergic Neuron Diversification. Nature 2021, 598, 151–158. [Google Scholar] [CrossRef] [PubMed]
- McConnell, S.K.; Ghosh, A.; Shatz, C.J. Subplate Neurons Pioneer the First Axon Pathway from the Cerebral Cortex. Science 1989, 245, 978–982. [Google Scholar] [CrossRef]
- Kostović, I. The Enigmatic Fetal Subplate Compartment Forms an Early Tangential Cortical Nexus and Provides the Framework for Construction of Cortical Connectivity. Prog. Neurobiol. 2020, 194, 101883. [Google Scholar] [CrossRef]
- Lickiss, T.; Cheung, A.F.P.; Hutchinson, C.E.; Taylor, J.S.H.; Molnár, Z. Examining the relationship between early axon growth and transcription factor expression in the developing cerebral cortex. J. Anat. 2012, 220, 201–211. [Google Scholar] [CrossRef]
- Miller, D.J.; Bhaduri, A.; Sestan, N.; Kriegstein, A. Shared and derived features of cellular diversity in the human cerebral cortex. Curr. Opin. Neurobiol. 2019, 56, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Bayer, S.A.; Altman, J. Development of Layer I and the Subplate in the Rat Neocortex. Exp. Neurol. 1990, 107, 48–62. [Google Scholar] [CrossRef]
- Molnár, Z.; Métin, C.; Stoykova, A.; Tarabykin, V.; Price, D.J.; Francis, F.; Meyer, G.; Dehay, C.; Kennedy, H. Comparative aspects of cerebral cortical development. Eur. J. Neurosci. 2006, 23, 921–934. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.F.; Pollen, A.A.; Tavare, A.; DeProto, J.; Molnár, Z. Comparative aspects of cortical neurogenesis in vertebrates. J. Anat. 2007, 211, 164–176. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.; Skarica, M.; Li, Q.; Xu, C.; Risgaard, R.D.; Tebbenkamp, A.T.N.; Mato-Blanco, X.; Kovner, R.; Krsnik, Ž.; de Martin, X.; et al. Molecular and cellular evolution of the primate dorsolateral prefrontal cortex. Science 2022, 377, eabo7257. [Google Scholar] [CrossRef]
- Van Eden, C.G.; Uylings, H.B.M. Cytoarchitectonic Development of the Prefrontal Cortex in the Rat. J. Comp. Neurol. 1985, 241, 253–267. [Google Scholar] [CrossRef]
- Kostović, I. Prenatal Development of Nucleus Basalis Complex and Related Fiber Systems in Man: A Histochemical Study. Neuroscience 1986, 17, 1047–1063. [Google Scholar] [CrossRef]
- Nobin, A.; Björklund, A. Topography of the Monoamine Neuron Systems in the Human Brain as Revealed in Fetuses. Acta Physiol. Scand. 1973, 988, 1–40. [Google Scholar]
- Zecevic, N.; Verney, C. Development of the Catecholamine Neurons in Human Embryos and Fetuses, with Special Emphasis on the Innervation of the Cerebral Cortex. J. Comp. Neurol. 1995, 351, 509–535. [Google Scholar] [CrossRef]
- Meyer, G.; González-Arnay, E.; Moll, U.; Nemajerova, A.; Tissir, F.; González-Gómez, M. Cajal-Retzius neurons are required for the development of the human hippocampal fissure. J. Anat. 2019, 235, 569–589. [Google Scholar] [CrossRef]
- McKenna, W.L.; Betancourt, J.; Larkin, K.A.; Abrams, B.; Guo, C.; Rubenstein, J.L.R.; Chen, B. Tbr1 and Fezf2 Regulate Alternate Corticofugal Neuronal Identities during Neocortical Development. J. Neurosci. 2011, 31, 549–564. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, Y.; Khalilov, I.; Represa, A.; Gozlan, H. Interneurons Set the Tune of Developing Networks. Trends Neurosci. 2004, 27, 422–427. [Google Scholar] [CrossRef] [PubMed]
- Jakovcevski, I.; Mayer, N.; Zecevic, N. Multiple Origins of Human Neocortical Interneurons Are Supported by Distinct Expression of Transcription Factors. Cereb. Cortex 2010, 21, 1771–1782. [Google Scholar] [CrossRef]
- Ma, T.; Wang, C.; Wang, L.; Zhou, X.; Tian, M.; Zhang, Q.; Zhang, Y.; Li, J.; Liu, Z.; Cai, Y.; et al. Subcortical Origins of Human and Monkey Neocortical Interneurons. Nat. Neurosci. 2013, 16, 1588–1597. [Google Scholar] [CrossRef]
- Bourgeois, J.P.; Goldman-Rakic, P.S.; Rakic, P. Synaptogenesis in the Prefrontal Cortex of Rhesus Monkeys. Cereb. Cortex 1994, 4, 78–96. [Google Scholar] [CrossRef] [PubMed]
- Kostović, I.; Radoš, M.; Kostović-Srzentić, M.; Krsnik, Ž. Fundamentals of the Development of Connectivity in the Human Fetal Brain in Late Gestation: From 24 Weeks Gestational Age to Term. J. Neuropathol. Exp. Neurol. 2021, 80, 393–414. [Google Scholar] [CrossRef] [PubMed]
- Hoerder-Suabedissen, A.; Molnár, Z. Development, Evolution and Pathology of Neocortical Subplate Neurons. Nat. Rev. Neurosci. 2015, 16, 133–146. [Google Scholar] [CrossRef]
- Thomason, M.E.; Grove, L.E.; Lozon, T.A.; Vila, A.M.; Ye, Y.; Nye, M.J.; Manning, J.H.; Pappas, A.; Hernandez-Andrade, E.; Yeo, L.; et al. Age-Related Increases in Long-Range Connectivity in Fetal Functional Neural Connectivity Networks in Utero. Dev. Cogn. Neurosci. 2015, 11, 96–104. [Google Scholar] [CrossRef]
- Kim, J.H.; De Asis-Cruz, J.; Cook, K.M.; Limperopoulos, C. Gestational Age-Related Changes in the Fetal Functional Connectome: In Utero Evidence for the Global Signal. Cereb. Cortex 2022. [Google Scholar] [CrossRef]
- Karolis, V.; Fitzgibbon, S.; Cordero-Grande, L.; Farahibozorg, R.; Price, A.; Hughes, E.; Fetit, A.; Kariakopoulou, V.; Pietsch, M.; Rutherford, M.; et al. Maturational Networks of Fetal Brain Activity. bioRxiv 2022. [Google Scholar] [CrossRef]
- Wess, J.M.; Isaiah, A.; Watkins, P.V.; Kanold, P.O. Subplate Neurons Are the First Cortical Neurons to Respond to Sensory Stimuli. Proc. Natl. Acad. Sci. USA 2017, 114, 12602–12607. [Google Scholar] [CrossRef]
- Rakic, P.; Bourgeois, J.P.; Eckenhoff, M.F.; Zecevic, N.; Goldman-Rakic, P.S. Concurrent Overproduction of Synapses in Diverse Regions of the Primate Cerebral Cortex. Science 1986, 232, 232–235. [Google Scholar] [CrossRef]
- Zecevic, N. Synaptogenesis in layer I of the human cerebral cortex in the first half of gestation. Cereb. Cortex 1998, 8, 245–252. [Google Scholar] [CrossRef]
- Nielsen, A.N.; Kaplan, S.; Meyer, D.; Alexopoulos, D.; Kenley, J.K.; Smyser, T.A.; Wakschlag, L.S.; Norton, E.S.; Raghuraman, N.; Warner, B.B.; et al. Maturation of Large-Scale Brain Systems over the First Month of Life. Cereb. Cortex 2022, bhac242. [Google Scholar] [CrossRef] [PubMed]
- Molnár, Z.; Rutherford, M. Brain Maturation after Preterm Birth. Sci. Transl. Med. 2013, 5, 168. [Google Scholar] [CrossRef] [PubMed]
- Huttenlocher, P.R. Dendritic and synaptic development in human cerebral cortex: Time course and critical periods. Dev. Neuropsychol. 1999, 16, 347–349. [Google Scholar] [CrossRef]
- Weinberger, D.R. Implications of Normal Brain Development for the Pathogenesis of Schizophrenia. Arch. Gen. Psychiatry 1987, 44, 660–669. [Google Scholar] [CrossRef]
- Kostović, I.; Judaš, M.; Sedmak, G. Developmental History of the Subplate Zone, Subplate Neurons and Interstitial White Matter Neurons: Relevance for Schizophrenia. Int. J. Dev. Neurosci. 2011, 29, 193–205. [Google Scholar] [CrossRef] [PubMed]
- Marchand, W.R. Cortico-Basal Ganglia Circuitry: A Review of Key Research and Implications for Functional Connectivity Studies of Mood and Anxiety Disorders. Brain Struct. Funct. 2010, 215, 73–96. [Google Scholar] [CrossRef]









| Primary antibodies | ||||
| Antibody | type | Cat. No | Supplier | Dilution |
| CELF1 (CUGBP1) | mouse monoclonal | sc-20003 | Santa Cruz biotechnology | 1:250 |
| CELF1 (CUGBP1) | rabbit polyclonal | ab129115 | Abcam | 1:1000 |
| TBR1 | rabbit polyclonal | ab31940 | Abcam | 1:150 |
| TBR2 | rabbit polyclonal | ab23345 | Abcam (RT) | 1:200 |
| CTIP2 | rat monoclonal | ab18465 | Abcam | 1:500 |
| TLE4 | mouse monoclonal | sc-365406 | Santa Cruz biotechnology | 1:50 |
| SOX5 | rabbit polyclonal | ab94396 | Abcam | 1:1000 |
| RELN | mouse monoclonal | MAB5366 | Millipore | 1:1000 |
| CALR | mouse monoclonal | 6B3 | Swant | 1:1000 |
| GAD67 | mouse monoclonal | MAB5406 | Millipore | 1:500 |
| DCX | mouse monoclonal | sc-271390 | Santa Cruz biotechnology | 1:50 |
| PAX6 | mouse monoclonal | AMAb91372 | Atlas Antibodies | 1:500 |
| TUBB3 | rabbit polyclonal | PRB-435P | BioLegend | 1:1000 |
| SMI312 | mouse monoclonal | 837901 | BioLegend | 1:1000 |
| Ki67 | mouse monoclonal | M7240 | Dako | 1:50 |
| Secondary antibodies | ||||
| Alexa Fluor 488 | goat anti-rabbit | A-32731 | Thermo Fisher Scientific | 1:1000 |
| Alexa Fluor 555 | goat anti-mouse | A-21422 | Thermo Fisher Scientific | 1:1000 |
| Alexa Fluor 555 | goat anti-rat | A-21434 | Thermo Fisher Scientific | 1:1000 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kopić, J.; Junaković, A.; Salamon, I.; Rasin, M.-R.; Kostović, I.; Krsnik, Ž. Early Regional Patterning in the Human Prefrontal Cortex Revealed by Laminar Dynamics of Deep Projection Neuron Markers. Cells 2023, 12, 231. https://doi.org/10.3390/cells12020231
Kopić J, Junaković A, Salamon I, Rasin M-R, Kostović I, Krsnik Ž. Early Regional Patterning in the Human Prefrontal Cortex Revealed by Laminar Dynamics of Deep Projection Neuron Markers. Cells. 2023; 12(2):231. https://doi.org/10.3390/cells12020231
Chicago/Turabian StyleKopić, Janja, Alisa Junaković, Iva Salamon, Mladen-Roko Rasin, Ivica Kostović, and Željka Krsnik. 2023. "Early Regional Patterning in the Human Prefrontal Cortex Revealed by Laminar Dynamics of Deep Projection Neuron Markers" Cells 12, no. 2: 231. https://doi.org/10.3390/cells12020231
APA StyleKopić, J., Junaković, A., Salamon, I., Rasin, M.-R., Kostović, I., & Krsnik, Ž. (2023). Early Regional Patterning in the Human Prefrontal Cortex Revealed by Laminar Dynamics of Deep Projection Neuron Markers. Cells, 12(2), 231. https://doi.org/10.3390/cells12020231






