The Nuclear Transporter Importin 13 Can Regulate Stress-Induced Cell Death through the Clusterin/KU70 Axis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Yeast-Two-Hybrid Screen and Functional Annotation Analyses
2.2. Plasmid Constructs
2.3. Cell Culture and Transfection/Immunofluorescence
2.4. Protein Expression and Purification
2.5. AlphaScreen Importin-Binding Assay
2.6. Confocal Laser Scanning Microscopy (CLSM) and Image Analysis
2.7. Fluorescence Recovery after Photobleaching (FRAP) Approach to Analyse Nuclear Transport
2.8. Co-Immunoprecipitation and Western Analysis
2.9. In-Gel Neutral Comet Assay
2.10. Flow Cytometry
2.11. Statistical Analysis
3. Results
3.1. The IPO13 Interactome
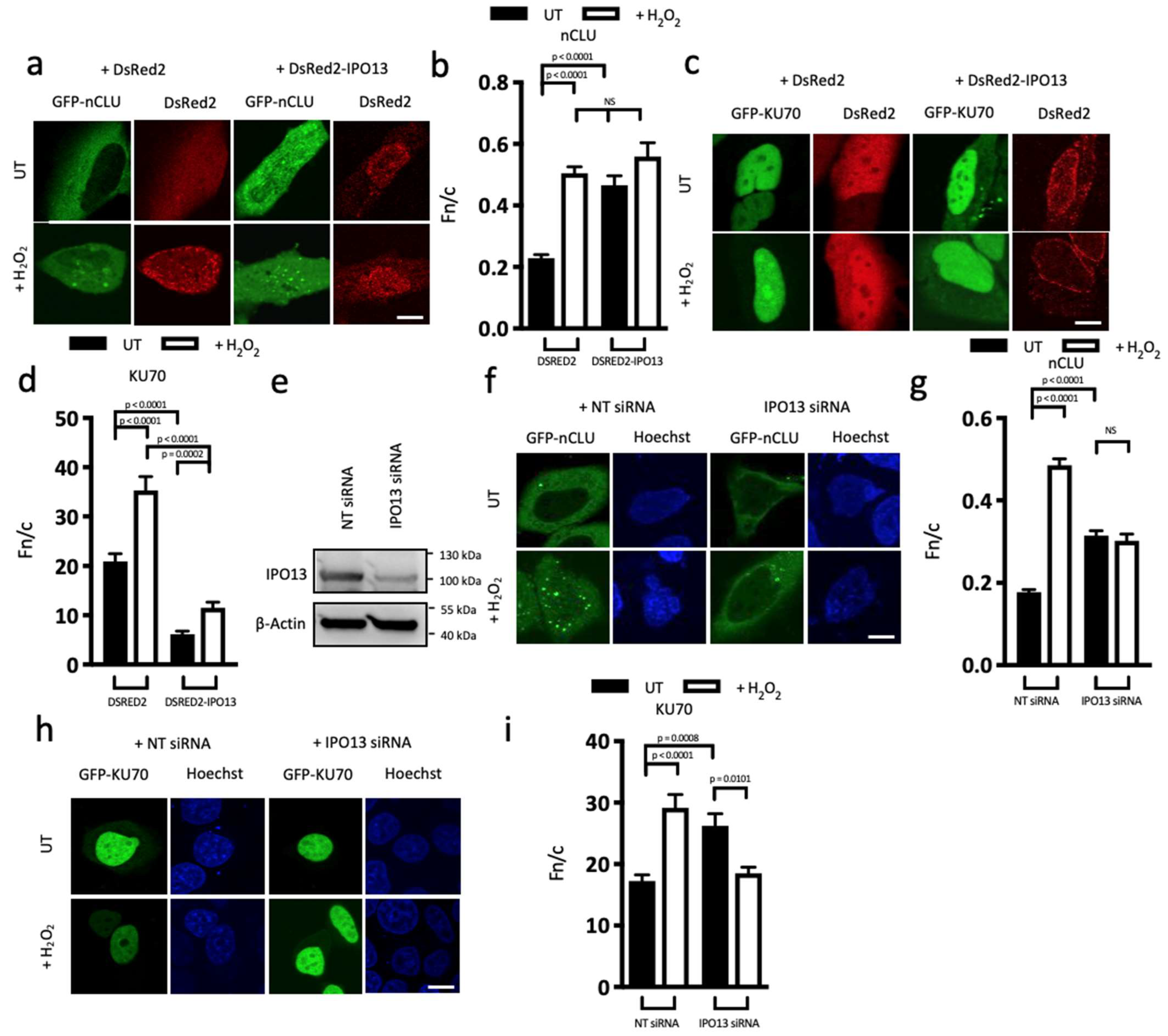
3.2. IPO13 Acts as a Nuclear Import Factor for nCLU and an Export Factor for KU70
3.3. FRAP Analysis Reveals IPO13 Facilitates Nuclear Import of nCLU under Stress Conditions and Export of KU70 under Steady State Conditions Which Are Downregulated by Stress
3.4. IPO13 Is a Key Regulator of DNA Repair
3.5. IPO13 Is a Key Regulator of Apoptosis
3.6. IPO13 Traffics Efficiently under Cellular Stress
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fried, H.; Kutay, U. Nucleocytoplasmic transport: Taking an inventory. Cell. Mol. Life Sci. 2003, 60, 1659–1688. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, K. Regulating Access to the Genome: Nucleocytoplasmic Transport throughout the Cell Cycle. Cell 2003, 112, 441–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldfarb, D.S.; Corbett, A.H.; Mason, D.A.; Harreman, M.T.; Adam, S.A. Importin α: A multipurpose nuclear-transport receptor. Trends Cell Biol. 2004, 14, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Pemberton, L.F.; Paschal, B.M. Mechanisms of Receptor-Mediated Nuclear Import and Nuclear Export. Traffic 2005, 6, 187–198. [Google Scholar] [CrossRef] [PubMed]
- Terry, L.J.; Shows, E.B.; Wente, S.R. Crossing the Nuclear Envelope: Hierarchical Regulation of Nucleocytoplasmic Transport. Science 2007, 318, 1412–1416. [Google Scholar] [CrossRef]
- Wagstaff, K.; Jans, D. Importins and Beyond: Non-Conventional Nuclear Transport Mechanisms. Traffic 2009, 10, 1188–1198. [Google Scholar] [CrossRef]
- Kodiha, M.; Stochaj, U. Nuclear Transport: A Switch for the Oxidative Stress—Signaling Circuit? J. Signal Transduct. 2011, 2012, 208650. [Google Scholar] [CrossRef] [Green Version]
- Himanen, S.V.; Sistonen, L. New insights into transcriptional reprogramming during cellular stress. J. Cell Sci. 2019, 132, jcs238402. [Google Scholar] [CrossRef]
- Stochaj, U.; Rassadi, R.; Chiu, J. Stress-mediated inhibition of the classical nuclear protein import pathway and nuclear accumulation of the small GTPase Gsp1p. FASEB J. 2000, 14, 2130–2132. [Google Scholar] [CrossRef]
- Miyamoto, Y.; Saiwaki, T.; Yamashita, J.; Yasuda, Y.; Kotera, I.; Shibata, S.; Shigeta, M.; Hiraoka, Y.; Haraguchi, T.; Yoneda, Y. Cellular stresses induce the nuclear accumulation of importin α and cause a conventional nuclear import block. J. Cell Biol. 2004, 165, 617–623. [Google Scholar] [CrossRef]
- Kodiha, M.; Chu, A.; Matusiewicz, N.; Stochaj, U. Multiple mechanisms promote the inhibition of classical nuclear import upon exposure to severe oxidative stress. Cell Death Differ. 2004, 11, 862–874. [Google Scholar] [CrossRef]
- Kelley, J.B.; Paschal, B.M. Hyperosmotic Stress Signaling to the Nucleus Disrupts the Ran Gradient and the Production of RanGTP. Mol. Biol. Cell 2007, 18, 4365–4376. [Google Scholar] [CrossRef] [Green Version]
- Kodiha, M.; Bański, P.; Ho-Wo-Cheong, D.; Stochaj, U. Dissection of the molecular mechanisms that control the nuclear accumulation of transport factors importin-α and CAS in stressed cells. Cell. Mol. Life Sci. 2008, 65, 1756–1767. [Google Scholar] [CrossRef]
- Chang, W.-L.; Tarn, W.-Y. A role for transportin in deposition of TTP to cytoplasmic RNA granules and mRNA decay. Nucleic Acids Res. 2009, 37, 6600–6612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahboubi, H.; Seganathy, E.; Kong, D.; Stochaj, U. Identification of Novel Stress Granule Components That Are Involved in Nuclear Transport. PLoS ONE 2013, 8, e68356. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.H.; Jans, D.A.; Bogoyevitch, M.A. Hyperosmotic stress sustains cytokine-stimulated phosphorylation of STAT3, but slows its nuclear trafficking and impairs STAT3-dependent transcription. Cell. Signal. 2014, 26, 815–824. [Google Scholar] [CrossRef] [PubMed]
- Ng, I.H.; Yeap, Y.Y.; Ong, L.S.; Jans, D.A.; Bogoyevitch, M.A. Oxidative stress impairs multiple regulatory events to drive persistent cytokine-stimulated STAT3 phosphorylation. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2014, 1843, 483–494. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ogawa, Y.; Imamoto, N. Nuclear transport adapts to varying heat stress in a multistep mechanism. J. Cell Biol. 2018, 217, 2341–2352. [Google Scholar] [CrossRef] [Green Version]
- Crampton, N.; Kodiha, M.; Shrivastava, S.; Umar, R.; Stochaj, U. Oxidative Stress Inhibits Nuclear Protein Export by Multiple Mechanisms That Target FG Nucleoporins and Crm1. Mol. Biol. Cell 2009, 20, 5106–5116. [Google Scholar] [CrossRef] [PubMed]
- Mingot, J.; Kostka, S.; Kraft, R.; Hartmann, E.; Görlich, D. Importin 13: A novel mediator of nuclear import and export. EMBO J. 2001, 20, 3685–3694. [Google Scholar] [CrossRef]
- Grünwald, M.; Lazzaretti, D.; Bono, F. Structural basis for the nuclear export activity of Importin13. EMBO J. 2013, 32, 899–913. [Google Scholar] [CrossRef] [Green Version]
- Poon, I.; Jans, D. Regulation of Nuclear Transport: Central Role in Development and Transformation? Traffic 2005, 6, 173–186. [Google Scholar] [CrossRef]
- Wente, S.R.; Rout, M.P. The Nuclear Pore Complex and Nuclear Transport. Cold Spring Harb. Perspect. Biol. 2010, 2, a000562. [Google Scholar] [CrossRef]
- Grünwald, M.; Bono, F. Structure of Importin13-Ubc9 complex: Nuclear import and release of a key regulator of sumoylation. EMBO J. 2010, 30, 427–438. [Google Scholar] [CrossRef] [PubMed]
- Ploski, J.E.; Shamsher, M.K.; Radu, A. Paired-Type Homeodomain Transcription Factors Are Imported into the Nucleus by Karyopherin 13. Mol. Cell. Biol. 2004, 24, 4824–4834. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoubridge, C.; Tan, M.H.; Fullston, T.; Cloosterman, D.; Coman, D.; McGillivray, G.; Mancini, G.M.; Kleefstra, T.; Gecz, J. Mutations in the nuclear localization sequence of the Aristaless related homeobox; sequestration of mutant ARX with IPO13 disrupts normal subcellular distribution of the transcription factor and retards cell division. PathoGenetics 2010, 3, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tao, T.; Lan, J.; Lukacs, G.L.; Haché, R.J.G.; Kaplan, F. Importin 13 Regulates Nuclear Import of the Glucocorticoid Receptor in Airway Epithelial Cells. Am. J. Respir. Cell Mol. Biol. 2006, 35, 668–680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatima, S.; Wagstaff, K.M.; Lieu, K.G.; Davies, R.G.; Tanaka, S.S.; Yamaguchi, Y.L.; Loveland, K.L.; Tam, P.P.; Jans, D.A. Interactome of the inhibitory isoform of the nuclear transporter Importin 13. Biochim. Biophys. Acta (BBA)—Mol. Cell Res. 2017, 1864, 546–561. [Google Scholar] [CrossRef]
- Gajewska, K.A.; Lescesen, H.; Ramialison, M.; Wagstaff, K.M.; Jans, D.A. Nuclear transporter Importin-13 plays a key role in the oxidative stress transcriptional response. Nat. Commun. 2021, 12, 5904. [Google Scholar] [CrossRef]
- Yamaguchi, Y.L.; Tanaka, S.S.; Yasuda, K.; Matsui, Y.; Tam, P.P. Stage-specific Importin13 activity influences meiosis of germ cells in the mouse. Dev. Biol. 2006, 297, 350–360. [Google Scholar] [CrossRef]
- You, P.; Peng, Z.; Wang, Y.; Tao, T. Expression and subcellular distribution of imp13 are regulated in brain development. Vitr. Cell. Dev. Biol.—Anim. 2013, 49, 346–353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gajewska, K.A.; Haynes, J.M.; Jans, D.A. Nuclear Transporter IPO13 Is Central to Efficient Neuronal Differentiation. Cells 2022, 11, 1904. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Sweezey, N.B.; Gagnon, S.; Muskat, B.; Koehler, D.; Post, M.; Kaplan, F. A Novel Karyopherin- β Homolog Is Developmentally and Hormonally Regulated in Fetal Lung. Am. J. Respir. Cell Mol. Biol. 2000, 22, 451–459. [Google Scholar] [CrossRef] [PubMed]
- Tao, T.; Lan, J.; Presley, J.F.; Sweezey, N.B.; Kaplan, F. Nucleocytoplasmic Shuttling of lgl2 Is Developmentally Regulated in Fetal Lung. Am. J. Respir. Cell Mol. Biol. 2004, 30, 350–359. [Google Scholar] [CrossRef]
- Gajewska, K.A.; Ramialison, M.; Wagstaff, K.M.; Jans, D.A. Transcriptomic profile dataset of embryonic stem cells (Wild-type and IPO13-Knock Out) with and without oxidative stress. Data Brief 2022, 42, 108099. [Google Scholar] [CrossRef] [PubMed]
- Leskov, K.S.; Klokov, D.Y.; Li, J.; Kinsella, T.J.; Boothman, D.A. Synthesis and Functional Analyses of Nuclear Clusterin, a Cell Death Protein. J. Biol. Chem. 2003, 278, 11590–11600. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.R.; Leskov, K.; Hosley-Eberlein, K.; Criswell, T.; Pink, J.J.; Kinsella, T.J.; Boothman, D.A. Nuclear clusterin/XIP8, an x-ray-induced Ku70-binding protein that signals cell death. Proc. Natl. Acad. Sci. USA 2000, 97, 5907–5912. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, C.-R.; Yeh, S.; Leskov, K.; Odegaard, E.; Hsu, H.-L.; Chang, C.; Kinsella, T.J.; Chen, D.J.; Boothman, D.A. Isolation of Ku70-binding proteins (KUBs). Nucleic Acids Res. 1999, 27, 2165–2174. [Google Scholar] [CrossRef] [Green Version]
- Formstecher, E.; Aresta, S.; Collura, V.; Hamburger, A.; Meil, A.; Trehin, A.; Reverdy, C.; Betin, V.; Maire, S.; Brun, C.; et al. Protein interaction mapping: A Drosophila case study. Genome Res. 2005, 15, 376–384. [Google Scholar] [CrossRef] [Green Version]
- Baliga, B.C.; Colussi, P.A.; Read, S.H.; Dias, M.M.; Jans, D.A.; Kumar, S. Role of Prodomain in Importin-mediated Nuclear Localization and Activation of Caspase-2. J. Biol. Chem. 2003, 278, 4899–4905. [Google Scholar] [CrossRef]
- Liu, X.; Lin, W.; Shi, X.; Davies, R.G.; Wagstaff, K.M.; Tao, T.; Jans, D.A. PKA-site phosphorylation of importin13 regulates its subcellular localization and nuclear transport function. Biochem. J. 2018, 475, 2699–2712. [Google Scholar] [CrossRef]
- Lieu, K.G.; Shim, E.-H.; Wang, J.; Lokareddy, R.K.; Tao, T.; Cingolani, G.; Zambetti, G.P.; Jans, D.A. The p53-induced factor Ei24 inhibits nuclear import through an importin β–binding–like domain. J. Cell Biol. 2014, 205, 301–312. [Google Scholar] [CrossRef] [Green Version]
- Wagstaff, K.; Jans, D. Intramolecular masking of nuclear localization signals: Analysis of importin binding using a novel AlphaScreen-based method. Anal. Biochem. 2006, 348, 49–56. [Google Scholar] [CrossRef]
- Forwood, J.K.; Harley, V.; Jans, D.A. The C-terminal Nuclear Localization Signal of the Sex-determining Region Y (SRY) High Mobility Group Domain Mediates Nuclear Import through Importin β1. J. Biol. Chem. 2001, 276, 46575–46582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hughes, M.L.; Liu, B.; Halls, M.L.; Wagstaff, K.M.; Patil, R.; Velkov, T.; Jans, D.A.; Bunnett, N.W.; Scanlon, M.J.; Porter, C.J. Fatty Acid-binding Proteins 1 and 2 Differentially Modulate the Activation of Peroxisome Proliferator-activated Receptor α in a Ligand-selective Manner. J. Biol. Chem. 2015, 290, 13895–13906. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lazinski, D.W.; Taylor, J.M. Relating structure to function in the hepatitis delta virus antigen. J. Virol. 1993, 67, 2672–2680. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nowsheen, S.; Xia, F.; Yang, E.S. Assaying DNA Damage in Hippocampal Neurons Using the Comet Assay. J. Vis. Exp. 2012, e50049. [Google Scholar] [CrossRef] [Green Version]
- Lorenzo, Y.; Costa, S.; Collins, A.; Azqueta, A. The comet assay, DNA damage, DNA repair and cytotoxicity: Hedgehogs are not always dead. Mutagenesis 2013, 28, 427–432. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat. Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Guo, Z.; Kozlov, S.; Lavin, M.F.; Person, M.D.; Paull, T.T. ATM Activation by Oxidative Stress. Science 2010, 330, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, K.; Wang, M.; Zhao, Y.; Sun, X.; Yang, Y.; Li, X.; Zhou, A.; Chu, H.; Zhou, H.; Xu, J.; et al. A redox mechanism underlying nucleolar stress sensing by nucleophosmin. Nat. Commun. 2016, 7, 13599. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amsel, A.D.; Rathaus, M.; Kronman, N.; Cohen, H.Y. Regulation of the proapoptotic factor Bax by Ku70-dependent deubiquitylation. Proc. Natl. Acad. Sci. USA 2008, 105, 5117–5122. [Google Scholar] [CrossRef] [Green Version]
- Leskov, K.S.; Araki, S.; Lavik, J.-P.; Gomez, J.A.; Gama, V.; Gonos, E.S.; Trougakos, I.P.; Matsuyama, S.; Boothman, D.A. CRM1 Protein-mediated Regulation of Nuclear Clusterin (nCLU), an Ionizing Radiation-stimulated, Bax-dependent Pro-death Factor. J. Biol. Chem. 2011, 286, 40083–40090. [Google Scholar] [CrossRef] [Green Version]
- Davis, A.J.; Chen, D.J. DNA double strand break repair via non-homologous end-joining. Transl. Cancer Res. 2013, 2, 130–143. [Google Scholar] [CrossRef] [PubMed]
- Jun, H.-O.; Kim, D.-H.; Lee, S.-W.; Lee, H.S.; Seo, J.H.; Kim, J.H.; Kim, J.H.; Yu, Y.S.; Min, B.H.; Kim, K.-W. Clusterin protects H9c2 cardiomyocytes from oxidative stress-induced apoptosis via Akt/GSK-3β signaling pathway. Exp. Mol. Med. 2011, 43, 53–61. [Google Scholar] [CrossRef] [Green Version]
- Trougakos, I.P.; Lourda, M.; Antonelou, M.H.; Kletsas, D.; Gorgoulis, V.G.; Papassideri, I.S.; Zou, Y.; Margaritis, L.H.; Boothman, D.A.; Gonos, E.S. Intracellular Clusterin Inhibits Mitochondrial Apoptosis by Suppressing p53-Activating Stress Signals and Stabilizing the Cytosolic Ku70-Bax Protein Complex. Clin. Cancer Res. 2008, 15, 48–59. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Park, S.Y.; Shin, E.; Lee, S.H.; Kim, Y.S.; Lee, N.H.; Roh, G.S.; Kim, H.J.; Kang, S.S.; Cho, G.J.; et al. Hypoxia inducible factor-1α directly regulates nuclear clusterin transcription by interacting with hypoxia response elements in the clusterin promoter. Mol. Cells 2014, 37, 178–186. [Google Scholar] [CrossRef] [Green Version]
- Davies, R.G.; Jans, D.A.; Wagstaff, K.M. Use of fluorescence photobleaching techniques to measure the kinetics of intracellular transport. In Microscopy: Science, Technology, Applications and Education; Formatex: Badajos, Spain, 2010; pp. 756–763. [Google Scholar]
- Jin, S.; Weaver, D.T. Double-strand break repair by Ku70 requires heterodimerization with Ku80 and DNA binding functions. EMBO J. 1997, 16, 6874–6885. [Google Scholar] [CrossRef] [Green Version]
- Mari, P.-O.; Florea, B.I.; Persengiev, S.P.; Verkaik, N.S.; Brüggenwirth, H.T.; Modesti, M.; Giglia-Mari, G.; Bezstarosti, K.; Demmers, J.; Luider, T.M.; et al. Dynamic assembly of end-joining complexes requires interaction between Ku70/80 and XRCC4. Proc. Natl. Acad. Sci. USA 2006, 103, 18597–18602. [Google Scholar] [CrossRef]
- García, C.P.; Videla Richardson, G.A.; Romorini, L.; Miriuka, S.G.; Sevlever, G.E.; Scassa, M.E. Topoisomerase I inhibitor, camptothecin, induces apoptogenic signaling in human embryonic stem cells. Stem Cell Res. 2014, 12, 400–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kose, S.; Furuta, M.; Imamoto, N. Hikeshi, a Nuclear Import Carrier for Hsp70s, Protects Cells from Heat Shock-Induced Nuclear Damage. Cell 2012, 149, 578–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Freedman, N.D.; Yamamoto, K.R. Importin 7 and Importin α/Importin β Are Nuclear Import Receptors for the Glucocorticoid Receptor. Mol. Biol. Cell 2004, 15, 2276–2286. [Google Scholar] [CrossRef]
- Vandevyver, S.; Dejager, L.; Libert, C. On the Trail of the Glucocorticoid Receptor: Into the Nucleus and Back. Traffic 2012, 13, 364–374. [Google Scholar] [CrossRef] [PubMed]
- Hakim, A.; Barnes, P.J.; Adcock, I.M.; Usmani, O.S. Importin-7 mediates glucocorticoid receptor nuclear import and is impaired by oxidative stress, leading to glucocorticoid insensitivity. FASEB J. 2013, 27, 4510–4519. [Google Scholar] [CrossRef]
- Trougakos, I.P.; Djeu, J.Y.; Gonos, E.S.; Boothman, D.A. Advances and Challenges in Basic and Translational Research on Clusterin. Cancer Res 2009, 69, 403–406. [Google Scholar] [CrossRef] [Green Version]
- Fisi, V.; Miseta, A.; Nagy, T. The Role of Stress-Induced O-GlcNAc Protein Modification in the Regulation of Membrane Transport. Oxidative Med. Cell. Longev. 2017, 2017, 1308692. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guinez, C.; Morelle, W.; Michalski, J.-C.; Lefebvre, T. O-GlcNAc glycosylation: A signal for the nuclear transport of cytosolic proteins? Int. J. Biochem. Cell Biol. 2005, 37, 765–774. [Google Scholar] [CrossRef]
- Bonello, S.; Zähringer, C.; BelAiba, R.S.; Djordjevic, T.; Hess, J.; Michiels, C.; Kietzmann, T.; Görlach, A. Reactive Oxygen Species Activate the HIF-1α Promoter Via a Functional NFκB Site. Arterioscler. Thromb. Vasc. Biol. 2007, 27, 755–761. [Google Scholar] [CrossRef] [Green Version]
- O’Sullivan, J.; Whyte, L.; Drake, J.; Tenniswood, M. Alterations in the post-translational modification and intracellular trafficking of clusterin in MCF-7 cells during apoptosis. Cell Death Differ. 2003, 10, 914–927. [Google Scholar] [CrossRef]









| Prey Protein | PBS Score * | SID # | Previously Identified | Subcellular Localisation; Functional Role |
|---|---|---|---|---|
| Zinc Finger AN1-Type-Containing 4 (ZFAND4;ANUBL1) | A (testis) | 28–119 | Cytoplasm/nucleus; contains an AN1-type zinc finger and one ubiquitin-like domain/paralog of ZFAND5, which is involved in proteasome regulation/NFκB regulation | |
| HIRA-Interacting Protein 3 (HIRIP3) | A (lung, testis) | 400–523 | Mackmull et al., 2017 | Nucleus; may function in chromatin and histone metabolism |
| Kinesin Family Member 5C (KIF5C) | A (brain) | 805–931 | Mackmull et al., 2017 | Cytoplasm/nucleus; microtubule motor protein, which may play a role in organelle transport |
| Protein Phosphatase 2 Regulatory Subunit B’Alpha (PPP2R5A) | A (brain, lung, testis) | 143–371 | Nucleus/cytoplasm; regulatory subunit of the phosphatase 2A serine/threonine phosphatase implicated in the negative control of cell growth and division | |
| GLI-Kruppel Zinc Finger Family Member (HKR1) | A (brain, lung, testis) | 237–410 | Nucleus/cytoplasm/Golgi/mitochondria; may be involved in transcriptional regulation | |
| Ribosomal Protein L31 (RPL31) | A (brain, testis) | 22–110 | Baade et al., 2018, Kimura et al., 2017 | Cytoplasm/EC; component of the 60 s ribosomal subunit |
| Ran-Binding Protein 2 (RanBP2; Nup358) | B (brain, lung, testis) | 1164–1489, 2890–3124 | Cytoplasmic filaments of the NPC; E3 ligase, which facilitates sumoylation of certain proteins/nuclear transport. NT. | |
| RANBP2-Like and GRIP Domain-Containing 8 (RGPD8) | B (testis) | 1335–1459 | NPC; may be involved in nuclear transport, particularly of RNA. | |
| RNA Polymerase II Subunit J2 (POLR2J2) | B (lung, testis) | 6–98 | Nucleus; component of the RNA polymerase II transcription machinery | |
| MGA, MAX Dimerization Protein (MGA) | B (brain, lung) | 744–978 | Nucleus/cytoplasm; dual-specificity transcription factor regulating the expression of both MAX-network and T-box family target genes | |
| RANBP2-Like and GRIP Domain-Containing 6 (RGPD6) | B (brain, lung) | 890–1208 | NPC; thought to play roles similar to RanBP2 due to homology/nuclear transport | |
| Zinc Finger Protein 106 (ZNF106) | B (brain, lung) | 145–392 | Cytoplasm/plasma membrane/nucleolus; may play a role in transcriptional regulation | |
| ADP Ribosylation Factor-Like GTPase 6 Interacting Protein 4 (ARL6IP4) | B (brain) | 179–241 | Kimura et al., 2017 | Nucleus/mitochondria; modulates alternative pre-mRNA splicing |
| Ran-Binding Protein 1 (RanBP1) | B (brain) | 1–158 | Mackmull et al., 2017, Baade et al., 2018 | Nucleus/cytoplasm/cytoskeleton; regulates nuclear transport by increasing GTP hydrolysis induced by the Ran GTPase-activating protein RANGAP1. NT. |
| Signal Recognition Particle 68 (SRP68) | B (brain) | 30–289 | Cytoplasm/ER/nucleus/mitochondria; subunit of the signal recognition particle, which transports secreted and membrane proteins to the endoplasmic reticulum for processing | |
| Clusterin (CLU) | B (brain, testis) | 248–348 | Extracellular/mitochondria/cytoplasm/ER/nucleus; suggested to be involved in several basic biological events, such as cell death, tumor progression, and neurodegenerative disorders. NT. | |
| Protein Phosphatase, Mg2+/Mn2+-Dependent 1E (PPM1E) | C (brain) | 557–755 | Nucleus/cytoplasm/mitochondria; serine/threonine phosphatase | |
| Activity-Dependent Neuroprotector Homeobox (ADNP) | D (testis, brain) | 206–500 | Nucleus/cytoskeleton/cytoplasm/EC; upregulated by vasoactive intestinal peptide and may be involved in its stimulatory effect on certain tumor cells/predicted transcription factor | |
| Amyotrophic Lateral Sclerosis 2 Chromosome Region Candidate 11 (ALS2CR11) | D (testis) | 3–337 | Nucleus/cytoplasm; contains a calcium-dependent membrane-targeting C2 domain, often found in proteins involved in membrane trafficking and signal transduction | |
| Chromosome 11 Open Reading Frame 63 (C11orf63) | D (testis) | 175–546 | Nucleus/cytoplasm/Golgi/mitochondria; unknown | |
| KAT8 Regulatory NSL Complex Subunit 1 (KANSL1; KIAA1267) | D (brain, lung, testis) | 514–712 | Nucleus/cytoplasm/cytoskeleton; involved in histone acetylation | |
| LCA5L, Lebercilin-Like (LCA5L) | D (testis) | 14–195 | Nucleus/cytoplasm/cilia; ciliary protein, precise function unknown | |
| Nucleoporin 153 (Nup153) | D (lung, testis) | 182–496 | NE/nucleus/cytoplasm; component of the NPC/contains an RNA-binding domain. NT. | |
| Splicing Factor 3b Subunit 2 (SF3B2) | D (testis) | 492–719 | Mackmull et al., 2017, Baade et al., 2018 | Nucleus/cytoplasm; RNA splicing |
| Zinc Finger Protein 479 (ZNF479) | D (testis) | 1–211 | Nucleus/cytoplasm; may be involved in transcriptional regulation | |
| Ankyrin Repeat and KH Domain-Containing 1 (ANKHD1) | D (lung) | 1549–1999 | Nucleus; scaffolding protein, which may play a role in apoptosis regulation | |
| Histone Deacetylase 9 (HDAC9) | D (lung) | 191–460 | Nucleus; histone deacetylase | |
| Cell-Cycle-Associated Protein 1 (CAPRIN1; M11S1) | D (lung) | 341–694 | Cytoplasm/plasma membrane/nucleus; may regulate the transport and translation of mRNAs involved in cell proliferation and migration | |
| MDS1 And EVI1 Complex Locus (MECOM) | D (lung) | 681–968 | Cytoplasm/nucleus/Golgi/EC; transcriptional regulator and oncoprotein that may be involved in hematopoiesis, apoptosis, development, and cell differentiation and proliferation | |
| Nucleoporin 58 (NUP58; NUPL1) | D (lung) | 33–372 | NPC; component of the NPC. NT. | |
| Proteoglycan 4 (PRG4) | D (lung) | 890–1066 | Extracellular, nucleus, cytoplasm; plays a role in boundary lubrication within articulating joints | |
| Retinoic Acid Receptor Gamma (RARG) | D (lung) | 72–270 | Nucleus; ligand-dependent transcriptional regulator | |
| Regulatory Factor X7 (RFX7; RFXDC2) | D (lung) | 100–475 | Nucleus/cytoplasm; transcription factor | |
| Zinc Finger Protein 280D (ZFN280D; SUHW4) | D (lung) | 52–394 | Nucleus/cytoskeleton/cytoplasm/Golgi; may function as a transcription factor | |
| Zinc Fingers and Homeoboxes 3 (ZHX3) | D (lung) | 292–627 | Mackmull et al., 2017 | Nucleus/mitochondria; may function as a transcriptional repressor in conjunction with the transcription factor NFY-A |
| ArfGAP with RhoGAP Domain, Ankyrin Repeat and PH Domain 2 (ARAP2) | D (brain) | 123–618 | Cytoplasm/nucleus/cytoskeleton; modulates actin cytoskeleton remodelling/plays a role in the regulation of focal adhesion dynamics | |
| ADP Ribosylation Factor 1 (ARF1) | D (brain) | 1–130 | Baade et al., 2018 | Cytoplasm/endosome/extracellular/Golgi/plasma membrane/cytoskeleton/ER/mitochondria/peroxisome/vacuole; ADP-ribosyltransferase involved in protein trafficking among different compartments |
| Coronin 2B (CORO2B) | D (brain) | 186–312 | Cytoskeleton/cytoplasm/nucleus/plasma membrane; may play a role in the reorganization of neuronal actin structure | |
| Filamin B (FLNB) | D (brain) | 2499–2578 | Baade et al., 2018, Kimura et al., 2017 | Cytoskeleton/cytoplasm/EC/plasma membrane/nucleus/Golgi; connects cell membrane constituents to the actin cytoskeleton |
| Ganglioside-Induced Differentiation-Associated Protein 1 (GDAP1) | D (brain) | 57–201 | Mitochondria/nucleus/cytoplasm; may play a role in neuronal development | |
| Human Immunodeficiency Virus Type I Enhancer-Binding Protein 1 (HIVEP1) | D (brain) | 1947–2377 | Mitochondria/nucleus/cytoplasm; transcription factor | |
| Heterogeneous Nuclear Ribonucleoprotein L (HNRPL) | D (brain) | 371–480 | EC/nucleus/cytoplasm; splicing factor | |
| Kinesin Family Member 5A (KIF5A) | D (brain) | 767–953 | Cytoskeleton/cytoplasm/nucleus; microtubule motor protein | |
| Microtubule–Actin Crosslinking Factor 1 (MACF1) | D (brain) | 4485–4695 | Cytoskeleton/Golgi/plasma membrane/cytoplasm; facilitates actin microtubule interactions | |
| Mitochondrial Ribosomal Protein S9 (MRPS9) | D (brain) | 100–396 | Mitochondria/nucleus/cytoplasm; mitochondrial protein synthesis | |
| PLAG1-Like Zinc Finger 1 (PLAGL1) | D (brain) | 1–215 | Golgi/nucleus; transcription factor that functions as a suppressor of cell growth | |
| RAN-Binding Protein 3-Like (RanBP3L) | D (brain) | 273–490 | Nucleus/cytoplasm; intracellular transport. NT. | |
| SH3 Domain-Binding Protein 5 (SH3BP5) | D (brain) | 56–125 | Mitochondria/nucleus/cytoplasm; inhibits the auto- and transphosphorylation activity of Bruton’s tyrosine kinase | |
| Tousled-Like Kinase 1 (TLK1) | D (brain) | 1–305 | Nucleus/cytoplasm; serine/threonine kinase, which may be involved in the regulation of chromatin assembly | |
| Zinc Finger Protein 419 (ZNF419) | D (brain) | 73–320 | Nucleus/cytoplasm; may be involved in transcriptional regulation | |
| Zinc Finger Protein 483 (ZNF483) | D (brain) | 520–681 | Nucleus/cytoplasm; may be involved in transcriptional regulation | |
| CREB/ATF BZIP Transcription Factor (CREBZF) | E (lung) | 183–304 | Nucleus/cytoplasm/mitochondria; transcription factor | |
| X-ray Repair Cross-Complementing 6 (KU70; XRCC6; G22P1) | E (brain, testis) | 477–609 | Baade et al., 2018 | Nucleus/cytoplasm/mitochondria; forms dimer with KU80, together act as the regulatory subunit involved in non-homologous end-joining required for DNA double-strand break repair/DNA helicase/suppression of apoptosis |
| Hes-Related Family BHLH Transcription Factor with YRPW Motif 2 (HEY2) | E (testis) | 18–187 | Nucleus/plasma membrane; transcriptional repressor implicated in cardiovascular development, neurogenesis, and somitogenesis/part of Notch signalling pathway. | |
| Protein Phosphatase 2 Regulatory Subunit B’Epsilon (PPP2R5E) | E (lung, testis) | 108–374 | Baade et al., 2018 | Cytoplasm/nucleus; regulatory subunit of the phosphatase 2A serine/threonine phosphatase implicated in the negative control of cell growth and division |
| Hes-Related Family BHLH Transcription Factor with YRPW Motif 1 (HEY1) | E (brain, lung) | 32–150 | Nucleus/plasma membrane; transcriptional repressor/part of Notch signalling pathway. | |
| High Mobility Group 20A (HMG20A) | E (brain, lung) | 31–198 | Fatima et al., 2017 | Nucleus; transcription factor |
| Zinc-Finger- and BTB Domain-Containing 38 (ZBTB38) | E (lung) | 431–784 | EC/nucleus; zinc-finger-containing transcriptional activator | |
| CXXC Finger Protein 5 (HSPC195) | E (brain) | 135–227 | Nucleus/cytoplasm; retinoid-inducible protein involved in myelopoiesis/required for DNA damage-induced p53 activation/regulates the differentiation of myoblasts into myocytes/negatively regulates cutaneous wound healing | |
| Paired Box 6 (PAX6) | E (brain) | 106–346 | Ploski et al., 2004 | Nucleus/cytoskeleton/cytoplasm/EC; regulator of transcription |
| Tripartite Motif-Containing 26 (TRIM26) | E (brain) | 123–284 | Nucleus/cytoplasm; may have DNA-binding activity | |
| Zinc Finger Protein 133 (ZNF133) | E (brain) | 129–398 | Nucleus/cytoplasm; may be involved in transcriptional regulation as a repressor |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gajewska, K.A.; Jans, D.A.; Wagstaff, K.M. The Nuclear Transporter Importin 13 Can Regulate Stress-Induced Cell Death through the Clusterin/KU70 Axis. Cells 2023, 12, 279. https://doi.org/10.3390/cells12020279
Gajewska KA, Jans DA, Wagstaff KM. The Nuclear Transporter Importin 13 Can Regulate Stress-Induced Cell Death through the Clusterin/KU70 Axis. Cells. 2023; 12(2):279. https://doi.org/10.3390/cells12020279
Chicago/Turabian StyleGajewska, Katarzyna A., David A. Jans, and Kylie M. Wagstaff. 2023. "The Nuclear Transporter Importin 13 Can Regulate Stress-Induced Cell Death through the Clusterin/KU70 Axis" Cells 12, no. 2: 279. https://doi.org/10.3390/cells12020279





