Role of Moonlighting Proteins in Disease: Analyzing the Contribution of Canonical and Moonlighting Functions in Disease Progression
Abstract
1. Introduction
2. Methods
3. Results
3.1. Function Relationship with Pathology
3.1.1. Canonical Functions Are the Cause but Moonlighting Functions Are the Effect of the Pathology to a Greater Extent
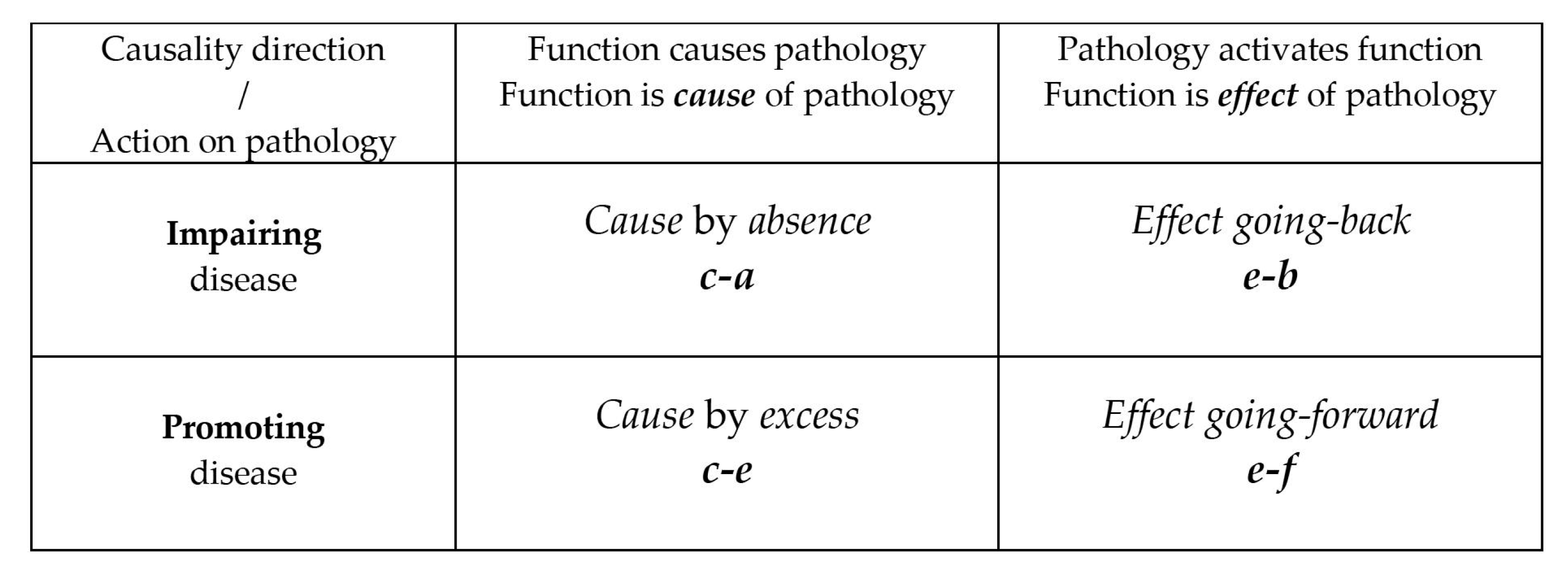
- Class 1—The pathology is caused by the absence of the function (hereinafter summarized as c-a).
- Class 2—The pathology is caused by an excess of function (hereinafter summarized as c-e).
- Class 3—The activation of the function is an effect of the pathology. The function tries to reverse the pathological condition that triggered it (and its consequences), back to the previous non-pathological state (henceforth summarized as e-b).
- Class 4—The activation of the function is an effect of the pathology that requires a more systemic response. The function contributes to the onset of new symptoms going forward in the pathological course (hereinafter summarized as e-f).
3.1.2. Preventive (e–b) Functions Are Predominant at Early Stages and Symptomatic (e–f) Functions at Later Stages of the Pathology
3.2. Dependence among the Multiple Functions of the Proteins in Disease
3.2.1. Mechanisms for Function Activation Mediated by Pathology
Function Activation: Multiple Functions of the Same Protein Are Linked to the Same Pathology, but They Are Activated at Different Stages
Function Activation: The Moonlighting Function Is Activated by Changes in Cellular Localization Mediated by Pathological Conditions
Function Activation: The Moonlighting Function Is Activated by Transcriptional and Post-Transcriptional Changes Mediated by Pathological Conditions
3.2.2. Types of Relationships among Canonical and Moonlighting Functions
Moon-Canonical Link: The Moonlighting Function Tries to Compensate for an Excess of the Canonical Function of the Same Protein
Moon-Moon Link: The Moonlighting Going Back Function Tries to Stop a Moonlighting Going Forward Function of the Same Protein
Moon-Moon Link: The Moonlighting Going Back Function Tries to Stop a Moonlighting Going Forward Function of a Different Protein
Moon-Canonical Link: The Moonlighting Going Forward Function (e–f) Is Activated when the Preventive Function Fails (c–a)
Moon-Canonical Link: Moonlighting Going Back Function Is Activated to Prevent Canonical-Function Failure in Adverse Conditions
3.3. Moonlighting Proteins in Wound-Healing, Cancer-Wound-Healing and Mesenchymal to Epithelial Transition (EMT)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huberts, D.H.; Ivan der Klei, I.J. Moonlighting proteins: An intriguing mode of multitasking. Biochim. Biophys. Acta 2010, 1803, 520–525. [Google Scholar] [CrossRef] [PubMed]
- Copley, S.D. Moonlighting is mainstream: Paradigm adjustment required. Bioessays 2012, 34, 578–588. [Google Scholar] [CrossRef] [PubMed]
- Jeffery, C.J. An introduction to protein moonlighting. Biochem. Soc. Trans. 2014, 42, 1679–1683. [Google Scholar] [CrossRef] [PubMed]
- Singh, N.; Bhalla, N. Moonlighting Proteins. Annu. Rev. Genet. 2020, 54, 265–285. [Google Scholar] [CrossRef]
- Franco-Serrano, L.; Hernandez, S.; Calvo, A.; Severi, M.A.; Ferragut, G.; Perez-Pons, J.; Pinol, J.; Pich, O.; Mozo-Villarias, A.; Amela, I.; et al. MultitaskProtDB-II: An update of a database of multitasking/moonlighting proteins. Nucleic Acids Res. 2018, 46, D645–D648. [Google Scholar] [CrossRef]
- Chen, C.; Liu, H.; Zabad, S.; Rivera, N.; Rowin, E.; Hassan, M.; De Jesus, S.M.G.; Santos, P.S.L.; Kravchenko, K.; Mikhova, M.; et al. MoonProt 3.0: An update of the moonlighting proteins database. Nucleic Acids Res. 2021, 49, D368–D372. [Google Scholar] [CrossRef]
- Ribeiro, D.; Briere, G.; Bely, B.; Spinelli, L.; Brun, C. MoonDB 2.0: An updated database of extreme multifunctional and moonlighting proteins. Nucleic Acids Res. 2019, 47, D398–D402. [Google Scholar] [CrossRef]
- Franco-Serrano, L.; Huerta, M.; Hernández, S.; Cedano, J.; Perez-Pons, J.; Piñol, J.; Mozo-Villarias, A.; Amela, I.; Querol, E. Multifunctional Proteins: Involvement in Human Diseases and Targets of Current Drugs. Protein J. 2018, 37, 444–453. [Google Scholar] [CrossRef]
- Hernández, S.; Ferragut, G.; Amela, I.; Perez-Pons, J.; Piñol, J.; Mozo-Villarias, A.; Cedano, J.; Querol, E. MultitaskProtDB: A database of multitasking proteins. Nucleic Acids Res. 2014, 42, D517–D520. [Google Scholar] [CrossRef]
- Sriram, G.; Martinez, J.A.; McCabe, E.R.; Liao, J.C.; Dipple, K.M. Single-gene disorders: What role could moonlighting enzymes play? Am. J. Hum. Genet. 2005, 76, 911–924. [Google Scholar] [CrossRef]
- Ovádi, J. Moonlighting proteins in neurological disorders. IUBMB Life 2011, 63, 453–456. [Google Scholar] [CrossRef]
- Jeffery, C.J. Proteins with neomorphic moonlighting functions in disease. IUBMB Life 2011, 63, 489–494. [Google Scholar] [CrossRef]
- Rybinski, B.; Franco-Barraza, J.; Cukierman, E. The wound healing, chronic fibrosis, and cancer progression triad. Physiol. Genom. 2014, 46, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Hamosh, A.; Amberger, J.S.; Bocchini, C.F.; Scott, A.; Rasmussen, S.A. Online Mendelian Inheritance in Man (OMIM(R)): Victor McKusick’s magnum opus. Am. J. Med. Genet. A 2021, 185, 3259–3265. [Google Scholar] [CrossRef] [PubMed]
- Maffucci, P.; Bigio, B.; Rapaport, F.; Cobat, A.; Borghesi, A.; Lopez, M.; Patin, E.; Bolze, A.; Shang, L.; Bendavid, M.; et al. Blacklisting variants common in private cohorts but not in public databases optimizes human exome analysis. Proc. Natl. Acad. Sci. USA 2019, 116, 950–959. [Google Scholar] [CrossRef]
- Dekoninck, S.; Blanpain, C. Stem cell dynamics, migration and plasticity during wound healing. Nature 2019, 21, 18–24. [Google Scholar] [CrossRef]
- Ge, Y.; Fuchs, E. Stretching the limits: From homeostasis to stem cell plasticity in wound healing and cancer. Nat. Rev. Genet. 2018, 19, 311–325. [Google Scholar] [CrossRef]
- Thorsson, V.; Gibbs, D.L.; Brown, S.D.; Wolf, D.; Bortone, D.S.; Ou Yang, T.-H.; Porta-Pardo, E.; Gao, G.F.; Plaisier, C.L.; Eddy, J.A.; et al. The Immune Landscape of Cancer. Immunity 2018, 48, 812–830.e14. [Google Scholar] [CrossRef]
- MacCarthy-Morrogh, L.; Martin, P. The hallmarks of cancer are also the hallmarks of wound healing. Sci. Signal. 2020, 13, eaay8690. [Google Scholar] [CrossRef]
- Rayagiri, S.S.; Ranaldi, D.; Raven, A.; Azhar, N.I.F.M.; Lefebvre, O.; Zammit, P.S.; Borycki, A.-G. Basal lamina remodeling at the skeletal muscle stem cell niche mediates stem cell self-renewal. Nat. Commun. 2018, 9, 1075. [Google Scholar] [CrossRef] [PubMed]
- Huerta, M.; Fernández-Márquez, J.; Cabello, J.L.; Medrano, A.; Querol, E.; Cedano, J. Analysis of gene expression for studying tumor progression: The case of glucocorticoid administration. Gene 2014, 549, 33–40. [Google Scholar] [CrossRef] [PubMed]
- Bustos, S.P.; Reithmeier, R.A.F. Protein 4.2 interaction with hereditary spherocytosis mutants of the cytoplasmic domain of human anion exchanger 1. Biochem. J. 2011, 433, 313–322. [Google Scholar] [CrossRef] [PubMed]
- Mora-Santos, M.; Castilla, C.; Herrero-Ruiz, J.; Giráldez, S.; Limón-Mortés, M.C.; Sáez, C.; Japón, M.; Tortolero, M.; Romero, F. A single mutation in Securin induces chromosomal instability and enhances cell invasion. Eur. J. Cancer 2013, 49, 500–510. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Li, C.; Elnwasany, A.; Sharma, G.; An, Y.A.; Zhang, G.; Elhelaly, W.M.; Lin, J.; Gong, Y.; Chen, G.; et al. PKM1 Exerts Critical Roles in Cardiac Remodeling Under Pressure Overload in the Heart. Circulation 2021, 144, 712–727. [Google Scholar] [CrossRef] [PubMed]
- Fan, H.C.; Chang, F.W.; Tsai, J.D.; Lin, K.M.; Chen, C.Z.; Lin, S.; Liu, C.A.; Harn, H.J. Telomeres and Cancer. Life 2021, 11, 1405. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; You, K.; Chen, C.; Zhong, H.; Jiang, Y.; Mo, H.; Song, J.; Qiu, X.; Liu, Y. High Pretreatment LDH Predicts Poor Prognosis in Hypopharyngeal Cancer. Front. Oncol. 2021, 11, 1075. [Google Scholar] [CrossRef]
- Hou, H.; Sun, D.; Zhang, X. The role of MDM2 amplification and overexpression in therapeutic resistance of malignant tumors. Cancer Cell Int. 2019, 19, 216. [Google Scholar] [CrossRef]
- Pinney, S.E.; Ganapathy, K.; Bradfield, J.; Stokes, D.; Sasson, A.; Mackiewicz, K.; Boodhansingh, K.; Hughes, N.; Becker, S.; Givler, S.; et al. Dominant Form of Congenital Hyperinsulinism Maps to HK1 Region on 10q. Horm. Res. Paediatr. 2013, 80, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Lackie, R.E.; Maciejewski, A.; Ostapchenko, V.G.; Marques-Lopes, J.; Choy, W.Y.; Duennwald, M.L.; Prado, V.F.; Prado, M.A.M. The Hsp70/Hsp90 Chaperone Machinery in Neurodegenerative Diseases. Front. Neurosci. 2017, 11, 254. [Google Scholar] [CrossRef]
- Hancock, D.B.; Martin, E.R.; Vance, J.M.; Scott, W.K. Nitric oxide synthase genes and their interactions with environmental factors in Parkinson’s disease. Neurogenetics 2008, 9, 249–262. [Google Scholar] [CrossRef]
- Davis, A.K.; Pratt, W.B.; Lieberman, A.P.; Osawa, Y. Targeting Hsp70 facilitated protein quality control for treatment of polyglutamine diseases. Cell. Mol. Life Sci. 2020, 77, 977–996. [Google Scholar] [CrossRef] [PubMed]
- Kaliatsi, E.G.; Argyriou, A.I.; Bouras, G.; Apostolidi, M.; Konstantinidou, P.; Shaukat, A.-N.; Spyroulias, G.A.; Stathopoulos, C. Functional and Structural Aspects of La Protein Overexpression in Lung Cancer. J. Mol. Biol. 2020, 432, 166712. [Google Scholar] [CrossRef] [PubMed]
- Cole-Ezea, P.; Swan, D.; Shanley, D.; Hesketh, J. Glutathione peroxidase 4 has a major role in protecting mitochondria from oxidative damage and maintaining oxidative phosphorylation complexes in gut epithelial cells. Free. Radic. Biol. Med. 2012, 53, 488–497. [Google Scholar] [CrossRef] [PubMed]
- Savina, N.V.; Nikitchenko, N.V.; Kuzhir, T.D.; Rolevich, A.I.; Krasny, S.A.; Goncharova, R.I. The Involvement of ERCC2/XPD and ERCC6/CSB Wild Type Alleles in Protection Against Aging and Cancer. Curr. Aging Sci. 2018, 11, 45–54. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Torras, S.; Vidal-Pla, A.; Cano-Soldado, P.; Huber-Ruano, I.; Mazo, A.; Pastor-Anglada, M. Concentrative nucleoside transporter 1 (hCNT1) promotes phenotypic changes relevant to tumor biology in a translocation-independent manner. Cell Death Dis. 2013, 4, e648. [Google Scholar] [CrossRef]
- Hajra, K.M.; Liu, J.R. Apoptosome dysfunction in human cancer. Apoptosis 2004, 9, 691–704. [Google Scholar] [CrossRef]
- Yip, J.; Wang, S.; Tan, J.; Lim, T.K.; Lin, Q.; Yu, Z.; Karmon, O.; Pines, O.; Lehming, N. Fumarase affects the deoxyribonucleic acid damage response by protecting the mitochondrial desulfurase Nfs1p from modification and inactivation. Iscience 2021, 24, 103354. [Google Scholar] [CrossRef]
- Singh, R.N.; Howell, M.D.; Ottesen, E.W.; Singh, N.N. Diverse role of survival motor neuron protein. Biochim. Biophys. Acta (BBA)-Gene Regul. Mech. 2017, 1860, 299–315. [Google Scholar] [CrossRef]
- Weng, T.-Y.; Wang, C.-Y.; Hung, Y.-H.; Chen, W.-C.; Chen, Y.-L.; Lai, M.-D. Differential Expression Pattern of THBS1 and THBS2 in Lung Cancer: Clinical Outcome and a Systematic-Analysis of Microarray Databases. PLoS ONE 2016, 11, e0161007. [Google Scholar] [CrossRef]
- Zhu, X.; Jiang, J.; Shen, H.; Wang, H.; Zong, H.; Li, Z.; Yang, Y.; Niu, Z.; Liu, W.; Chen, X.; et al. Elevated β1,4-Galactosyltransferase I in Highly Metastatic Human Lung Cancer Cells. J. Biol. Chem. 2005, 280, 12503–12516. [Google Scholar] [CrossRef]
- di Salvo, M.L.; Contestabile, R.; Paiardini, A.; Maras, B. Glycine consumption and mitochondrial serine hydroxymethyltransferase in cancer cells: The heme connection. Med. Hypotheses 2013, 80, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Massague, J.; Gomis, R.R. The logic of TGFbeta signaling. FEBS Lett. 2006, 580, 2811–2820. [Google Scholar] [CrossRef]
- Herrero, A.; Rojas, E.; Misiewicz-Krzeminska, I.; Krzeminski, P.; Gutiérrez, N. Molecular Mechanisms of p53 Deregulation in Cancer: An Overview in Multiple Myeloma. Int. J. Mol. Sci. 2016, 17, 2003. [Google Scholar] [CrossRef] [PubMed]
- Showeil, R.; Romano, C.; Valganon, M.; Lambros, M.; Trivedi, P.; Van Noorden, S.; Sriraksa, R.; El-Kaffash, D.; El-Etreby, N.; Natrajan, R.; et al. The status of epidermal growth factor receptor in borderline ovarian tumours. Oncotarget 2016, 7, 10568–10577. [Google Scholar] [CrossRef] [PubMed]
- Raab, M.S.; Breitkreutz, I.; Tonon, G.; Zhang, J.; Hayden, P.J.; Nguyen, T.; Fruehauf, J.H.; Lin, B.K.; Chauhan, D.; Hideshima, T.; et al. Targeting PKC: A novel role for beta-catenin in ER stress and apoptotic signaling. Blood 2009, 113, 1513–1521. [Google Scholar] [CrossRef] [PubMed]
- Du, W.; Liu, X.; Fan, G.; Zhao, X.; Sun, Y.; Wang, T.; Zhao, R.; Wang, G.; Zhao, C.; Zhu, Y.; et al. From cell membrane to the nucleus: An emerging role of E-cadherin in gene transcriptional regulation. J. Cell. Mol. Med. 2014, 18, 1712–1719. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Lee, A.S. The critical roles of endoplasmic reticulum chaperones and unfolded protein response in tumorigenesis and anticancer therapies. Oncogene 2013, 32, 805–818. [Google Scholar] [CrossRef] [PubMed]
- Tang, D.; Kang, R.; Zeh, H.J., 3rd; Lotze, M.T. High-mobility group box 1, oxidative stress, and disease. Antioxid. Redox Signal. 2011, 14, 1315–1335. [Google Scholar] [CrossRef]
- Qu, X.; Wang, C.; Zhang, J.; Qie, G.; Zhou, J. The Roles of CD147 and/or Cyclophilin A in Kidney Diseases. Mediat. Inflamm. 2014, 2014, 728673. [Google Scholar] [CrossRef]
- Motzik, A.; Nechushtan, H.; Foo, S.Y.; Razin, E. Non-canonical roles of lysyl-tRNA synthetase in health and disease. Trends. Mol. Med. 2013, 19, 726–731. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Faigle, M.; Knapp, S.; Karhausen, J.; Ibla, J.; Rosenberger, P.; Odegard, K.C.; Laussen, P.C.; Thompson, L.F.; Colgan, S.P. Endothelial catabolism of extracellular adenosine during hypoxia: The role of surface adenosine deaminase and CD26. Blood 2006, 108, 1602–1610. [Google Scholar] [CrossRef]
- Deák, M.; Hornung, Á.; Novák, J.; Demydenko, D.; Szabó, E.; Czibula, Á.; Fajka-Boja, R.; Kriston-Pál, É.; Monostori, É.; Kovács, L. Novel role for galectin-1 in T-cells under physiological and pathological conditions. Immunobiology 2015, 220, 483–489. [Google Scholar] [CrossRef]
- Camby, I.; Le Mercier, M.; Lefranc, F.; Kiss, R. Galectin-1: A small protein with major functions. Glycobiology 2006, 16, 137R–157R. [Google Scholar] [CrossRef] [PubMed]
- Thomas, G.E.; Egan, G.; García-Prat, L.; Botham, A.; Voisin, V.; Patel, P.S.; Hoff, F.W.; Chin, J.; Nachmias, B.; Kaufmann, K.B.; et al. The metabolic enzyme hexokinase 2 localizes to the nucleus in AML and normal haematopoietic stem and progenitor cells to maintain stemness. Nat. Cell Biol. 2022, 24, 872–884. [Google Scholar] [CrossRef] [PubMed]
- Cho-Vega, J.H.; Tsavachidis, S.; Do, K.-A.; Nakagawa, J.; Medeiros, L.J.; McDonnell, T.J. Dicarbonyl/l-Xylulose Reductase: A Potential Biomarker Identified by Laser-Capture Microdissection-Micro Serial Analysis of Gene Expression of Human Prostate Adenocarcinoma. Cancer Epidemiology Biomarkers Prev. 2007, 16, 2615–2622. [Google Scholar] [CrossRef][Green Version]
- Kang, J.; Brajanovski, N.; Chan, K.T.; Xuan, J.; Pearson, R.B.; Sanij, E. Ribosomal proteins and human diseases: Molecular mechanisms and targeted therapy. Signal Transduct. Target. Ther. 2021, 6, 323. [Google Scholar] [CrossRef]
- Zahra, K.; Dey, T.; Ashish; Mishra, S.P.; Pandey, U. Pyruvate Kinase M2 and Cancer: The Role of PKM2 in Promoting Tumorigenesis. Front. Oncol. 2020, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Piacentini, M.; D’Eletto, M.; Farrace, M.G.; Rodolfo, C.; Del Nonno, F.; Ippolito, G.; Falasca, L. Characterization of distinct sub-cellular location of transglutaminase type II: Changes in intracellular distribution in physiological and pathological states. Cell Tissue Res. 2014, 358, 793–805. [Google Scholar] [CrossRef]
- Min, K.W.; Liggett, J.L.; Silva, G.; Wu, W.W.; Wang, R.F.; Shen, R.F.; Eling, T.E.; Baek, S.J. NAG-1/GDF15 accumulates in the nucleus and modulates transcriptional regulation of the Smad pathway. Oncogene 2016, 35, 377–388. [Google Scholar] [CrossRef]
- Chandra, M.; Zang, S.; Li, H.; Zimmerman, L.J.; Champer, J.; Tsuyada, A.; Chow, A.; Zhou, W.; Yu, Y.; Gao, H.; et al. Nuclear translocation of type I transforming growth factor beta receptor confers a novel function in RNA processing. Mol. Cell. Biol. 2012, 32, 2183–2195. [Google Scholar] [CrossRef]
- Lo, H.-W.; Hung, M.-C. Nuclear EGFR signalling network in cancers: Linking EGFR pathway to cell cycle progression, nitric oxide pathway and patient survival. Br. J. Cancer 2006, 94, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Shang, S.; Hua, F.; Hu, Z.W. The regulation of beta-catenin activity and function in cancer: Therapeutic opportunities. Oncotarget 2017, 8, 33972–33989. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Y.; Yu, T.; Zhang, N.; Chen, J.; Zhang, P.; Li, S.; Luo, L.; Cui, Z.; Qin, Y.; Liu, F. Nuclear E-Cadherin Acetylation Promotes Colorectal Tumorigenesis via Enhancing beta-Catenin Activity. Mol. Cancer Res. 2019, 17, 655–665. [Google Scholar] [CrossRef] [PubMed]
- North, M.L.; Amatullah, H.; Khanna, N.; Urch, B.; Grasemann, H.; Silverman, F.; Scott, J.A. Augmentation of arginase 1 expression by exposure to air pollution exacerbates the airways hyperresponsiveness in murine models of asthma. Respir. Res. 2011, 12, 19. [Google Scholar] [CrossRef]
- Rodriguez, P.C.; Hernandez, C.P.; Quiceno, D.; Dubinett, S.M.; Zabaleta, J.; Ochoa, J.B.; Gilbert, J.; Ochoa, A.C. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 2005, 202, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Xie, Y.; Fan, J.; Sui, L.; Xu, Y.; Zhang, N.; Ma, Y.; Li, Y.; Kong, Y. HCG induces β1,4-GalT I expression and promotes embryo implantation. Int. J. Clin. Exp. Pathol. 2015, 8, 4673–4683. [Google Scholar]
- Zhang, Y.; Wang, J.; Yuan, Y.; Zhang, W.; Guan, W.; Wu, Z.; Jin, C.; Chen, H.; Zhang, L.; Yang, X.; et al. Negative regulation of HDM2 to attenuate p53 degradation by ribosomal protein L26. Nucleic Acids Res. 2010, 38, 6544–6554. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T.; Mager, A.; Küper, N.; Karcher, C.; Weissmüller, T.; Boengler, K.; Schulz, R.; Robson, S.C.; Colgan, S.P. ATP Release From Activated Neutrophils Occurs via Connexin 43 and Modulates Adenosine-Dependent Endothelial Cell Function. Circ. Res. 2006, 99, 1100–1108. [Google Scholar] [CrossRef]
- Santiago, J.-J.; McNaughton, L.J.; Koleini, N.; Ma, X.; Bestvater, B.; Nickel, B.E.; Fandrich, R.R.; Wigle, J.; Freed, D.H.; Arora, R.C.; et al. High Molecular Weight Fibroblast Growth Factor-2 in the Human Heart Is a Potential Target for Prevention of Cardiac Remodeling. PLoS ONE 2014, 9, e97281. [Google Scholar] [CrossRef]
- Li, L.; Zhang, Y.; Qiao, J.; Yang, J.J.; Liu, Z.-R. Pyruvate Kinase M2 in Blood Circulation Facilitates Tumor Growth by Promoting Angiogenesis. J. Biol. Chem. 2014, 289, 25812–25821. [Google Scholar] [CrossRef]
- Joruiz, S.M.; Bourdon, J.-C. p53 Isoforms: Key Regulators of the Cell Fate Decision. Cold Spring Harb. Perspect. Med. 2016, 6, a026039. [Google Scholar] [CrossRef] [PubMed]
- Min, K.-W.; Lee, S.-H.; Baek, S.J. Moonlighting proteins in cancer. Cancer Lett. 2016, 370, 108–116. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Jiang, Y.; Barnes, R.H., 2nd; Tokunaga, M.; Martinez-Santibanez, G.; Geletka, L.; Lumeng, C.N.; Buchner, D.A.; Chun, T. Thrombospondin 1 mediates high-fat diet-induced muscle fibrosis and insulin resistance in male mice. Endocrinology 2013, 154, 4548–4559. [Google Scholar] [CrossRef]
- Sanchez-Cuellar, S.; de la Fuente, H.; Cruz-Adalia, A.; Lamana, A.; Cibrian, D.; Giron, R.M.; Vara, A.; Sanchez-Madrid, F.; Ancochea, J. Reduced expression of galectin-1 and galectin-9 by leucocytes in asthma patients. Clin. Exp. Immunol. 2012, 170, 365–374. [Google Scholar] [CrossRef] [PubMed]
- Bosch, N.M.; Navarro, P. Targeting Galectin-1 in pancreatic cancer: Immune surveillance on guard. Oncoimmunology 2014, 3, e952201. [Google Scholar] [CrossRef] [PubMed]
- Zhang, M.; Wei, J.; Shan, H.; Wang, H.; Zhu, Y.; Xue, J.; Lin, L.; Yan, R. Calreticulin-STAT3 Signaling Pathway Modulates Mitochondrial Function in a Rat Model of Furazolidone-Induced Dilated Cardiomyopathy. PLoS ONE 2013, 8, e66779. [Google Scholar] [CrossRef]
- Gold, L.I.; Eggleton, P.; Sweetwyne, M.T.; Van Duyn, L.B.; Greives, M.R.; Naylor, S.M.; Michalak, M.; Murphy-Ullrich, J.E. Calreticulin: Non-endoplasmic reticulum functions in physiology and disease. FASEB J. 2010, 24, 665–683. [Google Scholar] [CrossRef]
- Kazama, H.; Ricci, J.E.; Herndon, J.M.; Hoppe, G.; Green, D.R.; Ferguson, T.A. Induction of Immunological Tolerance by Apoptotic Cells Requires Caspase-Dependent Oxidation of High-Mobility Group Box-1 Protein. Immunity 2008, 29, 21–32. [Google Scholar] [CrossRef]
- Nam, S.H.; Kang, M.; Ryu, J.; Kim, H.J.; Kim, D.G.; Kim, D.; Kwon, N.H.; Kim, S.; Lee, J.W. Suppression of lysyl-tRNA synthetase, KRS, causes incomplete epithelial-mesenchymal transition and ineffective cellextracellular matrix adhesion for migration. Int. J. Oncol. 2016, 48, 1553–1560. [Google Scholar] [CrossRef]
- Karmali, P.P.; Brunquell, C.; Tram, H.; Ireland, S.K.; Ruoslahti, E.; Biliran, H. Metastasis of Tumor Cells Is Enhanced by Downregulation of Bit1. PLoS ONE 2011, 6, e23840. [Google Scholar] [CrossRef]
- Song, G.; Xu, S.; Zhang, H.; Wang, Y.; Xiao, C.; Jiang, T.; Wu, L.; Zhang, T.; Sun, X.; Zhong, L.; et al. TIMP1 is a prognostic marker for the progression and metastasis of colon cancer through FAK-PI3K/AKT and MAPK pathway. J. Exp. Clin. Cancer Res. 2016, 35, 148. [Google Scholar] [CrossRef]
- Lee, H.; Overall, C.M.; McCulloch, C.A.; Sodek, J. A Critical Role for the Membrane-type 1 Matrix Metalloproteinase in Collagen Phagocytosis. Mol. Biol. Cell 2006, 17, 4812–4826. [Google Scholar] [CrossRef] [PubMed]
- Illemann, M.; Eefsen, R.H.L.; Bird, N.C.; Majeed, A.; Osterlind, K.; Laerum, O.D.; Alpízar-Alpízar, W.; Lund, I.K.; Høyer-Hansen, G. Tissue inhibitor of matrix metalloproteinase-1 expression in colorectal cancer liver metastases is associated with vascular structures. Mol. Carcinog. 2016, 55, 193–208. [Google Scholar] [CrossRef]
- Landstein, D.; Ulmansky, R.; Naparstek, Y. HSP60—A double edge sword in autoimmunity. Oncotarget 2015, 6, 32299–32300. [Google Scholar] [CrossRef] [PubMed]
- Cappello, F.; Zummo, G. HSP60 expression during carcinogenesis: A molecular "proteus" of carcinogenesis? Cell Stress Chaperones 2005, 10, 263–264. [Google Scholar] [CrossRef]
- Bhat, K.P.; Itahana, K.; Jin, A.; Zhang, Y. Essential role of ribosomal protein L11 in mediating growth inhibition-induced p53 activation. EMBO J. 2004, 23, 2402–2412. [Google Scholar] [CrossRef] [PubMed]
- Ocana, G.J.; Sims, E.K.; Watkins, R.; Ragg, S.; Mather, K.J.; Oram, R.G.; Mirmira, R.; DiMeglio, L.A.; Blum, J.S.; Evans-Molina, C. Analysis of serum Hsp90 as a potential biomarker of beta cell autoimmunity in type 1 diabetes. PLoS ONE 2019, 14, e0208456. [Google Scholar] [CrossRef]
- Gao, F.; Hu, X.Y.; Xie, X.J.; Xu, Q.Y.; Wang, Y.P.; Liu, X.B.; Xiang, M.X.; Sun, Y.; Wang, J. Heat shock protein 90 protects rat mesenchymal stem cells against hypoxia and serum deprivation-induced apoptosis via the PI3K/Akt and ERK1/2 pathways. J. Zhejiang Univ. Sci. B 2010, 11, 608–617. [Google Scholar] [CrossRef]
- Luengo, T.M.; Mayer, M.P.; Rüdiger, S.G. The Hsp70–Hsp90 Chaperone Cascade in Protein Folding. Trends Cell Biol. 2019, 29, 164–177. [Google Scholar] [CrossRef]
- Zhang, J.-Y.; Zhang, F.; Hong, C.-Q.; Giuliano, A.E.; Cui, X.-J.; Zhou, G.-J.; Zhang, G.-J.; Cui, Y.-K. Critical protein GAPDH and its regulatory mechanisms in cancer cells. Cancer Biol. Med. 2015, 12, 10–22. [Google Scholar] [CrossRef]
- Fésüs, L.; Szondy, Z. Transglutaminase 2 in the balance of cell death and survival. FEBS Lett. 2005, 579, 3297–3302. [Google Scholar] [CrossRef] [PubMed]
- Nurminskaya, M.; Recheis, B.; Nimpf, J.; Magee, C.; Linsenmayer, T. Transglutaminase factor XIIIA in the cartilage of developing avian long bones. Dev. Dyn. 2002, 223, 24–32. [Google Scholar] [CrossRef]
- Chen, R.; Kang, R.; Tang, D. The mechanism of HMGB1 secretion and release. Exp. Mol. Med. 2022, 54, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, D.; Poulogiannis, G.; Asara, J.M.; Boxer, M.B.; Jiang, J.-K.; Shen, M.; Bellinger, G.; Sasaki, A.T.; Locasale, J.W.; Auld, D.S.; et al. Inhibition of Pyruvate Kinase M2 by Reactive Oxygen Species Contributes to Cellular Antioxidant Responses. Science 2011, 334, 1278–1283. [Google Scholar] [CrossRef] [PubMed]
- Saleme, B.; Gurtu, V.; Zhang, Y.; Kinnaird, A.; Boukouris, A.E.; Gopal, K.; Ussher, J.R.; Sutendra, G. Tissue-specific regulation of p53 by PKM2 is redox dependent and provides a therapeutic target for anthracycline-induced cardiotoxicity. Sci. Transl. Med. 2019, 11, eaau8866. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wu, L.; Wang, J.; Li, G.; Feng, D.; Zhang, B.; Li, L.; Yang, J.; Ma, L.; Qin, H. Opposing Effects of PI3K/Akt and Smad-Dependent Signaling Pathways in NAG-1-Induced Glioblastoma Cell Apoptosis. PLoS ONE 2014, 9, e96283. [Google Scholar] [CrossRef]
- Conery, A.R.; Cao, Y.; Thompson, E.A.; Townsend, C.M., Jr.; Ko, T.C.; Luo, K. Akt interacts directly with Smad3 to regulate the sensitivity to TGF-beta induced apoptosis. Nat. Cell. Biol. 2004, 6, 366–372. [Google Scholar] [CrossRef]
- Pang, X.; Tang, Y.L.; Liang, X.H. Transforming growth factor-beta signaling in head and neck squamous cell carcinoma: Insights into cellular responses. Oncol. Lett. 2018, 16, 4799–4806. [Google Scholar]
- Butera, G.; Mullappilly, N.; Masetto, F.; Palmieri, M.; Scupoli, M.T.; Pacchiana, R.; Donadelli, M. Regulation of Autophagy by Nuclear GAPDH and Its Aggregates in Cancer and Neurodegenerative Disorders. Int. J. Mol. Sci. 2019, 20, 2062. [Google Scholar] [CrossRef]
- Mehta, K.; Han, A. Tissue Transglutaminase (TG2)-Induced Inflammation in Initiation, Progression, and Pathogenesis of Pancreatic Cancer. Cancers 2011, 3, 897–912. [Google Scholar] [CrossRef]
- Neve, R.; Chang, C.-H.; Scott, G.K.; Wong, A.; Friis, R.R.; Hynes, N.E.; Benz, C.C. The epithelium-specific Ets transcription factor ESX is associated with mammary gland development and involution. FASEB J. 1998, 12, 1541–1550. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, L.; Jin, L.; Yi, X.; Dang, E.; Yang, Y.; Li, C.; Gao, T. Oxidative Stress–Induced Calreticulin Expression and Translocation: New Insights into the Destruction of Melanocytes. J. Investig. Dermatol. 2014, 134, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Janko, C.; Filipovic, M.; Munoz, L.; Schorn, C.; Schett, G.; Ivanović-Burmazović, I.; Herrmann, M. Redox Modulation of HMGB1-Related Signaling. Antioxidants Redox Signal. 2014, 20, 1075–1085. [Google Scholar] [CrossRef]
- Multhoff, G.; Pockley, A.; Streffer, C.; Gaipl, U. Dual Role of Heat Shock Proteins (HSPs) in Anti-Tumor Immunity. Curr. Mol. Med. 2012, 12, 1174–1182. [Google Scholar] [CrossRef]
- Borges, T.J.; Wieten, L.; van Herwijnen, M.J.; Broere, F.; van der Zee, R.; Bonorino, C.; van Eden, W. The anti-inflammatory mechanisms of Hsp70. Front. Immunol. 2012, 3, 95. [Google Scholar] [CrossRef] [PubMed]
- Gammazza, A.M.; Tomasello, G.; Leone, A.; Jurjus, A. Hsp60 in Inflammatory Disorders. In Heat Shock Protein 60 in Human Diseases and Disorders; Springer: Cham, Switzerland, 2019; pp. 167–178. [Google Scholar] [CrossRef]
- Kumar, S.; Mehta, K. Tissue transglutaminase constitutively activates HIF-1alpha promoter and nuclear factor-kappaB via a non-canonical pathway. PLoS ONE 2012, 7, e49321. [Google Scholar] [CrossRef] [PubMed]
- Rudders, S.; Gaspar, J.; Madore, R.; Voland, C.; Grall, F.; Patel, A.; Pellacani, A.; Perrella, M.A.; Libermann, T.A.; Oettgen, P. ESE-1 is a novel transcriptional mediator of inflammation that interacts with NF-kappa B to regulate the inducible nitric-oxide synthase gene. J. Biol. Chem. 2001, 276, 3302–3309. [Google Scholar] [CrossRef]
- Grall, F.T.; Prall, W.C.; Wei, W.; Gu, X.; Cho, J.-Y.; Choy, B.K.; Zerbini, L.F.; Inan, M.S.; Goldring, S.R.; Gravallese, E.M.; et al. The Ets transcription factor ESE-1 mediates induction of the COX-2 gene by LPS in monocytes. FEBS J. 2005, 272, 1676–1687. [Google Scholar] [CrossRef]
- Kaur, A.; Raghavan, M. A Calreticulin Tail: C-terminal Mutants of Calreticulin Allow Cancer Cells to Evade Phagocytosis. Mol. Cell 2020, 77, 683–685. [Google Scholar] [CrossRef]
- Katsuno, Y.; Qin, J.; Oses-Prieto, J.; Wang, H.; Jackson-Weaver, O.; Zhang, T.; Lamouille, S.; Wu, J.; Burlingame, A.; Xu, J.; et al. Arginine methylation of SMAD7 by PRMT1 in TGF-beta-induced epithelial-mesenchymal transition and epithelial stem-cell generation. J. Biol. Chem. 2018, 293, 13059–13072. [Google Scholar] [CrossRef]
- Gordeeva, O. TGFbeta Family Signaling Pathways in Pluripotent and Teratocarcinoma Stem Cells’ Fate Decisions: Balancing Between Self-Renewal, Differentiation, and Cancer. Cells 2019, 8. [Google Scholar]
- Xu, J.; Lamouille, S.; Derynck, R. TGF-beta-induced epithelial to mesenchymal transition. Cell Res. 2009, 19, 156–172. [Google Scholar] [CrossRef]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of beta-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef] [PubMed]
- Nurminskaya, M.V.; Belkin, A.M. Cellular Functions of Tissue Transglutaminase. Int. Rev. Cell. Mol. Biol. 2012, 294, 1–97. [Google Scholar] [CrossRef] [PubMed]
- Ayinde, O.; Wang, Z.; Pinton, G.; Moro, L.; Griffin, M. Transglutaminase 2 maintains a colorectal cancer stem phenotype by regulating epithelial-mesenchymal transition. Oncotarget 2019, 10, 4556–4569. [Google Scholar] [CrossRef] [PubMed]
- Manavathi, B.; Rayala, S.; Kumar, R. Phosphorylation-dependent Regulation of Stability and Transforming Potential of ETS Transcriptional Factor ESE-1 by p21-activated Kinase 1. J. Biol. Chem. 2007, 282, 19820–19830. [Google Scholar] [CrossRef] [PubMed]
- Ye, C.; Li, H.; Li, Y.; Zhang, Y.; Liu, G.; Mi, H.; Li, H.; Xiao, Q.; Niu, L.; Yu, X. Hypoxia-induced HMGB1 promotes glioma stem cells self-renewal and tumorigenicity via RAGE. Iscience 2022, 25, 104872. [Google Scholar] [CrossRef] [PubMed]
- Deathridge, J.; Antolović, V.; Parsons, M.; Chubb, J.R. Live imaging of ERK signaling dynamics in differentiating mouse embryonic stem cells. Development 2019, 146, dev172940. [Google Scholar] [CrossRef]
- Hance, M.W.; Dole, K.; Gopal, U.; Bohonowych, J.E.; Jezierska-Drutel, A.; Neumann, C.A.; Liu, H.; Garraway, I.P.; Isaacs, J.S. Secreted Hsp90 Is a Novel Regulator of the Epithelial to Mesenchymal Transition (EMT) in Prostate Cancer. J. Biol. Chem. 2012, 287, 37732–37744. [Google Scholar] [CrossRef]
- Xu, X.; Zheng, L.; Yuan, Q.; Zhen, G.; Crane, J.L.; Zhou, X.; Cao, X. Transforming growth factor-beta in stem cells and tissue homeostasis. Bone Res. 2018, 6, 2. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Z.; Zhou, Q.; Wu, X.; Chen, X.; Li, J.; Zhu, Z.; Liu, B.; Su, L. Tissue transglutaminase-2 promotes gastric cancer progression via the ERK1/2 pathway. Oncotarget 2016, 7, 7066–7079. [Google Scholar] [CrossRef] [PubMed]
- Xu, D.; Liang, J.; Lin, J.; Yu, C. PKM2: A Potential Regulator of Rheumatoid Arthritis via Glycolytic and Non-Glycolytic Pathways. Front. Immunol. 2019, 10, 2919. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Guo, Y.; Wang, X.; Zhao, H.; Ji, Z.; Cheng, C.; Li, L.; Fang, Y.; Xu, D.; Zhu, H.H.; et al. WNT/beta-Catenin Directs Self-Renewal Symmetric Cell Division of hTERT(high) Prostate Cancer Stem Cells. Cancer Res. 2017, 77, 2534–2547. [Google Scholar] [CrossRef]
- Lee, G.; Espirito Santo, A.I.; Zwingenberger, S.; Cai, L.; Vogl, T.; Feldmann, M.; Horwood, N.J.; Chan, J.K.; Nanchahal, J. Fully reduced HMGB1 accelerates the regeneration of multiple tissues by transitioning stem cells to GAlert. Proc. Natl. Acad. Sci. USA 2018, 115, E4463–E4472. [Google Scholar]
- Chiosis, G.; Dickey, C.A.; Johnson, J.L. A global view of Hsp90 functions. Nat. Struct. Mol. Biol. 2013, 20, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Ren, Q.; Chen, J.; Liu, Y. LRP5 and LRP6 in Wnt Signaling: Similarity and Divergence. Front. Cell Dev. Biol. 2021, 9, 670960. [Google Scholar] [CrossRef] [PubMed]
- Antonyak, M.A.; Li, B.; Regan, A.D.; Feng, Q.; Dusaban, S.S.; Cerione, R.A. Tissue Transglutaminase Is an Essential Participant in the Epidermal Growth Factor-stimulated Signaling Pathway Leading to Cancer Cell Migration and Invasion. J. Biol. Chem. 2009, 284, 17914–17925. [Google Scholar] [CrossRef]
- Bagatur, Y.; Ilter Akulke, A.Z.; Bihorac, A.; Erdem, M.; Telci, D. Tissue transglutaminase expression is necessary for adhesion, metastatic potential and cancer stemness of renal cell carcinoma. Cell Adhes. Migr. 2018, 12, 138–151. [Google Scholar] [CrossRef]
- Wu, D.-S.; Chen, C.; Wu, Z.-J.; Liu, B.; Gao, L.; Yang, Q.; Chen, W.; Chen, J.-M.; Bao, Y.; Qu, L.; et al. ATF2 predicts poor prognosis and promotes malignant phenotypes in renal cell carcinoma. J. Exp. Clin. Cancer Res. 2016, 35, 108. [Google Scholar] [CrossRef]
- Snaebjornsson, M.T.; Schulze, A. Non-canonical functions of enzymes facilitate cross-talk between cell metabolic and regulatory pathways. Exp. Mol. Med. 2018, 50, 1–16. [Google Scholar] [CrossRef]
- Quintana, F.J.; Cohen, I.R. The HSP60 immune system network. Trends Immunol. 2011, 32, 89–95. [Google Scholar] [CrossRef] [PubMed]
- Kornberg, M.D.; Bhargava, P.; Kim, P.M.; Putluri, V.; Snowman, A.M.; Putluri, N.; Calabresi, P.A.; Snyder, S.H. Dimethyl fumarate targets GAPDH and aerobic glycolysis to modulate immunity. Science 2018, 360, 449–453. [Google Scholar] [CrossRef]
- Adela, R.; Banerjee, S.K. GDF-15 as a Target and Biomarker for Diabetes and Cardiovascular Diseases: A Translational Prospective. J. Diabetes Res. 2015, 2015, 670960. [Google Scholar] [CrossRef]
- Hu, H.H.; Chen, D.Q.; Wang, Y.N.; Feng, Y.L.; Cao, G.; Vaziri, N.D.; Zhao, Y.Y. New insights into TGF-beta/Smad signaling in tissue fibrosis. Chem. Biol. Interact. 2018, 292, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Eckert, R.L.; Kaartinen, M.T.; Nurminskaya, M.; Belkin, A.M.; Colak, G.; Johnson, G.V.; Mehta, K. Transglutaminase regulation of cell function. Physiol. Rev. 2014, 94, 383–417. [Google Scholar] [CrossRef] [PubMed]
- Loft, A.; Alfaro, A.J.; Schmidt, S.F.; Pedersen, F.B.; Terkelsen, M.K.; Puglia, M.; Chow, K.K.; Feuchtinger, A.; Troullinaki, M.; Maida, A.; et al. Liver-fibrosis-activated transcriptional networks govern hepatocyte reprogramming and intra-hepatic communication. Cell Metab. 2021, 33, 1685–1700.e9. [Google Scholar] [CrossRef]
- Tran, L.L.; Dang, T.; Thomas, R.; Rowley, D.R. ELF3 Mediates IL-1α Induced Differentiation of Mesenchymal Stem Cells to Inflammatory iCAFs. Stem Cells 2021, 39, 1766–1777. [Google Scholar] [CrossRef]
- Lan, J.; Luo, H.; Wu, R.; Wang, J.; Zhou, B.; Zhang, Y.; Jiang, Y.; Xu, J. Internalization of HMGB1 (High Mobility Group Box 1) Promotes Angiogenesis in Endothelial Cells. Arter. Thromb. Vasc. Biol. 2020, 40, 2922–2940. [Google Scholar] [CrossRef]
- Tomas, A.; Futter, C.E.; Eden, E.R. EGF receptor trafficking: Consequences for signaling and cancer. Trends Cell Biol. 2014, 24, 26–34. [Google Scholar] [CrossRef]
- Goeppert, B.; Stichel, D.; Toth, R.; Fritzsche, S.; Loeffler, M.A.; Schlitter, A.M.; Neumann, O.; Assenov, Y.; Vogel, M.N.; Mehrabi, A.; et al. Integrative analysis reveals early and distinct genetic and epigenetic changes in intraductal papillary and tubulopapillary cholangiocarcinogenesis. Gut 2022, 71, 391–401. [Google Scholar] [CrossRef]
- Dong, G.; Mao, Q.; Xia, W.; Xu, Y.; Wang, J.; Xu, L.; Jiang, F. PKM2 and cancer: The function of PKM2 beyond glycolysis. Oncol. Lett. 2016, 11, 1980–1986. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, S.; Zhou, R.; Li, W.; Zhang, G. Overexpression of E74-like transformation-specific transcription factor 3 promotes cellular proliferation and predicts poor prognosis in ovarian cancer. Oncol. Lett. 2021, 22, 710. [Google Scholar] [CrossRef] [PubMed]
- Wan, S.; Meyer, A.; Weiler, S.M.E.; Rupp, C.; Tóth, M.; Sticht, C.; Singer, S.; Thomann, S.; Roessler, S.; Schorpp-Kistner, M.; et al. Cytoplasmic localization of the cell polarity factor scribble supports liver tumor formation and tumor cell invasiveness. Hepatology 2018, 67, 1842–1856. [Google Scholar] [CrossRef]
- Kim, S.; Yoon, I.; Son, J.; Park, J.; Kim, K.; Lee, J.H.; Park, S.Y.; Kang, B.S.; Han, J.M.; Hwang, K.Y. Leucine-sensing mechanism of leucyl-tRNA synthetase 1 for mTORC1 activation. Cell. Rep. 2021, 35, 109031. [Google Scholar] [CrossRef] [PubMed]
- Ng, A.Y.-N.; Waring, P.; Ristevski, S.; Wang, C.; Wilson, T.; Pritchard, M.; Hertzog, P.; Kola, I. Inactivation of the transcription factor Elf3 in mice results in dysmorphogenesis and altered differentiation of intestinal epithelium. Gastroenterology 2002, 122, 1455–1466. [Google Scholar] [CrossRef]
- Chang, C.; Su, H.; Zhang, D.; Wang, Y.; Shen, Q.; Liu, B.; Huang, R.; Zhou, T.; Peng, C.; Wong, C.C.; et al. AMPK-Dependent Phosphorylation of GAPDH Triggers Sirt1 Activation and Is Necessary for Autophagy upon Glucose Starvation. Mol. Cell 2015, 60, 930–940. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.Y.; Yoon, J.Y.; Yun, J.H.; Cho, K.W.; Lee, S.H.; Rhee, Y.M.; Jung, H.S.; Lim, H.J.; Lee, H.; Choi, J.; et al. CXXC5 is a negative-feedback regulator of the Wnt/beta-catenin pathway involved in osteoblast differentiation. Cell. Death. Differ. 2015, 22, 912–920. [Google Scholar] [CrossRef] [PubMed]
- Griner, S.E.; Joshi, J.P.; Nahta, R. Growth differentiation factor 15 stimulates rapamycin-sensitive ovarian cancer cell growth and invasion. Biochem. Pharmacol. 2013, 85, 46–58. [Google Scholar] [CrossRef]
- Rada, A.; Merentes, E.; Rodríguez, M.; Anselmi, G.; Strauss, M. Human hepatoma cell line (HepG2) cellular response to hypothermic stress with recovery. Induction of Hsp70, Hsp60 and Hsf1 expression. Investig. Clínica 2010, 51, 479–488. [Google Scholar]
- Wu, T.C.; He, H.Z.; Tanguay, R.M.; Wu, Y.; Xu, D.G.; Currie, R.W.; Qu, S.; Feng, J.D.; Zhang, G.G. The combined effects of high temperature and carbon monoxide on heat stress response. J. Tongji Med. Univ. 1995, 15, 178–183. [Google Scholar]
- Pei, W.; Tanaka, K.; Huang, S.C.; Xu, L.; Liu, B.; Sinclair, J.; Idol, J.; Varshney, G.K.; Huang, H.; Lin, S.; et al. Extracellular HSP60 triggers tissue regeneration and wound healing by regulating inflammation and cell proliferation. npj Regen. Med. 2016, 1, 16013. [Google Scholar] [CrossRef] [PubMed]
- Ding, F.; Li, F.; Li, Y.; Hou, X.; Ma, Y.; Zhang, N.; Ma, J.; Zhang, R.; Lang, B.; Wang, H.; et al. HSP60 mediates the neuroprotective effects of curcumin by suppressing microglial activation. Exp. Ther. Med. 2016, 12, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Kilmartin, B.; Reen, D.J. HSP60 induces self-tolerance to repeated HSP60 stimulation and cross-tolerance to other pro-inflammatory stimuli. Eur. J. Immunol. 2004, 34, 2041–2051. [Google Scholar] [CrossRef]
- Shin, S.H.; Kim, H.S.; Jung, S.H.; Xu, H.D.; Jeong, Y.B.; Chung, Y.J. Implication of leucyl-tRNA synthetase 1 (LARS1) over-expression in growth and migration of lung cancer cells detected by siRNA targeted knock-down analysis. Exp. Mol. Med. 2008, 40, 229–236. [Google Scholar] [CrossRef] [PubMed]
- Quintavalle, C.; Meyer-Schaller, N.; Roessler, S.; Calabrese, D.; Marone, R.; Riedl, T.; Picco-Rey, S.; Panagiotou, O.A.; Uzun, S.; Piscuoglio, S.; et al. miR-579-3p Controls Hepatocellular Carcinoma Formation by Regulating the Phosphoinositide 3-Kinase–Protein Kinase B Pathway in Chronically Inflamed Liver. Hepatol. Commun. 2022, 6, 1467–1481. [Google Scholar] [CrossRef]
- Janiak, A.; Zemskov, E.A.; Belkin, A.M. Cell Surface Transglutaminase Promotes RhoA Activation via Integrin Clustering and Suppression of the Src–p190RhoGAP Signaling Pathway. Mol. Biol. Cell 2006, 17, 1606–1619. [Google Scholar] [CrossRef]
- Eckert, R.L. Transglutaminase 2 takes center stage as a cancer cell survival factor and therapy target. Mol. Carcinog. 2019, 58, 837–853. [Google Scholar] [CrossRef]
- Rappaport, N.; Twik, M.; Plaschkes, I.; Nudel, R.; Stein, T.I.; Levitt, J.; Gershoni, M.; Morrey, C.P.; Safran, M.; Lancet, D. MalaCards: An amalgamated human disease compendium with diverse clinical and genetic annotation and structured search. Nucleic Acids Res. 2017, 45, D877–D887. [Google Scholar] [CrossRef]
- Carvalho-Silva, D.; Pierleoni, A.; Pignatelli, M.; Ong, C.K.; Fumis, L.; Karamanis, N.; Carmona, M.; Faulconbridge, A.; Hercules, A.; McAuley, E.; et al. Open Targets Platform: New developments and updates two years on. Nucleic Acids Res. 2019, 47, D1056–D1065. [Google Scholar] [CrossRef]
- Khan, I.K.; Bhuiyan, M.; Kihara, D. DextMP: Deep dive into text for predicting moonlighting proteins. Bioinformatics 2017, 33, i83–i91. [Google Scholar] [CrossRef]
- Khan, I.; McGraw, J.; Kihara, D. MPFit: Computational Tool for Predicting Moonlighting Proteins. Methods Mol. Biol. 2017, 1611, 45–57. [Google Scholar] [CrossRef]
- Khan, I.K.; Kihara, D. Genome-scale prediction of moonlighting proteins using diverse protein association information. Bioinformatics 2016, 32, 2281–2288. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.K.; Kihara, D. Computational characterization of moonlighting proteins. Biochem. Soc. Trans. 2014, 42, 1780–1785. [Google Scholar] [CrossRef]
- Khan, I.K.; Chitale, M.; Rayon, C.; Kihara, D. Evaluation of function predictions by PFP, ESG, and PSI-BLAST for moonlighting proteins. BMC Proc. 2012, 6, S5. [Google Scholar] [CrossRef]
- Franco-Serrano, L.; Sánchez-Redondo, D.; Nájar-García, A.; Hernández, S.; Amela, I.; Perez-Pons, J.; Piñol, J.; Mozo-Villarias, A.; Cedano, J.; Querol, E. Pathogen Moonlighting Proteins: From Ancestral Key Metabolic Enzymes to Virulence Factors. Microorganisms 2021, 9, 1300. [Google Scholar] [CrossRef]
- Franco-Serrano, L.; Cedano, J.; Perez-Pons, J.A.; Mozo-Villarias, A.; Piñol, J.; Amela, I.; Querol, E. A hypothesis explaining why so many pathogen virulence proteins are moonlighting proteins. Pathog. Dis. 2018, 76, fty046. [Google Scholar] [CrossRef]
- Hernandez, S.; Franco, L.; Calvo, A.; Ferragut, G.; Hermoso, A.; Amela, I.; Gomez, A.; Querol, E.; Cedano, J. Bioinformatics and Moonlighting Proteins. Front. Bioeng. Biotechnol. 2015, 3, 90. [Google Scholar] [CrossRef]
- Hernandez, S.; Calvo, A.; Ferragut, G.; Franco, L.; Hermoso, A.; Amela, I.; Gomez, A.; Querol, E.; Cedano, J. Can bioinformatics help in the identification of moonlighting proteins? Biochem. Soc. Trans. 2014, 42, 1692–1697. [Google Scholar] [CrossRef] [PubMed]
- Gomez, A.; Hernandez, S.; Amela, I.; Pinol, J.; Cedano, J.; Querol, E. Do protein-protein interaction databases identify moonlighting proteins? Mol. Biosyst. 2011, 7, 2379–2382. [Google Scholar] [CrossRef]
- Gomez, A.; Domedel, N.; Cedano, J.; Pinol, J.; Querol, E. Do current sequence analysis algorithms disclose multifunctional (moonlighting) proteins? Bioinformatics 2003, 19, 895–896. [Google Scholar] [CrossRef]
- Shin, W.H.; Kihara, D. Virtual Ligand Screening Using PL-PatchSurfer2, a Molecular Surface-Based Protein-Ligand Docking Method. Methods Mol. Biol. 2018, 1762, 105–121. [Google Scholar] [PubMed]

| TISSUE ACTIVITY | Stress | Stress protection | HSPs [87,88,89], GAPDH [90], TG2 [91], FXIIIA [92], HMGB1 [93], PKM2 [94,95] | |
| WOUND HEALING CYCLE | TISSUE DESTRUCTION | Clearance | Apoptosis (intrinsic) | TGFBR1 [96,97,98], SMAD3 [96,97,98], GAPDH [99], TG2 [100], ESE-1 [101], CRT [102], HMGB1 [103] |
| Inflammation (cytotoxic and scavenger) | HSP70 [104,105], HSP60 [106], TG2 [107], ESE-1 [108,109], CRT [110], HMGB1 [103] | |||
| TISSUE CREATION | Niche creation | Stem cells (cell transformation) | TGFBR1 [111,112,113], SMAD3 [111,112,113], E-Cadherin [114], TG2 [115,116], ESE-1 [117], HMGB1 [118] | |
| ERK pathway activation | TGFBR1 [98], EGFR [119], HSP90 [120], E-Cadherin [121], TG2 [122], PKM2 [123] | |||
| Stem-cell self-renewal (symmetric proliferation) | β-Catenin [124], HMGB1 [125] | |||
| Invasiveness | TGFBR1 [113], SMAD3 [113], HSP90 [120,126], β-Catenin [127], TG2 [128,129], ESE-1 [117], ATF2 [130], FPK1 [131] | |||
| Extra-cellular matrix remodelling | Inflammation termination | HSP70 [104], HSP60 [132], GAPDH [133], HMGB1 [103], GDF-15 [134] | ||
| Fibrosis | SMAD3 [135], TG2 [122,136], FXIIIA [133], ESE-1 [137,138] | |||
| Angiogenesis | PKM2 [70], HMGB1 [139] | |||
| Re-epithelization | Epithelial proliferation | EGFR1 [140], β-Catenin [141], PKM2 [142] | ||
| mTOR pathway activation | ESE-1 [143], Scrib [144], GDF-15 [134], LARS1 [145], Aldolase [131] | |||
| Differentiation (epithelial) | ESE-1 [146] | |||
| Wound healing termination | GAPDH [147], β-Catenin [148] | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huerta, M.; Franco-Serrano, L.; Amela, I.; Perez-Pons, J.A.; Piñol, J.; Mozo-Villarías, A.; Querol, E.; Cedano, J. Role of Moonlighting Proteins in Disease: Analyzing the Contribution of Canonical and Moonlighting Functions in Disease Progression. Cells 2023, 12, 235. https://doi.org/10.3390/cells12020235
Huerta M, Franco-Serrano L, Amela I, Perez-Pons JA, Piñol J, Mozo-Villarías A, Querol E, Cedano J. Role of Moonlighting Proteins in Disease: Analyzing the Contribution of Canonical and Moonlighting Functions in Disease Progression. Cells. 2023; 12(2):235. https://doi.org/10.3390/cells12020235
Chicago/Turabian StyleHuerta, Mario, Luis Franco-Serrano, Isaac Amela, Josep Antoni Perez-Pons, Jaume Piñol, Angel Mozo-Villarías, Enrique Querol, and Juan Cedano. 2023. "Role of Moonlighting Proteins in Disease: Analyzing the Contribution of Canonical and Moonlighting Functions in Disease Progression" Cells 12, no. 2: 235. https://doi.org/10.3390/cells12020235
APA StyleHuerta, M., Franco-Serrano, L., Amela, I., Perez-Pons, J. A., Piñol, J., Mozo-Villarías, A., Querol, E., & Cedano, J. (2023). Role of Moonlighting Proteins in Disease: Analyzing the Contribution of Canonical and Moonlighting Functions in Disease Progression. Cells, 12(2), 235. https://doi.org/10.3390/cells12020235





