Sphingosylphosphorylcholine (SPC), a Causative Factor of SPC-Induced Vascular Smooth Muscle Cells Contraction, Is Taken Up via Endocytosis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Live-Cell Imaging
2.3. Flow Cytometry
2.4. Surface Plasmon Resonance (SPR) Analysis
2.5. Western Blotting
2.6. Exosome Isolation and Detection
2.7. Statistical Analysis
3. Results
3.1. Fisetin Prevents SPC-Induced Contraction by Directly Acting on HCASMCs
3.2. Microdomains Are Not Essential for the SPC-Induced Contractions of HCASMCs
3.3. SPCs Are Incorporated into Abnormally Contracting HCASMCs, Unrelated to Fisetin Treatment
3.4. Cellular Uptake of SPCs via Endocytosis
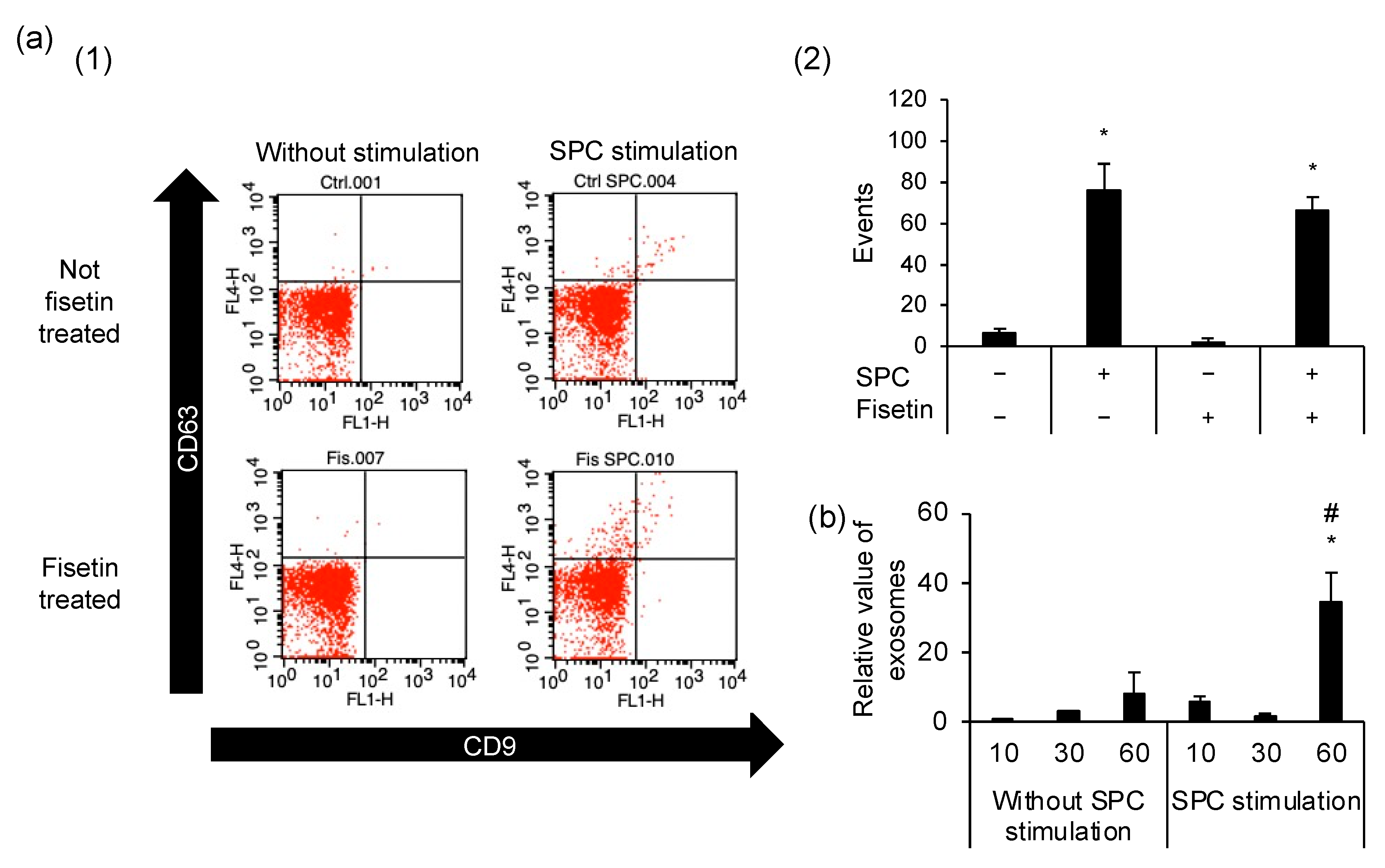
3.5. Exocytosis Is Caused by SPC-Induced Contractions but Is Unrelated to the Preventive Mechanism of Fisetin
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Agostoni, E.C.; Longoni, M. Migraine and cerebrovascular disease: Still a dangerous connection? Neurol. Sci. 2018, 39, 33–37. [Google Scholar] [CrossRef] [PubMed]
- Shirao, S.; Fujisawa, H.; Kudo, A.; Kurokawa, T.; Yoneda, H.; Kunitsugu, I.; Ogasawara, K.; Soma, M.; Kobayashi, S.; Ogawa, A.; et al. Inhibitory effects of eicosapentaenoic acid on chronic cerebral vasospasm after subarachnoid hemorrhage: Possible involvement of a sphingosylphosphorylcholine-rho-kinase pathway. Cerebrovasc. Dis. 2008, 26, 30–37. [Google Scholar] [CrossRef] [PubMed]
- Somlyo, A.P.; Somlyo, A.V. Signal transduction and regulation in smooth muscle. Nature 1994, 372, 231–236. [Google Scholar] [CrossRef] [PubMed]
- Lanza, G.A.; Careri, G.; Crea, F. Mechanisms of coronary artery spasm. Circulation 2011, 124, 1774–1782. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimokawa, H.; Sunamura, S.; Satoh, K. RhoA/Rho-kinase in the cardiovascular system. Circ. Res. 2016, 118, 352–366. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nixon, G.F.; Mathieson, F.A.; Hunter, I. The multi-functional role of sphingosylphosphorylcholine. Prog. Lipid Res. 2008, 47, 62–75. [Google Scholar] [CrossRef] [Green Version]
- Ge, D.; Yue, H.W.; Liu, H.H.; Zhao, J. Emerging roles of sphingosylphosphorylcholine in modulating cardiovascular functions and diseases. Acta Pharmacol. Sin. 2018, 39, 1830–1836. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shirao, S.; Kashiwagi, S.; Sato, M.; Miwa, S.; Nakao, F.; Kurokawa, T.; Todoroki-Ikeda, N.; Mogami, K.; Mizukami, Y.; Kuriyama, S.; et al. Sphingosylphosphorylcholine is a novel messenger for Rho-kinase-mediated Ca2+ sensitization in the bovine cerebral artery: Unimportant role for protein kinase C. Circ. Res. 2002, 91, 112–119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakao, F.; Kobayashi, S.; Mogami, K.; Mizukami, Y.; Shirao, S.; Miwa, S.; Todoroki-Ikeda, N.; Ito, M.; Matsuzaki, M. Involvement of Src family protein tyrosine kinases in Ca2+ sensitization of coronary artery contraction mediated by a sphingosylphosphorylcholine-Rho-kinase pathway. Circ. Res. 2002, 91, 953–960. [Google Scholar] [CrossRef] [Green Version]
- Morikage, N.; Kishi, H.; Sato, M.; Guo, F.; Shirao, S.; Yano, T.; Soma, M.; Hamano, K.; Esato, K.; Kobayashi, S. Cholesterol primes vascular smooth muscle to induce Ca2+ sensitization mediated by a sphingosylphosphorylcholine-Rho-kinase pathway: Possible role for membrane raft. Circ. Res. 2006, 99, 299–306. [Google Scholar] [CrossRef]
- Shirao, S.; Yoneda, H.; Shinoyama, M.; Sugimoto, K.; Koizumi, H.; Ishihara, H.; Oka, F.; Sadahiro, H.; Nomura, S.; Fujii, M.; et al. A novel trigger for cholesterol-dependent smooth muscle contraction mediated by the sphingosylphosphorylcholine-Rho-kinase pathway in the rat basilar artery: A mechanistic role for lipid rafts. J. Cereb. Blood Flow Metab. 2015, 35, 835–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sviridov, D.; Mukhamedova, N.; Miller, Y.I. Lipid rafts as a therapeutic target. J. Lipid Res. 2020, 61, 687–695. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuo, K.H.; Leo, J.M. Enhancement of vascular smooth muscle contractility by alterations of membranous architecture. Anat. Rec. 2019, 302, 186–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsurudome, N.; Minami, Y.; Kajiya, K. Fisetin, a major component derived from mulberry (Morus australis Poir.) leaves, prevents vascular abnormal contraction. BioFactors 2022, 48, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, M.; Lyu, B.; Kishi, H.; Kobayashi, S. Omega-3 and omega-6 DPA equally inhibit the sphingosylphosphorylcholine-induced Ca2+-sensitization of vascular smooth muscle contraction via inhibiting Rho-kinase activation and translocation. Sci. Rep. 2017, 7, 36368. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, Q.; Kishi, H.; Zhang, Y.; Morita, T.; Kobayashi, S. Hesperetin inhibits sphingosylphosphorylcholine-induced vascular smooth muscle contraction by regulating the Fyn/Rho-kinase pathway. J. Cardiovasc. Pharmacol. 2022, 79, 456–466. [Google Scholar] [CrossRef]
- van Ijzendoorn, S.C.; Zegers, M.M.; Kok, J.W.; Hoekstra, D. Segregation of glucosylceramide and sphingomyelin occurs in the apical to basolateral transcytotic route in HepG2 cells. J. Cell Biol. 1997, 137, 347–357. [Google Scholar] [CrossRef] [Green Version]
- Horii, K.; Omi, K.; Yoshida, Y.; Imai, Y.; Sakai, N.; Oka, A.; Masuda, H.; Furuichi, M.; Tanimoto, T.; Waga, I. Development of a sphingosylphosphorylcholine detection system using RNA aptamers. Molecules 2010, 15, 5742–5755. [Google Scholar] [CrossRef]
- Nguyen, H.H.; Park, J.; Kang, S.; Kim, M. Surface plasmon resonance: A versatile technique for biosensor applications. Sensors 2015, 15, 10481–10510. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Kishi, H.; Morita, T.; Kobayashi, S. Paxillin controls actin stress fiber formation and migration of vascular smooth muscle cells by directly binding to the active Fyn. FASEB J. 2021, 35, e22012. [Google Scholar] [CrossRef] [PubMed]
- Nishida-Aoki, N.; Tominaga, N.; Takeshita, F.; Sonoda, H.; Yoshioka, Y.; Ochiya, T. Disruption of circulating extracellular vesicles as a novel therapeutic strategy against cancer metastasis. Mol. Ther. 2017, 25, 181–191. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Muraoka, S.; Jedrychowski, M.P.; Iwahara, N.; Abdullah, M.; Onos, K.D.; Keezer, K.J.; Hu, J.; Ikezu, S.; Howell, G.R.; Gygi, S.P.; et al. Enrichment of neurodegenerative microglia signature in brain-derived extracellular vesicles isolated from Alzheimer’s disease mouse models. J. Proteome Res. 2021, 20, 1733–1743. [Google Scholar] [CrossRef] [PubMed]
- Saha, S.; Panieri, E.; Suzen, S.; Saso, L. The interaction of flavonols with membrane components: Potential effect on antioxidant activity. J. Membr. Biol. 2020, 253, 57–71. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.G.W. The caveolae membrane system. Annu. Rev. Biochem. 1998, 67, 199–225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, K.; Gerl, M.J. Revitalizing membrane rafts: New tools and insights. Nat. Rev. Mol. Cell Biol. 2010, 11, 688–699. [Google Scholar] [CrossRef] [PubMed]
- Kok, J.W.; Babia, T.; Klappe, K.; Hoekstra, D. Fluorescent, short-chain C6-NBD-sphingomyelin, but not C6-NBD-glucosylceramide, is subject to extensive degradation in the plasma membrane: Implications for signal transduction related to cell differentiation. Biochem. J. 1995, 309, 905–912. [Google Scholar] [CrossRef] [Green Version]
- Frick, M.; Bright, N.A.; Riento, K.; Bray, A.; Merrified, C.; Nichols, B.J. Coassembly of flotillins induces formation of membrane microdomains, membrane curvature, and vesicle budding. Curr. Biol. 2017, 17, 1151–1156. [Google Scholar] [CrossRef] [Green Version]
- Glebov, O.O.; Bright, N.A.; Nichols, B.J. Flotillin-1 defines a clathrin-independent endocytic pathway in mammalian cells. Nat. Cell Biol. 2006, 8, 46–54. [Google Scholar] [CrossRef]
- Chen, J.; Chen, J.; Cheng, Y.; Fu, Y.; Zhao, H.; Tang, M.; Zhao, H.; Lin, N.; Shi, X.; Lei, Y.; et al. Mesenchymal stem cell-derived exosomes protect beta cells against hypoxia-induced apoptosis via miR-21 by alleviating ER stress and inhibiting p38 MAPK phosphorylation. Stem Cell Res Ther. 2020, 11, 97. [Google Scholar] [CrossRef] [Green Version]
- Nabi, I.R.; Le, P.U. Caveolae/raft-dependent endocytosis. J. Cell Biol. 2003, 161, 673–677. [Google Scholar] [CrossRef]
- Riento, K.; Frick, M.; Schafer, I.; Nichols, B.J. Endocytosis of flotillin-1 and flotillin-2 is regulated by Fyn kinase. J. Cell Sci. 2009, 122, 912–918. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, D.; Kishi, H.; Kawamichi, H.; Kajiya, K.; Takada, Y.; Kobayashi, S. Involvement of Fyn tyrosine kinase in actin stress fiber formation in fibroblasts. FEBS Lett. 2007, 581, 5227–5233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, W.; Guo, J.; Gao, B.; Zhang, W.; Ling, L.; Xu, T.; Pan, C.; Li, L.; Chen, S.; Wang, H.; et al. Flotillin-mediated endocytosis and ALIX-syntenin-1-mediated exocytosis protect the cell membrane from damage caused by necroptosis. Sci. Signal. 2019, 12, eaaw3423. [Google Scholar] [CrossRef]
- Holthuis, J.C.M.; Menon, A.K. Lipid landscapes and pipelines in membrane homeostasis. Nature 2014, 510, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Jones, J.H.; Minshall, R.D. Lung endothelial transcytosis. Compr Physiol. 2020, 10, 491–508. [Google Scholar] [CrossRef] [Green Version]
- Li, N.; Zhang, Y.; Morita, T.; Kishi, H.; Kobayashi, S. Inhibitory mechanism of tangeretin, a citrus flavone on the sphingosylphosphorylcholine (SPC)-induced vascular smooth muscle contraction. J. Pharmacol. Sci. 2022, 149, 189–197. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Bi, J.; Huang, J.; Tang, Y.; Du, S.; Li, P. Exosome: A review of its classification, isolation techniques, storage, diagnostic and targeted therapy applications. Int. J. Nanomed. 2020, 15, 6917–6934. [Google Scholar] [CrossRef]
- Meldolesi, J. Exosomes and ectosomes in intercellular communication. Curr. Biol. 2018, 28, R435–R444. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tsurudome, N.; Minami, Y.; Kajiya, K. Sphingosylphosphorylcholine (SPC), a Causative Factor of SPC-Induced Vascular Smooth Muscle Cells Contraction, Is Taken Up via Endocytosis. Cells 2023, 12, 265. https://doi.org/10.3390/cells12020265
Tsurudome N, Minami Y, Kajiya K. Sphingosylphosphorylcholine (SPC), a Causative Factor of SPC-Induced Vascular Smooth Muscle Cells Contraction, Is Taken Up via Endocytosis. Cells. 2023; 12(2):265. https://doi.org/10.3390/cells12020265
Chicago/Turabian StyleTsurudome, Natsuko, Yuji Minami, and Katsuko Kajiya. 2023. "Sphingosylphosphorylcholine (SPC), a Causative Factor of SPC-Induced Vascular Smooth Muscle Cells Contraction, Is Taken Up via Endocytosis" Cells 12, no. 2: 265. https://doi.org/10.3390/cells12020265