Fabrication Scaffold with High Dimensional Control for Spheroids with Undifferentiated iPS Cell Properties
Abstract
:1. Introduction
2. Materials and Methods
2.1. Spheroid Culture Scaffold Fabrication
2.2. Resin Mask Fabrication for ECM Patterning
2.3. ECM Patterning
2.4. Human iPSC (hiPSC) Culture
2.5. Spheroid Preparation
2.6. Evaluation of Cell Patterning
2.7. Measurement of Spheroid Viability Using Trypan Blue Staining
2.8. Differentiation of iPSCs on Substrates
3. Results
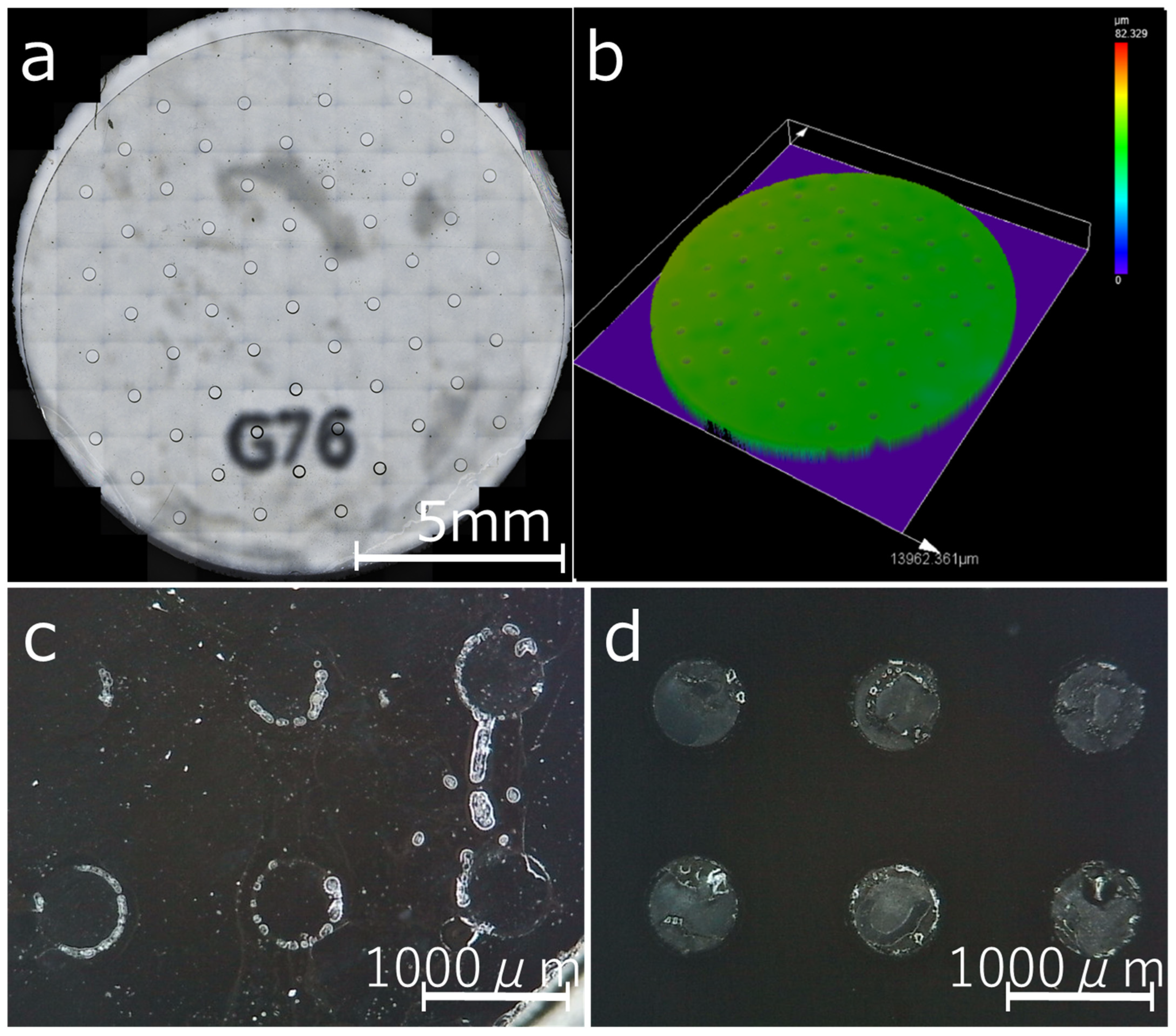
3.1. Dimensional Reproducibility of the Resin Mask
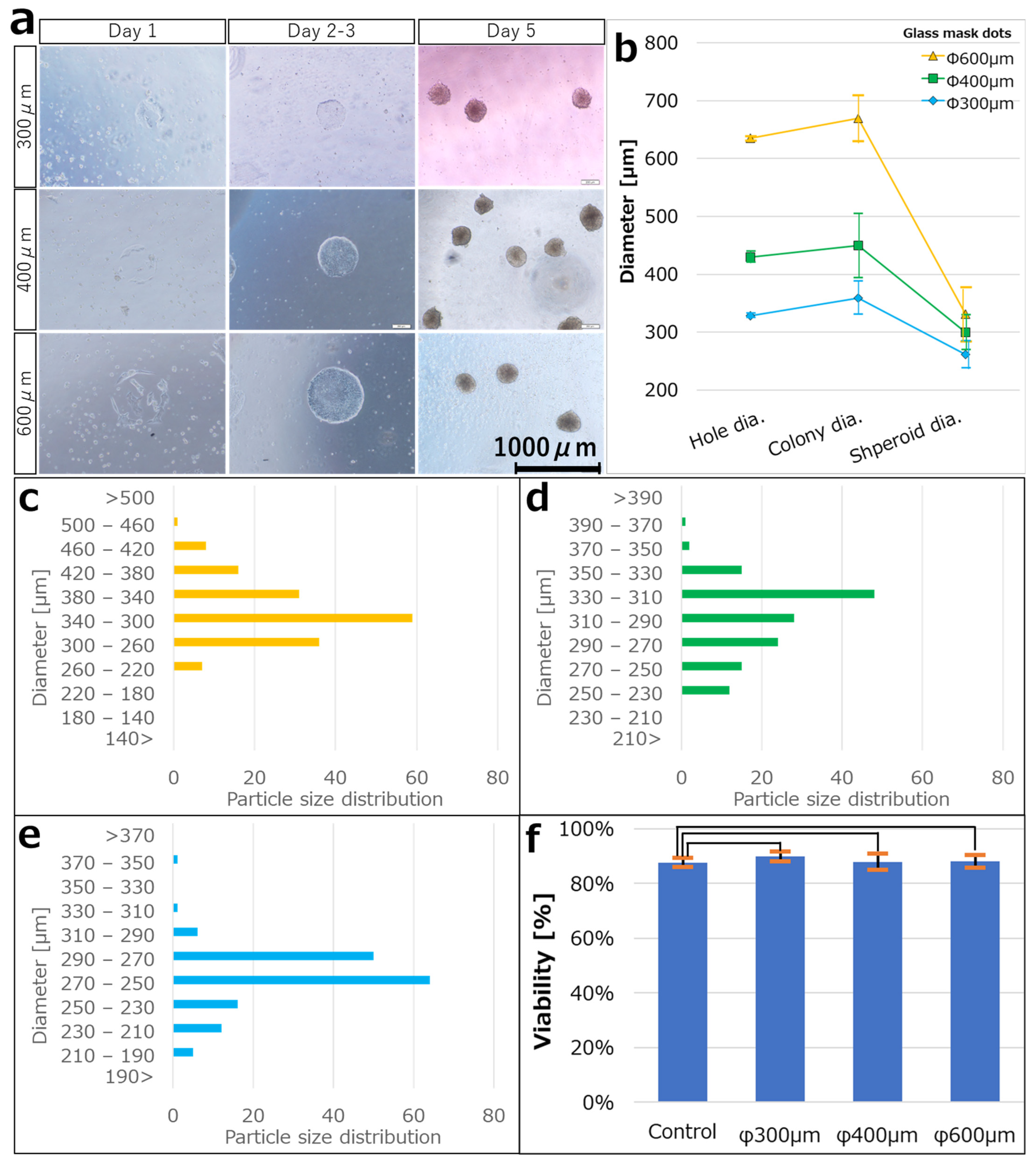
3.2. Culturing hiPSCs on the iMatrix Pattern and Confirming Viability
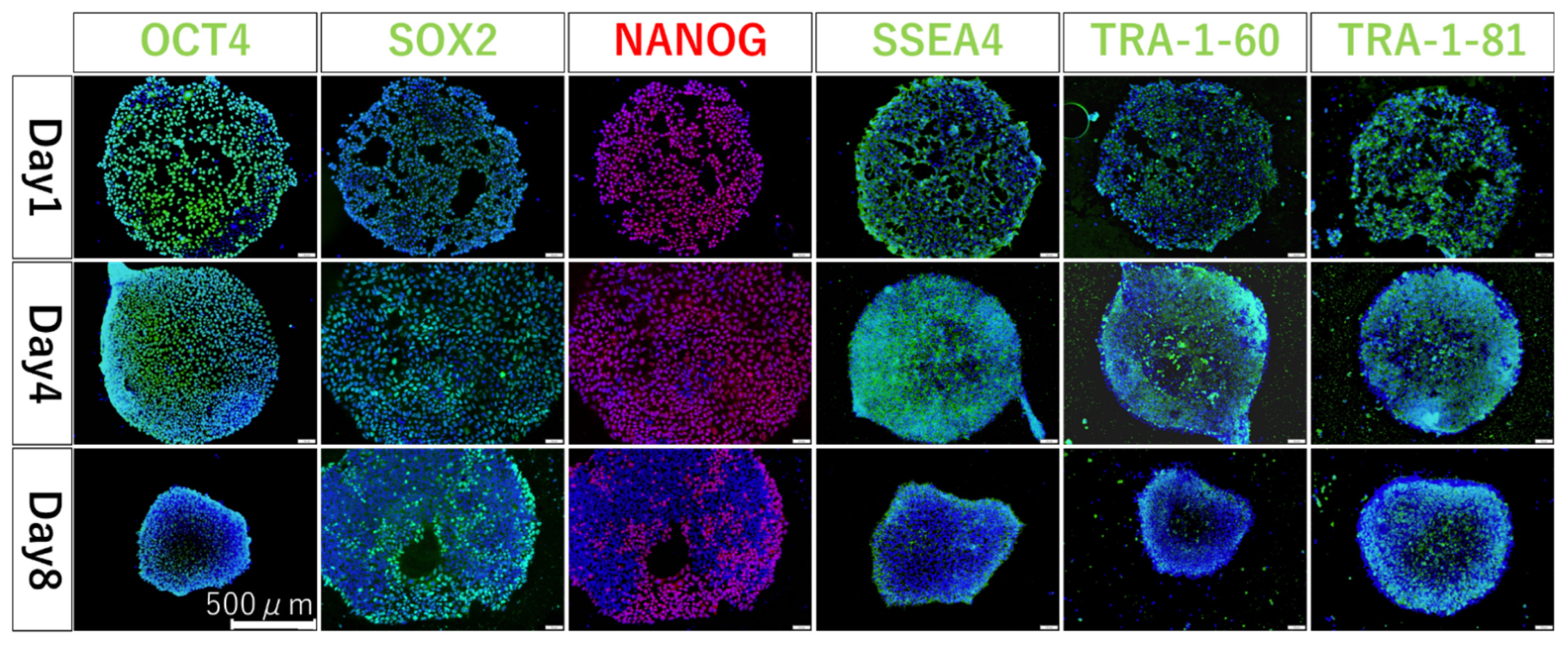
3.3. Confirming Undifferentiation in hiPSCs Seeded on iMatrix-Coated Substrates
3.4. Confirming Differentiation in hiPSCs Seeded on iMatrix-Coated Substrates
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- EU (2006) Regulation EC No. 1907/2006 of the European Parliament and of the Council of Concerning the Registration, Evaluation, Authorisation and Restriction of Chemicals (REACH), Establishing a European Chemicals Agency, Amending Directive 1999/45/EC and Repealing Council Regulation (EEC) No 793/93 and Commission Regulation (EC) No 1488/94 as Well as Council Directive 76/769/EEC and Commission Directives 91/155/EEC, 93/67/EEC, 93/105/EC and 2000/21/EC. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2007:136:0003:0280:en:PDF (accessed on 14 October 2022).
- ICH (2011) ICH Guideline, Guidance on Genotoxicity Testing and Data Interpretation for Pharmaceuticals Intended for Human Use s2(r1), Approval by the Steering Committee of S2(R1) under Step 4 and Recommendation for Adoption to the Three ICH Regulatory Bodies (9 November 2011). Available online: http://www.fda.gov/downloads/Drugs/Guidances/ucm074931.pdf (accessed on 14 October 2022).
- SCCS (2014) Addendum to the SCCS’s Notes of Guidance (NoG) for the Testing of Cosmetic Ingredients and Their Safety Evaluation 8th Revision, SCCS/1501/12. Available online: http://ec.europa.eu/health/scientific_committees/consumer_safety/docs/sccs_o_156.pdf (accessed on 14 October 2022).
- EU (2009) European Union 2009. Regulation (EC) No 1223/2009 of the European Parliament and of the Council of 30 November 2009 on Cosmetic Products (Recast). Official Journal L342, 22.12.2009. p. 59. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=uriserv:OJ.L_.2009.342.01.0059.01.ENG (accessed on 14 October 2022).
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef] [PubMed]
- Gomez, S.; Vlad, M.D.; Lopez, J.; Fernandez, E. Design and properties of 3D scaffolds for bone tissue engineering. Acta Biomater. 2016, 42, 341–350. [Google Scholar] [CrossRef]
- Raghunath, J.; Rollo, J.; Sales, K.M.; Butler, P.E.; Seifalian, A.M. Biomaterials and scaffold design: Key to tissue-engineering cartilage. Biotechnol. Appl. Biochem. 2007, 46, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Narayan, N.J.C.; Requena, D.; Lalazar, G.; Ramos-Espiritu, L.; Ng, D.; Levin, S.; Shebl, B.; Wang, R.; Hammond, W.J.; Saltsman, J.A., 3rd; et al. Human liver organoids for disease modeling of fibrolamellar carcinoma. Stem Cell Rep. 2022, 17, 1874–1888. [Google Scholar] [CrossRef]
- Tomofuji, K.; Fukumitsu, K.; Kondo, J.; Horie, H.; Makino, K.; Wakama, S.; Ito, T.; Oshima, Y.; Ogiso, S.; Ishii, T.; et al. Liver ductal organoids reconstruct intrahepatic biliary trees in decellularized liver grafts. Biomaterials 2022, 287, 121614. [Google Scholar] [CrossRef]
- Nunez-Nescolarde, A.B.; Nikolic-Paterson, D.J.; Combes, A.N. Human Kidney Organoids and Tubuloids as Models of Complex Kidney Disease. Am. J. Pathol. 2022, 192, 738–749. [Google Scholar] [CrossRef] [PubMed]
- Tekguc, M.; Gaal, R.C.V.; Uzel, S.G.M.; Gupta, N.; Riella, L.V.; Lewis, J.A.; Morizane, R. Kidney organoids: A pioneering model for kidney diseases. Transl. Res. 2022, 250, 1–17. [Google Scholar] [CrossRef]
- Trush, O.; Takasato, M. Kidney organoid research: Current status and applications. Curr. Opin. Genet. Dev. 2022, 75, 101944. [Google Scholar] [CrossRef] [PubMed]
- Allam-Ndoul, B.; Begin, F.; Houde, A.; Di Marzo, V.; Veilleux, A.; Silvestri, c.; Flamand, N.; Tinoco-Mar, B.A. Su1124 small intestine epithelial organoids as a model to investigate the role of the endocannabinoidome on intestinal paracellular permeability during inflammation. Gastroenterology 2020, 158, S-516. [Google Scholar] [CrossRef]
- Hou, X.; Barthel, E.R.; Speer, A.L.; Levin, D.E.; Grant, C.N.; Spurrier, R.G.; Garcia, S.; Grikscheit, T.C. Small Intestine Organoid Units Can Be Maintained in Long-Term Culture without Exogenous Growth Factors, with Subsequent Formation of Tissue-Engineered Small Intestine. J. Surg. Res. 2014, 186, 648. [Google Scholar] [CrossRef]
- Isani, M.A.; Schlieve, C.R.; Fowler, K.L.; Nucho, L.-M.A.; Grikscheit, T.C. Scaffold-Free Delivery of Organoid Units Forms Tissue-Engineered Intestine. J. Am. Coll. Surg. 2018, 227, e193. [Google Scholar] [CrossRef]
- Cui, X.; Hartanto, Y.; Zhang, H. Advances in multicellular spheroids formation. J. R. Soc. Interface 2017, 14, 20160877. [Google Scholar] [CrossRef] [Green Version]
- Yoshii, Y.; Waki, A.; Yoshida, K.; Kakezuka, A.; Kobayashi, M.; Namiki, H.; Kuroda, Y.; Kiyono, Y.; Yoshii, H.; Furukawa, T.; et al. The use of nanoimprinted scaffolds as 3D culture models to facilitate spontaneous tumor cell migration and well-regulated spheroid formation. Biomaterials 2011, 32, 6052–6058. [Google Scholar] [CrossRef] [PubMed]
- Yamazaki, H.; Gotou, S.; Ito, K.; Kohashi, S.; Goto, Y.; Yoshiura, Y.; Sakai, Y.; Yabu, H.; Shimomura, M.; Nakazawa, K. Micropatterned culture of HepG2 spheroids using microwell chip with honeycomb-patterned polymer film. J. Biosci. Bioeng. 2014, 118, 455–460. [Google Scholar] [CrossRef] [PubMed]
- Hurrell, T.; Ellero, A.A.; Masso, Z.F.; Cromarty, A.D. Characterization and reproducibility of HepG2 hanging drop spheroids toxicology in vitro. Toxicol. Vitr. 2018, 50, 86–94. [Google Scholar] [CrossRef] [Green Version]
- Snyman, C.; Elliott, E. An optimized protocol for handling and processing fragile acini cultured with the hanging drop technique. Anal. Biochem. 2011, 419, 348–350. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.; Zhao, Y.; Wu, W.; Zhao, Q.; Li, G. A superhydrophobic chip integrated with an array of medium reservoirs for long-term hanging drop spheroid culture. Acta Biomater. 2021, 135, 234–242. [Google Scholar] [CrossRef]
- Souza, G.R.; Molina, J.R.; Raphael, R.M.; Ozawa, M.G.; Stark, D.J.; Levin, C.S.; Bronk, L.F.; Ananta, J.S.; Mandelin, J.; Georgescu, M.M.; et al. Three-dimensional tissue culture based on magnetic cell levitation. Nat. Nanotechnol. 2010, 5, 291–296. [Google Scholar] [CrossRef] [Green Version]
- Yabe, S.G.; Fukuda, S.; Nishida, J.; Takeda, F.; Nashiro, K.; Okochi, H. Induction of functional islet-like cells from human iPS cells by suspension culture. Regen. Ther. 2019, 10, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Nguyen, H.N.; Song, M.M.; Hadiono, C.; Ogden, S.C.; Hammack, C.; Yao, B.; Hamersky, G.R.; Jacob, F.; Zhong, C.; et al. Brain-Region-Specific Organoids Using Mini-bioreactors for Modeling ZIKV Exposure. Cell 2016, 165, 1238–1254. [Google Scholar] [CrossRef]
- Hsiao, A.Y.; Torisawa, Y.S.; Tung, Y.C.; Sud, S.; Taichman, R.S.; Pienta, K.J.; Takayama, S. Microfluidic system for formation of PC-3 prostate cancer co-culture spheroids. Biomaterials 2009, 30, 3020–3027. [Google Scholar] [CrossRef] [Green Version]
- Sebastian, A.; Buckle, A.M.; Markx, G.H. Tissue engineering with electric fields: Immobilization of mammalian cells in multilayer aggregates using dielectrophoresis. Biotechnol. Bioeng. 2007, 98, 694–700. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.; Wu, M.; Guo, F.; Li, P.; Chan, C.Y.; Mao, Z.; Li, S.; Ren, L.; Zhang, R.; Huang, T.J. Rapid formation of size-controllable multicellular spheroids via 3D acoustic tweezers. Lab Chip 2016, 16, 2636–2643. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar, I.N.; Olivos, D.J., 3rd; Brinker, A.; Alvarez, M.B.; Smith, L.J.; Chu, T.G.; Kacena, M.A.; Wagner, D.R. Scaffold-free bioprinting of mesenchymal stem cells using the Regenova printer: Spheroid characterization and osteogenic differentiation. Bioprinting 2019, 15, e00050. [Google Scholar] [CrossRef] [PubMed]
- Daly, A.C.; Kelly, D.J. Biofabrication of spatially organised tissues by directing the growth of cellular spheroids within 3D printed polymeric microchambers. Biomaterials 2019, 197, 194–206. [Google Scholar] [CrossRef]
- Awaja, F.; Gilbert, M.; Kelly, G.; Fox, B.; Pigram, P.J. Adhesion of polymers. Prog. Polym. Sci. 2009, 34, 948–968. [Google Scholar] [CrossRef]
- Bacakova, L.; Filova, E.; Parizek, M.; Ruml, T.; Svorcik, V. Modulation of cell adhesion, proliferation and differentiation on materials designed for body implants. Biotechnol. Adv. 2011, 29, 739–767. [Google Scholar] [CrossRef]
- Roina, Y.; Auber, F.; Hocquet, D.; Herlem, G. ePTFE functionalization for medical applications. Mater. Today Chem. 2021, 20, 100412. [Google Scholar] [CrossRef]
- Wang, H.; Kwok, D.T.; Wang, W.; Wu, Z.; Tong, L.; Zhang, Y.; Chu, P.K. Osteoblast behavior on polytetrafluoroethylene modified by long pulse, high frequency oxygen plasma immersion ion implantation. Biomaterials 2010, 31, 413–419. [Google Scholar] [CrossRef]
- Togo, H.; Yoshikawa-Terada, K.; Hirose, Y.; Nakagawa, H.; Takeuchi, H.; Kusunoki, M. Development of a Simple Spheroid Production Method Using Fluoropolymers with Reduced Chemical and Physical Damage. Appl. Sci. 2021, 11, 10495. [Google Scholar] [CrossRef]
- Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef] [PubMed]
- Ghorbani-Dalini, S.; Azarpira, N.; Sangtarash, M.H.; Urbach, V.; Yaghobi, R.; Soleimanpour-Lichaei, H.R.; Sarshar, M. Optimization of 3D islet-like cluster derived from human pluripotent stem cells: An efficient in vitro differentiation protocol. Gene 2022, 845, 146855. [Google Scholar] [CrossRef] [PubMed]
- Ng, W.H.; Johnston, E.K.; Tan, J.J.; Bliley, J.M.; Feinberg, A.W.; Stolz, D.B.; Sun, M.; Wijesekara, P.; Hawkins, F.; Kotton, D.N.; et al. Co-differentiation and Co-maturation of Human Cardio-pulmonary Progenitors and Micro-Tissues from Human Induced Pluripotent Stem Cells. Elife 2022, 11, e67872. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Nakatsuji, N.; Suemori, H. Endodermal differentiation of human pluripotent stem cells to insulin-producing cells in 3D culture. Sci. Rep. 2014, 4, 4488. [Google Scholar] [CrossRef] [Green Version]
- Vinard, E.; Leseche, G.; Andreassian, B.; Costagliola, D. In vitro endothelialization of PTFE vascular grafts: A comparison of various substrates, cell densities, and incubation times. Ann. Vasc. Surg. 1999, 13, 141–150. [Google Scholar] [CrossRef]
- Wang, C.; Chen, J.-R. Studies on surface graft polymerization of acrylic acid onto PTFE film by remote argon plasma initiation. Appl. Surf. Sci. 2007, 253, 4599–4606. [Google Scholar] [CrossRef]
- Choi, Y.J.; Kim, M.S.; Noh, I. Surface modification of a polytetrafluoroethylene film with cyclotron ion beams and its evaluation. Surf. Coat. Technol. 2007, 201, 5724–5728. [Google Scholar] [CrossRef]
- Kitamura, A.; Satoh, T.; Koka, M.; Kobayashi, T.; Kamiya, T. Fabrication of micro-prominences on PTFE surface using proton beam writing. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2013, 306, 288–291. [Google Scholar] [CrossRef]
- Falah Toosi, S.; Moradi, S.; Kamal, S.; Hatzikiriakos, S.G. Superhydrophobic laser ablated PTFE substrates. Appl. Surf. Sci. 2015, 349, 715–723. [Google Scholar] [CrossRef]
- Riveiro, A.; Abalde, T.; Pou, P.; Soto, R.; del Val, J.; Comesaña, R.; Badaoui, A.; Boutinguiza, M.; Pou, J. Influence of laser texturing on the wettability of PTFE. Appl. Surf. Sci. 2020, 515, 145984. [Google Scholar] [CrossRef]
- Baquey, C.; Palumbo, F.; Porte-Durrieu, M.C.; Legeay, G.; Tressaud, A.; d’Agostino, R. Plasma treatment of expanded PTFE offers a way to a biofunctionalization of its surface. Nucl. Instrum. Methods Phys. Res. B 1999, 151, 255–262. [Google Scholar] [CrossRef]
- Song, H.; Yu, H.; Zhu, L.; Xue, L.; Wu, D.; Chen, H. Durable hydrophilic surface modification for PTFE hollow fiber membranes. React. Funct. Polym. 2017, 114, 110–117. [Google Scholar] [CrossRef]
- Rosado, D.; Meléndez-Ortiz, H.I.; Ortega, A.; Gallardo-Vega, C.; Burillo, G. Modification of poly(tetrafluoroethylene) with polyallylamine by gamma radiation. Radiat. Phys. Chem. 2020, 172, 108766. [Google Scholar] [CrossRef]
- Ahad, I.U.; Butruk, B.; Ayele, M.; Budner, B.; Bartnik, A.; Fiedorowicz, H.; Ciach, T.; Brabazon, D. Extreme ultraviolet (EUV) surface modification of polytetrafluoroethylene (PTFE) for control of biocompatibility. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. At. 2015, 364, 98–107. [Google Scholar] [CrossRef] [Green Version]
- Gumpenberger, T.; Heitz, J.; Bäuerle, D.; Kahr, H.; Graz, I.; Romanin, C.; Svorcik, V.; Leisch, F. Adhesion and proliferation of human endothelial cells on photochemically modified polytetrafluoroethylene. Biomaterials 2003, 24, 5139–5144. [Google Scholar] [CrossRef]
- Pérez-Calixto, M.; Diaz-Rodriguez, P.; Concheiro, A.; Alvarez-Lorenzo, C.; Burillo, G. Amino-functionalized polymers by gamma radiation and their influence on macrophage polarization. React. Funct. Polym. 2020, 151, 104568. [Google Scholar] [CrossRef]
- Nadzir, M.M.; Kino-oka, M.; Sugawara, K.; Taya, M. Effect of preservation conditions of collagen substrate on its fibril formation and rabbit chondrocyte morphology. J. Biosci. Bioeng. 2012, 114, 360–363. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Shi, L.; Deng, Y.; Zou, M.; Cai, B.; Song, Y.; Wang, Z.; Wang, L. Silk sericin-based materials for biomedical applications. Biomaterials 2022, 287, 121638. [Google Scholar] [CrossRef] [PubMed]
- Sonnleitner, D.; Sommer, C.; Scheibel, T.; Lang, G. Approaches to inhibit biofilm formation applying natural and artificial silk-based materials. Mater. Sci. Eng. C 2021, 131, 112458. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, F.; Xu, S.; Wang, R.; Chen, W.; Hou, K.; Tian, C.; Wang, F.; Yu, L.; Lu, Z.; et al. Genetically engineered bi-functional silk material with improved cell proliferation and anti-inflammatory activity for medical application. Acta Biomater. 2019, 86, 148–157. [Google Scholar] [CrossRef] [PubMed]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Togo, H.; Terada, K.; Ujitsugu, A.; Hirose, Y.; Takeuchi, H.; Kusunoki, M. Fabrication Scaffold with High Dimensional Control for Spheroids with Undifferentiated iPS Cell Properties. Cells 2023, 12, 278. https://doi.org/10.3390/cells12020278
Togo H, Terada K, Ujitsugu A, Hirose Y, Takeuchi H, Kusunoki M. Fabrication Scaffold with High Dimensional Control for Spheroids with Undifferentiated iPS Cell Properties. Cells. 2023; 12(2):278. https://doi.org/10.3390/cells12020278
Chicago/Turabian StyleTogo, Hidetaka, Kento Terada, Akira Ujitsugu, Yudai Hirose, Hiroki Takeuchi, and Masanobu Kusunoki. 2023. "Fabrication Scaffold with High Dimensional Control for Spheroids with Undifferentiated iPS Cell Properties" Cells 12, no. 2: 278. https://doi.org/10.3390/cells12020278
APA StyleTogo, H., Terada, K., Ujitsugu, A., Hirose, Y., Takeuchi, H., & Kusunoki, M. (2023). Fabrication Scaffold with High Dimensional Control for Spheroids with Undifferentiated iPS Cell Properties. Cells, 12(2), 278. https://doi.org/10.3390/cells12020278




